Abstract
Background: Erythropoietin is a naturally occurring hormone with multiple effects on a number of different cell types. Recent data have suggested neuroprotective and perhaps even neurotrophic roles for erythropoietin. We hypothesized that these functional effects could be demonstrable in standard models of peripheral nerve injury.
Methods: Experiments were undertaken to evaluate the effect of erythropoietin on the previously reported standard course of healing of sciatic injuries in mice. The injury groups included mice that were subjected to (1) sham surgery, (2) a calibrated sciatic crush injury, (3) transection of the sciatic nerve followed by epineural repair, or (4) a transection followed by burial of the proximal stump in the adjacent muscle tissue (neurectomy). Either erythropoietin or saline solution was administered to the mice in each of these experimental groups twenty-four hours preinjury, immediately after surgical creation of the injury, twenty-four hours postinjury, or one week postinjury. All mice were evaluated on the basis of the published model for recovery of sciatic nerve motor function by measuring footprint parameters at specific times after the injury. Immunohistochemistry was also performed to assess the erythropoietin-receptor expression profile at the site of injury.
Results: In general, the mice treated with erythropoietin recovered sciatic nerve motor function significantly faster than did the untreated controls. This conclusion was based on a sciatic function index that was 60% better in the erythropoietin-treated mice at seven days postinjury (p < 0.05). Although the group that had been given the erythropoietin immediately postinjury showed the best enhancement of recovery, the timing of the administration of the drug was not critical. Histological analysis demonstrated enhanced erythropoietin-receptor positivity in the nerves that recovered fastest, suggesting that accelerated healing correlates with expression of the receptor in nerve tissue.
Conclusions: Erythropoietin treatment of an acute sciatic nerve crush injury leads to an effect consistent with functional neuroprotection. This protective effect may have clinical relevance, especially since it was detectable even when erythropoietin had been administered up to one week after injury.
Clinical Relevance: Erythropoietin may represent a safe therapeutic agent to enhance nerve recovery in humans either after or before “nerve-at-risk” procedures as well as a useful adjunct to treatment of peripheral nerve crush injuries.
Peripheral nerve injuries, both acute and chronic, are commonly seen by physicians in all subspecialties of orthopaedic surgery. Acute peripheral nerve injuries are generally associated with trauma—such as brachial plexus injuries, traumatic lacerations, fractures, and crush injuries. Peripheral nerve injuries can also occur iatrogenically, arising in the course of routine elective orthopaedic procedures1-3.
Whatever the cause of a peripheral nerve injury, there are some generalities about its nature and prognosis. Specifically, it has long been known that the recovery potential is related to both patient and injury factors4. Recovery from the most mild neuropraxias is more reliable than recovery after complete laceration of a nerve, and older patients with poorer constitutions have the worst prognosis4-6.
Little is known about the pathophysiology of neuronal injury. The mechanism of chronic injuries to peripheral nerves, as in cubital tunnel and carpal tunnel syndromes, is believed to be ischemic7,8. Acute injuries that do not result in the complete severing of the epineurium seem to have a better prognosis4, and the rate of recovery from these injuries is believed to be governed by the logistics of axonal transport. Finally, a patient with a lacerated nerve is likely to benefit from a repair that orients the severed nerve fibers with respect to each other9,10. These considerations, although profound, have not ensured the reliable recovery of patients with peripheral nerve injuries.
Erythropoietin is a U.S. Food and Drug Administration-approved drug for use in the treatment of anemia with a minimal side-effect profile. Its proposed mechanism is anti-apoptotic and anti-inflammatory, which promotes cell survival11. Recent evidence has shown that erythropoietin also has an effect on cardiac, vascular, renal, and neural tissues12-14. Patients treated with erythropoietin show a reproducible improvement in peripheral neuronal function, even when peripheral neuropathy is not the chief symptom15.
Although the role of erythropoietin in both central and peripheral neurons is still under investigation, in vitro evidence of its neuroprotective and neurotrophic role has motivated its application to various nerve-tissue disorders16-19. Furthermore, investigators have shown a protective effect of erythropoietin in the central nervous system—following both stroke and spinal cord injury20-23. In addition, diabetic peripheral neuropathy in a rat model seems to be sensitive to the administration of erythropoietin24. Despite these new findings, little is known about the effect of erythropoietin on acute peripheral nerve injuries. This study was designed to evaluate the possible role of erythropoietin administration, including a possible temporal administration response, in the functional recovery following acute peripheral nerve injuries.
Materials and Methods
Animal Model of Peripheral Nerve Injury
The experimental design and protocols were approved by the Institutional Animal Care and Use Committee at our institution. Ten-week-old female C57BL6 mice weighing 20 to 25 g were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (4 mg/kg). The left hindlimb was shaved, washed with alcohol, and prepared with Betadine (povidone-iodine). A lateral skin incision along the length of the femur was made and a direct lateral approach through the iliotibial band was performed to expose the sciatic nerve lying directly posterior to the femur. The sciatic nerve was bluntly exposed, and then the mice underwent one of the following randomly assigned procedures: (1) wound closure without manipulation of the nerve (sham-surgery group), (2) a calibrated sciatic crush injury of thirty or sixty seconds' duration (crush-injury group), (3) a sciatic transection followed by epineural repair (transection/repair group), and (4) a sciatic transection followed by burial of the proximal nerve stump in the adjacent muscle tissue (neurectomy group). All injuries, regardless of their nature, were created proximal to the tibial and peroneal divisions, and all crush injuries were created with a smooth needle driver closed to the second notch. The skin was closed with surgical staples, which were removed fourteen days postoperatively. Buprenorphine (0.05 mg/kg) was used for postoperative analgesia on the day of surgery and was given every twelve hours until the mice were able to walk without signs of pain.
