Abstract
Objective
To evaluate the associations between dietary carbohydrate, glycemic index (GI), glycemic load (GL), and incident prostate cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort.
Methods
Between September 1993 and September 2000, 38,343 men were randomized to the screening arm of the trial at one of 10 PLCO centers. A food frequency questionnaire administered at baseline assessed usual dietary intake over the preceding 12 months. Prostate cancer was ascertained by medical follow-up of suspicious screening results and annual mailed questionnaires and confirmed with medical records. Cox proportional hazards regression was used to model the associations of carbohydrate, GI, and GL with prostate cancer risk.
Results
During follow-up (median = 9.2 years), 2,436 incident prostate cancers were identified among 30,482 eligible participants. Overall, there were no associations of baseline carbohydrate, GI, or GL with incident prostate cancer in minimally or fully adjusted models. There were no associations when the 228 advanced and 2,208 non-advanced cancers were analyzed separately.
Conclusions
Dietary carbohydrate, GI, and GL were not associated with incident prostate cancer in PLCO. The narrow range of GI in this cohort may have limited our ability to detect associations, an issue that future studies should address.
Keywords: Prostatic neoplasms, Dietary carbohydrates, Glycemic index
Introduction
Various dietary factors are purported to influence prostate cancer risk, including processed meat, milk and dairy products, and micronutrients such as calcium, lycopene, selenium, and vitamin E [1]. Although most studies of macronutrient intakes and prostate cancer have focused on dietary fat and protein, dietary carbohydrate also may play a role in the etiology of this cancer. Carbohydrates elicit a wide spectrum of blood glucose and insulin responses, influenced by both the quality and quantity of the carbohydrates consumed. Glycemic index (GI) is a ranking of carbohydrate-containing foods based on their postprandial blood glucose responses relative to a carbohydrate standard and is a measure of carbohydrate quality [2]. Generally, the lower the GI, the lower the rate of absorption of the carbohydrate and the smaller the rise in postprandial glucose and insulin concentrations [3]. Glycemic load (GL) is a measure that incorporates both the quality and quantity of dietary carbohydrates and is determined by multiplying the carbohydrate content of a given serving of the food by the food’s GI value. GL likely is the most informative exposure as far as carbohydrate intake and disease risk is concerned [4].
It has been proposed that high dietary GI and GL may affect cancer risk by increasing insulin concentrations; chronic hyperinsulinemia influences the insulin-like growth factor (IGF) axis, synthesis of sex hormone-binding globulin (SHBG), and circulating estrogen levels, all of which may increase prostate cancer risk [5–10]. While the potential associations between GI and GL and various cancers have been evaluated in previous studies and metaanalyses [11, 12], the associations of these dietary factors with prostate cancer have not been adequately studied. We are aware of only three published studies (one case–control study and two cohort studies) investigating the possible association of GI and/or GL with the risk of prostate cancer, which produced mixed results [13–15].
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), funded by the National Cancer Institute, was initiated in 1993 to investigate selected methods for the early detection of these four cancers, as well as to study cancer etiology. In this report, we present the results of an investigation into the possible associations between dietary carbohydrate, GI, and GL, assessed by a food frequency questionnaire (FFQ) administered at baseline, and the subsequent development of prostate cancer in PLCO participants.
Materials and methods
Study population
Participants in PLCO were enrolled from 10 screening centers throughout the US (Birmingham, AL; Denver, CO; Washington, DC; Honolulu, HI; Detroit, MI; Minneapolis, MN; St Louis, MO; Pittsburgh, PA; Salt Lake City, UT; and Marshfield, WI). Participants were assigned randomly to either a screening arm (in which subjects underwent various screening modalities for each of the four cancer sites) or a usual care arm. All study participants provided written informed consent, and the study was approved by institutional review boards at each participating institution.
