Abstract
This study aimed to determine the role of mitochondrial adenosine triphosphate-sensitive potassium (mitoKATP) channels and protein kinase C (PKC)-ε in the delayed protective effects of sevoflurane preconditioning using Langendorff isolated heart perfusion models. Fifty-four isolated perfused rat hearts were randomly divided into 6 groups (n=9). The rats were exposed for 60 min to 2.5% sevoflurane (the second window of protection group, SWOP group) or 33% oxygen inhalation (I/R group) 24 h before coronary occlusion. The control group (CON) and the sevoflurane group (SEVO) group were exposed to 33% oxygen and 2.5% sevoflurane for 60 min, respectively, without coronary occlusion. The mitoKATP channel inhibitor 5-hydroxydecanoate (5-HD) was given 30 min before sevoflurane preconditioning (5-HD+SWOP group). Cardiac function indices, infarct sizes, serum cardiac troponin I (cTnI) concentrations, and the expression levels of phosphorylated PKC-ε (p-PKC-ε) and caspase-8 were measured. Cardiac function was unchanged, p-PKC-ε expression was upregulated, caspase-8 expression was downregulated, cTnI concentrations were decreased, and the infarcts were significantly smaller (P<0.05) in the SWOP group compared with the I/R group. Cardiac function was worse, p-PKC-ε expression was downregulated, caspase-8 expression was upregulated, cTnI concentration was increased and infarcts were larger in the 5-HD+SWOP group (P<0.05) compared with the SWOP group. The results suggest that mitoKATP channels are involved in the myocardial protective effects of sevoflurane in preconditioning against I/R injury, by regulating PKC-ε phosphorylation before ischemia, and by downregulating caspase-8 during reperfusion.
Keywords: Ischemia, Reperfusion, mitoKatp channel, Preconditioning, Protein kinase C
Introduction
Myocardial ischemia/reperfusion (I/R) injury occurs not only in coronary heart disease but also as a complication of cardiac surgery performed to treat lethal reperfusion injury, myocardial stunning, reperfusion arrhythmias, and vascular injury. I/R injury is a significant factor in perioperative complications and increased mortality (1). Therefore, preventing myocardial I/R injury in noncardiac surgery patients and nonsurgical cardiac ischemia may decrease the incidence of cardiac complications.
Repeated short episodes of myocardial ischemia, called myocardial ischemic preconditioning (IPC), reduce the occurrence of myocardial I/R injury (2). However, ischemic pretreatment is difficult to implement clinically. Anesthetic preconditioning (APC) with inhaled anesthetics that are widely used in clinical anesthesia was first reported in 1997 to protect the myocardium from I/R injury (3). Since then, numerous studies have shown that inhaled anesthetics simulate ischemic preconditioning to reduce myocardial I/R injury, which has clinically significant implications (4-6). APC lowers the risk of myocardial ischemia, is readily available, and is convenient to apply, making it a good alternative to ischemic drugs and methods. APC is especially suitable for preoperative inhalational anesthetic pretreatment for myocardial I/R injury. Inhalational anesthetic pretreatment may trigger the release of a variety of endogenous bioactive substances in myocardial or brain tissue through cell membrane receptor binding or through direct activation of intracellular signaling pathways involving a variety of cell types and protective substances. Although numerous studies have focused on APC, its specific mechanism of action has not yet been fully elucidated (7). This study focused on the mechanism underlying the protective effect of APC.
Opening of mitochondrial ATP-sensitive potassium (mitoKATP) channels plays an important role in protecting against myocardial ischemic disease (8) and is known to occur during APC (9,10). Recent studies have found that mitoKATP channels are involved in the mitochondrial membrane cascade-signaling pathway during myocardial ischemic preconditioning (11). The mitoKATP channel-specific blocker 5-hydroxydecanoate (5-HD) blocks the myocardial protective effect of ischemic and pharmacological preconditioning (12,13). Pretreating nerve cells with diazoxide, a selective opener of mitoKATP channels, induces the phosphorylation of protein kinase C (PKC)-ε, as well as the opening of mitoKATP channels, thereby forming a system of positive feedback regulation (14). A recent study of the effects of theaflavins revealed that cardiac muscle contractility depends on PKC-ε concentration (15).
The delayed protective effects of sevoflurane preconditioning can effectively limit peri- and post-operative complications of cardiac surgery. This study utilized the Langendorff isolated heart perfusion model to investigate the effects of mitoKATP channel-mediated PKC-ε in relation to the delayed protective effects of sevoflurane preconditioning.
