Abstract
The aim of this study was to investigate the effects of resistance exercise training on hemodynamics and cardiac autonomic control in ovariectomized spontaneously hypertensive rats. Female rats were divided into 4 groups: sedentary control (SC), sedentary hypertensive (SH), sedentary hypertensive ovariectomized (SHO), and resistance-trained hypertensive ovariectomized (RTHO). Resistance exercise training was performed on a vertical ladder (5 days/week, 8 weeks) at 40-60% maximal load. Direct arterial pressure was recorded. Vagal and sympathetic tones were measured by heart rate (HR) responses to methylatropine (3 mg/kg, iv) and propranolol (4 mg/kg, iv). Ovariectomy resulted in additional increases in blood pressure in hypertensive rats and was associated with decreased vagal tone. Resistance exercise trained rats had lower mean arterial pressure than untrained rats (RTHO: 159±2.2 vs SHO: 177±3.4 mmHg), as well as resting bradycardia (RTHO: 332±9.0 vs SHO: 356±5 bpm). Sympathetic tone was also lower in the trained group. Moreover, sympathetic tone was positively correlated with resting HR (r=0.7, P<0.05). The additional arterial pressure increase in hypertensive rats caused by ovarian hormone deprivation was attenuated by moderate-intensity dynamic resistance training. This benefit may be associated with resting bradycardia and reduced cardiac sympathetic tone after training, which suggests potential benefits of resistance exercise for the management of hypertension after ovarian hormone deprivation.
Keywords: Resistance exercise training, Menopause, Hypertension, Sympathetic tone, Blood pressure
Introduction
Hypertension is a leading cause of cardiovascular disease in both genders. The incidence of cardiovascular disease (CVD) in women increases sharply after menopause, suggesting that ovarian hormones play a role in the regulation of arterial pressure (AP) (1). In a previous study, we found that ovarian hormone deprivation in rats resulted in an increase in AP values (2).
Resting bradycardia induced by aerobic exercise training has been well-documented in humans and animals. Studies have demonstrated resting bradycardia in young (3) and old (4) normotensive male rats, in young normotensive females rats (5), in female ovariectomized rats (6,7), in male and female hypertensive rats (8,9), and in humans (10). The mechanisms underlying the cardiac adaptive response to exercise training differ between species and genders (3,5,10). A recent report from our laboratory demonstrated that changes in the cardiac autonomic control in trained female rats were correlated with reduced basal heart rate (HR), which suggests that autonomic control of HR plays a role in the resting bradycardia observed in trained female rats (5).
Thus, it has been well established that aerobic exercise training causes decreases in basal AP and HR, which are associated with improvements in cardiovascular autonomic control (5,9) and baroreflex sensitivity (6,8,11). Recently, there has been growing clinical evidence showing the positive effects of resistance exercise training on the body composition and metabolic profile of both normal and diseased populations. In fact, medical associations now recommend resistance exercise training (at 40-60% of maximal load) as a complement to aerobic training for older adults and for the management of various chronic diseases, including hypertension (12,13). However, the cardiovascular effects of this type of training remain poorly understood. Silveira et al. reported bradycardia and a reduced intrinsic heart rate in Wistar normotensive OVX rats after high-intensity resistance exercise training (from 75% of body mass until maximal overload) on an adapted ladder (7). We recently standardized a moderate-intensity resistance exercise training protocol (at 40-60% of maximal load) on an adapted ladder to study the effects of this type of training in experimental models of disease. We applied this protocol to diabetic normotensive OVX rats and found improvements in body composition and hemodynamics (14). Given the high prevalence of hypertension in post-menopausal women, and the lack of studies investigating the cardiovascular effects of resistance training in this population, this was designed to test the hypothesis that dynamic moderate-intensity resistance exercise training will reduce AP and HR in association with positive cardiac autonomic changes in hypertensive OVX rats. Thus, the aim of our study was to investigate the effects of moderate-intensity resistance exercise training on hemodynamic parameters and cardiac autonomic control in hypertensive OVX rats.
