Abstract
Our purpose, by modification of standard bedside tilt–testing, was to search for lesser known but important initial orthostatic hypotension (IOH), occurring transiently within the first 30 seconds of standing, heretofore only detectable with sophisticated continuous photoplethysmographic monitoring systems, not readily available in most medical facilities. In screened outpatients over 60 years of age, supine blood pressure (BP) parameters were recorded. To achieve readiness for immediate BP after standing, the cuff was re–inflated prior to standing, rather than after. Immediate, 1–, and 3–minute standing BPs were recorded. One hundred fifteen patients were studied (mean age, 71.1 years; 50.5% male). Eighteen (15.6%) had OH, of whom 14 (12.1%) had classical OH, and four (3.5%) had IOH. Early standing BP detection time was 20.1 ± 5.3 seconds. Immediate transient physiologic systolic BP decline was detected in non–OH (−8.8 ± 9.9 mm Hg; P < .0001). In contrast to classical OH (with lesser but persistent orthostatic BP decrements), IOH patients had immediate mean orthostatic systolic/diastolic BP change of −32.8 (±13.8) mm Hg/−14.0 (±8.5) mm Hg (P < .02), with recovery back to baseline by 1 minute. Two of the four IOH patients had pre–syncopal symptoms. For the first time, using standard inflation–deflation BP equipment, immediate transient standing physiologic BP decrement and IOH were demonstrated. This preliminary study confirms proof of principle that manual BP cuff inflation prior to standing may be useful and practical in diagnosing IOH, and may stimulate direct comparative studies with continuous monitoring systems.
Keywords: Falls, orthostatic hypotension, pre-syncope, syncope
Introduction
Orthostatic hypotension (OH) may be associated with falls, injury, and sometimes death in the elderly and in patients with multiple co–morbidities.1–3 Prevalence appears to range from 5% beyond age 65 to over 20% in the very elderly.3–5 Blood pressure (BP) homeostasis upon standing depends on baseline peripheral vascular resistance and sympathetic reflex response, cardiac pump status and cardiac reflex response, volume status, and skeletal muscle pump (lower body muscles compressing veins). Underlying causes of OH thus may include baseline vasodilation (as may be seen with medications), autonomic dysfunction, pump failure, excessive venous pooling with deconditioning, or true volume depletion.6–8 By consensus guideline criteria, classical orthostatic hypotension (COH) is defined as a sustained reduction in systolic BP (SBP) of ≥20 mm Hg and/or diastolic BP (DBP) fall of ≥10 mm Hg within 3 minutes of standing, and is clinically important if there are associated symptoms of cerebral hypoperfusion.8
Initial orthostatic hypotension (IOH) is a lesser known form of consensus OH, described in adolescent patients and the elderly, which represents an aberration of normal physiologic events occurring upon active standing.9–13 It is defined as a reduction in SBP of >40 mm Hg and/or decrease in DBP of >20 mm Hg, presenting within the first 15 seconds of standing, and correcting within 30–60 seconds.8 As background, all humans may experience an immediate and occasionally symptomatic transient decrease in BP just after standing (nadir at 5–15 seconds), with recovery by 30 seconds. This normal physiologic phenomenon is not completely clarified but involves lower body muscle contraction and increased right atrial pressure (with initial neurovascular reflex changes) during the act of standing, immediate blood pooling below the diaphragm induced by gravity after completion of stand, and subsequent rapid sympathetic responses further affecting cardiac output and peripheral resistance. Thus, rarely does one experience pre–syncopal symptoms immediately after standing. However, if there is development of transient dramatic enhancement of early volume shifts associated with a temporal mismatch of cardiovascular compensation on standing, symptomatic IOH may occur consistently.9–11 Elderly patients with underlying IOH physiology may be particularly susceptible to pre–syncopal symptoms, since separate and unrelated volume and distributive issues are common.13,14
IOH has been previously discernible and defined only by use of continuous non–invasive beat–to–beat finger photoplethysmographic BP monitoring systems (Finometer/Finapres).12 The sophistication and expense of these devices has limited testing for IOH and awareness of this entity. The standard bedside tilt–test, using inflation–deflation methods, has been incapable of detecting immediate standing BP changes (IOH) due to time required to inflate the cuff after completion of standing.15 In addition, routine formal tilt–table testing does not detect IOH since the gradual rise of the table does not replicate the physiology of the acute act of standing.12 Hence, detection of IOH is problematic in most clinical settings. We sought to detect IOH by novel use of standard BP equipment involving supine cuff inflation prior to standing. Detection of an early transient fall in BP within physiologic parameters would serve as validation of this method. A dramatic early fall in BP upon standing, which corrects within 30–60 seconds, with or without symptoms, would be consistent with IOH.8 Symptomatic patients may respond dramatically to readily performed counter–pressure maneuvers (see below).
