Abstract
Background
Bone morphogenetic proteins (BMPs) play a sentinel role in osteoblastic differentiation, and their implementation into clinical practice can revolutionize cranial reconstruction. Preliminary data suggest a therapeutic role of adenoviral gene delivery of BMPs in murine calvarial defect healing. Poor transgene expression inherent in direct adenoviral therapy prompted investigation of cell-based strategies.
Objective
To isolate and immortalize calvarial cells as a potential progenitor source for osseous tissue engineering.
Materials & Methods
Cells were isolated from murine skulls, cultured, and transduced with a retroviral vector bearing the loxP-flanked SV40 large T antigen. Immortalized calvarial cells (iCALs) were evaluated via light microscopy, immunohistochemistry, and flow cytometry to determine whether the immortalization process altered cell morphology or progenitor cell profile. iCALs were then infected with adenoviral vectors encoding BMP-2 or GFP and assessed for early and late stages of osteogenic differentiation.
Results
Immortalization of calvarial cells did not alter cell morphology as demonstrated by phase contrast microscopy. Mesenchymal progenitor cell markers CD166, CD73, CD44, and CD105 were detected at varying levels in both primary cells and iCALs. Significant elevations in alkaline phosphatase activity, osteocalcin mRNA transcription, and matrix mineralization were detected in BMP-2 treated iCALs compared to GFP treated cells. Gross and histological analyses revealed ectopic bone production from treated cells compared to controls in an in vivo stem cell implantation assay.
Conclusion
We have established an immortalized osteoprogenitor cell line from juvenile calvarial cells that retain a progenitor cell phenotype and can successfully undergo osteogenic differentiation upon BMP-2 stimulation. These cells provide a valuable platform to investigate the molecular mechanisms underlying intramembranous bone formation and to screen for factors/small molecules that can facilitate the healing of osseous defects in the craniofacial skeleton.
Keywords: Bone morphogenetic proteins, bone morphogenetic protein–2, immortalized calvarial cells, mesenchymal progenitor cells
Introduction
The difficulties with bony regeneration in the adolescent and adult skull [1, 2] have prompted surgeons to seek new biomaterials to hasten the ossification process. A number of biomaterials such as demineralized bone, hydroxyapatite, polymer composites and acrylic resins have been utilized but with only limited success. Autologous bone transplantation has been the most widely used therapy to date. There is a limit, however, to how much bone can be harvested. The added risk of resorption of transplanted bone and the reduced biomechanical strength of grafts has driven the search for better strategies [3].
One such potential mode of therapy is the application of bone morphogenetic proteins (BMPs) to calvarial defects. BMPs are members of the TGF-β family of extracellular signaling molecules and activate the intracellular SMAD pathway via serine/threonine kinases [4]. Multiple studies have shown the osteogenic potential of BMPs, which ultimately led to the US Food and Drug Administration approval of recombinant human forms (rhBMP) of BMP-2 and BMP-7 for use in spinal fusions and tibial non-unions, respectively [5, 6]. Restricted approval exists currently for select indications in the craniofacial skeleton (e.g., BMP-2 in alveolar clefts). Although not yet fully approved for the entire craniofacial skeleton, in vitro and in vivo studies have been promising regarding the use of BMPs and their induction of osteogenesis of explanted calvarial cells and defect closure [7, 8]. Smith et al. demonstrated that the treatment of large scale calvarial defects in rabbits with rhBMP-2 induced complete resolution of defects within six weeks [8].
Although promising and seemingly effective, rhBMP therapy has multiple disadvantages: namely, the requirement of supraphysiologic concentrations and low biological activity due to high rates of clearance from the defect site [9]. High associated costs and difficulty of production are also potential factors limiting their use.
An alternative mode of delivering BMPs is via adenoviral vector technology. This form of gene therapy enables delivery of recombinant BMP DNA to cells in the defect site [10]. Engineered cells can then synthesize and secrete their own endogenous BMPs and supply the extracellular environment with a continuous concentration of osteo-inductive signaling factors without the need of reapplication. Multiple studies have shown the osteo-inductive ability of AdBMPs with Cheng et al. demonstrating AdBMP-2 and AdBMP-9 to be the most potent inducers of early and late markers of osteogenesis in osteoblastic progenitor cell lines [11].
Given this, our preliminary work focused on the use of AdBMP-2 in healing critical-sized calvarial defects. Direct transfer of AdBMP-2 into critically sized (4-mm) parietal defects yielded enhanced, yet suboptimal osseous healing of the defects 20-weeks post treatment compared to AdGFP-injected controls [12, 13]. The limitations of this tissue engineering strategy, which include poor viral uptake and transgene expression in native recipient site cells, the proinflammatory response of adenovirus [10], and lack of a suitable bioscaffold to promote osteoconduction, attributed to the marginal osteogenesis witnessed in vivo. Such preliminary results spawned investigation of cell-based strategies, which could potentially lead to a more stable delivery of osseous regeneration. Employing Tessier's concept of self-sufficiency [14, 15], we hypothesize that the calvarium itself would be a prime source of progenitor cells for tissue engineering of defects in the traumatized patient. We further postulate that these cells can be expanded, immortalized as osteoprogenitor cells, and modified ex vivo to confer a stable osteogenic phenotype.
