Abstract
Apolipoprotein E (APOE=gene, apoE=protein) is a known factor regulating the inflammatory response that may have regenerative effects during tissue recovery from injury. We investigated whether apoE deficiency reduces the healing effect of alanyl-glutamine (Ala-Gln) treatment, a recognized gut-trophic nutrient, during tissue recovery after 5-FU-induced intestinal mucositis. APOE-knockout (APOE-/-) and wild-type (APOE+/+) C57BL6J male and female mice (N=86) were given either Ala-Gln (100 mM) or phosphate buffered saline (PBS) by gavage 3 days before and 5 days after a 5-fluorouracil (5-FU) challenge (450 mg/kg, via intraperitoneal injection). Mouse body weight was monitored daily. The 5-FU cytotoxic effect was evaluated by leukometry. Intestinal villus height, villus/crypt ratio, and villin expression were monitored to assess recovery of the intestinal absorptive surface area. Crypt length, mitotic, apoptotic, and necrotic crypt indexes, and quantitative real-time PCR for insulin-like growth factor-1 (IGF-1) and B-cell lymphoma 2 (Bcl-2) intestinal mRNA transcripts were used to evaluate intestinal epithelial cell turnover. 5-FU challenge caused significant weight loss and leukopenia (P<0.001) in both mouse strains, which was not improved by Ala-Gln. Villus blunting, crypt hyperplasia, and reduced villus/crypt ratio (P<0.05) were found in all 5-FU-challenged mice but not in PBS controls. Ala-Gln improved villus/crypt ratio, crypt length and mitotic index in all challenged mice, compared with PBS controls. Ala-Gln improved villus height only in APOE-/- mice. Crypt cell apoptosis and necrotic scores were increased in all mice challenged by 5-FU, compared with untreated controls. Those scores were significantly lower in Ala-Gln-treated APOE+/+ mice than in controls. Bcl-2 and IGF-1 mRNA transcripts were reduced only in the APOE-/--challenged mice. Altogether our findings suggest APOE-independent Ala-Gln regenerative effects after 5-FU challenge.
Keywords: Apolipoprotein E, 5-Fluorouracil, Alanyl-glutamine, mucositis, mice
Introduction
5-Fluorouracil (5-FU) is the most frequently prescribed anticancer drug for clinical treatment of various types of cancer, including colorectal cancer (1,2). 5-FU may lead to intestinal mucositis, villus atrophy, and crypt necrosis as a result of mucosal tissue damage due to inflammatory cell infiltration, proinflammatory cytokine release and edema (3). A rapid increase in epithelial cell apoptosis and arrest of cell division within the intestinal crypts can lead to a breakdown of the intestinal epithelial barrier and continuous luminal bacterial translocation (4,5). 5-FU-induced intestinal mucositis is clinically relevant because it may disrupt the intestinal absorptive surface, causing nutrient malabsorption and a poor health condition that may lead to therapy failure if associated with diarrhea and vomiting (6). Such side effects may be aggravated by the presence of other simultaneous inflammatory bowel diseases (7).
Glutamine is considered to be a critical gut-trophic nutrient that has been shown to protect against intestinal injury in experimental models (8,9) and to be beneficial in clinical studies enrolling children with malnutrition and diarrhea in the developing world (9,10). Glutamine has been also implicated in the regulation of the innate immune system (11), the inflammatory process (12) and intestinal epithelial viability following Clostridium difficile toxin A (13) and 5-FU-challenge (14).
Recently, our group has documented anti-inflammatory and healing activities of the apolipoprotein E COG 133 mimetic peptide in a model of 5-FU-induced intestinal mucositis in vivo and in vitro. This suggests that apolipoprotein E (apoE), a cholesterol-carrier protein, is involved in the inflammatory process and intestinal epithelial restitution following 5-FU-induced tissue damage (15). In addition, we have demonstrated that APOE4 target replacement mice were protected against Cryptosporidium parvum infection by balancing the inflammatory responses (16). The APOE4 gene was also found to benefit the cognitive development of children with heavy diarrhea burdens (17) and may influence pro-cognition effects of gut-trophic nutrients in children at risk of diarrhea and malnutrition (18).
Alanyl-glutamine (Ala-Gln) is a glutamine dipeptide, but is more soluble and stable than glutamine. In order to assess whether apoE deficiency jeopardized the healing effects of Ala-Gln treatment during tissue recovery after 5-FU-induced intestinal mucositis, we treated 5-FU-challenged APOE knockout (APOE-/-) and wild-type (APOE+/+) mice with Ala-Gln (100 mM) and monitored parameters of intestinal surface recovery and crypt cell integrity and renewal.
Material and Methods
5-Fluorouracil
5-Fluorouracil (Eurofarma¯, Brazil) was obtained from the Laboratório de Farmacologia da Inflamação e do Câncer (LAFICA), Universidade Federal do Ceará (UFC) and used to induce intestinal mucositis. Alanyl-glutamine (Ala-Gln) was obtained from Rexim (Courbevoie, France). A 100 mM stock solution was prepared in phosphate buffered saline (PBS) consisting of 0.9% NaCl, 10 mM Na2HPO4, 10 mM NaH2PO4, pH 7.4.
Animals
C57BL6J wild-type and APOE-knockout (APOE-/-) male and female mice were obtained from the Universidade de São Paulo transgenic vivarium and housed in the Instituto de Biomedicina at the Universidade Federal do Ceará (UFC). When obtained, experimental mice weighed 20-25 g. All experimental protocols were in compliance with the Brazilian College for Animal Experimentation (COBEA) and the Animal Care and Use Committee Guidelines of the UFC. Experimental mice were kept in polyethylene boxes with free access to animal chow and water until sacrificed, and maintained on a 12-h light-dark cycle. The mice were randomly allocated to 6 treatment groups: 5-FU: (n=15 for APOE+/+ and n=14 for APOE-/- mice), Ala-Gln (n=16 for APOE+/+ and n=13 for APOE-/- mice), or PBS (n=15 for APOE+/+ and n=13 for APOE-/- mice).