Material and Methods
Animals
The Committee of the Medical College of Soochow University (Suzhou, China; License No. 20020008, Grade II) approved the use of healthy clean-grade male Sprague-Dawley (SD) rats that were maintained in their Experimental Animal Center for this study.
Experimental protocol
The Committee for Experimental Animals of the Medical College of Soochow University, Suzhou, China (Chairperson, Professor Zhi-mou Xue; No. SZULL-20090309) approved the study protocol on March 9, 2009.
A total of 54 rats were randomly assigned to 6 groups (n=9/group): Rats given 33% oxygen or 2.5% sevoflurane for 60 min without occlusion and reperfusion 24 h later served as the control (CON) and sevoflurane (SEVO) groups, respectively. Rats in the I/R group were given 33% oxygen for 60 min followed by a 30 min equilibration period, 30 min of occlusion, and 2 h of reperfusion 24 h later. Rats in the SWOP (second window of protection) group were given 2.5% sevoflurane for 60 min followed by a 30 min equilibration period, 30 min of occlusion, and 2 h of reperfusion 24 h later. Rats in the 5-HD+SWOP group were given an intraperitoneal injection of 10 mg/kg 5-HD (a selective mitoKATP channel antagonist; H135, Sigma, USA) 30 min before inhalation of 2.5% sevoflurane, followed by a 30 min equilibration period, 30 min of occlusion, and 2 h of reperfusion 24 h later. Rats in the 5-HD group were given an intraperitoneal injection of 10 mg/kg 5-hydroxydecanoate without inhalation of sevoflurane followed by a 30 min equilibration period, 30 min of occlusion, and 2 h of reperfusion 24 h later. The CON and SEVO groups did not undergo occlusion and reperfusion. The I/R, SWOP, 5-HD+SWOP, and 5-HD groups underwent occlusion and reperfusion.
Cardiac function indices of the rats in all groups were recorded. Myocardial infarct size was measured by triphenyl tetrazolium chloride (TTC) staining. Cardiac troponin I (cTnI) concentration in the coronary transudate was measured by an enzyme-linked immunosorbent assay (ELISA). The expression levels of phosphorylated PKC-ε (p-PKC-ε) and caspase-8 were assayed by Western blot. Rats that developed CO2 accumulation during sevoflurane preconditioning, refractory ventricular fibrillation, frequent recurrent arrhythmia, left ventricle systolic pressure (LVSP) <80 mmHg, or heart rate (HR) <200 bpm were excluded from the experiment.
Sevoflurane preconditioning
Rats were placed in a glass box connected to the gas flow inlet of a Sevotec 5 sevoflurane vaporizer (Drager Vapor 2000, Drager Medical, Germany) (16). The box was continuously infused with 2.5% sevoflurane (h20090714, Abbott Pharmaceutical, China) and 97.5% oxygen at a flow of 2 L/min. An anesthesia monitor (ULT-I type, Datex-Ohmeda, USA) was connected to monitor the concentrations of sevoflurane, O2, and CO2. The rats were placed in the glass box for 1 h after the sevoflurane concentration had reached approximately 1.0 MAC (monitored anesthesia care), after which they were returned to their cages and fed a normal diet.
Langendorff isolated heart perfusion model
Krebs-Henseleit (K-H) solution, consisting of 118.0 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PSO4, 25.0 mM NaHCO3, 1.2 mM MgSO4, 2.5 mM CaCl2, and 11.0 mM glucose was prepared. The pH was adjusted to 7.35-7.45, and the mixture was heated to 36.0°C on a temperature-controlled mechanical stirrer (90-3 type, Huxi Analysis Instrument, China). A pre-access test was conducted for 30 min to ensure that the 95% O2 and 5% CO2 gas was fully mixed with the K-H solution. A temperature-controlled water bath (ZC-10, Alcott Biotechnology Co., Ltd., China) was used to maintain the external circulation temperature at 36°C. The rats were intraperitoneally anesthetized with 50 mg/kg pentobarbital sodium (060928, Haitai Riel Biotechnology, China), followed by 500 U/kg of heparin. The rat hearts were quickly excised and mounted on a Langendorff apparatus via the aorta for retrograde perfusion with the K-H solution at a constant pressure of 10 kPa. The aorta of the hearts was tied with 5-0 silk to produce global ischemia. (17). The pump was adjusted to maintain a coronary flow rate of 12 mL/min. A cuff pressure transducer (YP200, SIA Industrial and Trade, China) was inserted into the left ventricle to monitor heart function. The cuff volume was adjusted to maintain a stable left ventricular end diastolic pressure (LVEDP) of 6-10 mmHg during initial equilibration.
