Abstract
High grade glioma is a rare brain cancer, incurable in spite of modern neurosurgery, radiotherapy, and chemotherapy. Novel approaches are in research, and immunotherapy emerges as a promising strategy. Clinical experiences with active specific immunotherapy demonstrate feasibility, safety and most importantly, but incompletely understood, prolonged long-term survival in a fraction of the patients. In relapsed patients, we developed an immunotherapy schedule and we categorized patients into clinically defined risk profiles. We learned how to combine immunotherapy with standard multimodal treatment strategies for newly diagnosed glioblastoma multiforme patients. The developmental program allows further improvements related to newest scientific insights. Finally, we developed a mode of care within academic centers to organize cell-based therapies for experimental clinical trials in a large number of patients.
Keywords: immunotherapy, malignant glioma, dendritic cell vaccines, immunomodulation, galectin-1, oncolytic viruses
Introduction
High grade gliomas (HGG) are brain tumors occurring in adults and children. The WHO grade IV HGG, called glioblastoma multiforme (GBM), is the most frequent brain cancer in adults with an incidence of 3–4 per 100,000 adults per year (1) and 2 per million children (2). The treatment for these patients consists primarily of maximal safe surgery in order to debulk the tumoral mass for symptomatic relief and to obtain tissue for histological diagnosis, followed by radiochemotherapy and maintenance chemotherapy to induce optimal local tumor control. In spite of improved surgery and radiotherapy, and the addition of temozolomide (TMZ) to the multimodal treatment strategy, the prognosis of patients with GBM remains poor: the median overall survival (OS) is about 15 months, with 88% of patients dying within 3 years (3, 4). Relapse is universal and is believed to be due to the extensive spread of tumor cells into surrounding regions of the brain (5, 6). At the time of relapse, the prognosis is particularly poor, with reports of 100% mortality within 18 months (7). A recent review pointed to the progression-free survival (PFS) at 6 month and median OS as most useful and accessible end points, the latter ranging between 5 and 13 months for relapsed GBM patients (8). The prognosis upon recurrence might be improving with the initiation of new multimodal treatment strategies (9–11). Most reports are not yet focusing on long-term survival. In spite of being an orphan disease, the tumor still causes the highest number of years of life lost due to cancer (12). One of the particular challenges with classical chemotherapeutic strategies is overcoming the blood–brain barrier. Therefore, preclinical research is focused on alternate approaches, such as targeted therapy (13) including anti-angiogenesis strategies (14), and especially immunotherapy. Treating cancer by means of immunotherapy (e.g., cancer vaccines, adoptive cell transfer, and checkpoint blockade) has slowly evolved over decades in a nowadays clinically applicable treatment in a number of cancer types (e.g., metastatic melanoma, renal cell carcinoma, non-small cell lung cancer, prostate cancer…).
Active specific immunotherapy with autologous mature dendritic cells (DCm) loaded with autologous tumor cell lysate (DCm-HGG-L) is an emerging and innovative treatment approach for patients with HGG. The development of DC therapy in HGG has started in 1999 in our center. Since then, we established a complete translational research program from bench to bed (Figure 1) including in vitro experiments (15, 16), in vivo experiments in the GL261 model (17–19), early clinical phase I/II clinical trials as part of the HGG-IMMUNO-2003 cohort comparison trial for relapsed HGG patients (20–26), a phase I/II clinical trial HGG-2006 for patients with newly diagnosed GBM (EudraCT 2006-002881-20) (27, 28), and the recently finished phase IIb prospective placebo-controlled double-blind randomized clinical trial (RCT) HGG-2010 (EudraCT 2009-018228-14). In parallel to this clinical program, advanced MRI studies have been performed on HGG, in particular to characterize immunotherapy-related changes (29–32). In this program, insights from preclinical research were translated into the HGG-IMMUNO-2003 cohort (A–D) comparison trial. Data from these cohorts were then used for integration into the multimodal treatment of patients with primary diagnosis of GBM. As such, the vaccination technology from cohort C was used for the HGG-2006 trial, while the technology from cohort D is now used for the RCT HGG-2010. In parallel, according to the evolving legislation, the preparation for the clinical applications was embedded into a Good Manufacturing Practice (GMP) facility within the University Hospitals Leuven. The translation back from bed to bench has been realized by samplings of tumor tissue and blood samples taken at defined vaccination time points. The new preclinical research perspectives in 2014 include galectin-1 targeting as a strategy for immunomodulation and oncolytic virus therapy.
Figure 1.
Immunotherapy for HGG: a translational research program.
The preclinical and clinical results, together with clinical results obtained independently by other research teams provide a strong rationale to continue exploration of immunotherapy in patients with HGG. We summarized our insights in several reviews and commentary papers (33–39). The emerging field of immunotherapy for HGG has been extensively reviewed by other researchers as well (40–43). A first meta-analysis on the available results in the literature show clear benefit of immunotherapy for OS (44). In this review, it is our intention to focus on our own experience.
Rationale for Active Specific Immunotherapy Against HGG
Theoretical concept of dendritic cell vaccination
Dendritic cells (DCs) are a subset of white blood cells, critical to most aspects of adaptive immunity due to their central role as specialized antigen-presenting cells (APCs) in the initiation phase of T cell responses (45). Typically DCs reside as immature cells in almost every organ and tissue at the interface of potential pathogen entry sites. Danger-triggered DCs start to mature: they up-regulate chemokine receptors, which guide them to draining lymph nodes. There, the mature DCs are capable of inducing primary T cell responses due to their high levels of major histocompatibility complex (MHC), adhesion and costimulatory molecule expression. As opposed to the other APC, DCs are able to present and cross-present the antigenic peptides in the context of both MHC Class II and Class I molecules, respectively (46, 47). In this way, they can prime not only CD4+ T helper cells, but also CD8+ cytotoxic T cells (CTLs) (48). Both effector cell types are believed to be necessary to induce an effective cell-mediated immune response (49).
Dendritic cells are not only sentinels in the adaptive immune response, but have also been shown to be strong activators of NK cells and NKT cells (50), thus linking the innate and adaptive immune responses. In this way, both tumor cells with and without expression of MHC class I molecules can theoretically be killed (51). All these particular characteristics make DCs a perfect adjuvant in active specific immunotherapeutic strategies, in which one aims to induce a specific immune response in vivo (52–55).
Justification of the use of dendritic cell technology in glioma therapy
Gliomas have been shown to express an impressive collection of glioma-associated antigens (GAAs) (56). Till today, antigen search is a field of interest (57) including even tumor-driving mechanisms (58). Up till now, however, identification of a universally expressed GAA with a critical downstream cell survival-related function has not been identified. Therefore, just targeting the known GAA using individual peptides would inherently lead to immune escape because of the positive clonal selection of antigen-loss variants (59, 60): those tumor cell clones that do not express the particular, targeted GAA (anymore), will escape from the immune rejection and thus have an important proliferation advantage as compared to the cell clones that do express the targeted GAA. That heterogeneity in GAA expression in gliomas represents the main reason to use whole tumor cell lysates as a source of GAAs to load the DC. In case, the GAAs are expressed not only exclusively on the tumor cells but also on normal healthy cells, tolerance and induction of auto-immunity are possible, both being theoretical hurdles to a beneficial immune response: in the former case, an antitumoral immune response cannot be induced because the GAA is considered a self-antigen and in the latter case, a pathological immune response against normal tissues is mounted.
In general, tumor vaccination strategies are not entirely new anymore (52). Especially for the spontaneously more immunogenic tumors like malignant melanoma (61), renal cell carcinoma (62), mesothelioma (63), leukemia (64), gynecological tumors (65–67) and prostate carcinoma (68), several vaccination strategies have been used in the past. Large-scale production of clinical grade DCs became possible (69), including the development of several closed culture systems to obtain large amounts of DCs for clinical use (70–72). DC vaccination for prostate cancer reached full marketing authorization (Provenge®).
The brain, once considered as immune privileged site (73), is a dynamic immunological environment. Astrocytes, microglia and infiltrating immune cells play a major role in the brain during host immunity to antigens (74). The question of immune privilege in the context of malignant glioma is fading (56, 75). Proof of the principle of immunotherapy has been demonstrated in in vitro experiments (15, 16) and in several rodent models (37). In these models, induction of protective immunity and immunological memory against syngeneic orthotopic gliomas have been shown after vaccination with DCs loaded with GAAs of different antigen sources.