Erythropoietin was administered at predetermined time points at a dose of 5000 U/kg. The time points were selected to establish a foundation for translation to treatments of human traumatic nerve injuries. The dosage was selected on the basis of previous studies of animals and humans11,24,25. Medical-grade erythropoietin was obtained from the hospital pharmacy and was kept refrigerated until it was used. A separate single-use vial was used at each time point. The erythropoietin was suspended in sterile saline solution and injected subcutaneously into the dorsal contralateral flank of each mouse in accordance with pharmacy and veterinary recommendations.
Experimental Design
To characterize the effect of injury on gait, animals were randomized to receive sham surgery, a crush injury (of thirty or sixty seconds in duration), sciatic transection and repair, or neurectomy with nerve burial in the gluteal muscle, as described above. In a second experiment, the mice received sham surgery or a sixty-second crush injury and were randomized to receive subcutaneous injection of erythropoietin (5000 U/kg) or saline solution (as a control) perioperatively. In the third experiment, the mice underwent sham surgery, a sixty-second crush injury, transection and repair, or neurectomy. These mice were given erythropoietin or saline solution twenty-four hours prior to the injury, perioperatively, twenty-four hours postinjury, or one week postinjury to evaluate whether the timing of administration of erythropoietin affects functional recovery of the sciatic nerve. The transection/repair and neurectomy groups were used to test, in this model, the ability of erythropoietin to overcome the effect of injuries from which recovery was hitherto not considered possible. All mice were evaluated at regular intervals for functional measures of gait as described previously26-29. The mice were also evaluated preoperatively and postoperatively to establish baseline and injury effects on gait parameters.
The number of mice chosen for each group was determined with a standard power analysis. We set the significance level (probability of a type-I error) at 5% (α = 0.05) and sought to choose a sample size that would minimize the probability of type-II error (β ≤ 10%). From our initial experiment, we obtained estimates of the expected effects of the sixty-second crush injury and variability for a given number of mice. On the basis of this analysis, we determined that a sample size of five animals in each treatment group at each time point would be sufficient to detect a 30% difference in the sciatic function index (described below) with a statistical power of 95%.
Walking Track Analysis
Walking track analysis was performed according to a published model that quantifies sciatic nerve injury and recovery26-29. Walking tracks were constructed as previously described with use of an open-ended narrow corridor that provided a darkened compartment26. Individual mouse footprints were obtained by painting each foot with ink (blue for the normal limb and black for the experimental limb). The mice walked without inducement or coaxing down the 50-cm path lined with paper. Each mouse walked until clear print marks during a walking gait were obtained. (Multiple attempts were often required.)
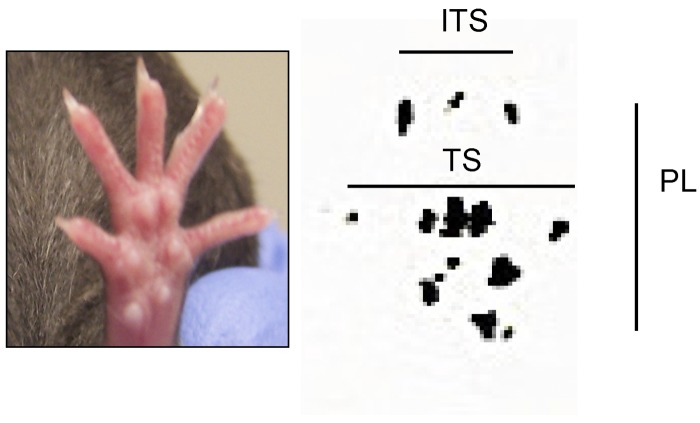
Walking tracks were measured in random order by two blinded observers using digital calipers. The samples were measured only after randomization of all completed sample data. Each observer made a separate set of paired measurements for each mouse—i.e., they each measured toe spread (first through fifth toes), print length, and intermediate toe spread (second, third, and fourth toes) of both the experimental (left) limb and the control (right) limb (Fig. 1). The two observers did not have to use the same footprint on each sample. The freedom to use any footprint on the sample did not introduce significant variability in the measurements when compared with measurements of samples on which the measured footprint had been predetermined. Each observer chose prints that clearly showed anatomical structures and represented steps within a walking gait.
Fig. 1.
Representative control footprint demonstrating measurements used to calculate the sciatic function index. These include toe spread (TS), intermediate toe spread (ITS), and print length (PL).
The measurements were compared directly with values derived from published norms (K1, K2, and K3) for either injured limbs or limbs that had undergone a sham operation26-32, and a sciatic function index was calculated with the equation: sciatic function index = K1 * toe spread + K2 * print length + K3 * intermediate toe spread. As was the case with the functional experiments, this assessment of the parameters was done in a blinded fashion. Once the parameters were recorded, they were unblinded in the analysis phase of the experiment, and the raw data were never again measured or tabulated (to ensure proper blinding).
Immunohistochemistry
The experimental and contralateral (uninjured) sciatic nerves from each test group were harvested at specific time points during healing and recovery. The nerves were bluntly dissected from the dorsal root ganglion to a point distal to the peroneal-tibial nerve divisions. All nerves were fixed in 10% neutral buffered saline solution for four days and embedded in paraffin to evaluate cross sections. Hematoxylin and eosin staining and erythropoietin-receptor immunohistochemistry were performed. Slides were pretreated with 0.01-M citrate buffer (pH 6.0) for antigen retrieval. Nonspecific blocking was performed with 1:20 diluted goat serum. Rabbit polyclonal erythropoietin-receptor antibodies (Santa Cruz Biotechnology, Santa Cruz, California) at a 1:50 dilution in 2% goat serum were incubated overnight. Secondary goat anti-rabbit antibody (Santa Cruz Biotechnology) at a 1:200 dilution was incubated for thirty minutes, and then color reaction was performed with Romulin AEC chromogen (Biocare Medical, Walnut Creek, California) for five minutes. Mouse brain, fixed and sectioned as described above, was used as a positive control for the erythropoietin-receptor immunohistochemistry on the basis of results described elsewhere25.
