Highlights
-
•
Brain responses to social exclusion were measured in ASD unaffected siblings.
-
•
Unaffected siblings show trait atypical activation in posterior temporal lobe.
-
•
Biological vulnerability moderates relationship between IQ and brain responses.
Keywords: Social exclusion, Autism, Unaffected siblings, Trait, fMRI
Abstract
Social exclusion elicits powerful feelings of negative affect associated with rejection. Additionally, experiencing social exclusion reliably recruits neural circuitry associated with emotion processing. Recent work has demonstrated abnormal neural responses to social exclusion in children and adolescents with autism spectrum disorders (ASD). However, it remains unknown to what extent these abnormalities are due to atypical social experiences versus genetic predispositions to atypical neural processing. To address this question, the current study investigated brain responses to social exclusion compared to a baseline condition of fair play in unaffected siblings of youth with ASD using functional magnetic resonance imaging. We identified common deviations between unaffected siblings and ASD probands that might represent trait-level abnormalities in processing Social Exclusion vs. Fair Play, specifically in the right anterior temporoparietal junction extending into posterior superior temporal sulcus. Thus, hypoactivation to Social Exclusion vs. Fair Play in this region may represent a shared genetic vulnerability to developing autism. In addition, we present evidence supporting the idea that one's status as an unaffected sibling moderates the relationship between IQ and neural activation to Social Exclusion vs. Fair Play in anterior cingulate cortex. These results are discussed in the context of previous literature on neural endophenotypes of autism.
1. Introduction
Social deficits are the cornerstone of behavioral symptoms in children with autism spectrum disorder (ASD; Wing and Gould, 1979). Such social deficits include abnormal eye contact or body language and difficulty engaging in normal back-and-forth conversation (APA, 2013). As one can imagine, these atypical social behaviors make children with ASD particularly vulnerable to ostracism by peers (Symes and Humphrey, 2010). However, because of deficits in understanding nonverbal communication, it is difficult to assess whether children with ASD process peer rejection (which is often communicated through actions instead of words) in a manner that it similar to typically developing youth.
The typical experience of being socially excluded has profound effects on basic psychological needs such as feelings of belonging, control, meaningful existence, and self-esteem (Williams et al., 2000; Williams, 2007). The distress of social exclusion has distinct neural correlates that are robust among healthy children, adolescents, and adults (Bolling et al., 2011a, Bolling et al., 2011c, Eisenberger et al., 2003, Krill and Platek, 2009, Masten et al., 2009, Moor et al., 2012, Onoda et al., 2009, Sebastian et al., 2011). Abnormal brain responses to social exclusion have been noted in children and adolescents with ASD (Bolling et al., 2011b, Masten et al., 2011). These abnormal brain responses to social exclusion (compared to social inclusion) manifest as hypoactivation in several regions including anterior insula and anterior cingulate cortex (ACC; Bolling et al., 2011b, Masten et al., 2011). However, it is unknown whether these atypical brain responses arise from the neurodevelopmental etiology of ASD or result from the unique social experiences afforded by growing up with ASD. Building on previously established evidence of neural hypoactivation in response to social exclusion in youth with ASD, the current study attempts to identify regions of trait differences, where the hypoactivation found in ASD is also found in a group of unaffected siblings (UAS) of children with ASD who share the genetic risk for developing ASD, but who have not experienced first-hand the social struggles faced by their brother or sister. In this way, we can dissociate biological from environmental influences on the neural response to social rejection in ASD.
It is likely that a history of atypical social experiences contributes to the abnormal brain activity observed in children with ASD during social interactions. Research examining the nature of social relationships in children and adolescents with ASD has found that compared to typically developing (TD) youth, children with ASD show higher rates of peer victimization (Little, 2001), as well as social rejection and bullying (Symes and Humphrey, 2010). Youth with ASD also report receiving less peer approval (Williamson et al., 2008) and experiencing more loneliness (Bauminger and Kasari, 2000). Children with ASD initiate social interactions less frequently than TD peers (Hauck et al., 1995). High-functioning children with ASD are both cognizant and distressed by social rejection (Ochs et al., 2001). Thus, by adolescence, individuals with ASD have likely endured a very different and profoundly difficult experience of peer relationships relative to their TD counterparts.
One's previous experiences and expectations of social interactions can influence immediate responses to peer rejection. For instance, the expectation of future social exclusion leads to emotional numbing to physical and social pain (DeWall and Baumeister, 2006). While adolescents with ASD exhibit normal anxiety and need threat responses to rejection, they also show decreased modulation of mood following exclusion compared to TD peers (Sebastian et al., 2009). This finding, along with two separate accounts of hypoactivation in brain regions typically responsive to social exclusion including ACC and anterior insula (Bolling et al., 2011b, Masten et al., 2011), led Masten et al. to hypothesize that reduced neural sensitivity to rejection in ASD may be a result of habituation to social exclusion or increased expectancy of being rejected by unfamiliar peers.
In contrast to the idea that experience accounts for abnormal brain responses to exclusion in ASD, other research suggests that endogenous, biological factors influence brain responses to social stimuli in children with ASD. Neuroimaging work has detected signs of a “neural endophenotype” of autism; atypical patterns of brain structure and function that are shared between children with ASD and their unaffected siblings (UAS; Barnea-Goraly et al., 2010; Belmonte et al., 2010; Dalton et al., 2007, Kaiser et al., 2010, Spencer et al., 2011). One study investigating brain responses to biological motion found trait-level hypoactivation shared between children with ASD and UAS in regions including right inferior temporal gyrus, left dorsolateral prefrontal cortex, and bilateral fusiform gyrus (Kaiser et al., 2010). Because UAS do not share in the ASD clinical phenotype or the experience of growing up with ASD, these common neural profiles are thought to be a result of the strong genetic basis for the disorder (for review see Gupta and State, 2007). Supporting this claim, behavioral assessments of UAS of children with ASD have found largely normal patterns of social support, social competence, and psychosocial development (Kaminsky and Dewey, 2002, Macks and Reeve, 2007, Pilowsky et al., 2004, Rodrigue and Geffken, 1993). Thus, while some neural profiles are common among the two groups, the experience of social victimization and isolation in youth with ASD does not seem to be shared by their healthy siblings.
To investigate atypical neural responses to social rejection in children with ASD and UAS that may represent trait-level biological vulnerabilities to developing autism, we used functional magnetic resonance imaging (fMRI) to measure brain responses to discrete periods of social exclusion. To this end, we used a modification of the Cyberball task (Williams et al., 2000) during which participants play an online ball-tossing game with two other ostensibly-real children. The game alternates between periods of fair play, where the participant receives the ball on one-third of the throws, and social exclusion, where the participant does not receive any throws. While it is extremely difficult to assess neural activation during actual peer rejection because of methodological constraints, this study utilized an experimental model of peer rejection that has been developed in an attempt to marry a naturalistic social experience of rejection with necessary controls on presentation (Williams et al., 2000). The hope is that for each participant, brain responses to the experience of social exclusion during Cyberball will mirror brain responses during a natural occurrence of peer rejection.
