Abstract
The feasibility of an innovative biomedical diagnostic technique, thermal photoacoustic (TPA) measurement, for non-ionizing and non-invasive assessment of bone health is investigated. Unlike conventional photoacoustic PA methods which are mostly focused on the measurement of absolute signal intensity, TPA targets the change in PA signal intensity as a function of the sample temperature, i.e. the temperature dependent Grueneisen parameter which is closely relevant to the chemical and molecular properties in the sample. Based on the differentiation measurement, the results from TPA technique are less susceptible to the variations associated with sample and system, and could be quantified with improved accurately. Due to the fact that the PA signal intensity from organic components such as blood changes faster than that from non-organic mineral under the same modulation of temperature, TPA measurement is able to objectively evaluate bone mineral density (BMD) and its loss as a result of osteoporosis. In an experiment on well-established rat models of bone loss and preservation, PA measurements of rat tibia bones were conducted over a temperature range from 37 °C to 44 °C. The slope of PA signal intensity verses temperature was quantified for each specimen. The comparison among three groups of specimens with different BMD shows that bones with lower BMD have higher slopes, demonstrating the potential of the proposed TPA technique in future clinical management of osteoporosis.
Osteoporosis is a major health problem worldwide, with healthcare costs of billions of dollars annually. In osteoporosis, the bone mineral density (BMD) reduces and bone microarchitecture deteriorates, leading to increased fracture susceptibility. Currently, clinically available diagnostic technologies for osteoporosis are based on the use of either X-rays, considered as the gold standard, or ultrasound (US). Dual X-ray absorptiometry (DXA) is the most commonly used technique to measure BMD. Unfortunately, X-ray based methods describes fracture risk incompletely, for it only provides a measure of BMD while other important parameters, such as bone microarchitecture, bone geometry and elastic properties, cannot be assessed [1]. Non-ionizing and non-invasive quantitative ultrasound (QUS) technologies provide a practical and low-cost surrogate for DXA. QUS has the ability to determine bone quality and provide information relate to not only BMD but also bone microstructure and elastic properties, which has been confirmed in many previous studies and has led to clinical instruments for evaluation of bone health [2].
Recently, photoacoustic (PA) imaging, an emerging hybrid technology involving both ultrasound and light, has been explored for potential application in the diagnosis of osteoporosis [3, 4]. Unlike QUS which is more sensitive to trabecular bone, PA signal is sensitive to the variations in bone density in both cortical and trabecular parts of the bone [3]. Multi-spectral PA signals from a biological sample can reflect the contents of the optically absorbing chemical components in the sample [4]. On the other hand, the frequency components of the PA signals from a sample are highly relevant to the histological microstructures in the sample, and can be quantitatively evaluated for tissue characterization [5]. Therefore, it is expected that, by adding PA to US, valuable complementary information about bone composition and structure, both relevant to bone strength, could be obtained.
Previous researches on PA based measurements of bone were mostly replying on the studies of the PA signal intensity in the time domain or the PA power spectrum in the frequency domain. Either one, however, is highly susceptible to sample and equipment-dependent variations and, hence, is difficult to lead to quantitative findings. As an example, the PA signal intensity as a function of optical wavelength is not only determined by the biochemical composition in the bone but also affected by the optical parameters of the soft tissue covering the bone which are highly variable for different patients and also difficult to be estimated. As another challenge although less significant, the individual differences in acoustic parameters in the soft tissue covering the bone could also affect the intensity and the frequency of the received PA signals from the target bone.
In this work, we developed a new method of thermal photoacoustic (TPA) evaluation of bone, with an ultimate goal of achieving quantitative diagnosis of osteoporosis by using the non-invasive and non-ionizing photoacoustic measurement. Unlike other PA techniques, TPA evaluates the tissue-specific Grueneisen parameter by quantifying the slope of the temperature-dependent PA signal intensity. Therefore, instead of relying on the measurement of absolute signal intensity, which is difficult to be quantified, TPA is focused on the relative changes in signal intensity as a function of temperature. By performing differentiation-based measurement, the impact from variations associated with sample and equipment on the accuracy of PA results could be effectively avoided.