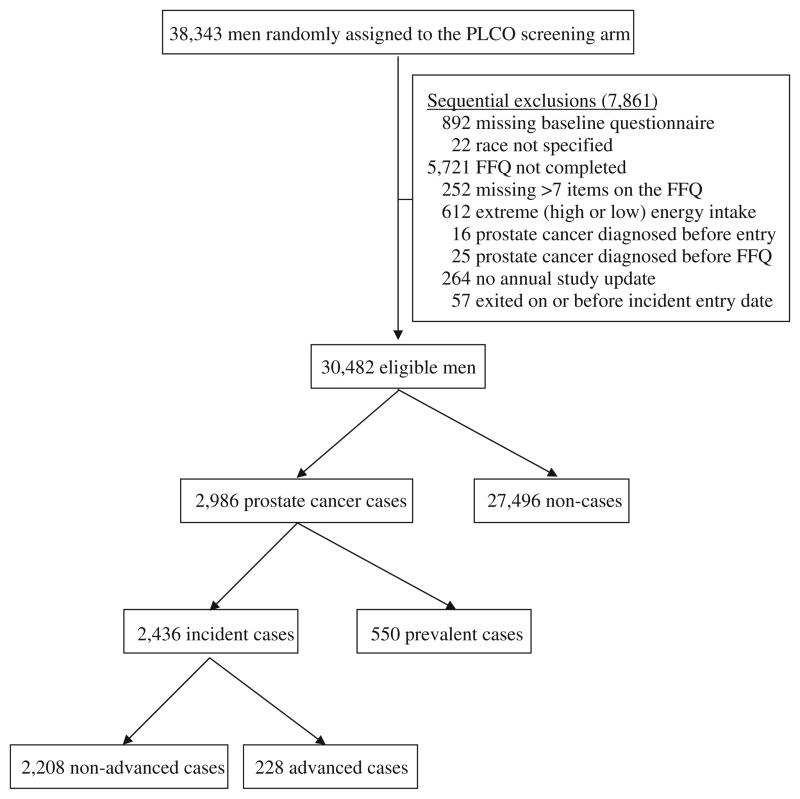
Between September 1993 and September 2000, 77,470 persons between 55 and 74 years of age were assigned randomly to the screening arm of PLCO, including 38,343 men. These men received a prostate-specific antigen (PSA) test—performed at one central laboratory using a standard assay [16]—and a digital rectal examination (DRE) at study entry and annually for 3 years, followed by two additional years of prostate cancer screening with PSA only. Of these participants, for the present analysis, we excluded sequentially 7,861 men for the following reasons: missing baseline risk factor questionnaire (n = 892); race not specified (n = 22); baseline FFQ not completed (n = 5,721); missing>7 food items on the FFQ (n = 252); extreme energy intake (intake in the highest or lowest 1%) (n = 612); prostate cancer diagnosed before study entry (n = 16); prostate cancer diagnosed prior to completing the FFQ (n = 25); no annual study update (ASU) completed (n = 264); and exited on or before incident entry date (n = 57). After these exclusions, data from 30,482 participants were available for analysis (Fig. 1).
Fig. 1.
Study inclusion scheme
Identification of prostate cancer cases
Prostate cancer was ascertained through two primary mechanisms. First, men with a PSA test result of>4 ng/ mL or a DRE suspicious for prostate cancer during a PLCO exam were referred to their medical providers for evaluation. Second, each participant was asked to complete a baseline questionnaire and ASU, which inquired about new cancer diagnoses in the past year. For men with suspected prostate cancer based on follow-up evaluation of abnormal clinical findings during a PLCO exam or with self-reported new prostate cancer on an ASU, medical records were requested to confirm the diagnosis and to obtain cancer stage and grade information. Death certificates, autopsy reports, and pathology reports were used to identify and confirm the diagnosis, stage, and grade in deceased participants. Only histologically confirmed cases of prostate cancer were included in the analysis. During follow-up through July 2008, 2,986 prostate cancer cases were identified among the 30,482 eligible participants, including 550 prevalent cases (defined as cases diagnosed up to 1 year after the baseline screen) and 2,436 incident cases (defined as cases diagnosed more than 1 year after the baseline screen). Only incident cases were included in this analysis. Clinical stage I and II tumors (stage I tumor = - occult or incidental finding; stage II tumor = confined to prostate) with Gleason score<8 were defined as nonadvanced. Clinical stage III and IV tumors (stage III tumor = localized to periprostatic area; stage IV tumor = metastatic disease) and/or tumors with Gleason score ≥8 were defined as advanced. Of the incident prostate cancer cases, 2,208 had non-advanced disease and 228 had advanced disease (Fig. 1).
Data collection
During initial screening, participants completed a risk factor questionnaire that included questions about sociodemographic factors, medical history, medication use, smoking history, physical activity, family history of cancer, recent history of screening examinations, height, and weight.
Dietary assessment
A 137-item FFQ was administered at baseline to assess usual dietary intake over the 12 months prior to enrollment in the study. Eighty-five percent of PLCO participants completed the FFQ on or before the day of their screening exam. Of the remaining 15%, 90% completed the FFQ within 1 month of the exam.
