Abstract
Dietary n-3 PUFAs have been shown to attenuate T-cell-mediated inflammation. To investigate whether dietary n-3 PUFAs promote activation-induced cell death (AICD) in CD4+ T-cells induced in vitro to a polarized T-helper1 (Th1) phenotype, C57BL/6 mice were fed diets containing either 5% corn oil (CO; n-6 PUFA control) or 4% fish oil (FO) plus 1% CO (n-3 PUFA) for 2 weeks. Splenic CD4+ T-cells were cultured with α-interleukin-4 (αIL-4), IL-12, and IL-2 for 2 days and then with recombinant (r) IL-12 and rIL-2 for 3 days in the presence of diet-matched homologous mouse serum (HMS) to prevent loss of cell membrane fatty acids, or with fetal bovine serum. After polarization, Th1 cells were reactivated and analyzed for interferon-γ and IL-4 by intracellular cytokine staining and for apoptosis by Annexin V/propidium iodide. Dietary FO enhanced Th1 polarization by 49% (P = 0.0001) and AICD by 24% (P = 0.0001) only in cells cultured in the presence of HMS. FO enhancement of Th1 polarization and AICD after culture was associated with the maintenance of eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3) in plasma membrane lipid rafts. In conclusion, n-3 PUFAs enhance the polarization and deletion of proinflammatory Th1 cells, possibly as a result of alterations in membrane micro-domain fatty acid composition.
Supplementary key words: docosahexaenoic acid, eicosapentaenoic acid, lipid rafts, interferon-γ
The differentiation of naive CD4+ T-cells into T-helper1 (Th1) or Th2 effector cells is a critical process during immune responses (1). Th1 cells are characterized by the production of interleukin-2 (IL-2), IFNγ, and tumor necrosis factor-β (TNF-β) and are required to mount a cell-mediated immunological response against intracellular pathogens (1). Th1 cells are proinflammatory and have been implicated in the pathogenesis of human inflammatory and autoimmune diseases such as rheumatoid arthritis, type I diabetes, and inflammatory bowel disease (2). In contrast, Th2 cells are characterized by the production of IL-4, IL-5, and IL-10 and are important in humoral immunity and defense against extracellular pathogens, but they can promote allergy (1). Additionally, Th1 and Th2 effectors apparently have different susceptibilities to activation-induced cell death (AICD), with Th1 cells reported to be AICD sensitive and Th2 cells reported to be AICD resistant (3). The selective death of Th1 cells has been attributed to a preferential requirement for phorbol ester-sensitive protein kinase C isoforms (4) and the upregulation of FasL expression (5). In contrast, the resistance of Th2 cells to AICD has been linked to the expression of c-FLIP and FAP-1, inhibitors of apoptosis (3), and the selective upregulation of phosphatidylinositol-3′-kinase activity (6).
AICD is a form of apoptosis resulting from chronic antigen stimulation and is responsible for the peripheral deletion of previously activated lymphocytes. Apoptosis is a highly regulated process resulting in cell death without an ensuing inflammatory response, thus playing an important role in maintaining lymphocyte homeostasis and a balanced T-cell repertoire (7). The inability of T-cells to undergo AICD is associated with a variety of immunopathological diseases. The induction of AICD in CD4+ T-cells is mediated by interactions between Fas and its ligand (FasL). The Fas death receptor is a member of the TNF/nerve growth factor receptor superfamily (8). The binding of Fas by FasL results in receptor trimerization, recruitment of the Fas-associated death domain (FADD) to the death domain of Fas, and binding of caspase 8 to the death-effector domain of FADD (9). This process results in the formation of the death-inducing signaling complex (DISC). Activation of caspase 8 leads to the induction of a cascade of caspases culminating in apoptosis.
Recent studies on nontransformed human T-cells have shown that Fas and components of the DISC are recruited to lipid rafts after Fas ligation and that raft structures are required for the efficient propagation of apoptotic signals (9). Rafts are highly organized plasma membrane micro-domains rich in cholesterol and sphingolipids, and their polar lipids are predominantly composed of saturated fatty acyl chains (10). These lipids form liquid-ordered membrane regions that are insoluble in nonionic detergents, facilitating raft isolation as detergent-resistant membrane domains (10). Lipid raft domains play an important role in the localization and distribution of key receptors and associated signal-transducing molecules (11) and are crucial in regulating the susceptibility of T-cells to AICD (12). Interestingly, Th1 and Th2 cells have distinct resident protein/lipid membrane microdomain compositions (13). This is likely to affect T-cell signaling and thereby modulate effector function, which may help explain the differential susceptibility to AICD in the two Th subsets.
We have recently shown that dietary n-3 PUFAs remodel the phospholipid composition of membrane rafts in murine T-cells (14). In addition, Stulnig et al. (15) have shown that Jurkat T-cells cultured in vitro with PUFAs are capable of modifying lipid rafts and suppressing signal transduction. The antiinflammatory properties of diets rich in n-3 PUFAs on T-cell function have been firmly established in human and animal models (16–18). The primary effector molecules are thought to be eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3). In general, consumption of diets rich in n-3 PUFAs is associated with a reduced proinflammatory T-cell response attributable, in part, to a decreased proliferative capacity resulting from a reduction in IL-2 production and/or function and an increase in T-cell AICD (19, 20). Previously, we have shown that n-3 PUFAs promote AICD in T-cells induced to secrete a biased cytokine pattern resembling Th1 cells. However, the effect of n-3 PUFAs on polarized Th1 cell function has not been determined. In addition, previous research has shown that cell culture conditions, with respect to the lipid content in the serum added to tissue culture medium, have a significant influence on cell fatty acid composition (21, 22). Using homologous mouse serum (HMS) in the cultures, we examined the effect of dietary n-3 PUFAs on Th1 AICD and membrane microdomain fatty acid composition. Our results indicate that dietary n-3 PUFAs enhance both the polarization and the deletion of proinflammatory Th1 cells, possibly as a result of alterations in membrane microdomain fatty acid composition.
EXPERIMENTAL PROCEDURES
Diet and animals
All experimental procedures using laboratory animals were approved by the University Laboratory Animal Care Committee of Texas A&M University. Female, pathogen-free weanling (12–14 g) C57BL/6 mice (Frederick National Cancer Research Facility, Frederick, MD) were housed in autoclaved polycarbonate microisolator cages and maintained at room temperature (~25°C) on a 12 h light/dark cycle. Mice were fed standard nonpurified diet (Teklad 9F Sterilizable Rodent Diet) during a 1 week acclimation period and had free access to autoclaved water and diet. Thereafter, animals were randomly assigned to one of two semi-purified diets (24–30 mice/diet group): corn oil (CO; n-6 PUFA) or an n-3 PUFA-enriched menhaden fish oil (FO)-CO mixture (4:1, w/w) at 50 g/kg diet for 14 days (20). The purified diets met National Research Council requirements and varied only in lipid composition (23). The diet composition, expressed in grams per kilogram of complete diet, was as follows: 200 g of casein, 420 g of sucrose, 219.8 g of cornstarch, 60 g of cellulose, 35 g of AIN-76 mineral mix, 10 g of AIN-76 vitamin mix, 3 g of DL-methionine, 2 g of choline chloride, 0.2 g of tertiary butyl hydroquinone, and 5 g of oil (20). Diets were divided into aliquots and stored at −80°C. Diets were provided ad libitum and were changed daily to prevent peroxidation. The fatty acid composition as assessed by gas chromatography, expressed in grams per kilogram of complete diet, is detailed in Table 1. The linoleic acid (18:2 n-6) content was 5.5% and 1.4% of total energy in the CO and FO diets, respectively, and thus met the minimum 1–2% essential fatty acid requirement for rodents (24). Vitamin A, D, and E levels were approximately equal and exceeded the minimum requirement (20). CO was obtained from Degussa Bioactives (Champaign, IL), and menhaden FO was provided by the National Institutes of Health Test Materials Program. There was no significant difference in food intake between dietary groups, and weight gain was similar in all groups (data not shown).