When a target soft tissue, is illuminated by a short laser pulse, the initial PA pressure from the tissue can be expressed as
| (1) |
Due to the difference in acoustic impedance between hard tissue and coupling medium, the initial PA pressure from the bone matrix illumined by light can be expressed as [3]
| (2) |
| (3) |
where Γ is the Grueneisen parameter, μa is the absorption coefficient, Φ is the local light fluence, B is the thermal coefficient of volume expansion, c is the speed of sound (SOS) of target tissue, Cp is the heat capacity at constant pressure, and ,ca cs and ρa, ρs are the SOS and density of bone matrix and coupling medium, respectively. Among these perameter determining the intial PA signal amplitude P0, the local light fluence Φ and the absorption coefficient μa in the tissue are both spatially distributed and difficult to be estimated. However, both of them do not change with the sample temperature as long as the target tissue is not thermally damaged and its status remains unchanged [6]. Since the tissue parameters including c, ρ, β and Cp are all temperature dependent, the Grueneisen parameter Γ as well as the PA signal intensity changes with the sample temperature. This phenomenon has been validated in previous studies and adapted to applications such as PA monitoring of thermal therapy including those based on highly intensity focused ultrasound (HIFU) [7-9]. The Grueneisen parameter Γ and its change as a function of the temperature are also highly dependent on the type and the status of the target tissue, due to the fact that the relevant parameters including c, β and Cp are all tissue specific. Containing various chemical components, different tissues show distinctive trends in PA signal intensity versus temperature. Therefore, quantifying the slope of temperature-dependent PA signal intensity, i.e. TPA measurement, offers a potentially new contrast for medical diagnosis and tissue characterization.
The schematic of the experiment system is shown in Fig. 1. An OPO system (Vibrant B, Opotek) pumped by an Nd: YAG laser (Brilliant B, Bigsky) was used to provide laser pulses with a repetition rate of 10Hz and a pulse width of 5.5 ns. The wavelength of the laser was continuously tunable during 680-900 nm. The laser beam with 2 mm in diameter illuminating the bone surface generates PA signals which were received by a 1 MHz unfocused transducer (A303S, Panametrics). To heat the sample uniformly and to realize a precise adjustment of the sample temperature, the sample as well as the transducer was immersed in a temperature controlled water bath (Thermo scientific, microprocessor controlled 280 series). About 10% of the energy of the laser beam was branched out by a glass plate and directed onto a black body which has a flat optical absorption spectrum over 680-900 nm. The PA signals from the black body were detected by another transducer (C310, Parametrics) and then recorded through the second channel of the oscilloscope (TDS 540B, Tektronics) for later normalization of the PA signals from the bone specimen.
Fig. 1.

Thermal photoacoustic measurement system.
Fresh human bone contains 70% non-organic mineral (hydroxyapatite) and 10% of water. Around 20% of bone is composed of organic compounds, including collagen, lipid and various non-collagenous proteins such as hemoglobin [10]. The percentage of non-organic mineral in total bone mass (or the ratio between non-organic mineral and organic compounds) is directly correlated with the BMD and the strength of the bone. Each of the non-organic and organic materials in the bone has its unique optical absorption spectrum and may contribute to the PA signal from the bone. Over the spectral region of 680-900 nm, the dominant absorbing components in the bone are hydroxyapatite and hemoglobin, while the optical absorption from other materials are relatively low.
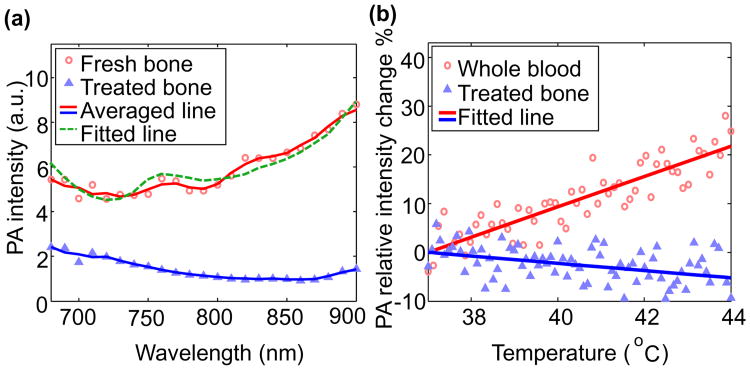
To understand the contributions of non-organic mineral and organic materials to the PA signal in the spectral range of 680-900 nm, we first conducted spectroscopic measurements of two different bone specimens: a) a fresh goat rib bone which contains both organic materials and non-organic mineral, and b) a treated goat rib bone that has been defatted by keeping in water for 2-3 weeks. In the treated bone, the organic components were mostly removed, and the dominant optically absorbing material was non-organic mineral. The spectroscopic PA curves from the two bone specimens, as shown in Fig. 2(a), have a good match with the results from previous studies [11, 12]. The PA signal from the fresh bone is higher than that from the treated bone over the entire spectrum of 680-900 nm, indicating that both organic tissue and non-organic mineral contribute to the PA signal. The PA spectrum from the fresh bone in Fig. 2(a) was decomposed into a sum of six chromophores including oxy-hemoglobin, deoxy-hemoglobin, mineral, collagen, lipid and water. For the best fit of the PA spectrum by using the least square method, as shown by the fitted line, the relative contributions from them were 13.6%, 38.0%, 45.2%, 2.6%, less than 1%, and less than 1%, respectively, at the wavelength of 685 nm. At 685-nm wavelength, the contributions from organic materials and non-organic mineral are similar (51.6 vs. 45.2%). This wavelength was chosen for later TPA measurements because it facilitates good sensitivity in assessing the ratio between organic and non-organic components in the bone.
