Abstract
Adaptive immunity is predicated on the ability of the T cell repertoire to have pre-existing specificity for the universe of potential pathogens. Recent findings suggest that TCR-self-pMHC interactions limit autoimmune responses while enhancing T cell response to foreign antigens. We review these findings here, placing them in context of the current understanding of how TCR-self-pMHC interactions regulate T cell activation thresholds, and suggest that TCR-self-pMHC interactions increase the efficiency of the T cell repertoire by giving a competitive advantage to peptide cross-reactive T cells. We propose that self-reactivity and peptide-cross-reactivity are controlled by particular CDR3 sequence motifs, which would allow thymic selection to contribute to solving the feat of broad pathogen-specificity by exporting T cells that are pre-screened by positive and negative selection for the ability to be ‘moderately’ peptide cross-reactive.
Keywords: T cell development, T cell homeostasis, TCR specificity, autoimmunity, CD5, dynamic tuning
T cell selectivity and ligand discrimination
To initiate an adaptive immune response, T cells scan antigen-presenting cells (APC) within the secondary lymphoid compartments for pathogen-derived peptides displayed on host-MHC proteins (pMHC). If a T cell-APC encounter results in intracellular signals that exceed a threshold, naïve T cells are triggered to undergo activation, clonal expansion and acquire effector cell functions that help orchestrate pathogen clearance [1]. Three antigen-recognition properties are sentinel to the ability of the T cell repertoire to create sterilizing immunity. First, individual T cell clonotypes need to be responsive to a limited set of peptides displayed by host-MHC. Second, to provide broad immunological coverage to the plethora of yet-to-be-seen pathogens, the collective T cell repertoire is required to have immense specificity for any and all of the unknown pathogens that might invade the host. Third, the T cell repertoire has to accomplish these feats of specificity and broad pathogen coverage using only several million T cell clonotypes [2–4].
Selective pressures that arise during T cell development contribute to solving these feats of antigen-specificity using a ‘goldilocks’ solution. TCR V(D)J somatic gene rearrangement creates sequence diversity at positions of the TCR CDR3 loops that directly engage pMHC, which gives rise to T cell clonotypes that have their own unique peptide specificity requirements [5]. T cell selection then seeds the mature repertoire with a few million unique T cell clonotypes that express only those TCRs that have a ‘moderate’ range of peptide cross-reactivity; TCRs which require the engagement of multiple side chain of the peptide to create a strong enough binding reaction to induce T cell activation [6, 7]. The structural features that allow post-selection TCRs to be self-tolerant and engage only a limited set of peptides include the pairing of particular TCR V gene combination with specific CDR3 sequences, as well as through the creation of TCRα to TCRβ inter-chain interactions that stabilize CDR3 loop conformations [8–10]. Whether there are generalizable structural or sequence feature of CDR3 loops that predispose TCRs to be self-reactive or control the rate of peptide cross-reactivity is poorly understood. The multiplicative effect of millions of T cell clonotypes specific for unique sets of similar (and sometimes dissimilar) peptides, allows the T cell repertoire to have broad pathogen specificity while still limiting dis-regulated autoimmune responses [4, 11–13].
The actual ‘rate’ of peptide cross-reactivity, and thus the likelihood that a given T cell clonotype will enter into an immune response, is a product of the ligand binding properties of the expressed TCR, the density in which the antigen is expressed, as well as T cell signaling thresholds. The signaling threshold for T cell activation is based on the quality and quantity of TCR interactions with pMHC displayed on APC, and is tunable based on TCR-self-pMHC interactions that occur during development and homeostasis. The ability to select the expressed TCRs and tune the threshold of T cell signaling ensures that a minimum but limited number of T cell clonotypes will respond to any given infection, providing T cell immunity while minimizing detrimental immune and autoimmune responses [14]. A number of recent findings have begun to shed light on how T cell signaling thresholds are set.
Setting TCR signaling thresholds
The consensus model of T cell ligand discrimination is based on the concept of kinetic proofreading [15, 16]. That is, the commencement of TCR signaling is not instantaneous following TCR engagement with pMHC, and requires the TCR to be bound for a period of time to allow the initiation of productive signaling (Box 1). The first step in this process requires TCR engagement of pMHC to result in the phosphorylation of TCR associated ITAM-containing TCRζ and CD3 chains by the Src family kinase, Lck [17]. How TCR signals are transmitted across the cell membrane is an area of intense research (Box 2).
Box 1. T cell activation: affinity, dwell time and force.