The current study utilized a two-step analysis approach to identify brain regions where both children with ASD and UAS showed differential activation to Social Exclusion vs. Fair Play compared to TD controls. First, the two groups of healthy children (the UAS and the TD controls) were compared in order to identify regions where UAS showed abnormal brain activation during social exclusion. Next, activation in each of these regions was assessed in a third group of children with ASD. Regions where the ASD group also significantly diverged from the TD controls in the same direction as the UAS were considered regions of trait-level difference. This two-step analysis strategy is fundamentally important. As it is tempting to only compare UAS and TD youth and conclude that differences reflect some sub-symptom genetic abnormality in the UAS, the potential remains that differences found between the two groups may be due to coincidental differences between the samples that prevented a full study replication. However, the use of a third, independent participant group (ASD) allowed us to confirm that certain regions where activation differed between TD and UAS groups represented meaningful, trait-level abnormalities.
2. Methods
2.1. Participants
Participants in the current study were children and adolescents ranging from 7 to 18 years of age. Individuals were excluded from participation in the current study if parents reported that the child had experienced neurological problems or abnormalities (unrelated to autism). In addition, if the child ever experienced seizures, epilepsy, hearing or vision loss, motor impairment, or severe allergies, then he or she was excluded from participation. Participants in the current study were recruited as three separate groups. Typically developing (TD) children had no parents, siblings, or half siblings with an autism spectrum disorder. In addition, these children had no diagnosis of an intellectual disability or learning disability. UAS were healthy children with a full sibling diagnosed with an autism spectrum disorder. One UAS was excluded from analyses when the diagnosis of the proband sibling (not in the current study) was not confirmed by study clinicians. Children with an ASD were diagnosed using one or both the autism diagnostic interview-revised (ADI-R; Lord et al., 1994) and the autism diagnostic observation schedule (ADOS; Lord et al., 2000), as well as expert clinical judgment based on DSM-IV-TR criteria (APA, 2000). All children (except for one ASD participant) had their social responsiveness level assessed by parent report using the social responsiveness scale (SRS; Constantino and Todd, 2003). Any TD child or UAS with an SRS standardized score within the “severe” range (t-score > 75) was excluded from the current study (two TD children were excluded for this reason). The data from the ASD group in the current study has been previously reported (Bolling et al., 2011b). In addition, data from the TD group in the current investigation is a subset of a sample, which has been previously reported (two TD children with elevated SRS scores were removed for the current investigation; Bolling et al., 2011a, Bolling et al., 2011b). The UAS in the current study are a novel group of participants, and thus the current study focuses on the UAS in comparison to TD and ASD children.
Following exclusions for head motion and task performance (thresholds described below), 19 TD controls (12.96 ± 2.7 years, 14 male), 16 youth with ASD (12.36 ± 4.2 years, 10 male), and 15 UAS (11.88 ± 3.2 years, 9 male) were included in analyses. Only 2 of the 15 UAS included in the current study had biological siblings in the ASD participant group. Comparing the three experimental groups, there was no main effect of age (F(2, 47) = 0.43, p = 0.65). A Pearson chi-square test additionally showed that gender ratios did not significantly differ by group (χ2 (2, N = 50) = 0.83, p = 0.66). All children in the current study (except for one ASD participant and one UAS) had their IQ assessed using the differential abilities scale (DAS-II; Elliott, 2007). Average IQ scores for all participants included in the study are reported in Table 1. There was a main effect of group on nonverbal IQ scores (F(2, 45) = 6.2, p = 0.004) and general conceptual ability (GCA) scores (F(2, 45) = 4.5, p = 0.016) but not verbal IQ scores (F(2, 45) = 2.94, p = 0.063). Post hoc Tukey's HSD tests revealed that for both nonverbal and GCA IQ scores, the UAS group significantly differed (UAS > TD and ASD) from both the TD and ASD youth (p's < 0.05). The TD and ASD groups did not differ in any IQ measure.
Table 1.
IQ and SRS scores.
| Measure | Group |
||
|---|---|---|---|
| TD | ASD | UAS | |
| DAS-II | |||
| n | 19 | 15 | 14 |
| Nonverbal | 97.32 (±14.5) | 100.47 (±18.7) | 117.93 (±19.7) |
| Verbal | 105.16 (±17.6) | 100.07 (±14.0) | 114.43 (±16.3) |
| Global | 100.84 (±17.1) | 100.87 (±15.6) | 117.07 (±18.2) |
| SRS | |||
| n | 19 | 15 | 15 |
| Raw total | 23.9 (±22.1) | 101.7 (±26.5) | 20.1 (±17.1) |
2.2. Cyberball
The Cyberball task (Williams et al., 2000, Bolling et al., 2011c) began with a mock Google® screen where the experimenter selected a link leading to the online game, followed by instructions for the participant to choose a playing glove that would represent him or her during the ball-toss. Instructions for the game were presented visually and auditorily, where participants were instructed to use buttons in each hand to throw a ball to one of two online players. After instructions, participants practiced playing the game for 16 throws. When an understanding of the game was confirmed, the scan began with a 10 s fixation where the participant viewed the pictures of the online players (matched on gender to the participant), followed by 5 min of a ball-toss interaction. The ball-toss alternated between 30 s periods of fair play where the participant received 1/3 of the throws, and social exclusion where the participant received no throws. There were 5 periods of each condition (Fair Play and Social Exclusion). The task concluded with another 10 s period of fixation. Immediately after the completion of Cyberball, scanning ceased and 10 statements about the participant's basic psychosocial needs (e.g. “I felt excluded”) were presented visually and auditorily. Participants rated each statement on a 1–5 Likert scale from “not at all” to “extremely”. Participants were able to communicate clarification questions to the experimenters during this period. When understanding was confirmed, participants responded verbally while still in the scanner and responses were recorded using E-Prime software (Psychological Software Tools, Inc., Pittsburgh, PA).
2.3. Imaging protocol
Images were collected on a Siemens 3 T Tim Trio scanner (Siemens Medical Solutions, Erlangen Germany) located in the Yale University Magnetic Resonance Research Center. Whole-brain T1-weighted anatomical images were acquired with an MPRAGE sequence (TR = 1900 ms; TE = 2.96 ms; flip angle = 9°; FOV = 256 mm; image matrix 256 mm2; voxel size =1 mm × 1 mm × 1 mm; 160 slices; NEX = 1). Whole-brain functional images were acquired with a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 64 mm2; voxel size = 3.4 mm × 3.4 mm × 4 mm; 34 slices) sensitive to blood oxygen level dependent (BOLD) contrast.
2.4. Data analysis
Data were processed and analyzed using BrainVoyager QX 2.0.8 (Brain Innovation, Maastricht, the Netherlands). The first 5 volumes acquired during functional scans (fixation) were discarded prior to pre-processing, in order to allow for T1 equilibrium. Pre-processing of functional data included slice time correction (using sinc interpolation), 3-D rigid-body motion correction (using trilinear–sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, linear trend removal, and temporal high-pass filtering (general linear model – GLM) with Fourier basis set, using two cycles per time course. The processed functional data sets were coregistered to within-session anatomical images, which were subsequently normalized to Talairach space.