Values for GI and GL were added to the PLCO FFQ nutrient database [17] according to the methods described for adding GI and GL values to the National Cancer Institute Diet History Questionnaire [18]. Briefly, the nutrient database for the PLCO FFQ is based on approximately 4,200 individual foods reported by adults in the 1994–1996 continuing survey of food intakes by individuals (CSFII). This list was condensed into 225 nutritionally similar groupings of individual foods. Using published GI values [19], GI values were linked to each of the individual CSFII foods in these food groups. Specifically, the published GI table was reviewed to identify those foods that, in the judgment of the investigators, were the best matches for each of the CSFII foods. In cases where CSFII foods did not correspond closely to foods with published GI values, a series of decision criteria were utilized to assign GI values [18]. GL values for each of the 225 food groups were calculated using the weighted mean method as described by Subar et al. [20]. These GL values were used in the PLCO FFQ nutrient database to calculate overall daily GL based on FFQ reported frequency and portion size across all items on the questionnaire. Because the intended use of GL is as an indicator of the overall glycemic effect of food, and glycemic effect is inherently a function of dietary carbohydrate that actually is digested and absorbed, we used available carbohydrate—defined as the United States Department of Agriculture (USDA) National Nutrient Database for Standard Reference [21] value for grams of carbohydrate per serving minus the USDA value for grams of dietary fiber per serving—in our calculations of GL.
Statistical analysis
Proportions of individuals within pre-specified ranges/categories were calculated for the following categorical variables at baseline: randomization year, center, age, race, marital status, body mass index (BMI), smoking status, vigorous physical activity in the past year, first-degree relatives with prostate cancer, any prostate problems, prior PSA test, prostate biopsy prior to entry, compliant for baseline screen, previous cancer (other than non-melanoma skin cancer), diabetes mellitus, and regular aspirin use in prior year. Descriptive statistics (means, standard deviations) were calculated for continuous variables, including exposure variables (available carbohydrate, GI, and GL), outcome measure (time until prostate cancer), and dietary covariates (energy, carbohydrate, total fat, calcium, vitamin D, vitamin E, lycopene, selenium, dairy, processed meat, and red meat). Cox proportional hazards regression was used to model the associations of the exposure variables with risk of prostate cancer. Prostate cancer-free survival time was calculated from the date of baseline prostate cancer screening to the date of prostate cancer diagnosis for cases, or to the date of death or the last completed annual follow-up questionnaire for those not developing prostate cancer. The median follow-up time for all participants was 9.2 years. Careful attention was paid to the proportionality assumption, and graphical examination of the plots did not indicate that the assumption was violated. To test for linear trends in the risk of prostate cancer, quintile ranks of the explanatory variables were included as continuous predictors in separate hazard regression models predicting time to: (1) any, (2) non-advanced, or (3) advanced prostate cancer. Associations were evaluated utilizing a standard added