A number of models have been proposed to explain the potency of pMHC ligands to induce T cell activation. T cells can only measure three parameters of the TCR – pMHC binding event: the number of TCRs engaged with a pMHC complex at any one time, the length of time in which each receptor is bound and the amount of force applied to the TCR-pMHC binding event. The number of TCR simultaneously engaged with a pMHC is dependent upon the equilibrium affinity (KD) of the TCR-pMHC interaction and the concentration of pMHC being presented. Evidence supporting KD-based receptor occupancy models of TCR signaling comes from studies showing a correlation between KD and ligand potency [75, 76], and from the fact that ligands can induce qualitatively distinct biological outcomes depending upon their concentration [77]. Kinetic proofreading models hypothesize that TCR must be engaged long enough to complete a series of signaling events, including co-receptor recruitment and TCR phosphorylation [15]. Increases in the dwell-time of the TCR-pMHC engagement raise the probability that any single TCR-pMHC engagement will surpass the threshold amount of time required to initiate T cell activation and undergo clonal expansion [16, 78]. More recently, the force that pMHC can apply to the TCR has been correlated with T cell activation [79]. Stronger TCR-pMHC binding events can stay bound for longer periods of time, allowing an accumulation of force to be applied to the TCR [80, 81]. The application of force may induce TCR constant domains to undergo allosteric conformational changes, allowing interactions with the extracellular portion of CD3 components to initiate the release of CD3 and TCRζ cytoplasmic domains from the plasma membrane [82–85]. As affinity, dwell time and force are not independent parameters; how the interplay of these variables allow for distinct biological outcomes including positive selection, T cell homeostasis and T cell antagonism, as well all clonal expansion and T cell exhaustion remains a work in progress.
Box 2. Transmitting TCR signals across the cell membrane.
Several non-mutually exclusive models have been proposed to explain how TCR engagement allows signals to be transmitted across the cell membrane, eventually resulting in the phosphorylation of the CD3ε and ζ cytoplasmic domains. Under non-activating conditions, the CD3ε and ζ cytoplasmic domains of the T cell receptor complex are thought to bind to the inner leaflet of the plasma membrane, resulting in the insertion of the aromatic tyrosine in the ITAMs to be buried within the plasma membrane [86, 87]. This sequestration of the ITAM, mediated by electrostatic interactions between acidic phospholipids and clusters of basic residues within the CD3ε and ζ cytoplasmic domains, may limit spurious phosphorylation of the TCR complex in the absence of pMHC binding [88]. When TCR engage agonist pMHC ligands, a mechanical shear force is applied to the TCR, due to the dynamic movement of these proteins bound to cell membranes. The force exerted on the TCR is thought to induce allosteric conformational changes within the constant domains, including changes to the A-B loop within the TCRα chain, as well as conformational changes within the FG loop of the TCRβ chain, allowing the TCR constant domains to change their interactions with CD3εγ heterodimer [79–85]. Within the cytosol, mechanical forces on the TCR, TCR multimerization and/or changes in the local environment may induce CD3ε and ζ cytoplasmic domains to be to be released from the plasma membrane, allowing the associated ITAM to be accessible for phosphorylation [86, 89]. Changes in the charge property of lipids have also been ascribed to increases in the Ca2+ concentration [90], however, the exact mechanisms involved is still incomplete as it has been noted that other divalent cations are able to induce this release of ITAMs, and that the magnesium concentrations in the cell far exceed the concentrations of free calcium [17]. Contrary to the idea that ITAMs need to first be released from the plasma membrane, it has also been suggested that the phosphorylation of TCRζ by Lck induces the dissociation of the TCRζ chain from the plasma membrane, which then facilitates TCR–CD3 clustering required for full T-cell activation [88]. These and other models and pathways not discussed here, indicate that although many details of TCR signaling are known, the question of “How does T cell receptor signaling begin?” is still not fully understood [17, 18].
Within the cytoplasm of T cells, the ability of the TCR complex to transduce activating signals stems from competing phosphorylation and dephosphorylation reactions. The T cell co-receptors, CD4 and CD8, are critical to this process as they enhance TCR signaling by delivering the Src family kinase Lck to pMHC bound TCRs, and for CD8, through stabilizing the TCR-pMHC interaction [17–19]. The phosphorylation status of Lck itself determines whether the kinase has enzymatic function. Lck has two major tyrosine phosphorylation regulatory sites. Tyr394, which when phosphorylated by trans-autophosphorylation or by Fyn, the other Src kinase, stabilize the active conformation. In contrast, the phosphorylation of Lck Tyr505 in the absence of phosphorylated Tyr394, promotes the inactive, auto-inhibited conformation of Lck. In unperturbed T cells and thymocytes, a basal level of active Lck is maintained. When the active form of Lck is recruited to the TCR complex, it initially phosphorylates the two tyrosines within the CD3 and TCRζ-chain ITAMs. Doubly phosphorylated TCRζ-chain ITAMs recruit ZAP70, a kinase that is subsequently activated by a second round of Lck-mediated phosphorylation, which then relays the signal downstream by phosphorylating LAT and SLP76 (thoroughly reviewed in refs. [17, 20]).