In all children in the current study, head motion in the duration of the scan did not exceed 4.0 mm or degrees from head placement at the initial volume of acquisition. Expanding on this motion threshold, the majority of participants included in the study had head motion that did not deviate from starting position by more than 2 mm or degrees (76% of participants). Any participant with isolated periods of excessive motion in the second half of the scan had experimental blocks with excessive motion removed prior to pre-processing, and only remaining data was analyzed. One TD control had the last 2 task blocks removed, one child with ASD had the last 5 task blocks removed, and one UAS had the last 3 task blocks removed, while another UAS had the final 80 s (2.67 blocks) removed. In addition, two UAS had only the last 6 volume acquisitions (12 s of data) removed from analysis, of which 10 s were fixation.
Single participant GLM-based analyses were performed for each experimental session. Prior to modeling the task predictors, events where the participant received the ball during fair play were modeled as a single predictor in a GLM. Activation corresponding to these ball throw events was regressed out of the single participant 4-dimensional data used for further analysis. Predictors were subsequently created for the two experimental conditions in the game of Cyberball (Fair Play, Social Exclusion) using boxcar functions with values of 1 during periods of the condition, and 0 otherwise. Boxcar functions were convolved with a double-gamma hemodynamic response function (HRF). In addition to the two task predictors, regressors for each of the six motion estimates were included as predictors of no interest, to additionally account for head motion.
Single-participant GLM analyses were combined into group level, random-effects analyses. All whole-brain analyses in the current study were limited to voxels within the extent of the MNI brain, normalized to Talairach space. For all analyses characterizing brain responses to Social Exclusion, the condition of Fair Play was used as a baseline. This is consistent with both previous fMRI investigations of Cyberball social exclusion in youth with ASD (Bolling et al., 2011b, Masten et al., 2011). Thus, all descriptions of neural activation to social exclusion in the current report are technically describing activation to Social Exclusion vs. Fair Play. Explicit referral to the control condition is minimized for brevity. For group analyses, we first conducted a whole-brain voxel-wise contrast of Social Exclusion and Fair Play in the UAS, the novel participant group in the current study. We then combined the UAS with the group of TD children who did not have a family member with ASD in a second random-effects GLM. To identify neural regions where activation to Social Exclusion (versus Fair Play) differed between these two healthy populations (UAS and TD), we conducted a Group X Condition ANOVA. Both whole-brain, voxel-wise analyses were assessed at a statistical threshold of p < 0.05, corrected for multiple comparisons at the cluster level. We used the BrainVoyager cluster threshold estimator plugin (which involves Monte-Carlo simulation) to calculate the probability of observing contiguous clusters of a given size in randomly generated parameter maps that are constrained by the spatial correlation characteristics of the activation map for each ANOVA. Using 1000 iterations of the plugin (restricted to voxels within our whole-brain mask), we estimated a cluster size threshold that would occur by chance with a probability of less than 5%, corresponding to α < 0.05 (FWHM = 1 functional voxel). For the Cyberball ANOVA, this cluster threshold was 35 functional voxels (applied as 945 mm3 to the interpolated map). For the within-group comparison of Social Exclusion and Fair Play in UAS, the estimated cluster threshold was 34 functional voxels (applied as 918 mm3 to the interpolated map; FWHM = 1 functional voxel).
For each region showing a Group (TD vs. UAS) X Condition (Social Exclusion vs. Fair Play) interaction, we extracted beta value parameter estimates averaged across all voxels within the region from TD and UAS individuals, as well as from our third participant group, the children and adolescents with ASD. We then compared brain activation in the ASD group in the contrast of Social Exclusion > Fair Play to parameter estimates of brain activation in the same contrast in each of the TD and UAS groups using independent-samples t-tests. Because the regions were defined on the basis that activation in the task contrast differed between TD and UAS groups, regions where brain activation in the ASD group differed significantly from the TD group (p < 0.05, uncorrected) but not from the UAS group were identified as “trait” regions.
3. Results
3.1. Behavioral
On the social exclusion questionnaire, 18 TD children (mean score 25.44 ± 8.1), 13 UAS (mean score 29.62 ± 7.8) and 14 children with ASD (mean score 29.57 ± 7.7) completed the self-report measure. A score of 10 would reflect no distress (responding “not at all” to all questions of exclusion-related negative affect e.g. “I felt rejected” and “extremely” on all reverse-scored items e.g. “I felt liked”). Conversely, a score of 50 would reflect maximal distress. Thus, we interpret average group scores all over 25 as significant experiences of distress (manipulation check). Importantly, average scores did not differ by group on the social exclusion questionnaire (F(2, 42) = 1.5, p = 0.2).
Scores on the SRS reflect parent-reported levels of social responsiveness, ranging from zero to 195. Higher scores imply greater levels of impairment in responsiveness. Group average SRS scores for all participants included in the study are reported in Table 1. The average score on the SRS in the TD group was 23.9 (±22.1). For the UAS, the average score was 20.1 (±17.1), and for children with ASD, the average score was 101.7 (±26.5). There was an expected main effect of group (F(2, 46) = 66.5, p < 0.001), with post hoc Tukey's HSD tests revealing that ASD youth had greater SRS scores than both the TD children and UAS (p's < 0.001).
3.2. Imaging
A group level, random-effects analysis contrasting Social Exclusion and Fair Play in the UAS revealed greater activation to Social Exclusion in several regions previously shown to be active during social rejection in healthy adults and children (Table 2, Fig. 1). These included bilateral posterior insula, bilateral hippocampus, ventromedial prefrontal cortex extending into ventral anterior cingulate cortex (vACC), left superior and inferior frontal gyri, right caudate, right inferior temporal gyrus, left middle temporal gyrus, and bilateral temporal pole. Activation was greater to Fair Play vs. Exclusion in right parietal cortex, right dorsolateral prefrontal cortex (dlPFC), right superior frontal gyrus, right cuneus, left precuneus, bilateral lateral occipital gyri, left supramarginal gyrus, and left cerebellum.
Table 2.
Task modulated neural activation during Cyberball in Unaffected Siblings (p < 0.05, k = 918 mm3). Statistics and coordinates refer to the voxel of maximum signal change within each region. Size refers to the region extent in structural voxels (1 mm3).
| Region | X | Y | Z | Size | t | p |
|---|---|---|---|---|---|---|
| Social Exclusion > Fair Play | ||||||
| Right posterior insula | 51 | −13 | 19 | 1718 | 4 | 0.001305 |
| Left posterior insula | −45 | −19 | 10 | 3114 | 4.15 | 0.000979 |
| Right hippocampus | 24 | −28 | 13 | 4209 | 4.46 | 0.000537 |
| Left hippocampus | −27 | −7 | −14 | 11186 | 5.8 | 0.000046 |
| Right temporal pole | 21 | −4 | −32 | 986 | 4.47 | 0.000528 |
| Left temporal pole | −24 | 2 | −29 | 1360 | 5.94 | 0.000036 |
| Right caudate | 21 | −25 | 22 | 1452 | 3.98 | 0.001371 |
| vACC/vmPFC | −3 | 32 | −14 | 3247 | 4.86 | 0.000251 |
| Right ITG | 48 | −7 | −17 | 972 | 3.5 | 0.003549 |
| Left SFG | −12 | 41 | 43 | 4064 | 4.22 | 0.000856 |
| Left IFG | −51 | 20 | 22 | 1896 | 4.65 | 0.000377 |
| Left MTG | −57 | −4 | −5 | 2618 | 6.27 | 0.00002 |
| Fair Play > Social Exclusion | ||||||
| Right parietal cortex | 51 | −40 | 34 | 15834 | −6 | 0.000032 |
| Right dlPFC | 36 | 44 | 34 | 5022 | −5.4 | 0.000094 |
| Right SFG | 6 | 23 | 61 | 3293 | −4.27 | 0.000771 |
| Right cuneus | 18 | −64 | 13 | 1275 | −3.68 | 0.002458 |
| Left precuneus | −12 | −46 | 52 | 1240 | −4.17 | 0.000946 |
| Left cerebellum | −33 | −49 | −26 | 4880 | −5.49 | 0.000079 |
| Right lateral occipital cortex | 39 | −64 | −2 | 1510 | −4.22 | 0.000852 |
| Left lateral occipital cortex | −42 | −64 | 4 | 1113 | −4.94 | 0.000218 |
| Left supramarginal gyrus | −60 | −46 | 28 | 1016 | −3.82 | 0.001862 |
Fig. 1.