last variable test in regression in three models to assess the potential for confounding by other covariates: (1) adjusted for age at entry, year of entry, race, and center; (2) adjusted for all variables in model 1 plus recognized prostate cancer risk factors, stratified as in Table 1; and (3) adjusted for all variables in model 2 plus dietary variables with putative associations with prostate cancer, entered in continuous form. Analyses were performed using SAS statistical software, version 9.1 (SAS Institute Inc., Cary, NC).
Table 1.
Baseline characteristics of eligible PLCO participants with and without incident prostate cancer
| Prostate cancer
|
No prostate cancer
|
|||
|---|---|---|---|---|
| n | % | n | % | |
| All | 2,436 | 100.0 | 27,496 | 100.0 |
| Randomization year | ||||
| 1993–1995 | 1,093 | 44.9 | 8,462 | 30.8 |
| 1996–1998 | 1,049 | 43.1 | 13,433 | 48.9 |
| 1999–2001 | 294 | 12.1 | 5,601 | 20.4 |
| Center | ||||
| Birmingham, AL | 42 | 1.7 | 822 | 3.0 |
| Denver, CO | 217 | 8.9 | 2,679 | 9.7 |
| Washington, DC | 189 | 7.8 | 1,730 | 6.3 |
| Honolulu, HI | 56 | 2.3 | 984 | 3.6 |
| Detroit, MI | 240 | 9.9 | 2,918 | 10.6 |
| Minneapolis, MN | 622 | 25.5 | 7,068 | 25.7 |
| St. Louis, MO | 193 | 7.9 | 2,492 | 9.1 |
| Pittsburgh, PA | 297 | 12.2 | 3,119 | 11.3 |
| Salt Lake City, UT | 259 | 10.6 | 2,024 | 7.4 |
| Marshfield, WI | 321 | 13.2 | 3,660 | 13.3 |
| Age (years) | ||||
| ≤59 | 492 | 20.2 | 8,969 | 32.6 |
| 60–64 | 867 | 35.6 | 8,656 | 31.5 |
| 65–69 | 706 | 29.0 | 6,323 | 23.0 |
| ≥70 | 371 | 15.2 | 3,548 | 12.9 |
| Race | ||||
| White, non-Hispanic | 2,234 | 91.7 | 24,963 | 90.8 |
| Black, non-Hispanic | 112 | 4.6 | 858 | 3.1 |
| Hispanic | 31 | 1.3 | 492 | 1.8 |
| Asian | 47 | 1.9 | 996 | 3.6 |
| Pacific Islander/American Indian | 12 | 0.5 | 187 | 0.7 |
| Marital status | ||||
| Married | 2,110 | 86.6 | 23,228 | 84.5 |
| Widowed | 76 | 3.1 | 924 | 3.4 |
| Divorced | 162 | 6.7 | 2,182 | 7.9 |
| Separated | 20 | 0.8 | 259 | 0.9 |
| Never married | 63 | 2.6 | 876 | 3.2 |
| BMI (kg/m2) | ||||
| <18.5 | 4 | 0.2 | 134 | 0.5 |
| 18.5 to<25.0 | 665 | 27.3 | 6,923 | 25.2 |
| 25.0 to<30.0 | 1,238 | 50.8 | 13,745 | 50.0 |
| ≥30.0 | 501 | 20.6 | 6,449 | 23.5 |
| Smoking status | ||||
| Never | 1,047 | 43.0 | 10,121 | 36.8 |
| Current | 195 | 8.0 | 2,994 | 10.9 |
| Former | 1,193 | 49.0 | 14,373 | 52.3 |
| Vigorous physical activity in past year (hours/week) | ||||
| None | 321 | 13.2 | 4,178 | 15.2 |
| <1 | 392 | 16.1 | 4,839 | 17.6 |
| 1 | 266 | 10.9 | 3,128 | 11.4 |
| 2 | 386 | 15.8 | 4,218 | 15.3 |
| 3 | 364 | 14.9 | 3,979 | 14.5 |
| ≥4 | 699 | 28.7 | 7,072 | 25.7 |
| First-degree relative with prostate cancer | ||||
| No | 2,103 | 86.3 | 24,867 | 90.4 |
| Yes | 275 | 11.3 | 1,956 | 7.1 |
| Any prostate problems | ||||
| No | 1,699 | 69.7 | 20,487 | 74.5 |
| Yes | 736 | 30.2 | 6,988 | 25.4 |
| Prior PSA test | ||||
| No | 949 | 39.0 | 12,590 | 45.8 |
| Yes—one | 905 | 37.2 | 9,958 | 36.2 |
| Yes—more than one | 374 | 15.4 | 2,581 | 9.4 |
| Does not know | 207 | 8.5 | 2,362 | 8.6 |
| Prostate biopsy prior to entry | ||||
| No | 2,175 | 89.3 | 25,547 | 92.9 |
| Yes | 193 | 7.9 | 1,131 | 4.1 |
| Compliant for baseline screen | ||||
| No | 56 | 2.3 | 636 | 2.3 |
| Yes | 2,380 | 97.7 | 26,860 | 97.7 |
| Previous cancer (other than non-melanoma skin cancer) | ||||
| No | 2,395 | 98.3 | 26,946 | 98.0 |
| Yes | 41 | 1.7 | 544 | 2.0 |
| Diabetes mellitus | ||||
| No | 2,214 | 90.9 | 24,289 | 88.3 |
| Yes | 137 | 5.6 | 2,415 | 8.8 |
| Regular aspirin use in prior year | ||||
