Abstract
Exposure to intense sounds often leads to loss of hearing of environmental sounds and hearing of a monotonous tonal sound not actually present, a condition known as tinnitus. Chronic physiological effects of exposure to intense tones have been reported for animals and should be accompanied by chemical changes present at long times after the intense sound exposure. By using a microdissection mapping procedure combined with high-performance liquid chromatography (HPLC), we have measured concentrations of nine amino acids, including those used as neurotransmitters, in the cochlear nucleus, inferior colliculus, medial geniculate, and auditory cortex of hamsters 5 months after exposure to an intense tone, compared with control hamsters of the same age. No very large differences in amino acid concentrations were found between exposed and control hamsters. However, increases of glutamate and γ-aminobutyrate (GABA) in some parts of the inferior colliculus of exposed hamsters were statistically significant. The most consistent differences between exposed and control hamsters were higher aspartate and lower taurine concentrations in virtually all regions of exposed hamsters, which reached statistical significance in many cases. Although these amino acids are not considered likely neurotransmitters, they indirectly have roles in excitatory and inhibitory neurotransmission, respectively. Thus, there is evidence for small, widespread, long-term increases in excitatory transmission and decreases in inhibitory transmission after a level of acoustic trauma previously shown to produce hearing loss and tinnitus.
Keywords: aspartate, glutamate, glycine, taurine, tinnitus
It is well known that exposure to high-intensity sound can produce damage to the cochlea that results in loss of hearing and tinnitus (Møller, 2000; Henry et al., 2005). In addition to the cochlear damage, there can be anatomical and activity changes in the cochlear nucleus (Kim et al., 2004; Feng et al., 2011; Kaltenbach, 2011; Vogler et al., 2011; Dehmel et al., 2012) and other central auditory regions (Ryan et al., 1992; Seki and Eggermont, 2003; Zhang et al., 2003; Mulders and Robertson, 2011) that may underlie related hearing disorders as well as tinnitus (Roberts et al., 2010). Once these changes occur, they may become chronic (Henry et al., 2005; Roberts et al., 2010). Increases in spontaneous activity following intense tone exposure, for example, endure for at least 6 months (Kaltenbach et al., 2000). They have been shown to be associated with behavioral evidence of tinnitus (Brozoski et al., 2002; Kaltenbach et al., 2004) and are therefore of clinical significance.
Chemical changes in the central auditory system are likely to be associated with these activity changes, and some have been reported in the first auditory brain center, the cochlear nucleus (Godfrey et al., 2008; Wang et al., 2009, 2011; Kraus et al., 2011), and in the inferior colliculus (Abbott et al., 1999; Milbrandt et al., 2000; Dong et al., 2010), at days, a month, or a few months after intense sound exposure. However, information on how amino acid metabolism is affected after long postex-posure recovery times is still lacking.
Here we extended our previous measurements of amino acid concentrations for the hamster cochlear nucleus after intense tone exposure (Godfrey et al., 2008) to 5 months, to gain further insight into the chemical changes that might underlie the chronic increases of spontaneous activity reported previously (Kaltenbach et al., 2000). We employed an intense tone exposure similar to that used in our previous study (Godfrey et al., 2008), which has been shown to produce behavioral evidence of tinnitus (Heffner and Harrington, 2002; Kaltenbach et al., 2004), cochlear damage, and upward threshold shifts of about 40–70 dB (Meleca et al., 1997; Kaltenbach et al., 1998). We also extended our measurements to include higher level auditory centers: the inferior colliculus, medial geniculate, and auditory cortex. We hypothesized that chemical changes associated with chronic effects of acoustic trauma, such as increases in spontaneous activity and tinnitus, should be present at extended times after the trauma. We employed the same methods as for our previous studies, including microdissection of samples from freeze-dried tissue sections for measurement of amino acid concentrations by high-performance liquid chromatography (HPLC). Others have reported decreased volume of ventral cochlear nucleus regions after acoustic trauma (Feng et al., 2011; Kraus et al., 2011), so we also checked for this possibility in our cochlear nucleus sections.
MATERIALS AND METHODS
Most of the procedures used in this study were similar to those described previously (Godfrey and Matschinsky, 1976; Ross et al., 1995; Godfrey et al., 2000, 2008).