Materials & Methods
Isolation and Culture Calvarial Cells
Calvariae were isolated from three-week old male CD-1 mice (Charles River, Wilmington, MA, USA). Mice were housed in standard cages in an experimental animal room (24°C, 55% humidity, 1atm, 12h light/dark cycle) and were fed a standard laboratory diet and water ad libitum. This investigation was approved by the Institutional Animal Care and Use Committee of the University of Chicago (Chicago, IL), and animal maintenance and experimental treatments were conducted in accordance with the ethical guidelines set forth by this committee. All procedures were conducted under sterile conditions.
Mice were sacrificed and calvariae were harvested by creating a mid-sagittal incision. The periosteum was incised to expose the calvarium on both sides of the midline. Soft tissue, dura and remaining periosteum were removed. The isolated calvaria were washed repeatedly in phosphate buffered saline (PBS) with 1% penicillin/streptomycin (p/s) solution, minced and transferred to 10mm2 wells containing regular DMEM supplemented with 10% fetal bovine serum (FBS) and 1% p/s solution (Sigma Aldrich, St. Louis, MO, USA). Cultures were incubated at 37°C, 95% humidified air, and 5% CO2. After approximately seven days, cells grew to 80% confluency (% of cells covering the plate) at which point they were passaged by enzymatic digestion (0.1% Trypsin, Sigma Aldrich) to 25cm2 flasks containing 8ml of DMEM with 10% FBS and 1% p/s for experimentation.
Immortalization of Primary Calvarial Cells
To allow for ease of culturing and preservation of cellular growth, harvested primary calvarial cells were allowed to grow in culture for 5 weeks and then underwent immortalization using a retroviral-mediated vector as previously described [16]. All further in vitro experimentation was performed using these immortalized calvarial cells (iCALs).
Recombinant Adenoviral Vectors
Recombinant adenoviruses were generated using AdEasy technology as previously described [17-20]. The coding regions of human BMP-2 were PCR amplified and cloned into an adenoviral shuttle vector and subsequently used to generate recombinant adenoviruses in HEK293 cells (American Type Culture Collection, Manassas, Virginia). The resulting adenoviruses were designated as AdBMP-2. Green fluorescence protein (GFP), which served as a marker of infection efficiency, was included in the vector. Analogous adenovirus expressing only monomeric GFP (AdGFP) was used as a control. Serial titrations of the adenoviruses were performed to determine the viral dosage that optimized viral infection and cell viability. All samples were exposed to virus for up to twenty-four hours prior to changing the medium. Immortalized cells were transduced with AdBMP-2 or AdGFP.
Immunohistochemistry
In order to confirm the identity of harvested cells as mesenchymal progenitors, immunohistochemical staining (IHC) for mesenchymal stem cell (MSC) markers CD-105 (Endoglin), CD-166 (ALCAM), and CD-133 was performed. Briefly, harvested cells were seeded onto 12-well cell culture plates and allowed to reach 100% confluency. They were fixed with 10% formalin and washed twice with PBS. Staining was performed using anti-CD105 mouse monoclonal antibody, goat anti-CD133 polyclonal antibody, and rabbit anti-CD166 polyclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Respective biotin-conjugated secondary antibodies were applied at a dilution of 1:600 (Santa Cruz Biotechnology) and incubation with horseradish peroxidase-strepavidin was performed at 1:300 dilution (Santa Cruz Biotechnology). The presence of the proteins of interest was visualized by DAB staining. Minus primary antibodies were used as negative controls.
Immunofluorescence Staining
Immunofluorescence staining was conducted on the immortalized mouse calvarial cells. Cells were seeded in 24-well plates and fixed with ice-cold methanol in -20°C for 15 min, washed twice with cold PBS and permeablized with 20% IGEPAL (in PBS) at room temperature for 10 mins. Cells were blocked 3%BSA at room temperature for 60 minutes, followed by incubation with specific mesenchymal stem cell marker antibodies including CD-44, CD-73, CD-90, and CD-105 (Santa Cruz Biotechnology) at room temperature for one hour, and then with anti-mouse-FITC secondary antibody at room temperature for 30 minutes. Fluorescence images were recorded under an inverted fluorescence microscope.
Flow Cytometry
Primary cells isolated from the calvariae of juvenile CD-1 mice and iCALs were used. Cells were plated at subconfluent conditions and harvested at 48h, washed twice with PBS, and stained with antibodies for mesenchymal stem cell markers including CD-44, CD-73, CD-90, and CD-105 (Santa Cruz Biotechnology). The stained cells were subjected to flow cytometry (Becton-Dickinson). Flow cytometry levels of each antigen were controlled by the IgG isotype control.
Alkaline Phosphatase (ALP) activity
Cells were seeded into 24-well cell culture plates at a density of 2x104 cells/cm2 and cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin for 3, 7, 9, 11 and 13 days with AdBMP-2 and AdGFP (control). ALP activity was assessed quantitatively with a modified assay using the Great Escape SEAP Chemiluminescence assay kit (BD Clontech, Mountain View, CA) and qualitatively with histochemical staining assay (using a mixture of 0.1 mg/mL napthol AS-MX phosphate and 0.6 mg/mL Fast Blue BB salt) as described [11, 21]. Each assay condition was performed in triplicate and the results were repeated in at least three independent experiments. ALP activity was normalized by total cellular protein concentrations among the samples.