Cardiac function
Cardiac function indices including LVSP, LVEDP, the maximal rate of rise of ventricular pressure (±dP/dtmax), and HR were monitored after 30 min of equilibration (T0), and then at 30 (T1), 60 (T2), 90 (T3), and 120 min (T4) after reperfusion using the Med Lab 6.0 software on a U/4C501H Med Lab biological signal acquisition and processing system (Mei Yi Technology, China).
Infarct size determination and cardiac troponin I
After 2 h of reperfusion, the heart was removed and cooled at -80°C for 5 min. The heart was then cut into 6 cross-sectional slices of 2-mm thickness that were incubated at 37°C for 15 min in 1% TTC (T8877, Sigma) in 0.1 M phosphate buffer, pH 7.4 and fixed overnight in 10% formaldehyde. The infarct size is reported as a percentage of the total range of the left ventricle area at risk (AAR) using the Alpha View gel image analysis software (Alpha Ease FC, USA). Coronary transudate (2 mL) was collected at T0 and T4, and centrifuged for 15 min at 1800 g and 4°C. cTnI concentrations in the coronary transudate were detected using cTnI ELISA kits (Xitang Biotechnology Company, China).
Protein extraction and Western blot analysis
The hearts were kept frozen at -80°C in liquid nitrogen until analysis. Lysis buffer and phosphatase inhibitor-extracted proteins were added, and the tissues were homogenized by low-temperature cracking using ultrasonication. The protein content of the samples was determined using a bicinchoninic acid assay kit (P0010, Biyuntian Biotechnology Company, China) and the protein content of the lysate was adjusted to the same concentration. The lysates were heated for 5 min in a 97°C water bath and 20 μg of each sample was electrophoresed in 12% polyacrylamide gels (Bio-Rad, USA) to isolate the proteins, which were then transferred onto nitrocellulose membranes (Millipore Company, USA). The proteins were blocked with 5% skim milk for 2 h. The samples were incubated overnight at 4°C with anti-p-PKC-ε (1:1000; ab63387, Abcam, USA) and anti-caspase-8 (1:1000; D35G2, Santa Cruz, USA) antibodies, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000; AG019, Biyuntian Biotechnology Company, China) as the internal reference. The membranes were rinsed three times with Tris-buffered saline containing Tween-20 and incubated with secondary antibodies for 2 h. An enhanced luminol-based chemoluminescence kit (34077, Thermo, USA) was used for secondary staining. The stained nitrocellulose membranes were placed into the light-emitting reagent solution (Western blot chemiluminescence reagent AB solution, 0609*025, Merck, Germany) and transferred to a dark room. Membranes were read by an automatic processor for color development and fixing. p-PKC-ε and caspase-8 expression are reported as gray density relative to that of GAPDH.
Statistical analysis
The Graph Pad Prism 4.00 statistical software was used for statistical processing. Data are reported as means±SD. The hemodynamic data were analyzed using analysis of variance (ANOVA) for repeated measures and one-way ANOVA followed by the Tukey multiple-comparison test to compare the differences among groups. P<0.05 was considered to be statistically significant.
Results
Rats with CO2 accumulation during sevoflurane preconditioning and those that developed refractory ventricular fibrillation, recurrent arrhythmia, LVSP <80 mmHg, HR<200 bpm after 30 min of equilibration were excluded from the experiment.
Cardiac function
LVSP, HR, and ±dP/dtmax decreased and LVEDP significantly increased in each group during reperfusion (P<0.05), compared with the levels during equilibration (Table 1). LVSP, HR, and ±dP/dtmax increased and LVEDP significantly decreased in the SWOP group (P<0.05), compared with those in the I/R group. LVSP, HR, and ±dP/dtmax significantly decreased, whereas the LVEDP significantly increased in the 5-HD+SWOP group (P<0.05), compared with those in the SWOP group. The preconditioning effects induced by sevoflurane were abolished by 5-HD (P<0.05) and 5-HD alone did not influence LVDP during reperfusion compared with its effect in the I/R group (P>0.05).

Myocardial infarct size
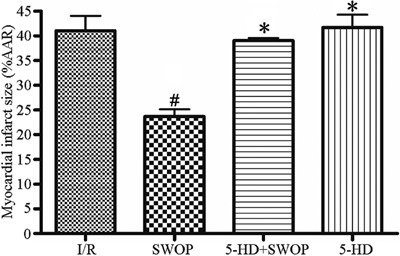
The myocardial infarcts in the SWOP group (25±4%) were smaller (P<0.05) than those in the I/R group (42±5%). The myocardial infarcts in the 5-HD+SWOP group (37±3%) were smaller (P<0.05) than those in the I/R group (42±5%), indicating that 5-HD negated the effects of sevoflurane. However, 5-HD itself did not influence the myocardial infarct size compared with the I/R group (P>0.05). The differences in size among the other groups were not statistically significant (P>0.05). The results are shown in Figure 1.