Animals and Intense Tone Exposure
Twelve male Syrian hamsters weighing 145–178 g, obtained from Charles River, were divided into two groups. Six hamsters were exposed to a 10-kHz intense tone in the free field at a level of 127 dB SPL for 4 hr. Six unexposed hamsters were placed in a sound-attenuation room for a similar period but in the absence of an exposure tone. All hamsters survived for 140–145 days after treatment with or without tone exposure. Treatment of animals was approved by and in accordance with existing policies and regulations of the Institutional Animal Care and Use Committee and the National Institutes of Health.
Isolation of Tissue Samples
Hamsters were euthanized by decapitation while deeply anesthetized with ketamine + xylazine (25 mg + 4 mg, i.m.); then, their brains were isolated and frozen within 3–4 min of decapitation in Freon (Fisher Friendly Freeze-It) chilled to its freezing point with liquid nitrogen. Frozen tissue blocks were stored in double airtight containers at −80°C until sectioning.
Transverse sections of frozen brains were cut 20 µm thick at −20°C in a cryostat. Sections were saved from the level of the caudalmost cochlear nucleus through the auditory cortex. At the cochlear nucleus level, every section was saved, including alternate ones for freeze drying and staining. Rostral to the cochlear nucleus, two of every six sections were saved, one for freeze drying and the other for staining. Sections for freeze drying were placed into aluminum racks (Lowry and Passonneau, 1972) that were kept on blocks of dry ice in the cryostat. After completion of sectioning, the racks were placed into a glass vacuum tube and into a freezer maintained at −40°C. The vacuum tube was attached to a vacuum pump through a dry ice trap for freeze drying overnight; then, the sections were stored under vacuum below −20°C. Adjacent sections were melted onto slides for staining of Nissl substance with thionin. Because of problems with sectioning, two of the control hamsters were not included in the study.
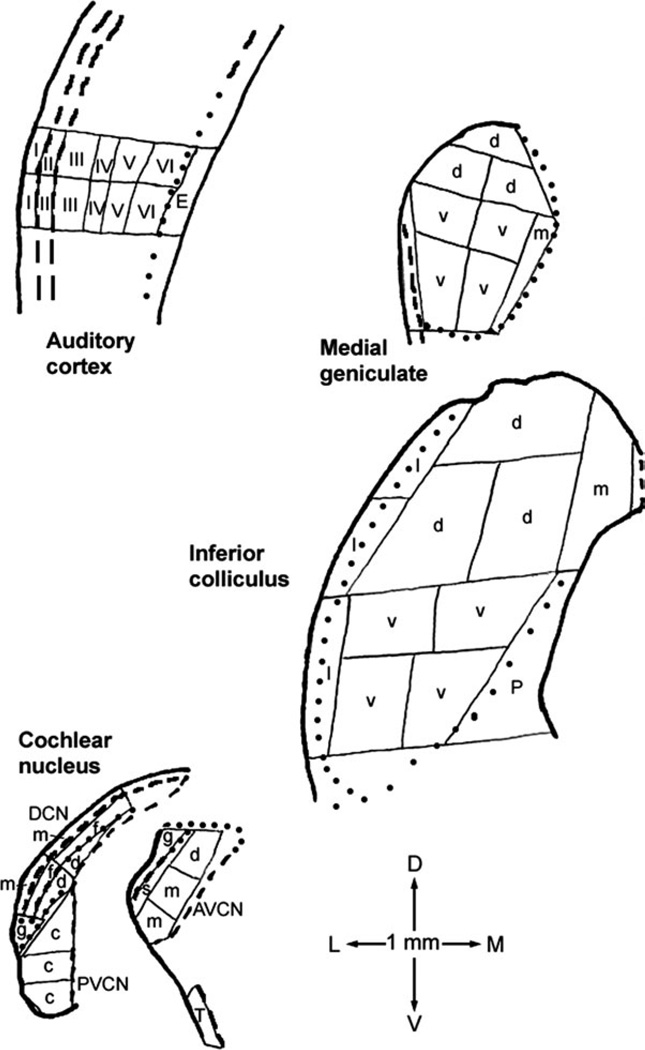
Dissection of freeze-dried tissue into samples for assay was performed at ×25 magnification, in a room with relative humidity maintained below 50%. A drawing attachment on a Wild dissecting microscope was used to map sample locations (Godfrey and Matschinsky, 1976). The regions sampled included the anteroventral (AVCN), posteroventral (PVCN), and dorsal (DCN) subdivisions of the cochlear nucleus; the inferior colliculus; the medial geniculate; and the auditory cortex. Sections for AVCN and PVCN dissections were chosen from more rostral and caudal parts, respectively. For the other regions, sections approximately halfway through their rostral– caudal extents were chosen for dissection. The dissection for each region followed the same plan for each hamster (Fig. 1) so that paired sample-by-sample comparisons could be made between exposed and control hamsters. Auditory cortex layers were discriminated by reference to adjacent thionin-stained sections and a published description of rat auditory cortex layers (Games and Winer, 1988), which appear similar to those for hamster based on one brief publication (Ravizza et al., 1976). Internal boundaries were not easily discerned within the medial geniculate and inferior colliculus, and available anatomical information for these regions in hamsters is limited (Morin and Wood, 2001; Fuentes-Santamaría et al., 2005), so the dissections were performed somewhat more objectively. The dissection plan for the medial geniculate approximated published regional definitions for rats (Paxinos and Watson, 1998; Winer et al., 1999) and cats (Morest, 1964). The dissection plan for the inferior colliculus less closely approximated published regional definitions for rats (Paxinos and Watson, 1998; Oliver et al., 1999; Loftus et al., 2008) and cats (Oliver et al., 1997; Loftus et al., 2008).