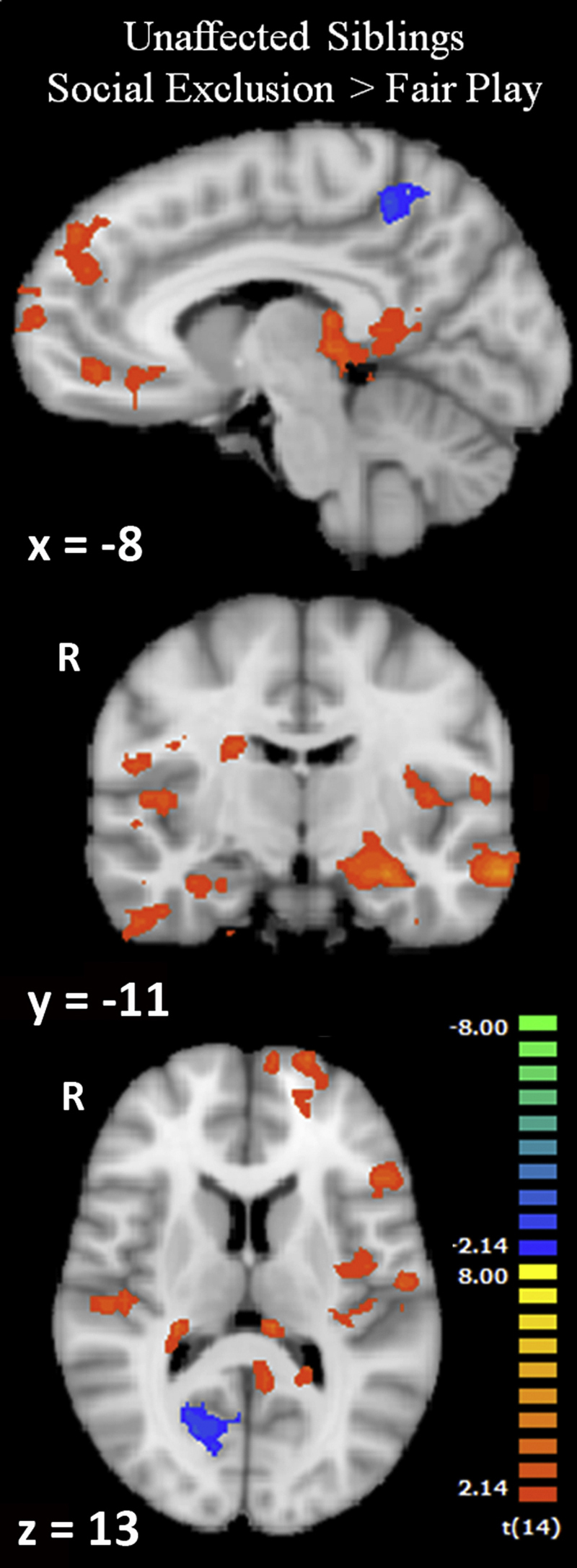
Social exclusion in UAS. Activation to Exclusion > Fair Play in UAS (p < 0.05, k = 918 mm). Images are displayed in radiologic convention. Coordinates are in Talairach space.
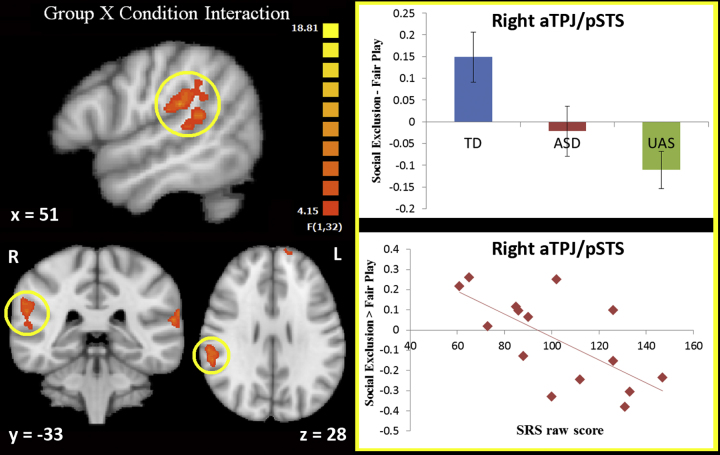
A Group (TD vs. UAS) X Condition (Social Exclusion X Fair Play) ANOVA revealed a significant interaction in eight regions (Table 3). These regions were right anterior temporoparietal junction extending into the superior temporal sulcus (aTPJ/pSTS), right anterior insula (rAI), bilateral precuneus, cuneus, left superior temporal gyrus (STG), left ventrolateral prefrontal cortex (vlPFC), and left posterior inferior temporal gyrus (pITG). All of these regions were defined as having a significant difference in activation to Social Exclusion vs. Fair Play in the TD vs. UAS groups. Of these 8 regions, only 1 showed a pattern of activation characteristic of a trait-level neural signature in the siblings (ASD group had activation in this region that significantly differed from the TD group (p < 0.05, uncorrected), but not the UAS). This trait region was right aTPJ/pSTS (Table 3, Fig. 2). Collapsed across groups, right aTPJ/pSTS activation did not show a significant correlation with age (r(48) = 0.07, p = 0.6). However, when assessing age correlations in each group separately, activation in right aTPJ/pSTS correlated significantly with age in UAS (r(13) = −0.597, p = 0.02), but not TD or ASD groups (p's > 0.3).
Table 3.
Group X Condition ANOVA comparing TD children and UAS in Cyberball (p < 0.05, k = 945 mm3). Asterisk denotes regions where activation in ASD youth significantly differed from TD children (p < 0.05) but not UAS (p > 0.2). Statistics and coordinates refer to the voxel of maximum signal change within each region. Size refers to the region extent in structural voxels (1 mm3).
| Region | X | Y | Z | Size | F | p |
|---|---|---|---|---|---|---|
| Right aTPJ/pSTS* | 57 | −25 | 25 | 3270 | 14.64 | 0.00057 |
| Right anterior insula | 39 | 8 | 1 | 1947 | 17.81 | 0.000188 |
| Cuneus | 6 | −73 | 10 | 8757 | 15.69 | 0.00039 |
| Right precuneus | 3 | −49 | 43 | 2517 | 11.55 | 0.001827 |
| Left precuneus | −12 | −49 | 55 | 1033 | 11.36 | 0.001973 |
| Left vlPFC | −11 | 65 | 22 | 972 | 8.6 | 0.006164 |
| Left ITG | −42 | −67 | 4 | 1212 | 15.9 | 0.000363 |
| Left STG | −60 | −28 | 13 | 1480 | 15.93 | 0.000359 |
Fig. 2.
