Abstract
Deregulated mucin expression is a hallmark of several inflammatory and malignant pathologies. Emerging evidence suggests that, apart from biomarkers, these deregulated mucins are functional contributors to pathogenesis in inflammation and cancer. Both overexpression and downregulation of mucins in various organ systems is associated with pathobiology of inflammation and cancer. Restoration of mucin homeostasis has become an important goal for therapy and management of such disorders and has fueled the quest for selective mucomodulators. With improved understanding of mucin regulation and mechanistic insights into their pathobiological roles, there is optimism to find selective non-toxic agents capable of modulating mucin expression and function. Recently, natural compounds derived from dietary sources have drawn attention due to their anti-inflammatory and anti-oxidant properties and low toxicity. Considerable efforts have been directed towards evaluating dietary natural products as chemopreventive and therapeutic agents; identification, characterization and synthesis of their active compounds; and improving their delivery and bioavailability. We describe the current understanding of mucin regulation, rationale for targeting mucins with natural products and discuss some natural products that modulate mucin expression and functions. We further discuss the approaches and parameters that should guide future research to identify and evaluate selective natural mucomodulators for therapy.
Keywords: Mucins, Inflammation, cancer, natural compounds, Curcumin, Guggulsterone, Apigenin, Glycyrrhizin, Thymoquinone, Silibinin, Scutellarin, Graviola, Quercetin, Ursolic acid, Resveratrol
Introduction
Mucins are high molecular weight glycoproteins primarily expressed by epithelial cells on apical surfaces for lubricating and protecting the epithelia of ducts and body lumens against harmful exogenous and endogenous agents like bacteria, drugs, toxins, digestive enzymes and acids [1–3]. In addition, they are also involved in nutrient and cofactor adsorption in the gut, gaseous exchange in the lungs, transparency at the ocular surface and chemical sensing [3]. Under physiological conditions, mucins mediate diverse biological functions like cell-cell adhesion, renewal and differentiation of the epithelium, inflammation and immune responses. Somewhat paradoxically, for what evolved as a protective mechanism for epithelial cells under normal physiological conditions, aberrant and deregulated expression of mucins in epithelial malignancies contributes to tumorigenesis and metastasis. These mucins either by physical interactions or by regulating signaling cascades, promote malignant transformation, cancer cell growth, cell invasiveness, metastasis, decreased immune surveillance and drug resistance [4–6]. Further, due to aberrant glycosylation in malignancies, mucins mediate cancer cell interactions with leukocytes, endothelial cells and platelets present in the tumor microenvironment during metastasis [3]. Owing to these above mentioned attributes, mucins have emerged as attractive targets for therapy and diagnosis [7].
Based on their structure, mucins are classified into transmembrane/membrane-bound (MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC16 and MUC17) and secreted/gel-forming mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC19) [8]. The bulk of membrane bound mucins is extracellular and comprised of several unique domains that modulate various biological properties by selective interactions with various ligands, cell-surface proteins and the components of extracellular matrix [8]. The C-terminal cytoplasmic tails of mucins contain several phosphorylation sites and are believed to be involved in signal transduction by serving as docking sites for scaffolding proteins. Secreted mucins on the other hand lack hydrophobic transmembrane domain and on secretion form mucus layer on the apical surfaces of healthy epithelial cells particularly in the aerodigestive and genitourinary system that are exposed to the external environmental stresses.
The promise of mucins as therapeutic targets can be appreciated by the ongoing clinical studies and has been described by us in previously published articles [7, 9]. Most mucin-targeted therapies have relied on the overexpression of mucins for eradication of cancer cells. These include immunotherapy to target epitopes presented by aberrantly expressed mucins on cancer cells, radioimmunotherapy to deliver cytotoxic radionuclides, or targeted therapy using anti-mucin antibodies or aptamers for delivering drugs toxins and nanoparticles specifically to mucin overexpressing cancer cells [2, 3]. Most of the current mucin-based therapeutic approaches have focused on MUC1, MUC2, MUC4, MUC5AC and MUC16, and target single mucin at a time [7, 9]. Since mucins are emerging as functional contributors to the pathobiology of inflammation and cancer and multiple mucins are expressed by tumor cells, therapeutic approaches based on comprehensive understanding of coordinated mucin regulation during inflammation and cancer can have significant impact. Therefore, it seems logical to use agents that can target multiple mucins simultaneously for more pronounced anti-cancer effects. In this context, development of novel small molecule inhibitors and natural product based formulations may result in simultaneous modulation of various mucins and mucin mediated diverse signaling pathways.
Due to their structural diversity, natural products derived from medicinal plants and microorganisms provide “privileged scaffolds” [10] and have thus been significant contributors to drug development. Some of the earliest natural product-based drugs including actinomycin, anthracycline, taxol, campthothecin and vinca alkaloids were developed and approved between 1964 to 1997. Then followed a decade of lull from 1997–2007, when no new natural product based anti-cancer drug was approved, due to the success of the genome project that shifted the focus towards targeted therapies like antibodies that generally inhibit signaling pathways by targeting a single gene product like EGFR, HER-2, VEGF. However, the existence of redundant signaling pathways and adaptive mechanisms leading to resistance, in combination with high cost and limited benefit of such targeted therapies, have shifted the focus back on natural products for anti-cancer drugs. Since 2007, several natural product derivatives including rapamycin, vinflunine, trabecedine, carfilzomib have been approved and marketed for the treatment of various malignancies (Reviewed in [11]). Recently, natural compounds derived from dietary sources like spices, fruits, vegetables and beverages have generated interest as chemopreventive agents due to their anti-oxidative and anti-inflammatory effects. Numerous dietary active compounds including curcumin, genistein, and resveratrol have been identified, characterized and evaluated for anti-inflammatory and anti-cancer effects in preclinical and clinical studies (reviewed in [12]) and have been demonstrated to modulate signaling pathways that are implicated in mucin dysregulation. Importantly, several of these compounds have recently been shown to modulate mucin expression, secretion or function in vitro and in vivo in models of inflammation and cancer.
In this review article, we provide a brief overview of the functional implications of mucins in epithelial malignancies, discuss the interplay of mucins with inflammation, and describe current understanding of mucin regulation, with a goal to define the rationale for targeting mucins with natural products. Subsequently, recent studies of natural products that modulate mucin expression and function are described. We further discuss the strategies and considerations for future research to identify and evaluate natural product derivatives as selective mucomodulators for mucin-targeted therapies.
Pathobiological implications of mucins
Deregulated expression and aberrant glycosylation of mucins is a prominent characteristic of inflammatory diseases and malignancies and contributes to disease progression and pathogenesis [2, 3]. MUC1 and MUC4 are the two most studied membrane associated mucins. Both have many unique domains, which enhance or inhibit various signaling pathways involved in cellular proliferation and cell death [13]. Both MUC1 and MUC4 physically interact with and stabilize ErbB family of growth factor receptor tyrosine kinases (RTKs) and potentiate ErbB-dependent signal transduction including extracellular signal regulated kinases (ERK1/2), MAPK and attenuate genotoxic stress induced apoptosis [4, 14]. ErbB family members particularly Her2 mediated activation of downstream mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K)/Akt and c-Src/FAK family kinase pathways regulate cell proliferation and metastasis [15]. Previous studies in breast, ovarian and pancreatic cancer (PC) have established that the effects of MUC4 on these processes are mediated by PI3K/Akt, ERK1/2 and Src/FAK signaling pathways [16]. Both MUC1 and MUC4 suppress apoptosis through the regulation of various pathways. MUC4 mediated phosphorylation of Bad results in its interaction with 14-3-3ζ and its sequestration in the cytoplasm away from mitochondria leading to its anti-apoptotic effects [17, 18]. Anti-apoptotic effects of MUC1 are mediated by phosphorylation and subsequent degradation of IκB leading to constitutive activation of nuclear factor-κB (NF-κB) [19].
CA125/MUC16 mucin is overexpressed in the majority of serous ovarian carcinomas but not in normal ovarian epithelium [20]. Although little is known about the signaling pathways regulated by CA125/MUC16, recently it was shown to modulate epidermal growth factor receptor (EGFR) and its downstream targets Akt and ERK1/2 to promote metastasis via enhanced cell motility and epithelial to mesenchymal transition [21]. In addition, cytoplasmic domain of MUC16 is involved in cytoskeleton reorganization through its interaction with ezrin/radixin/moesin proteins [22]. We have recently reported that MUC16 physically interacts with ERM domain-containing Jak2 protein and activates STAT3 and c-jun signaling to promote proliferation of breast cancer cells [23]. Akita K et al. have recently reported association and phosphorylation of cytoplasmic MUC16 (Tyr-22142) by SFKs such as c-Src and c-Yes [24].
MUC2 and MUC5AC are the most well-studied secretory gel-forming mucins which impart the characteristic viscoelastic property to mucus [25]. Normally, MUC5AC is produced by goblet cells [26], but is deregulated in many cancers [27]. Its overexpression is associated with early post-operative metastasis in non-small cell lung cancer patients [28–30], and worse prognosis for lung adenocarcinoma [29], cholangiocarcinoma [31, 32] and PC patients [27]. Although MUC5AC-mediated molecular mechanisms are unknown, it has been shown to promote cell invasion and migration of human PC and colon cancer cells [33, 34] by regulating α3, α9 and β3 integrins, VEGF and MMP-3 expression [35]. Interestingly, similar to other mucins, MUC5AC knockdown was associated with decreased ERK1/2 signaling as evidenced by decreased pERK1/2 expression.
MUC2 is predominantly expressed in the colon and forms a two-layered mucus system, with an inner dense and attached mucus layer adherent to intestinal epithelial cells (IECs) that is impervious to bacteria and an outer loose and unattached mucus inhabited by the microbiota [36]. MUC2 enhances gut homeostasis and oral tolerance by conditioning dendritic cells (DCs) and intestinal epithelial cells (IECs) [37]. However its deregulated expression has been associated with colorectal cancer development [38]. As opposed to other mucins, inhibition of MUC2 expression in LS174 colon cancer cells was associated with increased cell proliferation and invasion/metastasis both in vitro and in vivo [38], suggesting its protective role in colon cancer. MUC2 also plays a protective role in the intestinal epithelium and MUC2 loss is generally associated with inflammatory bowel disease [39]. Besides this, MUC2 overexpression has been observed in many lung adenocarcinoma cell lines and its expression correlates with the development of some types of lung cancer [40].