Methods
Study approval was obtained from the Charleston Area Medical Center/West Virginia University HSC, Charleston Division Institutional Review Board. This was a prospective study of outpatients, who were recruited at clinic sign–in to participate in screening for BP changes upon standing. They were not initially told about what kind of BP changes might be expected, and possible orthostatic symptoms were not discussed prior to the procedure to avoid patient bias in reporting. Patients were excluded who were under 60 years of age, non–ambulatory (ie, those not able to walk or stand on their own), or who had history of orthostatic hypotension. Records were reviewed regarding basic demographics. The study was conducted during clinic hours, duplicating real–world conditions. Wall–mounted WelchAllyn Tycos aneroid sphygmomanometry was utilized. Large cuffs were used appropriately in obese patients. Investigators were trained in basic uniform BP recording technique.15,16 Following informed consent, patients were maintained in the supine position for between 5 and 10 minutes. Baseline lying and standing BP and heart rate (HR) were recorded in the right arm with midline of cuff located in line with the brachial artery. A pulse oximeter device was used to provide HR throughout the standing process. To achieve readiness for BP measurement just after standing, the cuff was re–inflated in the supine position to 180 mm Hg. Patients were then asked to stand, with some assistance if necessary since right arm was extended. Immediately following completion of stand, BP, HR, and time to completion were recorded. Subsequent BP and HR were recorded at approximately 1 and 3 minutes. Patients were then queried for any symptoms such as “dizziness, wooziness, or faintness” occurring upon standing. Patients were grouped as non–OH (normal), COH, or IOH based on BP changes by the above criteria. Patients meeting COH criteria were evaluated for underlying causes and managed appropriately. Those meeting IOH criteria were provided with information on physical counter–pressure maneuvers to minimize orthostatic symptoms.
Statistical Analysis
To evaluate supine cuff inflation for detecting early standing BP changes, statistical differences were assessed assuming zero change in initial BP after standing. Basic descriptive statistics such as means and standard deviations for continuous variables and proportions and frequencies for categorical variables were used to analyze the data. The paired samples t–test was conducted to compare BPs and HRs from baseline to immediate, 1–minute, and 3–minute intervals. Comparisons were made for differences in baseline BP and HR in non–OH versus COH and non– OH versus IOH; for standing BP changes from baseline in all groups; and for differences in symptoms in non–OH versus OH (COH plus IOH) upon standing. Data are reported as mean (standard deviation [SD]). All data were analyzed using SAS 9.3 software. A P value of < .05 was used to determine statistical significance.
Results
One hundred fifteen patients were studied (mean age, 71.1 years; 50.5% male). Thirty–one percent had type 2 diabetes mellitus, 80% had hypertension, and the mean number of medications prescribed per patient was nine. Table 1 summarizes baseline BP data. Ninety–seven patients (84.3%) had no OH, and 18 (15.6%) had OH. Of the OH patients, 14 (12.1%) had COH, and four (3.5%) had IOH (Table 2). Recent falls were reported in four (22.2%) of OH patients and 17 (17.5%) of non–OH patients (P = .63). Mean time to completion of early BP determination after active stand was 20.1 ±5.3 seconds (Figure 1). In non–OH, immediate mean change in (Δ) SBP was −8.8 (±9.9) mm Hg (P < .0001), with recovery toward baseline by 1 and 3 minutes to −2.0 (±9.1) mm (P < .04), and +0.9 (±8.4) mm (P = .30). No statistically significant immediate decline in DBPs was detected. Those with COH had mean immediate, 1–minute, and 3–minute DSBP/DDBP of −25.7 (±15.5) mm/−7.4 (±9.2) mm; −25.8 (±13.8) mm/−6.8 (±9.5) mm; and −21.4 (±14.5) mm/−6.8 (±9.6) mm (P < .0001 from baseline for all). Patients with IOH had immediate mean ΔSBP/ΔDBP from baseline of −32.8 mm (±13.8)/−14.0 (±8.5) mm (P < .02). One–minute and 3–minute ΔSBP/ΔDBP was not statistically different from baseline. Two of the four IOH patients had pre–syncopal symptoms on standing. Of outpatients screened, six (33.3%) OH patients (COH plus IOH) had pre–syncopal symptoms on standing versus 14 (14.4%) non–OH patients (P < .05).