The signal activation cascade that occurs when the TCR engages strongly activating ligands results in the increase in intracellular Ca2+, activation of the Carma1/Bcl10/Malt1 (CBM) and Ras-ERK pathways. Consequently NFAT and NF-κB translocate to the nucleus and AP-1 becomes phosphorylated to induce gene transcription. Competitive to the activating signal are phosphatases, such as CD45, SHP-1 and PTPN22, which dephosphorylate Lck at Tyr394, ZAP-70, Lat, SLP-76 and Vav1, as well as kinases such as CSK, which phosphorylates Lck at Tyr505 [17, 21]. Weak TCR-pMHC binding events are thought to trigger a negative feedback loop leading to rapid recruitment of the phosphatase SHP-1, followed by receptor desensitization, through the inactivation of Lck kinase and through a still being defined THEMIS:GRB2:SHP1 complex [21–24].
Creating an ‘autoimmunity buffer’
Mature T cells have tremendous selectivity; T cell activating signals can be generated from as few as 1–10 pMHC ligands within the T cell-APC contact area [25, 26]. These findings raise a specificity conundrum. If thymocytes can, and indeed are required by positive selection, to functionally engage as few 1–10 self-pMHC complexes, why doesn’t the mature T cell repertoire chronically induce fulminant autoimmunity?
Part of the answer to this riddle was observed twenty five years ago by Yagi and Janeway who showed that developing thymocytes are 30–100 times more sensitive to antigen than mature T cells [27]. These early experiments using super-antigens were followed up using TCR transgenic mice and sets of altered peptide ligands (APLs) to show that mature T cells become desensitized to both ligand potency and ligand density [28–30]. Allowing thymocytes to undergo positive selection on very weak ligands as well as somewhat stronger ligands ensures that the subsequent T cell repertoire has a range of self-pMHC reactivity profiles. The ability of thymocytes to incorporate different strengths of self-pMHC signals appears to be critical for T cells to differentiate into distinct T cell lineages. Selection of conventional naïve T cells require the least amount of signaling derived from self-pMHC to undergo positive selection and be maintained through homeostasis. In contrast, anti-inflammatory regulatory T cells, unconventional innate-like T cells, including NKT cells and CD8aa IELs are thought to require stronger signals derived be selected on stronger ‘agonist’ self-pMHC complexes [31].
The desensitization of mature T cells creates an ‘autoimmunity buffer’ that may be critical to avoid autoimmunity. For thymocytes to undergo positive selection and be exported into the mature T cell repertoire, the TCR-self-pMHC binding event has to propagate TCR signal transduction. The TCR-pMHC potency or dwell-time threshold that separates positive selection from negative selection is quite narrow [19, 32]. Without T cell signaling desensitization, the narrow window between positive and negative selection could pose great risk for the development of autoimmunity. This would be particularly true for autoreactive T cells that express self-reactive TCRs that are near the threshold for negative selection [33], target antigens that are expressed at much higher levels in the peripheral tissues as compared to the thymus (such as myelin) or target self-antigens that have temporal expression patterns [34]. However, based on in vitro assays, T cell signaling desensitization ensures that a self-pMHC ligand would need to be presented minimally at a 30–100-fold greater density in a peripheral tissue, as compared to the thymus, for a T cell to avoid negative selection and cause autoimmunity [27–30].
Difference in TCR signaling thresholds and pathways between thymocytes and mature T cells arise from both developmentally programmed changes and through dynamic tuning of T cell signal thresholds. Developmentally programmed changes include the differential expression of miRNAs, short non-coding RNAs that alter gene expression by targeting specific mRNA molecules for degradation or translational repression. The expression of miR-181a, for example, correlates with changes in ligand sensitivity as it is highly expressed in pre-selection thymocytes and is down-regulated following TCR signaling and differentiation into mature T cells [35]. miR-181a amplifies TCR signaling by repressing multiple negative regulators in the TCR signaling pathway, including non-receptor type tyrosine phosphatase SHP-2, PTPN22 and the ERK-specific phosphatases, dual specificity phosphatases (DUSP5 and DUSP6). Repression of miR-181a targets is required for proper thymocyte positive and negative selection in vitro, due to altered TCR-signaling thresholds, while the inhibition of miR-181a results in the development of mature T cells that are overtly self-reactive [36]. Analysis and interpretation of deficiency of the entire miR-181 family is somewhat complicated: the miR-181 family is composed of six mature miRNAs which are encoded in three independent paralog precursor transcripts on three separate chromosomes, and is a critical regulator of cellular metabolism [37].