Mucositis induction, Ala-Gln treatment and tissue collection
For the induction of intestinal mucositis, a single intraperitoneal (ip) dose of 5-FU (450 mg/kg) was administered to both wild-type and APOE-/- mice on the 1st day of the experiment; control animals received PBS. Experimental mice received either 100 mM of Ala-Gln (0.5 mL/animal) or PBS control solution given by oral gavage 3 days prior to 5-FU administration and for 5 days afterwards. Animals were weighed daily and were euthanized on day 6 with an overdose of ketamine/xylazine solution. After sacrifice, 1-cm-long ileal segments were harvested, immediately frozen in liquid nitrogen and stored in a -80°C freezer until use. Additional 1-cm ileal segments were fixed in formaldehyde and stored for further histology processing.
Blood leukocyte counts
In order to evaluate 5-FU cytotoxicity, blood leukocyte counts were carried out in Neubauer chambers. Mice were lightly anesthetized with ether, and peripheral blood was drawn up in a capillary tube inserted into the orbital plexus. The blood was mixed with a 3% acetic acid solution (Turk's solution) at a 1:20 ratio for loading into the chambers; leukocyte counts were recorded as cells/mm3.
Analyses of intestinal surface recovery
Intestinal morphometric analysis
Morphometric analysis was carried out in low magnification photomicrographs of hematoxylin and eosin (H&E)-stained tissue using the Image J software 1.4 (National Institutes of Health, USA) after proper calibration. Villus height was measured in at least 10 villus longitudinal sections (4 animals per group). Intestinal crypts were severely affected by 5-FU treatment in our regimen protocol; therefore crypt length measurements were not considered.
Villin protein expression by immunoblotting
Western blots of ileal villin were used to evaluate the brush border of the intestinal absorptive surface. In brief, intestinal segments were harvested and immediately frozen in liquid nitrogen. Thawed specimens were pulverized in glass homogenizers containing lysis buffer and then transferred to test tubes with protease inhibitor and centrifuged at 17,530 g for 10 min. Supernatants were assayed using the bicinchoninic acid method (BCA Protein Assay Kit; Pierce, USA) to standardize 50 μg of protein product in each well. Samples were loaded onto 10% denaturing polyacryamide gels (Amersham Biosciences, UK). The gels were then transferred onto nitrocellulose membranes. Membranes were blocked overnight (5% fat-free milk solution), incubated with rabbit villin (1:500) or β-actin (1:1000) antibody for 1 h, rinsed 3 times in rinsing buffer, incubated in a biotinylated secondary antibody (horseradish peroxidase, 1:1000), and then rinsed as described above. Each membrane was washed with coumaric acid, luminol, Tris, and H2O2 and exposed to Kodak X-Omat AR film (Kodak, USA). Western blot bands were identified and the densitometry analyzed by Image J (Media Cybernetics, USA), and are reported as villin/β-actin ratio.
Crypt integrity and function
Mitotic and apoptotic indexes
To evaluate the role of Ala-Gln in healing of the injured small intestinal mucosa, we determined the mitotic index by counting well-defined mitotic figures in the crypt bases. An investigator who was blinded to the study treatment did the counts. The number of mitotic figures per crypt were scored in 20 H&E stained longitudinal crypt sections from each mouse intestine (n=4 mice per group). The crypts were selected by identifying the proliferative glands. The absolute values were averaged to produce the mitotic index of each group. The apoptotic index was investigated by blindly counting the apoptotic bodies per crypt in at least 20 H&E stained longitudinal crypt sections from each mouse intestine (n=4 mice per group). Measurements were made by light microscopy (Olympus CX3, Japan) using an immersion objective (1000×) and image acquisition (Q-Color 3, Olympus). Crypt mitotic figures included classical mitosis phases or cytokinesis; apoptotic figures were identified as eosinophilic bodies with a fragmented nucleus and were generally found near or inside the intestinal gland lumen, as described elsewhere (5).
Crypt necrotic scores
Crypt necrotic scores were determined by light microscopy (Olympus CX31) in H&E-stained slides at both low and high magnification by an experienced histologist who was blinded to the experimental treatments. Table 1 lists the criteria used for evaluation and scoring of intestinal crypts. A lower score indicates normal crypt architecture with secretory cells having conspicuous cytoplasmic granules.
IGF-1 and BCL-2 quantitative real-time PCR (qPCR)
Samples were taken immediately from necropsied animals and frozen in liquid nitrogen. After freezing, the samples were stored at -80°C until analysis. Samples were thawed and total RNA was extracted using Qiagen RNeasy mini kit (QIAGEN Biotecnologia Brasil Ltda, Brazil) according to the manufacturer's instructions. RNA concentration was quantified and checked spectrophotometrically (Biophotometer; Eppendorf, Germany) for purity by UV absorbance at 260 nm and 280 nm (A260:280 ratio). Synthesis of cDNA by reverse transcriptase PCR was performed using the SuperScript III First-Strand Synthesis System SuperMix (Invitrogen, Life Technologies, Brazil) with the use of oligo (dT) as primers. cDNA was used in qPCR for measuring IGF-1 and BCL-2 expression compared with actin expression (used as the housekeeping gene). The primers for murine IGF-1 and BCL-2 were purchased from Invitrogen and their nucleotide sequences are shown in Table 2. Amplification consisted of 10 min at 95°C, followed by 40 cycles of 25 s at 95°C, 25 s at the respective annealing temperature for each pair of primers (60°C), and 20 s at 72°C, followed by 40 cycles of 10 s starting at 75°C, with 0.5°C increments for the melt curve. Fluorescence was measured during the annealing step of each cycle. The relative gene expression was determined using the 2−ΔΔCt method.