Table 1. Baseline supine blood pressure (BP) and heart rate (HR) characteristics.
| Diagnosis | No. (%) | Systolic BP mm Hg Mean (SD) | Diastolic BP mm Hg Mean (SD) | HR bpm Mean (SD) |
|---|---|---|---|---|
| Non–OH | 97 (84.3) | 139.9 (±18.5)* | 79.1 (±11.7) | 72.6 (±11.3) |
| COH | 14 (12.2) | 151.1 (±17.9) | 81.8 (±11.1) | 71.3 (±11.1) |
| IOH | 4 (3.5) | 164.3 (±14.6) | 84.0 (±13.6) | 75.5 (±13.9) |
bpm, beats per minute; COH, classical orthostatic hypotension; HR, heart rate; IOH, initial orthostatic hypotension; Non–OH, no orthostatic hypotension; SD, standard deviation.
Non–OH systolic BP was statistically divergent from COH (P = .04) and IOH (P < .01).
Table 2. Blood pressure (BP) and heart rate (HR) changes on standing from supine position.
| Diagnosis | No. (%) | Time | ΔSystolic BP mm Hg Mean (SD) | P Value | Time | ΔHR bpm Mean (SD) | P Value |
|---|---|---|---|---|---|---|---|
| Non–OH | 97 (84.3) | ||||||
| <20 sec | −8.8 (±9.9)* | <.0001 | <20 sec | +4.4 (±7.8) | <.0001 | ||
| 1 min | −2.0 (±9.1) | .04 | 1 min | +6.0 (±6.6) | <.0001 | ||
| 3 min | ±0.9 (±8.4) | .30 | 3 min | +5.3 (+7.8) | <.0001 | ||
| COH | 14 (12.2) | ||||||
| <20 sec | −25.7 (±15.5) | <.0001 | <20 sec | +6.6 (±7.8) | .006 | ||
| 1 min | −25.8 (±13.8) | <.0001 | 1 min | +6.0 (±8.6) | .02 | ||
| 3 min | −21.4 (±14.5) | <.0001 | 3 min | +6.3 (±7.2) | .006 | ||
| IOH | 4 (3.5) | ||||||
| <20 sec | −32.8 (±13.8)† | .02 | <20 sec | +8.5 (±13.1) | .29 | ||
| 1 min | −3.0 (±6.8) | .44 | 1 min | +4.0 (±5.7) | .25 | ||
| 3 min | +3.8 (±6.6) | .34 | 3 min | +4.0 (±3.3) | .09 | ||
bpm, beats per minute; COH, classical orthostatic hypotension; HR, heart rate; IOH, initial orthostatic hypotension; Non–OH, no orthostatic hypotension; SD, standard deviation.
Immediate physiologic BP decrement in non–OH.
Two with IOH met systolic criteria, two met diastolic criteria.
Figure 1.
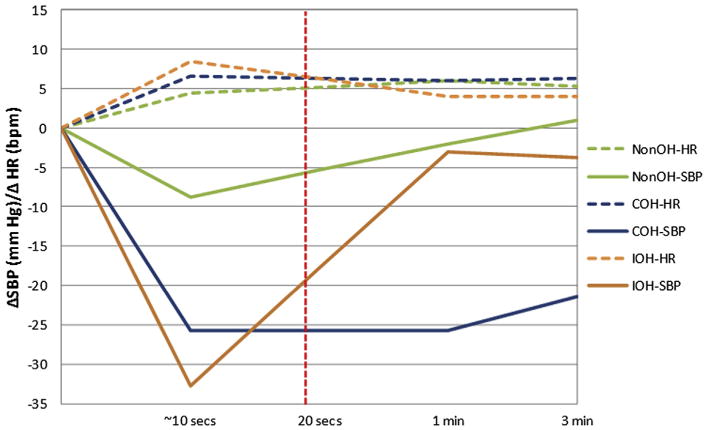
Recorded change in systolic blood pressure (ΔSBP) and heart rate (ΔHR) within 20 seconds of active standing. bpm, beats per minute; COH, classical orthostatic hypotension; IOH, initial orthostatic hypotension; Non–OH, non–orthostatic hypotension.