Alizarin S Red Staining
Immortalized calvarial cells were plated into 12-well tissue culture plates at a density of 2 x 104 cells/cm2 and cultured for 14 and 21 days with DMEM containing ascorbic acid (50μg/mL) and β-glycerophosphate (10mM) with AdBMP-2 and AdGFP treatments. Fresh culture medium was added to each well every 4 days and nodule formation was routinely checked by phase contrast microscopy. The presence of mineralized nodules was determined by Alizarin red staining as described by Cheng et al. [11]. Briefly, cells were washed with 1% PBS and fixed with 2.5% glutaraldehyde at room temperature for 10 minutes. Fixed cells were incubated with 0.4% alizarin red S (Sigma-Aldrich) for fifteen minutes, followed by washing with distilled water. Stained cells were further incubated with PBS for 5 minutes. Mineralization was assessed via bright field microscopy.
RNA isolation and semi-quantitative RT-PCR
Total RNA was isolated using TRIZOL Reagents (Invitrogen, Carlsbad, CA, USA) and cDNA was generated utilizing reverse transcription with random hexamers and M-MuLV Reverse Transcriptase (New England Biolabs, Ipswich, MA). First strand cDNA products were further diluted 5- to 10-fold and used as PCR templates. Semi-quantitative RT-PCR was carried out using qPCR primers (∼18 bps) designed by using primer sets for osteocalcin (OCN) and GAPDH. GAPDH was used as a control (approximately 150-180 bps). Primers used were as follows: OCN 5′-CCAAGCAGGAGGGCAATA-3′; antisense, 5′-TCGTCACAAGCAGGGTCA-3′; GAPDH: sense, 5′- ACCCAGAAGACTGTGGATGG-3′; antisense, 5′-CACATTGGGGGTAGGAACAC-3′. PCR amplification was carried out with the following program: denature at 92°C for 20 seconds, annealing at 55°C, 17 cycles for GAPDH and 22 cycles for OCN. The PCR products were separated via a 1% agarose gel with 3 μl of ethidium bromide (10 mg/ml solution) for 15 min at room temperature at 80 V. Resulting bands were analyzed in a Kodak image station 440-CF using Kodak 1D 3.6 software.
Osteopontin (OPN) IHC Staining
To confirm the presence of OPN upregulation in AdBMP-2 treated iCALs, IHC staining on post-infection day 8 was performed as described above. Specifically, rabbit anti-OPN polyclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used.
Stem Cell Implantation Assay
iCALs were infected with adenovirus encoding BMP-2 or GFP as indicated. At 16 hours post-infection, cells were harvested, resuspended in PBS, and injected (5 x 106 cells/injection) into the flanks of athymic (nu/nu) mice (4-6 week-old males, Harlan Sprague-Dawley). At 6 weeks post-implantation, the mice were euthanized and the implantation sites were harvested and analyzed via histochemical staining.
Histology
Retrieved tissues were fixed in 10% formalin (decalcified, if necessary) and embedded in paraffin. Serial sections of the embedded specimens were stained with hematoxylin and eosin (H&E). Paraffin-embedded sections were deparaffinized and then rehydrated in a graduated fashion. The deparaffinized samples were subjected to antigen retrieval and fixation. The sections were stained with H&E and Masson's trichrome. For calculating the percentage of trabecular bone area over total area for each treatment group, at least three to five random high-powered fields (HPFs) for each treatment group were analyzed using the NIH ImageJ software.
Statistical Analysis
Statistical analysis was performed using SPSS software (IBM, Chicago, IL, USA). All values are reported as mean ± standard deviation. Differences between two groups were compared using a student's unpaired t-test, and more than two groups were compared with single factor ANOVA. P<0.05 was considered statistically significant.
Results
Characterization of the Immortalized Mouse Calvarial Cells (iCALs)
Primary calvarial cells were observed migrating outwards from the bony pieces at approximately 3 days. Initially, the cells were small, round and loosely attached to the plate. As culture time increased, the cells attached to the plate, grew larger and developed multiple projections. Cells proliferated to reach a peak confluence of 80-100% after 10-12 days (Figures 1A-D). To allow for ease of culturing and preservation of cellular growth, harvested primary calvarial cells were allowed to grow in culture for 5 weeks and then underwent immortalization using a retrovirus-based SV40 large T antigen expression vector (Figure 2A). Primary calvarial cells (pre-transduction) and immortalized calvarial cells (post-transduction) exhibited similar morphology on phase microscopy (Figure 2B).
Figure 1. Phase contrast micrographs of cell isolates from a mouse calvarium.
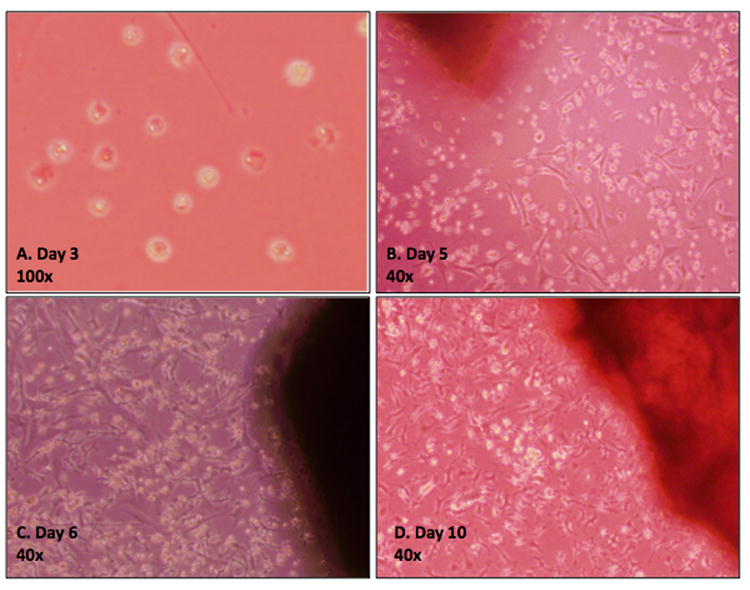
A) Day 3 image demonstrating the migration of cuboidal-shaped cells from harvested bone. B) Day 5 image demonstrating adherence of the cells to the plastic culture plates and acquisition of a stromal cell morphology. C) Day 7 and D) Day 10-12 images demonstrating 50% and 80-100% confluency, respectively. Cells were subsequently passaged into 25mm2 flasks.