Statistical analysis
Results are reported as means±SEM, except for Table 3 where data are reported as means±SD. ANOVA followed by the Bonferroni correction was applied to compare multiple groups. The unpaired Student's t-test was used for two-group comparisons. The nonparametric Mann-Whitney test was used to determine the significance of differences in crypt necrosis scores. P<0.05 indicated statistically significant differences.
Results
Weight curves
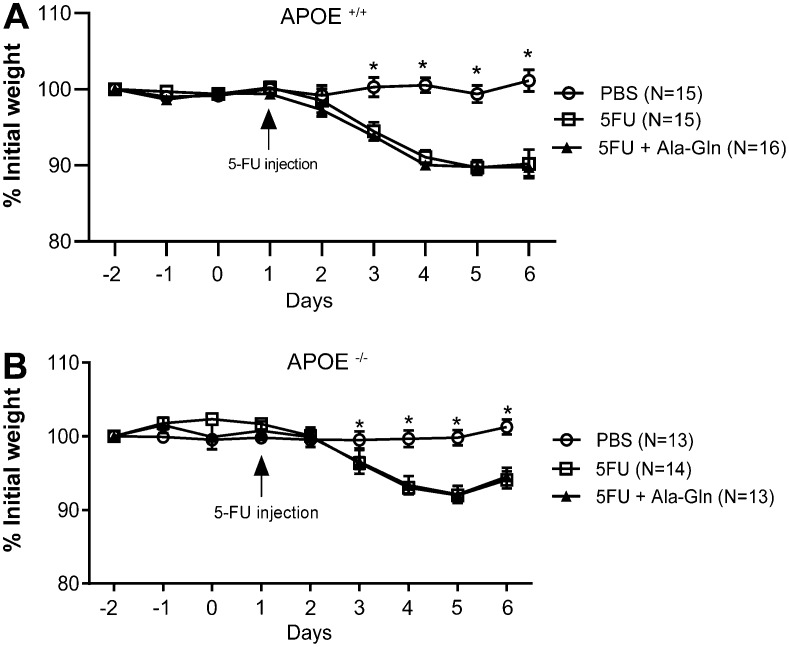
Following the 5-FU challenge (a single 450 mg/kg ip dose), body weight significantly decreased in all experimental groups and both mouse strains, as early as 3 days post-injection (P<0.05). Ala-Gln treatment (100 mM) did not restore weight to the level of the unchallenged control group. APOE-/- mice were slightly less affected than the wild-type controls following the 5-FU challenge (Figure 1).
Figure 1. Weight curves of wild-type (APOE+/+) (A) and APOE knockout (APOE-/-) mice (B) following 5-fluorouracil (5-FU)-induced mucositis and of unchallenged controls receiving phosphate buffered saline (PBS). Data are reported as means±SEM. *P<0.001 vs other groups (one-way ANOVA, followed by the Bonferroni multiple test).
Blood leukocyte counts
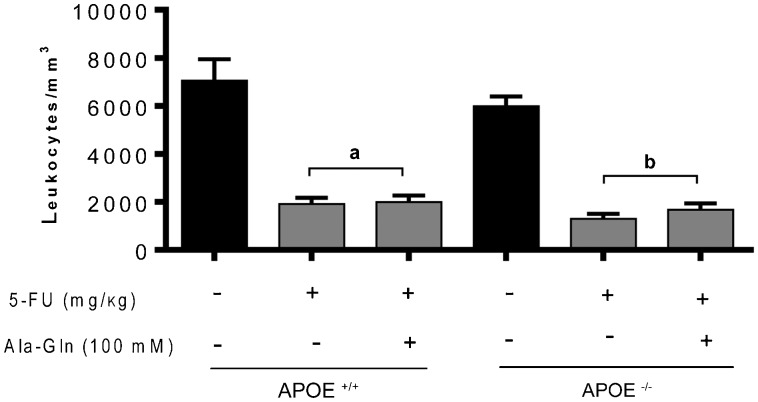
A significant leukopenia (reduction in the number of leukocytes/mm3) was seen by 5 days following the 5-FU injection, regardless of the mouse genetic background (P<0.001), when compared with unchallenged wild-type controls. This finding confirms the cytotoxic effect of 5-FU. This leukopenic effect was not altered nor prevented by Ala-Gln administration (P>0.05; Figure 2).
Figure 2. Leukometry results. Mice were anesthetized and blood samples were collected 5 days after 5-fluorouracil (5-FU) challenge. The total number of white cells was determined after dilution in Turk's solution using a Neubauer chamber. Data are reported as means±SEM of the number of leukocytes per mL of sample (n=7 animals/group). aP<0.001 vs unchallenged wild-type (APOE+/+) mice. bP<0.001 vs unchallenged APOE knockout (APOE-/-) mice (one-way ANOVA, followed by the Bonferroni multiple test).
