Abstract
Background
Severe falciparum malaria may be complicated by haemolysis after parasite clearance, however the mechanisms remain unclear. Recent reports describe a pattern of delayed onset haemolysis among non-immune travellers with hyperparasitaemia treated with intravenous artesunate, termed post-artesunate delayed haemolysis (PADH). The occurrence and clinical impact of PADH following severe malaria infections in areas of unstable transmission are unknown.
Case
A 45-year-old Bangladeshi male was initially admitted to a local hospital with severe falciparum malaria complicated by hyperparasitaemia and treated with intravenous artesunate. Twenty days from his first presentation he was readmitted with delayed onset haemolytic anaemia and acute kidney injury. Multiple blood transfusions and haemodialysis were required. Renal biopsy revealed acute tubular injury and haem pigment nephropathy. His haemoglobin and renal function recovered to baseline after 62 days from his second admission.
Discussion
This case highlights the differential diagnosis of post-malaria delayed onset haemolysis, including the recently described syndrome of post-artemisinin delayed haemolysis. The pathophysiology contributing to acute kidney injury in this patient and the limited treatment options are discussed.
Conclusions
This report describes PADH complicated by acute kidney injury in an adult patient living in a malaria hypoendemic region who subsequently required blood transfusions and haemodialysis. This case emphasizes the importance of routine follow up of haemoglobin and renal function in artesunate-treated patients who have recovered from severe malaria.
Keywords: Falciparum malaria, Delayed haemolysis, Acute kidney injury
Background
Delayed onset haemolytic anaemia is a recognized but infrequent complication during recovery after severe falciparum malaria infection. The course of anaemia following severe malaria is often protracted and, in those treated with quinine, haemoglobin may not return to baseline until 28 days after infection [1]. Prior to the introduction of artesunate, prolonged haemolysis contributed to this delayed haemoglobin recovery in less than 7% of patients [1]. The mechanisms of continued haemolysis after parasite clearance are unclear, with both auto-immune and non-immune processes implicated in the absence of artesunate treatment [2–4]. Recent reports describe a syndrome termed post-artemisinin delayed haemolysis (PADH) following treatment with intravenous artesunate [5–11]. PADH usually occurs 1–3 weeks after artesunate is administered to non-immune travellers with high parasitaemia. The different patterns of haemolysis following malaria infection and artesunate treatment highlight the complexity of malaria anaemia.
Massive haemolysis is frequently associated with haemoglobinuria and acute kidney injury (AKI) [12–15]. When haemoglobin and haem scavengers, haptoglobin and haemopexin, are saturated the haemoglobin is filtered through the glomerulus. It is then re-absorbed and catabolized in the proximal tubule. Haemoglobinuria results when re-absorption capacity is reached [16]. Studies implicate cell-free haemoglobin in the mechanism of AKI in haemolytic conditions [17, 18]. This report describes the first case of a patient from an unstable malaria transmission area presenting with delayed-onset haemolysis complicated by acute kidney injury following treatment of severe malaria with intravenous artesunate and follow-on oral artemether lumefantrine combination treatment.
Case presentation
A 45-year-old, previously well, Bangladeshi male presented to Chittagong Medical College Hospital with a 4-day history of fever, headache, prostration, myalgias, nausea, and anorexia. This center is the main referral hospital for the Chittagong Division in southern Bangladesh, which is endemic for malaria with unstable transmission. One day prior he was diagnosed with malaria based on a positive rapid diagnostic test (RDT).
There was no significant past medical history, specifically no prior malaria infections, chronic illness, jaundice, liver or kidney disease, transfusions, malignancy, or auto-immune disease. There was no history of passing red or black urine. He was not taking any regular prescription or traditional medications and had no prior exposure to artemisinins.
On admission (day 0), his temperature was 40.3°C, Glasgow Coma Score (GCS) 15/15, blood pressure (BP) 117/62 mmHg; heart rate (HR) 102 beats per minute (bpm) and respiratory rate 36 breaths per minute with 98% oxygen saturation. He appeared well with mild dehydration and scleral icterus, but otherwise unremarkable examination. He was diagnosed with uncomplicated malaria on admission and received one dose of oral artemether/lumefantrine combination (AL) 80/480 mg (Novartis) since he had no clinical criteria for severe malaria, no initial blood tests and only an RDT-positive test.