Developmentally programmed changes in TCR signaling also arise from the differential expression of T cell signaling molecules. This has been documented for the signal transduction cascade leading to NF-κB activation: while thymocytes mature by and large normally in animals deficient in PKCθ, Carma1, Bcl10 or Malt1, peripheral T cells carrying these deficiencies are unable to respond to strong TCR stimuli, indicating that positive and negative thymocyte selection minimally involve the CBM complex that is necessary for peripheral responsiveness [38–41]. Conversely, the adaptor molecules, thymocyte-expressed positive selection–associated-1 (Tespa1), and thymocyte-expressed molecule involved in selection (Themis) and a voltage-gated Na+ channel (VGSC) are highly expressed in double negative and immature TCRlo DP thymocytes, and their expression levels are reduced or absent in mature single positive thymocytes and in mature T cells [42–47]. The expression of both Tespa1 and VGSC provides mechanisms that allow weak positive selection signals to induce sustained Ca2+ signals that are required for CD4+ T cell development [47, 48]. Themis, however, is thought to be a negative regulator of TCR-signaling, based on the observation that Themis-deficient thymocytes respond to positively selecting self-pMHC in a fashion similar to that observed in wild-type thymocytes interacting with higher-affinity ligands and from observations that the expression of Themis desensitizes TCR-signaling in mature T cells [24, 42]. Thus, the developmental changes in TCR signaling thresholds is a product of differentially expressed positive and negative regulators of signaling, the balance of which is required to allow T cells to be less sensitive to self-pMHC ligands presented in the periphery, as well as to allow T cells to develop into different lineages.
Dynamic tuning of TCR sensitivity is initially set during T cell selection and continues to occur during mature T cell homeostasis. During development, the expression level of negative-regulators of TCR signaling can be adjusted following thymocyte interactions with endogenous self-pMHC to fine-tune the TCR signaling sensitivity of mature T cells. For example, T cells that more strongly recognize self-pMHC increase the expression of CD5, a cell-surface molecule that can negatively regulate TCR signals through association with the phosphatase SHP-1, whereas T cells that have weak interactions with self-pMHC express lower levels of CD5 [12, 14, 31, 49–51]. Dynamic tuning of TCR sensitivity continues to occur post-selection. During homeostasis, T cell interactions with self-pMHC can regulate the expression of negative regulatory molecules, influence cytokine responsiveness and limit autoimmune responses [14, 52–54]. However, it is clear that T cell interactions with self-pMHC do not solely result in limiting T cell reactivity. Continuous TCR-self-pMHC interactions are required for proper lymphocyte homeostasis, and to maintain the effector functions. These processes ensure that peripheral T cells remain capable of recognizing peptides displayed by host-MHC molecules [55]. Moreover, recent findings suggest that the quality (signal strength) of TCR-self-pMHC interactions may be important in determining the T cell’s response to foreign antigens
TCR-self-pMHC interactions prepare T cells for effector responses
Once exported from the thymus, the naïve T cell repertoire continually interacts with self-pMHC ligands displayed on APC. At the repertoire level, these TCR-self-pMHC interactions are required for naïve T cells to undergo homeostatic proliferation, regulate cytokine responsiveness and endow T cells with a heightened sensitivity towards foreign antigens through inducing partial phosphorylation of the TCRζ-chain [31, 55–58]. TCR-self-pMHC interactions are also required for CD4+ FoxP3+ T cells to properly function, through the regulation of mTOR signaling and the expression of, amongst others, IRF4 and CTLA-4 [59, 60].
It has been evident since the earliest experiments that individual naïve T cell clonotypes likely interpret self-pMHC interactions differently. By transferring polyclonal T cell populations into lymphopenic hosts, it was observed that some naïve T cells failed to undergo homeostatic proliferation. Similar studies using monoclonal and polyclonal T cell populations demonstrated that the capacity of self-pMHC ligands to regulate T cell homeostasis is clonotype specific and that the peripheral expression of ligands that induce T cell positive selection can provide T cell homeostatic signals [55, 61, 62]. Likewise, competition among CD4+ T cells in unmanipulated hosts is probably caused by the limited availability of particular self-pMHC complexes [63]. However, deciphering this heterogeneity in naïve T cell reactivity to self-pMHC is intrinsically challenging; the interaction of self-pMHC complexes with the clone-specific TCR is of very low affinity and only a few self-peptides have been identified which induce positive selection of T cells or affect on peripheral T cell function.