Analysis of the intestinal surface recovery
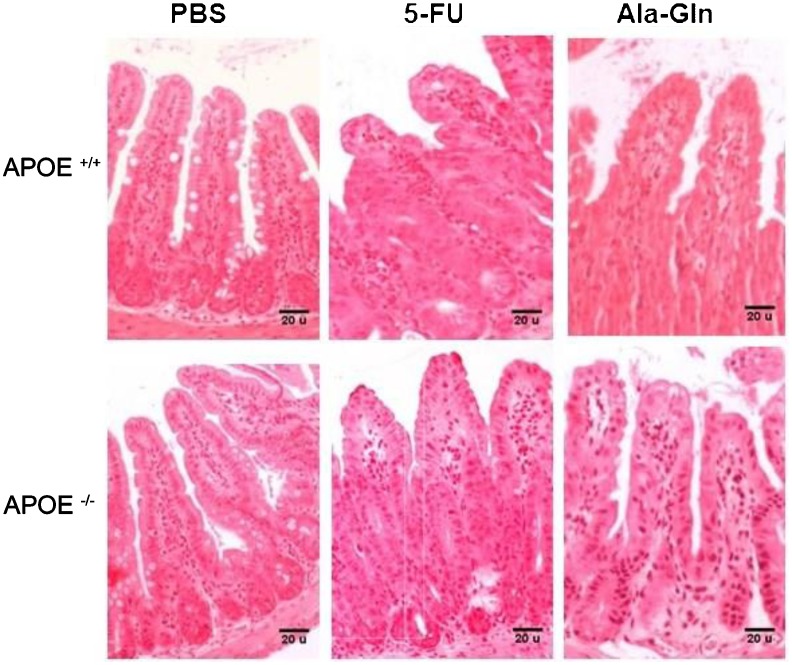
The 5-FU challenge (450 mg/kg) caused a significant reduction in villus height (P<0.001) in both APOE+/+ and APOE-/- animals compared with their respective PBS controls. Treatment with Ala-Gln (100 mM) enhanced villus height (P<0.01), regardless of apoE deficiency, by 5 days after the 5-FU challenge. However, the villus histology in Ala-Gln-treated mice still included few goblet cells and did not yet resemble that of the unchallenged controls (Figure 3 and Table 3). The crypts of both wild-type (P<0.001) and APOE-/- (P<0.01) mice given the 5-FU challenge were significantly longer than those of PBS controls, a finding that was partially reversed (P<0.001) or improved (P<0.0001) by Ala-Gln administration (Figure 3). As expected, 5-FU induced a significant reduction (P<0.0001) in the morphometric villus/crypt ratio compared with the PBS controls, a finding that was partially reversed (P<0.0001) by Ala-Gln treatment, regardless of the mouse strain (Table 3). No between-group differences were found in total ileal villin expression 5 days after 5-FU challenge (data not shown), suggesting that microvilli recovery precedes return of villus height.
Figure 3. Representative histology of H&E-stained ileal tissue (100×) from experimental mice with 5-fluorouracil-induced intestinal mucositis (5-FU, 450 mg/kg, ip, single dose), and mice treated with alanyl-glutamine (Ala-Gln, 100 mM) or phosphate buffered saline (PBS) via gavage on day 6 post-challenge.
Mitotic and apoptotic indexes
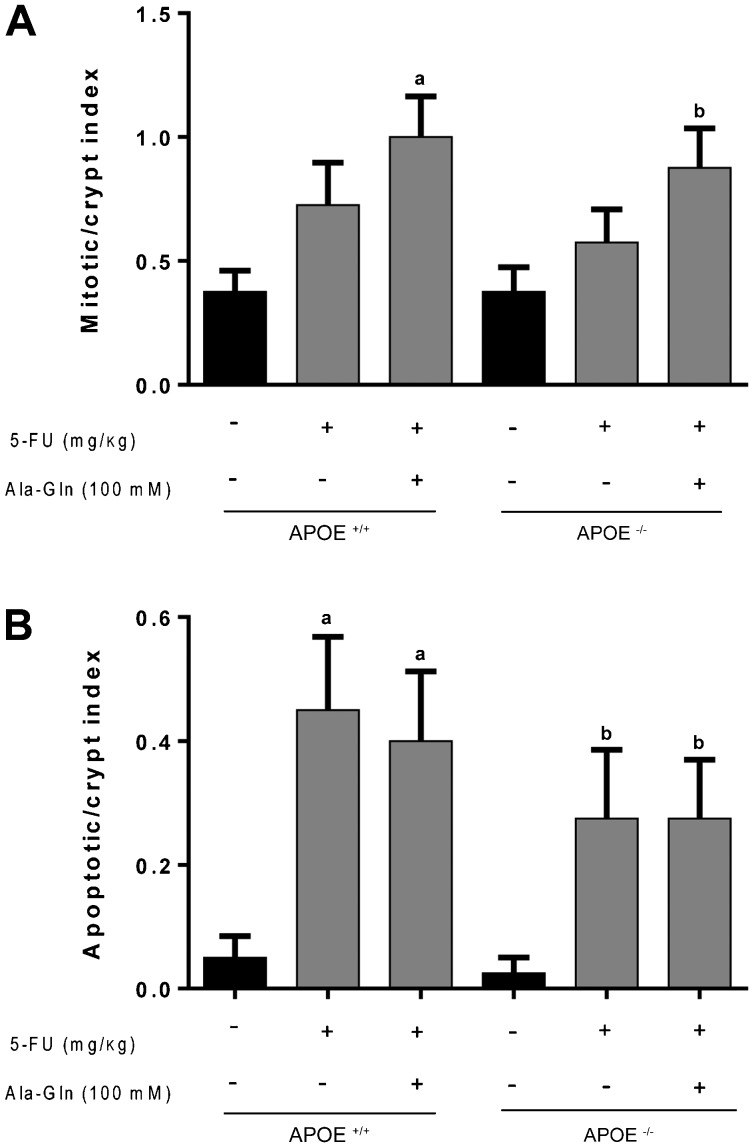
Albeit we found enhanced mitotic crypt index 6 days-following 5-FU-injection, this effect was not significantly different compared to the unchallenged controls both in the wild-type and APOE(-/-) mice. Ala-Gln was not able to improve crypt mitotic index compared with the untreated challenged mice in both mouse strains. However, Ala-Gln improved crypt mitotic index only when compared with the unchallenged control (p <0.01). 5-FU challenge remarkably increased crypt apopotic index regardless of the mouse genetic background (P <0.05). Ala-Gln was unable to reduce the crypt apoptotic index following 5-FU challenge (Figure 4).