In addition to their functional roles in tumorigenesis and metastasis, various mucins contribute to resistance to chemotherapy [6]. Several studies have reported a positive correlation between mucin expression and resistance to chemotherapeutic agents like 5-fluorouracil (5-FU) or methotrexate [41–43]. We and others have demonstrated that overexpression of MUC4 is associated with resistance to several chemotherapeutic agents [17, 44, 45] and downregulation of MUC4 results in chemosensitization of PC cells. Wissniowski et al have also shown that downregulation of mucins, particularly MUC4, sensitizes PC cells to bortezomib and gemcitabine [46]. Similarly, MUC1 overexpression in breast cancer cells has been reported to induce Herceptin®, paclitaxel (Taxol®), doxorubicin and cyclophosphamide resistance [47]. Silencing the MUC1 or MUC4 gene reverses resistance to trastuzumab in HER2/ErbB2-positive gastric cancers [48, 49]. Thus, therapeutic strategies targeting mucins can not only inhibit mucin-mediated tumor progression, but can also result in the sensitization of tumor cells to other chemotherapeutic agents.
Mucins and inflammatory network in tumor microenvironment
Chronic inflammation is considered to be precursor to malignant transformation and contributes to tumor growth and progression. The presence of inflammatory elements including infiltrating immune cells and cytokines and growth factors in the preneoplastic and early neoplastic lesions in various malignancies underscore the central involvement of inflammation in carcinogenesis [50]. Overexpression of chemotactic factors by tumor cells recruit hematopoietic cells such as lymphocytes, monocytes (macrophages), and neutrophils into the tumor microenvironment [51]. Activation of several transcription factors like NF-κB, HIF-1β, and STAT3 [52] during neoplastic transformation induces expression of pro-inflammatory cytokines, chemokines, prostaglandins and nitric oxide in both tumor and stromal cells and create a tumor promoting inflammatory microenvironment [53]. These inflammatory mediators function individually or cumulatively to trigger and sustain chronic inflammation, promote proliferation of tumor and stromal cells and inhibit immunosurveillance [51]. Several inflammation and hypoxia associated factors result in mucin deregulation in a variety of inflammatory and malignant diseases [13]. In turn, these mucins facilitate oncogenic signaling promoting tumor cell proliferation, EMT and metastasis and contribute to the formation of tumor microenvironment (TME) by recruiting and/or interacting with stromal compartments including stromal cells, extra cellular matrix (ECM) and several growth factors (reviewed in [3]). The nature of the cytokines in the milieu, the degree and duration of inflammation determines the overall mucin expression and therefore the extent and type of mucin dependent oncogenic cell signaling and immunomodulation [51].
Previous studies have shown that a large array of pro-inflammatory cytokines and interleukins like IL-4, IL-9, IL-13 and tumor necrosis factor-α (TNF-α) secreted by lymphocytes during injury or infection results in aberrant mucin expression [54, 55]. Our studies and others have shown that MUC4 is transcriptionally regulated by inflammatory cytokines like interferon-γ (IFN-γ) [56] whereas MUC1/MUC5AC is regulated by neutrophil elastase respectively [57], a neutrophil secreted serine protease during inflammation [58]. MUC1 expression is also induced by inflammatory cytokines, including TNFα, IFN-γ and IL-6 [59]. Anti-inflammatory cytokine interleukin-10−/− (Il-10−/−) deficient mice showed defective colonic MUC2 synthesis leading to colonic inflammation and ulcerative colitis [60, 61]. Inflammatory cytokines like IL-13, IL-1β and TNFα have been shown to induce secretion and expression of MUC5AC and orchestrate respiratory mucus hypersecretion [62, 63]. In addition, TNF-α, IL-1β, IL-6 and IL-8 upregulate major gel-forming MUC19 transcript in the human middle ear [64]. Not only expression, mucin glycosylation patterns are also altered in response to pro-inflammatory conditions in cancer cells [55].
While inflammatory cytokines contribute to mucin deregulation during carcinogenesis, studies have also documented mucin-mediated deregulation of various cytokines and transcription factors during inflammation induced cancer progression. Yokoigawa et al., demonstrated that mucins secreted by the cancer cells induce IL-6 and PEG2 expression from peripheral blood monocytes/macrophages through activation of orphan receptor [65]. MUC1-CT has been implicated in the activation of NF-κB family member RelA by direct interaction with nuclear factor-κB kinase-β (IKKβ) [59, 65] thereby increasing the expression of IL-2, IFN-γ, and TNF-α [65, 66]. Transgenic mice expressing human MUC1-CT specifically in the pancreas, exhibit overexpression of COX-2 that converts arachidonic acid to prostaglandin (PG) which is a strong immune modulator and immune suppressor [67]. It is becoming increasingly evident that the intricate association between mucins, inflammation and tumor microenvironment contribute significantly to tumor development and progression. Therefore, targeting this intricate network can provide novel avenues for cancer prevention and therapy.
Regulation of mucin expression
Normal tissue homeostasis requires tight regulation of mucin expression. Biologically diverse molecules including cytokines, growth factors and transcription factors regulate mucin expression in a defined tissue, time and developmental state specific manner [13, 56]. However, several environmental and/or intrinsic insults compromise this regulation leading to their aberrant expression resulting in diverse inflammatory and pathological disorders including cancer [2, 3]. Therefore, it is critical to understand the molecular mechanisms of mucin deregulation for devising appropriate therapeutic modalities to combat mucin associated pathologies.
The gene promoters play a very critical role in transcriptional regulation. MUC1 overexpression is considered to contribute to several epithelial malignancies and its transcriptional regulation has extensively been studied [54, 55]. The MUC1 promoter is GC-rich and contains numerous binding sites for specificity protein-1 (Sp1), activator protein-1 (AP-1), AP-2, AP-3, nuclear factor-1 (NF-1), estrogen receptor (ER) and signal transducers and activators of transcription (STAT) transcription factors [68]. In human lung adenocarcinoma cells (A549) pro-inflammatory cytokines like TNF-α stimulate de novo expression of MUC1 through mitogen-activated protein kinase kinase (MAPKK/MAP2K), extracellular signal-regulated kinase (ERK1/2)/SP1 and NF-κB pathway [69]. In addition, MUC1 promoter also contains four STAT binding cis-elements which are downstream of interferon signaling pathway [70]. IFN-γ and IL-6 activate STAT-1 and STAT-3 transcription factors respectively, which interact with their cis-elements on MUC1 promoter to upregulate its expression in tumor cells. In addition, binding of Hypoxia Inducible Factor (HIF)-1α to HIF responsive elements (HRE) at -1488/-1485 and at -1510/-1507 of MUC1 promoter upregulates its expression.
MUC4 promoter is 3.7 kb long and includes four transcriptional start sites, one in proximal promoter (TATA-less) at -199 and three at the distal promoter at -2603, 2604 and -2605 with TATA a box located at (-2672/-2668) from translational start site [70]. While the proximal promoter has potential binding sites for SP1, CACCC box, glucocorticoid receptor element, AP-1, polyomavirus enhancer activator-3 (PEA3) and Med-1; distal promoter has binding sites for Sp1, AP-1, AP-4, GATA and cyclic adenosine monophosphate responsive element binding protein (CREB) transcription factors [70]. MUC4 promoter also contains numerous putative binding sites for transcription factors downstream of protein kinase A (PKA), PKC, cAMP signaling pathways [70]. Inflammatory factors like IFN-γ and TNF-α upregulate MUC4 expression by activating Jak/STAT1 and NF-κB pathways [71]. In addition, interleukins like IL4/IL9 and IL6 through Jak/STAT pathway regulate MUC4 expression in normal airways [72]. Besides IFN-γ and TNF-α, TGF-β in cooperation with SMAD2 or SMAD4 transcription factors upregulates MUC4 expression [73]. Interestingly, all-trans-retinoic acid (RA) treatment has also been shown to increase expression of TGF-β2 to increase MUC4 expression [74]. IFN-γ and RA synergy has been observed to induce MUC4 expression in PC cells [75] and gastric cancer respectively [76]. Besides, MUC4 expression in intestinal cells are also regulated by hepatocyte nuclear factors (HNF-1/-4), forkhead box A FOXA1/A2), GATA-4/-5/-6 and caudal-related homeobox (CDX-1/-2) TFs [77]. Bile acids through PI3K signaling activate HNF1-α transcription factor to upregulate MUC4 expression in esophageal cancer [78].
Similarly, secretory mucin MUC5AC that is known to be overexpressed during airway inflammatory conditions, inflammatory diseases of the colon, different malignancies such as PC, lung cancer and colorectal cancer is extensively regulated by inflammatory cytokines (e.g., TNF-α, IL-1β, IL-4, IL-6, IL-9, IL-13, IL-17), bacterial products (LPS, LTA, peptidoglycans, flagellin), growth factors (EGF, TGF-α, RA, thyroid hormones), proteases (neutrophil elastase), environmental pollutants (cigarette smoke extracts, acrolein, ozone) and viral mediators (respiratory syncytial virus, rhinovirus) [79]. Transforming growth factor-α (TGF-α)-Epidermal growth factor receptor (EGFR) signaling pathway are being implicated in regulating MUC5AC over expression in many diseases including pulmonary mucoepidermoid carcinoma [40].
Overall, looking at the simultaneous expression of different mucins in various tissues and their co-stimulation by certain reagents along with extensive analysis of individual MUC gene promoters suggests common regulatory mechanisms for various mucins. This includes the inflammatory mediators such as cytokines IL-1β, IL-4, IL-6, IL-9, IL-12, IL-13, TNF-α, EGF, TGF-α and functional binding sites for transcription factors induced during inflammatory conditions such as NF-κB, STAT-1, STAT-3, STAT-6, Sp1, Sp3, CREB, AP-1, c-Myc, n-Myc and SMADs. Thus, knowledge of mucin regulation and identification of common regulators of mucin expression can be utilized to repress or control their expression through products that have effects on these pathways and factors as an effective therapeutic strategy in many pathological conditions.
Natural products modulating mucin expression: potential therapeutic agents
Fruits, vegetables, spices and beverage like tea contain a heterogeneous class of molecules called phytochemicals such as vitamins (carotenoids) and polyphenols that include flavonoids, phytoalexins, phenolic acids indoles and sulfur rich compounds (reviewed in [80]). Initially promoted as chemopreventive agents, these plant derived chemicals have shown promise as potent anti-cancer and anti-inflammatory agents through their significant inhibitory impact on cancer cell growth, proliferation, progression and metastasis by affecting a large array of proteins involved in various signaling cascades. Encouraged by the results of these in vitro and in vivo studies, several clinical trials have been undertaken in cancers and other human diseases (reviewed in [12]). Recently, several studies have demonstrated the ability of natural product derivatives to modulate mucin expression in various cell types in vitro and organ systems in vivo (summarized in Table 1) and have raised hopes of developing selective and safe mucomodulators for inflammatory and neoplastic disorders. Herein we discuss selective natural products that have demonstrated to alter mucin expression in cellular and animal models of inflammation and cancer.