Figure 2. Retroviral transduction of calvarial cells with loxP-flanked SV40/large T antigen.
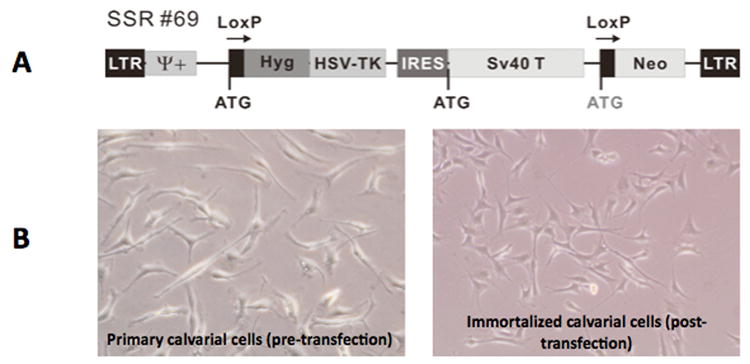
A) Sequence map. B) Phase contrast micrographs of calvarial cells before and after transduction.
Immunohistochemistry performed on day 21 of culture confirmed positive staining for MSC markers CD105, CD133, and CD166 in the harvested calvarial cells compared to the negative control group (Figure 3). Additionally, to determine the level of contaminating osteoclastogenic cells, a TRAP assay was performed on day 21 post-harvest, which did not reveal a significant population of differentiated osteoclasts (data not shown). Further survey for constitutive expression of cell surface markers revealed other findings consistent with a progenitor cell phenotype. Compared to unstained controls, immortalized calvarial cells stained with antibodies for CD-44, CD-73, and CD-105 expressed increased immunofluorescence (Figure 4). Immortalized calvarial cells stained with antibodies for CD-90 did not demonstrate increased immunofluorescence compared to negative controls (Figure 4).
Figure 3. Immunohistochemical staining of immortalized calvarial cells (iCALs) for stem cell markers.
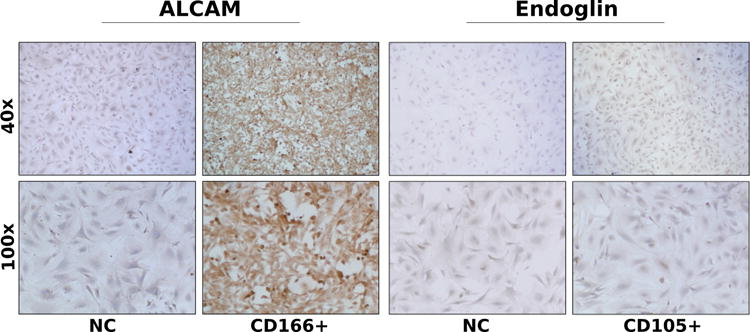
Representative images of anti-CD166 (ALCAM) staining (left) and anti-CD105 (Endoglin) staining (right). NC, negative control.
Figure 4. Immunofluorescent staining of iCALs for stem cell markers.
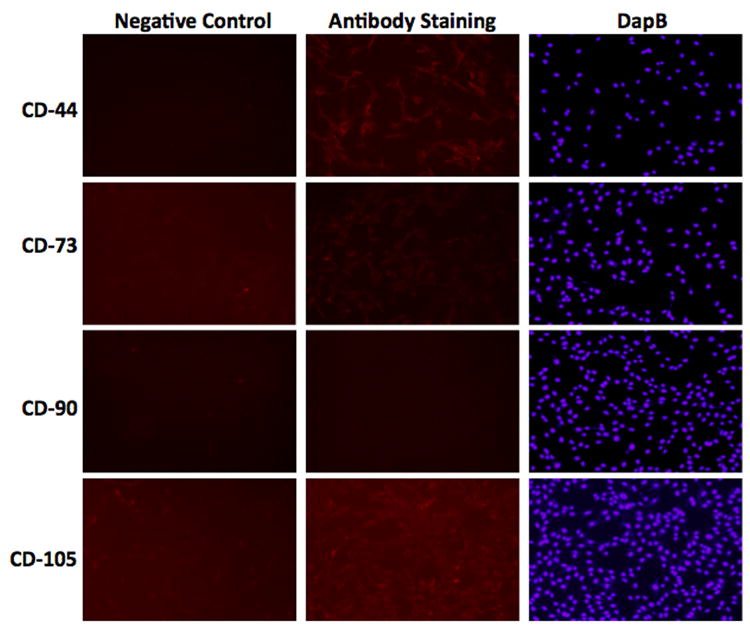
Representative images of anti-CD44, anti-CD73, anti-CD90, and anti-CD105 staining. Left column denotes negative control (background, no antibody; NC); center column denotes experimental group (antibody staining); right column denotes DapB intranuclear staining.