| No | 1,189 | 48.8 | 13,112 | 47.7 |
| Yes | 1,229 | 50.5 | 14,254 | 51.8 |
|
| ||||
| Dietary intakes | Mean | ±SDa | Mean | ±SDa |
|
| ||||
| Energy (kJ/day) | 9,688 | 3,475 | 9,797 | 3,571 |
| Carbohydrate (g/day) | 274.2 | 103.0 | 274.1 | 102.2 |
| Glycemic index | 55.2 | 3.8 | 55.2 | 4.0 |
| Glycemic load (g/day) | 151.5 | 59.0 | 151.4 | 58.1 |
| Total fat (g/day) | 78.1 | 35.3 | 79.9 | 36.3 |
| Calciumb (mg/day) | 1,160 | 555 | 1,165 | 582 |
| Vitamin D (calciferol)b (μg/day) | 10.8 | 8.0 | 11.0 | 8.5 |
| Vitamin E (α-tocopherol)b (mg/day) | 68.4 | 103.2 | 74.6 | 109.0 |
| Seleniumb (μg/day) | 120.2 | 45.0 | 123.1 | 47.1 |
| Lycopene (μg/day) | 11,216 | 7,032 | 11,747 | 8,743 |
| Dairy (cup equivalents/day) | 1.95 | 1.40 | 1.95 | 1.49 |
| Processed meat (g/day) | 17.0 | 20.5 | 17.6 | 20.2 |
| Red meat (g/day) | 95.3 | 71.2 | 101.0 | 74.5 |
SD standard deviation
Includes intake from dietary supplements
Results
Cases tended to be older than non-cases at study entry and included a higher percentage of blacks and lower percentage of Hispanics and Asians than non-cases (Table 1). While there was no difference in the prevalence of over-weight subjects between the groups, a lower proportion of cases than non-cases were obese (20.6% vs. 23.5%, respectively; p = 0.001). There was a slightly lower prevalence of smoking in cases compared to non-cases (8.0% vs. 10.9%, respectively; p<0.001), and cases tended to be more physically active, with 28.7% of cases reporting at least 4 h of vigorous physical activity per week during the past year compared to 25.7% of non-cases (p = 0.001). A higher percentage of cases compared to non-cases reported a first-degree relative with prostate cancer, a history of any prostate problems, more than one previous PSA test, and a history of prostate biopsy prior to study entry (all p values<0.001). The prevalence of diabetes was lower in cases compared to non-cases (5.6% vs. 8.8%, respectively; p<0.001). The only meaningful difference in dietary intakes was an 8% lower intake of vitamin E in cases compared to non-cases (68.4 mg/day vs. 74.6 mg/day, respectively; p = 0.007). Cases and non-cases did not differ on mean daily GI (55.2 ± 3.8 and 55.2 ± 4.0, respectively), GL (151.5 ± 59.0 and 151.4 ± 58.1 g, respectively), or available carbohydrate intake (274.2 ± 103.0 and 274.1 ± 102.2 g, respectively).
Overall, there were no associations of baseline available carbohydrate, GI, or GL with incident prostate cancer in the minimally adjusted model including study design and demographic factors (model 1), the model adjusted additionally for recognized prostate cancer risk factors (model 2), or the model adjusted additionally for putative dietary risk factors (model 3) (Table 2). Hazard ratios and 95% confidence intervals for the highest versus lowest quintiles in the fully adjusted models were 0.86 (0.67–1.10), 0.95 (0.82–1.09), and 0.89 (0.71–1.12) for available carbohydrate, GI, and GL, respectively. In addition, none of the tests for trend were statistically significant. When analyzing non-advanced and advanced prostate cancer separately with full adjusted models, there were no apparent associations of available carbohydrate, GI, or GL with incident prostate cancer (Table 3). Additional analyses stratifying on categories of BMI (<25.0, 25.0 to<30.0, and ≥30.0 kg/m2) and age (<65 and ≥65 years) failed to show any statistically significant associations of available carbohydrate, GI, or GL with incident prostate cancer among these subgroups.
Table 2.