Table 1.
Effect of natural compounds on mucin expression in human diseases.
| Natural compound | Mucin type | Effect | Disease/site of action | Models used | Reference No. |
|---|---|---|---|---|---|
| Curcumin | MUC5AC | Down | Lung cancer | Airway epithelial NCI-H292cells | [84, 86] |
| MUC2 | Up | Colitis and Colon Cancer | Core-3−/− NIH:Swiss mice | [89] | |
| MUC2 | Down | mucinous colon carcinoma | LiM6 cells | [92] | |
| MUC1 | Down | Pancreatic cancer | HPAF-II and Panc-1 | [93] | |
| Apigenin | MUC2 | Up | Gastric Epithelium | KN45 cells | [96] |
| MUC1 C-Ter | Down | Breast Cancer | MCF10A cells | [97] | |
| MUC5AC | Down | Lung cancer | NCI-H292 cells | [100] | |
| Glycyrrhizin | MUC5AC | Down | Goblet airway cells | Cell lines and animal models | [102] |
| MUC5AC | Down | Lung cancer | NCI-H292 cells and Male ICR mice | [102, 103] | |
| MUC5AC | Down | Bronchial epithelial cells | Human Bronchial epithelial cells 16 (HBE16) | [104] | |
| MUC2 | Up | Colon Cancer | Wistar rats | [106] | |
| Guggulsterone | MUC2 | Up | Gastric cancer | Normal rat gastric epithelial cells RGM-1 | [110] |
| MUC1 | Down | Pancreatic cancer | CD18 and Capan1 cells | [18] | |
| MUC4 | Down | Pancreatic cancer | CD18 and Capan1 cells | [18] | |
| MUC16 | Down | Pancreatic cancer | CD18 and Capan1 cells | [18] | |
| Thymoquinone | MUC4 | Down | Pancreatic cancer | COLO357 and CD18 cells | [118] |
| Silibinin | MUC5AC | Down | Lung Cancer | NCI-H292 cells | [121] |
| Scutellarin | MUC5AC | Down | Lung Cancer | HBE16 cells | [125] |
| Muc5ac | Down | Rat lungs | Cell lines and animal models | [126] | |
| Graviola | MUC4 | Down | Pancreatic cancer | COLO357, CD18 cells and Orthotopic mouse models | [128] |
| Quercetin | MUC5AC | Down | Airway epithelium | HBE16 and NCI-H292 cells | [130] |
| Muc5ac | Down | Airway epithelium airway | NCI-H292 cells and Sprague Dawley rats | [132-133] | |
| Ursolic acid | MUC5AC | Down | Lung epithelium and | NCI-H292 and HTSE cells | [135, 136] |
| Resveratrol | MUC1 | Down | Colon cancer | Albino Wistar rats | [138] |
| MUC2 | Up | Colon cancer | Albino Wistar rats | [138] | |
| MUC5AC | Down | Lung epithelia | NCI-H292 cells | [139] |
Curcumin
Curcumin (diferuloylmethane), a naturally occurring polyphenol derived from Curcuma longa is one of the most studied natural products for medicinal use. The pharmacological activities of curcumin are attributed to its antioxidant, antimicrobial, anti-inflammatory and immunomodulatory effects [81]. Extensive research addressing the pharmacokinetics, safety and efficacy of this highly pleiotropic compound has demonstrated its utility to modulate multiple cell signaling pathways in various pro-inflammatory diseases including arthritis, crohn’s disease, ulcerative colitis, irritable bowel disease, pancreatitis, gastric ulcer, gastric inflammation, psoriasis, atherosclerosis, diabetes and cancer (pancreatic, colon, rectal, osteosarcoma, multiple myeloma and oral) [82]. Several in vitro and in vivo studies have shown that curcumin decreases cyclooxygenase-2 (COX-2), lipoxygenase and inducible nitric oxide synthase (iNOS) enzymes activity by suppressing a transcription factor NF-κB [83]. Besides affecting a plethora of molecules, curcumin has shown to impact mucin expression in several cell models [84]. In one of the earliest studies, curcumin increased the secretion of mucins in primary hamster tracheal surface epithelial (HTSE) cells [85]. However, subsequent studies by the same research group demonstrated that curcumin decreased EGF-induced MUC5AC expression in lung cancer NCI-H292 cells both at protein and transcript level [84]. Similarly, curcumin pretreatment down-regulated IL-1β induced MUC5AC expression in lung airway epithelial H292 cells [86]. In addition, modulation of mucin expression by curcumin was also reported in gastrointestinal epithelial cells. MUC2 expression is generally decreased in colorectal adenocarcinoma (reviewed in [87]), and Muc2−/− mice spontaneously developed colitis and colorectal cancer [88] suggesting its tumor suppressor role in colon cancer. Interestingly, in Citrobacter rodentium and dibenzazepine (DBZ) induced murine model of colitis and hyperplasia, dietary curcumin restored mucin production by modulating Notch and Wnt/β-catenin signaling pathways and facilitated crypt regeneration [89]. In contrast to its loss in the majority of colon cancers, MUC2 expression is preserved in subset of mucinous type colon cancer and is associated with advanced-stage disease. Bile acids have been shown to induce mucin secretion [90, 91] and promote colorectal carcinogenesis. In this context, using mucinous colon carcinoma cell line LiM6, bile acid was shown to upregulate MUC2 expression by activating AP-1 transcription factor [92]. However, curcumin treatment reduced both basal and bile acid-induced MUC2 expression both at the protein and transcript level by inhibiting AP1 [92]. Similarly, curcumin encapsulated nanoparticles were found to decrease oncogenic MUC1 protein in human PC cells and exhibit anti-tumor effects both in vitro and in vivo [93]. Not only curcumin modulates mucin expression, it can also affect mucin glycosylation by targeting the enzymes involved. Ozen et al, have shown that curcumin decreased both the sialic acid and sialidase activity in ascites fluids of Ehrlich ascites tumor bearing mice [94].
Apigenin
Apigenin, a plant flavone (4, 5, 7,-trihydroxyflavone), has gained considerable interest as a beneficial agent for human health due to its low intrinsic toxicity on normal cells, anti-inflammatory and anti-mutagenic effects. Cancer preventive and/or therapeutic properties of apigenin has been investigated in Breast, colon, pancreatic, prostate, lung and oral squamous cell cancer [95] cells and are attributed to its ability to modulate cell growth, inhibit cell cycle progression, diminish oxidative stress, improve the efficacy of detoxification enzymes, induce apoptosis and stimulate the immune system. Recently, Wang et al. have shown that apigenin abrogated Helicobacter pylori induced inflammation of gastric epithelial MKN45 cells and concomitantly restored the expression of protective mucin MUC2 [96]. Apigenin treated cells exhibited significantly increased levels of IκBα expression and thus reduced NF-κB signaling along with decrease in the expression of downstream inflammatory mediators including COX-2, ICAM-1, ROS, IL-6 and IL-8) following H. pylori infection[96]. MUC2 suppresses inflammation in the intestinal tract and inhibits intestinal tumor development [13] supporting the potential utility of apigenin to suppress inflammation and gastric cancer development. Apigenin was also demonstrated to modulate mucin function in breast cancer cells by inhibiting the dimerization of MUC1-C terminal subunit and its subsequent nuclear translocations [97]. Through MUC1-C terminal mediated auto-inductive loop, apigenin was also found to decrease MUC1 mRNA levels [97]. The same study also demonstrated that apigenin-mediated MUC1 downregulation was associated with increased apoptosis and decreased clonogenic potential. EGF through EGFR signaling stimulates MUC5AC expression in a human airway epithelial cell line [98] and its hypersecretion is associated with severe pulmonary disorders like asthma, chronic bronchitis, cystic fibrosis and bronchiectasis [99]. Apigenin treatment was shown to decrease both de-novo [100] as well as EGF-induced MUC5AC expression [100] in lung cancer NCI-H292 cells. Molecular mechanism of MUC5AC downregulation revealed that apigenin inhibits phosphorylation of EGFR and its downstream signaling molecules MEK1/2 and ERK1/2 [101].
Glycyrrhizin
The roots of Glycyrrhiza glabra (licorice) have been used in traditional medicine to treat peptic ulcers, liver disorders, bronchitis and asthma. Glycyrrhizic acid or glycyrrhizin, the active ingredient in the licorice root extract is triterpene glycoside, that exhibits anti-inflammatory, antiviral and anti-cancer properties. Recent studies have documented the impact of glycyrrhizin on mucin expression in airway and colonic epithelial cells. In LPS- and IL-4-induced murine models of airway goblet cell hyperplasia, glycyrrhizin attenuated goblet cell hyperplasia and significantly reduced LPS and IL-4 induced MUC5AC protein and transcripts in vivo [102]. Further, glycyrrhizin attenuated TGF-α-, EGF- or phorbol ester-induced MUC5AC protein and mRNA in carcinoma-derived H292 airway epithelial cells and reduced MUC5AC promoter activity in A549 cells in vitro in a dose dependent manner [102, 103]. Similarly, glycyrrhizin reduced neutrophil elastase-induced expression of MUC5AC protein and transcripts in bronchial epithelial cells (HBE16) by augmenting p38-NF- κB p65/IκBα pathway [104]. Glycyrrhizin also reduced IL-13-induced MUC5AC hypersecretion and production along with reduced oxidative stress in vivo in Sprague Dawley rats and in vitro in HBE16 [105]. Khan et al. evaluated the chemopreventive effect of glycyrrhizin in DMH induced colon carcinogenesis in a rat model. Mucin depleted foci (MDF) and aberrant crypt foci (ACF), the two well recognized preneoplastic lesions of colon cancer are observed in this model following DMH treatment. MDF are known to exhibit numerous preneoplastic characteristics including reduced expression of MUC2 (a mucin abundantly expressed in the normal colon). Glycyrrhizin supplementation resulted in a significant decrease in the number of DMH-induced MDF and attenuated the depletion of the mucous layer. While the study did not investigate any alteration in the expression of any specific mucin gene, it was noted that glycyrrhizin prevented the shifting of sulphomucin (present in normal colon) to sialomucin (associated with colon cancer) following DMH treatment. This study indicated that glycyrrhizin in addition to modulating mucin expression, can also impact post-translation modifications of mucins [106].