Immunotherapy for Patients with Relapsed HGG
Overview of different cohorts
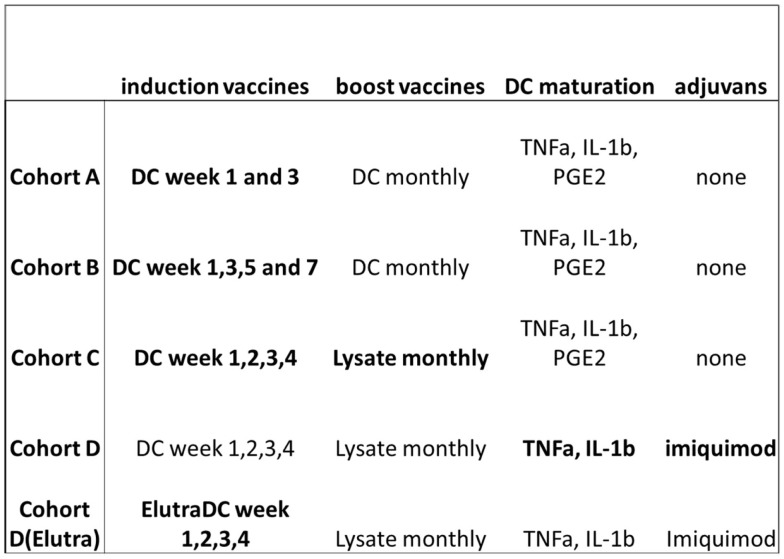
We started in 2001 to implement preclinical insights into clinical practice after obtaining approval of the local Ethics Committee. Since 2003, we initiated the HGG-IMMUNO-2003 study protocols consisting of sequential therapy-optimalization protocols in consecutive cohorts for patients with relapsed HGG. It is aimed to prove the feasibility and explore the efficacy of immune therapy for HGG, and to “dissect” different aspects of the immune therapy in order to find a putative ideal vaccination strategy. Cohorts have been built up on the most recent insights in vaccination strategy available at time of preparation of the cohort protocol (Figure 2).
Figure 2.
HGG-IMMUNO-2003 cohorts.
Cohort A. The DC vaccination schedule existed of five intradermal injections of autologous mature DC loaded with autologous tumor antigens. DC maturation was induced with the classical cytokine cocktail (IL-1b, TNF-a, PGE2). The latter cytokine cocktail was based primarily on the so-called Jonuleit cocktail (76). Already from the beginning, we omitted IL-6 out of the cocktail. IL-6 was known to play a major role in the induction of a Th17 phenotype of T cell response (77). Injections were administered at week 1, 3, 7, 11, 15.
Cohort B. Based on the observations made in the patient group treated according to the vaccination schedule in cohort A, injections with autologous mature DC loaded with tumor-derived antigens were administered at week 1, 3, 5, 7, (9) and further each 4 weeks.
Cohort C. Based on further observations made in the patient groups treated according to both prior vaccination schedules and based on recent insights in in vivo models upon priming with DC and boosting with lysate instead of DC (78), patients were treated with 4 weekly DC-HGG-L injections followed by monthly boosting with HGG-L.
Cohort D. In this cohort, we omitted PGE2 out of the maturation cocktail. PGE2 was already long time ago linked to the induction of a DC2-type (79). Because of its importance for the induction mainly of the mobility of DC (80), it was kept in the classical maturation cocktail. However, PGE2 was later-on also shown to induce IDO activity in human DC, thereby creating a tolerizing DC phenotype (81). Moreover, PGE2 upregulated CD25 on DC, as such believed as a marker of strong DC maturation, but a marker, of which was shown that it was shed in the surrounding thereby consuming the IL-2 needed for autocrine T cell activation. Because not-fully maturated DC themselves play a role in tolerance induction (82), we wanted to apply a method to induce with imiquimod in vivo DC maturation after injection (83–86). Imiquimod binds to Toll-like receptor 7 and induces strong DC maturation and activation. Moreover, its role in generating immune responses in a preclinical in vivo model of HGG has been described (85). Based on this rationale, PGE2 ex vivo maturation was replaced by local application of imiquimod to increase in vivo maturation and activation of loaded DC. Within this cohort, we switched at a certain time point from the open cell culture methodology toward a closed cell culture methodology. This group of patients was defined as cohort D(e). The monocytes were isolated with Elutra instead of plastic adherence. Elutriation allows for fast and easy enrichment of monocytes within a closed system, and is superior to other GMP-approved methods (87–89). DCs were cultured in VueLife tissue culture bags instead of Falcon culture flasks. The cytokines used for differentiation and maturation were GMP-certified. Finally, four batches of GMP-DCm-HGG-L were produced at the same time, of which the first was injected immediately as vaccine, while the three other batches were frozen until use. For each of the three remaining induction vaccinations, a batch was thawed and washed once before injection. Of note, the open cell culture methodology continued to include children with relapsed HGG, because the closed culture systems could not be applied to the leukapheresis product of children.
Updated clinical results
Patients suspected of a relapse of HGG, who could be taken into consideration for immunotherapy, were re-operated upon to maximally remove the tumor and in order to obtain tissue as a source of tumor proteins. Part of the tumor was provided for pathology diagnosis, part was placed immediately in a sterile vial, to be stored at −80°C. Because of the large amount of tumor tissue needed for vaccine production, in rare cases it was impossible for the pathologist to unequivocally prove the recurrent pathology: in these cases, radiological evolution and sometimes amino acid PET scan results were consulted to conclude a relapsing, progressive HGG.
Patients with relapsed HGG were entered into the trial. About 40% of the included patients combined or consecutively applied neurosurgery and immunotherapy with other types of treatment like re-irradiation or chemotherapy upon decision of the referring physician. We obtained clinical results from 366 patients (48 children younger than 18 years and 318 adults above the age of 18 years). These patients belong to the “as treated” group from whom also the RPA was estimated and who received new resection and only immunotherapy till the next event. Median PFS of these children and adults were 3.8 and 2.6 months, respectively; median OS was both 10.6 months. Most importantly, the 2-year OS for these patients with relapsed HGG was 20% (SEM = 6) for children and 22% (SEM = 2) for adults. When the subgroup of 33 children and 247 adults with relapsed GBM was taken separately, median PFS was 2.5 months for children and 2.6 months for adults, median OS was 8 and 9.9 months with a 2-year OS of 10% (SEM = 6) and 17% (SEM = 3), respectively. Thirteen percent (SEM = 8) of adults with relapsed GBM remained free of recurrence for more than 18 months, and 10% (SEM = 2) lived longer than 3 years. Although hard to compare with literature data, the tail of the OS curve seems beneficial to data published on repeated re-operations combined with drug-based adjuvant therapies (11). Our data are difficult to compare to published data on PFS and OS upon new chemotherapy (8) or radiochemotherapy (9, 10). To compare future clinical trials, data should be presented according to prognostic models as has been published after radio(chemo)therapy (90) or immunotherapy (25). Moreover, besides PFS at 6 months and median OS, we believe that long-term OS (2 years or more) should also be considered as further outcome of patients with relapsed HGG in the context of immunotherapy.
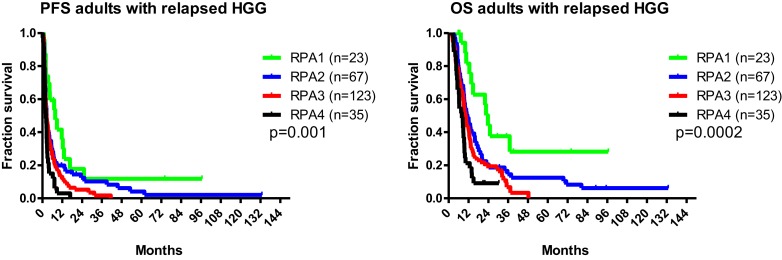
Having included a large series of patients with relapsed HGG and treated with neurosurgery and immunotherapy, it became indeed obvious that clinical risk factors were influencing the prognosis of the patients. This was considered as very important for counseling of the patients and for stratification while designing future RCTs for such patients. Therefore, a novel recursive partitioning analysis (RPA IMMUNO) classification was developed for adults above the age of 18 years with relapsed HGG, and survival data were analyzed on the 117 first included adult patients (25). The RPA classification was based on the age of the patient, the grading of the relapsed tumor (grade III or grade IV), the Karnofsky Self Performance Scale and the estimated mental status. We internally validated the RPA IMMUNO in an extended group of 251 adults with relapsed HGG treated in patient cohorts of the HGG-IMMUNO-2003 protocol and from whom we could retrieve the data for RPA classification. These patients were equally distributed into the four cohorts of patients. Patient characteristics are described in Table 1. As shown in Figure 3, the PFS and the OS of patients belonging to the different RPA risk classes were significantly different.
Table 1.
Patient characteristics.
| HGG-IMMUNO-2003 | HGG-2006 | |
|---|---|---|
| Age (median, range) | 49 (18–77) | 57 (27–70) |
| Sex (M/F) | 161/90 | 49/28 |
| Grade III/IV/no grading tumors | 43/205/3 | 0/77/0 |
| Number of events (median, range) | 2 (2–7) | 1 |
| Number of vaccines | 6 (4–24) | 8 (0–30) |
| Cohort A/B/C/D/D(e) | 11/15/26/72/127 | – |
Figure 3.
PFS and OS of adults with relapsed HGG.
The immunotherapy was feasible without major treatment-related toxicities. Almost all patients were treated in an ambulatory setting.