Hazard ratios and 95% CI for prostate cancer by available carbohydrate, glycemic index, and glycemic load
| Cases | Model 1a | Model 2b | Model 3c | |
|---|---|---|---|---|
| Hazard ratio(95% CId) | Hazard ratio (95% CId) | Hazard ratio (95% CId) | ||
| Available carbohydrate (g/day) | ||||
| Q1 (≤188.6) | 491 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (188.7–236.0) | 472 | 0.93 (0.82–1.06) | 0.91 (0.80–1.04) | 0.90 (0.79–1.03) |
| Q3 (236.1–284.6) | 510 | 1.01 (0.89–1.14) | 0.97 (0.86–1.10) | 0.95 (0.82–1.11) |
| Q4 (284.7–350.0) | 483 | 0.95 (0.84–1.08) | 0.91 (0.80–1.03) | 0.88 (0.73–1.05) |
| Q5 (≥350.1) | 480 | 0.95 (0.84–1.08) | 0.90 (0.80–1.03) | 0.86 (0.67–1.10) |
| p for trend | 0.59 | 0.16 | 0.27 | |
| Glycemic index | ||||
| Q1 (≤52.1) | 472 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (52.2–54.1) | 516 | 1.05 (0.92–1.18) | 1.03 (0.91–1.17) | 1.02 (0.90–1.16) |
| Q3 (54.2–55.8) | 496 | 1.01 (0.89–1.15) | 1.00 (0.88–1.14) | 0.98 (0.86–1.12) |
| Q4 (55.9–58.0) | 497 | 1.04 (0.91–1.17) | 1.03 (0.90–1.16) | 0.99 (0.87–1.14) |
| Q5 (≥58.1) | 455 | 0.99 (0.87–1.13) | 0.99 (0.87–1.13) | 0.95 (0.82–1.09) |
| p for trend | 0.88 | 0.89 | 0.41 | |
| Glycemic load (g/day) | ||||
| Q1 (≤103.2) | 493 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (103.3–129.4) | 458 | 0.90 (0.80–1.03) | 0.88 (0.78–1.00) | 0.88 (0.77–1.01) |
| Q3 (129.5–156.7) | 510 | 1.01 (0.89–1.14) | 0.97 (0.85–1.09) | 0.96 (0.83–1.11) |
| Q4 (156.8–193.9) | 498 | 0.98 (0.86–1.11) | 0.94 (0.83–1.06) | 0.93 (0.79–1.10) |
| Q5 (≥194.0) | 477 | 0.95 (0.84–1.08) | 0.90 (0.79–1.02) | 0.89 (0.71–1.12) |
| p for trend | 0.86 | 0.30 | 0.61 | |
Adjusted for age at entry, year of entry, race, and center
Adjusted for age at entry, year of entry, race, center, compliant for baseline screen, marital status, BMI, vigorous physical activity, smoking, history of diabetes, history of cancer (other than non-melanoma skin cancer), aspirin use, family history of prostate cancer, any prostate problems, prior PSA test, and prostate biopsy prior to entry
Adjusted for age at entry, year of entry, race, center, compliant for baseline screen, marital status, BMI, vigorous physical activity, smoking, history of diabetes, history of cancer (other than non-melanoma skin cancer), aspirin use, family history of prostate cancer, any prostate problems, prior PSA test, prostate biopsy prior to entry, and dietary factors—energy, total fat, red meat, processed meat, dairy, calcium, vitamin D, vitamin E, lycopene, and selenium
CI confidence interval
Table 3.
Hazard ratios and 95% CI for prostate cancer by available carbohydrate, glycemic index, and glycemic load in advanced and nonadvanced cases
| Advanced casesa (n = 228)
|
Non-advanced casesb (n = 2,208)
|
|||
|---|---|---|---|---|
| n | Hazard ratioc (95% CId) | n | Hazard ratioc (95% CId) | |
| Available carbohydrate (g/day) | ||||
| Q1 (≤188.6) | 42 | 1.00 (ref) | 449 | 1.00 (ref) |
| Q2 (188.7–236.0) | 48 | 1.09 (0.70–1.71) | 424 | 0.88 (0.77–1.02) |
| Q3 (236.1–284.6) | 49 | 1.12 (0.67–1.87) | 461 | 0.94 (0.80–1.10) |
| Q4 (284.7–350.0) | 57 | 1.27 (0.70–2.32) | 426 | 0.84 (0.70–1.02) |
| Q5 (≥350.1) | 32 | 0.70 (0.29–1.69) | 448 | 0.87 (0.67–1.13) |
| p for trend | 0.81 | 0.22 | ||
| Glycemic index | ||||
| Q1 (≤52.1) | 39 | 1.00 (ref) | 433 | 1.00 (ref) |
| Q2 (52.2–54.1) | 54 | 1.18 (0.77–1.79) | 462 | 1.00 (0.88–1.15) |
| Q3 (54.2–55.8) | 51 | 1.08 (0.70–1.66) | 445 | 0.97 (0.85–1.12) |
| Q4 (55.9–58.0) | 43 | 0.92 (0.58–1.45) | 454 | 1.00 (0.87–1.15) |
| Q5 (≥58.1) | 41 | 0.86 (0.53–1.39) | 414 | 0.95 (0.82–1.11) |
| p for trend | 0.27 | 0.59 | ||
| Glycemic load (g/day) | ||||
| Q1 (≤103.2) | 44 | 1.00 (ref) | 449 | 1.00 (ref) |
| Q2 (103.3–129.4) | 44 | 0.99 (0.63–1.53) | 414 | 0.87 (0.76–1.00) |
| Q3 (129.5–156.7) | 50 | 1.13 (0.70–1.83) | 460 | 0.95 (0.81–1.10) |
| Q4 (156.8–193.9) | 54 | 1.25 (0.72–2.18) | 444 | 0.91 (0.76–1.08) |
| Q5 (≥194.0) | 36 | 0.84 (0.39–1.80) | 441 | 0.89 (0.71–1.13) |
| p for trend | 0.73 | 0.52 | ||
Stage ≥ III and Gleason score ≥8
Stage<III and Gleason score<8
Adjusted for age at entry, year of entry, race, center, compliant for baseline screen, marital status, BMI, vigorous physical activity, smoking, history of diabetes, history of cancer (other than non-melanoma skin cancer), aspirin use, family history of prostate cancer, any prostate problems, prior PSA test, prostate biopsy prior to entry, and dietary factors—energy, total fat, red meat, processed meats, dairy, calcium, vitamin D, vitamin E, lycopene, and selenium