Figure 4. Mean mitotic (A) and apoptotic (B) indexes of H&E-stained ileal crypts from C57BL6J ApoE-knockout (APOE-/-) and wild-type (APOE+/+) mice following 5-fluorouracil (5-FU)-induced intestinal mucositis (450 mg/kg, ip, single dose) and treated with the alanyl-glutamine (Ala-Gln, 100 mM) or phosphate buffered saline (PBS, control) via gavage. At least 10 crypts per animal were analyzed (n=4 animals/group) on the 6th day post-challenge. Data are reported as means±SEM. aP<0.001 vs unchallenged APOE+/+ mice; bP<0.05 vs unchallenged APOE-/- mice (one-way ANOVA and the Bonferroni test).
Necrotic crypt scores
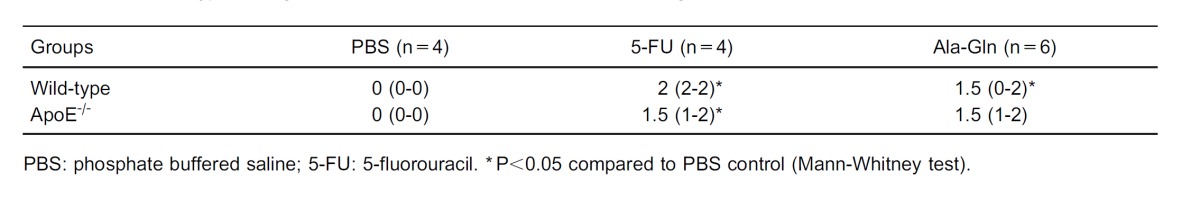
As expected, unchallenged PBS mice met the zero degree score criteria established for necrotic crypts (see Tables 1 and 4). After being challenged by 5-FU (450 mg/kg), both the wild-type and APOE-/- mice had a significant increase in ileal necrotic crypt scores (P<0.028) when compared with PBS controls. No differences in crypt necrotic scores were observed between mouse strains after 5-FU challenge. Ala-Gln treatment significantly reduced the necrotic crypt scores only in wild-type mice (P<0.04) (Table 4).
IGF-1 and BCL-2 quantitative real-time PCR (qPCR)
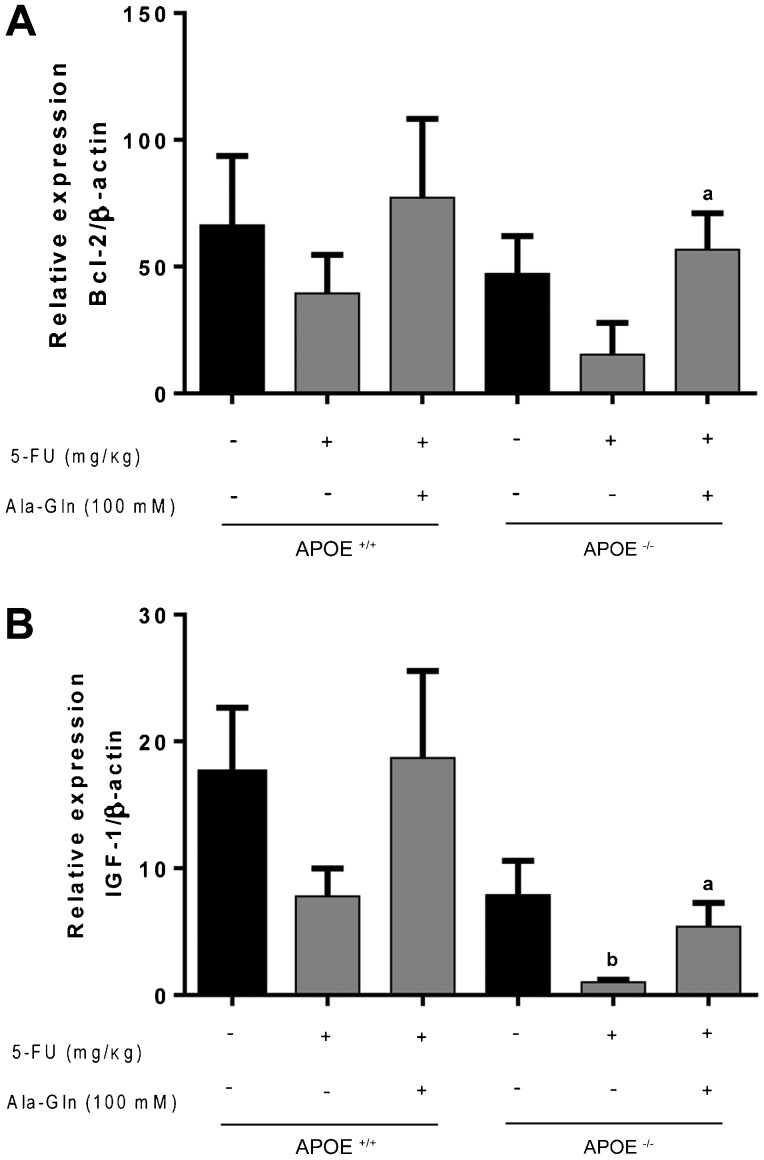
No statistical differences were found in the ileal IGF-1 and Bcl-2 mRNA transcripts 5 days after 5-FU injection in APOE+/+ mice compared with the unchallenged controls. Ala-Gln treatment raised ileal Bcl-2 transcripts following 5-FU challenge in the APOE-/- mice. Noteworthy, 5-FU caused a significant reduction in the ileal IGF-1 mRNA transcripts at 5 days post-challenge only in the APOE-/- mouse strain (P<0.05), an effect that was improved by Ala-Gln (100 mM) treatment (see Figure 5).