Immunotherapy for Patients with Newly Diagnosed GBM
HGG-2006 phase I/II trial
Rationale
As next step in our program, we wanted to integrate immunotherapy within the multimodal standard treatment for adults with newly diagnosed and histologically proven GBM (3, 4). A complex rationale was elaborated for the design. (1) Leukapheresis was scheduled after the surgical resection and before radiochemotherapy. After resection of GBM, a functional immune system is normally recovered within 1 week (91). Pro-inflammatory activity after irradiation might influence the activation state of monocytes and hence their differentiation capacity toward DC (92). Moreover, although grade III and IV hematologic toxic effects after radiochemotherapy were minimal (3), mild reduction of the monocyte count cannot be excluded. (2) The four induction vaccines were administered immediately after the radiochemotherapy. The immune suppression after 6 weeks concomitant TMZ was shown to be minimal but still might exist (3). The concept of tumor-specific immunization at time of immune reconstitution after chemotherapy has been demonstrated in several animal models (93, 94) and in clinical practice (95). Moreover, besides the induction of pro-inflammation (92), local radiotherapy might remove suppressor T cells, thus permitting a more effective T cell stimulation in loco (96). Another important reason to immunize prior to maintenance TMZ was the finding that the sensitivity of GBM to chemotherapeutics, among which TMZ, after prior vaccination was significantly increased (97, 98). (3) We further continued the boost vaccines during the TMZ maintenance therapy. Injection of lysate-loaded DCs for the priming, followed by boosts with tumor cell lysate alone generated the most effective antitumor effects in a preclinical model. The protocol allowed better CTL responses and also triggered an antitumor humoral response (78). The experiences in cohort C with induction vaccines with DCm-HGG-L and boost vaccines with HGG-L as immunotherapeutic strategy supported the concept for the HGG-2006 trial.
Updated Clinical Results
The first aim of this study was to assess the feasibility/toxicity to integrate tumor vaccination within the global treatment plan for an adult patient with newly diagnosed and GBM WHO grade IV, which could at least subtotally be removed. The major primary aim was the PFS at 6 months after diagnosis. To fulfill both the aims of (1) monitoring toxicity (phase I) of this treatment in the newly diagnosed patients and (2) detecting a potential benefit as a treatment strategy (phase II), we included a “STOP and GO” design.
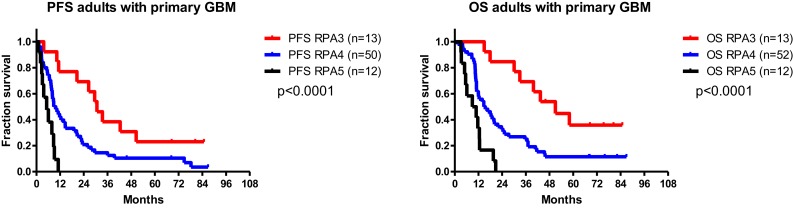
The results of the pilot phase and the full trial phase have been published recently (27, 28). The trial was feasible without major immunotherapy-related toxicities. The integrated immunotherapy did not affect quality of life. We here present the last updated results (31 July 2014) of the PFS and OS of patients from the HGG-2006 study, divided into the EORTC RPA risk profiles three to five (Figure 4). Patient characteristics are described in Table 1. The data represent the intent-to-treat analysis. The 5-year OS for the EORTC RPA class III and class IV patients was 35.9% (asymmetrical CI95%: +25.4, −24.2) and 11.5% (asymmetrical CI95%: +10.2, −6.9), respectively. As compared to the historical control data of patients belonging to the same EORTC RPA risk profiles (4), patients from EORTC RPA class III had a better OS when immunotherapy was added to the standard treatment. These data were used to power the HGG-2010 trial.
Figure 4.
PFS and OS of adults with primary diagnosis of GBM.
HGG-2010 prospective placebo-controlled double blind randomized clinical trial
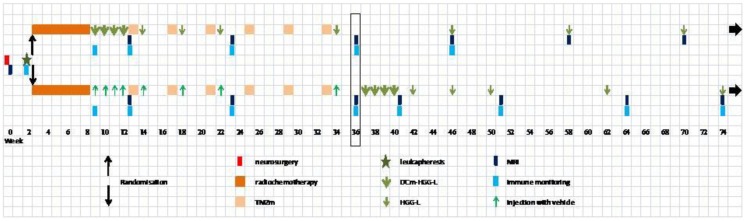
A prospective placebo-controlled double-blind phase IIb RCT was designed to explore the benefit of immunotherapy as fourth treatment modality to be included within the standard primary treatment strategy for patients with GBM (Figure 5). Supported by our experiences with patients included in HGG-2006, the design of the experimental arm (immunotherapy) is almost similar to HGG-2006. DCm-HGG-L is prepared and maturation is induced similar to Cohort D of the HGG-IMMUNO-2003 trial, using TNF-a, IL-1b, and Imiquimod skin preparation (aimed for TLR7-mediated DC activation). The design of the control arm is the current standard primary treatment: surgery, radiochemotherapy with TMZ, and maintenance chemotherapy with TMZ (3, 4). Randomization is performed with age as stratification variable (99). MGMT (O(6)-methylguanine DNA methyltransferase) methylation is not used for stratification. There is emerging evidence that other cytogenetic abnormalities outside MGMT methylation are of strong prognostic value as well (100–102). Primary endpoint of the trial is the PFS after six cycles of maintenance chemotherapy with TMZ. Secondary endpoints are quality of life assessments, OS, and induction of immune responses in both arms.
Figure 5.
Outline of the phase IIb randomized clinical trial HGG-2010.
Patients are unblinded after the assessment of disease status at time of MRI after the sixth cycle of TMZ or at time of progression if earlier progression occurred before the end of the sixth cycle of TMZ. Patients treated in the placebo arm and not yet relapsed (or with a compatible salvage treatment and no steroids after relapse) are treated with the immunotherapy regimen at this later stage, allowing to compare with immunomonitoring early vaccination efficacy during multimodal therapy with late vaccination after multimodal therapy.
The data of this RCT will be subject to the consortium Computational Horizons in Cancer (www.chic-vph.eu) to develop a hypermodel based on granular hypomodels in order to predict for which patient immunotherapy might be of added value. Clinical, radiological, immunological, and molecular data at diagnosis and at early evolution upon the radiochemotherapy will serve as incoming data into the different hypomodels.
New Preclinical Research Perspectives in 2014
Targeting galectin-1 as strategy for immunomodulation
GL261 Orthotopic Mouse Model
Galectin-1 is a glycan-binding protein which is involved in the aggressive nature of GBM by stimulating angiogenesis, cell migration, and proliferation. In different cancer models, galectin-1 has been demonstrated to play a pivotal role in tumor-mediated immune evasion especially by modulating cells of the adaptive immune system. It was unknown, however, whether the absence or presence of galectin-1 within the glioma microenvironment also causes qualitative or quantitative differences in innate and/or adaptive antitumor immune responses. We explored the role of galectin-1 in the orthotopic GL261 mouse glioma model (19). Stable galectin-1 knockdown was achieved via transduction of parental GL261 tumor cells with a lentiviral vector encoding a galectin-1-targeting miRNA. We demonstrated that the absence of tumor-derived but not of host-derived galectin-1 significantly prolonged the survival of glioma-bearing mice as such and in combination with DC-based immunotherapy. Both flow cytometric and pathological analysis revealed that the silencing of glioma-derived galectin-1 significantly decreased the amount of brain-infiltrating macrophages and myeloid-derived suppressor cells (MDSCs) in tumor-bearing mice. Additionally, we demonstrated a pro-angiogenic role for galectin-1 within the glioma microenvironment. The data provided in this study point to a pivotal role for glioma-derived galectin-1 in the regulation of myeloid cell accumulation within the glioma microenvironment, the most abundant immune cell population in HGG. Furthermore, the prolonged survival observed in untreated and DC-vaccinated glioma-bearing mice upon the silencing of tumor-derived galectin-1 strongly suggests that the in vivo targeting of tumor-derived galectin-1 might offer a promising and realistic adjuvant treatment modality in patients diagnosed with GBM.
Galectin-1 in the Serum of Patients
In parallel to this preclinical work, we questioned whether increased galectin-1 expression levels were exclusively found at the tumor site or whether galectin-1 could also be detected in the serum of HGG patients. Galectin-1 serum levels were analyzed in a prospective dataset of 43 healthy controls and 125 patients with newly diagnosed or recurrent HGG (103). Samples were taken at the moment of surgical resection and/or 2–3 weeks after surgery. Galectin-1 serum levels were determined using an ELISA for galectin-1. Galectin-1 serum levels depended significantly on age and sex in the control group. Age- and sex-adjusted galectin-1 serum levels were significantly higher in all patient subgroups compared to healthy controls with a high discriminative ability that increased with age. We did not observe a significant decrease in the galectin-1 serum levels upon surgical resection of the tumor. Collectively, the data may represent a first step to establish galectin-1 as a serum biomarker in HGG disease monitoring.