Subsequent blood tests confirmed a diagnosis of severe falciparum malaria based on hyperparasitaemia (13.8% erythrocytes infected; 659,000 parasites/μL) and hyperbilirubinaemia (bilirubin 80 μmol/L). He was promptly switched to intravenous artesunate 120 mg, 2.4 mg/kg body weight, (Guilin No. 2, Pharmaceuticals, China) and received three doses at 12-h intervals. He was also randomized to the normal saline arm of a randomized controlled trial of adjunctive l-arginine in severe malaria (ACTRN12612000571875). After 24 h he defervesced and was switched to oral AL for 3 days (total of 480 mg artemether and 2,880 mg lumefantrine). His recovery was unremarkable except for a delayed parasite clearance (estimated half life 8.2 h). Due to this persistent parasitaemia, he received a second 3-day course of AL and his parasites cleared at 148 h. In total he received 13 doses of AL (total dose of 1,040 mg of artemether and 6,240 mg of lumefantrine) and three doses of intravenous artesunate (total dose of 360 mg). He was discharged 8 days later with full recovery, a stable haematocrit and creatinine at a baseline of 86 μmol/L.
At follow-up on day 14 after admission he was asymptomatic, however his white blood count revealed a significant leukocytosis and severe normocytic anaemia (haemoglobin of 60 g/L; Table 1). He declined re-admission and subsequently experienced fever, rigors, headache, jaundice, abdominal pain, and vomiting. He had not taken any antibiotics, non-steroidal anti-inflammatories or proton pump inhibitors during this intercurrent period after treatment of the initial malaria episode.
Table 1.
Investigations
| Parameter | Day 0 | Day 3 | Day 14 | Day 20 | Day 44 | Normal range |
|---|---|---|---|---|---|---|
| Plasma haemoglobin (ng/mL)a | 177,839 | – | – | 127,146 | – | <124,000 |
| Haemoglobin (g/L) | 141 | 136 | 60 | 49 | 102 | 140–180 |
| Haematocrit (%) | 43.6 | 40 | 18.7 | 16 | 27.1 | 47–54 |
| Mean corpuscule volume (fL) | 86.3 | – | 86.2 | 94.7 | 85.2 | 76–96 |
| Plasma haptoglobin (μg/mL)a | 1,160 | – | – | 5 | – | 300–2,000 |
| Platelets (×103/μL) | 28 | – | 228 | 478 | 320 | 150–00 |
| White blood cells (×103/μL) | 6.23 | – | 19.9 | 9.8 | 10.5 | 4–10 |
| Neutrophils (%) | 83 | – | 70 | 60 | 40–70 | |
| Creatinine (μmol/L) | 97 | 86 | – | 1,769 | 132 | 53–115 |
| Potassium (μmol/L) | 3.7 | 4.4 | – | 8.4 | 4.8 | 3.5–4.9 |
| Venous pH | 7.41 | – | – | 7.2 | – | 7.31–7.41 |
| Venous bicarbonate (mmol/L) | 19.5 | – | – | 8.7 | – | 23–28 |
| Venous carbon dioxide (mmHg) | 31.2 | – | – | 23.5 | – | 41–51 |
| Venous base excess (mmol/L) | −5 | – | – | −20 | – | (−2) to (+3) |
| Venous lactate (mmol/L) | 2.36 | – | – | 0.58 | – | 0.9–1.7 |
| Anion gap (mmol/L) | 13 | – | – | 6 | – | 10–20 |
| Total bilirubin (μmol/L) | 80 | – | – | – | – | 3–21 |
| Indirect bilirubin (μmol/L) | 26 | – | – | – | – | 2–14 |
| Lactate dehydrogenase (U/L) | 592 | – | – | 887 | – | 140–280 |
| International normalization ratio | – | 1.3 | – | – | – | 0.8–1.2 |
| Partial thromboplastin time (seconds) | – | 15 | – | – | – | <30 |
aSpecialized tests performed on samples sent to Darwin, Australia.
He was re-admitted on day 20 feeling unwell with generalized oedema, particularly of the face and lower extremities. Vital signs were stable: temperature 36.5°C, GCS 15, BP 130/74 mmHg and HR 78 bpm. He appeared sick, anaemic and had periorbital oedema. Cardiac examination revealed a systolic flow murmur at the left upper sternal border with no radiation. There was mild abdominal tenderness on palpation and bilateral lower limb pitting oedema. Biochemistry revealed AKI with a creatinine increased to 1,769 μmol/L, hyperkalaemia, a non-anion gap hyperchloraemic metabolic acidosis and severe anaemia with 4+ haemoglobinuria (Table 1), with other investigations outlined below. Bladder catheterization did not suggest urinary retention with a residual volume of less than 50 mL of dark orange urine. He promptly received calcium gluconate and insulin for hyperkalaemia, empiric ceftriaxone, and was referred for haemodialysis and blood transfusion. Following five rounds of haemodialysis and three units whole blood transfusion, his metabolic acidosis, hyperkalaemia, renal function, and anaemia improved (Figure 1).