Fig. 1.
Examples of microdissection maps for coronal sections through the four major auditory regions sampled. A similar dissection strategy was used for all hamsters. The 1-mm scale at the bottom also shows dorsal (D), ventral (V), lateral (L), and medial (M) directions. Thin solid lines are cut-sample boundaries; thick solid lines are outside sectional boundaries; thick dashed lines are internal boundaries traced in the freeze-dried sections themselves; and dotted lines are internal boundaries traced from adjacent thionin-stained sections. Letters and numerals within samples identify those for which data were averaged for Tables I and II. Abbreviations are, for cochlear nucleus, AVCN, anteroventral cochlear nucleus, including dorsal (d), main (m), subgranular (s), and granular (g) regions; DCN, dorsal cochlear nucleus, including molecular (m), fusiform soma (f), and deep (d) layers; and PVCN, posteroventral cochlear nucleus, including caudal portion (c) and granular region (g); for inferior colliculus, dorsal (d), ventral (v), lateral (l), and medial (m) parts and periaqueductal gray (P); for medial geniculate, dorsal (d), ventral (v), and medial (m) parts; and for auditory cortex, layers I through VI and underlying external capsule (E). Compared with inferior colliculus subdivisions as defined for rats (Paxinos and Watson, 1998; Loftus et al., 2008), our most dorsal ‘‘d’’ sample, medial ‘‘d’’ sample of the second row, and ‘‘m’’ sample collectively approximate its dorsal cortex; the ventral part plus the lateral ‘‘d’’ sample of the second row its central nucleus; and the lateral part its lateral, or external, cortex. The dorsal, ventral, and medial parts of the medial geniculate as defined here approximately correspond to the same-named regions as defined for rats (Paxinos and Watson, 1998; Winer et al., 1999). The most lateral, unlabeled sample location in the medial geniculate map included some optic tract and some marginal zone of the medial geniculate (Paxinos and Watson, 1998; Morin and Wood, 2001).
Samples were weighed on quartz-fiber microbalances (Lowry and Passonneau, 1972) and then loaded into 300-µl-capacity glass tube inserts for HPLC measurement of amino acid concentrations. Comparably located samples from both control and exposed hamsters were always included in the same assays to minimize chances of finding differences that resulted from technical factors rather than real differences between the two groups.
Amino Acid Assay
For HPLC assay of free amino acid concentrations, 16 µl of 50% (vol/vol) methanol containing β-(2-thienyl)-DL-serine as an internal standard (to control for variations in injection volume) were added to the samples in all tubes to extract the amino acids. Subsequently, 8-µl aliquots were withdrawn by a WISP autosampler and derivatized with 8 µl ortho-phthaldialdehyde solution, and the fluorescent derivatized amino acids were separated by reversed-phase chromatography on a C8 column, using gradient elution. The eluate was passed through a Spectrovision fluorescence detector, and the peaks of fluorescence were quantified in Millennium software. Amino acids were identified by their retention times, and their concentrations were calculated by comparison with calibrated amino acid standard solutions included in the same assays (Hill et al., 1979; Ross et al., 1995; Godfrey et al., 2000). Standards were prepared from Standard H supplemented with calibrated amounts of asparagine, glutamine, taurine, and GABA. Although measurements were made for 12 amino acids, data for asparagine, alanine, and tyrosine are not reported because they were not sufficiently reliable in the assays for this study. Occasional samples showed contamination, recognized by an unusually high serine concentration, probably resulting from a contaminated tube. Such contamination affected some amino acids more than others, serine and glycine being greatly affected and glutamate, glutamine, taurine, and GABA much less affected. Contaminated data were omitted from the results.