TABLE 1.
Fatty acid composition of experimental diets
| Fatty Acid | CO | FO |
|---|---|---|
| g/100 g fatty acids | ||
| 14:0 | tr | 7.4 |
| 16:0 | 12.4 | 16.4 |
| 16:1 (n-7) | tr | 9.2 |
| 18:0 | 2.2 | 3.0 |
| 18:1 (n-7 + n-9) | 32.8 | 11.0 |
| 18:2 (n-6) | 50.2 | 12.5 |
| 18:3 (n-3) | 1.1 | 1.2 |
| 20:5 (n-3) | tr | 12.2 |
| 22:5 (n-3) | tr | 2.1 |
| 22:6 (n-3) | tr | 9.2 |
Only the major fatty acids are listed. CO, 5 g/100 g corn oil; FO, 4 g/100 g fish oil + 1 g/100 g corn oil; tr, trace amount (<0.1 g/100 g).
Splenocyte isolation and CD4+ T-cell enrichment
Mice were euthanized by CO2 asphyxiation. Spleens were placed in 3 ml of RPMI complete medium [RPMI 1640 with 25 mmol/l HEPES (Irvine Scientific, Santa Ana, CA) supplemented with 5% heat-inactivated fetal bovine serum (Irvine Scientific), 1 × 105 U/l penicillin and 100 mg/l streptomycin (Irvine Scientific), 2 mmol/l L-glutamine, and 10 μmol/l 2-mercaptoethanol]. Spleens were dispersed with glass homogenizers and passed through a 149 μm wire mesh filter to create single-cell suspensions. Total lymphocytes were initially enriched by density gradient centrifugation using Lympholyte-M (Cedarlane, Toronto, Canada) as previously described (20). Subsequently, ~120 × 106 mononuclear cells were loaded onto negative-selection CD4+ T-cell purification columns (R&D Systems, Minneapolis, MN). Nonadherent cells were eluted for assay. The purity of the T-cell population as analyzed by flow cytometry was determined to be 90.3 ± 1.4% (n = 3) (19).
Th1 polarization
Th1 cells were induced in vitro using standardized polarization methodology (25). In general, CD4+ T-cells (5 × 106 cells/well) were cultured in the presence of 1 ng/ml phorbol-12-myristate-13-acetate (PMA) (Sigma, St. Louis, MO) with 500 nM Ionomycin (Calbiochem-Novabiochem, San Diego, CA) combined with 10 μg/ml αIL-4 monoclonal antibody (National Cancer Institute), 20 ng/ml recombinant IL-2 (R&D Systems), and 5 ng/ml recombinant IL-12 (BD PharMingen, San Diego, CA) (Th1 cocktail) in either 5% FBS complete medium (FBS) or 2.5% HMS plus 2.5% FBS complete medium for 2 days at 37°C, 5% CO2. Cells were harvested and recultured in 20 ng/ml recombinant (r) IL-2 and 5 ng/ml rIL-12 for an additional 3 days at 37°C, 5% CO2 in the presence of FBS or HMS. The serum was changed each time cytokines and antibodies were provided (i.e., on days 2 and 3). Polarized Th1 cells were subsequently analyzed for intracellular cytokine expression and AICD.
Intracellular cytokine staining
After Th1 polarization, cultures were harvested, dead cells were removed by density gradient centrifugation using Lympholyte-M, and 1 × 106 cells/well were reactivated with PMA/Ionomycin with 3 μM monensin for 5 h at 37°C, 5% CO2. Cells were then washed, fixed, and permeabilized using a Cytofix/Cytoperm Kit (BD PharMingen) before staining with αIL-4-phycoerythrin (PE) (BD PharMingen) and αIFNγ-FITC (BD PharMingen). Cytokine staining was assessed via flow cytometry (FACScan; Becton Dickinson, Bedford, MA) as previously described (20).
Induction of AICD
After Th1 polarization, cultures were harvested, dead cells were removed by density gradient centrifugation using Lympholyte-M, and 1 × 106 cells/well were reactivated with PMA/Ionomycin for 5 h at 37°C, 5% CO2. Cells were then washed with cold 1× PBS and stained with 5 μl of FITC-conjugated Annexin V and 2 μl of propidium iodide (PI) for 15 min followed by flow cytometry analysis. Controls consisted of single staining for Annexin V-FITC only and PI only.
Density gradient centrifugation and isolation of lipid rafts
Raft microdomains were isolated from CD4+ T-cells as previously described by Tamir et al. (26) and Fan at al. (14) with slight modification immediately after isolation (day 0), after 5 days in culture with FBS, or after 5 days in culture with HMS. Cells were resuspended in lysis buffer [100 mmol/l NaCl, 2 mmol/l EDTA, 0.14 mmol/l 4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.2 mmol/l Na3VO4, 50 μmol/l aprotinin, 88 μmol/l leupeptin, 160 μmol/l bestatin, 60 μmol/l pepstatin A, and 56 μmol/l E-64, pH 6.9] supplemented with 1% Brij-58. Cell lysates were passed through a 27 gauge needle twice, followed by a 30 min incubation on ice. An 850 g/l sucrose solution in lysis buffer was added to the homogenate and mixed by pipetting to generate a 450 g/l sucrose lysate. Cell lysates were transferred to the bottom of a 2 ml polyallomer ultracentrifuge tube, which was subsequently overlaid with 350 and 50 g/l sucrose. After centrifugation at 200,000 g (Beckman Coulter Optima Max-E Ultracentrifuge, TLS 55 rotor) for 16 h at 4°C, aliquots from the top (low density detergent-insoluble glycerolipid-enriched raft fraction, liquid-ordered membrane rafts) and bottom (cytosol-high density membrane detergent-soluble fraction, liquid-disordered soluble fractions) of each tube were collected for lipid analysis. We have previously shown that the protein distribution patterns are consistent with the features of lipid rafts (i.e., enrichment of ganglioside GM-1 and exclusion of CD3, etc.) (14).
Analysis of phospholipid fatty acid composition
Total lipids in liquid-ordered membrane rafts and liquid-disordered soluble fractions were extracted by the method of Folch, Lees, and Sloane Stanley (27). Total phospholipids were separated by TLC on silica gel 60 G plates using chloroform-methanol-acetic acid-water (90:8:1:0.8, v/v) as the developing solvent. Bands were detected under UV light after spraying with 0.1% 8-anilino-naphthalene-sulfonic acid. Total phospholipids were scraped from the plates, spiked with heptadecanoic acid (17:0), and transesterified in the presence of 6% methanolic HCl (14). Fatty acid methyl esters were extracted using hexane and 0.1 M potassium chloride and analyzed by capillary gas chromatography as previously described (14).
Cholesterol analysis
After lipid extraction, cholesterol was analyzed using the Amplex Red Cholesterol Assay Kit (Molecular Probes, Eugene, OR). Total lipids in liquid-ordered membrane rafts and liquid-disordered soluble fractions were dried down under nitrogen and redissolved in reaction buffer. Samples were assayed in duplicate using a 300 μM solution of Amplex Red reagent mix containing 2 U/ml horseradish peroxidase, 2 U/ml cholesterol oxidase, and 2 U/ml cholesterol according to kit instructions.