Further longitudinal evaluation is required and ongoing to investigate the value of galectin-1 serum levels in HGG patients as an additional diagnostic marker, but more importantly as a predictor of treatment response and prognosis. Furthermore, galectin-1 serum levels can also provide an important tool for the identification of HGG patients that can benefit from galectin-1-directed therapies that are currently under development.
Oncolytic virus therapy
The oncolytic features of several naturally occurring oncolytic viruses have been shown on GBM cell lines and in (subcutaneous) xenotransplant models (104). However, orthotopic glioma studies in immunocompetent animals were lacking. We investigated Newcastle disease virus (NDV) in the orthotopic, syngeneic murine GL261 glioma model (105). Seven days after tumor induction, mice were treated intratumorally with NDV. Treatment significantly prolonged median survival of treated animals and 50% showed long-term survival versus none in the control group. We demonstrated immunogenic cell death (ICD) induction in GL261 cells after NDV infection, comprising of calreticulin surface exposure, release of HMGB1 and increased expression of PMEL17 cancer antigen. Uniquely, we found absence of secreted ATP. NDV-induced ICD in GL261 cells was shown to occur through programmed necrosis or necroptosis. In vivo, elevated infiltration of IFN-γ+ T cells was observed in NDV-treated tumors, along with reduced accumulation of myeloid derived suppressor cells. The importance of a functional adaptive immune system in this paradigm was demonstrated in immunodeficient Rag2−/− mice, in which NDV induced a slight prolongation of survival, but failed to induce long-term survival. After secondary tumor induction in mice surviving long-term after NDV treatment, protection against glioma outgrowth was seen in 80% of animals, demonstrating induction of long-term antitumor immune memory after NDV therapy. We thus demonstrated for the first time that NDV has therapeutic activity against GL261 tumors, evidenced in an orthotopic mouse model. The therapeutic effect relies on the induction of a unique ICD route in the tumor cells, which primes adaptive antitumor immunity. The data change the paradigm that the use of oncolytic viruses for anti-cancer therapies should be performed in combination with suppression of potential antiviral immune responses. These insights are of high importance when using oncolytic viruses in combination with tumor vaccines within a multimodal treatment strategy.
Clinical Experiences on Immunotherapy Obtained in Other Centers
Active specific immunotherapy has been widely studied in many centers in phase I and/or phase II trials. Reviewing 37 reports on DC vaccines between 2000 and 2014, the patient number in each report was in median 15 ranging from 1 to 146. All these trials have been designed in different ways making read-outs hardly comparable. Moreover technologies for the vaccine production and administration routes were different as well. Characteristics of these trials are described in Table 2. Besides, the methodology to perform immune monitoring was variable: DTH tests, relative immune phenotypes of circulating lymphocytes, T cell proliferation and CTL assays, NK cell assays, IFN-γ production (serum, ELISPOT, mRNA expression, FACS), and recent thymic emigrant assay. In spite of all these differences, some general conclusions can be made. Immunotherapy for patients with (relapsed) HGG is feasible, and is safe. Only two immunotherapy-related serious adverse reactions have been reported: an overwhelming inflammatory reaction in a patient with large residual disease (21) and a cutaneous GBM growth after DTH testing of tumor cells which were presumably radio-resistant (106). Induction of autoimmune reactions has not been observed at all, in spite of the fact that crude lysate of tumor tissue used in several trials contained also normal tissue antigens. In most of the trials, an effect is observed being long-term surviving patients and/or immune responses. Immune monitoring data were hardly correlated with clinical data. Most importantly for the further development, a first meta-analysis on the available data shows clear clinical benefit of DC-based immunotherapy for patients with HGG (44).
Table 2.
Overview of DC-based clinical trials.
| Study phase | Case report | (20, 148) |
| Phase I | (21, 27, 149–161) | |
| Phase I/II | (22–26, 28, 162–171) | |
| Phase II | (106, 172) | |
| HGG grade | Grade III | (24, 148) |
| Grade III and IV | (23, 25, 106, 149–151, 153, 154, 158, 160, 162, 164–169) | |
| Grade IV | (20–22, 26–28, 97, 152, 155–157, 159, 161, 163, 170, 172) | |
| Disease status | Relapse (R) | (20–26, 148, 150, 151, 160–162, 165–167, 171) |
| New diagnosis (ND) | (27, 28, 97, 149, 152, 155, 156, 159, 169, 170, 172) | |
| R and ND | (106, 153, 154, 157, 158, 163, 164, 168) | |
| Tumor antigen | Lysate | (20–28, 97, 106, 153, 155, 158, 161–164, 166, 169) |
| Peptides | (97, 148, 149, 152, 156, 160, 167, 171, 173) | |
| Tumor cell mRNA | (151) | |
| Cancer stem cell mRNA | (159) | |
| Tumor cell suspension | (154) | |
| IFN-g-treated tumor cells | (168) | |
| Apoptotic tumor cells | (170, 172) | |
| Fusions | (150, 165) | |
| Route | ID | (20–28, 148, 150, 152–154, 156–161, 165) |
| SC | (97, 106, 149, 164, 168, 170, 172) | |
| ID + intratumoral | (162, 166) | |
| ID + IV | (151) | |
| Intranodal | (167) |
Modulation to Escape Immune Evasion Mechanisms
There are numerous factors that are responsible for HGG immune evasion (107). Intrinsic mechanisms include low expression of MHC class I and MHC class II molecules on the HGG tumor cells, microglia cells that produce IL-10 and IL-6, and an unbalance of the Th1/Th2 ratio in favor of Th2. Moreover Tenascin-C in the extracellular matrix in glioma prevents efficient immune cell to tumor cell contact. HGG cells produce a lot of immunosuppressive factors like TGF-b and PGE-2. Tumor cells lack costimulatory signals and might induce T cell anergy upon recognition. Moreover, stat-3 expression in the tumor cells promotes tumor immune evasion by inhibiting pro-inflammatory cytokine signaling and by amplifying Tregs. The PD-1L-1 expression on HGG is identified as a strong inhibitor of CD4+ and CD8+ T cell activation. The expression of HLA-E, HLA-G, and the presence of TGF-b and lectin-like transcript 1 are responsible for the absence of an NK attack to HGG. HGG cells express fas and fasL as well as CD70, and produce gangliosides and galectin-1. All these mechanisms are responsible for apoptosis of immune cells. Immune checkpoint blockade in combination with immunotherapy for glioma is therefore an emerging area of research (108). The most important immune evasion mechanisms are, however, the presence of myeloid-derived suppressor cells and especially Tregs.
The presence of Tregs in HGG tumors was found for the first time in 2006 (109). The number of Tregs infiltrating the brain was correlated with the WHO grade of the glioma (110). The suppressive activity of HGG-derived Tregs was demonstrated (109, 111–113). In preclinical research, we clearly showed the role of Tregs not only to block the antitumoral immune response (18) but also to change the inflammatory tumor microenvironment (114). Tregs have been shown to play a role on M2 macrophage differentiation (115) and MDSC functioning (116) in rodents. Tregs are particularly recruited into HGG by the production of CCL2 and CCL22 (117). Moreover, Tregs in HGG patients have a higher expression of the CCL2 receptor CCR4 as compared to controls. In the peripheral blood, a relative increase of the Treg fraction in the CD4 compartment as compared to controls was also described (118). Functional studies on Tregs from HGG patients became possible through isolation and characterization of this population as CD4 + CD127dim cells (119). These clinical data clearly show the presence and function of Tregs within the tumor microenvironment and even systemically.
Treg depletion and Treg inhibition are a widely discussed strategy in cancer (120). TLR ligands have been shown in preclinical models to inhibit Treg function and enhance in vivo tumor immunity (121, 122). Also TMZ (117, 123, 124) and gemcitabine (125) have been found to affect Treg infiltration in rodent models. Treatment with Sunitinib (126–128) or low dose paclitaxel (129) decreased the number of Tregs in cancer patients. Specific Treg depletion strategies have been performed in humans with anti-CD25 mAb daclizumab or with IL-2 diphtheria toxin conjugate denileukin diftitox (Ontak) (130–132). Treg depletion and immunological benefits could be obtained, especially with daclizumab. However, a trial had to be stopped because of availability of the product (130). The most important depleting strategy is the metronomic use of CPM (133–140). CPM suppresses in vitro induction of Tregs (141). The Treg depleting activity of CPM has been demonstrated in murine models in the context of vaccines (142). Some studies in humans have shown improvement of T cell effector function associated with a reduction in Treg numbers after low dose CPM (135). The timing and dose are critical for a robust CPM-based protocol able to induce significant ablation of Treg inhibitory functions in patients. Because the Treg depletion is aimed to be performed shortly after neurosurgery, potential interaction with used corticosteroids as described in mice should be taken into account (143).