Fig. 2.
(a) Spectroscopiic photoacoustic curves of a fresh goat bone and a treated goat bone. Scattered symbols are PA measurements. Solid lines are 3-point averaging. (b) Photoacoustic signal intensity as a function of temperature for two different specimens: 1) whole blood and 2) treated bone.
To validate that organic materials and non-organic mineral have different trends of PA signal intensity versus temperature, the slopes of temperature-dependent PA signal intensity were quantified for two different samples: a) porcine whole blood filled in a transparent tube (I.D. 23 mm), and b) treated goat rib bone which contains mostly non-organic mineral. The percentage changes in PA signal intensity at 685 nm as a function of temperature from 37 °C to 44 °C for the two samples are shown in Fig. 2(b) for comparison. With the temperature change from 37-44 C, for blood as well as other organic tissues containing large amount of water, the thermal coefficient of volume expansion β changes (increase >10%) much faster with the temperature T in comparison with the heat capacity at constant pressure Cp (increase <1%) and the speed of sound c (increase < 1%) [13]. When T is higher, β is higher leading to stronger PA signal. For mineral in response to the temperature change from 37-44 C, Cp (increase >10%) changes much faster compared to the other parameters including β, ca, cs,ρa , and ρs (all < 2%) [13-16]. When T is higher, Cp is higher leading to weaker PA signal. This result in Fig. 2(b) demonstrates distinctive TPA trends between organic materials and non-organic mineral, and suggests that bones with different BMD should also show different TPA outcomes.
To validate the feasibility of the TPA technique in evaluating of osteoporosis, an experiment was conducted on well-established rat models of bone loss and preservation. Tibia from three groups of rats subject to 1) ovariectomy-induced bone loss (OVX, N=4), 2) preservation of bone mass with Zoledronic Acid (OVX+ZOL, N=4), and 3) normal controls (Sham, N=4) provides bone specimens with low, high, and normal BMD, respectively. The FDA requires the use of the ovariectomized rat model for evaluation of agents used to treat or prevent postmenopausal osteoporosis, and the model has been validated based on the early bone turnover that produces bone loss following estrogen withdrawal. PA measurement was performed ex vivo on the proximal tibia (metaphysis) underwent 3 months of bone loss or preservation. Temperature controllable water bath was used to heat bone precisely from 37 °C to 44 °C. The PA signal was averaged 50 times and recorded for every 0.1°C of temperature change.
Besides PA measurement, the tibia bones were also imaged with a microCT (GE Healthcare eXplore Locus RS) used as a gold standard to assess bone mass. The microCT images, as shown in Fig. 3, demonstrate that the non-organic mineral of OVX rats diminished significantly, resulting in a 49% reduction in trabecular BMD; while for OVX+ZOL rats, the BMD increased about 26% over the sham controls.
Fig. 3.
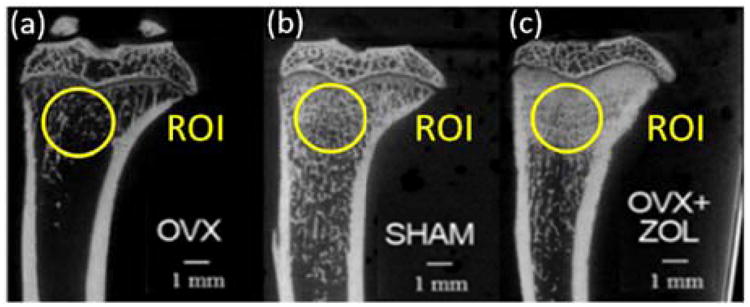
MicroCT images of tibia bones of female rats subject to a) OVX, b) sham, and c) OVX+ZOL. OVX induces a 49% reduction in trabecular BMD, while OVX+ZOL makes BMD increase 26% over sham controls.