Figure 1. Myocardial infarct size after 2 h of reperfusion. AAR: area at risk, I/R: ischemia/reperfusion; SWOP: second window of protection; 5-HD: 5-hydroxydecanoate. #P<0.05 vs I/R group; *P<0.05 vs SWOP group (one-way ANOVA).
Concentration of cTnI
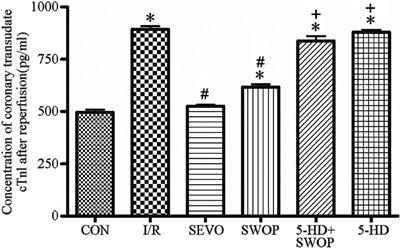
The cTnI concentration in coronary transudate was higher in the I/R (892±27), SWOP (617±29), and 5-HD+SWOP groups (837±26) than in the CON group (498±26). The cTnI concentration in the coronary transudate from the I/R group (892±27) was higher than that in the SWOP (617±29) and in the SEVO groups (501±23; P<0.05). The cTnI concentration in the coronary transudate from the 5-HD+SWOP group (837±26) was higher (P<0.05) than that in the SWOP group (617±29), indicating that 5-HD negated the effects of sevoflurane; however, 5-HD alone did not influence the coronary transudate cTnI concentration compared with the I/R group (P>0.05). Statistically significant differences were not observed among the other groups (P>0.05). The results are shown in Figure 2.
Figure 2. Concentration of coronary transudate cTnI after 2 h of reperfusion. cTnI: cardiac troponin I; CON: control; I/R: ischemia/reperfusion; SEVO: sevoflurane; SWOP: second window of protection; 5-HD: 5-hydroxydecanoate. *P<0.05 vs CON group; #P<0.05 vs I/R group; +P<0.05 vs SWOP group (one-way ANOVA).
Western blots
Before development of ischemia, p-PKC-ε expression was upregulated in the SEVO and SWOP groups compared with the CON and I/R groups (P<0.05), and p-PKC-ε was downregulated in the 5-HD+SWOP group (P<0.05) compared with the SWOP group. 5-HD downregulated p-PKC-ε expression, but 5-HD alone did not influence p-PKC-ε expression compared with expression in the I/R group (P>0.05). Caspase-8 expression before ischemia did not differ significantly among the other groups (P>0.05). The results are shown in Figures 3 and 4.
Figure 3. p-PKC-ε expression before ischemia (A,C) and 2 h after reperfusion (B,D). p-PKC-ε: phosphorylated protein kinase C-ε; CON: control; I/R: ischemia/reperfusion; SEVO: sevoflurane; SWOP: second window of protection; 5-HD: 5-hydroxydecanoate. *P<0.05 vs CON; #P<0.05 vs I/R group; +P<0.05 vs SWOP group (one-way ANOVA).
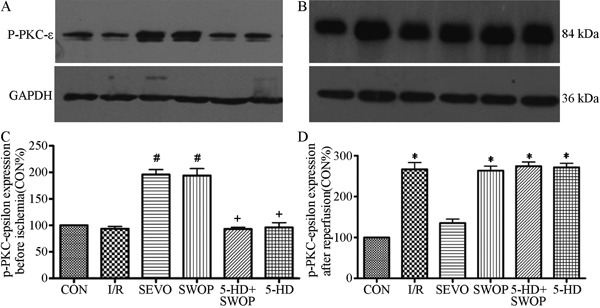
Figure 4. Caspase-8 protein expression before ischemia (A,C) and 2 h after reperfusion (B,D). CON: control; I/R: ischemia/reperfusion; SEVO: sevoflurane; SWOP: second window of protection; 5-HD: 5-hydroxydecanoate. *P<0.05 vs CON; #P<0.05 vs I/R group; +P<0.05 vs SWOP group (one-way ANOVA).