Statistical Methods
With the above-mentioned power criteria, we made comparisons using standard statistical software to probe for significant differences in quantitative results. All statistical tests were performed on standard tabulated sciatic function index values after analyses were performed to show that results were independent of constants chosen within one order of magnitude. Standard two-way analysis of variance was performed to compare averaged data from each experimental group at each time point.
Results
Effect of Sciatic Injury on Sciatic Function Index
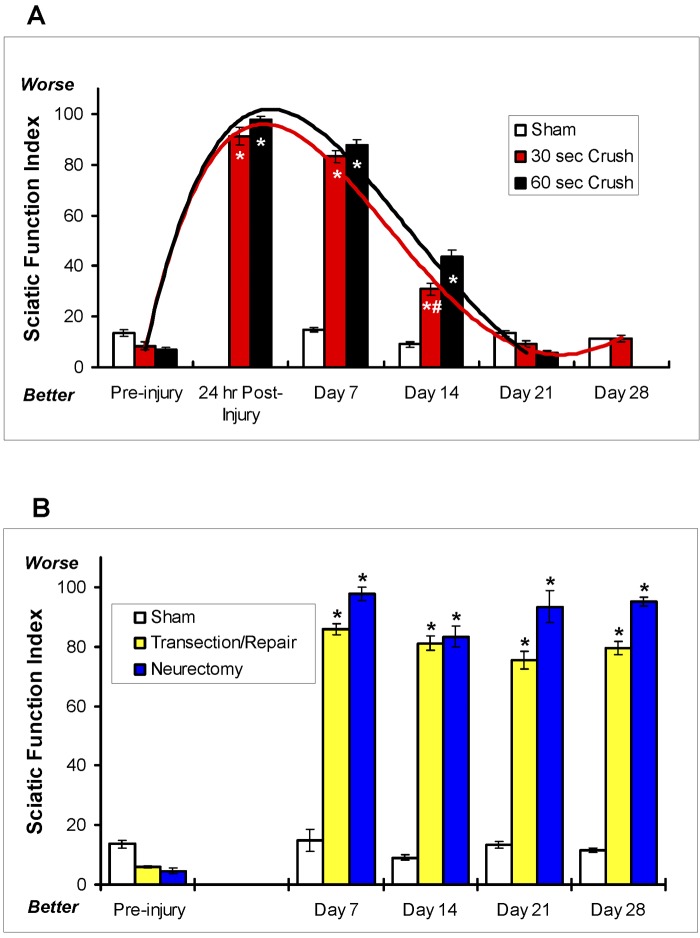
The natural recovery from the crush injuries did not differ significantly between the groups with the different durations of crushing (Fig. 2, A). Specifically, direct comparison of the sciatic function indices between the mice that had undergone the crush injury for thirty seconds and those that had undergone it for sixty seconds showed no significant difference in the time course to full recovery (p > 0.5). However, the incremental recovery achieved at fourteen days was greater in the mice that had undergone the crushing for a shorter duration (p < 0.005). Overall, the mice subjected to the crush injury for thirty or sixty seconds had full recovery of sciatic nerve function, as measured by the sciatic function index, by twenty-one days (Fig. 2, A). Comparison of the two crush regimens demonstrated that full axonotmesis could be achieved with either duration of crushing.
Fig. 2.
Effects of sciatic crush injury on the sciatic function index. (The various types of sciatic injury are described in the Materials and Methods section.) The bars represent the mean and standard error of the mean for each group (n = 8), and the asterisks denote a significant difference (p < 0.05) compared with the sham-operation group at the same time point as determined by analysis of variance. A: Direct comparison between the sham-operation group (white bars) and the groups subjected to crushing of the nerve for thirty seconds (red bars) or sixty seconds (black bars). (Data were not collected for the sixty-second-crush group on Day 28.) Curves fit to the data demonstrate that there was no significant difference in the sciatic function index over the recovery period between the thirty and sixty-second-crush groups at any time point except at fourteen days (pound sign; p < 0.005). B: A negative control experiment was performed to verify that transection and repair (yellow bars) and neurectomy (blue bars) resulted in irreversible injury of the sciatic nerve and complete abrogation of recovery of sciatic function (as measured with the sciatic function index) in mice. The sciatic function index in these groups was compared with that in the sham-operation control group (white bars).
More severe transection injuries were created as controls to show complete abrogation of sciatic function that could not be recovered, as demonstrated by the sciatic function index. As described in the Materials and Methods section, operations were performed to either transect and repair the nerve with a suture or transect the nerve and bury the proximal stumps in a muscle flap (neurectomy). There was a significant worsening of the sciatic function indices in those groups, as compared with the crush-only groups, at seven days postinjury (Fig. 2, B) that was comparable with that in the thirty and sixty-second crush-injury groups at the same time point (Fig. 2, A). As expected, neither injury group (transection/repair or neurectomy) showed significant recovery of sciatic function at twenty-eight days (Fig. 2, B). This finding was in contrast to the significant recovery that occurred in the crush-injury groups (Fig. 2, A). These results confirm that the sciatic function index is completely dependent on the integrity of the sciatic nerve.