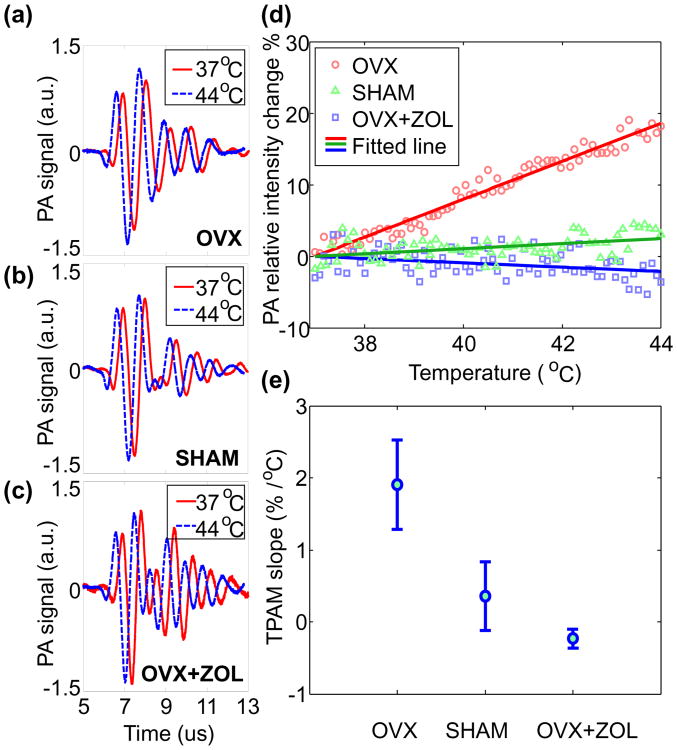
Example radio-frequency PA signals from the three groups of bone specimens are compared in Figs. 4(a)-(c). For bones in OVX group with low BMD, enhancement in PA signal intensity as a result of temperature rise can be seen. For bones in the OVX+ZOL group with high BMD, the PA signal intensity decreased after the same temperature rise. Fig. 4(d) shows example TPA results from the three sample groups. Bone specimens containing different BMD show distinctive trends in PA signal intensity as a function of temperature. For the TPA result from each sample, a linear fit can be made. Then a TPA slope defined as the percentile change of PA signal intensity for every degree of increase in temperature can be quantified. The averages and the standard deviations of the quantified TPA slopes are 1.91±0.62%/°C, 0.36±0.48%/°C, and -0.23±0.13%/°C for OVX, Sham, and OVX+ZOL groups, respectively. The result in Fig. 4(e) shows that the TPA slope is higher for bone specimen with lower BMD, which matches well with our expectation. Based on the quantified TPA slope, the separation between the OVX (i.e. osteoporosis) group and the other two groups is successful, although there is a mild overlap between the Sham and the OVX+ZOL groups.
Fig. 4.
(a)(b)(c) PA signal of OVX, SHAM, OVX+ZOL samples at 37 °C and 44 °C (with 0.2-μs delay in order to show it clearly), respectively. (d) The PA signal intensity vs. temperature for the three groups of rat tibia bones (OVX with low BMD, sham with normal BMD, and OVX+ZOL with high BMD), respectively. The numbers of recorded temperature points are 74, 67 and 75, respectively. The solid lines are the linear fit for each group (p<0.01). (e) Quantified TPA slopes for the three groups of bones.
The result from the rat models of bone loss and preservation suggests the potential of the TPA technique for future clinical assessment of osteoporosis and treatment. TPA measurement is based the new contrast of temperature dependent Grueneisen parameter which is highly sensitive to the chemical compositions in biological tissues. As validated in this research, because non-organic mineral and organic materials in the bone show distinctive trends in PA signal intensity as a function of temperature, the quantified slope from the TPA measurement could assess the ratio between them and the change as a result of osteoporosis and treatment. Unlike established technologies such as QUS which focused mainly on bone mineral, TPA is sensitive to both mineral and other chemical components (e.g. hemoglobin) in the bone. There are additional parameters beyond BMD which reflect the makeup of the extracellular matrix which may have potential for monitoring fracture risk. Therefore TPA assessment may offer a better tool for evaluating bone health.
To achieve future clinical applications in completely non-invasive manner, the temperature modulation in tissue required for TPA needs to be precisely controlled. The localized temperature modulation in a target tissue, e.g. bone, may be realized using high intensity focused ultrasound (HIFU). For clinical use, the modulated temperature in the bone should be kept below 44 °C. According to a former study, 47 °C treatment for 1 minute is the threshold level for bone survival [17], which is equivalent to the thermal dose of 8 minutes at 44 °C. It has been validated by our study on rat models that the range of temperature modulation from 37 °C to 44 °C is sufficient for TPA evaluation of bone health and can separate the three groups of bone specimens with different BMD.