Figure 5. Quantitative real-time PCR for B-cell lymphoma 2 (Bcl-2) and insulin-like growth factor-1 (IGF-1) mRNA transcripts from C57BL6J ApoE-knockout (APOE-/-) and wild-type (APOE+/+) mice challenged by 5-fluorouracil (5-FU)-induced intestinal mucositis (450 mg/kg, ip, single dose) on the day 6 post-challenge and treated with alanyl-glutamine (Ala-Gln, 100 mM) or phosphate buffered saline (PBS, control) via gavage. Data are reported as means±SEM. aP<0.05 vs 5-FU-challenged group. bP<0.05 vs unchallenged control (one-way ANOVA and the Bonferroni test).
Discussion
Recently, we documented a protective effect of the apoE mimetic peptide COG133 in 5-FU-induced intestinal mucositis, with reduction in the inflammatory cytokine response and improvements in intestinal villus height. We have also confirmed a healing effect of the apoE COG133 in the rat jejunal IEC-6 wound model after 5-FU exposure (15). Ala-Gln is a glutamine compound with increased solubility and stability. It is known to be a key gut-tropic nutrient (19), and has been found to improve healing in the IEC-6 model (14). In this study, we addressed whether Ala-Gln requires apoE for improving intestinal mucosal recovery following 5-FU-induced tissue damage. To test this hypothesis, we used APOE-/- and wild-type (APOE+/+) mice with or without 5-FU challenge.
5-FU induces weight loss in mice mainly due to intestinal mucositis and systemic changes, affecting crypt cell turnover, and ultimately leading to reduced villus absorptive surface area (20). 5-FU-challenged mice develop dehydration, anorexia and exhibit a 20% weight loss 6 days after 5-FU injection, in addition to profound leukopenia (4). It is recognized that myelotoxicity is one of the major side effects in patients receiving 5-FU treatment (21). Leukopenia and neutropenia are significantly correlated with the pharmacokinetic parameters of 5-FU metabolism, with increase in 5-FU exposure leading to greater toxicity. Moreover, leukopenia and neutropenia are inversely correlated with peripheral blood mononuclear cell telomere length (22). As shown previously by our earlier study, Ala-Gln treatment could not promote weight gain in 5-FU challenged mice (5). In this study, C57BL6J APOE+/+ and APOE-/- mice showed significant weight loss compared with PBS controls by day 3 after 5-FU challenge. Likewise, we could not find a benefit of Ala-Gln treatment in improving weight gain following 5-FU challenge, independent of the genetic background of the tested mice. In support of our findings, one small clinical trial using intravenous alanyl-glutamine infusion in patients with neck and head cancer undergoing chemoradiotherapy (radiotherapy daily up to 70 Gy, plus cisplatin/5-fluoruracil once a week) found improvement of oral mucositis after Ala-Gln treatment, but without change in weight (23).
Soares et al. (20) challenged Wistar rats with the same 450 mg/kg 5-FU dose used in this study, and reported prolonged weight decrements up to 15 days after 5-FU challenge, compared with unchallenged controls. Therefore, improvements in intestinal mucositis precede weight catch-up; however it seems that even with improved villus height in Ala-Gln-treated mice by the sixth post-challenge day, nutrient absorption may still have been too low to support weight recovery. Although villi were taller at that time, villus histology did not yet resemble that of the controls. Goblet cells (in the villus and crypts) and Paneth cells (in the crypts) were still scarce at 6 days post-challenge in the 5-FU-treated mice regardless of the presence of the APOE gene.
In a study by Carneiro-Filho et al. (19), treatment with Ala-Gln (100 mM), but not with Gln (100 mM), ameliorated intestinal morphometry (Ala-Gln improved villous area) and increased the crypt mitotic index following 5-FU challenge. Our findings did not include an increase in crypt mitotic index following Ala-Gln treatment. This discrepancy may be due to the difference in the mouse strain used. Also, the higher 5-FU dose used in our study (450 mg/kg ip, single dose) may have lowered the crypt mitotic rate for a longer time.
The crypt hyperplasia present on day 6 after 5-FU challenge represents a compensatory phenomenon to renew the intestinal epithelium and restore the absorptive surface compromised by chemotherapy. Ala-Gln treatment improved villus height and villus/crypt ratio in both APOE+/+ and APOE-/- mice, suggesting that the Ala-Gln mucosal healing effect occurs regardless of apoE deficiency.
Villin is a structural component of microvilli forming the brush border of the small intestine and plays a key role in maintaining brush border organization by binding to F-actin in a network of filaments (24). The brush border acts to increase the absorptive surface of the enterocytes. Although villus blunting was seen in 5-FU-challenged mice, the villin/actin ratio was not significantly different between groups, suggesting sufficient protein recovery 5 days after the administration of 5-FU. Protein recovery precedes the recovery of the villus height.