Whether TCR-self-pMHC signal strength, and not simply the presence or absence of a positively selecting ligand, regulates naïve T cell survival and function has been addressed by over-expressing positively selecting ligands and through the use of TCR signaling reporters and surrogates. Allen and colleagues have recently provided direct evidence using gp250, a self-peptide that induces the positive selection of AND TCR transgenic T cells, that the expression level of a positively selecting ligand influences the frequency in which T cells with a particular specificity undergo positive selection, as well as the frequency in which these T cells are present in the mature T cell repertoire [64, 65]. Experiments using reporters of TCR signaling further support the model that the quality of the TCR signals generated by self-pMHC regulates T cell function. Of particular value is the expression of CD5, which may function as a rheostat to weaken or strengthen TCR signaling [12, 31, 50, 51, 66]. Paradoxically, increasing levels of CD5, which one might expect to reduce TCR signals, positively correlate with the degree of basal phosphorylation of TCRζ, and following TCR stimulation, the rapid induction of Erk phosphorylation and production of IL-2 [67, 68]. Consistent with these findings, CD5hi CD4+ and CD8+ T cells express higher levels of GFP than do CD5lo naïve T cells in TCR signaling reporter Nurr77-gfp mice. Naïve CD5hi T cells also show increased expression of multiple genes involved in T cell activations, including Eomes, T-bet, Helios and Id3 [69]. These functional and transcriptional changes have led several groups to test the hypothesis that responses to foreign antigens may be influenced by the quality of a T cell clonotype’s interactions with self-pMHC complexes.
Self-pMHC reactivity biases increase the efficiency of T cell responses
By co-transferring polyclonal CD5hi and CD5lo T cells into recipient mice, the groups of Germain and Jameson have recently demonstrated that polyclonal CD5hi CD4 and CD5hi CD8 T cells, respectively, outcompete CD5lo T cells in primary responses to multiple pathogen challenges. For CD8 T cells, the immunodominance of CD5hi T cells was maintained at the memory phase and during recall responses. The immunodominance of CD5hi T cells appears not to be due to a greater intrinsic ability of these T cells to undergo TCR-induced proliferation, as both CD5lo and CD5hi T cells proliferate similarly to in vitro activation using α-CD3 and α-CD28 [67, 69]. Although there is a strong consensus regarding the increased basal TCR signaling and improved functional characteristics, clonal analyses of the CD5hi versus CD5lo T cell subsets suggests the mechanisms which underlie differences in the T cell response during pathogen challenge are diverse. Using pMHC tetramer staining as a measure of TCR-pMHC binding strength, Mandl et al argue that CD5hi T cells express TCRs that are intrinsically of higher affinity for both self-peptides and foreign-peptides, and that this higher affinity for foreign-peptides gives these T cells a competitive advantage during clonal expansion [67]. Alternative to this TCR-intrinsic affinity model, Fulton et al suggest a T cell-intrinsic model in which increasing strength of TCR-self-pMHC interactions more efficiently poise naïve T cells to proliferate and integrate pro-inflammatory signals following pathogen challenge [69]. These findings are somewhat of a paradox to the idea that strong T cell interactions with self-pMHC dampen T cell reactivity and limit autoimmunity [52]. It is possible however, that the different experimental approaches elucidated different aspects of peripheral T cell interactions with self-pMHC; stronger sub-threshold interactions with self-pMHC enhance T cell responses until the threshold is met and receptor desensitization, anergy and deletion occur.
The complexities of immune responses and the importance of maintaining T cell diversity, suggest that the response of individual T cell clonotypes to pathogen challenge may diverge from the general features of the polyclonal repertoire. Indeed, study of two CD4 T cells specific for an identical epitope from Listeria monocytogenes (LLO190–205), in which one is CD5hi and the other is CD5lo, demonstrated that the CD5lo clonotype undergoes greater clonal expansion during a primary immune response. This occurred despite the two TCR having near identical affinities for the IAb-LLO peptide complex and the CD5hi clonotype having increased basal levels of phosphorylated TCRζ and ERK [68, 70]. Immune response dynamics and functional heterogeneity likely reconcile these differences. Consistent with all of the models of CD5 expression, the CD5hi T cells produced greater IL-2 responses following antigenic and non-specific stimulation, arguing there are intrinsic differences in the responsiveness of the two T cell lines to antigen receptor stimulation. Although this might portend the CD5hi T cells to undergo greater clonal expansion, the CD5hi T cells in fact showed a greater disposition to undergo apoptosis, potentially through IL-2-mediated activation induced cell death. Nevertheless, some of the CD5hi T cells were maintained and indeed dominate the immune response during a secondary challenge. Thus, during polyclonal T cell response to pathogens, the immune system has multiple mechanisms in place to limit clonal dominance and maintain immunological diversity (Box 3).
Box 3. Ensuring immunological diversity.