Activation in right temporal cortex. Left panel: the region of right aTPJ/pSTS where activation to Exclusion vs. Fair Play differed between TD and UAS. Images are displayed in radiologic convention. Coordinates are in Talairach space. Right panel: bar graph depicts average parameter estimates from the right TPJ/pSTS for each group. Error bars depict SEM. Scatterplot depicts the relationship between SRS raw score and activation to Social Exclusion > Fair Play in the ASD group.
To assess if there existed anatomically distinct areas of activation within our aTPJ/pSTS cluster, we searched for coordinates of local maxima within the region. We identified voxels of maximum statistical significance within the aTPJ/pSTS cluster, with the constraint that they must be at least 15 mm apart, must have no adjacent voxels (voxels sharing a face, edge or vertex) with higher statistical values, and must not have any adjacent voxels outside of the cluster. The identified local maxima included Talairach coordinates: (57, −25, 25; also the absolute maximum) and (51, −37, 16).
To control for differences in nonverbal and overall IQ scores between UAS vs. ASD and TD groups, groups comparisons of aTPJ/pSTS ROI activation were recalculated with IQ scores regressed out. Regardless of sub-scores used (nonverbal, verbal, global), the difference in activation between UAS and TD children by which the region was delineated remained significant (p < 0.05), while the difference in activation between UAS and ASD participants remained non-significant (p > 0.05). We did not control for IQ in the comparison between activation in ASD and TD participants, as these two groups did not significantly differ on any IQ scales.
4. Discussion
The current study identified regions of the brain where activation in response to Social Exclusion vs. Fair Play in typically developing children and adolescents differed as a function of their biological vulnerability to autism. A group of healthy children defined as having a biological sibling with autism showed activity to social rejection that differed from the other TD youth in several brain regions. Most interestingly, activation in UAS mirrored abnormal neural activation to exclusion in ASD youth in the right aTPJ, extending into right pSTS. This pattern of activation is potentially suggestive of a trait deficit in neural processing during an experimental elicitation of social exclusion (relative to fair play), which is independent of autism symptomatology.
The notion of trait hypoactivation in right aTPJ is intriguing given the temporoparietal junction's well-established roles in both social and attentional processes (for reviews see Carter and Huettel, 2013, Decety and Lamm, 2007). Due to the social nature of the task utilized in the current study (Cyberball), we will first discuss our results in the context of TPJ function in social cognition. Attentional theories of TPJ activation as they relate to interpretations of the current results are discussed later. Right TPJ is active across several social domains, including theory of mind (Saxe and Kanwisher, 2003, Saxe and Wexler, 2005, Saxe et al., 2009, Young et al., 2010b), moral reasoning (Young et al., 2010a) and empathy (Jackson et al., 2006). In addition, abnormal activation in right TPJ has been found in individuals with ASD during gaze processing (Pitskel et al., 2011, von dem Hagen et al., 2014), imitation (Williams et al., 2006), theory of mind (Castelli et al., 2002, Kana et al., 2014, Mason et al., 2008) and moral judgments (Koster-Hale et al., 2013). While substantial research has examined theory of mind in autism (Baron-Cohen, 2000), investigations of theory of mind in UAS is comparatively scant. One such study reported poorer performance in UAS on a behavioral test of mind-reading, suggesting that difficulties in theory of mind may be shared to some extent between ASD probands and siblings (Dorris et al., 2004). Although we did not explicitly measure theory of mind/mentalization during social exclusion, one could speculate that the shared hypoactivation between ASD probands and siblings in right aTPJ during the experience of social exclusion in the current study may reflect abnormalities in social cognitive processing (i.e. theory of mind), representing a trait-level neurocognitive profile of ASD vulnerability. Further work is necessary to support the interpretation of right aTPJ activation during social exclusion as subserving mentalization processes where the excluded participant is thinking about the intentions of the excluders.
Trait hypoactivation in the right aTPJ included a ventral extension into the right pSTS. Posterior STS, like the adjacent TPJ, holds an important role in both low-level social perception of biologically relevant stimuli such as faces, as well as high-level processing of the thoughts and intentions of others (for review see Allison et al., 2000). Consistent with the integral role of the pSTS in social cognition, abnormal activation of this region is often found in individuals with ASD (Pelphrey and Carter, 2008).
A study of neuroendophenotypes of social processing in youth with ASD reported that a region of right pSTS showed hypoactivation in response to viewing point-light displays of biological motion (Kaiser et al., 2010). Interestingly, this pattern of hypoactivation in ASD probands was not shared with UAS. Participants in the current study partially overlap with those reported on by Kaiser et al. (2010); however, the neural profile identified in the current study is inconsistent with previous results. Specifically, we found that hypoactivation in the right pSTS was shared between ASD probands and siblings, not specific to probands as was previously reported. Consistent with the literature, the ASD group in both studies showed abnormal activation in the right pSTS. In contrast, the current study also revealed atypical pSTS activation to social exclusion in UAS. It is possible that the experience of social exclusion requires more complex and elaborate socio-emotional reasoning, and it is only during more complex tasks involving social and emotional processing that abnormal activation is revealed in the UAS. During passive viewing of point-light displays of biological motion, participants are not required to engage in any actions. In contrast, during Cyberball, participants are required to perform socially-contingent actions in the form of deciding to which virtual player to throw the ball. Further, in Cyberball, the actions of the virtual players are directly relevant to the participant, in that the participant is only included in the game if the other players throw to him/her. However, the actions of a point-light display of biological motion are not directly relevant to the participant viewing the lights, since there is no interaction (real or virtual) between them. Finally, the social experience of playing Cyberball is designed to elicit negative emotions. In contrast, point-light displays are not intended to elicit strong emotional responses. The interactive nature of the Cyberball task makes it more likely to require participants to engage in reasoning about the actions and intentions of others (in this case, the virtual players), both to decide to whom to throw (social) and to regulate negative feelings in response to exclusion (emotional). Similar to the findings of the current investigation, another emotionally-valenced study by Spencer et al. (2011) found that UAS differed from TD adolescents in right STS activation to emotional faces, with UAS showing no significant difference from their ASD siblings in this region.
Research in ASD and typical populations has highlighted a link between temporal lobe activation and social behavioral profiles. Social responsiveness (as measured by the SRS) has been found to be negatively correlated with right pSTS responses to social stimuli in children with ASD (Kaiser et al., 2010). Another measure of autistic traits, the autism quotient (AQ; Baron-Cohen et al., 2001), predicts variability in both structure and function of the pSTS in healthy adults (von dem Hagen et al., 2011) and correlates with pSTS responses to social touch in healthy adults (Voos et al., 2013). In the current study, 15 children with ASD had parent-reported measures of social responsiveness (SRS). A post hoc Pearson correlation revealed that activation in the trait-defined right aTPJ/pSTS showed a significant negative correlation with SRS score, as predicted (r(13) = −0.7, p < 0.05, two-tailed; Fig. 2). This means that within our ASD sample, increased impairment in social responsiveness was related to decreased activation in right aTPJ/pSTS during social exclusion compared to fair play. This is consistent with the finding that compared to TD youth with no social impairment, children with ASD showed significantly less activation in right aTPJ/pSTS.