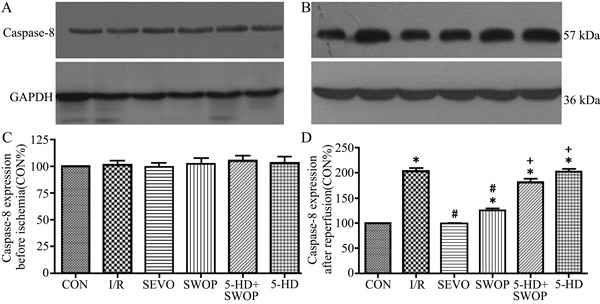
After reperfusion, p-PKC-ε expression was upregulated in the SEVO group compared with the CON group (P<0.05), but there were no statistically significant differences in expression among the I/R, SWOP, and 5-HD+SWOP groups (P>0.05). Caspase-8 expression was downregulated during reperfusion in the SWOP group (P<0.05) compared with the I/R group. Caspase-8 expression was upregulated during reperfusion in the 5-HD+SWOP group (P<0.05) compared with the SWOP group. 5-HD alone did not influence caspase-8 expression compared with that in the I/R group (P>0.05). The results are shown in Figures 3 and 4.
Discussion
This study showed that delayed sevoflurane preconditioning reduced myocardial infarct sizes and protected against myocardial ischemia/reperfusion (I/R) injury in isolated rats. The mitoKATP channel played an important role in triggering the upregulation of p-PKC-ε as well as the downregulation of the anti-apoptotic protein caspase-8, thereby decreasing the severity of myocardial I/R injury.
APC occurs within a few hours (2-3 h in the early stages) to within a few days before ischemia, reaches a peak in approximately 24 to 48 h and persists for 72 h (18). Konia (19) reported that inhalation of 2.5% sevoflurane for 1 h reduces I/R injury in rats. The results of the blood gas analysis prior to sevoflurane preconditioning confirmed that the rats in this study had normal blood gas concentrations. The transudate cTnI concentrations increased during reperfusion compared with those in the equilibrium phase and their infarct sizes remained within a certain range of difference, which indicated the successful establishment of the Langendorff isolated heart perfusion model.
Sevoflurane is a new inhalational anesthetic that is widely used clinically and has been recommended for routine use during noncardiac surgery for patients with a risk of myocardial infarction. Sevoflurane affects the dynamics of human blood, heart rate, and sympathetic nerve activity, and it has a low blood-gas partition coefficient (0.63). Recovery after discontinuation is rapid, but with respiratory tract irritation. Sevoflurane is highly stable and superior to other anesthetics. Lutz (20) reported that 2.5% sevoflurane pretreatment before myocardial ischemia significantly improves cardiac function. Chiari et al. (21) reported that intravenous emulsified sevoflurane has both an early and a delayed myocardial protective effect; however, the mechanism of this delayed protective effect is unclear (19).
LVEDP reflects left ventricular compliance, whereas LVSP reflects left ventricular systolic function. LVEDP was decreased, whereas LVDP, LVSP, HR, and ±dP/dtmax were increased during reperfusion in the SWOP group compared with those in the I/R group, which suggest that the protective effect of delayed sevoflurane preconditioning significantly improves both diastolic and systolic cardiac function. LVEDP was increased, whereas LVDP, LVSP, HR, and ±dP/dtmax were decreased during reperfusion in the 5-HD group compared with those in the SWOP group, which suggest that mitoKATP is involved in the protective effect of delayed sevoflurane preconditioning.
Myocardial infarct size is the gold standard for measuring myocardial I/R injury. The infarcts in the SWOP group were smaller than those in the I/R group, which indicates that sevoflurane pretreatment has a delayed protective effect against I/R injury in isolated perfused rat hearts. The infarcts in the SWOP group were larger than those in the 5-HD+SWOP group, which suggests that mitoKATP is involved in the protective effect of delayed sevoflurane preconditioning.
mitoKATP channels are present in the mitochondrial membranes of myocardial cells, and they function during tissue ischemia as hypoxia protection effectors (9). mitoKATP channels act as influx rectifier potassium channels activated by intracellular nucleoside diphosphate, whereas intracellular ATP inhibits their opening. Under normal physiological conditions, the KATP channel is closed, but myocardial ischemia, decreased intracellular ATP concentration, and ischemic metabolite accumulation causes membrane hyperpolarization cardiac action potential duration (APD) shortening, calcium currents, and decreased myocardial contractility that trigger the opening of KATP channels. mitoKATP channels are present in the mitochondrial membrane, and are activated by hypoxia metabolites known as hypoxia-induced effectors to protect tissues from injury. Calcium release reduced apoptosis after ischemia-reperfusion injury (22). Studies have shown that the opening of mitoKATP channels is triggered and/or mediated during the early stages of inhalational anesthetic preconditioning (23-25). In the current study, 5-HD, a specific inhibitor of the mitoKATP channels, weakened the protective effect of sevoflurane, which demonstrates that mitoKATP channels are involved in the protective effect of sevoflurane. cTnI is a cardiac myocyte-specific marker for myocardial injury that occurs at different concentrations. cTnI is a sensitive indicator of myocardial injury. The concentrations of coronary transudate cTnI were higher, and myocardial infarct sizes were larger, in the 5-HD+SWOP group than in the SWOP group. This result shows that inhibition of mitoKATP channels increases the cTnI transudate concentration and the myocardial infarct size. Therefore, mitoKATP channels triggered the protective effect of sevoflurane preconditioning.