Guggulsterone
Gum Guggul derived from the bark of the plant Commiphora wightii is used from centuries to treat various inflammation associated diseases like hypercholesterolemia, atherosclerosis, rheumatism, and obesity. The crude ethyl acetate extract of gum guggul has been shown to suppress inflammatory mediators such as IFN-γ, IL-12, TNF-α and IL-1β. Recently, Guggulsterone E and Guggulsterone Z the active components from the gum guggul has been shown to exhibit anti-inflammatory activities by suppressing cytokines expression [107, 108] and inhibit tumor growth both in vitro and in vivo by modulating numerous survival and proapototic signaling cascades [107]. By affecting growth factors, growth factor receptors and several transcription factors implicated in inflammation like NF-κB, STAT1/3, C/EBPα, androgen and glucocorticoid receptors, guggulsterone (GS) modulates the expression of antiapoptotic proteins (IAP1, XIAP, Bcl-2, cFLIP, survivin), inhibits cell survival, cell proliferation associated proteins (cyclin D1, c-Myc), abrogates angiogenesis, and metastasis (MMP-9, COX-2, VEGF) in tumor cells [107]. GS has also been demonstrated to inhibit the transcriptional regulator farnesoid X receptor (FXR) and is involved in maintaining cholesterol/bile acid homeostasis, triglyceride and glucose metabolism [107].
Bile reflux into the stomach results in induction of metaplasia and gastric carcinogenesis. Secretory mucin MUC2 is not expressed in normal gastric epithelium but a significant de novo expression of MUC2 is observed during gastric carcinogenesis [109]. While studying the impact of bile acids on MUC2 expression in normal rat gastric epithelial (RGM-1) cells, Xu et al., demonstrated the ability of GS to modulate mucin expression. By antagonizing FXR, GS abolished chenodeoxycholic acid (CDCA) induced expression of Cdx2 and MUC2 expression at both the mRNA and protein levels suggesting its utility as a potential therapeutic modality in gastric carcinogenesis [110]. In a recent study, we observed that GS downregulates the expression of oncogenic mucins MUC1, MUC4 and MUC16 in PC cells [18]. MUC4 downregulation was associated with decreased proliferation, migratory capabilities and increased apoptosis of PC cells. In PC cells, the molecular mechanism of GS mediated MUC4 down-regulation was independent of FXR and involved inhibition of Janus Kinase- signal transduction and activator transcription (JAK-STAT) pathway and modulation of inflammation-induced transcription factors like STAT1 and STAT3 on MUC4 promoter [18].
Thymoquinone
Thymoquinone (TQ) constitutes about 30–48% of essential oils extracted from Nigella sativa seeds and is extensively investigated at preclinical level for its antioxidant, anti-inflammatory and anticancer activities [111]. TQ also preserves the activity of various anti-oxidant enzymes such as catalase, glutathione peroxidase and glutathione-S-transferase thus acting as free radical and superoxide scavenger [112]. TQ modulates numerous molecular targets (p53, p73, PTEN, STAT3, PPAR-γ), activates caspases and generates ROS that are implicated in tumor cell proliferation, apoptosis, cell cycle arrest, metastasis and angiogenesis [113] in different drug resistant cancers like osteosarcoma, uterine sarcoma and leukemia [114]. Nigella sativa oil was found to exhibit protective effects against ethanol-induced gastric ulcers in rats and result in increased gastric mucin content [115]. Recently, gastroprotective effects of TQ were demonstrated in the rat ischemia/reperfusion model of gastric ulcers. TQ alone at high doses, and in combination with omeprazole at low doses, resulted in decreased acid secretion and neutrophil infiltration and increased mucin production and secretion following ischemia/reperfusion [116]. In the PC cells, TQ has been shown to be 4- to 5-fold more cytotoxic compared to normal cells [117]. TQ also showed anti-inflammatory properties and sensitized the PC cells to conventional chemotherapeutic drugs [113, 117]. Our recent studies demonstrated that TQ treatment resulted in decreased levels of MUC4 protein in PC cells. While TQ treatment had no impact on MUC4 transcripts, TQ promoted proteasomal degradation of MUC4 suggesting that the TQ modulates MUC4 levels post-translationally [118]. TQ can potentially modulate the mucin function by altering their glycosylaion particularly by removing sialic acid. In an interesting study, TQ treatment resulted in increased activity of Neuraminadase 4 (sialidase) present on the surface of macrophages, dendritic cells, fibroblasts, cancer cells and enhanced desialylation of gangliosides and mucins [119]. Given the significance of sialic acid in receptor recognition (selectins), involvement of mucins in cell-cell interactions during growth and metastasis, enrichment of macrophages, fibroblast in TME and surface localization of sialidases and mucins, it will be of interest to determine the impact of TQ on mucin glycosylation.
Silibinin
Silibinin, a naturally occurring polyphenolic antioxidant, is a major constituent of the dietary supplement milk thistle (Silybum marianum L.) extract and has been extensively studied for anti-inflammatory and anti-neoplastic effects [120]. In airway epithelial cells, silibinin was demonstrated to inhibit mucin production and secretion. Silibinin attenuated EGF, PMA or TNF-α induced expression of the MUC5AC at the transcript and protein levels in NCI-H292 cells. Further, silibinin resulted in a significant reduction of ATP-induced mucin secretion in cultured rat tracheal surface epithelial (RTSE) cells [121]. Transcription factor Cdx2, has been shown to directly bind MUC2 and MUC4 promoters and upregulate their expression [122, 123]. Recently, Sangeetha et al. demonstrated the ability of silibinin to modulate mucin expression in colonic epithelial cells. In the DMH-induced model of colorectal carcinogenesis in rats characterized by reduced goblet cell mucin and increase in the number of MDF, silibinin resulted in increased mucin content in the goblet cells following DMH treatment [124]. Like guggulsterone, silibinin prevented the suppression of Cdx2, both at mRNA and protein level [124], suggesting that silibinin has a great potential in modulating mucin expression through Cdx2 regulation.
Scutellarin
Scutellarin is a flavonoid extracted from traditional Chinese herbs, Erigeron breviscapus and Scutellaria barbata. Mucomodulatory effects of scutellarin were recently demonstrated in airway epithelium. Pretreatment of human bronchial epithelial 16 (HBE16) cells with scutellarin inhibited IL-13 or human neutrophil elastase (HNE)-induced MUC5AC expression in a dose-dependent manner [125]. Similarly, neutrophil elastase-induced MUC5AC mRNA, protein expression and goblet cell hyperplasia were significantly decreased in rats treated with scutellarin [126]. Modulation of MUC5AC expression by scutellarin appeared to be due to inhibition of PKC and ERK1/2 signaling and was independent of STAT-6 [125, 126].
Graviola
Leaves of Annona Muricata, also known as Graviola or Soursop, have been used for a wide range of human diseases including inflammatory conditions, neuralgia, diabetes, cystitis and cancer [127]. The potent bioactive anticancer components were recently identified to be annonaceous acetogenins. We recently evaluated the therapeutic efficacy of Graviola leaves extract (GLE) on growth and metastasis of PC cells [128]. GLE significantly inhibited the expression of MUC4 mucin (in vitro and in vivo) and its downstream signaling regulating cell cycle, survival and metastasis of the PC cells [128]. Besides, significant inhibition of cell proliferation and invasive behavior, GLE induced acute metabolic stress leading to ATP depletion and exhibited in vivo therapeutic efficacy.
Quercetin
Quercetin is a plant flavonoid that occurs in numerous vegetables, fruits and grains including capers, radish, onions, cranberry and exhibits potent antioxidant, anti-angiogenic, anti-proliferative and pro-apoptotic functions [129]. Several studies have also revealed its anti-inflammatory potential in airway pathologies that are usually associated with mucus hypersecretion. Using airway epithelial cells (HBE16), quercetin treatment was shown to reduce neutrophil elastase induced MUC5AC expression both at RNA and protein level through PKC/EGFR/ERK signal transduction pathway [130]. In addition, quercetin also attenuated IL-1β-induced MUC5AC expression by modulating ERK and p38 MAPK phosphorylation [131]. Besides in vitro studies, quercetin has been shown to attenuate cigarette smoke [132] and elastase/LPS [133] induced Muc5ac expression in mouse models.
Ursolic acid
Ursolic acid, a pentacyclic triterpenoid, is widely found in fruits and medicinal herbs with hypoglycemic, anti-obesity and anti-cancer properties [134]. Ursolic acid also showed anti-inflammatory potential by inhibiting EGF and PMA induced MUC5AC expression in the lung epithelial cells (NCI-H292)[135]. Furthermore, ursolic acid inhibited ATP induced secretion of MUC5AC in hamster tracheal surface epithelial cells (HTSE) [136].
Resveratrol
Resveratrol (3, 5, 4′-trihydroxy-trans-stilbene), is a phytoelaxin and a natural phenol produced by many plants when attacked by pathogens. Usually abundant in berries, grape, peanuts and wine, resveratrol exhibits strong anti-tumor activities by modulating cell proliferation, apoptosis, invasion, metastasis and enhances host immune system to kill tumor cells [137]. Interestingly, resveratrol has been shown to decrease MUC1 expression while it upregulated MUC2 expression in a carcinogen induced colon cancer rat model [138]. Besides affecting mucin expression, resveratrol also decreased the expression of inflammatory mediator COX-2 [138]. Moreover, resveratrol suppressed the EGF, PMA and TNF-α induced production and secretion of MUC5AC in NCI-H292 cells. In addition, resveratrol also suppressed the ATP-induced MUC5AC secretion in primary rat tracheal surface epithelial (RTSE) cells [139]. Several studies have reported a positive correlation between mucins expression and resistance to chemotherapy [41-43]. Resveratrol has been shown to sensitization of tumor cells to many chemotherapeutic drugs by modulating multiple cell-signaling molecules, including drug transporters and members of the NF –κB, SP1and STAT3 signaling pathways [140]. Our lab and others have conclusively established the involvement of NF-κB and STAT3 signaling pathways in regulating mucins expression particularly MUC1 and MUC4 (reviewed in[54, 55].While these studies suggest that resveratrol mediated chemosensitization of cancer cells may be attributed to the modulation of mucin expression, a more direct association needs to be explored.