Material and Methods
Seven normotensive female Wistar rats and 21 spontaneously hypertensive (SHR; 3 months) female rats were obtained from the Animal Facilities of Institute of Cardiology of Rio Grande do Sul. The animals were provided with standard laboratory chow and water ad libitum, and were housed in temperature-controlled rooms (22°C) with a 12-h dark-light cycle. The rats were assigned to one of four groups (n=7 each): sedentary normotensive (SC), sedentary hypertensive (SH), sedentary hypertensive ovariectomized (SHO), and resistance-trained hypertensive ovariectomized (RTHO). All surgical procedures and protocols were approved by the Ethics Committee of Universidade Nove de Julho (Protocol #0035/2011) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
At 12 weeks of age, the animals were anesthetized with ketamine (80 mg/kg, ip) and xylazine (12 mg/kg, ip) and ovariectomy was performed. Two dorsolateral skin and muscle incisions were made and the ovaries were located. The oviducts, including the ovarian blood vessels, were ligated and cut and the ovaries were removed. The skin and muscle wall were then closed with absorbable suture. After surgery, the animals received an injection of 40,000 U/kg penicillin G procaine, im (2,6,9).
Resistance exercise training (RT) was performed on a ladder adapted for rats with 54 vertical steps spaced 0.5 cm apart. The steps had a small rat cage at the top, which was covered with a cloth to create a dark environment for the animal to rest in between climbs. All animals were habituated to the act of climbing for 5 consecutive days before the maximal load test. The test consisted of an initial load of 75% of the body weight, which was attached to the base of the rat tail. The load was progressively increased by 50 g increments in subsequent climbs as previously described (14). The resistance exercise training was then performed using the normalized value of the individual maximal load (load of the last complete climb/body weight) for each rat, and was adjusted weekly according to the body weight of the animal. Resistance exercise was performed 5 days per week for 8 weeks at moderate intensity (1st-2nd week: 30-40% of maximal load; 3rd-5th week: 40-50% of maximal load; 6th-8th week: 40-60% of maximal load). The rats performed 15 climbs per session with a 1-min time interval between climbs as previously described (14).
On the day after the last exercise session, the rats were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (20 mg/kg) and two polyethylene-tipped Tygon cannulas filled with heparinized saline were implanted, one into the right carotid artery and one into the jugular vein, for direct measurement of arterial pressure and for drug administration, respectively. The free ends of the cannulas were tunneled subcutaneously and exteriorized at the base of the skull. To avoid effects of detraining (period without exercise training), hemodynamic measurements were made with the rats conscious and freely moving in their home cages. The arterial cannula was connected to a transducer (Blood Pressure XDCR, Kent¯ Scientific, USA), and AP signals were recorded for a 30-min period using a microcomputer equipped with an analog-to-digital converter (CODAS, 2Kz, DATAQ Instruments, USA). The recorded data were analyzed on a beat-to-beat basis to quantify changes in mean AP (MAP) and HR.
After the basal HR recording, vagal and sympathetic tone, and intrinsic heart rate (IHR) were measured by determining the response to methylatropine (3 mg/kg, iv) and propranolol (4 mg/kg, iv) with a maximum volume of 0.2 mL per injection. Because the HR response to these drugs reaches its peak within 3 to 5 min, this time interval was allowed to elapse between the drug administration and the HR measurement. Methylatropine was injected first, and the HR response recorded, and propranolol was injected 10 min after methylatropine and the intrinsic heart rate (IHR) was evaluated. On the following day, the sequence of the injections was reversed (first propranolol and then methylatropine), and the IHR was again evaluated after simultaneous blockade with propranolol and methylatropine. Sympathetic tone was determined as the difference between maximum HR after methylatropine injection and IHR. Vagal tone was measured as the difference between the lowest HR after propranolol injection and IHR (3,5).
Data are reported as means ± SE. Levene's test was used to assess variance homogeneity. Comparisons between the four groups were performed using one-way ANOVA, followed by Student-Newmann-Keuls post hoc tests. Pearson's correlation coefficients were determined to establish the associations between variables. The significance level was set at P<0.05.
Results
At the beginning of the protocol, rats in the SC group had a higher mean body weight than rats in the hypertensive groups (SC: 216±5 g vs SH: 188±2 g; SHO: 191±2 g, and RTHO: 190±1 g, P<0.05). Body weight did not significantly differ between the hypertensive groups. All groups showed a significant increase in body weight over the study period (SC: 280±5; SH: 197±2; SHO: 264±3, and RTHO: 240±4 g, P>0.05); however, RTHO rats (240±4 g) weighed significantly less than SHO rats at the end of the study (264±3 g, P<0.05).