Figure 1.
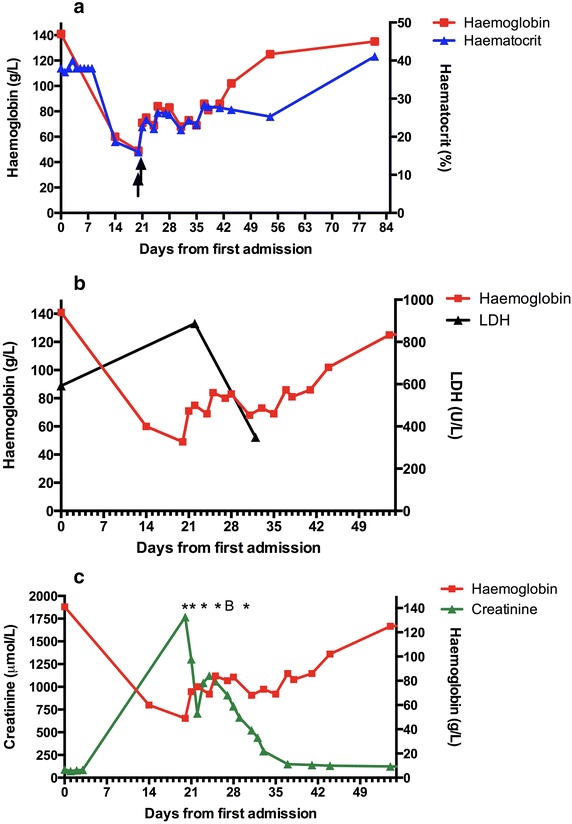
Haemoglobin, haematocrit, creatinine, and lactate dehydrogenase profiles from both hospital admissions. The first admission for severe malaria with hyperparasitaemia and hyperbilirubinaemia from day 0 to day 8; second hospital admission for severe haemolytic anaemia and acute kidney injury from day 20 to day 44. a Haemoglobin and haematocrit profile during hospital admissions. Haematocrit was stable during first hospital admission. Haemoglobin dropped by 57 and 65% on days 14 and 20, respectively, and recovered by day 81. Arrows indicate timing of whole blood transfusions on days 20 and 21; b haemoglobin and lactate dehydrogenase (LDH) profile during hospital admissions; c haemoglobin and creatinine profile during hospital admissions. Acute kidney injury, with a creatinine increase by 21× on second hospital admission, classified as stage 3 according to AKIN criteria and ‘failure’ by RIFLE criteria. Presumed baseline creatinine from day 1 was 74 μmol/L. His creatinine returned close to baseline by day 81 (106 μmol/L). Asterisks indicate haemodialysis on days 20, 21, 23, 25, and 30; ‘B’ indicates renal biopsy on day 27. AKIN acute kidney injury network, RIFLE risk, injury, failure, loss, end-stage kidney disease.
He was discharged recovering well on day 44 with stable serum creatinine and haemoglobin. He was followed up weekly thereafter, and by day 81 his kidney function improved further towards baseline (creatinine 115 μmol/L), and his haemoglobin recovered (135 g/L).
Investigations
Investigations are summarized in Table 1. Samples sent to Thailand and Australia for specialized tests are indicated by an asterisk. On day 20, reticulocytes accounted for 3% of erythrocytes (reticulocyte production index 0.4). The direct antiglobulin test (DAT; anti-IgG and anti-C3d) and indirect antiglobulin test (IAT) were negative. Plasma cell-free haemoglobin* was elevated (127,000 ng/mL; normal range <124,000 ng/mL), and haptoglobin* decreased (5 μg/mL; normal range: 300–2,000 μg/mL). A 24-h urine collection revealed 525 mg of protein (normal range <150 mg/day). Urine microscopy showed red blood cells casts and dysmorphic red cells. Urine biochemistry*: osmolarity 283 mOsm/kg, sodium 84 mmol/L, urine anion gap of 8, urine osmolar gap 21, and transtubular potassium gradient 1.8. Anti-nuclear antibodies were absent and C3/C4 levels were normal. HIV and hepatitis C antibodies, hepatitis B surface and envelope antigen were negative. Blood cultures and bacterial 16S* ribosomal DNA amplification performed to exclude bacteraemia as a cause of the leukocytosis were negative. Repeat peripheral blood smears for malaria were negative and no schistocytes were found. Glucose-6-phosphate dehydrogenase (G6PD) was qualitatively normal on day 0.