Tea Polyphenols and Flavins
Epicatechin-3-gallate (ECG), Epicatechin (EC), Epigallocatechin-3-gallate (EGCG) and Epigallocatechin (EGC) are the polyphenol components of green tea (Camellia sinensis) with potent anti-oxidant and anti-inflammatory effects [141]. In context with its effect on mucin expression, EGCG was recently shown to attenuate MUC5AC expression by modulating EGFR signaling in normal human nasal epithelial (NHNE) cells [142]. Further, EGCG was demonstrated to suppress IL-1β induced expression and secretion of MUC5AC via inhibition of the phosphorylation of ERK/MAP kinase and MSK1 [143]. Importantly, Lu et al have shown that EGCG decreased MUC1 expression in hepatocellular carcinoma cells (HepG2) that was associated with reduced invasive potential [144]. In addition, theaflavins isolated from the black tea have also been shown to decrease smoking induced MUC5AC expression and decrease airway mucus hypersecretion by modulating the EGFR signaling [145].
Conclusions and Perspectives
Considering deregulated and aberrant expression of mucins in inflammation and cancer, and involvement in the epithelial protection, there has been an ongoing interest in identifying agents that can modulate their expression and functions in pathobiology. Recent studies involving cell culture, animal models and human tissues and advances in molecular biology have enhanced our understanding of mucin gene regulation. The promoters of several mucin genes have been characterized and their regulatory pathways have been studied. Mucin promoters are enriched in binding elements for regulatory proteins responsive to signaling pathways involved in differentiation and inflammation and mechanisms of deregulated mucin expression under pathological conditions are beginning to be understood. Given the role of inflammation in mucin gene regulation and the anti-inflammatory effects of plant-derived natural products, it is not surprising that several of these compounds have been found to modulate mucin expression. Importantly, despite the similarity in the promoters, the regulation of mucins by natural products is context dependent. A compound can result in the restoration of protective mucin under pathological conditions where the given mucin is typically lost; on the other hand the same compound can downregulate the expression of an aberrantly overexpressed mucin contributing to pathobiology in another disease. Although the utility of natural products in restoring mucin homeostasis and function is only beginning to be realized, the promise of these compounds in developing mucin targeted therapies is immense.
Imbalance in intestinal microflora is associated with and contributes to pathogenesis of inflammatory bowel diseases (IBD) [146]. Many natural compounds have been shown to affect both the intestinal microflora as well as IBD. Natural compounds by modulating intestinal flora and inflammation can impact mucin expression during pathogenesis of intestinal cancers. Although there is currently no direct evidence suggesting the modulation of mucin expression by natural compounds via impact on intestinal microflora, it will be interesting to explore this link in future studies.
Epigenetic alterations including DNA methylation, histone modifications and micro-RNAs (miRNAs) have been shown to regulate mucin expression in various diseases including cancer (reviewed in [147]. Use of demethylating agent 5-aza-20-deoxycytidine upregulated MUC5AC, MUC5B expression while use of histone deacetylases (HDAC) inhibitor Trichostatin (TSA) downregulated MUC6 expression in adenocarcinomatous cells respectively [148]. Recent studies have indicated that various natural compounds including curcumin, resveratrol, folates, and polyphenols can impact epigenetic modifications by targeting histone acetyl transferases (HATs), DNA methyl transferees (DNMTs) and HDACs [149]. The impact of natural compounds on the epigenetic modifications on the promoters of mucins and mucin-regulating genes (cytokines, growth factor receptors and miRNAs) need to be thoroughly studied and characterized to fully exploit their potential as meaningful mucomodulators.
The discovery of compounds capable of regulating mucin expression should be accelerated by the increasing availability of libraries of natural and synthetic compounds and development of mucin promoter reporter constructs. Similarly, screening strategies to identify compounds capable of regulating mucin function should be devised for mucins whose mechanism(s) of action or interacting partners are known. An encouraging first step in this direction was the employment of a MUC1 cytoplasmic domain dimerization-based approach for identifying MUC1 function-blocking compounds [96]. Some of the oncogenic functions of MUC1 are attributed to the dimerization of its cytoplasmic domain that triggers nuclear translocation [150]. Yang et al., screened four libraries containing known bioactives (ICCB3, NINDS2, Prestwick1, Microsource1) and two libraries of natural product extracts (NCDDG8, MMV6) for identifying compounds capable of inhibiting MUC1-cytoplasmic domain dimerization. Of the several compounds found to inhibit MUC1-cyptoplasmic domain dimerization, apigenin was further validated in mammary epithelial cells for its ability to augment the nuclear translocation of MUC1. Some of pro-tumorigenic functions of mucins have been attributed to their ability to interact with various cell surface proteins and several mucin-interacting proteins have been identified. These include growth factor receptors [151], selectins, galectin-3 and mesothelin. It will be of interest to develop assays for screening compounds capable of disrupting the interaction of mucins with their functional partners.
Although mucomodulatory effects of several natural compounds have been documented and demonstrated, their clinical utility is compromised due to poor bioavailability and pharmacokinetics, and administration of some of these compounds at higher doses could induce non-target toxicity. Extensive research is underway to modify these natural compounds by either acylating, alkylating or glycosylating their reactive hydroxy or methylene groups[152], and formulate the natural products to overcome these limitations. Curcumin has been encapsulated in a variety of nanoformulations including magnetic nanoparticles, polymer micelles, nanogels
[153]. Similarly, while there is limited data on the bioavailability and other pharmacokinetic parameters of thymoquinone, novel thymoquinone analogs and nanoparticle formulations have been developed and found to exhibit greater anti-cancer and anti-oxidant activities than TQ [154]. Various nanoparticle formulations have also been developed for apigenin, glycyrrhizin and silibinin [153, 155-160]. Given the emerging role of mucins in resistance to chemotherapy and the ability of several mucomodulatory agents to modulate pathways associated with chemoresistance, it will also be of interest to evaluate mucomodulators as chemosensitizers.
In conclusion, while the research regarding the utility of natural products and modulators of mucin expression and function is still in early stages, it holds promise for developing novel and non-toxic strategies to combat inflammation and cancer.
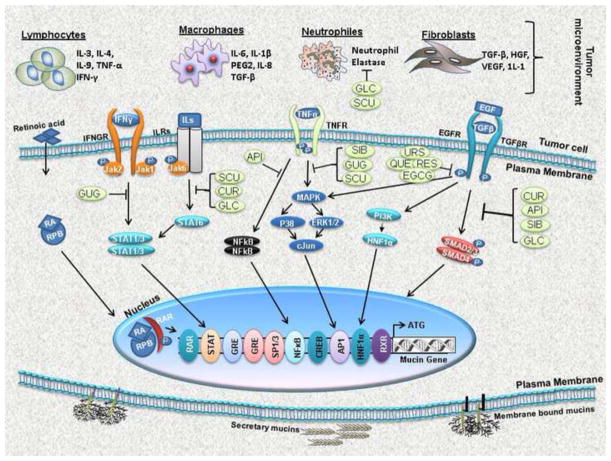
Figure 1.
Schematic representation of natural compounds that target signaling pathways regulating expression of mucins. Cytokines, interleukins and the growth factors secreted by the tumor cells or other cells of tumor microenvironment (lymphocytes, macrophages, neutrophils and fibroblasts) activate various signaling cascades like PI3K-AKT, Jak/STAT, MAPK/ERK/P38 and SMAD pathways involved in mucin regulation. Several dietary natural product derivatives modulate these signaling pathways at various steps to regulate mucin expression. Abbreviations: NFB, nuclear factor kappa B; P, phosphate; PI3K, phosphatidylinositol-3-kinase; STAT3, signal transducer and activator of transcription 3; TNF: tumor necrosis factor, IL: interleukin, IFN: interferon, TGF: transforming growth factor, MEK: mitogen-activated protein kinase (MAPK) extracellular signal regulated kinase, ERK: extracellular signal regulated kinase, STAT: signal transducers and activators of transcription, Sp: specificity protein, HRE: hypoxia response element, ER: estrogen receptor, AP: activator protein; CUR, Curcumin; GUG, Guggulsterone, API, Apigenin; GLC, Glycyrrhizin; THY, Thymoquinone; SIL, Silibinin; SCU, Scutellarin; GRA, Graviola, QUE, Quercetin; URS, Ursolic acid; RES Resveratrol and EGCG, Epigallocatechin gallate.
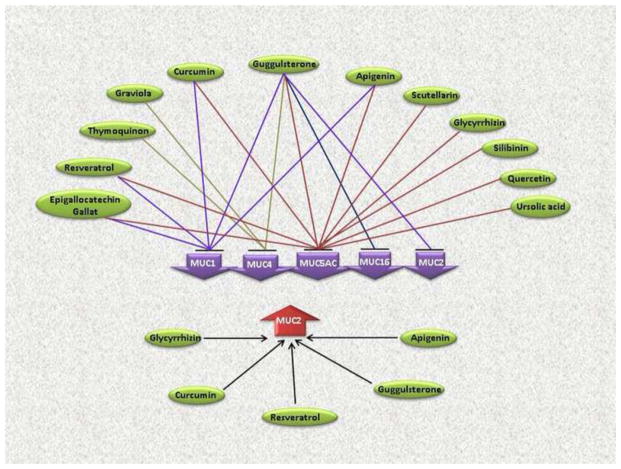
Figure 2.
Modulation of mucin expression by natural compounds. Mucins represented in downward purple arrows are downregulated by the indicated natural compounds, while MUC2 shown in upward red arrow is upregulated by glycyrrhizin, curcumin, guggulsterone and apigenin.
Highlights.
Deregulated mucin expression is a hallmark of inflammation and cancer.
Due to their functional involvement, mucins are potential therapeutic targets for cancer and inflammatory disorders.
Inflammatory and oncogenic signaling networks that result in deregulated mucin expression have been characterized.
Natural products are potential mucomodulators as they can modulate signaling pathways involved in mucin dysregulation.
Recently several natural products have been demonstrated to modulate mucin expression and function in vitro and in vivo.
Acknowledgments
The authors on this manuscript, in part, were supported by the grants from National Institutes of Health (P20 GM103480, RO1 CA183459, EDRN UO1CA111294, SPORE P50 CA 127297, R21 CA156037, R03 CA167342 and U54 CA163120).