Statistical analysis
Membrane lipid data were analyzed using a robust ANOVA method (28). A cell mean model was fitted using S-PLUS software (Insightful, Seattle, WA). Huber’s weights were implemented to downweight potentially outlying observations. This approach enables all data points to be considered without the disadvantage of having one or few outlying data points dominate and bias the outcomes. For the group containing no outlying observations, the cell means are identical to those obtained by the regular ANOVA. The corresponding t/F-tests were then used to test the existence of differences between treatment groups. The remaining data were analyzed using one-way ANOVA. Differences of P < 0.05 were considered significant.
RESULTS
Dietary lipids differentially affect Th1 polarization
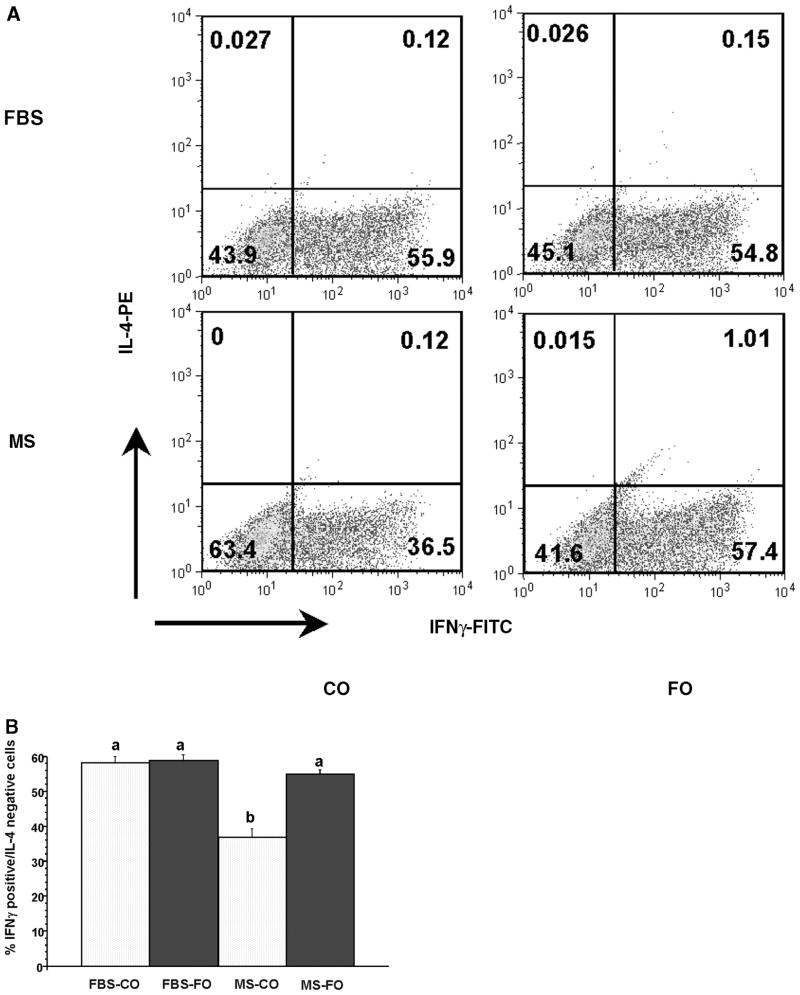
Th1 cells were induced in vitro using standardized polarization methodology (25). To verify that CD4+ T-cells were polarized toward a Th1 phenotype, cells were analyzed by flow cytometry for coexpression of INFγ and IL-4 using intracellular cytokine staining. Representative two parameter flow cytometric histograms of IFNγ-FITC- and IL-4-PE-labeled cells are shown in Fig. 1A. The numbers in each quadrant represent the percentage of cells positive for INFγ and/or IL-4. Figure 1B shows the effect of diet and culture conditions on cells positively expressing IFNγ but not IL-4. A majority of the cells were IFNγ+IL-4−, and less than 1% of the cells were IL-4+IFNγ− producing T-cells, indicating successful Th1 polarization. With respect to the effect of dietary treatment on Th1 polarization status, CD4+ T-cells from mice fed CO had significantly fewer IFNγ+ cells than FO-fed mice (36.9% in HMS-CO vs. 55.1% in HMS-FO; P = 0.0001), but the change was only observed in cells cultured in the presence of HMS (Fig. 1).
Fig. 1.
Diet-induced alteration of T-helper1 (Th1) cell polarization is observed only in the presence of homologous mouse serum [HMS (MS)]. Splenic CD4+ T-cells were isolated from mice fed corn oil (CO)- or fish oil (FO)-containing diets for 2 weeks followed by polarization toward Th1 in the presence of HMS or FBS. After 5 days in culture, cells were reactivated with phorbol-12-myristate-13-acetate (PMA)/Ionomycin in the presence of monensin and assessed for the coexpression of IFNγ and interleukin-4 (IL-4) by intracellular cytokine staining as described in Experimental Procedures. A: Representative two parameter flow cytometric histograms of HMS- versus FBS-cultured cells. Numbers in each quadrant represent the percentage of cells positive for IFNγ and/or IL-4. B: Bars with different letters denote significant differences (P < 0.05). Data represent means ± SEM; n = 6 replicates per diet group, and 4 mice were pooled per analysis.
The fatty acid composition of HMS and FBS, as shown in Table 2, revealed that FBS was relatively devoid of n-3 PUFAs (20:5n-3, 22:5n-3, 22:6n-3). With respect to the effect of dietary source on HMS, serum from FO- versus CO-fed mice had an increased n-3/n-6 PUFA molar ratio, markedly lower levels of saturated and monounsaturated fatty acids and n-6 PUFAs (18:2n-6, 20:4n-6), and was highly enriched in 20:5n-3.
TABLE 2.
Fatty acid composition of sera used for cell culture
| Fatty Acid | FBS | HMS-CO | HMS-FO |
|---|---|---|---|
| μg fatty acid/ml serum | |||
| 16:0 | 1.71 ± 0.02 | 14.20 ± 0.55 | 5.03 ± 0.22 |
| 18:0 | 0.80 ± 0.02 | 7.93 ± 0.20 | 1.84 ± 0.07 |
| 18:1n-9/n-7 | 1.63 ± 0.05 | 15.54 ± 0.22 | 3.82 ± 0.15 |
| 18:2n-6 | 0.40 ± 0.01 | 17.72 ± 0.37 | 2.10 ± 0.09 |
| 20:4n-6 | 0.53 ± 0.01 | 12.32 ± 0.63 | 1.12 ± 0.07 |
| 20:5n-3 | tr | tr | 2.99 ± 0.07 |
| 22:5n-3 | 0.15 ± 0.0 | tr | 0.22 ± 0.02 |
| 22:6n-3 | 0.17 ± 0.01 | 2.24 ± 0.24 | 2.22 ± 0.13 |
| n-3/n-6 | 0.34 ± 0.00 | 0.07 ± 0.01 | 0.76 ± 0.04 |
Homologous mouse serum (HMS) from CO- and FO-fed mice was extracted and analyzed to assess fatty acid composition. FBS values are shown for comparison. Values represent means ± SEM; n = 3. tr, trace amount (<0.1 μg/ml).
n-3 PUFAs promote Th1 AICD
To assess the effect of diet on AICD after Th1 polarization, cells were reactivated and analyzed for apoptosis using Annexin V-FITC/PI labeling. Annexin V binding is an excellent marker for apoptotic cells because the early distribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus (12, 29, 30). To rule out the possibility of primary necrosis, Fas-Fc, an inhibitor of the Fas death receptor, and z-VAD-fmk (50 μmol/l), a pan-caspase inhibitor, were added to select cultures (20). Unreactivated CD4+ T-cells from both diet groups had equivalent levels of apoptosis (13.53 ± 0.77% in FO vs. 13.32 ± 0.72% in CO treatments; n = 36 per diet) after 3 days in culture. Upon re-stimulation (reactivation), apoptosis was increased 2-fold (data not shown), consistent with our previously published results (20). These data indicate that T-cells were not compromised in any way before restimulation.