At the beginning of the experiment, the maximal load in the ladder test was similar among the hypertensive groups (SH: 315±19.2 g; SHO: 320±13 g, and RTHO: 300±10.4 g, P>0.05). However, the hypertensive groups carried a greater maximal load when compared with the SC animals (128.5±5.9 g, P<0.05). After 8 weeks of resistance exercise training, the RTHO group demonstrated an increase in maximal load when compared with other groups (RTHO: 490±10.3 g vs SC: 204±11.4 g; SH: 360±20.4 g, and SHO: 335±37.2 g, P<0.05).
Ovariectomized hypertensive rats had a higher MAP than non-ovariectomized hypertensive rats (SHO vs SH group). Rats that underwent resistance exercise training had a lower MAP relative to those in both the SH and SHO groups. Resistance exercise training also resulted in resting bradycardia in the RTHO group (Figure 1).
Figure 1. Hemodynamic and cardiac autonomic control in the studied groups. SC: sedentary control; SH: sedentary hypertensive; SHO: sedentary hypertensive ovariectomized; RTHO: resistance-trained hypertensive ovariectomized. *P<0.05 vs SC; #P<0.05 vs SH; +P<0.05 vs SHO (one-way ANOVA, followed by Student-Newmann-Keuls test).
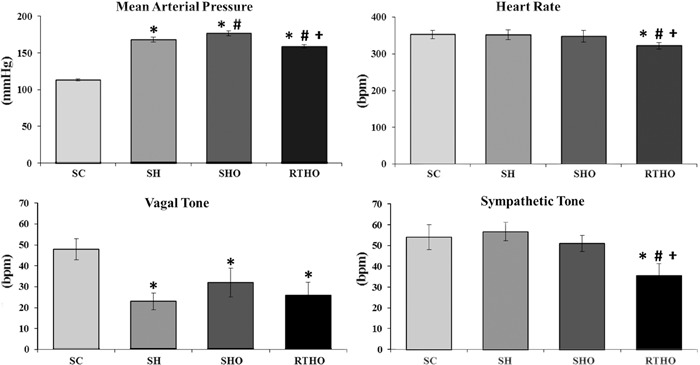
Assessment of the autonomic control of HR demonstrated that the hypertensive groups had reduced vagal tone when compared with the SC group (SH: 21.5±5 bpm; SHO: 26±6 bpm, and RTHO 23.7±4 bpm vs SC: 48±5 bpm, P<0.05). Resistance exercise training decreased sympathetic tone in hypertensive OVX rats (RTHO: 35.6±5.7 bpm vs SC: 54±6 bpm; SH: 56.7±4.4 bpm, and SHO: 50.9±3.9 bpm, P<0.05; Figure 1). The IHR was lower in the hypertensive groups (SH: 325±10 bpm; SHO: 312±6 bpm, and RTHO: 314±7 bpm) when compared with the SC group (368±8 bpm, P<0.05).
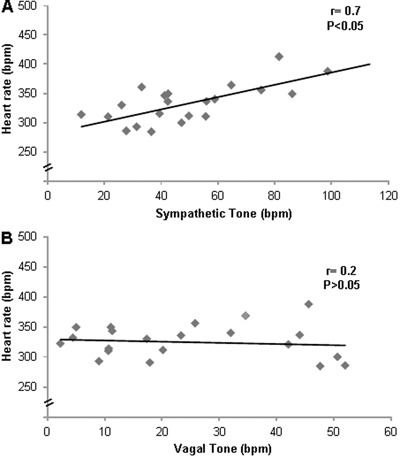
Correlation analysis involving all hypertensive rats (SH, SHO and RTHO groups) showed a significant positive correlation between sympathetic tone and heart rate (r=0.7, P<0.05; Figure 2A). There was no correlation between vagal tone and heart rate (r=0.2, P>0.05; Figure 2B).
Figure 2. Correlations between A, resting heart rate and sympathetic tone (r=0.7, P<0.05) and B, resting heart rate and vagal tone (r=0.2, P>0.05). Pearson's correlation coefficients were determined using data from all hypertensive groups.
Discussion
In this study, we used an experimental model of menopause with associated hypertension to demonstrate that resistance exercise training minimizes the long-term effects of hypertension. There were two major findings of this study: first, the RTHO rats had a lower AP and HR at rest than the rats in the sedentary hypertensive groups; second, resistance exercise training seemed to reduce the sympathetic tone, which is associated with resting bradycardia.