Toward a New Health Care Model for Advanced Therapy Treatments
Autologous mature DCs loaded with autologous tumor lysate belong to the category of advanced therapy medicinal products (ATMP). According to EU Regulation 2007/1394/EC, ATMP for human use means (1) a gene therapy medicinal product as defined in Part IV of Annex I to Directive 2001/83/EC; (2) a somatic cell therapy medicinal product as defined in Part IV of Annex I to Directive 2001/83/EC; or (3) a tissue engineered product. In that context, DCs differentiated out of monocytes are defined as ATMPs. The boost vaccines consisting of HGG-L are regulated by the Directive 2004/23/EC. ATMPs in academic hospitals can be produced under the hospital exemption clausule. Hospital exemption means preparation of ATMPs on a non-routine basis according to specific quality standards, and used within the same Member State in a hospital under the exclusive professional responsibility of a medical practitioner in order to comply with an individual medical prescription for a custom-made product for an individual patient.
The production and administration of personalized ATMPs together with other anti-cancer therapies in a multimodal treatment approach for very diseased patients should be considered as Advanced Therapy Treatment for these patients, preferentially performed in centers of excellence by fully equipped specialty teams with particular multidisciplinary knowledge on basic, translational, and clinical science around the ATMP within the given clinical context. From the beginning of the translational research program, the working model was organized as a multicentre collaboration. The goal was to make this experimental treatment strategy in clinical trials easily accessible for all potential patients in and outside the country. By doing this, a multiple “win” situation was created: the accessibility to immunotherapy program was easy for each patient, the referring specialist remained involved in the patient care (vaccination in ambulant setting) and in the scientific evolutions of the program, and the vaccination center obtained large series of patients so that experience could be maximized and scientific data generated within short periods. It might take time before patient-specific ATMPs that are used within a very complex clinical context, will reach industrialization for their production. In their report to the European Parliament and the Council in March 2014, the reporters from the European Commission pointed to creating a more favorable environment for ATMP developers working in an academic or non-for-profit setting, including by promoting early contacts with the authorities through the application of the fee reduction for scientific advice and by extending the existing certification scheme to these developers (144). Nevertheless, the DCVax®-L vaccine is developed by Northwest Biotherapeutics as an adjunct to the treatment of GBM, and is currently under evaluation in a phase III trial (145).
Obviously, the use of autologous ex vivo cultured mature loaded DCs is labor-intensive and expensive. This means a small-scale production for each individual patient as well as an adapted health care model to develop and provide such technologies. Meanwhile, strategies are searched for targeting DCs in the patient themselves. Appropriate pattern recognition receptors ligands are bound to tumor antigens to provide necessary adjuvant immune signals. Antigens are bound to antibodies which target particular receptors on DCs for internalization of the antigen and subsequent presentation (146). Besides antibody-based DC targeting, nanoparticles are rapidly emerging as new vehicles for delivering vaccines. Nanoparticles are a platform for co-encapsulating TLR ligands with the tumor antigen, and for targeting DCs through monoclonal antibodies or carbohydrate ligands (147).
Conclusion
Immunotherapy for HGG is feasible and has shown promising clinical results in a subgroup of patients without major adverse events. Decisive scientific results from large randomized trials are needed and awaited before the true position of DC vaccination in the therapy of HGG can be established. In parallel, patients who can benefit from this technology are characterized and defined. With current available basic science knowledge, further improvements of techniques and treatment strategies are reachable. However, administrative burdens to produce individualized vaccines remain a major threat, so that research focusses on as much as possible standardized off-the-shelf consumables for their production.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work has been supported by the Olivia Hendrickx Research Fund (www.olivia.be). Support was also obtained from the Herman Memorial Research Fund (www.hmrf.be), the James E. Kearney Foundation, Leuven’s ARCH fund, CAF Belgium, Baxter, and gifts from private families, service clubs and organizations. Additionally, grants were obtained from “Stichting tegen Kanker,” IWT (TBM projects), the Stem Cell Institute Leuven, the Emmanuel van der Schueren Fund, the International Union against Cancer, the klinisch Onderzoeksfonds UZ Leuven and the Fund for Scientific Research – Flanders (FWO-V). The HGG-2010 project has received funding from the European Union’s Seventh Framework Program for research, technological development and demonstration under grant agreement No 600841. I am very grateful for the technical assistance from the technicians from the Immunotherapy Platform Leuven (www.itpl.be). I thank the neuro-oncology team in the University Hospital Leuven for fruitful patient discussion, and the staff of the Laboratory of Experimental Immunology for basic scientific discussions.
References
- 1.Fleury A, Menegoz F, Grosclaude P, Daures JP, Henry Amar M, Raverdy N, et al. Descriptive epidemiology of cerebral gliomas in France. Cancer (1997) 79:1195–202. [DOI] [PubMed] [Google Scholar]
- 2.Tamber MS, Rutka JT. Pediatric supratentorial high-grade gliomas. Neurosurg Focus (2003) 14 Available from: http://www.medscape.com/viewarticle/449870 [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoom MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med (2005) 352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol (2009) 10:459–66. 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 5.Kleihues P, Soylemezoglu F, Schauble B, Scheithauer BW, Burger PC. Histopathology, classification, and grading of gliomas. Glia (1995) 15:211–21. 10.1002/glia.440150303 [DOI] [PubMed] [Google Scholar]
- 6.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol (2007) 114:443–58. 10.1007/s00401-007-0293-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol (2001) 12:259–66. 10.1023/A:1008382516636 [DOI] [PubMed] [Google Scholar]
- 8.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma – are we there yet? Neuro Oncol (2013) 15:4–27. 10.1093/neuonc/nos273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenspoon JN, Sharieff W, Hirte H, Overholt A, Devillers R, Gunnarsson T, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Onco Targets Ther (2014) 7:485–90. 10.2147/OTT.S60358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholtyssek F, Zwiener I, Schlamann A, Seidel C, Meixensberger J, Bauer M, et al. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol (2013) 8:161. 10.1186/1748-717X-8-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg (2013) 118:812–20. 10.3171/2012.9.JNS1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life lost (YLL) from cancer is an important measure of population burden – and should be considered when allocating research funds. Br J Cancer (2005) 92:241–5. 10.1038/sj.bjc.6602321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller M, Stupp R, Hegi M, Wick W. Individualized targeted therapy for glioblastoma: fact or fiction? Cancer J (2012) 18:40–4. 10.1097/PPO.0b013e318243f6c9 [DOI] [PubMed] [Google Scholar]
- 14.De Fazio S, Russo E, Ammendola M, Donato Di PE, De Sarro G. Efficacy and safety of bevacizumab in glioblastomas. Curr Med Chem (2012) 19:972–81. 10.2174/092986712799320646 [DOI] [PubMed] [Google Scholar]
- 15.De Vleeschouwer S, Arredouani M, Ade M, Cadot P, Vermassen E, Ceuppens JL, et al. Uptake and presentation of malignant glioma tumor cell lysates by monocyte-derived dendritic cells. Cancer Immunol Immunother (2005) 54:372–82. 10.1007/s00262-004-0615-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vleeschouwer S, Spencer L, I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol (2007) 84:131–40. 10.1007/s11060-007-9362-y [DOI] [PubMed] [Google Scholar]
- 17.Maes W, Deroose C, Reumers V, Krylyshkina O, Gijsbers R, Ceuppens J, et al. In vivo bioluminescence imaging in an experimental mouse model for dendritic cell based immunotherapy against malignant glioma. J Neurooncol (2009) 91:127–39. 10.1007/s11060-008-9691-5 [DOI] [PubMed] [Google Scholar]
- 18.Maes W, Galicia Rosas G, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, et al. DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol (2009) 11:529–42. 10.1215/15228517-2009-004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verschuere T, Toelen J, Maes W, Poirier F, Boon L, Tousseyn T, et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int J Cancer (2014) 134:873–84. 10.1002/ijc.28426 [DOI] [PubMed] [Google Scholar]