MitoKATP channels mediate the effects of inhaled anesthetic preconditioning by activating G protein-coupled receptors and the intracellular signal transduction pathway, as well as the translocation of PKC-ε (9). Studies have shown that G protein-coupled receptor agonists such as adenosine, opioids, and bradykinin reduce the cell infarction size range (26). Ischemic and pharmacological preconditioning activates PKC (27), protein tyrosine kinase (PTK) (26), and mitogen-activated protein kinases (MAPK) (28). APC occurs in organs such as the brain, heart, liver, small intestines, skeletal muscles, kidneys, and lungs. The possible mechanisms of APC include glutamate, adenosine receptor activation mechanisms, mitoKATP channels, nitric oxide, oxidative stress, and low temperature mechanisms. The low temperature mechanisms include those that involve protein kinase C, which is released by a series of signal transduction mechanisms. As a novel protein kinase C, PKC-ε plays an important role in the development of ischemic tolerance. Myocardial ischemia tolerance is dependent on PKC activation, especially on the activation of PKC-ε. Novalija et al. (29) reported that the PKC-ε inhibitor PP-149 counteracts the myocardial protective effects of sevoflurane pretreatment in Langendorff models, confirming the role of sevoflurane in PKC-ε-mediated myocardial protection. Weber et al. (30) reported that xenon exerts its myocardial protective effect by increasing PKC-ε expression. That effect is blocked by 5-HD, which suggests that PKC-ε is the downstream target of mitoKATP channels. The degree of PKC-ε phosphorylation was lower before myocardial ischemia, which suggests that mitoKATP channels trigger the delayed protective effect of sevoflurane by increasing PKC-ε phosphorylation.
Myocardial ischemia causes cell necrosis, which delays myocardial apoptosis. Delayed anesthetic preconditioning protects against myocardial infarction by downregulating caspase-3 (31). Apoptosis is gene-controlled autonomous programmed cell death (32) and is regulated by genes and proteins such as Bcl-2, caspase-3, and caspase-8. Caspases are essential in the apoptotic process. Caspase-8 is usually secreted as a zymogen. Once activated, caspase-8 causes the cleavage and activation of caspase-3, caspase-6, and caspase-7, resulting in apoptosis (2). Apoptosis is an irreversible cascade of limited substrate hydrolysis of caspases (33,34). Sevoflurane inhibits the production of reactive oxygen species and activates Bcl-2 by stimulating KATP channel opening, thereby preventing apoptosis (35). The pro-apoptotic protein caspase-8 is an upstream promoter that controls the death receptor-mediated apoptosis pathway (36). Considering that 5-HD concentrations were increased before sevoflurane preconditioning, caspase-8 protein expression was elevated during reperfusion. Inhibition of mitoKATP channels promoted myocardial cell apoptosis. Thus, caspase-8 is an ideal target for inhibiting apoptosis.
We only used sevoflurane in the current study; thus, we do not know whether all of the volatile anesthetics are equally effective for IPC. A previous study has demonstrated the efficacy of IPC in human surgical series (2). Most of the study focused on the acute phase of protection, not on delayed protection against I/R injury, which occurs 12 to 24 h after preconditioning. Two types of KATP channels are present in myocardial cells, membrane-bound channels or cell membrane KATP channel (sarcKATP) and mitochondrial membrane-bound channels or mitoKATP. We used only the mitoKATP channel inhibitor 5-HD in the current study, not the sarcKATP inhibitor HMR-1098 because HMR-1098 is not yet commercially available. We did not find any significant difference in the protective effects of the two channels and did not distinguish between the specific mechanisms of the two channels. Finally, we did not investigate the pharmacokinetics and pharmacodynamics of 5-HD and only based the dosage and timing of 5-HD on previously published studies.
In summary, sevoflurane preconditioning exerted a delayed protective effect against myocardial I/R injury in isolated hearts. The mechanism involved mitoKATP channels, which regulate the phosphorylation of p-PKC-ε before ischemia and downregulate caspase-8 during reperfusion.
Acknowledgments
Research supported by the Natural Science Foundation of Jiangsu Province (grant #BK20141187 to C. Wang), and by the Technology Bureau of Suzhou, China (grant #SYS201473 to S.G. Qiao). C. Wang also received support from the Project of Gusu Health Key Talent.