Abdominal ultrasound on day 22 showed normal kidney size with increased parenchymal echogenicity and poor corticomedullary differentiation, with no pelvicalyceal system dilatation.
A renal biopsy performed on day 27 revealed severe and recovering acute tubular injury, with interstitial oedema but only minimal tubulointerstitial inflammation (Figure 2). Tubules showed acute tubular dilatation, injury and regenerative features. Several red cell casts and amorphous casts were present. Vessels were normal. Glomeruli were morphologically normal with no active glomerulonephritis, negative immunoperoxidase staining for immunoglobulins and C3, and no immune complex deposition or basement membrane abnormalities on electron microscopy. A myoglobin immunostain was negative in casts. However, a Perl’s stain for iron showed both diffuse tubular staining and increased deposition in granular deposits within the cytoplasm of damaged tubular epithelial cells.
Figure 2.
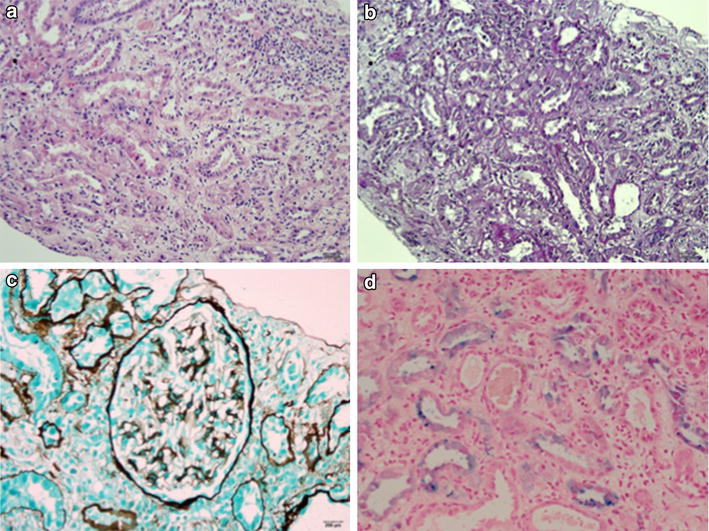
Renal biopsy. Left kidney ultrasound guided biopsy performed on day 27. Photomicrographs to show histopathological findings; a acute tubular necrosis with dilation, epithelial thinning, tubular cell necrosis and regeneration, interstitial oedema, and minimal inflammation (haematoxylin and eosin stain ×200); b acute tubular injury with PAS staining showing loss of brush border in the dilated injured proximal tubules (Periodic acid Schiff ×200); c a normal glomerulus (×400 Jones methanamine silver stain), and d a Perls stain for iron showing particulate and diffuse staining in damaged proximal tubules (×400). Specialized PAS, methanamine, and Perls straining were performed in Bangkok, Thailand since only haematoxylin and eosin stain was available in Bangladesh.
Discussion
This report describes a case of post-artesunate delayed-onset haemolysis complicated by acute kidney injury after recovery from severe falciparum malaria with hyperparasitaemia. Identifying the cause of haemolytic anaemia following malaria infection is challenging, as its mechanism is multifactorial and the broad differential diagnosis requires specialized testing that is often unavailable in resource-limited settings. The timing of haemolysis in this case 14 days after the first dose of artesunate accompanied by a >10% drop in haemoglobin (from day 7 haemoglobin), haptoglobin <0.1 g/L and lactate dehydrogenase >390 U/L is consistent with the case definition of PADH [5, 7, 8]. Since 2012, PADH is proposed to have occurred in at least 38 patients [19, 20], however these reports do not strictly adhere to the optimized definition of PADH recently published in 2014 [5, 7]. Given this limitation it is difficult to accurately determine the frequency and clinical impacts of PADH. A recent review of the reported cases with an alternative PADH case definition estimates that among severe malaria patients treated with intravenous artesunate PADH occurs in 13% and blood transfusions are required in 9% [20]. Of these PADH cases, 31 were non-immune returning travellers, six were immune African children under 5 years, and one was an immune West African adult [6, 9–11, 20–26]. The case reported here describes PADH in a non-immune adult Bangladeshi patient who recovered from an episode of severe malaria acquired while living in an unstable malaria transmission region.