Menopause has been associated with reduced muscle strength and bone mineral density, as well as with weight gain (15). In the present study, resistance exercise training reduced the body weight of hypertensive ovariectomized rats. Several previous studies have failed to demonstrate any reduction in body weight with aerobic training of hypertensive male rats (8) or female hypertensive OVX rats (9). However, the results of several other studies suggest that progressive resistance training is a promising intervention for positively changing body composition. In fact, despite no change in body weight, we have previously observed a decrease in adipose tissue and an increase in muscle mass after resistance training on an adapted ladder in male normotensive rats. Moreover, resistance training has been demonstrated to slow the development of sarcopenia and the overall deterioration of muscle structure associated with menopause (16,17). The significant gain (∼46%) in the maximum load on the ladder test that we observed in hypertensive OVX rats after training supports this, and the magnitude of the gain that we found was similar (∼50%) to that seen in diabetic normotensive OVX rats, as we previously reported (14). Using a similar resistance training protocol, Grans et al. (18) reported a more pronounced gain in strength (∼80%) in male normotensive rats than the gain that we observed in females. Taken together, these data suggest that gender and ovarian hormone deprivation both probably play a role in the response to dynamic resistance training.
The incidence of hypertension rises after menopause (1), and this increase involves changes in AP and its regulation, which are associated with estrogen loss. In the present study, we showed an additional increase in AP in spontaneously hypertensive OVX rats, thus corroborating previously published research undertaken by our group (2). Importantly, we demonstrated that 8 weeks of moderate-intensity dynamic resistance exercise training resulted in a reduction in AP values in ovarian hormone-deprived hypertensive rats. There are several mechanisms that may account for the reduction in blood pressure seen after aerobic exercise training in hypertensive humans; these include reduced cardiac output (19) and/or peripheral vascular resistance (20). The reduction in AP values seen in this study may be associated with a decrease in sympathetic tone, resulting in resting bradycardia after 8 weeks of moderate-intensity resistance exercise training. However, 10 weeks (3 times/week) of high-intensity resistance training on a ladder led to a reduction in resting HR and in intrinsic heart rate, but did not change either AP or cardiac autonomic tone in normotensive OVX rats (7). In male SHR rats, moderate-intensity treadmill exercise training reduced AP, HR, and sympathetic tone. Moreover, the reduced resting HR correlated with the reduced sympathetic tone observed in female Wistar rats after moderate-intensity aerobic treadmill training (5). In another study, we previously reported that resting bradycardia may be the candidate mechanism for AP reduction in OVX rats undertaking aerobic treadmill training (6). In the present study, we observed a positive correlation between sympathetic tone and resting HR (r=0.7), which further suggests that reduced cardiac sympathetic tone plays a critical role in the induction of resting bradycardia in hypertensive animals.
In conclusion, ovarian hormone deprivation in hypertensive rats resulted in an additional increase in AP, which was attenuated by moderate-intensity dynamic resistance exercise training. Our results suggest that this benefit may be associated with resting bradycardia and reduced cardiac sympathetic tone after training. These findings indicate that moderate-intensity dynamic resistance exercise training may be beneficial in the management of hypertension after ovarian hormone deprivation.
Acknowledgments
This research was supported by FAPESP (#2011/16441-0; #2012/02023-5; #2013/07869-2; #2012/20141-5), CNPq and CAPES (#88881.062178/2014-01). M.C. Irigoyen and K. De Angelis are recipients of CNPq-BPQ fellowships.
Footnotes
First published online.