Circulating factors, such as IGF-1, have been implicated in the control of intestinal epithelial proliferation (25,26) through interactions with gut trophic nutrients, multiple hormones, and growth factors (27). Furthermore, oral IGF-1 treatment has been shown to improve Gln transport in piglet enterocytes (28). APOE-/- (but not wild-type) mice challenged by 5-FU (450 mg/kg) had reduced IGF-1 mRNA transcription, suggesting that apoE could influence intestinal IGF-1 levels. This effect was reversed by Ala-Gln treatment. At this stage, this result remains unexplained. An earlier study from our group documented impaired intestinal IGF-1 expression following refeeding in APOE-/- subjected to maternal-offspring separation (29).
One explanation for the apoE effects on IGF-1 levels may be related to a potential apoE protective effect on the liver. ApoE may redirect lipopolysaccharide (LPS) (from the leaky gut) from Kupffer cells to liver parenchymal cells, improving LPS clearance from circulation via bile (30), which may improve liver and intestinal IGF-1 levels. That hypothesis should be further explored in future studies using the 5-FU induced-intestinal mucositis model. Another interesting approach would be to assess whether the intestinal microbiome is changed by Ala-Gln and how much that is affected by apoE deficiency. Probiotics have been used recently to improve 5-FU intestinal mucositis (31) and Gln has been shown to activate the innate immune system through the intestinal microbiome (11).
Cool et al. (32) investigated a 7-day infusion (4.3 mg/kg daily) of IGF-I to Sprague-Dawley rats challenged by 5-FU (150 mg/kg, ip). They reported that 5-FU reduced the villus height in the duodenum by 23%, in the jejunum by 20%, and in the ileum by 30% at 48-h post-challenge, with an 87-times increase in the crypt apoptotic rate. These effects were substantially less pronounced in IGF-I pretreated rats.
An increase in the apoptotic rate in the crypts (which was not improved by the administration of Gln or Ala-Gln) has been found on day 1, and to a lesser extent on day 6, after 5-FU injection, compared with a PBS control (5). In addition, Yasuda et al. (33) have documented that the number of apoptotic, caspase-3- and caspase-8-activated cells increased 24 h after the first 5-FU administration.
In agreement with those results, we found a significant increase in the apoptotic crypt index at day 6 post-challenge in both APOE-/- and wild-type mice, an effect that was not reversed by Ala-Gln treatment. To evaluate this effect at the molecular level, we assessed the antiapoptotic Bcl-2 mRNA transcripts by q PCR. Ala-Gln enhanced the Bcl-2 transcription activity compared with the control only in APOE-/- mice challenged by 5-FU. It is noteworthy that Gln deprivation can lead to increased intestinal apoptosis with involvement of specific caspases (34-36).
In summary, our overall findings suggest an apoE-independent Ala-Gln effect on intestinal mucosa improvement following 5-FU challenge because this nutrient improved the 5-FU intestinal tissue damage in ApoE-genetically depleted mice. The potential synergistic effect of ApoE and Ala-Gln supplementation warrants further studies.
Acknowledgments
The authors would like to thank Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) and CNPq for funding support.
Footnotes
First published online
References
- 1.Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–637. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham JS, Cassidy J. Adjuvant therapy in colon cancer. Expert Rev Anticancer Ther. 2012;12:99–109. doi: 10.1586/era.11.189. [DOI] [PubMed] [Google Scholar]
- 3.Soares PM, Mota JM, Souza EP, Justino PF, Franco AX, Cunha FQ, et al. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine. 2013;61:46–49. doi: 10.1016/j.cyto.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Han X, Qin S, Zheng Q, Wang Z, Xiang D, et al. Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-fluorouracil in a mouse mucositis model. Biomed Pharmacother. 2011;65:339–344. doi: 10.1016/j.biopha.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Carneiro-Filho BA, Oriá RB, Wood RK, Brito GA, Fujii J, Obrig T, et al. Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition. 2004;20:934–941. doi: 10.1016/j.nut.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 7.Goessling W, Mayer RJ. Systemic treatment of patients who have colorectal cancer and inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:713–727. doi: 10.1016/j.gtc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Drozdowski L, Thomson AB. Intestinal mucosal adaptation. World J Gastroenterol. 2006;12:4614–4627. doi: 10.3748/wjg.v12.i29.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–261. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 10.Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lonnerdal B, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. Am J Clin Nutr. 2004;79:457–465. doi: 10.1093/ajcn/79.3.457. [DOI] [PubMed] [Google Scholar]
- 11.Ren W, Duan J, Yin J, Liu G, Cao Z, Xiong X, et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. 2014;46:2403–2413. doi: 10.1007/s00726-014-1793-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Wu X, Yin Y, Zhang C, He L. Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids. 2012;43:813–821. doi: 10.1007/s00726-011-1137-2. [DOI] [PubMed] [Google Scholar]
- 13.Brito GA, Carneiro-Filho B, Oriá RB, Destura RV, Lima AA, Guerrant RL. Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig Dis Sci. 2005;50:1271–1278. doi: 10.1007/s10620-005-2771-x. [DOI] [PubMed] [Google Scholar]