Effective immune responses occur when polyclonal T cells target the invading pathogen. However, during immune responses there is a competitive advantage for T cells with a strong reactivity for the pathogen over T cells with a weak reactivity for the pathogen. Thus, T cell competition based on antigen-reactivity could result in the entire immune response being dominated by progeny of just a few T cell clones. Although a focused T cell response may initially be successful in attacking cells harboring the invader, pathogens often have the ability to escape narrow oligoclonal T cell response through clonal exhaustion or through deleterious mutations within the T cell epitope [91–95]. To limit these effects, several additional layers of T cell competition ensure clonal diversity of the overall naïve T cell repertoire, as well as during immune responses [96]. During homeostasis, the mature T cell repertoire is subject to intraclonal competition, likely for access to self-pMHC ligands presented by APC and cytokines that provide survival signals [53, 55]. This form of competition ensures that there are relatively few numbers of any individual clonotype, allowing the space for a large number of unique T cell clonotypes to exist [4]. During immune responses, T cell intrinsic and extrinsic mechanisms limit oligoclonality and ensure individual clones do not overly dominate the T cell response during the priming phase. These include co-inhibitory molecules, including CTLA4, selective apoptosis of T cell clonotypes and T cell competition [21, 68, 97, 98]. Intrinsic T cell signaling, T cell competition for self-ligands and cytokines further impacts the transition from activated to memory T cell formation [99].
Concluding Remarks
How might self-pMHC reactivity benefit the efficiency of the T cell repertoire? An effective adaptive immune system is predicated on creating a T cell repertoire that is capable of providing immunological coverage to the universe of pathogens, an impossible feat if each TCR were overly specific for a particular antigen. By requiring thymocytes to functionally engage self-pMHC complexes, the very nature of positive selection itself biases the mature T cell repertoire to be peptide cross-reactive. Thais occurs because thymocytes expressing increasingly peptide cross-reactive TCRs will have a higher likelihood of recognizing one of the 103–105 self-peptides presented on host MHC, and thus receive a positive selecting signal [71–74].
Thymic and peripheral T cell recognition of self-pMHC complexes, leading to the modulation of CD5 levels and basal levels of phosphorylated TCRζ and ERK, is likely to follow the same general rules that govern mature T cells engaging foreign-antigens. Increases in CD5 levels will undoubtedly be associated with increases in the TCR-self-pMHC binding strength and the density in which the self-pMHC is expressed. Increases in CD5 levels could also occur from T cells being able to functionally engage multiple distinct self-peptide presented on MHC molecules (Figure 1).
Figure 1.
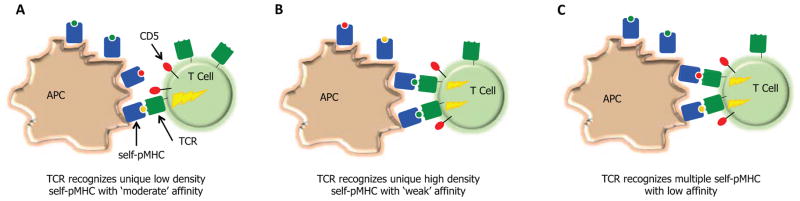
Does homeostasis increase the efficiency of the mature T cell repertoire through promoting T cell cross-reactivity? T cell interactions with self-pMHC ligands can promote responsiveness of T cell towards foreign-antigens through inducing the partial phosphorylation of TCRζ, allowing for the rapid induction of Erk phosphorylation and production of IL-2 following TCR recognition of pMHC. The nature of the self-pMHC ligands that drive this process are not well defined. Three possibilities exist: T cells may generate homeostatic signals from (A) ‘moderate’ affinity interactions with self-pMHC that are expressed at a low density, (B) ‘weak’ affinity interactions with self-pMHC that are expressed at a high density, or (C) ‘weak’ affinity interactions with multiple self-pMHC. Because thymic development selects against T cells that have stronger interactions with self-pMHC (depicted in A), and there are relatively few high-density self-MHC ligands presented in the periphery (depicted in B), cross-reactive T cell that can interact weakly with multiple self-pMHC (depicted in C) would have a competitive advantage to over T cells that are more peptide specific.
T cell efficiency gains through increasing peptide cross-reactivity, however, have to be balanced to minimize overt self-reactivity within the pro-inflammatory T cell repertoire. Restraining the development and clonal dominance of CD5hi T cells in the mature T cell repertoire is thymic selection. T cell developmental processes instruct developing thymocytes to undergo positive or negative selection based on the affinity or dwell-time of TCR-self-pMHC interactions. Reflective of this, thymocytes that express TCR with increasingly stronger binding strength for a particular self-pMHC ligand have a higher likelihood of being eliminated in the thymus by negative selection. Thus, CD5hi T cells may be skewed towards recognizing high-density, weak affinity/short dwell time self-pMHC ligands or have the ability to weakly recognize multiple unique self-pMHC ligands. Because there are relatively few high-density self-pMHC available to induce homeostatic signals, one consequence of peripheral TCR-self-pMHC interactions may be to increase the efficiency of the T cell repertoire by allowing T cells with increased peptide cross-reactivity to outcompete more peptide-specific T cells. These peripheral selection processes may also underpin the limiting of T cell diversity in aged individuals following thymic involution.