CI confidence interval
Discussion
It has been hypothesized that elevated serum insulin levels resulting from chronically high-GI/GL diets [6] may increase prostate cancer risk through several mechanisms, including: inhibiting hepatic synthesis of SHBG, with a resulting greater bioavailability of testosterone [7]; increasing levels of circulating estrogen, which binds to estrogen receptors and to mutated androgen receptors in the prostate, increasing cell proliferation [8]; and down-regulating levels of IGF binding proteins, increasing the bioactivity and bioavailability of IGF, which has proliferative, cell differentiation, and antiapoptotic actions [9, 10]. However, we observed no associations between dietary available carbohydrate, GI, or GL and incident prostate cancer in this cohort of PLCO participants. Also, we did not detect any associations for either advanced or nonadvanced prostate cancer cases.
Previous studies of the associations between GI and GL and specific cancers have produced mixed results. A recent meta-analysis of cohort studies showed no association between dietary GI or GL and the risk of colorectal (five studies), pancreatic (five studies), endometrial (two studies), and gastric (one study) cancers; although utilizing data from five studies, there was a modest positive association between GI and breast cancer risk [12]. In another metaanalysis of GI, GL, and cancer risk that included 15 case– control and 24 cohort studies, both GL and GI were significantly and positively associated with an increased risk of colorectal and endometrial cancer [11]. Risk of breast cancer also was positively associated with GL, although this association disappeared when publication bias was taken into account. In contrast to the positive association seen with risk of specific cancers in these two meta-analyses, a recent study of GI, GL, and risk of cancer in the prospective National Institutes of Health (NIH)—American Association of Retired Persons (AARP) Diet and Health Study found that GL was inversely and significantly associated with risk of all cancer in both women and men, with a 10 and 7% reduced risk, respectively, for the highest GL quintile relative to the lowest [14].
To our knowledge, there have been only three studies of GI and GL and risk of prostate cancer reported in the literature. GI and GL were positively associated with prostate cancer risk in a multi-center case–control study conducted in Italy between 1991 and 2002. The multivariable-adjusted odds ratio (OR) for the highest versus lowest quintile of GI was 1.57 (95% confidence interval (CI): 1.19–2.07), while the OR for the highest versus lowest quintile of GL was 1.41 (95% CI: 1.04–1.89), with positive trends in risk (p<0.01) with both GI and GL [13]. The advantage of our study (and prospective cohort studies in general) was that diet was assessed at baseline (prior to the diagnosis of prostate cancer), eliminating the potential for bias in the recall of dietary intake based on prostate cancer status. In the second study—the prospective NIH-AARP Diet and Health Study, which included 15,949 incident cases of prostate cancer—there were no associations between GI or GL and prostate cancer, in agreement with our results [14]. Finally, in a study using data from more than 5,000 participants in the Health Professionals Follow-up Study cohort, dietary GI or GL were not associated with risk of total or subgroups of prostate cancer, again in agreement with the results presented here [15].
The strengths of this study included the large sample size, the detailed information on diet and prostate cancer risk factors obtained at baseline, careful estimation of dietary GI and GL, histologic confirmation of prostate cancer cases, the ability to distinguish between advanced and non-advanced prostate cancer cases, and excellent participant follow-up (98.7% of participants were either known dead or filled out an ASU within the previous 2 years). A possible limitation of the study was the rather narrow range of dietary GI observed in this cohort of predominantly older, white men. 95% of mean dietary GI values were between 51 and 59, which limited our ability to detect the effects of diets substantially different in their ability to modulate insulin and glucose levels. Finally, while the addition of GI/GL values to the PLCO FFQ nutrient database was done in a systematic and well-documented manner [17], most FFQs in common use were not designed to assess GI and GL and thus may be unable to capture the true range of GI and GL [11].