Effect of Erythropoietin on Recovery of the Sciatic Function Index
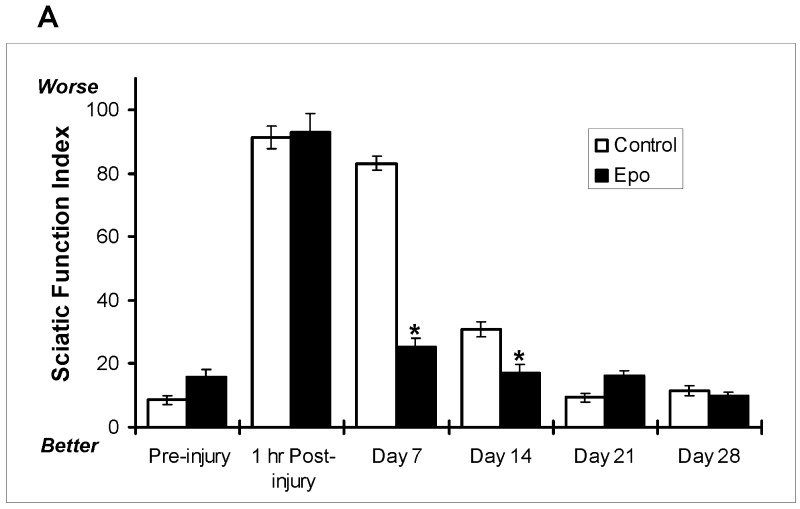
As demonstrated in Figure 2, A, mice subjected to a crush injury alone seemed to recover their normal footprint parameters within one month after the original injury. With this as a benchmark, it was determined that mice should be evaluated for the effects of erythropoietin on recovery within this period. A sixty-second sciatic crush injury was administered as described in the Materials and Methods section. Mice treated with erythropoietin (a single dose of 5000 U/kg administered subcutaneously immediately after the injury) had a significantly (60%) better sciatic function index than the saline-solution-treated control mice at seven and fourteen days postinjury (Fig. 3, A). This difference was visually obvious seven days postinjury as depicted in the photographs presented in Figure 3, B. The top pair of photographs show a representative digit pattern in an untreated mouse (no administration of erythropoietin) immediately following injury (B1) and seven days postinjury (B2), and the bottom pair of photographs depict a representative digit pattern in an erythropoietin-treated mouse immediately following injury (B3) and seven days later (B4). These photographs demonstrate the change in digit orientation that is quantified by the sciatic function indices in Figure 3, A. It should be noted that mice in the neurectomy group showed no improvement in any functional parameter. These results provide evidence that administration of erythropoietin affects functional recovery of damaged sciatic nerves, raising the possibility of therapeutic effects.
Fig. 3.
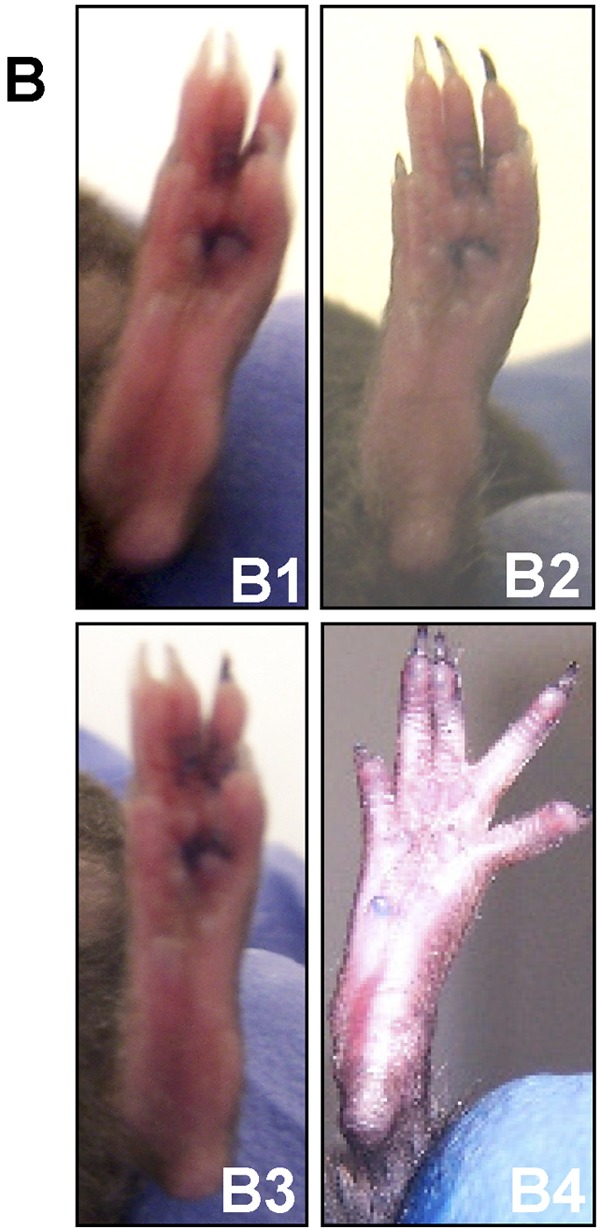
Effect of erythropoietin (5000 U/kg administered by subcutaneous injection immediately postinjury), as compared with saline solution (control), on the sciatic function index in mice subjected to a sciatic crush injury (for a sixty-second duration) as described in the Materials and Methods section. A: The sciatic function index preinjury and at one hour and seven, fourteen, twenty-one, and twenty-eight days postinjury for the erythropoietin-treated (Epo; black bars) and saline-solution-treated (Control; white bars) mice. The bars represent the mean and standard error of the mean for each group (n = 8), and the asterisks denote a significant difference (p < 0.05) compared with the control group at the same time point as determined with analysis of variance. B: Representative photographs of the foot position of a control (saline-solution-treated) mouse immediately after the surgical crush injury described in A (B1), the same foot one week after the surgical crush injury (B2), an erythropoietin-treated mouse (as described in A) immediately after the surgical crush injury (B3), and the same foot one week after the surgical crush injury (B4).