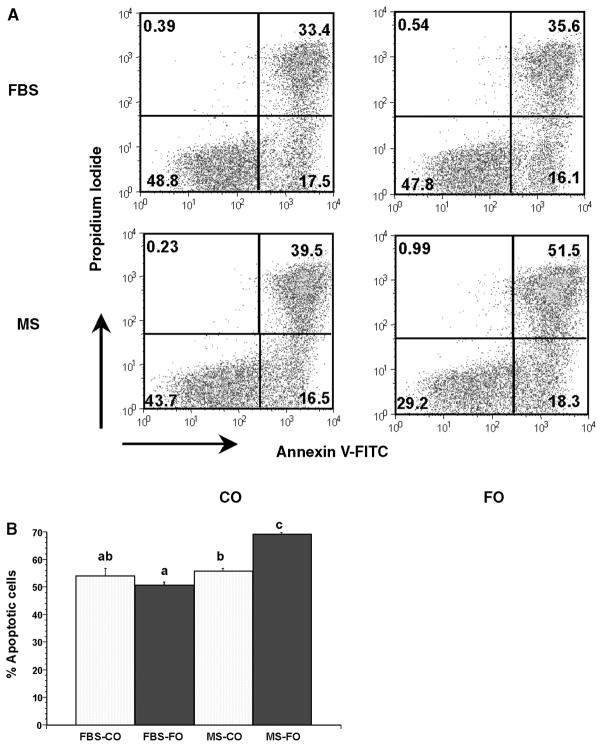
Representative two parameter flow cytometric histograms of Annexin V-FITC- and PI-labeled cells are shown in Fig. 2A. The numbers in each quadrant represent the percentage of cells positive for Annexin V-FITC and/or PI. The relative number of apoptotic cells (indicated by Annexin V positive, PI negative and Annexin V positive, PI positive) from CD4+ T-cells from CO- and FO-fed mice polarized in FBS or HMS is detailed in Fig. 2B. CD4+ T-cells from FO-fed mice exhibited a statistically significant enhancement in AICD (55.9% in HMS-CO vs. 69.3% in HMS-FO; P = 0.0001). Similar to the polarization results illustrated above (Fig. 1), this effect only occurred in HMS-cultured cells, as this phenotype was lost when cells were cultured in FBS. In other words, dietary changes in CD4+ T-cell function were maintained in cells cultured in HMS but were lost when cells were cultured in FBS during the 5 day polarization period.
Fig. 2.
Dietary n-3 PUFAs promote CD4+ T-cell activation-induced cell death (AICD) only in the presence of HMS (MS). Splenic CD4+ T-cells were isolated from mice fed CO- or FO-containing diets for 2 weeks followed by polarization toward Th1 in the presence of HMS or FBS. After 5 days in culture, cells were reactivated with PMA/Ionomycin and assessed for apoptosis by Annexin V-FITC and propidium iodide (PI) staining as described in Experimental Procedures. A: Representative two parameter flow cytometric histograms of HMS- versus FBS-cultured cells. Numbers in each quadrant represent the percentage of cells positive for Annexin V-FITC and/or PI. B: Bars with different letters denote significant differences (P < 0.05). Data represent means ± SEM; n = 6 replicates per diet group, and 4 mice were pooled per analysis.
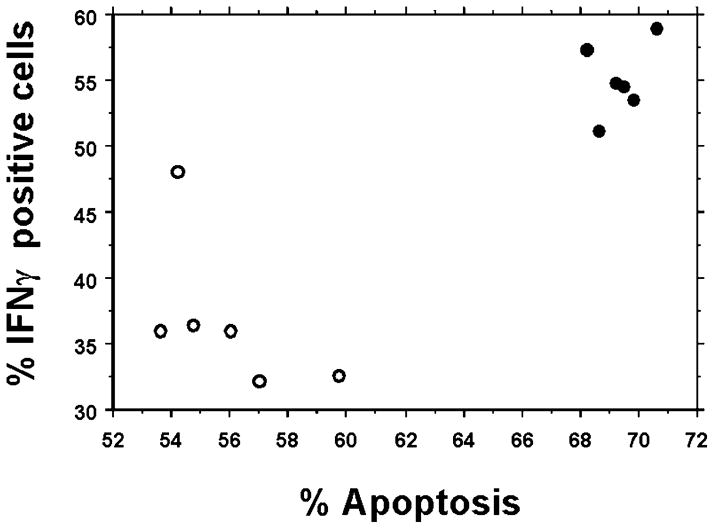
To determine the relationship between cell polarization status and the amount of AICD induced, a correlation analysis of IFNγ+ cells versus apoptotic cells was performed. Figure 3 shows the correlation of HMS-CO and HMS-FO CD4+ T-cells. Both the Spearman and Pearson correlation tests indicated that there was a significant positive correlation (P = 0.0119 and P < 0.0005, respectively) between IFNγ+ cells and the number of apoptotic cells when the data from the HMS-CO and HMS-FO cultures were analyzed together. There was also a statistically significant positive correlation, P = 0.0139 (Spearman test) and P = 0.0073 (Pearson test), in FBS-cultured cells (data not shown).
Fig. 3.
AICD and Th1 differentiation are highly correlated in mouse serum-cultured CD4+ T-cells. The relationship between AICD and intracellular IFNγ levels was examined by both Spearman and Pearson tests of IFNγ+ cells versus apoptotic cells. Closed circles represent HMS-cultured cells isolated from FO-fed animals, and open circles represent HMS-cultured cells isolated from CO-fed animals. Data represent means ± SEM; n = 6 replicates per diet group, and 4 mice were pooled per analysis (P < 0.05).
Effect of culture conditions on Th1 cell membrane microdomains
To clarify how dietary n-3 PUFA membrane enrichment is influenced by the 5 day culture period necessary for Th1 polarization, we examined lipid raft (liquid-ordered) and soluble (liquid-disordered) membrane fractions from CD4+ T-cells immediately after isolation (day 0) and after 5 days in culture with either FBS or HMS. Table 3 shows the effect of diet and culture conditions on the fatty acid composition of raft and soluble membrane domains. Consistent with previous reports (15, 31), the raft membrane fraction had a 36% greater cholesterol-to-phospholipid molar ratio compared with the soluble fractions (P = 0.07) (data not shown). In addition, the total phospholipid unsaturation index was lower in rafts than in soluble membrane fractions (Table 3). Collectively, these data indicate that proper fractionation was accomplished.
TABLE 3.