The mechanism of PADH is hypothesized to be due to pitting of ring staged parasitized red cells, where the spleen removes the dead parasite from the red cell akin to the removal of Howell–Jolly bodies [5]. These pitted cells are formed quickly after treatment with artesunate and have a shortened red cell survival [27–29]. Destruction of the once-infected pitted cells manifests as delayed onset of haemolysis. However, in this case and other reported cases of PADH [30] the magnitude of the drop in haemoglobin exceeds the fall expected if only the once-infected pitted cells are destroyed. This suggests that additional artesunate-associated mechanisms of delayed haemolysis involving uninfected red cells must occur. Inhibition of erythropoiesis has also been proposed to contribute to the anaemia as it has been suggested that patients receiving higher doses of artesunate are at higher risk of bone marrow suppression [7]. This may have contributed to the anaemia in the presented case as he received a total dose of 7.2 mg/kg artesunate and 20.8 mg/kg artemether, and a day 20 reticulocyte production index of <2% supported an inadequate bone marrow response to his haemolysis. The young ring-stage activity of artesunate is one characteristic that confers its life-saving benefit over quinine. Artesunate remains first-line anti-malarial for the treatment of severe malaria since the benefit of survival outweighs the low probability of non-life-threatening delayed haemolysis. Recent guidelines recommend weekly monitoring malaria patients for indicators of haemolysis for 1 month following treatment with artesunate [30].
This case of delayed haemolysis was complicated by severe AKI, which has not been reported in the setting of PADH. The presence of excess haem pigment in tubular cells may be due to re-absorption from tubular red cells or haemoglobinuria. The role of haemoglobinuria has been associated with AKI in a spectrum of diseases including blackwater fever, babesiosis, paroxysmal nocturnal haemoglobinuria, massive transfusion and post-cardiopulmonary bypass [12–15, 17, 18, 31]. While not all cases of haemoglobinuria result in AKI, up to 64% of patients with blackwater fever develop renal dysfunction [14]. In this case the features suggested direct tubular epithelial toxicity due to free haem rather than a cast nephropathy. The pathophysiology of haem-pigment AKI is not completely defined but vasoconstriction; direct tubular cell injury and tubular obstruction are thought to contribute [32]. There is accumulating evidence that haemoproteins induce oxidative renal damage by lipid peroxidation [17, 33]. The redox cycling of the haem group initiates lipid peroxidation, releasing potent oxidative compounds that cause renal vasoconstriction [33]. This mechanism is demonstrated in post-operative AKI after post-cardiopulmonary bypass where high cell-free haemoglobin and oxidative stress were associated with kidney injury [17, 18]. Cell-free haemoglobin is also likely to cause vasoconstriction through its quenching of endothelial nitric oxide (NO) in severe falciparum malaria, where it has been associated with impairment in NO-dependent endothelial function [34]. The renal pathology in this case is consistent with haem-pigment induced AKI with clinical evidence of non-anion gap hyperchloraemic metabolic acidosis with distal tubule dysfunction likely secondary to hypovolaemia and vasoconstriction.
The differential diagnosis for haemolysis in this case is broad and the possible contribution of an intercurrent bacterial infection between admissions was considered given his leukocytosis on day 14. Infections may cause haemolysis by precipitating oxidative stress in patients with underlying red blood cell (RBC) disorders, inducing haemolytic pathologic responses, or from the antimicrobial therapy itself [35]. Limited diagnostics suggested no underlying RBC abnormality: normal qualitative G6PD, mean corpuscular volume (MCV) and peripheral smear. Negative DAT and IAT argued against an immune-mediated haemolytic anaemia. Infections characterized by haemolysis include: malaria, babesiosis, Carrion’s disease, and typhoid. The peripheral smear on second admission revealed no intra-erythrocytic organisms. Negative blood cultures and bacterial 16S ribosomal DNA amplification were not conclusive but argued against infection as a cause of the haemolysis. Furthermore, the leukocytosis observed on day 14 had resolved by day 20 in the absence of antibiotic treatment.
The differential diagnosis for the etiology of AKI included (1) pre-renal, and (2) intrinsic renal causes including: acute tubular injury (ATI) due to ischaemia or direct toxin-mediated injury by cell-free haemoglobin, acute interstitial nephritis (AIN) secondary to β-lactams, microangiopathy, or post-infectious glomerulonephritis (PIGN). The urinary osmolarity <350 mOsm/kg and fractional excretion of sodium >1% did not suggest a pre-renal cause. The absence of systemic eosinophils and eosinophil-rich tubulo-interstitial inflammation on renal biopsy argued against a diagnosis of AIN, although recovering AIN can be pauci-inflammatory. The normal platelets, lack of schistocytes, negative blood cultures and 16S rRNA amplification, and normal renal vessels and glomeruli on biopsy were not consistent with haemolytic uraemia syndrome/thrombotic thrombocytopenic purpura or PIGN.