Tissue Density
To check whether gradients of amino acid concentrations in some regions were related to variations in lipid content, measurements of tissue densities were made. The density of freeze-dried brain tissue, as dry weight per volume, is strongly related to lipid content, particularly the lipid in myelin (Godfrey and Matschinsky, 1976). Because the concentrations of the amino acids are expressed per dry weight and because they are associated predominantly with the nonlipid portions of tissue, apparent gradients in amino acid concentrations could reflect merely variations in tissue density. Tissue density, as dry weight per volume, was determined by dividing the dry weight of each sample by its volume, measured as area of the dissected sample times the 20-µm section thickness. A source of error in measurements of sample volume, which makes them less reliable than sample dry weight, is variations of section thickness, which has been only an occasional problem with our current microtome.
Volumes of Cochlear Nucleus Regions
To check for any changes in size of cochlear nucleus regions after intense tone exposure, boundaries of the regions were traced in all available thionin-stained sections. The tracings were scanned in Adobe Photoshop, and then the boundaries were traced over and digitized in Neurolucida, which calculated the area of each region in each section. Multiplying the areas by the distances between sections gave the regional volumes.
Data Presentation
Concentrations of amino acids (as mmol/kg dry weight) were plotted onto the maps of the dissected sections. Also, the data for all samples within defined regions (Fig. 1) were averaged for each group. Differences from control values were evaluated for statistical significance by t-tests, and those with P < 0.05 were considered statistically significant.
Materials
Glass vacuum tubes for freeze drying and for storage of freeze-dried sections were from Ace Glass (Vineland, NJ). Glass tube inserts were from Waters Corporation (Milford, MA), Kimble (Vineland, NJ), or Microliter Analytical Supplies (Suwanee, GA). HPLC equipment and Millennium software were from Waters Corporation. HPLC columns were from Mac-Mod Analytical (Chadds Ford, PA). Amino Acid Standard H was from Pierce Protein Research Products (a division of Thermo Fisher Scientific, Rockford, IL). Other chemicals were from Sigma (St. Louis, MO) or Fisher Scientific.
RESULTS
The data for the cochlear nucleus and auditory cortex can be adequately represented in Tables I and II, whereas additional details about the results for the inferior colliculus and medial geniculate can be appreciated from their maps.