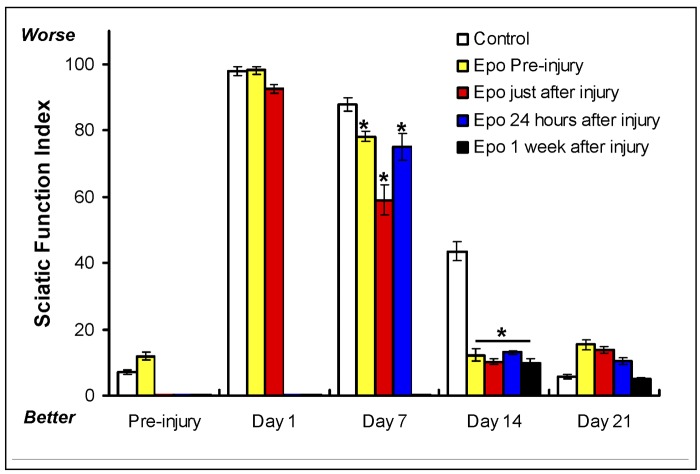
To evaluate whether the timing of administration of erythropoietin affects functional recovery of the sciatic nerve, as demonstrated by the sciatic function index, a separate cohort of mice was treated with erythropoietin at various times relative to the injury. A sixty-second crush injury to the sciatic nerve was surgically induced and a single dose of erythropoietin (5000 U/kg) was administered subcutaneously twenty-four hours prior to the injury, perioperatively (immediately following the injury), twenty-four hours after the injury, or one week after the injury. While the group that had received the erythropoietin twenty-four hours postinjury had the most improved sciatic function index at seven days postinjury, all of the erythropoietin-treated groups showed robust improvement at the fourteen-day time point (Fig. 4). Interestingly, when data from the groups that received the erythropoietin preoperatively or did not receive erythropoietin at all were removed from the statistical analysis, there was no significant difference between the group that received the erythropoietin at twenty-four hours and the group that received it at one week; only the group treated immediately postinjury was found to be significantly different from the other treatment groups. Furthermore, two-way analysis of variance of these data showed a significant effect of treatment with erythropoietin (p < 0.0001); however, other than immediate administration, the timing of the drug administration did not have a significant effect (Fig. 4). These results not only support our earlier finding that erythropoietin enhances recovery of sciatic function in injured mice, but it also suggests that the timing of postinjury administration of the drug is not critical. The wide window of therapeutic efficacy seen in this model suggests that, if erythropoietin were to be found useful in clinical situations, the timing of administration might be flexible.
Fig. 4.
Effect of timing of erythropoietin administration (5000 U/kg administered by subcutaneous injection) on the sciatic function index in mice subjected to a sciatic crush injury (for a sixty-second duration) as described in the Materials and Methods section. The sciatic function index was determined preinjury and at one, seven, fourteen, and twenty-one days postinjury for the control group (no erythropoietin, white bars) and the groups treated with erythropoietin (Epo) preinjury (yellow bars), immediately after injury (red bars), twenty-four hours after injury (blue bars), and one week after injury (black bars). (The sciatic function index was not measured on Day 1 for the mice given erythropoietin at twenty-four hours or one week. Similarly, it was not measured on Day 7 for the mice given erythropoietin at one week.) The bars represent the mean and standard error of the mean for each group (n = 8), and the asterisks denote a significant difference (p < 0.05) compared with the control group at the same time point as determined with analysis of variance.
Erythropoietin-Receptor Expression in the Sciatic Nerve
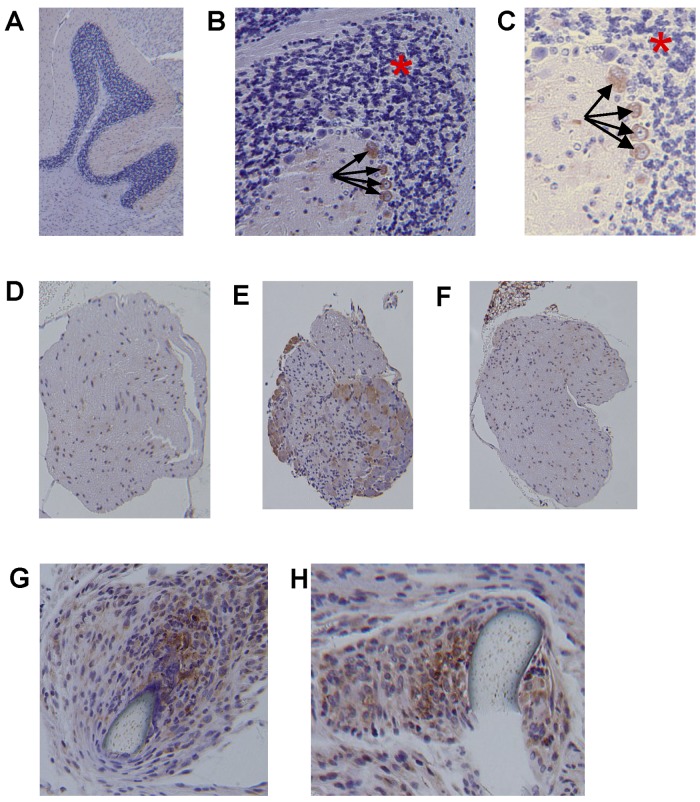
Immunohistochemical analysis of nerves recovered from mice killed after the experiments revealed that the nerve could be stained and analyzed in isolation from the surrounding muscle tissue if it had been harvested carefully. With use of standard hematoxylin staining as a localizer, successive slices of sciatic nerve and mouse cerebellum (the control) underwent immunohistochemical staining for the presence of the erythropoietin receptor. Confirming antibody detection of cells expressing erythropoietin receptors, cerebellar tissue showed intense staining in the climbing fibers (black arrows in Fig. 5, B and C) and absence of staining in receptor-negative cerebellar granule cells (red asterisk in Fig. 5, B and C). This distribution of erythropoietin receptors (Fig. 5, A, B, and C) is consistent with that found in previously published studies25. Not only was the erythropoietin receptor found in the sciatic nerves from the mice that had undergone the sham operation (Fig. 5, D), positivity in the nerves from the erythropoietin-treated mice was enhanced seven days after the crush injury (Fig. 5, E). Interestingly, while erythropoietin-receptor expression was not enhanced seven days postoperatively in the nerves that had been subjected to a crush injury but not treated with erythropoietin (Fig. 5, F), higher-power magnification showed strong expression in neurons proximal to sutures in nerves that had been transected and repaired (Fig. 5, G and H). Overall, these results support the concept that cellular responsiveness to erythropoietin is preserved and possibly enhanced in injured nerves and that administration of erythropoietin in general does not lead to detectable negative regulation of receptor expression.