Treatment for established AKI is limited to renal replacement therapy. Research towards prevention and alternative treatments is vital since dialysis is frequently unavailable. Currently, there is no targeted treatment for cell-free haemoglobin-induced AKI, however results from experimental models and case series are encouraging. Recent studies have shown that paracetamol inhibits haemoprotein-induced lipid peroxidation, resulting in decreased oxidative kidney injury and improved renal function [36, 37]. Administration of haptoglobin infusions is shown to attenuate cell-free haemoglobin-mediated AKI in paroxysmal nocturnal haemoglobinuria and an experimental model of stored blood transfusion [38, 39].
Conclusions
Malaria treatment guidelines should consider recommending that renal function be checked if a drop in haemoglobin is detected during monitoring of severe malaria patients after treatment with artesunate. This may be logistically difficult in limited-resource countries but where it can be done, monitoring the creatinine should be a part of severe malaria management to detect and avoid renal complications.
Learning points
Delayed onset haemolytic anaemia following parasite clearance in severe malaria infection has a broad differential diagnosis to consider.
Delayed onset haemolysis following severe malaria may occur following artesunate treatment, and be complicated by AKI.
Severe malaria patients treated with artesunate should be monitored weekly for decline of haemoglobin and renal function.
There are limited treatment options to treat haemoprotein-mediated AKI.
Authors’ contributions
All authors contributed to the content of this case report. KP performed the clinical assessments, data collection and drafted manuscript and figures. MSH performed clinical assessments, guided dialysis and performed renal biopsy. HK, TY and NMA performed clinical assessments. AG and AH were responsible for clinical care of the patient. GDHT reviewed the renal biopsy, and assisted in drafting the manuscript and figures. HK, TWY, AMD, GDHT, and NMA critically interpreted the clinical case, and revised manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgements
We thank the patient, medical residents, attending physicians, and support staff at Chittagong Medical College Hospital for their assistance and collaboration with the Mahidol-Oxford Tropical Medicine Research Unit, Kim Piera for laboratory assistance, and Dr. Claire Ling for molecular analysis. This work was supported by the Australian National Health and Medical Research Council (grant number 605807 and Fellowships to NMA and TWY). Mahidol-Oxford Tropical Medicine Research Unit is funded by the Wellcome Trust of Great Britain through grant number 089275/Z/09/Z. KP was supported by the University of British Columbia Clinician Investigator Program, Vancouver, Canada.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Consent for publication Ethical approval was obtained from the Ministry of Health in Bangladesh, Menzies Human Research Ethics Committee in Australia, and the Oxford Tropical Research Ethics Committee, Oxford, UK. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of Malaria Journal.
Abbreviations
- AIN
acute interstitial nephritis
- AKI
acute kidney injury
- AL
artemether lumefantrine
- ATI
acute tubular injury
- BP
blood pressure
- bpm
beats per minute
- DAT
direct antiglobulin test
- G6PD
glucose-6-phosphate dehydrogenase
- GCS
Glasgow coma score
- HIV
human immunodeficiency virus
- HR
heart rate
- IAT
indirect antiglobulin test
- MCV
mean corpuscular volume
- NO
nitric oxide
- PADH
post-artesunate delayed haemolysis
- PIGN
post-infectious glomerulonephritis
- RBC
red blood cell
- RDT
rapid diagnostic test
Contributor Information
Katherine Plewes, Email: katherine@tropmedres.ac.
Md Shafiul Haider, Email: dr_rusho@hotmail.com.
Hugh W F Kingston, Email: hugh@tropmedres.ac.
Tsin W Yeo, Email: Tsin.Yeo@menzies.edu.au.
Aniruddha Ghose, Email: anrdghs@yahoo.com.
Md Amir Hossain, Email: amir_hossain_ctg@yahoo.com.
Arjen M Dondorp, Email: Arjen@tropmedres.ac.
Gareth D H Turner, Email: Gareth.Turner@fhft.nhs.uk.
Nicholas M Anstey, Email: Nicholas.Anstey@menzies.edu.au.