Fatty acid composition in raft and soluble membrane fractions of CO- and FO-fed mouse CD4+ T-cells
| Fatty Acid | Raft
|
Soluble
|
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 5 (FBS) | Day 5 (HMS) | Day 0 | Day 5 (FBS) | Day 5 (HMS) | |
| mol% | ||||||
| CO fed | ||||||
| SFA | 70.57 ± 0.76 | 69.33 ± 2.04 | 68.97 ± 1.30 | 55.13 ± 0.81 | 53.69 ± 1.46 | 54.88 ± 6.25 |
| MUFA | 10.72 ± 0.43a | 20.30 ± 1.79b | 12.57 ± 0.38a | 10.27 ± 1.60a | 31.09 ± 2.19b | 14.82 ± 1.43a |
| PUFA | 18.70 ± 1.01b | 10.38 ± 1.26a | 18.46 1.58b | 34.59 ± 1.87b | 15.22 ± 1.87a | 30.30 ± 5.15b |
| n-6 PUFA | 15.65 ± 2.43b | 8.58 ± 1.45a | 15.29 ± 1.71b | 28.07 ± 1.27b | 12.78 ± 1.66a | 25.67 ± 5.45b |
| n-3 PUFA | 3.05 ± 2.50 | 1.79 ± 0.30 | 3.17 ± 0.71 | 6.52 ± 1.38b | 2.44 ± 0.35a | 4.64 ± 0.36a,b |
| n-3/n-6 | 0.26 ± 0.23 | 0.23 ± 0.06 | 0.21 ± 0.06 | 0.23 ± 0.05 | 0.19 ± 0.03 | 0.21 ± 0.06 |
| UId | 54.79 ± 3.44a,b | 36.81 ± 3.62a | 65.35 ± 4.36b | 124.24 ± 8.25b | 53.47 ± 5.80a | 99.58 ± 17.26b |
| 18:2n-6 | 3.76 ± 1.96 | 2.61 ± 0.42 | 4.82 ± 0.69 | 6.44 ± 0.33b | 3.52 ± 0.49a | 12.74 ± 0.77c |
| 20:3n-6 | 3.32 ± 1.50b | 0.59 ± 0.35a | 2.05 ± 0.61a,b | 8.36 ± 0.94b | 2.48 ± 1.2a | 1.11 ± 0.56a |
| 20:4n-6 | 4.69 ± 0.68 | 3.72 ± 0.45 | 6.49 ± 0.58 | 11.43 ± 1.88b | 5.14 ± 0.26a | 8.33 ± 3.39ab |
| 20:5n-3 | tr | tr | 0.29 ± 0.16 | 0.45 ± 0.45 | tr | tr |
| 22:6n-3 | 0.61 ± 0.31 | 1.01 ± 0.17 | 1.95 ± 0.12 | 2.42 ± 0.50a,b | 1.29 ± 0.14a | 3.80 ± 0.24b |
| FO fed | ||||||
| SFA | 71.22 ± 3.07 | 76.52 ± 5.01 | 71.02 ± 1.58 | 65.16 ± 1.57 | 53.15 ± 0.83 | 54.39 ± 2.79 |
| MUFA | 12.64 ± 2.51 | 13.50 ± 4.07 | 13.62 ± 1.66 | 10.87 ± 0.79a | 30.23 ± 0.29c | 19.16 ± 3.61b |
| PUFA | 16.14 ± 1.25 | 9.98 ± 1.22 | 15.36 ± 0.35 | 23.97 ± 1.37a,b | 16.63 ± 0.67a | 26.45 ± 6.37b |
| n-6 PUFA | 13.93 ± 1.39b | 7.80 ± 1.20a | 9.03 ± 0.15a,b | 16.28 ± 0.64 | 12.52 ± 0.56 | 14.26 ± 2.93 |
| n-3 PUFA | 2.21 ± 0.29a | 2.18 ± 0.04a | 6.34 ± 0.49b | 7.69 ± 1.92a | 4.10 ± 0.68a | 12.19 ± 3.44b |
| n-3/n-6 | 0.17 ± 0.03a | 0.30 ± 0.05a | 0.70 ± 0.07b | 0.48 ± 0.13a | 0.33 ± 0.06a | 0.82 ± 0.09b |
| UI | 54.24 ± 3.67 | 36.96 ± 3.99 | 60.75 ± 2.21 | 97.36 ± 10.03b | 63.42 ± 3.92a | 109.55 ± 27.50b |
| 18:2n-6 | 3.39 ± 0.28 | 2.00 ± 0.46 | 4.15 ± 0.55 | 4.78 ± 1.48a,b | 2.78 ± 0.42a | 6.01 ± 1.02b |
| 20:3n-6 | 2.43 ± 0.85 | 1.42 ± 0.22 | 0.81 ± 0.81 | 0.82 ± 0.42 | 1.66 ± 0.09 | 1.04 ± 0.52 |
| 20:4n-6 | 5.20 ± 2.02 | 3.48 ± 0.68 | 2.54 ± 0.20 | 10.30 ± 0.86b | 6.43 ± 0.74a,b | 5.56 ± 1.27a |
| 20:5n-3 | 0.83 ± 0.66 | tr | 0.26 ± 0.14 | 1.14 ± 0.62 | 0.26 ± 0.13 | 0.96 ± 0.50 |
| 22:6n-3 | 2.47 ± 1.27a,b | 1.17 ± 0.06a | 3.61 ± 0.38b | 4.54 ± 0.34b | 1.92 ± 0.04a | 6.26 ± 1.31c |
Splenic CD4+ T-cells were isolated from mice fed CO- or FO-containing diets for 2 weeks followed by membrane preparation (day 0) or cultured in the presence of either 5% FBS complete medium or 5% HMS complete medium for 5 days. Rafts and soluble membrane fractions were isolated, and fatty acid composition was analyzed. Values represent means ± SEM; n = 3, and 10 mice were pooled per analysis. SFA, saturated fatty acids; tr, trace amounts (<0.1 mol%).
Letters indicate significant differences between culture conditions of the same diet and membrane fraction (P < 0.05).
Unsaturation index (UI) represents the summed mol% multiplied by the number of double bonds.
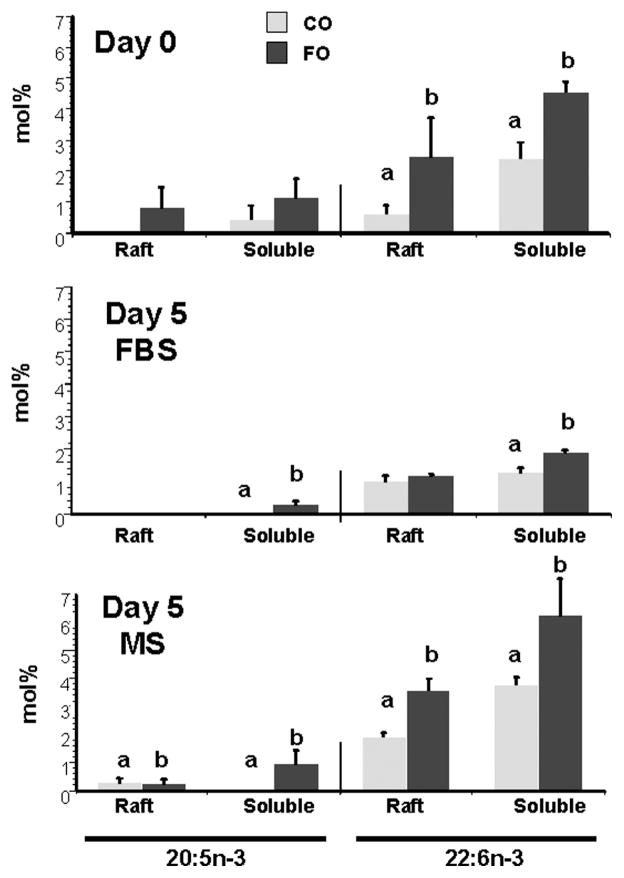
Gas chromatographic analysis of the fatty acid composition of cell phospholipids showed that the amount of DHA (22:6n-3) in both raft and soluble fractions was decreased in FBS cultures of CD4+ T-cells from FO-fed mice 5 days after culture (Table 3). In contrast, the inclusion of HMS prevented the culture-induced rearrangement of T-cell membrane lipids. This phenomenon was observed with respect to other PUFA and fatty acid classes in both diets and both membrane fractions (Table 3). Examination of day 0 and day 5 membrane microdomain distributions of EPA (20:5n-3) and DHA (22:6n-3) from CO- and FO-fed mouse CD4+ T-cells (Fig. 4) revealed that the significant enhancement of these two n-3 PUFAs in the CD4+ T-cells of FO-fed, compared with CO-fed, mice was lost in the rafts from CD4+ T-cells in the FBS cultures.