Fig. 5.
Immunohistochemical staining of erythropoietin receptors in sciatic nerve tissue harvested from mice subjected to sciatic injury or sham surgery with or without erythropoietin administration. A, B, and C: Representative images of cerebellum, used as a control, are shown depicting the expected red staining of climbing fibers with erythropoietin-receptor antibodies (black arrows) and the expected absence of receptor positivity in cerebellar granule cells (area denoted with a red asterisk) (magnification, ×4 for A, ×10 for B, and ×20 for C). D, E, and F: Representative images of sciatic nerve cross sections showing erythropoietin-receptor positivity seven days postinjury in the sham-operation group not treated with erythropoietin (D), the group subjected to a sixty-second crush injury and treated with erythropoietin (E), and the group subjected to a sixty-second crush injury and not treated with erythropoietin (F) (magnification, ×4 for all). G and H: Sections of the repair site after transection and repair, showing staining for erythropoietin receptor around the repair stitch (magnification, ×20 for both).
Discussion
There is little doubt that erythropoietin has a wider spectrum of effects on a larger number of tissues than was previously believed12-14. Recent reviews of the literature have suggested three principal areas of erythropoietin-mediated protection: neuroprotection, cardioprotection, and erythroid support12,13. These effects likely point to a teleologic role of erythropoietin as a pleuriprotective agent12,13. Thus, there is an expectation that hormones that are triggered by hemorrhage may play a protective role in many tissue systems.
There is specific evidence that points to erythropoietin as a potent neuromodulator. Studies have confirmed neuroprotective21-23, neurotrophic33-35, and neuromodulatory34 effects that are directly attributable to erythropoietin. Some would argue that this effect is not surprising as neurons express receptors for erythropoietin both centrally and peripherally34,35.
Of specific interest is the idea that erythropoietin might have effects on nerve repair and regeneration in cases of peripheral nerve injury. Such effects have direct clinical importance because the safety of therapeutic doses of erythropoietin makes it an attractive adjunct to current treatments. Recovery is unpredictable following injuries to peripheral nerves sustained traumatically or iatrogenically. These injuries often require difficult procedures such as neurorrhaphy, neurolysis, neurotization, direct repair, and conduit-assisted repair36,37.
To our knowledge, erythropoietin has not been investigated in all accepted models of peripheral nerve regeneration and repair. Although a number of models have been used to investigate the theoretical efficacy of treatments for peripheral nerve injury38,39, the rodent sciatic nerve model has some advantages. First, it is a well-established model both in the rat24,26,28,29 and the mouse15,27,40. Second, and perhaps more importantly, it allows a functional assay of motor nerve regeneration and therefore is of particular clinical relevance. The murine model also opens the door to molecular and genetic studies if a candidate agent proves successful.
There is already evidence to suggest that erythropoietin should have a positive effect on nerve regeneration. Bianchi et al. found improvement following administration of erythropoietin in a rat model for diabetic neuropathy24. Of particular interest in that study was the use of a functional assay of sensory function, which is the relevant deficit experienced by patients with diabetic neuropathy. Other studies have demonstrated, rather convincingly, that the erythropoietin receptor is present on central dopaminergic33 and peripheral nerves34,41 as well as the supporting structures of the nerves. Campana and Myers even showed an upregulation of erythropoietin receptors in Schwann cells surrounding an injured rat sciatic nerve34. Finally, on the intracellular side, Tanaka et al. implicated tetrahydrobiopterin in the cascade of mediators responsible for the neurotrophic effects of erythropoietin38. These findings are in the face of a fair amount of data suggesting that erythropoietin acts both directly on neurons and preferentially on the microvasculature to prevent neuronal cell death and bolster microcirculation at the site of an injury34,38,41. Both of these effects occur over a time course that argues for an erythroid-precursor-independent and neuronally specific role for erythropoietin.
The cellular level mechanism of peripheral nerve injury and its response to erythropoietin are poorly understood, but certain mechanisms are being elucidated. In vitro primary cultures of rat sciatic nerve Schwann cells show increased expression of erythropoietin receptor when the cultures are stressed (low-serum cultures)42. Furthermore, Schwann cell cultures under stress show increased proliferation after exogenous administration of erythropoietin when compared with controls42. The underlying intracellular signaling response to erythropoietin that might transduce these phenotypic responses in target cells is only now being defined. Such intracellular events have great importance as they represent essential steps for Schwann cell proliferation and development41,42. It is possible that these types of signaling responses participate in the direct effects of erythropoietin on the sciatic function index in our injury model.