Cells were also analyzed by flow cytometry (Table 1). Using fluorescence-activated cell sorting (FACS), 2.67% and 9.01% of primary calvarial cells expressed CD-44 and CD-73, respectively. Primary calvarial cells did not significantly express CD-90 or CD-105. Also using FACS, 23.8% and 4.06% of iCALs expressed CD-44 and CD-73, respectively. Similar to primary calvarial cells, immortalized calvarial cells did not significantly express CD-90 or CD-105 on FACS analysis. Although discrepancies exist between our immunohistochemical, immunofluorescence and flow cytometry results in terms of CD-90 and -105 expression, these results strongly suggest that the iCAL population is heterogeneous, and most likely bears cell types along the entire osteoblastic lineage.
Table 1. % of cells expressing mesenchymal stem cell markers using fluorescence-activated cell sorting.
| CD44 | CD73 | CD90 | CD105 | |
|---|---|---|---|---|
| Primary calvarial cells | 2.67 | 9.01 | .89 | .43 |
| Immortalized calvarial cells | 23.8 | 4.06 | .09 | .35 |
Adenovirus-mediated transgene expression
All adenovirally treated cells showed equal GFP expression at 24 hours post infection, which verifies uptake of the DNA of interest via the adenoviral vectors (Figure 5A). Control group cells that were not treated with adenovirus did not express GFP (data not shown).
Figure 5. Detection of early and late markers of osteogenic differentiation.
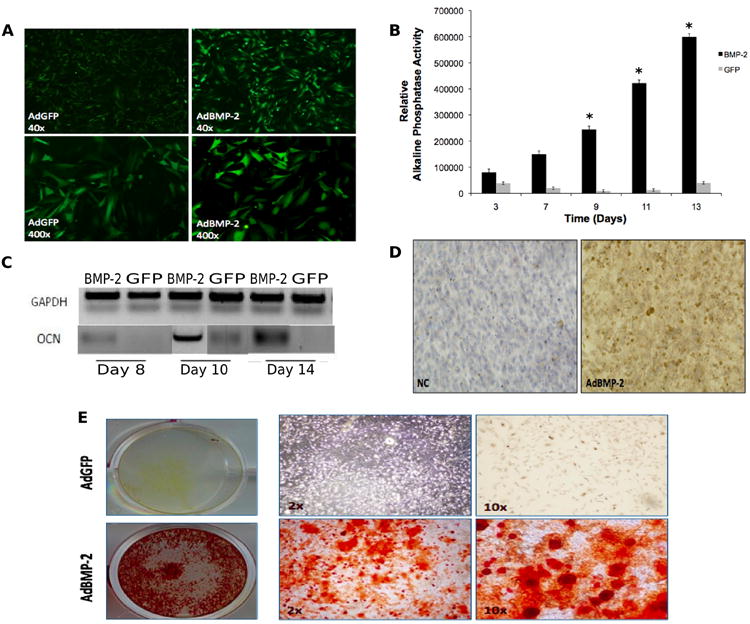
A) Fluorescent microscopy imaging of green fluorescent protein (GFP) expression at 24 hours post-adenoviral infection of calvarial cells in GFP and BMP-2 groups. B) Average relative alkaline phosphatase activity in calvarial cells on days 3, 7, 9, 11 and 13 post-adenoviral infection with BMP-2 (black bars) or GFP alone (grey bars). Experiments were performed in triplicate. Error bars represent S.D. Asterisks denote statistical significance between AdBMP-2 versus AdGFP-treated cells. C) mRNA expression levels of osteocalcin in harvested calvarial cells on days 8, 10 and 14 post-adenoviral infection with BMP-2 or GFP (control). Internal control with GAPDH is also shown. D) Immunohistochemical staining for osteopontin in AdBMP-2 and negative control (NC) groups 10 days post adenoviral infection. E) Matrix mineralization (Alizarin red staining) on day 14 post infection in AdGFP-treated (top panel), and AdBMP-2-treated (bottom panel) cells.
Alkaline Phosphatase (ALP) Activity
ALP activity in cells tranduced with AdBMP-2, which was measured for up to 13 days in culture, increased gradually during the experimental period (Figure 5B). By day 9, the AdBMP-2 group showed a significant difference compared to control cells (p<0.05).
Effect of AdBMP-2 on Expression of Secreted Extracellular Proteins
To confirm that the harvested and infected osteoblastic cells produced extracellular matrix upon AdBMP-2 stimulation, the mRNA level of late osteogenic marker gene osteocalcin (OCN) was assessed via RT-PCR. Levels of OCN mRNA were elevated in the AdBMP-2 group by day 14 (Figure 5C). Additionally, osteopontin (OPN) protein expression was elevated in the AdBMP-2 group by day 8 (Figure 5D).
Mineralized Nodule Formation
To examine the mineralization capacity of the osteoblastic cells, the last step in osteogenic cell differentiation, mineral nodule formation, was assessed at day 14 of culture in cells grown in the presence of AdBMP-2 or AdGFP. Alizarin red intensity of mineralized nodules was higher by day 14 in the AdBMP-2 group compared to AdGFP controls (Figure 5E).