Footnotes
First published online.
References
- 1.Kertai MD, Klein J, Bax JJ, Poldermans D. Predicting perioperative cardiac risk. Prog Cardiovasc Dis. 2005;47:240–257. doi: 10.1016/j.pcad.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-I. [DOI] [PubMed] [Google Scholar]
- 3.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Engelhard K, Werner C, Reeker W, Lu H, Mollenberg O, Mielke L, et al. Desflurane and isoflurane improve neurological outcome after incomplete cerebral ischaemia in rats. Br J Anaesth. 1999;83:415–421. doi: 10.1093/bja/83.3.415. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Lei B, Popp S, Meng F, Cottrell JE, Kass IS. Sevoflurane immediate preconditioning alters hypoxic membrane potential changes in rat hippocampal slices and improves recovery of CA1 pyramidal cells after hypoxia and global cerebral ischemia. Neuroscience. 2007;145:1097–1107. doi: 10.1016/j.neuroscience.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, et al. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–237. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- 7.De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg. 2005;100:1584–1593. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- 8.Fujita A, Kurachi Y. Molecular aspects of ATP-sensitive K+ channels in the cardiovascular system and K+ channel openers. Pharmacol Ther. 2000;85:39–53. doi: 10.1016/S0163-7258(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Rajapakse N, Shimizu K, Kis B, Snipes J, Lacza Z, Busija D. Activation of mitochondrial ATP-sensitive potassium channels prevents neuronal cell death after ischemia in neonatal rats. Neurosci Lett. 2002;327:208–212. doi: 10.1016/S0304-3940(02)00413-5. [DOI] [PubMed] [Google Scholar]
- 10.Hu H, Sato T, Seharaseyon J, Liu Y, Johns DC, O'Rourke B, et al. Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels. Mol Pharmacol. 1999;55:1000–1005. [PubMed] [Google Scholar]
- 11.Papp Z, Csapo K, Pollesello P, Haikala H, Edes I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc Drug Rev. 2005;23:71–98. doi: 10.1111/j.1527-3466.2005.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 12.Schultz JE, Qian YZ, Gross GJ, Kukreja RC. The ischemia-selective KATP channel antagonist, 5-hydroxydecanoate, blocks ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 1997;29:1055–1060. doi: 10.1006/jmcc.1996.0358. [DOI] [PubMed] [Google Scholar]
- 13.Ockaili R, Emani VR, Okubo S, Brown M, Krottapalli K, Kukreja RC. Opening of mitochondrial KATP channel induces early and delayed cardioprotective effect: role of nitric oxide. Am J Physiol. 1999;277:H2425–H2434. doi: 10.1152/ajpheart.1999.277.6.H2425. [DOI] [PubMed] [Google Scholar]
- 14.Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606:1–21. doi: 10.1016/S0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Yang C, Chen Y, Tian J, Liu L, Dai Q, et al. Identification of a PKC-epsilon-dependent regulation of myocardial contraction by epicatechin-3-gallate. Am J Physiol Heart Circ Physiol. 2008;294:H345–H353. doi: 10.1152/ajpheart.00785.2007. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Liu K, Chan JY. Anesthetic preconditioning confers acute cardioprotection via up-regulation of manganese superoxide dismutase and preservation of mitochondrial respiratory enzyme activity. Shock. 2008;29:300–308. doi: 10.1097/SHK.0b013e3181454295. [DOI] [PubMed] [Google Scholar]
- 17.Varadarajan SG, An J, Novalija E, Stowe DF. Sevoflurane before or after ischemia improves contractile and metabolic function while reducing myoplasmic Ca(2+) loading in intact hearts. Anesthesiology. 2002;96:125–133. doi: 10.1097/00000542-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Parra VM, Macho P, Domenech RJ. Late cardiac preconditioning by exercise in dogs is mediated by mitochondrial potassium channels. J Cardiovasc Pharmacol. 2010;56:268–274. doi: 10.1097/FJC.0b013e3181eb3049. [DOI] [PubMed] [Google Scholar]
- 19.Konia MR, Schaefer S, Liu H. Nuclear factor-[kappa]B inhibition provides additional protection against ischaemia/reperfusion injury in delayed sevoflurane preconditioning. Eur J Anaesthesiol. 2009;26:496–503. doi: 10.1097/EJA.0b013e328324ed2e. [DOI] [PubMed] [Google Scholar]