Fig. 4.
Membrane microdomain distribution of eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) in CO- and FO-fed mouse CD4+ T-cells. Splenic CD4+ T-cell membrane fractions were isolated from mice fed CO- or FO-containing diets for 2 weeks either immediately (day 0) or after culture in the presence of either 5% FBS complete medium or HMS for 5 days. Raft and soluble membrane fractions were isolated, and EPA and DHA were analyzed. Data represent means ± SEM; n = 3 replicates per diet group, and 10 mice were pooled per analysis. Letters indicate significant differences between diets for the same membrane fraction and fatty acid (P < 0.05).
Because cholesterol is critical for raft integrity (10), we examined the cholesterol level by assessing the molar cholesterol-to-phospholipid ratio in the various cell membrane fractions. Figure 5 shows the effect of diet and culture conditions on the molar ratio of cholesterol to total phospholipid. In both diets groups, the cholesterol-to-phospholipid ratio decreased significantly from day 0 to day 5 (P < 0.02) in CD4+ T-cell membrane rafts. However, in cultures of CD4+ T-cells from FO-fed, but not CO-fed, mice, the addition of HMS prevented the culture-induced loss of cholesterol. In comparison, in soluble membrane fractions, a significant reduction (P < 0.05) in the cholesterol-to-phospholipid ratio occurred over time regardless of culture conditions (Fig. 5).
Fig. 5.
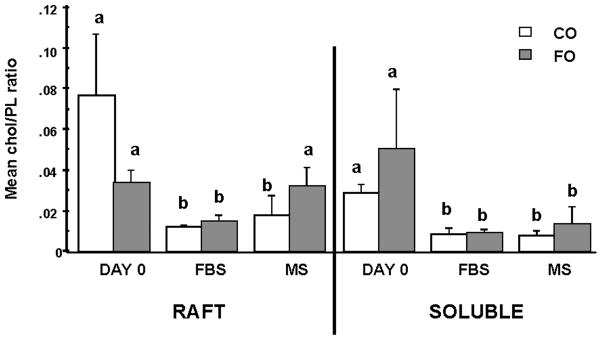
CD4+ T-cells from n-3 PUFA-fed mice cultured in the presence of homologous HMS maintain the cholesterol content of lipid rafts. Splenic CD4+ T-cells were isolated from mice fed CO- or FO-containing diets for 2 weeks followed by membrane isolation (day 0) or after culture in the presence of either 5% FBS complete medium (FBS) or HMS (MS) for 5 days. Raft and soluble membrane fractions were isolated, and total phospholipid (PL) and cholesterol (chol) levels were expressed as a mol% ratio. Data represent means ± SEM; n = 3 replicates per diet group, and 10 mice were pooled per analysis. Letters indicate significant differences between culture conditions for the same membrane fraction and diet (P < 0.05).
DISCUSSION
Among dietary factors, there is overwhelming evidence for a protective effect of n-3 PUFAs on autoimmune/inflammatory diseases (16–18). In contrast, dietary lipids rich in n-6 PUFAs can be deleterious with respect to the incidence and severity of inflammatory diseases. This is significant because the typical Western diet contains 10–20 times more n-6 than n-3 PUFAs (32). Additionally, the immunosuppressive effects of diets rich in n-3 PUFAs on a variety of T-cell functions have been firmly established in both human and animal models (16–18, 33–35). With respect to the physiological relevance of the diets used in our study, the FO diet contained ~1.4% and 1.0% of energy as EPA and DHA, respectively. As a point of reference, the Japanese typically consume n-3 PUFAs at 1–2% of energy in their diet (36), whereas most European countries and the United States consume 0.1–0.2% of energy as n-3 PUFAs (37). Therefore, the n-3 PUFA content of the experimental diets used in this study was well within the range consumed by humans.
We have observed previously that the dietary effect of n-3 PUFAs on T-cell proliferation and apoptosis depended upon the propensity of the in vitro stimulus to bias T-cells toward a predominantly IL-2/IFNγ-producing population (a putative Th1-like subset) or an IL-4/IL-10-producing population (a putative Th2-like subset) (19, 20). Specifically, n-3 PUFAs increased proliferation only in CD4+ T-cells stimulated with αCD3/PMA, a Th2-biasing stimulus (19). With respect to apoptosis, we have previously shown that n-3 PUFAs enhanced AICD in T-cells induced to secrete a biased cytokine pattern, resembling Th1 cells, after stimulation with PMA/Ionomycin (20). Therefore, we hypothesized that n-3 PUFAs would promote AICD in polarized Th1 cells. CD4+ T-cells were driven to differentiate in vitro toward a Th1 cytokine pole using standard polarization methodology (25). Because of the extended time in culture to achieve polarization (5 days), a set of cells from each diet group was cultured in diet-matched HMS, rather than fetal bovine serum, to prevent the culture-induced loss of n-3 PUFAs from membrane phospholipids. Previous research has shown that cell culture conditions have a significant influence on lymphocyte bulk membrane fatty acid composition (21, 22). Yaqoob, Newsholme, and Calder (21) reported that culturing lymphocytes in medium containing autologous serum, which had a fatty acid composition closely resembling that of the diet, allowed the maintenance of diet-induced changes in fatty acid composition. In addition, culturing in autologous or homologous serum was shown to have a significant effect on lymphocyte function. Yaqoob, Newsholme, and Calder (38) and Pompos and Fritsche (22) reported that n-3 PUFAs decreased T-cell proliferation when cells were cultured in autologous/homologous serum but had little effect when the same cells were cultured in standard medium. These results provided the rationale to include HMS in our cultures.
To verify that CD4+ T-cells were polarized toward a Th1 phenotype, cells were analyzed for the coexpression of IFNγ and IL-4. Consistent with previous reports (25), a majority of the cells were IFNγ+IL-4−, indicating a predominant Th1 phenotype (Fig. 1). A noteworthy outcome from these analyses was that dietary CO suppressed Th1 polarization relative to FO; however, this effect only occurred in cells cultured in the presence of HMS (Fig. 1). These results are not consistent with findings from other researchers who have shown that n-3 PUFAs decrease IFNγ (39, 40). Specifically, Fritsche, Byrge, and Feng (39) reported decreased serum IFNγ and splenic IFNγ mRNA levels in mice fed FO compared with n-6 PUFA control diets. In contrast, other studies indicate that n-3 PUFAs increase IFNγ production (41–43). There are several reasons for these apparent discrepancies. Fritsche, Byrge, and Feng (39) and Wallace et al (40), who also reported decreased IFNγ after FO feeding, analyzed whole splenocyte populations and cultured cells in the presence of fetal bovine serum before IFNγ analysis. In contrast, Oarada et al. (41) and Fritsche, Feng, and Berg (42) detected enhanced IFNγ when samples were assayed directly from n-3 PUFA-fed animals (i.e., cells were not maintained in culture). The fact that a number of in vivo experiments show an increase in IFNγ production after n-3 PUFA feeding supports the conclusion that diet-induced changes in T-cell cytokine production can be masked by culture conditions.