TABLE I.
Concentrations of Transmitter-Related Amino Acids in Central Auditory Regions of Control and Intense-Tone-Exposed Hamsters†
| Aspartate | Glutamate | GABA | Glycine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Control | Exposed | Control | Exposed | Control | Exposed | Control | Exposed | Dry wt/Vol (cont and exp) |
| AVCN main (9,14) | 13.0 ± 0.7* | 15.2 ± 0.6 | 24.0 ± 1.5 | 25.6 ± 1.1 | 4.2 ± 0.4 | 4.3 ± 0.2 | 15.2 ± 1.4 | 14.4 ± 0.6 | 349 ± 11 (12) |
| AVCN dorsal (4,6) | 15.5 ± 0.5 | 16.6 ± 1.4 | 24.9 ± 1.6 | 24.6 ± 1.6 | 5.3 ± 0.8 | 5.3 ± 0.6 | 16.8 ± 1.4 | 16.2 ± 1.2 | 321 ± 12 (12) |
| AVCN subgr (4,6) | 14.1 ± 0.5 | 16.0 ± 0.9 | 26.4 ± 2.1 | 27.2 ± 1.1 | 5.6 ± 0.4 | 5.4 ± 0.5 | 16.4 ± 0.9 | 16.2 ± 1.3 | 319 ± 6 (24) |
| AVCN gran (6,11) | 10.8 ± 0.7* | 13.3 ± 0.7 | 30.5 ± 1.8 | 32.1 ± 1.4 | 6.6 ± 0.5 | 6.5 ± 0.5 | 16.5 ± 1.0 | 14.7 ± 0.9 | 279 ± 8 (24) |
| PVCN caudal (13,15) | 11.2 ± 0.7** | 15.2 ± 1.0 | 24.2 ± 1.0* | 27.9 ± 1.3 | 3.8 ± 0.3 | 3.9 ± 0.2 | 14.1 ± 0.7 | 13.9 ± 0.5 | 347 ± 11 (16) |
| PVCN gran (3,4) | 9.5 ± 0.7 | 10.2 ± 1.3 | 31.8 ± 1.4 | 31.5 ± 2.7 | 8.4 ± 0.6 | 7.2 ± 0.5 | 26.9 ± 2.2 | 21.4 ± 1.1 | 307 ± 10 (14) |
| DCN deep (11,12) | 11.7 ± 0.8 | 14.1 ± 1.0 | 24.4 ± 1.3 | 28.2 ± 1.6 | 6.0 ± 0.6 | 6.0 ± 0.4 | 22.8 ± 1.5 | 22.8 ± 1.4 | 344 ± 7 (34) |
| DCN fus (11,12) | 12.2 ± 0.4 | 13.9 ± 0.8 | 29.7 ± 1.3 | 32.6 ± 1.7 | 9.1 ± 0.5 | 8.9 ± 0.4 | 29.6 ± 1.0 | 26.8 ± 1.1 | 300 ± 6 (34) |
| DCN mol (11,12) | 9.5 ± 0.3 | 10.2 ± 0.5 | 40.8 ± 1.4 | 42.4 ± 1.7 | 10.4 ± 0.5 | 10.7 ± 0.4 | 26.9 ± 0.9 | 25.0 ± 0.7 | 259 ± 5 (35) |
| Trapezoid body (4,5) | 3.5 ± 0.3** | 5.3 ± 0.3 | 9.4 ± 1.0 | 10.2 ± 1.0 | 0.6 ± 0.1 | 0.6 ± 0.1 | 4.8 ± 0.7 | 3.8 ± 0.8 | 560 ± 21 (17) |
| IC dorsal (18,18) | 15.7 ± 0.6** | 17.6 ± 0.4 | 32.6 ± 1.2* | 36.5 ± 1.1 | 11.0 ± 0.3 | 11.9 ± 0.3 | 9.6 ± 0.3 | 9.3 ± 0.3 | 294 ± 4 (36) |
| IC ventral (24,24) | 14.8 ± 0.5* | 16.3 ± 0.4 | 24.3 ± 0.7* | 26.8 ± 0.7 | 8.9 ± 0.3* | 9.9 ± 0.3 | 11.8 ± 0.3 | 12.5 ± 0.4 | 346 ± 5 (48) |
| IC lateral (16,17) | 11.4 ± 0.4* | 12.7 ± 0.3 | 27.5 ± 1.0 | 29.3 ± 0.8 | 8.3 ± 0.3* | 9.2 ± 0.3 | 7.1 ± 0.2 | 7.0 ± 0.2 | 336 ± 7 (33) |
| IC medial (6,6) | 14.0 ± 0.4 | 15.0 ± 0.7 | 42.2 ± 2.5 | 43.5 ± 0.8 | 12.4 ± 0.5 | 12.1 ± 0.7 | 10.0 ± 0.6 | 8.7 ± 0.4 | 267 ± 6 (12) |
| MG dorsal (15,18) | 9.2 ± 0.3** | 10.4 ± 0.3 | 52.8 ± 1.5 | 56.1 ± 1.3 | 8.2 ± 0.5 | 7.7 ± 0.4 | 6.9 ± 0.3 | 6.3 ± 0.4 | 320 ± 5 (27) |
| MG ventral (20,24) | 10.0 ± 0.2** | 11.2 ± 0.3 | 46.4 ± 1.8 | 47.3 ± 1.4 | 7.6 ± 0.6 | 7.7 ± 0.5 | 6.2 ± 0.3 | 5.7 ± 0.2 | 333 ± 4 (36) |