Abbreviations
- ECM
extracellular matrix
- TGF-α
Transforming growth factor-α
- EGFR
Epidermal growth factor receptor
- STAT
signal transducer and activation of transcription
- VEGF
vascular endothelial growth factor
- TME
tumor microenvironment
- IKKβ
Inhibitor of nuclear factor-κB kinase-β
- PG
prostaglandin
- HIF-1α
Hypoxia Inducible Factor-1α
- HRE
HIF responsive elements
- PEA3
polyomavirus enhancer activator-3
- CREB
cyclic adenosine monophosphate responsive element binding protein
- PKA
protein kinase A
- COX-2
cyclooxygenase-2
- iNOS
inducible nitric oxide synthase
- ACF
aberrant crypt foci
- MDF
Mucin depleted foci
- HNF1-α
hepatocyte nuclear factors-α
- RA
all-trans-retinoic acid
- EGCG
Epigallocatechin gallate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 3.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–1638. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonckheere N, Skrypek N, Van SI. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142–151. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472–2481. doi: 10.2174/13816128112092472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonckheere N, Skrypek N, Frenois F, Van SI. Membrane-bound mucin modular domains: from structure to function. Biochimie. 2013;95:1077–1086. doi: 10.1016/j.biochi.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509–527. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, et al. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 11.Basmadjian C, Thuaud F, Ribeiro N, Desaubry L. Flavaglines: potent anticancer drugs that target prohibitins and the helicase eIF4A. Future Med Chem. 2013;5:2185–2197. doi: 10.4155/fmc.13.177. [DOI] [PubMed] [Google Scholar]
- 12.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. 195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 15.Raina D, Kharbanda S, Kufe D. The MUC1 oncoprotein activates the anti-apoptotic phosphoinositide 3-kinase/Akt and Bcl-xL pathways in rat 3Y1 fibroblasts. J Biol Chem. 2004;279:20607–20612. doi: 10.1074/jbc.M310538200. [DOI] [PubMed] [Google Scholar]
- 16.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, et al. Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett. 2013;341:166–177. doi: 10.1016/j.canlet.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, et al. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol. 2007;9:1419–1427. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi G, Fujita N, Noda Y, Kimura K, Watanabe H, Chonan A, et al. Study of cases of mucin producing tumors of the pancreas showing penetration into other organs. Nihon Shokakibyo Gakkai Zasshi. 1993;90:3081–3089. [PubMed] [Google Scholar]
- 21.Comamala M, Pinard M, Theriault C, Matte I, Albert A, Boivin M, et al. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer. 2011;104:989–999. doi: 10.1038/bjc.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 23.Lakshmanan I, Ponnusamy MP, Das S, Chakraborty S, Haridas D, Mukhopadhyay P, et al. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene. 2012;31:805–817. doi: 10.1038/onc.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akita K, Tanaka M, Tanida S, Mori Y, Toda M, Nakada H. CA125/MUC16 interacts with Src family kinases, and over-expression of its C-terminal fragment in human epithelial cancer cells reduces cell-cell adhesion. Eur J Cell Biol. 2013;92:257–263. doi: 10.1016/j.ejcb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 26.Buisine MP, Devisme L, Copin MC, Durand-Reville M, Gosselin B, Aubert JP, et al. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999;20:209–218. doi: 10.1165/ajrcmb.20.2.3259. [DOI] [PubMed] [Google Scholar]
- 27.Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M, et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950–2955. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitazaki T, Soda H, Doi S, Nakano H, Nakamura Y, Kohno S. Gefitinib inhibits MUC5AC synthesis in mucin-secreting non-small cell lung cancer cells. Lung Cancer. 2005;50:19–24. doi: 10.1016/j.lungcan.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Yu CJ, Yang PC, Shun CT, Lee YC, Kuo SH, Luh KT. Overexpression of MUC5 genes is associated with early post-operative metastasis in non-small-cell lung cancer. Int J Cancer. 1996;69:457–465. doi: 10.1002/(SICI)1097-0215(19961220)69:6<457::AID-IJC7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Yu CJ, Shih JY, Lee YC, Shun CT, Yuan A, Yang PC. Sialyl Lewis antigens: association with MUC5AC protein and correlation with post-operative recurrence of non-small cell lung cancer. Lung Cancer. 2005;47:59–67. doi: 10.1016/j.lungcan.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Aishima S, Kuroda Y, Nishihara Y, Taguchi K, Taketomi A, Maehara Y, et al. Gastric mucin phenotype defines tumour progression and prognosis of intrahepatic cholangiocarcinoma: gastric foveolar type is associated with aggressive tumour behaviour. Histopathology. 2006;49:35–44. doi: 10.1111/j.1365-2559.2006.02414.x. [DOI] [PubMed] [Google Scholar]
- 32.Boonla C, Wongkham S, Sheehan JK, Wongkham C, Bhudhisawasdi V, Tepsiri N, et al. Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer. 2003;98:1438–1443. doi: 10.1002/cncr.11652. [DOI] [PubMed] [Google Scholar]
- 33.Inaguma S, Kasai K, Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene. 2011;30:714–723. doi: 10.1038/onc.2010.459. [DOI] [PubMed] [Google Scholar]
- 34.Truant S, Bruyneel E, Gouyer V, De WO, Pruvot FR, Mareel M, et al. Requirement of both mucins and proteoglycans in cell-cell dissociation and invasiveness of colon carcinoma HT-29 cells. Int J Cancer. 2003;104:683–694. doi: 10.1002/ijc.11011. [DOI] [PubMed] [Google Scholar]
- 35.Yamazoe S, Tanaka H, Sawada T, Amano R, Yamada N, Ohira M, et al. RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res. 2010;29:53. doi: 10.1186/1756-9966-29-53.:53-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bu X, Li L, Li N, Tian X, Huang P. Suppression of mucin 2 enhances the proliferation and invasion of LS174T human colorectal cancer cells. Cell Biol Int. 2011;35:1121–1129. doi: 10.1042/CBI20100876. [DOI] [PubMed] [Google Scholar]
- 39.Boltin D, Perets TT, Vilkin A, Niv Y. Mucin function in inflammatory bowel disease: an update. J Clin Gastroenterol. 2013;47:106–111. doi: 10.1097/MCG.0b013e3182688e73. [DOI] [PubMed] [Google Scholar]
- 40.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther. 2009;121:332–348. doi: 10.1016/j.pharmthera.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahiya R, Lesuffleur T, Kwak KS, Byrd JC, Barbat A, Zweibaum A, et al. Expression and characterization of mucins associated with the resistance to methotrexate of human colonic adenocarcinoma cell line HT29. Cancer Res. 1992;52:4655–4662. [PubMed] [Google Scholar]
- 42.Lesuffleur T, Porchet N, Aubert JP, Swallow D, Gum JR, Kim YS, et al. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993;106(Pt 3):771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- 43.Leteurtre E, Gouyer V, Rousseau K, Moreau O, Barbat A, Swallow D, et al. Differential mucin expression in colon carcinoma HT-29 clones with variable resistance to 5-fluorouracil and methotrexate. Biol Cell. 2004;96:145–151. doi: 10.1016/j.biolcel.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagy P, Friedlander E, Tanner M, Kapanen AI, Carraway KL, Isola J, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 46.Wissniowski TT, Meister S, Hahn EG, Kalden JR, Voll R, Ocker M. Mucin production determines sensitivity to bortezomib and gemcitabine in pancreatic cancer cells. Int J Oncol. 2012;40:1581–1589. doi: 10.3892/ijo.2012.1337. [DOI] [PubMed] [Google Scholar]
- 47.Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118:113–124. doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- 48.Deng M, Jing DD, Meng XJ. Effect of MUC1 siRNA on drug resistance of gastric cancer cells to trastuzumab. Asian Pac J Cancer Prev. 2013;14:127–131. doi: 10.7314/apjcp.2013.14.1.127. [DOI] [PubMed] [Google Scholar]
- 49.Shi M, Yang Z, Hu M, Liu D, Hu Y, Qian L, et al. Catecholamine-Induced beta2-adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J Immunol. 2013;190:5600–5608. doi: 10.4049/jimmunol.1202364. [DOI] [PubMed] [Google Scholar]
- 50.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantovani G, Madeddu C. Cyclooxygenase-2 inhibitors and antioxidants in the treatment of cachexia. Curr Opin Support Palliat Care. 2008;2:275–281. doi: 10.1097/spc.0b013e32830f47e4. [DOI] [PubMed] [Google Scholar]
- 53.Atsumi T, Singh R, Sabharwal L, Bando H, Meng J, Arima Y, et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014;74:8–14. doi: 10.1158/0008-5472.CAN-13-2322. [DOI] [PubMed] [Google Scholar]
- 54.Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem. 2007;102:1103–1116. doi: 10.1002/jcb.21539. [DOI] [PubMed] [Google Scholar]
- 55.Wu YM, Nowack DD, Omenn GS, Haab BB. Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J Proteome Res. 2009;8:1876–1886. doi: 10.1021/pr8008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrianifahanana M, Singh AP, Nemos C, Ponnusamy MP, Moniaux N, Mehta PP, et al. IFN-gamma-induced expression of MUC4 in pancreatic cancer cells is mediated by STAT-1 upregulation: a novel mechanism for IFN-gamma response. Oncogene. 2007;26:7251–7261. doi: 10.1038/sj.onc.1210532. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Perelman JM, Kolosov VP, Zhou X. Neutrophil elastase induces MUC5AC secretion via protease-activated receptor 2. Mol Cell Biochem. 2013;377:75–85. doi: 10.1007/s11010-013-1572-3. [DOI] [PubMed] [Google Scholar]
- 58.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, et al. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res. 2009;69:7013–7021. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwerbrock NM, Makkink MK, Van der Sluis M, Buller HA, Einerhand AW, Sartor RB, et al. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, et al. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L320–L330. doi: 10.1152/ajplung.00440.2002. [DOI] [PubMed] [Google Scholar]
- 63.Koo JS, Kim YD, Jetten AM, Belloni P, Nettesheim P. Overexpression of mucin genes induced by interleukin-1 beta, tumor necrosis factor-alpha, lipopolysaccharide, and neutrophil elastase is inhibited by a retinoic acid receptor alpha antagonist. Exp Lung Res. 2002;28:315–332. doi: 10.1080/01902140252964393. [DOI] [PubMed] [Google Scholar]