Transduction of iCALs with AdBMP2 enhances bone formation in vivo
Using the well-established stem cell implantation assay (Figure 6A), we tested the hypothesis that BMP-2 would induce a prominent osseous response in vivo when compared to control conditions. The ability of the immortalized osteoprogenitor cells to sustain their terminally differentiated state in vivo was tested. Somewhat consistent with our in vitro studies, GFP-infected cells produced minimal or miniscule bone nodule formations in implantation sites recovered 6 weeks post cell transfer (Figure 6B). In contrast, BMP2-infected cells produced bony masses that were noticeably larger compared to the control group (Figure 6B). Furthermore, histological survey of these bony masses revealed more mature bone and thicker trabeculae in the BMP-2-infected cell group (Figure 6C). ImageJ analysis of representative histologic samples revealed that bony masses derived from iCALs transduced with AdBMP-2 had a percentage of trabecular bone area to total area of 71.4% whereas bony masses derived from iCALs transduced with AdGFP had a percentage of trabecular bone area to total area of 60.2% (p <0.05). Masson's trichrome staining also demonstrated increased matrix mineralization in the bony masses formed by BMP-2 induced cells relative to GFP-infected controls (Figure 6C).
Figure 6. Stem cell implantation assay with AdBMP-transduced iCALs leads to formation of ectopic bone.
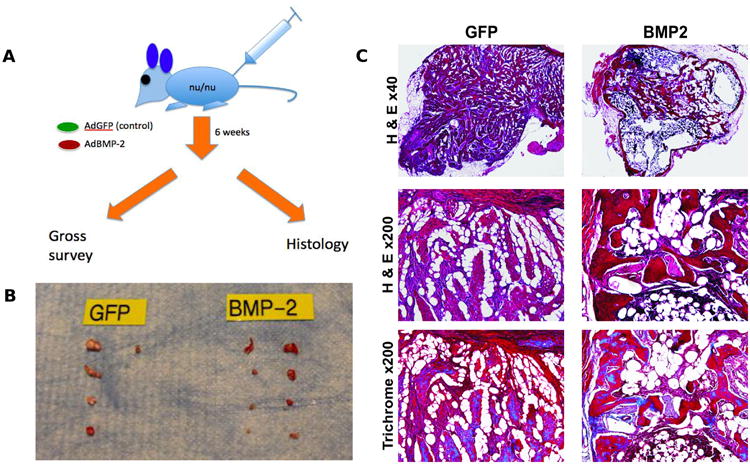
A) Schematic diagram of experimentation. B) Digital photography of specimens retrieved. C) Representative hematoxylin and eosin staining and trichrome staining of 0.1mm cross sections of harvested specimens reveal dense trabecular bone in experimental group versus fibrous cell reaction present in control group.
Discussion
The use of biological reagents in bony healing has the potential to revolutionize the way osseous injuries and congenital defects are managed. Currently, recombinant BMP-2 and -7 are FDA approved for the treatment of spinal fusions and long bone non-unions, respectively [9]. However, little has been reported on the use of AdBMPs in the craniofacial skeleton. Our pilot in vivo studies strongly suggest a promising therapeutic role of AdBMP therapy in the healing of critical sized defects in this area [12, 13].
To establish a cell-based strategy for gene delivery, we investigated the effects of AdBMPs on isolated calvarial cells in vitro. As mentioned above, in order to confirm the expression of adenoviral BMP DNA, the AdBMP vectors contained the Green Fluorescent Protein (GFP) gene. Based on previous studies, we can assume cells displaying GFP fluorescence are also expressing their respective BMPs [20].
To assess early osteogenic differentiation, we measured the ALP activity of treated cells. By day 9, the average ALP activity in the BMP-2 treated cells was significantly higher than the GFP group (p<0.05). This increased ALP activity suggests that treated cells are entering the very early stages of osteogenesis [11, 22]. Our data suggest relatively rapid entry of cells infected by AdBMP-2, and resemble our findings with established MSC lines, such as C3H10 T1/2 [11], lending further credence to the progenitor cell phenotype of the immortalized calvarial cells we have characterized.
It is well known that extracellular matrix proteins such as OCN and OPN are produced via BMP activation of osteogenic transcription factors. Our RT-PCR data indicates the upregulation of OCN mRNA levels in AdBMP-2 treated cells by day 14. IHC staining for OPN in treatment groups also confirms the presence of these proteins. Assessment for upregulation of other proteins in the BMP signaling pathway, such as Smad1, Runx2 and Osterix are also of interest and will drive future experiments.
In addition, previous studies have established that BMP treated cells can produce positive Alizarin red S staining for calcium nodule formation [7, 11, 23]. The observance of positive staining in the AdBMP-2 group by day 14 is indicative of cells undergoing the final steps of osteogenesis, which is further evidence that AdBMP-2 can induce complete osteogenesis in iCALs in vitro.
The major limitation of direct adenoviral-mediated BMP therapy to critically-sized murine calvarial defects was postulated to be the low uptake of the virus by resident cells in the defect, which in turn led to sub-therapeutic expression and secretion of BMP into the local environment. To ultimately address this deficiency, we have established a reproducible method of harvesting calvarial cells as biological agents for bone tissue engineering. Immunological survey of these cells, as discussed below, demonstrates a potentially rich source of osteoprogenitor cells at varying stages of differentiation.
A potential drawback to the use of primary progenitor cells for basic and translational research, however, is their limited lifespan. Because isolation of these cells is labor-intensive and time-consuming, the development of progenitor cells with permanent growth features is attractive. To this end, recent investigation from our laboratory has demonstrated that mouse embryonic fibroblasts, which also serve as a rich source of mesenchymal progenitor cells, can be safely and efficiently immortalized in reversible fashion using the large T/SV40 antigen system [24]. We applied this system to harvested primary calvarial cells.