Discussion
Orthostatic hypotension is a common cause of falls, injury, and risk for death. Presentation is particularly hazardous in the elderly and patients with multiple co– morbidities.1–3,13,14 On occasion, patients will present with orthostatic symptoms but negative bedside tilt–testing and formal tilt–table testing. This discrepancy may be explained by the presence of IOH with exaggerated initial standing physiologic BP decline (involving acute volume fluxes and compensatory timing).13,14 Delineation of IOH is important because physical counter–pressure maneuvers can be utilized that may prevent symptoms and possible falls.17–19 These include leg extension exercises before rising, and leg and lower body muscle tensing continued beyond initial period of standing, to minimize lower body venous pooling. In addition, even without symptoms, detection of IOH BP changes may nonetheless portend falls if patients develop even minor distributive or absolute volume changes and/or autonomic dysfunction.
In this prospective cohort of consecutive elderly patients offered screening, OH prevalence (COH and IOH) was 15.6%, consistent with previous studies.3–5 Those with positive OH screening had significantly more symptomatology, with about one in three having pre–syncopal symptoms versus one in seven with negative OH screening. Of OH patients, 3.4% were found to have IOH, with BP correction (unlike COH) by first recordable repeat reading at approximately 1 minute. With an abnormally large initial BP reduction, these screened patients were felt to be at increased fall risk, and, in fact, two of the four IOH patients were symptomatic. They were instructed on physical counter–pressure maneuvers.17–19 The remaining patients had COH (12.2%), with persistent decrement in BP at 1 and 3 minutes. Evaluation for underlying causes was undertaken, and patients were appropriately managed.
IOH has only been described and defined by the use of expensive continuous finger photoplethysmographic monitoring systems (Finometer/Finapres),12 not available in most offices and hospitals. Standard inflation–deflation BP systems (mercury and aneroid) heretofore have not been shown to detect changes within the first 20 seconds of standing due to time required for cuff inflation at completion of stand. Digital oscillatory systems have not been studied for this application, but may be an area for future research. Our purpose, by simple inflation of an aneroid cuff system before standing, was to evaluate this method and search for what might be the relative screened prevalence of IOH in an older outpatient population. Confirmation of a significant initial physiologic BP decrement upon standing in non–OH patients seems to substantiate this method. Hence, such an approach may be satisfactory and practical to confirm possible IOH in patients with orthostatic symptoms but negative standard 1– and 3–minute bedside tilt–testing. In addition, detection of IOH may obviate further costly and unnecessary diagnostics, including formal tilt–table testing, which may be unrevealing in this context.
Limitations
This approach for detection of early standing BP changes has not been directly compared with continuous monitoring systems. A pre–inflated cuff method might be satisfactory for detecting the exaggeration of the initial BP fall in some patients with IOH, though less sensitive without beat–to–beat data points. Also, in this study, there were differences in baseline SBP between groups, which might affect comparative timing of SBP nadir detection upon standing, and could make IOH findings less pronounced proportionally in comparison to the other groups. In addition, DBP nadir was detected later in initial BP recording cycle (completed at about 20 seconds) and thus may show a smaller decrement from baseline due to some degree of BP recovery at that point. Thus sensitivity in detecting diastolic IOH may be adversely affected. A pre–set inflation of 20 mm Hg above baseline BP (rather than 180 mm Hg) may have been preferable in order to shorten detection time and better standardize technique. Repetition of the full cycle of orthostatic BP determinations was not consistently accomplished due to time constraints in a screening study necessarily carried out during active clinic hours, and reflecting real–world conditions. Statistical accuracy may thus have been affected. A precise history of timing and duration of symptoms, which may have provided suspicion of IOH versus COH, was not consistently obtained. We did see a significant statistical correlation between non–specific reported standing pre–syncopal symptoms and detected consensus OH (COH plus IOH). Finally, there is a brief learning curve involved in obtaining orthostatic BPs by this method.