- 20.De Vleeschouwer S, Van Calenbergh F, Demaerel P, Flamen P, Rutkowski S, Kaempgen E, et al. Transient local response and persistent tumor control of recurrent malignant glioma treated with combination therapy including dendritic cell therapy. J Neurosurg (2004) 100:492–7. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JE, Kuhl J, Demaerel P, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer (2004) 91:1656–62. 10.1038/sj.bjc.6602195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res (2008) 14:3098–104. 10.1158/1078-0432.CCR-07-4875 [DOI] [PubMed] [Google Scholar]
- 23.Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, et al. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer (2010) 54:519–25. 10.1002/pbc.22319 [DOI] [PubMed] [Google Scholar]
- 24.Elens I, De Vleeschouwer S, Pauwels F, Van Gool SW. Resection and immunotherapy for recurrent grade III glioma. ISRN Immunol (2012) 2012:1–9. 10.5402/2012/530179 [DOI] [Google Scholar]
- 25.De Vleeschouwer S, Ardon H, Van Calenbergh F, Sciot R, Wilms G, Van Loon J, et al. Stratification according to HGG-IMMUNO RPA model predicts outcome in a large group of patients with relapsed malignant glioma treated by adjuvant postoperative dendritic cell vaccination. Cancer Immunol Immunother (2012) 61:2105–12. 10.1007/s00262-012-1271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyrich M, Schreiber SC, Rachor J, Krauss J, Pauwels F, Hain J, et al. Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy (2014) 16:946–64. 10.1016/j.jcyt.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 27.Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol (2010) 99:261–72. 10.1007/s11060-010-0131-y [DOI] [PubMed] [Google Scholar]
- 28.Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother (2012) 61:2033–44. 10.1007/s00262-012-1261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrabec M, Van Cauter S, Himmelreich U, Van Gool SW, Sunaert S, De Vleeschouwer S, et al. MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology (2011) 53:721–31. 10.1007/s00234-010-0802-6 [DOI] [PubMed] [Google Scholar]
- 30.Van Cauter S, Veraart J, Sijbers J, Peeters RR, Himmelreich U, De Keyzer F, et al. Gliomas: diffusion kurtosis MR imaging in grading. Radiology (2012) 263:492–501. 10.1148/radiol.12110927 [DOI] [PubMed] [Google Scholar]
- 31.Van Cauter S, Sima DM, Luts J, Ter BL, Ribbens A, Peeters RR, et al. Reproducibility of rapid short echo time CSI at 3 Tesla for clinical applications. J Magn Reson Imaging (2013) 37:445–56. 10.1002/jmri.23820 [DOI] [PubMed] [Google Scholar]
- 32.Van Cauter S, De Keyzer F, Sima DM, Croitor Sava A, D’Arco F, Veraart J, et al. Integrating diffusion kurtosis imaging, dynamic susceptibility-weighted contrast-enhanced MRI, and short echo time chemical shift imaging for grading gliomas. Neuro Oncol (2014) 16(7):1010–21. 10.1093/neuonc/not304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vleeschouwer S, Van Gool SW, Van Calenbergh F. Immunotherapy for malignant gliomas: emphasis on strategies of active specific immunotherapy using autologous dendritic cells. Childs Nerv Syst (2005) 21:7–18. 10.1007/s00381-004-0994-3 [DOI] [PubMed] [Google Scholar]
- 34.De Vleeschouwer S, Rapp M, Sorg RV, Steiger HJ, Stummer W, Van Gool S, et al. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery (2006) 59:988–99. 10.1227/01.NEU.0000245595.38957.3E [DOI] [PubMed] [Google Scholar]
- 35.Van Gool SW, Maes W, Ardon H, Verschuere T, Van Cauter S, De Vleeschouwer S. Dendritic cell therapy of high grade gliomas. Brain Pathol (2009) 19:694–712. 10.1111/j.1750-3639.2009.00316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verschuere T, De VS, Lefranc F, Kiss R, Van Gool SW. Galectin-1 and immunotherapy for brain cancer. Expert Rev Neurother (2011) 11:533–43. 10.1586/ern.11.40 [DOI] [PubMed] [Google Scholar]
- 37.Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother (2011) 60:153–60. 10.1007/s00262-010-0946-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Gool S, De Vleeschouwer S. Should dendritic cell-based tumor vaccination be incorporated into standard therapy for newly diagnosed glioblastoma patients? Expert Rev Neurother (2012) 12:1173–6. 10.1586/ern.12.107 [DOI] [PubMed] [Google Scholar]
- 39.Vandenberk L, Van Gool SW. Treg infiltration in glioma: a hurdle for antiglioma immunotherapy. Immunotherapy (2012) 4:675–8. 10.2217/imt.12.64 [DOI] [PubMed] [Google Scholar]
- 40.Jadavji NM, Deng L, Leclerc D, Malysheva O, Bedell BJ, Caudill MA, et al. Severe methylenetetrahydrofolate reductase deficiency in mice results in behavioral anomalies with morphological and biochemical changes in hippocampus. Mol Genet Metab (2012) 106:149–59. 10.1016/j.ymgme.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 41.Marsh JC, Goldfarb J, Shafman TD, Diaz AZ. Current status of immunotherapy and gene therapy for high-grade gliomas. Cancer Control (2013) 20:43–8. [DOI] [PubMed] [Google Scholar]
- 42.Jackson C, Ruzevick J, Brem H, Lim M. Vaccine strategies for glioblastoma: progress and future directions. Immunotherapy (2013) 5:155–67. 10.2217/imt.12.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev (2013) 39:891–907. 10.1016/j.ctrv.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 44.Cao JX, Zhang XY, Liu JL, Li D, Li JL, Liu YS, et al. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: evidence from a meta-analysis. PLoS One (2014) 9:e107173. 10.1371/journal.pone.0107173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol (2001) 19:47–64. 10.1146/annurev.immunol.19.1.47 [DOI] [PubMed] [Google Scholar]
- 46.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science (1990) 249:918–21. 10.1126/science.2392683 [DOI] [PubMed] [Google Scholar]
- 47.Rock KL, Clark K. Analysis of the role of MHC class II presentation in the stimulation ofcytotoxic T lymphocytes by antigens targeted into the exogenous antigen-MHCclass I presentation pathway. J Immunol (1996) 156:3721–6. [PubMed] [Google Scholar]
- 48.Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol (2000) 67:607–14. [DOI] [PubMed] [Google Scholar]
- 49.Levitsky HI, Lazenby A, Hayashi RJ, Pardoll DM. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med (1994) 179:1215–24. 10.1084/jem.179.4.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer (2004) 109:893–9. 10.1002/ijc.20050 [DOI] [PubMed] [Google Scholar]
- 51.Basse PH, Whiteside TL, Chambers W, Herberman RB. Therapeutic activity of NK cells against tumors. Int Rev Immunol (2001) 20:439–501. 10.3109/08830180109054416 [DOI] [PubMed] [Google Scholar]
- 52.Anguille S, Smits EL, Lion E, Van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol (2014) 15:e257–67. 10.1016/S1470-2045(13)70585-0 [DOI] [PubMed] [Google Scholar]
- 53.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity (2013) 39:38–48. 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi H, Appel S. Current status and future perspectives of dendritic cell-based cancer immunotherapy. Scand J Immunol (2013) 78:167–71. 10.1111/sji.12060 [DOI] [PubMed] [Google Scholar]
- 55.Kalinski P, Muthuswamy R, Urban J. Dendritic cells in cancer immunotherapy: vaccines and combination immunotherapies. Expert Rev Vaccines (2013) 12:285–95. 10.1586/erv.13.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin Cancer Res (2014) 20:5620–9. 10.1158/1078-0432.CCR-14-0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu ZB, Qiu C, Zhang AL, Cai L, Lin SJ, Yao Y, et al. Glioma-associated antigen HEATR1 induces functional cytotoxic T lymphocytes in patients with glioma. J Immunol Res (2014) 2014:131494. 10.1155/2014/131494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuessler A, Walker DG, Khanna R. Cellular immunotherapy directed against human cytomegalovirus as a novel approach for glioblastoma treatment. Oncoimmunology (2014) 3:e29381. 10.4161/onci.29381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother (1998) 46:82–7. 10.1007/s002620050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol (2010) 28:4722–9. 10.1200/JCO.2010.28.6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res (2012) 18:5460–70. 10.1158/1078-0432.CCR-11-3368 [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Liao L, Tan J. Dendritic cell-based vaccination for renal cell carcinoma: challenges in clinical trials. Immunotherapy (2012) 4:1031–42. 10.2217/imt.12.107 [DOI] [PubMed] [Google Scholar]
- 63.Hegmans JP, Veltman JD, Lambers ME, de Vries IJ, Figdor CG, Hendriks RW, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med (2010) 181:1383–90. 10.1164/rccm.200909-1465OC [DOI] [PubMed] [Google Scholar]
- 64.Van Tendeloo VF, Van De Velde AL, Van Driessche A, Cools N, Anguille S, Ladell K, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A (2010) 107:13824–9. 10.1073/pnas.1008051107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coosemans A, Wolfl M, Berneman ZN, Van Tendeloo V, Vergote I, Amant F, et al. Immunological response after therapeutic vaccination with WT1 mRNA-loaded dendritic cells in end-stage endometrial carcinoma. Anticancer Res (2010) 30:3709–14. [PubMed] [Google Scholar]
- 66.Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman ZN, et al. Wilms’ tumor gene 1 (WT1) – loaded dendritic cell immunotherapy in patients with uterine tumors: a phase I/II clinical trial. Anticancer Res (2013) 33:5495–500. [PubMed] [Google Scholar]
- 67.Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman Z, et al. Immunological response after WT1 mRNA-loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Res (2013) 33:3855–9. [PubMed] [Google Scholar]