Acknowledgments
This research was supported by National Institute of Health under grant numbers R01AR060350 and R01CA186769, and R21AR061594, National Natural Science Foundation of China under grants number 61201425, Natural Science Foundation of Jiangsu Province under grants number BK20131280 and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Contributor Information
Jie Yuan, Email: yuanjie@nju.edu.cn.
Cheri X. Deng, Email: cxdeng@umich.edu.
Xueding Wang, Email: xdwang@umich.edu.
References for review
- 1.Pisani P, Renna MD, Conversano F, Casciaro E, Muratore M, Quarta E, Di Paola M, Casciaro S. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World journal of radiology. 2013;5:398. doi: 10.4329/wjr.v5.i11.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laugier P. Quantitative ultrasound instrumentation for bone in vivo characterization. Springer; 2011. [Google Scholar]
- 3.Lashkari B, Mandelis A. Coregistered photoacoustic and ultrasonic signatures of early bone density variations. Journal of biomedical optics. 2014;19:036015–036015. doi: 10.1117/1.JBO.19.3.036015. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg I, Eyal A, Gannot I. SPIE BiOS. International Society for Optics and Photonics; 2013. Multispectral photoacoustic method for the early detection and diagnosis of osteoporosis; pp. 85656G-85656G–85659. [Google Scholar]
- 5.Xu G, Meng ZX, Lin JD, Yuan J, Carson PL, Joshi B, Wang X. The Functional Pitch of An Organ: Quantification of Tissue Texture with Photoacoustic Spectrum Analysis. Radiology. 2014;271:248–254. doi: 10.1148/radiol.13130777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao YS, Wang X, Deng CX. Dual-wavelength photoacoustic technique for monitoring tissue status during thermal treatments. Journal of biomedical optics. 2013;18:067003–067003. doi: 10.1117/1.JBO.18.6.067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pramanik M, Wang LV. Thermoacoustic and photoacoustic sensing of temperature. Journal of biomedical optics. 2009;14:054024-054024–054027. doi: 10.1117/1.3247155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Emelianov S. Thermal intravascular photoacoustic imaging. Biomedical optics express. 2011;2:3072–3078. doi: 10.1364/BOE.2.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khokhlova TD, Pelivanov IM, Sapozhnikov OA, Solomatin VS, Karabutov AA. Opto-acoustic diagnostics of the thermal action of high-intensity focused ultrasound on biological tissues: the possibility of its applications and model experiments. Quantum Electronics. 2006;36:1097. [Google Scholar]
- 10.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Analytical and bioanalytical chemistry. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 11.Pifferi A, Torricelli A, Taroni P, Bassi A, Chikoidze E, Giambattistelli E, Cubeddu R. Optical biopsy of bone tissue: a step toward the diagnosis of bone pathologies. Journal of biomedical optics. 2004;9:474–480. doi: 10.1117/1.1691029. [DOI] [PubMed] [Google Scholar]
- 12.Coelho T, Nogueira E, Steimacher A, Medina A, Weinand W, Lima W, Baesso M, Bento A. Characterization of natural nanostructured hydroxyapatite obtained from the bones of Brazilian river fish. Journal of applied physics. 2006;100:094312. [Google Scholar]
- 13.Duck FA. Physical properties of tissues: a comprehensive reference book. Academic Press; 1990. [Google Scholar]
- 14.Southard J, Milner R. Low Temperature Specific Heats. V. The Heat Capacity of Tricalcium Phosphate between 15 and 298° K. Journal of the American Chemical Society. 1935;57:983–984. [Google Scholar]
- 15.McCarthy RN, Jeffcott LB, McCartney RN. Ultrasound speed in equine cortical bone: effects of orientation, density, porosity and temperature. Journal of biomechanics. 1990;23:1139–1143. doi: 10.1016/0021-9290(90)90006-o. [DOI] [PubMed] [Google Scholar]
- 16.Milosevski M, Bossert J, Milosevski D, Gruevska N. Preparation and properties of dense and porous calcium phosphate. Ceramics International. 1999;25:693–696. [Google Scholar]
- 17.Eriksson A, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. The Journal of prosthetic dentistry. 1983;50:101–107. doi: 10.1016/0022-3913(83)90174-9. [DOI] [PubMed] [Google Scholar]