Importantly, immortalization of the cells harvested in the current study with the large T/SV40 antigen system did not significantly alter the transfectants compared to their primary cell counterparts. In fact, flow cytometry analysis (FACS) revealed that immortalized cells contained 23% of CD44+ cells, compared to 3% in primary isolates, which suggests an enrichment of these cells during several passages and the immortalization process. Immunohistochemistry demonstrated expression of classic markers in harvested cells well known to signify mesenchymal progenitor cells in bone marrow [25-27] in both untreated (primary) and treated (immortalized) groups: CD166 (ALCAM), CD105 (Endoglin), and CD133 were found to stain positive in immunohistochemical assays, whereas CD44, CD73 (SH3/SH4), and CD105 stained positive via immunofluorescence. In addition to characterizing the harvested cell population and monitoring that the immortalization process did not alter the physical and morphological properties of our cell population, FACS analysis provided information regarding the osteogenic potential of cells.
Harvested calvarial cells likely consisted of a pooled population of cells including mesenchymal stem cells (MSCs), cells at various stages of the osteoblastic lineage, and adipocytes. While we are not able to quantify the osteogenic potential of cells based on their marker profile, we can utilize cell surface markers to demonstrate which cells do in fact have osteogenic potential. The presence and expression levels of CD44, CD73, and CD105 are critical for osteogenic differentiation of MSCs [28-30]. The hyaluronate receptor CD44, for example, is necessary for osteoblastic migration and differentiation and can be used as a marker for osteoblasts [31, 32]. CD73, an ecto-5′-nucleotidase, is downregulated during chondrogenic differentiation of MSCs; additionally, it is thought to be important for osteogenic differentiation based on the finding that in the setting of CD73 antagonist treatment, there is decreased expression of osteogenic differentiation factors and mineral matrix deposition of MSCs [28]. Many of our cells expressed these markers; subsequently, these cells matured toward the osteogenic lineage as demonstrated by markers of early and late stages of osteogenic differentiation. Our findings compare to similar marker studies of human MSCs derived from bone marrow, which corroborate that MSCs cultivated and expanded ex vivo retain critical properties necessary for therapeutic applications [33]. Interestingly, CD90 gene transcripts were virtually absent in both primary and transduced (immortalized) calvarial cells, suggesting that an undefined post-translational modification of this cell surface antigen is a hallmark of both cell types. The phenotypic profiling of these cells, however, is somewhat incomplete, as we did not investigate the specific subsets of cells in our immortalized pool. Also, we did not assay for MHC-II (HLA-DR) negativity, another feature of MSCs.
A further limitation in this part of our study relates to the clinical relevance of this type of cell in therapeutic application. It would be fitting, to make this a more translational concept, to investigate progenitor cells that can be isolated from the adult cranium, as it is more likely that critical-sized cranial defects would be found in the adult population. Additionally, while our studies are primarily at in vitro and animal model stages, it is conceivable that a virally-transduced cell approach (e.g., ex vivo cell-based therapy) can be used clinically. This approach would depend on the successful harvesting of primary calvarial progenitor cells from individual patients. Short of evaluating the efficacy of virally-transduced primary calvarial cells harvested from human subjects in the clinical setting, the ultimate comparative test for osteogenic potential of this cell population would be their use in critical-size bony defect or similar animal models. Several investigations within our laboratory to determine the in vivo utility of these cells are ongoing, including their biocompatibility with nanoscaffolds and their efficacy in repairing cranial defects in murine models when stimulated with various osteogenic factors. Despite the use of iCALs and related approaches not being immediately useful in the clinical setting, these cells do indeed provide a valuable platform to investigate the molecular mechanisms underlying intramembranous bone formation and to screen for factors/small molecules that can facilitate the healing of osseous defects in the craniofacial skeleton as demonstrated by the current study.
A further concern arises from the concept of introducing viral agents into humans. The theoretical issue of iatrogenic viral infection should not prove to be a significant concern as cells would be infected/transduced in vitro (i.e., ex vivo), allowing for any excess virus to be removed during culturing.
Conclusion
We have established a reliable method for isolating and immortalizing primary calvarial cells in vitro. Immunological screening of these cells by immunohistochemical staining and flow cytometry reveals a mixed progenitor cell type and therefore a prime resource for cell-based tissue engineering. Further studies should be performed to comprehensively test the differential capacity of the various BMPs to induce osteogenesis of these cells, and eventually test the ability of modified cells to heal osseous defects.
Clinical relevance.
A limitation of cranial defect repair is the finite supply of bone available. As such, other methods to produce bony healing must be explored. In this manuscript, we report the results of a study aimed at establishing a reliable method for isolating and immortalizing calvarial cells in vitro. The establishment of long-term cell culture of primary osteoprogenitor cells can facilitate their use in basic and translational research, as well as for potential clinical applications. For example, a strategy involving the infection of primary osteoprogenitor cells with adenoviral vector-delivered bone morphogenetic proteins may facilitate increased autologous bone formation.
Acknowledgments
The reported work was supported in part by an NIH/NIDCR K08 Career Development Award (#1K08DE020140-01; RRR) and the 2009 Maxillofacial Surgeons Foundation of the American Society of Maxillofacial Surgeons Research Award (RRR).
Footnotes
Presented at the 3rd European Plastic Surgery Research Council, Hamburg, Germany, August, 2011
Financial Disclosures: The authors have received no financial support from and have no interest in any commercial source that is related directly or indirectly to the scientific work presented in this article.