- 68.Shore ND, Mantz CA, Dosoretz DE, Fernandez E, Myslicki FA, McCoy C, et al. Building on sipuleucel-T for immunologic treatment of castration-resistant prostate cancer. Cancer Control (2013) 20:7–16. [DOI] [PubMed] [Google Scholar]
- 69.Thurner B, Roder C, Dieckmann D, Heuer H, Kruse M, Glaser A, et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods (1999) 223:1–15. 10.1016/S0022-1759(98)00208-7 [DOI] [PubMed] [Google Scholar]
- 70.Mu LJ, Gaudernack G, Saeboe-Larssen S, Hammerstad H, Tierens A, Kvalheim G. A protocol for generation of clinical grade mRNA-transfected monocyte-derived dendritic cells for cancer vaccines. Scand J Immunol (2003) 58:578–86. 10.1046/j.1365-3083.2003.01333.x [DOI] [PubMed] [Google Scholar]
- 71.Sorg RV, Ozcan Z, Brefort T, Fischer J, Ackermann R, Muller M, et al. Clinical-scale generation of dendritic cells in a closed system. J Immunother (2003) 26:374–83. 10.1097/00002371-200307000-00010 [DOI] [PubMed] [Google Scholar]
- 72.Tuyaerts S, Noppe SM, Corthals J, Breckpot K, Heirman C, De Greef C, et al. Generation of large numbers of dendritic cells in a closed system using cell factories. J Immunol Methods (2002) 264:135–51. 10.1016/S0022-1759(02)00099-6 [DOI] [PubMed] [Google Scholar]
- 73.Weller RO, Engelhardt B, Phillips MJ. Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol (1996) 6:275–88. 10.1111/j.1750-3639.1996.tb00855.x [DOI] [PubMed] [Google Scholar]
- 74.Huber AK, Duncker PC, Irani DN. Immune responses to non-tumor antigens in the central nervous system. Front Oncol (2014) 4:328. 10.3389/fonc.2014.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery (2012) 71:201–22. 10.1227/NEU.0b013e31824f840d [DOI] [PubMed] [Google Scholar]
- 76.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, et al. Proinflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal-calf serum-free conditions. Eur J Immunol (1997) 27:3135–42. 10.1002/eji.1830271209 [DOI] [PubMed] [Google Scholar]
- 77.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity (2006) 24:677–88. 10.1016/j.immuni.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 78.Jouanneau E, Poujol D, Gulia S, Le Mercier I, Blay JY, Belin MF, et al. Dendritic cells are essential for priming but inefficient for boosting antitumour immune response in an orthotopic murine glioma model. Cancer Immunol Immunother (2006) 55(3):254–67 10.1007/s00262-005-0040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today (1999) 20:561–7. 10.1016/S0167-5699(99)01547-9 [DOI] [PubMed] [Google Scholar]
- 80.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood (2002) 100:1354–61. 10.1182/blood-2001-11-0017 [DOI] [PubMed] [Google Scholar]
- 81.Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood (2006) 108:228–37. 10.1182/blood-2005-08-3507 [DOI] [PubMed] [Google Scholar]
- 82.Moser M. Dendritic cells in immunity and tolerance – do they display opposite functions? Immunity (2003) 19:5–8. 10.1016/S1074-7613(03)00182-1 [DOI] [PubMed] [Google Scholar]
- 83.Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, et al. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol (2003) 171:6275–82. 10.4049/jimmunol.171.11.6275 [DOI] [PubMed] [Google Scholar]
- 84.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol (2005) 174:2476–80. 10.4049/jimmunol.174.5.2476 [DOI] [PubMed] [Google Scholar]
- 85.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol (2006) 176:157–64. 10.4049/jimmunol.176.1.157 [DOI] [PubMed] [Google Scholar]
- 86.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med (2007) 204:1441–51. 10.1084/jem.20070021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berger TG, Strasser E, Smith R, Carste C, Schuler-Thurner B, Kaempgen E, et al. Efficient elutriation of monocytes within a closed system (Elutra) for clinical-scale generation of dendritic cells. J Immunol Methods (2005) 298:61–72. 10.1016/j.jim.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 88.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology (2005) 114:204–12. 10.1111/j.1365-2567.2004.02076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dohnal AM, Graffi S, Witt V, Eichstill C, Wagner D, Ul-Haq S, et al. Comparative evaluation of techniques for the manufacturing of dendritic cell-based cancer vaccines. J Cell Mol Med (2009) 13:125–35. 10.1111/j.1582-4934.2008.00304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Combs SE, Edler L, Rausch R, Welzel T, Wick W, Debus J. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol (2013) 52:147–52. 10.3109/0284186X.2012.692882 [DOI] [PubMed] [Google Scholar]
- 91.Rapp M, Ozcan Z, Steiger HJ, Wernet P, Sabel MC, Sorg RV. Cellular immunity of patients with malignant glioma: prerequisites for dendritic cell vaccination immunotherapy. J Neurosurg (2006) 105:41–50. 10.3171/jns.2006.105.1.41 [DOI] [PubMed] [Google Scholar]
- 92.Petrini B, Andersson B, Strannegard O, Wasserman J, Blomgren H, Glas U. Monocyte release and plasma levels of interleukin-6 in patients irradiated for cancer. In vivo (1992) 6:531–4. [PubMed] [Google Scholar]
- 93.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A (2002) 99:931–6. 10.1073/pnas.022634999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pritchard-Jones K, Spendlove I, Wilton C, Whelan J, Weeden S, Lewis I, et al. Immune responses to the 105AD7 human anti-idiotypic vaccine after intensive chemotherapy, for osteosarcoma. Br J Cancer (2005) 92:1358–65. 10.1038/sj.bjc.6602500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol (2011) 13:324–33. 10.1093/neuonc/noq157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.North RJ. Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother (1984) 16:175–81. 10.1007/BF00205425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res (2004) 10:5316–26. 10.1158/1078-0432.CCR-04-0497 [DOI] [PubMed] [Google Scholar]
- 98.Liu G, Akasaki Y, Khong HT, Wheeler CJ, Das A, Black KL, et al. Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene (2005) 24:5226–34. 10.1038/sj.onc.1208519 [DOI] [PubMed] [Google Scholar]
- 99.Mirimanoff RO, Gorlia T, Mason W, van den Bent MJ, Kortmann RD, Fisher B, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol (2006) 24:2563–9. 10.1200/JCO.2005.04.5963 [DOI] [PubMed] [Google Scholar]
- 100.Hassler M, Seidl S, Fazeny-Doerner B, Preusser M, Hainfellner J, Rossler K, et al. Diversity of cytogenetic and pathohistologic profiles in glioblastoma. Cancer Genet Cytogenet (2006) 166:46–55. 10.1016/j.cancergencyto.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 101.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci (2009) 100:2235–41. 10.1111/j.1349-7006.2009.01308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med (2009) 360:765–73. 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verschuere T, van WM, Fieuws S, Lefranc F, Mathieu V, Kiss R, et al. Altered galectin-1 serum levels in patients diagnosed with high-grade glioma. J Neurooncol (2013) 115:9–17. 10.1007/s11060-013-1201-8 [DOI] [PubMed] [Google Scholar]
- 104.Alkassar M, Gartner B, Roemer K, Graesser F, Rommelaere J, Kaestner L, et al. The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo. J Neurooncol (2011) 104:715–27. 10.1007/s11060-011-0606-5 [DOI] [PubMed] [Google Scholar]
- 105.Koks CA, Garg AD, Ehrhardt M, Riva M, De Vleeschouwer S, Agostinis P, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer (2014) 136:e313–25. 10.1002/ijc.29202 [DOI] [PubMed] [Google Scholar]
- 106.Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res (2008) 68:5955–64. 10.1158/0008-5472.CAN-07-5973 [DOI] [PubMed] [Google Scholar]
- 107.Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol (2012) 746:53–76. 10.1007/978-1-4614-3146-6_5 [DOI] [PubMed] [Google Scholar]
- 108.Ahn BJ, Pollack IF, Okada H. Immune-checkpoint blockade and active immunotherapy for glioma. Cancers (Basel) (2013) 5:1379–412. 10.3390/cancers5041379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol (2006) 8:234–43. 10.1215/15228517-2006-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol (2010) 225:195–9. 10.1016/j.jneuroim.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 111.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol (2006) 8:261–79. 10.1215/15228517-2006-008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol (1997) 159:4415–25. [PubMed] [Google Scholar]
- 113.Roszman TL, Brooks WH. Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol (1980) 39:395–402. [PMC free article] [PubMed] [Google Scholar]
- 114.Maes W, Verschuere T, Van HA, Boon L, van GS. Depletion of regulatory T cells in a mouse experimental glioma model through anti-CD25 treatment results in the infiltration of non-immunosuppressive myeloid cells in the brain. Clin Dev Immunol (2013) 2013:952469. 10.1155/2013/952469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol (2011) 89:130–42. 10.1038/icb.2010.70 [DOI] [PubMed] [Google Scholar]
- 116.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol (2011) 186:807–15. 10.4049/jimmunol.1001483 [DOI] [PubMed] [Google Scholar]
- 117.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother (2008) 57:123–31. 10.1007/s00262-007-0336-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res (2006) 66:3294–302. 10.1158/0008-5472.CAN-05-3773 [DOI] [PubMed] [Google Scholar]