How might TCRs be created which have increased frequencies of self-reactivity and peptide cross-reactivity? TCR expressed on mature T cells are selected in part on their ability to be peptide cross-reactive. To create TCRs which have a beneficial range of peptide cross-reactivity, thymic selection equips mature T cells with TCR that carry structural features that allow TCRs to have ‘moderate rates’ of peptide-cross-reactivity [6]. Somatic gene recombination creates TCR with variations in pMHC specificity and rates of peptide cross-reactivity by pairing different TCR Vα and Vβ gene segments with rearranged CDR3 V(D)J sequences, with the vast a majority of TCR diversity and thus control of ligand specificity arising from CDR3 sequences. Because CDR3 sequences are the hot spot of TCR diversity [5], we predict that the control of peptide cross-reactivity will derived from these sequences. In particular, we hypothesize that thymic selection equips mature T cells with TCR that carry particular types of amino acids at the tips of CDR3 loops that allow TCRs to have ‘moderate rates’ of peptide-cross-reactivity. Unique CDR3 sequences may also create loops with greater or lessor flexibility, regulating the number peptide features CDR3 residues can engage. Future experiments will be needed to determine if CDR3 self-reactivity and peptide cross-reactivity sequence motifs can be identified, and how these recognition properties regulate the development of inflammatory and anti-inflammatory T cells, as well as immune and autoimmune T cell responses.
Highlights.
T cell interactions with self-pMHC tune antigen receptor signaling thresholds
Desensitization of TCR signaling in mature T cells creates an autoimmunity buffer
T cell homeostatic signals ready T cells to respond to foreign pathogens
Competition during homeostasis may promote T cell peptide cross-reactivity
Acknowledgments
The authors would like to thank Drs. Reinhard Obst, Brian Stadinski and Rajat Varma for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corse E, et al. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 2.Arstila TP, et al. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 3.Robins HS, et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Science translational medicine. 2010;2:47ra64. doi: 10.1126/scitranslmed.3001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins MK, et al. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2010;28:275–294. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- 5.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 6.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Huseby ES, et al. Interface-disrupting amino acids establish specificity between T cell receptors and complexes of major histocompatibility complex and peptide. Nat Immunol. 2006;7:1191–1199. doi: 10.1038/ni1401. [DOI] [PubMed] [Google Scholar]
- 8.Stadinski BD, et al. A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity. 2011;35:694–704. doi: 10.1016/j.immuni.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadinski BD, et al. Effect of CDR3 sequences and distal V gene residues in regulating TCR-MHC contacts and ligand specificity. J Immunol. 2014;192:6071–6082. doi: 10.4049/jimmunol.1303209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borg NA, et al. The CDR3 regions of an immunodominant T cell receptor dictate the 'energetic landscape' of peptide-MHC recognition. Nat Immunol. 2005;6:171–180. doi: 10.1038/ni1155. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum ME, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014 doi: 10.1016/j.cell.2014.03.047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrisekoop N, et al. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity. 2014;41:181–190. doi: 10.1016/j.immuni.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson RW, et al. T cell receptor cross-reactivity between similar foreign and self peptides influences naive cell population size and autoimmunity. Immunity. 2015;42:95–107. doi: 10.1016/j.immuni.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman Z, Paul WE. Dynamic Tuning of Lymphocytes: Physiological Basis, Mechanisms, and Function. Annu Rev Immunol. 2015 doi: 10.1146/annurev-immunol-032712-100027. [DOI] [PubMed] [Google Scholar]
- 15.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lever M, et al. Phenotypic models of T cell activation. Nat Rev Immunol. 2014;14:619–629. doi: 10.1038/nri3728. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty AK, Weiss A. Insights into the initiation of TCR signaling. Nat Immunol. 2014;15:798–807. doi: 10.1038/ni.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhns MS, Davis MM. TCR Signaling Emerges from the Sum of Many Parts. Front Immunol. 2012;3:159. doi: 10.3389/fimmu.2012.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepanek O, et al. Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell. 2014;159:333–345. doi: 10.1016/j.cell.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito T. Fundamental Immunology. Lippincott Williams & Wilkins; 2013. Mechanisms of T-lymphocyte signaling and activation. [Google Scholar]
- 21.Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- 22.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanova I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 24.Paster W, et al. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J. 2015;34:393–409. doi: 10.15252/embj.201387725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sykulev Y, et al. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 26.Irvine DJ, et al. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 27.Yagi J, Janeway CA., Jr Ligand thresholds at different stages of T cell development. Int Immunol. 1990;2:83–89. doi: 10.1093/intimm/2.1.83. [DOI] [PubMed] [Google Scholar]
- 28.Pircher H, et al. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 29.Davey GM, et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas B, et al. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 31.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function. Nat Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alam SM, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 33.Koehli S, et al. Optimal T-cell receptor affinity for inducing autoimmunity. Proc Natl Acad Sci U S A. 2014;111:17248–17253. doi: 10.1073/pnas.1402724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huseby ES, et al. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 35.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Ebert PJ, et al. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henao-Mejia J, et al. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Z, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 39.Egawa T, et al. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Current biology : CB. 2003;13:1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 40.Ruland J, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 41.Ruland J, et al. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity. 2003;19:749–758. doi: 10.1016/s1074-7613(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 42.Fu G, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013;504:441–445. doi: 10.1038/nature12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu G, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lesourne R, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson AL, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, et al. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat Immunol. 2012;13:560–568. doi: 10.1038/ni.2301. [DOI] [PubMed] [Google Scholar]