References
- 1.Camacho LH, Gordeuk VR, Wilairatana P, Pootrakul P, Brittenham GM, Looareesuwan S. The course of anaemia after the treatment of acute, falciparum malaria. Ann Trop Med Parasitol. 1998;92:525–537. doi: 10.1080/00034989859221. [DOI] [PubMed] [Google Scholar]
- 2.Adner MM, Altstatt LB, Conrad ME. Coombs’-positive hemolytic disease in malaria. Ann Intern Med. 1968;68:33–38. doi: 10.7326/0003-4819-68-1-33. [DOI] [PubMed] [Google Scholar]
- 3.Omodeo-Sale F, Motti A, Dondorp A, White NJ, Taramelli D. Destabilisation and subsequent lysis of human erythrocytes induced by Plasmodium falciparum haem products. Eur J Haematol. 2005;74:324–332. doi: 10.1111/j.1600-0609.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff AW, Ansdell VE, Pettitt LE. Cause of anaemia in malaria. Lancet. 1979;1:1055–1057. doi: 10.1016/S0140-6736(79)92952-0. [DOI] [PubMed] [Google Scholar]
- 5.Jaureguiberry S, Ndour PA, Roussel C, Ader F, Safeukui I, Nguyen M, et al. Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood. 2014;124:167–175. doi: 10.1182/blood-2014-02-555953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreeftmeijer-Vegter AR, van Genderen PJ, Visser LG, Bierman WF, Clerinx J, van Veldhuizen CK, et al. Treatment outcome of intravenous artesunate in patients with severe malaria in the Netherlands and Belgium. Malar J. 2012;11:102. doi: 10.1186/1475-2875-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Experts Group Meeting on delayed anaemia following treatment with injectable artesunate, Wien Austria. (http://www.mmv.org/sites/default/files/uploads/docs/events/2013/InjectableArtesunateExpertGroupMeeting.pdf)
- 8.Paczkowski MM, Landman KL, Arguin PM. Update on cases of delayed hemolysis after parenteral artesunate therapy for malaria—United States, 2008 and 2013. MMWR Morb Mortal Wkly Rep. 2014;63:753–755. [PMC free article] [PubMed] [Google Scholar]
- 9.Rolling T, Agbenyega T, Issifou S, Adegnika AA, Sylverken J, Spahlinger D, et al. Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria—a double-center prospective study. J Infect Dis. 2014;209:1921–1928. doi: 10.1093/infdis/jit841. [DOI] [PubMed] [Google Scholar]
- 10.Rolling T, Wichmann D, Schmiedel S, Burchard GD, Kluge S, Cramer JP. Artesunate versus quinine in the treatment of severe imported malaria: comparative analysis of adverse events focussing on delayed haemolysis. Malar J. 2013;12:241. doi: 10.1186/1475-2875-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoller T, Junghanss T, Kapaun A, Gjorup I, Richter J, Hugo-Persson M, et al. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis. 2011;17:771–777. doi: 10.3201/eid1705.101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum S, Gattringer R, Haschke E, Walochnik J, Tschurtschenthaler G, Lang F, et al. The case: hemolysis and acute renal failure. Babesiosis. Kidney Int. 2011;80:681–683. doi: 10.1038/ki.2011.184. [DOI] [PubMed] [Google Scholar]
- 13.Clark DA, Butler SA, Braren V, Hartmann RC, Jenkins DE., Jr The kidneys in paroxysmal nocturnal hemoglobinuria. Blood. 1981;57:83–89. [PubMed] [Google Scholar]
- 14.Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, et al. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin Infect Dis. 1996;23:1274–1281. doi: 10.1093/clinids/23.6.1274. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CW, Wu VC, Lin WC, Huang JW, Wu MS. Acute renal failure in a patient with paroxysmal nocturnal hemoglobinuria. Kidney Int. 2007;71:1187. doi: 10.1038/sj.ki.5002179. [DOI] [PubMed] [Google Scholar]
- 16.Bunn HF, Jandl JH. The renal handling of hemoglobin. II. Catabolism. J Exp Med. 1969;129:925–934. doi: 10.1084/jem.129.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billings FT, Ball SK, Roberts LJ, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 19.Raffray L, Receveur MC, Beguet M, Lauroua P, Pistone T, Malvy D. Severe delayed autoimmune haemolytic anaemia following artesunate administration in severe malaria: a case report. Malar J. 2014;13:398. doi: 10.1186/1475-2875-13-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehman K, Lotsch F, Kremsner PG, Ramharter M. Haemolysis associated with the treatment of malaria with artemisinin derivatives: a systematic review of current evidence. Int J Infect Dis. 2014;29:268–273. doi: 10.1016/j.ijid.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Caramello P, Balbiano R, De Blasi T, Chiriotto M, Deagostini M, Calleri G. Severe malaria, artesunate and haemolysis. J Antimicrob Chemother. 2012;67:2053–2054. doi: 10.1093/jac/dks139. [DOI] [PubMed] [Google Scholar]