References
- 1.Greenwald JA, Mehrara BJ, Spector JA, et al. Biomolecular mechanisms of calvarial bone induction: immature versus mature dura mater. Plast Reconstr Surg. 2000;105:1382–92. doi: 10.1097/00006534-200004040-00018. [DOI] [PubMed] [Google Scholar]
- 2.Cei S, Mair B, Kandler B, et al. Age-related changes of cell outgrowth from rat calvarial and mandibular bone in vitro. J Craniomaxillofac Surg. 2006;34:387–94. doi: 10.1016/j.jcms.2006.07.856. [DOI] [PubMed] [Google Scholar]
- 3.Cho YR, Gosain AK. Biomaterials in craniofacial reconstruction. Clin Plast Surg. 2004;31:377–85. doi: 10.1016/j.cps.2004.03.001. v. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 5.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 6.Administration USFaD. INFUSE Bone Graft. 2009 [Google Scholar]
- 7.Hasegawa Y, Shimada K, Suzuki N, et al. The in vitro osteogenetic characteristics of primary osteoblastic cells from a rabbit calvarium. J Oral Sci. 2008;50:427–34. doi: 10.2334/josnusd.50.427. [DOI] [PubMed] [Google Scholar]
- 8.Smith DM, Afifi AM, Cooper GM, et al. BMP-2-based repair of large-scale calvarial defects in an experimental model: regenerative surgery in cranioplasty. J Craniofac Surg. 2008;19:1315–22. doi: 10.1097/SCS.0b013e3181843369. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Sun MH, Kang Q, et al. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5:167–79. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 10.Breyer B, Jiang W, Cheng H, et al. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149–62. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–52. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Seitz IA, Spiguel L, Teven CM, et al. ISCFS XIII Biennial International Congress Volume S29.A.3. Oxford, UK: 2009. The differential effects of BMP-2 and -9 in critical-sized, murine cranial defects. [Google Scholar]
- 13.Shenaq DS, Teven CM, He TC, Reid RR. The osteogenic characteristics of immortalized calvarial cells. Plast Reconstr Surg. 2011;128:624. [Google Scholar]
- 14.Tessier P, Kawamoto H, Posnick J, et al. Taking calvarial grafts--tools and techniques: VI. The splitting of a parietal bone “flap”. Plast Reconstr Surg. 2005;116:74S–88S. doi: 10.1097/01.prs.0000177277.36391.28. discussion 92S-4S. [DOI] [PubMed] [Google Scholar]
- 15.Tessier P, Kawamoto H, Posnick J, et al. Taking calvarial grafts, either split in situ or splitting of the parietal bone flap ex vivo--tools and techniques: V. A 9650-case experience in craniofacial and maxillofacial surgery. Plast Reconstr Surg. 2005;116:54S–71S. doi: 10.1097/01.prs.0000173949.51391.d4. discussion 92S-4S. [DOI] [PubMed] [Google Scholar]
- 16.Westerman KA, Leboulch P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci U S A. 1996;93:8971–6. doi: 10.1073/pnas.93.17.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Q, Sun MH, Cheng H, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 18.He TC, Zhou S, da Costa LT, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang Q, Song WX, Luo Q, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18:545–59. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Deng ZL, Luo X, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–47. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhang H, Zhang W, et al. Bone Morphogenetic Protein-9 (BMP9) Effectively Induces Osteo/Odontoblastic Differentiation of the Reversibly Immortalized Stem Cells of Dental Apical Papilla. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho JY, Lee WB, Kim HJ, et al. Bone-related gene profiles in developing calvaria. Gene. 2006;372:71–81. doi: 10.1016/j.gene.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Malladi P, Zhou D, Longaker MT. Molecular and cellular characterization of mouse calvarial osteoblasts derived from neural crest and paraxial mesoderm. Plast Reconstr Surg. 2007;120:1783–95. doi: 10.1097/01.prs.0000279491.48283.51. [DOI] [PubMed] [Google Scholar]
- 24.Huang E, Yang B, Jiang W, et al. Conditionally Immortalized Mouse Embryonic Fibroblasts Retain Proliferative Activity without Compromising Multipotent Differentiation Potential. PLoS ONE. 2012 doi: 10.1371/journal.pone.0032428. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.in 't Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–52. [PubMed] [Google Scholar]
- 27.Shenaq DS, Rastegar F, Petkovic D, et al. Mesenchymal Progenitor Cells and Their Orthopedic Applications: Forging a Path towards Clinical Trials. Stem Cells Int. 2010;2010:519028. doi: 10.4061/2010/519028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ode A, Schoon J, Kurtz A, et al. CD73/5′-ecto-nucleotidase acts as a regulatory factor in osteo-/chondrogenic differentiation of mechanically stimulated mesenchymal stromal cells. Eur Cell Mater. 2013;25:37–47. doi: 10.22203/ecm.v025a03. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, Kim JH, Abbas AA, Yoon TR. Alendronate enhances osteogenic differentiation of bone marrow stromal cells: a preliminary study. Clin Orthop Relat Res. 2009;467:3121–8. doi: 10.1007/s11999-008-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson P, Carrillo-Galvez AB, Garcia-Perez A, Cobo M, Martin F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS ONE. 2013;8:e76979. doi: 10.1371/journal.pone.0076979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamal HH, Aubin JE. CD44 expression in fetal rat bone: in vivo and in vitro analysis. Exp Cell Res. 1996;223:467–77. doi: 10.1006/excr.1996.0103. [DOI] [PubMed] [Google Scholar]
- 32.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–34. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 33.Kulterer B, Friedl G, Jandrositz A, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:70. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]