- 20.Lutz M, Liu H. Inhaled sevoflurane produces better delayed myocardial protection at 48 versus 24 hours after exposure. Anesth Analg. 2006;102:984–990. doi: 10.1213/01.ane.0000198568.79079.4c. [DOI] [PubMed] [Google Scholar]
- 21.Chiari PC, Pagel PS, Tanaka K, Krolikowski JG, Ludwig LM, Trillo RA, Jr, et al. Intravenous emulsified halogenated anesthetics produce acute and delayed preconditioning against myocardial infarction in rabbits. Anesthesiology. 2004;101:1160–1166. doi: 10.1097/00000542-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Pomerantz BJ, Robinson TN, Morrell TD, Heimbach JK, Banerjee A, Harken AH. Selective mitochondrial adenosine triphosphate-sensitive potassium channel activation is sufficient to precondition human myocardium. J Thorac Cardiovasc Surg. 2000;120:387–392. doi: 10.1067/mtc.2000.107521. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Cone J, Liu Y. Dual roles of mitochondrial K(ATP) channels in diazoxide-mediated protection in isolated rabbit hearts. Am J Physiol Heart Circ Physiol. 2001;280:H246–H255. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Takashi E, Xu M, Ayub A, Ashraf M. Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation. 2001;104:85–90. doi: 10.1161/01.CIR.104.1.85. [DOI] [PubMed] [Google Scholar]
- 25.Yao Z, McPherson BC, Liu H, Shao Z, Li C, Qin Y, et al. Signal transduction of flumazenil-induced preconditioning in myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1249–H1255. doi: 10.1152/ajpheart.2001.280.3.H1249. [DOI] [PubMed] [Google Scholar]
- 26.Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol Heart Circ Physiol. 2002;283:H2322–H2330. doi: 10.1152/ajpheart.00474.2002. [DOI] [PubMed] [Google Scholar]
- 27.Fryer RM, Wang Y, Hsu AK, Gross GJ. Essential activation of PKC-delta in opioid-initiated cardioprotection. Am J Physiol Heart Circ Physiol. 2001;280:H1346–H1353. doi: 10.1152/ajpheart.2001.280.3.H1346. [DOI] [PubMed] [Google Scholar]
- 28.Fryer RM, Hsu AK, Gross GJ. ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Res Cardiol. 2001;96:136–142. doi: 10.1007/s003950170063. [DOI] [PubMed] [Google Scholar]
- 29.Novalija E, Kevin LG, Camara AK, Bosnjak ZJ, Kampine JP, Stowe DF. Reactive oxygen species precede the epsilon isoform of protein kinase C in the anesthetic preconditioning signaling cascade. Anesthesiology. 2003;99:421–428. doi: 10.1097/00000542-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Weber NC, Toma O, Damla H, Wolter JI, Schlack W, Preckel B. Upstream signaling of protein kinase C-epsilon in xenon-induced pharmacological preconditioning. Implication of mitochondrial adenosine triphosphate dependent potassium channels and phosphatidylinositol-dependent kinase-1. Eur J Pharmacol. 2006;539:1–9. doi: 10.1016/j.ejphar.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Qiao S, Xie H, Wang C, Wu X, Liu H, Liu C. Delayed anesthetic preconditioning protects against myocardial infarction via activation of nuclear factor-kappaB and upregulation of autophagy. J Anesth. 2013;27:251–260. doi: 10.1007/s00540-012-1494-3. [DOI] [PubMed] [Google Scholar]
- 32.Harvey NL, Kumar S. The role of caspases in apoptosis. Adv Biochem Eng Biotechnol. 1998;62:107–128. doi: 10.1007/BFb0102307. [DOI] [PubMed] [Google Scholar]
- 33.Gurtl B, Kratky D, Guelly C, Zhang L, Gorkiewicz G, Das SK, et al. Apoptosis and fibrosis are early features of heart failure in an animal model of metabolic cardiomyopathy. Int J Exp Pathol. 2009;90:338–346. doi: 10.1111/j.1365-2613.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Horm Metab Res. 2007;39:672–676. doi: 10.1055/s-2007-985823. [DOI] [PubMed] [Google Scholar]
- 35.Lu X, Liu H, Wang L, Schaefer S. Activation of NF-kappaB is a critical element in the antiapoptotic effect of anesthetic preconditioning. Am J Physiol Heart Circ Physiol. 2009;296:H1296–H1304. doi: 10.1152/ajpheart.01282.2008. [DOI] [PubMed] [Google Scholar]
- 36.Gervais FG, Singaraja R, Xanthoudakis S, Gutekunst CA, Leavitt BR, Metzler M, et al. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat Cell Biol. 2002;4:95–105. doi: 10.1038/ncb735. [DOI] [PubMed] [Google Scholar]