However, this logic fails when applied to the UAS. While the UAS show no social impairment (SRS scores equivalent to the TD group), activation in the right aTPJ/pSTS in UAS represented trait hypoactivation shared with the ASD probands. The dissociation between brain and behavior implies that neural processing of social exclusion in UAS is special. One can put forth two potential explanations for this discrepancy. First, trait patterns in processing social exclusion shared between ASD and UAS groups reflect shared abnormalities in social behavior that were undetected in UAS in the current study, suggesting that the neural activation in UAS is reflective of a less-severe social phenotype of autistic traits. Second, UAS share some aspects of the neural profile seen in ASD but are able to maintain normal levels of social functioning.
We will expound upon the second explanation, that the UAS share some neural abnormalities with their autistic counterparts, however are able to maintain normal levels of social functioning. This explanation is supported by our careful characterization of the UAS group with the goal of excluding siblings displaying the broad autism phenotype. The conclusion that the unaffected siblings display normal social functioning is also supported by our failure to differentiate TD and UAS based on brain activation in many regions repeatedly implicated in typical processing of social exclusion. The regions identified in the within-group comparison of Social Exclusion > Fair Play in UAS were strikingly similar to activation patterns found in TD youth. One region of specific interest where TD and UAS groups did not diverge was the ACC. Both groups showed robust activation in ACC, an area found to be active in many tasks eliciting the experience of social rejection (Bolling et al., 2011a, Bolling et al., 2011c, Masten et al., 2009, Moor et al., 2012, Sebastian et al., 2011). Importantly, two independent investigations have reported hypoactivation of this region during social exclusion in youth with ASD (Bolling et al., 2011b, Masten et al., 2011). With respect to the ACC, the UAS demonstrated typical neural responses to social rejection. Posterior temporal lobe activation to social exclusion is often undiscussed, in favor of prefrontal and midline emotion-related neural circuitry such as the ACC.
Despite the intriguing nature of temporal lobe activation in ASD symptomatology, caution must be taken when interpreting the potential psychological correlates of abnormal activation in aTPJ/pSTS identified in the current study. The region identified as aTPJ/pSTS is somewhat anterior to typical TPJ coordinates reported in social cognition and mentalization research (TPJ: (50, −55, 25), pSTS: (50, −55, 10); Van Overwalle and Baetens, 2009). The absolute maximum of our reported aTPJ/pSTS cluster is questionably representative of our anatomical label. However, the location of the next-most significant local maximum within the cluster better reflects this label.
Multiple studies of the TPJ have found that the region serves both social and attentional functions (for reviews see Carter and Huettel, 2013, Decety and Lamm, 2007). However, within the TPJ, attention/reorienting processes tend to lie more anterior than social processes, with the former being centralized near the supramarginal gyrus and the latter being located near the angular gyrus. Thus, with the finding that our group differences in aTPJ/pSTS activation to social exclusion lie anterior to typical TPJ coordinates, it is possible that this activation is better characterized by attentional reorienting processes occurring during the Cyberball game. It has been hypothesized that the TPJ serves a role in contextual updating, a relatively late process in response to attentional cues (Geng and Vossel, 2013). This hypothesis predicts that activation in TPJ is increased in situations of expectancy violation, because events that violate one's predictions lead to shifts in mental context models. With this hypothesis in mind, it is possible that aTPJ/pSTS activation to social exclusion in the current study reflects a contextual updating process in response to a violation of the expectancy of social inclusion. Thus, hypoactivation in UAS and ASD groups may represent atypical social expectancies within these groups, or a deficit in contextual updating in response to experiencing unpredicted exclusion. It is of note that there still exists uncertainty over whether different cognitive functions activating the TPJ (e.g. social vs. attentional) represent distinct sub-regional functions in this brain area, or a unifying function that underlies the common cognitive domains subserved by the region (Lee and McCarthy, 2014).
To test the generalizability of our aTPJ/pSTS results to broader literature on the social functions of these regions, we created spherical regions of interest based on TPJ and pSTS coordinates reported by Van Overwalle and Baetens (2009). We delineated 10 mm radius spheres around the TPJ and pSTS Talairach coordinates (50, −55, 25) and (50, −55, 10), respectively. We then calculated beta values for the contrast of Social Exclusion > Fair Play for each participant, and averaged these values for each group (TD, ASD, UAS). Pairwise group comparisons as were conducted for our functionally defined regions did not reveal any significant group differences in either ROI (TD vs. ASD, TD vs. UAS, ASD vs. UAS all p's > 0.5, 2-tailed). Thus, we conclude from this analysis that the pattern of group differences we report are specific to the functional region defined by our comparison of the TD and UAS groups, and must be interpreted with caution when making comparisons to structural labels such as TPJ and pSTS.
Although the design of the current study is based on the social typicality of the unaffected siblings of children with ASD, we cannot expect that the lives of these siblings are in no way affected by having a brother or sister with autism. Utilizing unaffected siblings of patient populations is a commonly employed method for dissociating genetic traits versus disease states in psychopathology (Gottesman and Gould, 2003). However, in the current study where the variable in question is neural responses to social exclusion, the ability to dissociate genetics and environment is complicated by the possibility that having a sibling with ASD may influence one's experiences of peer rejection and acceptance. Siblings of children with ASD have been found to report feeling lonelier and having more peer problems than other healthy youth (Bågenholm and Gillberg, 1991). Notably, several studies do not support this finding (Kaminsky and Dewey, 2002, Macks and Reeve, 2007, Pilowsky et al., 2004, Rodrigue and Geffken, 1993). However, one might formulate an alternate hypothesis; instead of having unaffected peer experiences, the sibling group may represent an intermediary between the typical peer experiences of control children and the significantly negative peer experiences of children with ASD.