Given our goal of showing a functional response to erythropoietin treatment, we chose a model that would reveal regeneration of motor function. Critical to this model is the ability to record actual gait patterns of mice as neurons regenerate. The sciatic crush model ensures a reliable recovery in untreated mice—an advantageous feature in the study of nerve regeneration. The results of actual repairs after transection in this model are reliably poor, probably because of the difficulty in orienting repairs to allow fascicular congruity.
One interesting result of our study is that both functional experiments yielded similar courses of recovery despite our use of a longer crush time in the second experiment. This might be explained by inferring that both durations of crushing are sufficient to provide complete axonotmesis. If this were the case, then it would theoretically not matter how long a nerve is crushed as it must regenerate along the same path distal to the injury. Indeed, our goal as well as that of others has been to crush the nerve completely, leaving only the outer epineurium intact. Thus, our findings provide a strong basis for choosing a single crush duration of sixty seconds for future work with this sciatic crush-injury mouse model.
The results presented in Figures 3 and 4 show an acceleration of functional recovery attributable to erythropoietin treatment. Additional studies are under way to further define the quantitative difference in healing rates. Moreover, this result seemed to have little dependence on the exact time of erythropoietin administration within a week after the injury with the exception of a slight, but significant, advantage in the mice that had received the erythropoietin at the time of the injury. The fact that erythropoietin can have a positive effect on the functional outcome even when it is administered well after the injury may point to multiple roles for erythropoietin-mediated neuroprotection in this model.
There were limitations to this study. A crush injury to a mixed (motor and sensory) nerve must result in a mixed loss of function. Although we did observe a loss of sensory function that anecdotally coincided with the motor loss, we did not measure this sensory deficit directly. Sensory recovery is an important part of the functional recovery needed following a human peripheral nerve injury. Classically, insensate limbs have contributed to morbidity even in the setting of adequate motor function35. We did not control for the possibility that erythropoietin-mediated recovery may be preferential to one or another cell type in mixed nerves, a fact that would be relevant to fully characterize the effect of erythropoietin. This limitation notwithstanding, data from a study of erythropoietin, albeit involving a different species of animal with a different injury profile, do suggest a neuroprotective role for erythropoietin in diabetic neuropathy24. Perhaps future functional experiments could be directed at quantitatively describing the time course of erythropoietin-mediated sensory and motor recovery in the same injury model.
The model for sciatic nerve injury is useful in that it provides a reliable recovery in a predictable time period. Although critical to the purposes of this study, the recovery period does limit the ability to fully describe the erythropoietin-mediated healing potential. For example, we could not include a group in which erythropoietin was administered beginning at fourteen days postinjury because the untreated mice had made a great stride toward healing by then in this model. We do not know of any nerve injury in rodents that heals reliably well over a time course of months. It is just such a model that would be needed to completely describe the time window for erythropoietin's protective effects on murine peripheral nerves. It is likely that larger animals with more complex nerves will serve better in this regard. A related difficulty lies in the characterization of erythropoietin-mediated effects on complex nerve repair. We cannot rule out the possibility that erythropoietin has effects on function following a classic or fascicular nerve repair because our chosen model does not provide a reproducible recovery following repair with which recovery in erythropoietin-treated animals can be compared.
Taken together, our data suggest a neuroprotective role for erythropoietin in the setting of crush injury of mixed peripheral nerves. Histological analysis of relevant structures showed receptor expression comparable with that found in previous studies of erythropoietin in nervous tissue41,42, suggesting that the observed rescue may at least partially depend on the direct influence of erythropoietin on the nerve. Since erythropoietin has a favorable side-effect profile, we believe that it may be a candidate agent for the treatment of nerve injuries. This is especially true in the patient population with concomitant anemia. Examples of such patients include adults who have undergone major reconstructive procedures and total joint arthroplasties complicated by traction nerve injury as well as those who have sustained polytrauma. The benefits of erythropoietin treatment for these patients may be multifold. Finally, it is possible that nerve transfer surgery, an emerging and exciting application of nerve repair principles outlined by Mackinnon et al.43-45 and Bianchi et al.24, might provide an interesting venue for the investigation of the clinical efficacy of adjunctive erythropoietin treatment to enhance nerve healing.
Footnotes
Disclosure: The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at the Department of Orthopaedics and Rehabilitation and the Center for Musculoskeletal Research, University of Rochester, Rochester, New York
References
- 1.Idusuyi OB, Morrey BF. Peroneal nerve palsy after total knee arthroplasty. Assessment of predisposing and prognostic factors. J Bone Joint Surg Am. 1996;78:177-84. [DOI] [PubMed] [Google Scholar]
- 2.Farrell CM, Springer BD, Haidukewych GJ, Morrey BF. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2619-25. [DOI] [PubMed] [Google Scholar]
- 3.DeHart MM, Riley LH Jr. Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg. 1999;7:101-11. [DOI] [PubMed] [Google Scholar]
- 4.Sunderland S. Nerve injuries and their repair: a critical appraisal. New York: Churchill Livingstone; 1991. [Google Scholar]