| MG medial (5,6) | 10.4 ± 0.5 | 11.6 ± 0.7 | 36.6 ± 1.6 | 35.8 ± 1.5 | 7.5 ± 0.8 | 8.5 ± 0.5 | 6.9 ± 0.6 | 6.3 ± 0.2 | 355 ± 6 (9) |
| AC layer I (10,12) | 14.9 ± 0.6 | 16.7 ± 0.7 | 61.4 ± 1.3 | 65.2 ± 2.8 | 10.5 ± 0.4 | 10.6 ± 0.5 | 7.6 ± 0.6 | 7.7 ± 0.5 | 284 ± 11 (11) |
| AC layer II (10,12) | 16.3 ± 1.0 | 18.3 ± 0.7 | 63.3 ± 2.2 | 67.3 ± 2.4 | 10.2 ± 0.4 | 10.3 ± 0.5 | 7.7 ± 0.6 | 7.3 ± 0.5 | 274 ± 7 (12) |
| AC layer III (11,13) | 18.2 ± 1.2 | 20.0 ± 0.6 | 61.0 ± 2.2 | 64.8 ± 3.0 | 10.3 ± 0.4 | 10.9 ± 0.4 | 6.9 ± 0.5 | 7.1 ± 0.3 | 284 ± 8 (12) |
| AC layer IV (10,12) | 19.2 ± 1.1 | 20.6 ± 1.0 | 57.8 ± 2.7 | 62.3 ± 3.1 | 10.5 ± 0.4 | 10.7 ± 0.4 | 6.8 ± 0.8 | 7.4 ± 0.5 | 285 ± 7 (12) |
| AC layer V (11,12) | 16.2 ± 0.7* | 18.8 ± 0.7 | 57.3 ± 2.4 | 61.6 ± 2.9 | 8.3 ± 0.4 | 8.9 ± 0.4 | 6.2 ± 0.4 | 6.1 ± 0.3 | 292 ± 7 (12) |
| AC layer VI (11,15) | 12.0 ± 0.8* | 14.5 ± 0.7 | 50.8 ± 2.0 | 54.9 ± 2.2 | 6.7 ± 0.3 | 6.9 ± 0.3 | 5.1 ± 0.4 | 5.1 ± 0.3 | 318 ± 8 (12) |
| Ext capsule (5,5) | 3.7 ± 0.3* | 5.1 ± 0.4 | 21.8 ± 0.5 | 24.5 ± 2.5 | 1.5 ± 0.1 | 1.7 ± 0.3 | 3.4 ± 0.4 | 3.2 ± 0.4 | 421 ± 9 (10) |
Data for amino acids (mmol/kg dry wt) are presented as mean ± SEM, based on numbers of samples from four control and six intense-tone-exposed hamsters, except three control and four exposed for PVCN granular and four control and five exposed for PVCN caudal.
AC, auditory cortex; AVCN, anteroventral cochlear nucleus; DCN, dorsal cochlear nucleus; Ext, external; fus, fusiform soma layer; gran, granular region; IC, inferior colliculus; mol, molecular layer; MG, medial geniculate; PVCN, posteroventral cochlear nucleus; subgr, subgranular region.
Numbers of samples assayed for control and exposed hamsters, respectively, are given in parentheses after each region name. In some cases, the number for a given amino acid was less because of contamination (see Materials and Methods): AVCN gran, 10 for glycine for exposed; MG ventral, 19 for all except GABA for control and 23 for aspartate and glycine for exposed; AC layer IV, nine for all except GABA for control and 11 for aspartate and glycine for exposed. Data for dry weight per volume are grams per liter presented as mean ± SEM for numbers of measurements (in parentheses) from both control and exposed hamsters; measurements for exposed hamsters showed no statistically significant differences from control except for layers II–VI of auditory cortex. Ratios of exposed to control dry weight per volume and P values, as denoted below, for these layers were II, 1.10*; III, 1.13**; IV, 1.09*, V, 1.09*, VI, 1.10*.
P < 0.05 control vs. exposed.
P < 0.01 control vs. exposed.
TABLE II.