In summary, the results of this large cohort study do not support the hypothesis that dietary carbohydrate, GI, or GL are important risk factors for prostate cancer. The narrow range of mean dietary GI observed may have limited our ability to detect modest associations, an issue that future studies should address.
Acknowledgments
Supported by the US National Cancer Institute (grant no. N01CN75022).
The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff of the PLCO Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., and Ms. Barbara O’Brien and staff, Westat, Inc. Most importantly, we acknowledge PLCO participants for their contributions to making this study possible.
Contributor Information
James M. Shikany, Email: jshikany@dopm.uab.edu, Division of Preventive Medicine, School of Medicine, University of Alabama at Birmingham, 1530 3rd Ave. S., MT 610, Birmingham, AL 35294, USA
Andrew P. Flood, Division of Epidemiology and Community Health, Department of Food Science and Nutrition, University of Minnesota, St. Paul, MN 55454, USA
Cari M. Kitahara, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD 20852, USA
Ann W. Hsing, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD 20852, USA
Tamra E. Meyer, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD 20852, USA
Bradley J. Willcox, Pacific Health Research Institute, The Queen’s Medical Center, Honolulu, HI 96813, USA
David T. Redden, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, AL 35294, USA
Regina G. Ziegler, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD 20852, USA
References
- 1.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund/American Institute for Cancer Research; Washington, DC: 2007. [Google Scholar]
- 2.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 3.Wolever TM. The glycaemic index. World Rev Nutr Diet. 1990;62:120–185. [PubMed] [Google Scholar]
- 4.Liu S, Manson JE, Stampfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73:560–566. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 5.Biddinger SB, Ludwig DS. The insulin-like growth factor axis: a potential link between glycemic index and cancer. Am J Clin Nutr. 2005;82:277–278. doi: 10.1093/ajcn.82.2.277. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Wolever TM, Collier GR, et al. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr. 1987;46:968–975. doi: 10.1093/ajcn/46.6.968. [DOI] [PubMed] [Google Scholar]
- 7.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone binding-globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 8.Castagnetta LA, Miceli MD, Sorci CM, et al. Growth of LNCaP human prostate cancer cells is stimulated by estradiol via its own receptor. Endocrinology. 1995;136:2309–2319. doi: 10.1210/endo.136.5.7536668. [DOI] [PubMed] [Google Scholar]
- 9.Pollak M. Insulin-like growth factors and prostate cancer. Epidemiol Rev. 2001;23:59–66. doi: 10.1093/oxfordjournals.epirev.a000796. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 11.Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. 2008;87:1793–1801. doi: 10.1093/ajcn/87.6.1793. [DOI] [PubMed] [Google Scholar]
- 12.Barclay AW, Petocz P, McMillan-Price J, et al. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 13.Augustin LSA, Galeone C, Dal Maso L, et al. Glycemic index, glycemic load and risk of prostate cancer. Int J Cancer. 2004;112:446–450. doi: 10.1002/ijc.20416. [DOI] [PubMed] [Google Scholar]
- 14.George SM, Mayne ST, Leitzmann MF, et al. Dietary glycemic index, glycemic load, and risk of cancer: a prospective cohort study. Am J Epidemiol. 2009;169:462–472. doi: 10.1093/aje/kwn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimptsch K, Kenfield S, Jensen MK, et al. Dietary glycemic index, glycemic load, insulin index, fiber and whole-grain intake in relation to risk of prostate cancer. Cancer Causes Control. 2011;22:51–61. doi: 10.1007/s10552-010-9671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinsky PF, Kramer BS, Crawford ED, et al. Prostate volume and prostate-specific antigen levels in men enrolled in a large screening trial. Urology. 2006;68:352–356. doi: 10.1016/j.urology.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Flood A, Peters U, Jenkins DJA, et al. for the Prostate, Lung Colorectal Ovarian (PLCO) Project Team. Carbohydrate, glycemic index, and glycemic load and colorectal adenomas in the prostate, lung, colorectal, and ovarian screening study. Am J Clin Nutr. 2006;84:1184–1192. doi: 10.1093/ajcn/84.5.1184. [DOI] [PubMed] [Google Scholar]
- 18.Flood A, Subar AF, Hull SG, Zimmerman TP, Jenkins DJ, Schatzkin A. Methodology for adding glycemic load values to the National Cancer Institute Diet History Questionnaire database. J Am Diet Assoc. 2006;106:393–402. doi: 10.1016/j.jada.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152:279–286. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Agriculture, Agricultural Research Service. USDA national nutrient database for standard reference, release 18. Nutrient data laboratory home page. 2005 http://www.nal.usda.gov/fnic/foodcomp.