References
- 1.Vokonas PS, Kannel WB, Cupples LA. Epidemiology and risk of hypertension in the elderly: the Framingham Study. J Hypertens Suppl. 1988;6:S3–S9. [PubMed] [Google Scholar]
- 2.Flues K, Paulini J, Brito S, Sanches IC, Consolim-Colombo F, Irigoyen MC, et al. Exercise training associated with estrogen therapy induced cardiovascular benefits after ovarian hormones deprivation. Maturitas. 2010;65:267–271. doi: 10.1016/j.maturitas.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Negrao CE, Moreira ED, Santos MC, Farah VM, Krieger EM. Vagal function impairment after exercise training. J Appl Physiol. 1992;72:1749–1753. doi: 10.1152/jappl.1992.72.5.1749. [DOI] [PubMed] [Google Scholar]
- 4.De Angelis KL, Oliveira AR, Werner A, Bock P, Bello-Klein A, Fernandes TG, et al. Exercise training in aging: hemodynamic, metabolic, and oxidative stress evaluations. Hypertension. 1997;30:767–771. doi: 10.1161/01.HYP.30.3.767. [DOI] [PubMed] [Google Scholar]
- 5.Sanches IC, Sartori M, Jorge L, Irigoyen MC, De Angelis K. Tonic and reflex cardiovascular autonomic control in trained-female rats. Braz J Med Biol Res. 2009;42:942–948. doi: 10.1590/S0100-879X2009001000011. [DOI] [PubMed] [Google Scholar]
- 6.Irigoyen MC, Paulini J, Flores LJ, Flues K, Bertagnolli M, Moreira ED, et al. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats. Hypertension. 2005;46:998–1003. doi: 10.1161/01.HYP.0000176238.90688.6b. [DOI] [PubMed] [Google Scholar]
- 7.Silveira LC, Tezini GC, Schujmann DS, Porto JM, Rossi BR, Souza HC. Comparison of the effects of aerobic and resistance training on cardiac autonomic adaptations in ovariectomized rats. Auton Neurosci. 2011;162:35–41. doi: 10.1016/j.autneu.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Gava NS, Veras-Silva AS, Negrao CE, Krieger EM. Low-intensity exercise training attenuates cardiac beta-adrenergic tone during exercise in spontaneously hypertensive rats. Hypertension. 1995;26:1129–1133. doi: 10.1161/01.HYP.26.6.1129. [DOI] [PubMed] [Google Scholar]
- 9.Sanches IC, de Oliveira BJ, Candido GO, da Silva DD, Jorge L, Irigoyen MC, et al. Cardiometabolic benefits of exercise training in an experimental model of metabolic syndrome and menopause. Menopause. 2012;19:562–568. doi: 10.1097/gme.0b013e3182358c9c. [DOI] [PubMed] [Google Scholar]
- 10.Smith ML, Hudson DL, Graitzer HM, Raven PB. Exercise training bradycardia: the role of autonomic balance. Med Sci Sports Exerc. 1989;21:40–44. doi: 10.1249/00005768-198902000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Bertagnolli M, Campos C, Schenkel PC, de Oliveira V, De Angelis K, Bello-Klein A, et al. Baroreflex sensitivity improvement is associated with decreased oxidative stress in trained spontaneously hypertensive rat. J Hypertens. 2006;24:2437–2443. doi: 10.1097/01.hjh.0000251905.08547.17. [DOI] [PubMed] [Google Scholar]
- 12.Williams AD, Almond J, Ahuja KD, Beard DC, Robertson IK, Ball MJ. Cardiovascular and metabolic effects of community based resistance training in an older population. J Sci Med Sport. 2011;14:331–337. doi: 10.1016/j.jsams.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 14.Sanches IC, Conti FF, Sartori M, Irigoyen MC, De Angelis K. Standardization of resistance exercise training: effects in diabetic ovariectomized rats. Int J Sports Med. 2014;35:323–329. doi: 10.1055/s-0033-1351254. [DOI] [PubMed] [Google Scholar]
- 15.Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Med. 2004;34:753–778. doi: 10.2165/00007256-200434110-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bocalini DS, Serra AJ, dos Santos L, Murad N, Levy RF. Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health. 2009;21:519–527. doi: 10.1177/0898264309332839. [DOI] [PubMed] [Google Scholar]
- 17.Fjeldstad C, Palmer IJ, Bemben MG, Bemben DA. Whole-body vibration augments resistance training effects on body composition in postmenopausal women. Maturitas. 2009;63:79–83. doi: 10.1016/j.maturitas.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Grans CF, Feriani DJ, Abssamra ME, Rocha LY, Carrozzi NM, Mostarda C, et al. Resistance training after myocardial infarction in rats: its role on cardiac and autonomic function. Arq Bras Cardiol. 2014;103:60–68. doi: 10.5935/abc.20140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagberg JM, Montain SJ, Martin WH, III, Ehsani AA. Effect of exercise training in 60- to 69-year-old persons with essential hypertension. Am J Cardiol. 1989;64:348–353. doi: 10.1016/0002-9149(89)90533-X. [DOI] [PubMed] [Google Scholar]
- 20.Jennings GL, Deakin G, Korner P, Meredith I, Kingwell B, Nelson L. What is the dose-response relationship between exercise training and blood pressure? Ann Med. 1991;23:313–318. doi: 10.3109/07853899109148066. [DOI] [PubMed] [Google Scholar]