Because dietary lipids are incorporated into T-cell membrane phospholipids (20), we investigated the effect of culture conditions on CD4+ T-cell membranes. Recent data generated in our laboratory have shown that n-3 PUFAs remodel T-cell lipid rafts (14); therefore, we examined plasma membrane microdomains (i.e., rafts). Interestingly, only in rafts isolated from CD4+ T-cells from FO-fed mice did the addition of HMS prevent the culture-induced loss of cholesterol (Fig. 5). These data are noteworthy because perturbations in lipid raft integrity/composition directly mediate T-cell AICD (12). Rafts play an important role in cell signaling, particularly through the organization and distribution of surface receptors, including Fas, at specific sites in the plasma membrane (12, 44). Recent studies have shown that the formation of macromolecular complexes containing the T-cell receptor, CD4, and CD45 is believed to contribute to sustained T cell receptor (TCR) interaction with its ligand (11). Lipid rafts are important for the formation and stabilization of these TCR signaling complexes, acting as platforms that facilitate intramolecular associations and the propagation of signal transduction cascades (11). Interestingly, conditions that modify raft structure can disrupt early steps in T-cell activation (45). Rafts appear to differ depending on the developmental state of the T-cell, and these differences probably contribute to markedly different outcomes of signaling (13). Effector and memory T-cells have more surface rafts compared with naïve T-cells, and activated Th1 cells differ from activated Th2 cells in raft organization. Stimulation of Th1 cells results in a stable association of TCR components with raft domains, whereas Th2 stimulation fails to form these signaling complexes (13). Therefore, our data are noteworthy because this is one of the first studies to examine the effect of diet on raft modification and its relationship to T-cell activation.
With respect to AICD, raft structures are required for the efficient propagation of apoptotic signals. The death receptor and primary initiator of AICD, Fas, has been shown to require clustering and capping at the membrane to effectively signal to downstream apoptotic molecules (46). This clustering and capping occurs in rafts, a location that best facilitates the trapping of Fas, recruitment of additional intracellular molecules of the DISC, and exclusion of inhibitory pathways. Th1 and Th2 cells have different susceptibilities to AICD that are possibly explained, in part, by their distinct lipid raft compositions (13). Our analysis showed that CD4+ T-cells from FO-fed mice cultured in the presence of HMS exhibited significantly enhanced AICD (Fig. 2), i.e., the diet and culture conditions that promoted the greatest number of Th1 cells also enhanced AICD to the greatest extent. These data extend our earlier findings in which we demonstrated that alterations in dietary lipid composition can directly influence AICD (20). In these experiments, after a 2 week feeding period, T-cells were isolated from mice on diet and subsequently cultured for 3 days in the presence of FBS. Only T-cells from n-3 PUFA-fed mice had increased levels of AICD. These observations clearly indicate that the tendency to undergo AICD is already established in vivo. Therefore, the use of HMS does not create a new phenotype. Interestingly, there was a significant positive correlation between the polarization status of the cell and the amount of AICD induced in HMS cells (Fig. 3). The enhancement of AICD was not observed in FBS cultures in which IFNγ was suppressed. Because Th1 cells are susceptible to AICD (3), our data suggest that n-3 PUFAs may indirectly enhance AICD via the promotion of a Th1 phenotype.
It has been reported that IFNγ is required for T-cell AICD (47). IFNγ signaling results in the activation of Stat-1, which translocates to the nucleus and induces the expression of caspase 8, the initiator caspase associated with the DISC. Thus, IFNγ and Stat-1 are involved in apoptosis mediated by death receptors. Refaeli et al. (47) showed that T-cells from IFNγ−/− or Stat-1−/− mice are unable to undergo apoptosis. Our data indicating that dietary FO enhances the number of IFNγ-producing CD4+ T-cells relative to dietary CO (Fig. 1) suggest that IFNγ may mediate the n-3 PUFA enhancement of AICD.
The IFNγ receptor (IFNγR) has recently been found to be recruited to raftlike domains in T-cells after IFNγ stimulation (48). IFNγ binding and receptor relocalization to rafts is followed by IFNγR endocytosis and translocation of the IFNγ/IFNγR/Stat-1 complex to the nucleus (48). Because our data clearly show that dietary n-3 PUFAs remodel CD4+ T-cell lipid rafts (Figs. 4, 5, Table 3), it is possible that n-3 PUFAs modulate IFNγ signaling by enhancing IFNγR raft localization as a result of alterations in raft composition. Experiments are in progress to test this hypothesis.
Alterations in the methylation status of the IFNγ promoter play a critical role in regulating IFNγ production (49). For example, DNA methylation may interfere with transcription by preventing the recruitment of acetylase to the local chromatin, thereby inhibiting acquisition of the open conformation suitable for the recruitment of transcription factors (49). Recently, it has been shown that the IFNγ promoter undergoes differential methylation during in vitro differentiation, with the promoter being in a hypomethylated state in Th1 cells and hypermethylated in Th2 cells (49). Interestingly, prostaglandin E2 (PGE2) inhibits IFNγ promoter hypomethylation (50). This is noteworthy because n-3 PUFAs decrease PGE2 production (51), whereas n-6 PUFAs promote the synthesis of PGE2 (52). Overall, these data suggest that the reduced IFNγ expression in n-6 PUFA-fed mice in our studies (Fig. 1) might be attributable to reduced IFNγ hypomethylation mediatedby PGE2. Additional studies to address the involvement of this mechanism are in progress.
Another mechanism by which n-3 PUFAs could alter CD4+ T-cell polarization might involve peroxisome proliferator-activated receptors (PPARs), specifically PPARγ. PPARγ has been shown to inhibit IL-4 production in CD4+ T-cells (53). However, PPARγ also reduces IFNγ and IL-2 in human T-cells (54), indicating that PPARγ is not selective in T-cell subset repression. Additionally, PPARγ binds both n-3 and n-6 PUFAs with equal affinity and lacks fatty acid class specificity (55, 56). Therefore, the effects of n-3 PUFAs observed in this study are likely not mediated by PPARs.
In conclusion, our data support the hypothesis that dietary n-3 PUFAs promote AICD in CD4+ T-cells that are polarized toward the Th1 phenotype in vitro. Susceptibility to AICD in T-cells from FO-fed mice appears to be an indirect result of the propensity of those cells to be induced to acquire a Th1 phenotype (i.e., to produce IFNγ). We demonstrated the requirement for HMS in long-term cultures to preclude the loss of diet-derived n-3 PUFAs from lipid rafts. Furthermore, we have shown for the first time that the maintenance of diet-induced membrane alterations in vitro is required to observe the biological impact of diet on CD4+ T-cell function.
Acknowledgments
The authors thank Roger Smith for assistance with flow cytometry analyses and Lan Ly for assistance with the collection and purification of T-cells and the establishment of in vitro cultures. This work was supported by National Institutes of Health Grants DK-53055 and P30 ES-09106 and by U.S. Department of Agriculture Grant 2003-35200-13338.