Conclusion
Screened orthostatic hypotension was common in this prospective cohort of consecutive elderly outpatients, and included both COH and IOH. Lesser known IOH is important, and has previously not been detectable by standard methods. This preliminary study confirms proof of principle that manual BP cuff inflation prior to standing can detect this entity. If IOH is diagnosed, specific physical counter–pressure maneuvers may prevent falls and injury, and further diagnostic testing may be avoided. Future research may refine the diagnostic yield of this BP recording concept by comparison of continuous monitoring systems with both aneroid sphygmomanometry and digital oscillatory systems.
Footnotes
Conflict of interest: none.
References
- 1.Benvenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24:135–44. doi: 10.1038/ajh.2010.146. [DOI] [PubMed] [Google Scholar]
- 2.Mussi C, Ungar A, Salvioli G, Menozzi C, Bartoletti A, Giada F, et al. Orthostatic hypotension as a cause of syncope in patients older than 65 years admitted to emergency departments for transient loss of consciousness. J Gerontol A Biol Sci Med Sci. 2009;64:801–6. doi: 10.1093/gerona/glp028. [DOI] [PubMed] [Google Scholar]
- 3.Low A. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18(Suppl 1):8–13. doi: 10.1007/s10286-007-1001-3. [DOI] [PubMed] [Google Scholar]
- 4.Masaki KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–5. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 5.Rutan GH, Hermanson R, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The cardiovascular health study. CHS Collaborative Research Group. Hypertension. 1992;19(6 pt 1):508–19. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 6.Mukai S, Lipsitz LA. Orthostatic hypotension. Clin Geriatr Med. 2002;18:253–68. doi: 10.1016/s0749-0690(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 7.Lanier JB, Mote MB, Clay EC. Evaluation and management of orthostatic hypotension. Am Fam Phys. 2011;84:527–36. [PubMed] [Google Scholar]
- 8.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally related syncope, and postural tachycardia syndrome. Auton Neurosci. 2011;161:46–8. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Wieling W, Dambrink JH, Borst C. Cardiovascular effects of arising suddenly. N Engl J Med. 1984;310:1189. doi: 10.1056/nejm198405033101816. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JM, Clark D. “He's dizzy when he stands up”: an introduction to initial orthostatic hypotension. J Pediatr. 2011;158:499–504. doi: 10.1016/j.jpeds.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka H, Yamaguchi H, Matushima R, Tamai H. Instantaneous orthostatic hypotension in children and adolescents: a new clinical entitiy of orthostatic intolerance. Pediatr Res. 1999;46:691–6. doi: 10.1203/00006450-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Wieling W, Krediet CTP, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 2007;112:157–65. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 13.Imholz BPM, Dambrink JHA, Karenaker JM, Wieling W. Orthostatic circulatory control in the elderly evaluated by continuous non-invasive blood pressure measurement. Clin Sci (Lond) 1990;79:73–9. doi: 10.1042/cs0790073. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Ortuno R, Med L, Cogan L. Continuous non-invasive blood pressure measurements and their relationship with orthostatic intolerance, falls and frailty in older people. J Am Geriatr Soc. 2011;59:655–65. doi: 10.1111/j.1532-5415.2011.03352.x. [DOI] [PubMed] [Google Scholar]
- 15.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals, part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 16.Beevers G, Lip GYH, O'Brien E. ABC of hypertension: blood pressure measurement part II – conventional sphymomanometry: technique of auscultatory blood pressure measurement. BMJ. 2001;322:1043–7. doi: 10.1136/bmj.322.7293.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krediet CT, Go-Schon IK, Kim Y, Linzer M, Van Lieshout JJ, Wieling W. Management of initial orthostatitic hypotension: lower body muscle contraction attenuates the transient arterial blood pressure decrease upon standing from squatting. Clin Sci (Lond) 2007;113:401–7. doi: 10.1042/CS20070064. [DOI] [PubMed] [Google Scholar]
- 18.vanDijk N, deBruin IGJM, Gisolf J, Rianne de Bruin-Bon HACM, Linzer M, van Lieshout JJ, et al. Hemodynamic effects of leg crossing in patients with vaso-vagal syncope. J Appl Physiol. 2005;98:584–90. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]
- 19.van Lieshout JJ, Pott F, Madsen PL, van Goudoever J, Secher NH. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. 2001;32:1546–51. doi: 10.1161/01.str.32.7.1546. [DOI] [PubMed] [Google Scholar]