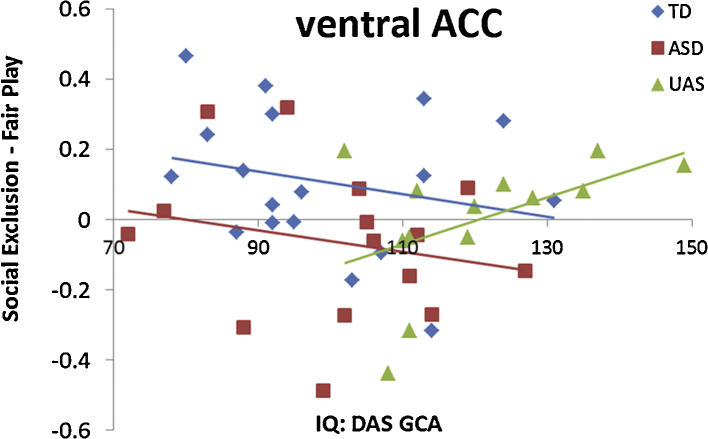
One intriguing characteristic of the current UAS sample is that on average, the siblings had higher IQ scores than both the ASD and TD groups. While regression analyses demonstrated that IQ differences could not explain the pattern of trait activation identified in aTPJ/pSTS, IQ may be a mechanism by which UAS maintain typical activation to social exclusion. We tested this hypothesis in an ROI where TD and UAS showed comparable activation to exclusion, the ACC. In a post hoc exploratory analysis, we extracted parameter estimates of activation to Social Exclusion > Fair Play from a 5 mm sphere centered on the voxel within ACC which previous work had found to show the largest difference in processing exclusion between TD and ASD youth (Bolling et al., 2011b; Talairach coordinates: [0,26, 10]). Consistent with our whole brain analyses in the current study, TD (n = 19) and UAS (n = 15) did not differ in activation in this ROI (t(32) = 1.61, p > 0.1). After excluding one TD child and one UAS for having IQ scores more than 2 standard deviations from their respective group means, we attempted to explain variance in vACC activation to Social Exclusion > Fair Play using linear regression. Predictor variables entered into the regression included IQ, group (ASD and UAS dummy coded in comparison to TD reference group), the interaction between IQ and ASD group membership, and the interaction between IQ and UAS group membership. Continuous variables for IQ and vACC activation were mean-centered. This 5 predictor model was able to account for 20.6% of the variance in vACC activation, F(5,40) = 2.07, p = 0.09. IQ did not significantly predict vACC activation (β = −0.26, p = 0.33). As expected by the method used to define the vACC region (TD > ASD), ASD group membership had significant partial effects in the model (β = −0.77, p = 0.04). UAS group membership was only marginally significant (β = −0.87, p = 0.07). Furthermore, the interaction between IQ and UAS group status was marginally significant (β = 0.81, p = 0.07), while the interaction between IQ and ASD group status was negligible (β = 0.01, p = 0.98). The TD and ASD groups each showed negative-sloping correlations, while the UAS showed a positive correlation between vACC activation and IQ (Fig. 3). This finding provides preliminary evidence that UAS group membership moderates the relationship between IQ and activation to social exclusion in vACC. Thus, high IQ may serve as a mechanism facilitating normal social cognition in UAS.
Fig. 3.
Correlation between activation to Social Exclusion > Fair Play and IQ measured by the DAS (GCA) in TD (blue), ASD (red), and UAS (green) youth (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Collapsed across groups, vACC activation did not show a significant correlation with age (r(48) = 0.23, p = 0.1). However, when assessing these correlations in each group separately, activation in the vACC ROI sphere correlated significantly with age in TD youth (r(17) = 0.464, p = 0.045), but not UAS or ASD groups (p's > 0.3). Developmental effects on brain responses to social exclusion in the TD participants of the current study have been reported elsewhere (Bolling et al., 2011a).
The current study has several limitations. First, the study design utilized a baseline condition (Fair Play) in order to maximize experimental specificity within our construct of interest (Social Exclusion). Utilizing a control condition of Fair Play (social inclusion) in contrast to Social Exclusion is standard for fMRI studies using a Cyberball manipulation (Cacioppo et al., 2013). This contrast is ideal, given that the two conditions differ minimally except for the variable of interest (rejection). Consequently, all results where we demonstrate hypoactivation to social exclusion could alternatively be interpreted as hyperactivation to fair play. However, the directionality in the current presentation of the results is precedented by previous research. Second, the limited sample size of the current investigation makes it difficult to detect significant group differences. Brain regions where TD and UAS differed in activation to social exclusion were subjected to a secondary analysis where activation in children with ASD was compared to TD and UAS groups in order to determine which region(s) showed a pattern of “trait” activation. We presented results that were uncorrected for the number of regions (8) which we analyzed in this way. Thus, we interpret our results cautiously, in order to generate hypotheses about the nature of social cognition in UAS. Similarly, IQ correlations were also limited in sample size and as such must be taken as propositional information, rather than confirmatory evidence. Finally, due to the anatomical variability in TPJ activation in studies of social cognition, it is difficult to make inferences about the psychological correlates of our temporal lobe activations. Thus, the interpretations discussed should not be considered an exhaustive list of the possible explanations for the current study's findings.
5. Conclusions
The results of this study generate two important hypotheses. First, we present evidence suggesting that neural endophenotypes of ASD defined as common atypical brain responses between probands and siblings may vary based on the complexity of the social task employed. The investigation of neural processing in UAS during a realistic social interaction (social exclusion) revealed a trait deficit in right aTPJ/pSTS, in contrast to previous work demonstrating typical pSTS responses to more basic social stimuli in UAS. More ecologically valid social experiences may reveal trait deficits in social processing which go undetected in comparatively more simple social tasks. Second, we present evidence suggesting that IQ could be a mechanism by which UAS maintain relatively normal brain responses to social exclusion in ACC. While neuroimaging research generally aims to compare participant groups matched on factors such as age and IQ, the current study took advantage of a group disparity in IQ to investigate potential compensatory mechanisms during social processing in UAS. It is important in attempting to match participant groups, that one does not ignore meaningful differences in the characteristics of the populations being studied. Future research will benefit from further investigation of the potential compensatory nature of elevated IQ in unaffected siblings of children with ASD.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The research presented was supported by grants from the National Institute of Mental Health (R01 MH084080), the John Merck Scholars fund, and the Simons Foundation. DZB was supported by NINDS T32 training grant (T32 NS07224).