- 64.Kerschner JE, Khampang P, Erbe CB, Kolker A, Cioffi JA. Mucin gene 19 (MUC19) expression and response to inflammatory cytokines in middle ear epithelium. Glycoconj J. 2009;26:1275–1284. doi: 10.1007/s10719-009-9245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoigawa N, Takeuchi N, Toda M, Inoue M, Kaibori M, Yanagida H, et al. Enhanced production of interleukin 6 in peripheral blood monocytes stimulated with mucins secreted into the bloodstream. Clin Cancer Res. 2005;11:6127–6132. doi: 10.1158/1078-0432.CCR-05-0292. [DOI] [PubMed] [Google Scholar]
- 66.Cascio S, Zhang L, Finn OJ. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-kappaB p65 and binding to cytokine promoters: importance of extracellular domain. J Biol Chem. 2011;286:42248–42256. doi: 10.1074/jbc.M111.297630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tinder TL, Subramani DB, Basu GD, Bradley JM, Schettini J, Million A, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181:3116–3125. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abe M, Siddiqui J, Kufe D. Sequence analysis of the 5′ region of the human DF3 breast carcinoma-associated antigen gene. Biochem Biophys Res Commun. 1989;165:644–649. doi: 10.1016/s0006-291x(89)80014-2. [DOI] [PubMed] [Google Scholar]
- 69.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, et al. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol. 2007;293:L693–L701. doi: 10.1152/ajplung.00491.2006. [DOI] [PubMed] [Google Scholar]
- 70.Jonckheere N, Van SI. The membrane-bound mucins: From cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 2010;92:1–11. doi: 10.1016/j.biochi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Perrais M, Pigny P, Ducourouble MP, Petitprez D, Porchet N, Aubert JP, et al. Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinflammatory cytokines for its regulation in pancreatic cancer cells. J Biol Chem. 2001;276:30923–30933. doi: 10.1074/jbc.M104204200. [DOI] [PubMed] [Google Scholar]
- 72.Damera G, Xia B, Ancha HR, Sachdev GP. IL-9 modulated MUC4 gene and glycoprotein expression in airway epithelial cells. Biosci Rep. 2006;26:55–67. doi: 10.1007/s10540-006-9000-5. [DOI] [PubMed] [Google Scholar]
- 73.Jonckheere N, Perrais M, Mariette C, Batra SK, Aubert JP, Pigny P, et al. A role for human MUC4 mucin gene, the ErbB2 ligand, as a target of TGF-beta in pancreatic carcinogenesis. Oncogene. 2004;23:5729–5738. doi: 10.1038/sj.onc.1207769. [DOI] [PubMed] [Google Scholar]
- 74.Choudhury A, Singh RK, Moniaux N, El-Metwally TH, Aubert JP, Batra SK. Retinoic acid-dependent transforming growth factor-beta 2-mediated induction of MUC4 mucin expression in human pancreatic tumor cells follows retinoic acid receptor–alpha signaling pathway. J Biol Chem. 2000;275:33929–33936. doi: 10.1074/jbc.M005115200. [DOI] [PubMed] [Google Scholar]
- 75.Andrianifahanana M, Agrawal A, Singh AP, Moniaux N, Van SI, Aubert JP, et al. Synergistic induction of the MUC4 mucin gene by interferon-gamma and retinoic acid in human pancreatic tumour cells involves a reprogramming of signalling pathways. Oncogene. 2005;24:6143–6154. doi: 10.1038/sj.onc.1208756. [DOI] [PubMed] [Google Scholar]
- 76.Mejias-Luque R, Peiro S, Vincent A, Van SI, de BC. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim Biophys Acta. 2008;1783:1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 77.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–1640. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu X, Price-Schiavi SA, Carraway KL. Extracellular regulated kinase (ERK)-dependent regulation of sialomucin complex/rat Muc4 in mammary epithelial cells. Oncogene. 2000;19:4354–4361. doi: 10.1038/sj.onc.1203781. [DOI] [PubMed] [Google Scholar]
- 79.Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008;70:405–429. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins (Basel) 2010;2:517–551. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 82.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol Adv. 2014 doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009;23:1458–1461. doi: 10.1002/ptr.2801. [DOI] [PubMed] [Google Scholar]
- 85.Lee CJ, Lee JH, Seok JH, Hur GM, Park YC, Seol IC, et al. Effects of baicalein, berberine, curcumin and hesperidin on mucin release from airway goblet cells. Planta Med. 2003;69:523–526. doi: 10.1055/s-2003-40655. [DOI] [PubMed] [Google Scholar]
- 86.Chang JH, Song KJ, Kim HJ, Kim JH, Kim NH, Kim KS. Dietary polyphenols affect MUC5AC expression and ciliary movement in respiratory cells and nasal mucosa. Am J Rhinol Allergy. 2010;24:e59–e62. doi: 10.2500/ajra.2010.24.3447. [DOI] [PubMed] [Google Scholar]
- 87.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 88.Kawashima H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol Pharm Bull. 2012;35:1637–1641. doi: 10.1248/bpb.b12-00412. [DOI] [PubMed] [Google Scholar]
- 89.Ahmed I, Chandrakesan P, Tawfik O, Xia L, Anant S, Umar S. Critical roles of Notch and Wnt/beta-catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection. Infect Immun. 2012;80:3107–3121. doi: 10.1128/IAI.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klinkspoor JH, Mok KS, Van Klinken BJ, Tytgat GN, Lee SP, Groen AK. Mucin secretion by the human colon cell line LS174T is regulated by bile salts. Glycobiology. 1999;9:13–19. doi: 10.1093/glycob/9.1.13. [DOI] [PubMed] [Google Scholar]
- 91.Shekels LL, Lyftogt CT, Ho SB. Bile acid-induced alterations of mucin production in differentiated human colon cancer cell lines. Int J Biochem Cell Biol. 1996;28:193–201. doi: 10.1016/1357-2725(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 92.Song S, Byrd JC, Koo JS, Bresalier RS. Bile acids induce MUC2 overexpression in human colon carcinoma cells. Cancer. 2005;103:1606–1614. doi: 10.1002/cncr.21015. [DOI] [PubMed] [Google Scholar]
- 93.Yallapu MM, Ebeling MC, Khan S, Sundram V, Chauhan N, Gupta BK, et al. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol Cancer Ther. 2013;12:1471–1480. doi: 10.1158/1535-7163.MCT-12-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ozen N, Uslu E, Ozen M, Aydin S, Altug T, Belce A, et al. Curcumin’s effects on sialic acid level and sialidase activity in Ehrlich ascites tumor bearing mice. Tohoku J Exp Med. 2002;197:221–227. doi: 10.1620/tjem.197.221. [DOI] [PubMed] [Google Scholar]
- 95.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang YC, Huang KM. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem Toxicol. 2013;53:376–383. doi: 10.1016/j.fct.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Y, Rajabi H, Kufe D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol Pharmacol. 2011;79:886–893. doi: 10.1124/mol.110.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burgel PR, Cardell LO, Ueki IF, Nadel JA. Intranasal steroids decrease eosinophils but not mucin expression in nasal polyps. Eur Respir J. 2004;24:594–600. doi: 10.1183/09031936.04.00014404. [DOI] [PubMed] [Google Scholar]
- 99.Rogers DF. Mucoactive drugs for asthma and COPD: any place in therapy? Expert Opin Investig Drugs. 2002;11:15–35. doi: 10.1517/13543784.11.1.15. [DOI] [PubMed] [Google Scholar]
- 100.Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, et al. Phorbol ester or epidermal growth-factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012;26:1784–1788. doi: 10.1002/ptr.4650. [DOI] [PubMed] [Google Scholar]
- 101.Sikder MA, Lee HJ, Ryu J, Park SH, Kim JO, Hong JH, et al. Apigenin and Wogonin Regulate Epidermal Growth Factor Receptor Signaling Pathway Involved in MUC5AC Mucin Gene Expression and Production from Cultured Airway Epithelial Cells. Tuberc Respir Dis (Seoul) 2014;76:120–126. doi: 10.4046/trd.2014.76.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nishimoto Y, Hisatsune A, Katsuki H, Miyata T, Yokomizo K, Isohama Y. Glycyrrhizin attenuates mucus production by inhibition of MUC5AC mRNA expression in vivo and in vitro. J Pharmacol Sci. 2010;113:76–83. doi: 10.1254/jphs.09344fp. [DOI] [PubMed] [Google Scholar]
- 103.Lee HJ, Lee SY, Bae HS, Kim JH, Chang GT, Seok JH, et al. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine. 2011;18:743–747. doi: 10.1016/j.phymed.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Xiao Q, Zhou X. Glycyrrhizin inhibits human neutrophil elastase-induced mucin 5AC overproduction in human bronchial epithelial cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:252–257. doi: 10.11817/j.issn.1672-7347.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Tong J, Zhou XD, Kolosov VP, Perelman J. Effects of glycyrrhizin on airway mucus hypersecretion induced by interleukin-13 in rats. Zhonghua Yi Xue Za Zhi. 2013;93:2225–2229. [PubMed] [Google Scholar]
- 106.Khan R, Khan AQ, Lateef A, Rehman MU, Tahir M, Ali F, et al. Glycyrrhizic acid suppresses the development of precancerous lesions via regulating the hyperproliferation, inflammation, angiogenesis and apoptosis in the colon of Wistar rats. PLoS One. 2013;8:e56020. doi: 10.1371/journal.pone.0056020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res. 2008;28:3647–3664. [PubMed] [Google Scholar]
- 108.Almazari I, Surh YJ. Cancer chemopreventive and therapeutic potential of guggulsterone. Top Curr Chem. 2013;329:35–60. doi: 10.1007/128_2012_344. [DOI] [PubMed] [Google Scholar]
- 109.Ilhan O, Han U, Onal B, Celik SY. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk J Gastroenterol. 2010;21:345–352. doi: 10.4318/tjg.2010.0119. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y, Watanabe T, Tanigawa T, Machida H, Okazaki H, Yamagami H, et al. Bile acids induce cdx2 expression through the farnesoid x receptor in gastric epithelial cells. J Clin Biochem Nutr. 2010;46:81–86. doi: 10.3164/jcbn.09-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nagi MN, Almakki HA. Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: possible role in protection against chemical carcinogenesis and toxicity. Phytother Res. 2009;23:1295–1298. doi: 10.1002/ptr.2766. [DOI] [PubMed] [Google Scholar]