- 22.Corpolongo A, De Nardo P, Ghirga P, Gentilotti E, Bellagamba R, Tommasi C, et al. Haemolytic anaemia in an HIV-infected patient with severe falciparum malaria after treatment with oral artemether–lumefantrine. Malar J. 2012;11:91. doi: 10.1186/1475-2875-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Nardo P, Oliva A, Giancola ML, Ghirga P, Mencarini P, Bibas M, et al. Haemolytic anaemia after oral artemether–lumefantrine treatment in a patient affected by severe imported falciparum malaria. Infection. 2013;41:863–865. doi: 10.1007/s15010-013-0451-x. [DOI] [PubMed] [Google Scholar]
- 24.Eder M, Farne H, Cargill T, Abbara A, Davidson RN. Intravenous artesunate versus intravenous quinine in the treatment of severe falciparum malaria: a retrospective evaluation from a UK centre. Pathog Glob Health. 2012;106:181–187. doi: 10.1179/2047773212Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis JN, Coltart CE, Pule M, Chiodini PL, Doherty T. Artemisinin therapy and severe delayed haemolysis. Lancet. 2013;382:180. doi: 10.1016/S0140-6736(13)60812-0. [DOI] [PubMed] [Google Scholar]
- 26.Kano S. Artemisinin-based combination therapies and their introduction in Japan. J Infect Chemother. 2010;16:375–382. doi: 10.1007/s10156-010-0077-1. [DOI] [PubMed] [Google Scholar]
- 27.Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. In vivo removal of malaria parasites from red blood cells without their destruction in acute falciparum malaria. Blood. 1997;90:2037–2040. [PubMed] [Google Scholar]
- 28.Chotivanich K, Udomsangpetch R, Dondorp A, Williams T, Angus B, Simpson JA, et al. The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J Infect Dis. 2000;182:629–633. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 29.Newton PN, Chotivanich K, Chierakul W, Ruangveerayuth R, Teerapong P, Silamut K, et al. A comparison of the in vivo kinetics of Plasmodium falciparum ring-infected erythrocyte surface antigen-positive and -negative erythrocytes. Blood. 2001;98:450–457. doi: 10.1182/blood.V98.2.450. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) (2013) Published reports of delayed hemolytic anemia after treatment with artesunate for severe malaria—worldwide, 2010–2012. MMWR Morb Mortal Wkly Rep 62:5–8 [PMC free article] [PubMed]
- 31.Rubin H. Paroxysmal nocturnal hemoglobinuria with renal failure. JAMA. 1971;215:433–436. doi: 10.1001/jama.1971.03180160033008. [DOI] [PubMed] [Google Scholar]
- 32.Heyman SN, Rosen S, Fuchs S, Epstein FH, Brezis M. Myoglobinuric acute renal failure in the rat: a role for medullary hypoperfusion, hypoxia, and tubular obstruction. J Am Soc Nephrol. 1996;7:1066–1074. doi: 10.1681/ASN.V771066. [DOI] [PubMed] [Google Scholar]
- 33.Moore KP, Holt SG, Patel RP, Svistunenko DA, Zackert W, Goodier D, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273:31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 34.Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis. 2009;200:1522–1529. doi: 10.1086/644641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkowitz FE. Hemolysis and infection: categories and mechanisms of their interrelationship. Rev Infect Dis. 1991;13:1151–1162. doi: 10.1093/clinids/13.6.1151. [DOI] [PubMed] [Google Scholar]
- 36.Boutaud O, Moore KP, Reeder BJ, Harry D, Howie AJ, Wang S, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci USA. 2010;107:2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janz DR, Bastarache JA, Peterson JF, Sills G, Wickersham N, May AK, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41:784–790. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibasaki T, Matsuda H, Furuya K. Haptoglobin therapy during pregnancy for paroxysmal nocturnal hemoglobinuria with renal failure. Int J Gynaecol Obstet. 2007;98:267–268. doi: 10.1016/j.ijgo.2007.03.021. [DOI] [PubMed] [Google Scholar]