Footnotes
Available online 30 April 2015
References
- Allison T., Puce A., McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. Pervasive developmental disorders. [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Bågenholm A., Gillberg C. Psychosocial effects on siblings of children with autism and mental retardation: a population-based study. J. Intellect. Disabil. Res. 1991;35:291–307. doi: 10.1111/j.1365-2788.1991.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Lotspeich L.J., Reiss A.L. Similar white matter aberrations in children with autism and their unaffected siblings. A diffusion tensor imaging study using tract-based spatial statistics. Arch. Gen. Psychiatry. 2010;67:1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Theory of mind and autism: a review. Int. Rev. Res. Ment. Retard. 2000;23:169–184. [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism spectrum quotient AQ: evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bauminger N., Kasari C. Loneliness and friendship in high-finctioning children with autism. Child Dev. 2000;71:447–456. doi: 10.1111/1467-8624.00156. [DOI] [PubMed] [Google Scholar]
- Belmonte M.K., Gomot M., Baron-Cohen S. Visual attention in autism families: ‘unaffected’ sibs share atypical frontal activation. J. Child Psychol. Psychiatry. 2010;51:259–276. doi: 10.1111/j.1469-7610.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., Mayes L.C., Pelphrey K.A. Development of neural systems for processing social exclusion from childhood to adolescence. Dev. Sci. 2011;14:1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., McPartland J.C., Kaiser M.D., Vander Wyk B.C., Wu J., Mayes L.C., Pelphrey K.A. Enhanced neural responses to rule violation in children with autism: a comparison to social exclusion. Dev. Cogn. Neurosci. 2011;1:280–294. doi: 10.1016/j.dcn.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., McPartland J.C., Mayes L.C., Pelphrey K.A. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S., Frum C., Asp E., Weiss R.M., Lewis J.W., Cacioppo J.T. A quantitative meta-analysis of functional imaging studies of social rejection. Sci. Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Huettel S.A. A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 2013;17:328–336. doi: 10.1016/j.tics.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Frith C., Happé F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Constantino J.N., Todd R.D. Autistic traits in the general population: a twin study. Arch. Gen. Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Alexander A.L., Davidson R.J. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol. Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Decety J., Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- DeWall C.N., Baumeister R.F. Alone but feeling no pain: effects of social exclusion on physical pain tolerance and pain threshold, affective forecasting, and interpersonal empathy. J. Pers. Soc. Psychol. 2006;91:1–15. doi: 10.1037/0022-3514.91.1.1. [DOI] [PubMed] [Google Scholar]
- Dorris L., Espie C.A.E., Knott F., Salt J. Mind-reading difficulties in the siblings of people with Asperger's syndrome: evidence for a genetic influence in the abnormal development of a specific cognitive domain. J. Child Psychol. Psychiatry. 2004;45:412–418. doi: 10.1111/j.1469-7610.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Elliott C.D. second ed. Harcourt Assessment; San Antonio, TX: 2007. Differential Ability Scales. [Google Scholar]
- Geng J.J., Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci. Biobehav. Rev. 2013;37:2608–2620. doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gupta A.R., State M.W. Recent advances in the genetics of autism. Biol. Psychiatry. 2007;61:429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Hauck M., Fein D., Waterhouse L., Feinstein C. Social initiations by autistic children to adults and other children. J. Autism Dev. Disord. 1995;25:579–595. doi: 10.1007/BF02178189. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Brunet E., Meltzoff A.N., Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Hudac C.M., Shultz S., Lee S.M., Cheung C., Berken A.M., Deen B., Pitskel N.B., Sugrue D.R., Voos A.C., Saulnier C.A., Ventola P., Wolf J.M., Klin A., Vander Wyk B.C., Pelphrey K.A. Neural signatures of autism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky L., Dewey D. Psychosocial adjustment in siblings of children with autism. J. Child Psychol. Psychiatry. 2002;43:225–232. doi: 10.1111/1469-7610.00015. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Libero L.E., Hu C.P., Deshpande H.D., Colburn J.S. Functional brain networks and white matter underlying theory of mind in autism. Soc. Cogn. Affect. Neurosci. 2014;9:98–105. doi: 10.1093/scan/nss106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J., Saxe R., Dungan J., Young L.L. Decoding moral judgments from neural representations of intentions. Proc. Natl. Acad. Sci. U.S.A. 2013;110:5271–5272. doi: 10.1073/pnas.1207992110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill A., Platek S.M. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Front. Evol. Neurosci. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., McCarthy G. Functional heterogeneity and convergence in the right temporoparietal junction. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little L. Peer victimization of children with Asperger spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:995–996. doi: 10.1097/00004583-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macks R.J., Reeve R.E. The adjustment of non-disabled siblings of children with autism. J. Autism Dev. Disord. 2007;37:1060–1067. doi: 10.1007/s10803-006-0249-0. [DOI] [PubMed] [Google Scholar]
- Mason R.A., Williams D.L., Kana R.K., Minshew N., Just M.A. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Colich N.L., Rudie J.D., Bookheimer S.Y., Eisenberger N.I., Dapretto M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Dev. Cogn. Neurosci. 2011;1:260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J.C., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor B.G., Güroğlu B., Op de Macks Z.A., Rombouts S.A.R.B., Van der Molen M.W., Crone E.A. Social exclusion and punishment of the excluders: neural correlates and developmental trajectories. Neuroimage. 2012;59:708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Ochs E., Kremer-Sadlik T., Solomon O., Sirota K.G. Inclusion as social practice: views of children with autism. Soc. Dev. 2001;10:399–419. [Google Scholar]
- Onoda K., Okamoto Y., Nakashima K., Mittono H., Ura M., Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc. Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Carter E.J. Brain mechanisms for social perception: lessons from autism and typical development. Ann. N. Y. Acad. Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky T., Yirmiya N., Doppelt O., Gross-Tsur V., Shalev R.S. Social and emotional adjustment of siblings of children with autism. J. Child Psychol. Psychiatry. 2004;45:855–865. doi: 10.1111/j.1469-7610.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Hudac C.M., Lantz S.D., Minshew N.J., Vander Wyk B.C., Pelphrey K.A. Brain mechanisms for processing direct and averted gaze in individuals with autism. J. Autism Dev. Disord. 2011;41:1686–1693. doi: 10.1007/s10803-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue J.R., Geffken G.R. Perceived competence and behavioral adjustment of siblings of children with autism. J. Autism Dev. Disord. 1993;23:665–674. doi: 10.1007/BF01046108. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. People thinking about people: the role of the temporo-parietal junction in theory of mind. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R., Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Saxe R.R., Whitfield-Gabrieli S., Scholz J., Pelphrey K.A. Brain regions for perceiving and reasoning about other people in school-aged children. Child Dev. 2009;80:1197–1209. doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Blakemore S-J., Charman T. Reactions to ostracism in adolescents with autism spectrum conditions. J. Autism Dev. Disord. 2009;39:1122–1130. doi: 10.1007/s10803-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., Tan G.C.Y., Roiser J.P., Viding E., Dumontheil I., Blakemore S-J. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Spencer M.D., Holt R.J., Chura L.R., Suckling J., Calder A.J., Bullmore E.T., Baron-Cohen S. A novel functional brain imaging endophenotype of autism: the neural response to facial expression of emotion. Transl. Psychiatry. 2011;1:e19. doi: 10.1038/tp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes W., Humphrey N. Peer-group indicators of social inclusion among pupils with autistic spectrum disorders ASD in mainstream secondary schools: a comparative study. Sch. Psychol. Int. 2010;31:478–494. [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- von dem Hagen E.A., Nummenmaa L., Yu R., Engell A.D., Ewbank M.P., Calder A.J. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb. Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen E.A.H., Stoyanova R.S., Rowe J.B., Baron-Cohen S., Calder A.J. Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cereb. Cortex. 2014;24:1485–1492. doi: 10.1093/cercor/bht003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos A.C., Pelphrey K.A., Kaiser M.D. Autistic traits are associated with diminished neural response to affective touch. Soc. Cogn. Affect. Neurosci. 2013;8:378–386. doi: 10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Gilchrist A., Perrett D.I., Murray A.D., Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams K.D. Ostracism. Annu. Rev. Psychol. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Cheung C.K.T., Choi W. Cyberostracism: effects of being ignored over the internet. J. Pers. Soc. Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williamson S., Craig J., Slinger R. Exploring the relationship between measures of self-esteem and psychological adjustment among adolescents with Asperger syndrome. Autism. 2008;12:391–402. doi: 10.1177/1362361308091652. [DOI] [PubMed] [Google Scholar]
- Wing L., Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Young L., Camprodon J.A., Hauser M., Pascual-Leone A., Saxe R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6753–6758. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L., Dodell-Feder D., Saxe R. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia. 2010;48:2658–2664. doi: 10.1016/j.neuropsychologia.2010.05.012. [DOI] [PubMed] [Google Scholar]