- 113.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–451. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 114.Salim LZ, Mohan S, Othman R, Abdelwahab SI, Kamalidehghan B, Sheikh BY, et al. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules. 2013;18:11219–11240. doi: 10.3390/molecules180911219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Dakhakhny M, Barakat M, El-Halim MA, Aly SM. Effects of Nigella sativa oil on gastric secretion and ethanol induced ulcer in rats. J Ethnopharmacol. 2000;72:299–304. doi: 10.1016/s0378-8741(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 116.Magdy MA, Hanan E, Nabila E. Thymoquinone: Novel gastroprotective mechanisms. Eur J Pharmacol. 2012;697:126–131. doi: 10.1016/j.ejphar.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 117.Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11:373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9:1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finlay TM, Jayanth P, Amith SR, Gilmour A, Guzzo C, Gee K, et al. Thymoquinone from nutraceutical black cumin oil activates Neu4 sialidase in live macrophage, dendritic, and normal and type I sialidosis human fibroblast cells via GPCR Galphai proteins and matrix metalloproteinase-9. Glycoconj J. 2010;27:329–348. doi: 10.1007/s10719-010-9281-6. [DOI] [PubMed] [Google Scholar]
- 120.Ting H, Deep G, Agarwal R. Molecular mechanisms of silibinin-mediated cancer chemoprevention with major emphasis on prostate cancer. AAPS J. 2013;15:707–716. doi: 10.1208/s12248-013-9486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim KD, Lee HJ, Lim SP, Sikder A, Lee SY, Lee CJ. Silibinin regulates gene expression, production and secretion of mucin from cultured airway epithelial cells. Phytother Res. 2012;26:1301–1307. doi: 10.1002/ptr.3727. [DOI] [PubMed] [Google Scholar]
- 122.Jonckheere N, Vincent A, Perrais M, Ducourouble MP, Male AK, Aubert JP, et al. The human mucin MUC4 is transcriptionally regulated by caudal-related homeobox, hepatocyte nuclear factors, forkhead box A, and GATA endodermal transcription factors in epithelial cancer cells. J Biol Chem. 2007;282:22638–22650. doi: 10.1074/jbc.M700905200. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300:813–818. doi: 10.1016/s0006-291x(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 124.Sangeetha N, Nalini N. Silibinin modulates caudal-type homeobox transcription factor (CDX2), an intestine specific tumor suppressor to abrogate colon cancer in experimental rats. Hum Exp Toxicol. 2014 doi: 10.1177/0960327114530741. [DOI] [PubMed] [Google Scholar]
- 125.Jiang DP, Perelman JM, Kolosov VP, Zhou XD. Effects of scutellarin on MUC5AC mucin production induced by human neutrophil elastase or interleukin 13 on airway epithelial cells. J Korean Med Sci. 2011;26:778–784. doi: 10.3346/jkms.2011.26.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang DP, Li Q, Yang J, Perelman JM, Kolosov VP, Zhou XD. Scutellarin attenuates human-neutrophil-elastase-induced mucus production by inhibiting the PKC-ERK signaling pathway in vitro and in vivo. Am J Chin Med. 2011;39:1193–1206. doi: 10.1142/S0192415X11009494. [DOI] [PubMed] [Google Scholar]
- 127.Dvorkin-Camiel L, Whelan JS. Tropical American plants in the treatment of infectious diseases. J Diet Suppl. 2008;5:349–372. doi: 10.1080/19390210802519648. [DOI] [PubMed] [Google Scholar]
- 128.Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, et al. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, et al. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. 2014;159:185–205. doi: 10.1007/978-3-642-38007-5_11. [DOI] [PubMed] [Google Scholar]
- 130.Li N, Li Q, Zhou XD, Kolosov VP, Perelman JM. The effect of quercetin on human neutrophil elastase-induced mucin5AC expression in human airway epithelial cells. Int Immunopharmacol. 2012;14:195–201. doi: 10.1016/j.intimp.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 131.Kwon SH, Nam JI, Kim SH, Kim JH, Yoon JH, Kim KS. Kaempferol and quercetin, essential ingredients in Ginkgo biloba extract, inhibit interleukin-1beta-induced MUC5AC gene expression in human airway epithelial cells. Phytother Res. 2009;23:1708–1712. doi: 10.1002/ptr.2817. [DOI] [PubMed] [Google Scholar]
- 132.Yang T, Luo F, Shen Y, An J, Li X, Liu X, et al. Quercetin attenuates airway inflammation and mucus production induced by cigarette smoke in rats. Int Immunopharmacol. 2012;13:73–81. doi: 10.1016/j.intimp.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 133.Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, Burgess JR, et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res. 2010;11:131. doi: 10.1186/1465-9921-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem Pharmacol. 2013;85:1579–1587. doi: 10.1016/j.bcp.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Cho K, Lee HJ, Lee SY, Woo H, Lee MN, Seok JH, et al. Oleanolic acid and ursolic acid derived from Cornus officinalis Sieb. et Zucc. suppress epidermal growth factor- and phorbol ester-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2011;25:760–764. doi: 10.1002/ptr.3488. [DOI] [PubMed] [Google Scholar]
- 136.Heo HJ, Kim C, Lee HJ, Kim YS, Kang SS, Seo UK, et al. Carbenoxolone and triterpenoids inhibited mucin secretion from airway epithelial cells. Phytother Res. 2007;21:462–465. doi: 10.1002/ptr.2102. [DOI] [PubMed] [Google Scholar]
- 137.Yang X, Li X, Ren J. From French Paradox to Cancer Treatment: Anti-cancer Activities and Mechanisms of Resveratrol. Anticancer Agents Med Chem. 2014;14:806–825. doi: 10.2174/1871520614666140521121722. [DOI] [PubMed] [Google Scholar]
- 138.Sengottuvelan M, Deeptha K, Nalini N. Influence of dietary resveratrol on early and late molecular markers of 1,2-dimethylhydrazine-induced colon carcinogenesis. Nutrition. 2009;25:1169–1176. doi: 10.1016/j.nut.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 139.Lee SY, Lee HJ, Sikder MA, Shin HD, Kim JH, Chang GT, et al. Resveratrol inhibits mucin gene expression, production and secretion from airway epithelial cells. Phytother Res. 2012;26:1082–1087. doi: 10.1002/ptr.3701. [DOI] [PubMed] [Google Scholar]
- 140.Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butt MS, Ahmad RS, Sultan MT, Nasir Qayyum MM, Naz A. Green tea and anticancer perspectives: Updates from last decade. Crit Rev Food Sci Nutr. 2013 doi: 10.1080/10408398.2012.680205. [DOI] [PubMed] [Google Scholar]
- 142.Kim HJ, Ryu JH, Kim CH, Lim JW, Moon UY, Lee GH, et al. Epicatechin gallate suppresses oxidative stress-induced MUC5AC overexpression by interaction with epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2010;43:349–357. doi: 10.1165/rcmb.2009-0205OC. [DOI] [PubMed] [Google Scholar]
- 143.Kim HJ, Park SH, Park SY, Moon UY, Lee BD, Yoon SH, et al. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced MUC5AC gene expression and MUC5AC secretion in normal human nasal epithelial cells. J Nutr Biochem. 2008;19:536–544. doi: 10.1016/j.jnutbio.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 144.Lu L, Liu HM, Tang WX. Effect of epigallocatechin-3-gallate on the invasiveness of hepatocarcinoma cells in vitro. Zhonghua Gan Zang Bing Za Zhi. 2007;15:825–827. [PubMed] [Google Scholar]
- 145.Wu H, Li Q, Zhou X, Kolosov VP, Perelman JM. Theaflavins extracted from black tea inhibit airway mucous hypersecretion induced by cigarette smoke in rats. Inflammation. 2012;35:271–279. doi: 10.1007/s10753-011-9314-8. [DOI] [PubMed] [Google Scholar]
- 146.Tojo R, Suarez A, Clemente MG, de Los Reyes-Gavilan CG, Margolles A, Gueimonde M, et al. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamada N, Kitamoto S, Yokoyama S, Hamada T, Goto M, Tsutsumida H, et al. Epigenetic regulation of mucin genes in human cancers. Clin Epigenetics. 2011;2:85–96. doi: 10.1007/s13148-011-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vincent A, Perrais M, Desseyn JL, Aubert JP, Pigny P, Van SI. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene. 2007;26:6566–6576. doi: 10.1038/sj.onc.1210479. [DOI] [PubMed] [Google Scholar]
- 149.Supic G, Jagodic M, Magic Z. Epigenetics: a new link between nutrition and cancer. Nutr Cancer. 2013;65:781–792. doi: 10.1080/01635581.2013.805794. [DOI] [PubMed] [Google Scholar]
- 150.Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236–245. doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guo ZR. Modification of natural products for drug discovery. Yao Xue Xue Bao. 2012;47:144–157. [PubMed] [Google Scholar]
- 153.Sun M, Su X, Ding B, He X, Liu X, Yu A, et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine (Lond) 2012;7:1085–1100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 154.Banerjee S, Azmi AS, Padhye S, Singh MW, Baruah JB, Philip PA, et al. Structure-activity studies on therapeutic potential of Thymoquinone analogs in pancreatic cancer. Pharm Res. 2010;27:1146–1158. doi: 10.1007/s11095-010-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deep G, Agarwal R. Targeting tumor microenvironment with silibinin: promise and potential for a translational cancer chemopreventive strategy. Curr Cancer Drug Targets. 2013;13:486–499. doi: 10.2174/15680096113139990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pooja D, Babu Bikkina DJ, Kulhari H, Nikhila N, Chinde S, Tiwari AK. Fabrication, characterization and bioevaluation of silibinin loaded chitosan nanoparticles. Int J Biol Macromol. 2014 doi: 10.1016/j.ijbiomac.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 157.Wang W, Luo M, Fu Y, Wang S, Efferth T, Zu Y. Glycyrrhizic acid nanoparticles inhibit LPS-induced inflammatory mediators in 264. 7 mouse macrophages compared with unprocessed glycyrrhizic acid. Int J Nanomedicine. 2013;8:1377–1383. doi: 10.2147/IJN.S37788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mishra D, Jain N, Rajoriya V, Jain AK. Glycyrrhizin conjugated chitosan nanoparticles for hepatocyte-targeted delivery of lamivudine. J Pharm Pharmacol. 2014 doi: 10.1111/jphp.12235. [DOI] [PubMed] [Google Scholar]
- 159.Ding B, Chen H, Wang C, Zhai Y, Zhai G. Preparation and in vitro evaluation of apigenin loaded lipid nanocapsules. J Nanosci Nanotechnol. 2013;13:6546–6552. doi: 10.1166/jnn.2013.7763. [DOI] [PubMed] [Google Scholar]
- 160.Das S, Das J, Samadder A, Paul A, Khuda-Bukhsh AR. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol Lett. 2013;223:124–138. doi: 10.1016/j.toxlet.2013.09.012. [DOI] [PubMed] [Google Scholar]