- 47.Lo WL, et al. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol. 2012;13:880–887. doi: 10.1038/ni.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuzaki H, et al. Tespa1 is a novel inositol 1,4,5-trisphosphate receptor binding protein in T and B lymphocytes. FEBS open bio. 2012;2:255–259. doi: 10.1016/j.fob.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 50.Tarakhovsky A, et al. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 51.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhandoola A, et al. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 53.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 54.Han S, et al. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci U S A. 2010;107:20453–20458. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Stefanova I, et al. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 57.Cho JH, et al. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer MJ, et al. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8(+) T cells. Immunol Cell Biol. 2011;89:581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vahl JC, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Levine AG, et al. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ernst B, et al. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 62.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hataye J, et al. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 64.Lo WL, et al. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo WL, et al. T cell immunodominance is dictated by the positively selecting self-peptide. eLife. 2014;3:e01457. doi: 10.7554/eLife.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong P, et al. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandl JN, et al. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Persaud SP, et al. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014;15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fulton RB, et al. The TCR's sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol. 2015;16:107–117. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber KS, et al. Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc Natl Acad Sci U S A. 2012;109:9511–9516. doi: 10.1073/pnas.1202408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Detours V, et al. Deriving quantitative constraints on T cell selection from data on the mature T cell repertoire. J Immunol. 2000;164:121–128. doi: 10.4049/jimmunol.164.1.121. [DOI] [PubMed] [Google Scholar]
- 72.Engelhard VH. Structure of peptides associated with class I and class II MHC molecules. Annu Rev Immunol. 1994;12:181–207. doi: 10.1146/annurev.iy.12.040194.001145. [DOI] [PubMed] [Google Scholar]
- 73.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 74.Nikolich-Zugich J, et al. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 75.Stone JD, et al. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zehn D, et al. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashton-Rickardt PG, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 78.Vanguri V, et al. Viral antigen density and confinement time regulate the reactivity pattern of CD4 T-cell responses to vaccinia virus infection. Proc Natl Acad Sci U S A. 2013;110:288–293. doi: 10.1073/pnas.1208328110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Depoil D, Dustin ML. Force and affinity in ligand discrimination by the TCR. Trends Immunol. 2014;35:597–603. doi: 10.1016/j.it.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu B, et al. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 2014;157:357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Das DK, et al. Force-dependent transition in the T-cell receptor beta-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc Natl Acad Sci U S A. 2015;112:1517–1522. doi: 10.1073/pnas.1424829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Judokusumo E, et al. Mechanosensing in T lymphocyte activation. Biophys J. 2012;102:L5–7. doi: 10.1016/j.bpj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim ST, et al. The alphabeta T cell receptor is an anisotropic mechanosensor. J Biol Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beddoe T, et al. Antigen ligation triggers a conformational change within the constant domain of the alphabeta T cell receptor. Immunity. 2009;30:777–788. doi: 10.1016/j.immuni.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Robert P, et al. Kinetics and mechanics of two-dimensional interactions between T cell receptors and different activating ligands. Biophys J. 2012;102:248–257. doi: 10.1016/j.bpj.2011.11.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nature structural biology. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 87.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H, et al. Basic residues in the T-cell receptor zeta cytoplasmic domain mediate membrane association and modulate signaling. Proc Natl Acad Sci U S A. 2011;108:19323–19328. doi: 10.1073/pnas.1108052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gagnon E, et al. Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3epsilon cytoplasmic domain. J Exp Med. 2012;209:2423–2439. doi: 10.1084/jem.20120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi X, et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493:111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- 91.Pircher H, et al. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 92.Ciurea A, et al. CD4+ T-cell-epitope escape mutant virus selected in vivo. Nat Med. 2001;7:795–800. doi: 10.1038/89915. [DOI] [PubMed] [Google Scholar]
- 93.Barouch DH, et al. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 94.Gaiha GD, et al. Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity. 2014;41:1001–1012. doi: 10.1016/j.immuni.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kedl RM, et al. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 97.Corse E, et al. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willis RA, et al. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc Natl Acad Sci U S A. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang JT, et al. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]