Concentrations of Other Amino Acids in Central Auditory Regions of Control and Intense-Tone-Exposed Hamsters†
| Taurine | Glutamine | Arginine | Threonine | Serine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Control | Exposed | Control | Exposed | Control | Exposed | Control | Exposed | Control | Exposed |
| AVCN main (9,14) | 13.9 ± 1.0 | 12.7 ± 0.6 | 26.2 ± 1.8 | 25.6 ± 1.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 3.7 ± 0.1 | 4.1 ± 0.1 | 4.4 ± 0.5 | 3.8 ± 0.4 |
| AVCN dorsal (4,6) | 14.0 ± 1.4 | 13.5 ± 0.9 | 29.8 ± 2.9 | 27.5 ± 1.9 | 1.1 ± 0.1 | 1.2 ± 0.1 | 4.2 ± 0.4 | 4.4 ± 0.2 | 4.2 ± 0.3 | 3.3 ± 0.6 |
| AVCN subgr (4,6) | 17.2 ± 1.4 | 15.0 ± 1.0 | 30.2 ± 1.8 | 28.5 ± 1.3 | 1.3 ± 0.1 | 1.4 ± 0.2 | 4.6 ± 0.3 | 4.5 ± 0.1 | 4.0 ± 0.6 | 4.1 ± 0.6 |
| AVCN gran (6,11) | 27.8 ± 1.8 | 25.0 ± 1.4 | 38.0 ± 1.6 | 37.0 ± 1.6 | 1.7 ± 0.2 | 2.0 ± 0.2 | 6.0 ± 0.4 | 6.7 ± 0.5 | 5.4 ± 0.9 | 6.2 ± 0.5 |
| PVCN caudal (13,15) | 17.0 ± 0.8* | 14.8 ± 0.6 | 25.9 ± 1.0 | 26.6 ± 0.8 | 1.7 ± 0.1 | 1.6 ± 0.1 | 4.7 ± 0.2 | 4.9 ± 0.2 | 4.6 ± 0.1* | 4.2 ± 0.1 |
| PVCN gran (3,4) | 31.6 ± 1.7* | 22.3 ± 2.5 | 34.5 ± 2.6 | 30.6 ± 1.7 | 1.7 ± 0.3 | 1.4 ± 0.2 | 7.0 ± 0.3* | 6.0 ± 0.2 | 6.3 ± 0.8 | 4.8 ± 0.3 |
| DCN deep (11,12) | 16.3 ± 0.8 | 14.6 ± 0.6 | 24.9 ± 1.0 | 25.0 ± 0.9 | 1.6 ± 0.1 | 1.5 ± 0.1 | 5.0 ± 0.2 | 5.0 ± 0.2 | 4.1 ± 0.3 | 3.7 ± 0.1 |
| DCN fus (11,12) | 22.2 ± 1.0 | 19.5 ± 0.8 | 31.0 ± 0.9 | 30.0 ± 0.7 | 1.6 ± 0.1 | 1.4 ± 0.1 | 6.2 ± 0.3 | 6.0 ± 0.2 | 4.8 ± 0.3 | 4.0 ± 0.3 |
| DCN mol (11,12) | 33.8 ± 0.8 | 32.3 ± 1.5 | 40.2 ± 1.4 | 40.0 ± 1.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 6.8 ± 0.3 | 6.7 ± 0.2 | 6.0 ± 0.4 | 5.7 ± 0.3 |
| Trapezoid body (4,5) | 8.9 ± 0.7 | 8.2 ± 0.6 | 10.4 ± 1.0 | 10.2 ± 0.5 | 1.3 ± 0.2 | 1.5 ± 0.2 | 2.0 ± 0.1 | 2.1 ± 0.3 | 2.2 ± 0.5 | 3.1 ± 0.3 |
| IC dorsal (18,18) | 14.0 ± 0.5* | 12.3 ± 0.4 | 29.4 ± 1.2 | 30.0 ± 1.3 | 1.0 ± 0.03 | 1.0 ± 0.03 | 8.4 ± 0.5 | 8.3 ± 0.5 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| IC ventral (24,24) | 11.5 ± 0.2* | 10.8 ± 0.2 | 21.0 ± 0.5 | 21.6 ± 0.6 | 1.1 ± 0.02 | 1.1 ± 0.04 | 4.8 ± 0.2 | 4.8 ± 0.2 | 1.8 ± 0.04 | 1.8 ± 0.05 |
| IC lateral (16,17) | 14.2 ± 0.3 | 13.4 ± 0.5 | 25.1 ± 1.0 | 25.8 ± 1.0 | 1.1 ± 0.1 | 1.1 ± 0.02 | 6.6 ± 0.4 | 6.6 ± 0.4 | 2.3 ± 0.1 | 2.3 ± 0.1 |