- 14.Braga-Neto MB, Warren CA, Oriá RB, Monteiro MS, Maciel AA, Brito GA, et al. Alanyl-glutamine and glutamine supplementation improves 5-fluorouracil-induced intestinal epithelium damage in vitro . Dig Dis Sci. 2008;53:2687–2696. doi: 10.1007/s10620-008-0215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azevedo OG, Oliveira RA, Oliveira BC, Zaja-Milatovic S, Araujo CV, Wong DV, et al. Apolipoprotein E COG 133 mimetic peptide improves 5-fluorouracil-induced intestinal mucositis. BMC Gastroenterol. 2012;12:35. doi: 10.1186/1471-230X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azevedo OG, Bolick DT, Roche JK, Pinkerton RF, Lima AA, Vitek MP, et al. Apolipoprotein E plays a key role against cryptosporidial infection in transgenic undernourished mice. PLoS One. 2014;9:e89562. doi: 10.1371/journal.pone.0089562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oriá RB, Patrick PD, Blackman JA, Lima AA, Guerrant RL. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses. 2007;68:1099–1107. doi: 10.1016/j.mehy.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitter SS, Oriá RB, Kvalsund MP, Pamplona P, Joventino ES, Mota RM, et al. Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clinics. 2012;67:11–18. doi: 10.6061/clinics/2012(01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carneiro-Filho BA, Bushen OY, Brito GA, Lima AA, Guerrant RL. Glutamine analogues as adjunctive therapy for infectious diarrhea. Curr Infect Dis Rep. 2003;5:114–119. doi: 10.1007/s11908-003-0046-2. [DOI] [PubMed] [Google Scholar]
- 20.Soares PM, Mota JM, Gomes AS, Oliveira RB, Assreuy AM, Brito GA, et al. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol. 2008;63:91–98. doi: 10.1007/s00280-008-0715-9. [DOI] [PubMed] [Google Scholar]
- 21.Malet-Martino M, Jolimaitre P, Martino R. The prodrugs of 5-fluorouracil. Curr Med Chem Anticancer Agents. 2002;2:267–310. doi: 10.2174/1568011023354146. [DOI] [PubMed] [Google Scholar]
- 22.Garg MB, Lincz LF, Adler K, Scorgie FE, Ackland SP, Sakoff JA. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br J Cancer. 2012;107:1525–1533. doi: 10.1038/bjc.2012.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerchietti LC, Navigante AH, Lutteral MA, Castro MA, Kirchuk R, Bonomi M, et al. Double-blinded, placebo-controlled trial on intravenous L-alanyl-L-glutamine in the incidence of oral mucositis following chemoradiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;65:1330–1337. doi: 10.1016/j.ijrobp.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 24.Athman R, Louvard D, Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am J Physiol Gastrointest Liver Physiol. 2002;283:G496–G502. doi: 10.1152/ajpgi.00207.2002. [DOI] [PubMed] [Google Scholar]
- 25.Gordon PV, Paxton JB, Herman AC, Carlisle EM, Fox NS. Igf-I accelerates ileal epithelial cell migration in culture and newborn mice and may be a mediator of steroid-induced maturation. Pediatr Res. 2004;55:34–41. doi: 10.1203/01.PDR.0000100461.00878.75. [DOI] [PubMed] [Google Scholar]
- 26.Chen K, Nezu R, Wasa M, Sando K, Kamata S, Takagi Y, et al. Insulin-like growth factor-1 modulation of intestinal epithelial cell restitution. JPEN J Parenter Enteral Nutr. 1999;23:S89–S92. doi: 10.1177/014860719902300522. [DOI] [PubMed] [Google Scholar]
- 27.Bortvedt SF, Lund PK. Insulin-like growth factor 1: common mediator of multiple enterotrophic hormones and growth factors. Curr Opin Gastroenterol. 2012;28:89–98. doi: 10.1097/MOG.0b013e32835004c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander AN, Carey HV. Insulin-like growth factor-I stimulates Na+-dependent glutamine absorption in piglet enterocytes. Dig Dis Sci. 2002;47:1129–1134. doi: 10.1023/A:1015010728696. [DOI] [PubMed] [Google Scholar]
- 29.Oriá RB, Vieira CMG, Pinkerton RC, De Castro-Costa CM, Lopes MB, Hussaini I, et al. Apolipoprotein E knockout mice have accentuated malnutrition with mucosal disruption and blunted insulin-like growth factor responses to refeeding. Nutrition Res. 2007;26:427–435. doi: 10.1016/j.nutres.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rensen PC, Oosten M, Bilt E, Eck M, Kuiper J, Berkel TJ. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats In vivo . J Clin Invest. 1997;99:2438–2445. doi: 10.1172/JCI119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justino PF, Melo LF, Nogueira AF, Costa JV, Silva LM, Santos CM, et al. Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice. Br J Nutr. 2014;111:1611–1621. doi: 10.1017/S0007114513004248. [DOI] [PubMed] [Google Scholar]
- 32.Cool JC, Dyer JL, Xian CJ, Butler RN, Geier MS, Howarth GS. Pre-treatment with insulin-like growth factor-I partially ameliorates 5-fluorouracil-induced intestinal mucositis in rats. Growth Horm IGF Res. 2005;15:72–82. doi: 10.1016/j.ghir.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda M, Kato S, Yamanaka N, Iimori M, Matsumoto K, Utsumi D, et al. 5-HT(3) receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cells. Br J Pharmacol. 2013;168:1388–1400. doi: 10.1111/bph.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papaconstantinou HT, Hwang KO, Rajaraman S, Hellmich MR, Townsend CM, Jr, Ko TC. Glutamine deprivation induces apoptosis in intestinal epithelial cells. Surgery. 1998;124:152–159. doi: 10.1016/S0039-6060(98)70115-1. [DOI] [PubMed] [Google Scholar]
- 35.Carneiro BA, Fujii J, Brito GA, Alcantara C, Oriá RB, Lima AA, et al. Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro . Infect Immun. 2006;74:81–87. doi: 10.1128/IAI.74.1.81-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Jr, et al. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. doi: 10.1016/S1091-255X(00)80022-0. [DOI] [PubMed] [Google Scholar]