- 119.Ardon H, Verbinnen B, Maes W, Beez T, Van Gool S, De Vleeschouwer S. Technical advancement in regulatory T cell isolation and characterization using CD127 expression in patients with malignant glioma treated with autologous dendritic cell vaccination. J Immunol Methods (2009) 352(1–2):169–73. 10.1016/j.jim.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 120.Pere H, Tanchot C, Bayry J, Terme M, Taieb J, Badoual C, et al. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology (2012) 1:326–33. 10.4161/onci.18852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol (2006) 27:387–93. 10.1016/j.it.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 122.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science (2005) 309:1380–4. 10.1126/science.1113401 [DOI] [PubMed] [Google Scholar]
- 123.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother (2009) 58:1627–34. 10.1007/s00262-009-0671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim TG, Kim CH, Park JS, Park SD, Kim CK, Chung DS, et al. Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin Vaccine Immunol (2010) 17:143–53. 10.1128/CVI.00292-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, et al. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer (2013) 133(1):98–107. 10.1002/ijc.27990 [DOI] [PubMed] [Google Scholar]
- 126.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res (2008) 14:6674–82. 10.1158/1078-0432.CCR-07-5212 [DOI] [PubMed] [Google Scholar]
- 127.Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother (2010) 33:991–8. 10.1097/CJI.0b013e3181f4c208 [DOI] [PubMed] [Google Scholar]
- 128.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood (2008) 111:5610–20. 10.1182/blood-2007-02-075945 [DOI] [PubMed] [Google Scholar]
- 129.Chen CA, Ho CM, Chang MC, Sun WZ, Chen YL, Chiang YC, et al. Metronomic chemotherapy enhances antitumor effects of cancer vaccine by depleting regulatory T lymphocytes and inhibiting tumor angiogenesis. Mol Ther (2010) 18:1233–43. 10.1038/mt.2010.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Akasaki Y, Kikuchi T, Irie M, Yamamoto Y, Arai T, Tanaka T, et al. Cotransfection of poly(I: C) and siRNA of IL-10 into fusions of dendritic and glioma cells enhances antitumor T helper type 1 induction in patients with glioma. J Immunother (2011) 34:121–8. 10.1097/CJI.0b013e3181e5c278 [DOI] [PubMed] [Google Scholar]
- 131.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci (2009) 1174:99–106. 10.1111/j.1749-6632.2009.04939.x [DOI] [PubMed] [Google Scholar]
- 132.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood (2008) 112:610–8. 10.1182/blood-2008-01-135319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Vries IJ, Castelli C, Huygens C, Jacobs JF, Stockis J, Schuler-Thurner B, et al. Frequency of circulating Tregs with demethylated FOXP3 intron 1 in melanoma patients receiving tumor vaccines and potentially Treg-depleting agents. Clin Cancer Res (2011) 17:841–8. 10.1158/1078-0432.CCR-10-2227 [DOI] [PubMed] [Google Scholar]
- 134.Chu CS, Boyer J, Schullery DS, Gimotty PA, Gamerman V, Bender J, et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol Immunother (2012) 61:629–41. 10.1007/s00262-011-1081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother (2007) 56:641–8. 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol (2011) 33:369–83. 10.1007/s00281-011-0245-0 [DOI] [PubMed] [Google Scholar]
- 137.Vermeij R, Leffers N, Hoogeboom BN, Hamming IL, Wolf R, Reyners AK, et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: a single-arm phase II study. Int J Cancer (2012) 131(5):E670–80. 10.1002/ijc.27388 [DOI] [PubMed] [Google Scholar]
- 138.Huijts CM, Santegoets SJ, van den Eertwegh AJ, Pijpers LS, Haanen JB, de Gruijl TD, et al. Phase I-II study of everolimus and low-dose oral cyclophosphamide in patients with metastatic renal cell cancer. BMC Cancer (2011) 11:505. 10.1186/1471-2407-11-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother (2011) 61(3):353–62. 10.1007/s00262-011-1106-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res (2011) 71:661–5. 10.1158/0008-5472.CAN-10-1259 [DOI] [PubMed] [Google Scholar]
- 141.Kan S, Hazama S, Maeda K, Inoue Y, Homma S, Koido S, et al. Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro. Anticancer Res (2012) 32:5363–9. [PubMed] [Google Scholar]
- 142.Peng S, Lyford-Pike S, Akpeng B, Wu A, Hung CF, Hannaman D, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer Immunol Immunother (2013) 62(1):171–82. 10.1007/s00262-012-1322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moraes-Fontes MF, Rebelo M, Caramalho I, Zelenay S, Bergman ML, Coutinho A, et al. Steroid treatments in mice do not alter the number and function of regulatory T cells, but amplify cyclophosphamide-induced autoimmune disease. J Autoimmun (2009) 33:109–20. 10.1016/j.jaut.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 144.Available from: http://ec.europa.eu/health/files/advtherapies/2014_atmp/atmp_en.pdf
- 145.Polyzoidis S, Keyoumars A. DCVax(R)-L-developed by northwest biotherapeutics. Hum Vaccin Immunother (2014) 10(11):3139–45. 10.4161/hv.29276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kastenmuller W, Kastenmuller K, Kurts C, Seder RA. Dendritic cell-targeted vaccines – hope or hype? Nat Rev Immunol (2014) 14:705–11. 10.1038/nri3727 [DOI] [PubMed] [Google Scholar]
- 147.Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett (2014) 162(1 Pt A):59–67. 10.1016/j.imlet.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liau LM, Black KL, Martin NA, Sykes SN, Bronstein JM, Jouben-Steele L, et al. Treatment of a glioblastoma patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides: case report. Neurosurg Focus (2000) 9(6):e8. 10.3171/foc.2000.9.6.9 [DOI] [PubMed] [Google Scholar]
- 149.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res (2001) 61:842–7. [PubMed] [Google Scholar]
- 150.Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother (2001) 50:337–44. 10.1007/s002620100205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol (2004) 6:236–46. 10.1215/S1152851703000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res (2005) 11:5515–25. 10.1158/1078-0432.CCR-05-0464 [DOI] [PubMed] [Google Scholar]
- 153.Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med (2007) 5:67. 10.1186/1479-5876-5-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci (2008) 15:114–21. 10.1016/j.jocn.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 155.Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med (2008) 359:539–41. 10.1056/NEJMc0804818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther (2009) 8:2773–9. 10.1158/1535-7163.MCT-09-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, et al. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS One (2012) 7:e32614. 10.1371/journal.pone.0032614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lasky JL, III, Panosyan EH, Plant A, Davidson T, Yong WH, Prins RM, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res (2013) 33:2047–56. [PMC free article] [PubMed] [Google Scholar]
- 159.Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother (2013) 62(9):1499–509. 10.1007/s00262-013-1453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Akiyama Y, Oshita C, Kume A, Iizuka A, Miyata H, Komiyama M, et al. Alpha-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. BMC Cancer (2012) 12:623. 10.1186/1471-2407-12-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pellegatta S, Eoli M, Frigerio S, Antozzi C, Bruzzone MG, Cantini G, et al. The natural killer cell response and tumor debulking are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates. Oncoimmunology (2013) 2:e23401. 10.4161/onci.23401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer (2003) 89:1172–9. 10.1038/sj.bjc.6601268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, et al. Thymic CD8(+) T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol (2003) 171:4927–33. 10.4049/jimmunol.171.9.4927 [DOI] [PubMed] [Google Scholar]
- 164.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res (2004) 64:4973–9. 10.1158/0008-5472.CAN-03-3505 [DOI] [PubMed] [Google Scholar]
- 165.Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother (2004) 27:452–9. 10.1097/00002371-200411000-00005 [DOI] [PubMed] [Google Scholar]
- 166.Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res (2005) 11:4160–7. 10.1158/1078-0432.CCR-05-0120 [DOI] [PubMed] [Google Scholar]
- 167.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol (2011) 29:330–6. 10.1200/JCO.2010.30.7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci (2011) 18:1048–54. 10.1016/j.jocn.2010.11.034 [DOI] [PubMed] [Google Scholar]
- 169.Fadul CE, Fisher JL, Hampton TH, Lallana EC, Li Z, Gui J, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother (2011) 34:382–9. 10.1097/CJI.0b013e318215e300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jie X, Hua L, Jiang W, Feng F, Feng G, Hua Z. Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem Biophys (2012) 62:91–9. 10.1007/s12013-011-9265-6 [DOI] [PubMed] [Google Scholar]
- 171.Iwami K, Shimato S, Ohno M, Okada H, Nakahara N, Sato Y, et al. Peptide-pulsed dendritic cell vaccination targeting interleukin-13 receptor alpha2 chain in recurrent malignant glioma patients with HLA-A*24/A*02 allele. Cytotherapy (2012) 14(6):733–42. 10.3109/14653249.2012.666633 [DOI] [PubMed] [Google Scholar]
- 172.Cho DY, Yang WK, Lee HC, Hsu DM, Lin HL, Lin SZ, et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: a phase II clinical trial. World Neurosurg (2011) 77(5–6):736–44. 10.1016/j.wneu.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 173.Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuno MA, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother (2013) 62:125–35. 10.1007/s00262-012-1319-0 [DOI] [PMC free article] [PubMed] [Google Scholar]