Abbreviations
- AICD
activation-induced cell death
- CO
corn oil
- DHA
docosahexaenoic acid
- DISC
death-inducing signaling complex
- EPA
eicosapentaenoic acid
- FADD
Fas-associated death domain
- FO
fish oil
- HMS
homologous mouse serum
- IFNγR
interferon-γ receptor
- IL
interleukin
- PGE2
prostaglandin E2
- PI
propidium iodide
- PMA
phorbol-12-myristate-13-acetate
- PPAR
peroxisome proliferator-activated receptor
- Th
T-helper
- TNF-β
tumor necrosis factor-β
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Brunner T, Carter L, Dutton RW, Rogers P, Bradley L, Sato T, Reed JC, Green D, Swain SL. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahata T, Abe N, Yahata C, Ohmi Y, Ohta A, Iwakabe K, Habu S, Yagita H, Kitamura H, Matsuki N, Nakui M, Sato M, Nishimura T. The essential role of phorbol ester-sensitive protein kinase C isoforms in activation-induced cell death of Th1 cells. Eur J Immunol. 1999;29:727–732. doi: 10.1002/(SICI)1521-4141(199903)29:03<727::AID-IMMU727>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 6.Varadhachary AS, Edidin M, Hanlon AM, Peter ME, Krammer PH, Salgame P. Phosphatidylinositol 3′-kinase blocks CD95 aggregation and caspase-8 cleavage at the death-inducing signaling complex by modulating lateral diffusion of CD95. J Immunol. 2001;166:6564–6569. doi: 10.4049/jimmunol.166.11.6564. [DOI] [PubMed] [Google Scholar]
- 7.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 8.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 9.Scheel-Toellner D, Wang K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun. 2002;297:876–879. doi: 10.1016/s0006-291x(02)02311-2. [DOI] [PubMed] [Google Scholar]
- 10.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 11.Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and Th2 cells. Immunity. 2001;15:729–738. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 12.Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- 13.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signaling complex and membrane microdomains during T cell development and activation. Semin Immunol. 2001;13:129–138. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]
- 14.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 15.Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 16.Calder PC. Dietary fatty acids and the immune system. Nutr Rev. 1998;56(Suppl):70–83. doi: 10.1111/j.1753-4887.1998.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapkin RS, McMurray DN, Jolly CA. Dietary n-3 polyunsaturated fatty acids modulate T cell lymphocyte activation: clinical relevance in treating diseases of chronic inflammation. In: Gershwin ME, German B, Keen C, editors. Nutrition and Immunology: Principles and Practice. Plenum; New York: 1999. pp. 121–134. [Google Scholar]
- 18.Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71(Suppl):349–351. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- 19.Arrington JL, Chapkin RS, Switzer KC, Morris JS, McMurray DN. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin Exp Immunol. 2001;125:499–507. doi: 10.1046/j.1365-2249.2001.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Switzer KC, McMurray DN, Morris JS, Chapkin RS. (n-3) polyunsaturated fatty acids promote activation-induced cell death in murine T lymphocytes. J Nutr. 2003;133:496–503. doi: 10.1093/jn/133.2.496. [DOI] [PubMed] [Google Scholar]
- 21.Yaqoob P, Newsholme EA, Calder PC. Influence of cell culture conditions on diet-induced changes in lymphocyte fatty acid composition. Biochim Biophys Acta. 1995;1255:333–340. doi: 10.1016/0005-2760(94)00251-s. [DOI] [PubMed] [Google Scholar]
- 22.Pompos LJ, Fritsche KL. Antigen-driven murine CD4+ T lymphocyte proliferation and interleukin-2 production are diminished by dietary (n-3) polyunsaturated fatty acids. J Nutr. 2002;132:3293–3300. doi: 10.1093/jn/132.11.3293. [DOI] [PubMed] [Google Scholar]
- 23.Arrington JL, McMurray DN, Switzer KC, Fan YY, Chapkin RS. Docosahexaenoic acid suppresses function of the CD28 costimulatory membrane receptor in primary murine and Jurkat T cells. J Nutr. 2001;131:1147–1153. doi: 10.1093/jn/131.4.1147. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council. Nutrient requirements for laboratory animals. 4. National Academic Press; Washington, D.C: 1995. [Google Scholar]
- 25.Yamashita M, Katsumata M, Iwashima M, Kimura M, Shimizu C, Kamata T, Shin T, Seki N, Suzuki S, Taniguchi M, Nakayama T. T cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the inter-leukin 4 receptor signaling complex. J Exp Med. 2000;191:1869–1879. doi: 10.1084/jem.191.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 28.Rousseeuw PJ, Leroy AM. Robust Regression and Outlier Detection. Wiley; New York: 1987. [Google Scholar]
- 29.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacso Z, Everson RB, Eliason JF. The DNA of annexin V-binding apoptotic cells is highly fragmented. Cancer Res. 2000;60:4623–4628. [PubMed] [Google Scholar]
- 31.Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T, Heemers H, Heyns W, Verhoeven G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 32.Spector AA. Essentiality of fatty acids. Lipids. 1999;34(Suppl):1–3. doi: 10.1007/BF02562220. [DOI] [PubMed] [Google Scholar]
- 33.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 34.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 35.Yaqoob P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 2003;24:639–645. doi: 10.1016/j.it.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156:824–831. doi: 10.1093/aje/kwf118. [DOI] [PubMed] [Google Scholar]
- 37.Gibney MJ. Incorporation of n-3 polyunsaturated fatty acids into processed foods. Br J Nutr. 1997;78:193–195. doi: 10.1079/bjn19970138. [DOI] [PubMed] [Google Scholar]
- 38.Yaqoob P, Newsholme EA, Calder PC. The effect of dietary lipid manipulation on rat lymphocyte subsets and proliferation. Immunology. 1994;82:603–610. [PMC free article] [PubMed] [Google Scholar]
- 39.Fritsche KL, Byrge M, Feng C. Dietary omega-3 poly-unsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol Lett. 1999;65:167–173. doi: 10.1016/s0165-2478(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 40.Wallace FA, Miles EA, Evans C, Stock TE, Yaqoob P, Calder PC. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J Leukoc Biol. 2001;69:449–457. [PubMed] [Google Scholar]
- 41.Oarada M, Tsuduki T, Suzuki T, Miyazawa T, Nikawa T, Hong-quan G, Kurita N. Dietary supplementation with docosahexaenoic acid, but not with eicosapentaenoic acid, reduces host resistance to fungal infection in mice. Biochim Biophys Acta. 2003;1622:151–160. doi: 10.1016/s0304-4165(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 42.Fritsche KL, Feng C, Berg JN. Dietary fish oil enhances circulating interferon-gamma in mice during listeriosis without altering in vitro production of this cytokine. J Interferon Cytokine Res. 1997;17:271–277. doi: 10.1089/jir.1997.17.271. [DOI] [PubMed] [Google Scholar]
- 43.Fritsche KL, Anderson M, Feng C. Consumption of eicosapentaenoic acid and docosahexaenoic acid impair murine interleukin-12 and interferon-gamma production in vivo. J Infect Dis. 2000;182(Suppl 1):54–61. doi: 10.1086/315925. [DOI] [PubMed] [Google Scholar]
- 44.Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002;3:190–196. doi: 10.1093/embo-reports/kvf022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 46.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 47.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramaniam PS, Johnson HM. Lipid microdomains are required sites for the selective endocytosis and nuclear translocation of IFN-gamma, its receptor chain IFN-gamma receptor-1, and the phosphorylation and nuclear translocation of STAT1alpha. J Immunol. 2002;169:1959–1969. doi: 10.4049/jimmunol.169.4.1959. [DOI] [PubMed] [Google Scholar]
- 49.Yano S, Ghosh P, Kusaba H, Buchholz M, Longo DL. Effect of promoter methylation on the regulation of IFN-gamma gene during in vitro differentiation of human peripheral blood T cells into a Th2 population. J Immunol. 2003;171:2510–2516. doi: 10.4049/jimmunol.171.5.2510. [DOI] [PubMed] [Google Scholar]
- 50.Katamura K, Fukui T, Kiyomasu T, Iio J, Tai G, Ueno H, Heike T, Mayumi M, Furusho K. IL-4 and prostaglandin E2 inhibit hypomethylation of the 5′ regulatory region of IFN-gamma gene during differentiation of naive CD4+ T cells. Mol Immunol. 1998;35:39–45. doi: 10.1016/s0161-5890(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 51.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, Schaefer EJ, Wolff SM, Dinarello CA. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 52.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung SW, Kang BY, Kim TS. Inhibition of interleukin-4 production in CD4+ T cells by peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands: involvement of physical association between PPAR-gamma and the nuclear factor of activated T cells transcription factor. Mol Pharmacol. 2003;64:1169–1179. doi: 10.1124/mol.64.5.1169. [DOI] [PubMed] [Google Scholar]
- 54.Marx N, Kehrle B, Kohlhammer K, Grub M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan YY, Spencer TE, Wang N, Moyer MP, Chapkin RS. Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis. 2003;24:1541–1548. doi: 10.1093/carcin/bgg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]