- 5.Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771-84. [DOI] [PubMed] [Google Scholar]
- 6.Seddon HJ. Surgical disorders of the peripheral nerves. New York: Churchill Livingstone; 1972. [Google Scholar]
- 7.Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1976;39:615-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellon AL, Mackinnon SE. Human ulnar neuropathy at the elbow: clinical, electrical, and morphometric correlations. J Reconstr Microsurg. 1988;4:179-84. [DOI] [PubMed] [Google Scholar]
- 9.Diao E, Vannuyen T. Techniques for primary nerve repair. Hand Clin. 2000;16:53-66, viii. [PubMed] [Google Scholar]
- 10.Kutz JE, Shealy G, Lubbers L. Interfascicular nerve repair. Orthop Clin North Am. 1981;12:277-86. [PubMed] [Google Scholar]
- 11.Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl. 2002;91:36-42. [DOI] [PubMed] [Google Scholar]
- 12.Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538-48. [DOI] [PubMed] [Google Scholar]
- 13.Bogoyevitch MA. An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res. 2004;63:208-16. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin: new uses for an old hormone. Nephrology (Carlton). 2006;11:306-12. [DOI] [PubMed] [Google Scholar]
- 15.Bommer J, Kugel M, Schwobel B, Ritz E, Barth HP, Seelig R. Improved sexual function during recombinant human erythropoietin therapy. Nephrol Dial Transplant. 1990;5:204-7. [DOI] [PubMed] [Google Scholar]
- 16.Montero M, Poulsen FR, Noraberg J, Kirkeby A, van Beek J, Leist M, Zimmer J. Comparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice cultures. Exp Neurol. 2007;204:106-17. [DOI] [PubMed] [Google Scholar]
- 17.Mennini T, De Paola M, Bigini P, Mastrotto C, Fumagalli E, Barbera S, Mengozzi M, Viviani B, Corsini E, Marinovich M, Torup L, Van Beek J, Leist M, Brines M, Cerami A, Ghezzi P. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med. 2006;12:153-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meloni BP, Tilbrook PA, Boulos S, Arthur PG, Knuckey NW. Erythropoietin preconditioning in neuronal cultures: signaling, protection from in vitro ischemia, and proteomic analysis. J Neurosci Res. 2006;83:584-93. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101-10. [DOI] [PubMed] [Google Scholar]
- 20.Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Bührer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177-87. [DOI] [PubMed] [Google Scholar]
- 22.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26-32. [DOI] [PubMed] [Google Scholar]
- 23.Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tataroglu C, Cerami A, Ghezzi P. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101:823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbayraktar S, Grasso G, Sfacteria A, Xie QW, Coleman T, Kreilgaard M, Torup L, Sager T, Erbayraktar Z, Gokmen N, Yilmaz O, Ghezzi P, Villa P, Fratelli M, Casagrande S, Leist M, Helboe L, Gerwein J, Christensen S, Geist MA, Pedersen LØ, Cerami-Hand C, Wuerth JP, Cerami A, Brines M. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci U S A. 2003;100:6741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634-43. [DOI] [PubMed] [Google Scholar]
- 27.Inserra MM, Bloch DA, Terris DJ. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery. 1998;18:119-24. [DOI] [PubMed] [Google Scholar]
- 28.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129-38. [DOI] [PubMed] [Google Scholar]
- 29.Brown CJ, Evans PJ, Mackinnon SE, Bain JR, Makino AP, Hunter DA, Hare G. Inter- and intraobserver reliability of walking-track analysis used to assess sciatic nerve function in rats. Microsurgery. 1991;12:76-9. [DOI] [PubMed] [Google Scholar]
- 30.Hare GM, Evans PJ, Mackinnon SE, Best TJ, Bain JR, Szalai JP, Hunter DA. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plast Reconstr Surg. 1992;89:251-8. [PubMed] [Google Scholar]
- 31.Dellon AL, Mackinnon SE. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery. 1989;10:220-5. [DOI] [PubMed] [Google Scholar]
- 32.Hare GM, Evans PJ, Mackinnon SE, Best TJ, Midha R, Szalai JP, Hunter DA. Walking track analysis: utilization of individual footprint parameters. Ann Plast Surg. 1993;30:147-53. [DOI] [PubMed] [Google Scholar]
- 33.Csete M, Rodriguez L, Wilcox M, Chadalavada S. Erythropoietin receptor is expressed on adult rat dopaminergic neurons and erythropoietin is neurotrophic in cultured dopaminergic neuroblasts. Neurosci Lett. 2004;359:124-6. [DOI] [PubMed] [Google Scholar]
- 34.Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci. 2003;18:1497-506. [DOI] [PubMed] [Google Scholar]
- 35.McLean J, Batt J, Doering LC, Rotin D, Bain JR. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase sigma knock-out mice. J Neurosci. 2002;22:5481-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canale ST, editor. Campbell's operative orthopaedics. 10th ed. St. Louis: Mosby; 2003. [Google Scholar]
- 37.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243-52. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka J, Koshimura K, Sohmiya M, Murakami Y, Kato Y. Involvement of tetrahydrobiopterin in trophic effect of erythropoietin on PC12 cells. Biochem Biophys Res Commun. 2001;289:358-62. [DOI] [PubMed] [Google Scholar]
- 39.Tonge D, Edström A, Ekström P. Use of explant cultures of peripheral nerves of adult vertebrates to study axonal regeneration in vitro. Prog Neurobiol. 1998;54:459-80. [DOI] [PubMed] [Google Scholar]
- 40.Yao M, Inserra MM, Duh MJ, Terris DJ. A longitudinal, functional study of peripheral nerve recovery in the mouse. Laryngoscope. 1998;108:1141-5. [DOI] [PubMed] [Google Scholar]
- 41.Campana WM, Myers RR. Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. FASEB J. 2001;15:1804-6. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Gonias SL, Campana WM. Schwann cells express erythropoietin receptor and represent a major target for Epo in peripheral nerve injury. Glia. 2005;51:254-65. [DOI] [PubMed] [Google Scholar]
- 43.Nath RK, Mackinnon SE. Nerve transfers in the upper extremity. Hand Clin. 2000;16:131-9, ix. [PubMed] [Google Scholar]
- 44.Mackinnon SE, Novak CB. Nerve transfers. New options for reconstruction following nerve injury. Hand Clin. 1999;15:643-66, ix. [PubMed] [Google Scholar]
- 45.Dvali L, Mackinnon S. Nerve repair, grafting, and nerve transfers. Clin Plast Surg. 2003;30:203-21. [DOI] [PubMed] [Google Scholar]