| IC medial (6,6) | 18.8 ± 0.7 | 17.3 ± 1.2 | 40.9 ± 2.5 | 39.3 ± 1.7 | 1.0 ± 0.05 | 1.0 ± 0.04 | 12.4 ± 0.8 | 11.8 ± 0.8 | 2.8 ± 0.2 | 2.8 ± 0.1 |
| MG dorsal (15,18) | 17.1 ± 0.8 | 15.2 ± 0.8 | 38.1 ± 0.8 | 38.1 ± 1.1 | 0.8 ± 0.03 | 0.8 ± 0.02 | 7.9 ± 0.4 | 8.0 ± 0.3 | 3.2 ± 0.1 | 3.3 ± 0.2 |
| MG ventral (20,24) | 14.5 ± 0.3*** | 12.7 ± 0.3 | 35.7 ± 1.0 | 34.3 ± 1.0 | 0.8 ± 0.03 | 0.8 ± 0.03 | 6.7 ± 0.3 | 6.7 ± 0.2 | 3.0 ± 0.1 | 3.0 ± 0.1 |
| MG medial (5,6) | 12.2 ± 0.5 | 11.7 ± 0.3 | 28.4 ± 1.2 | 27.5 ± 1.6 | 0.9 ± 0.1* | 1.1 ± 0.1 | 5.7 ± 0.4 | 5.8 ± 0.1 | 3.3 ± 0.7 | 2.7 ± 0.1 |
| AC layer I (10,12) | 37.3 ± 1.4 | 36.3 ± 1.0 | 48.1 ± 1.4 | 50.4 ± 1.7 | 0.9 ± 0.1 | 1.0 ± 0.1 | 13.7 ± 0.7 | 14.1± 0.7 | 6.6 ± 0.3 | 6.0 ± 0.7 |
| AC layer II (10,12) | 35.9 ± 1.2 | 34.6 ± 1.6 | 47.7 ± 1.6 | 49.9 ± 2.2 | 1.0 ± 0.1 | 0.9 ± 0.1 | 14.2 ± 0.7 | 14.8 ± 0.9 | 6.9 ± 0.5 | 6.3 ± 0.6 |
| AC layer III (11,13) | 33.2 ± 0.9 | 31.7 ± 1.3 | 45.6 ± 1.3 | 46.1 ± 2.0 | 0.8 ± 0.1 | 1.0 ± 0.1 | 13.1 ± 1.4 | 12.9 ± 0.9 | 6.0 ± 0.3 | 5.7 ± 0.6 |
| AC layer IV (10,12) | 32.1 ± 1.5 | 30.7 ± 1.1 | 44.1 ± 2.0 | 45.9 ± 2.0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 13.3 ± 1.8 | 12.9 ± 0.9 | 5.5 ± 0.7 | 5.9 ± 0.7 |
| AC layer V (11,12) | 29.2 ± 1.5 | 27.5 ± 1.1 | 43.6 ± 1.6 | 45.7 ± 2.1 | 0.5 ± 0.1 | 0.7 ± 0.05 | 10.9 ± 0.7 | 11.7 ± 0.7 | 5.2 ± 0.3 | 5.1 ± 0.4 |
| AC layer VI (11,15) | 28.6 ± 1.4 | 26.1 ± 0.7 | 42.7 ± 1.6 | 43.1 ± 1.5 | 0.7 ± 0.1 | 0.8 ± 0.04 | 10.8 ± 0.6 | 10.8 ± 0.5 | 5.5 ± 0.3 | 5.1 ± 0.3 |
| Ext capsule (5,5) | 22.1 ± 1.2 | 22.2 ± 1.3 | 19.1 ± 0.8 | 21.4 ± 2.2 | 1.1 ± 0.1 | 1.2 ± 0.1 | 5.7 ± 0.4 | 6.5 ± 0.6 | 4.9 ± 0.3 | 4.5 ± 0.5 |
Data presentation, numbers of hamsters represented, abbreviations, and numbers of samples assayed as in Table I. Cases with less than the full number of samples are: AVCN gran, eight for serine for exposed; MG ventral, 19 for all except taurine for control and 23 for all except taurine and glutamine for exposed; AC layer II, 11 for serine for exposed; AC layer III, 12 for serine for exposed; AC layer IV, nine for all except taurine for control and 11 for arginine and threonine and 10 for serine for exposed.
P < 0.05 control vs. exposed.
P < 0.001 control vs. exposed.
Tissue Densities
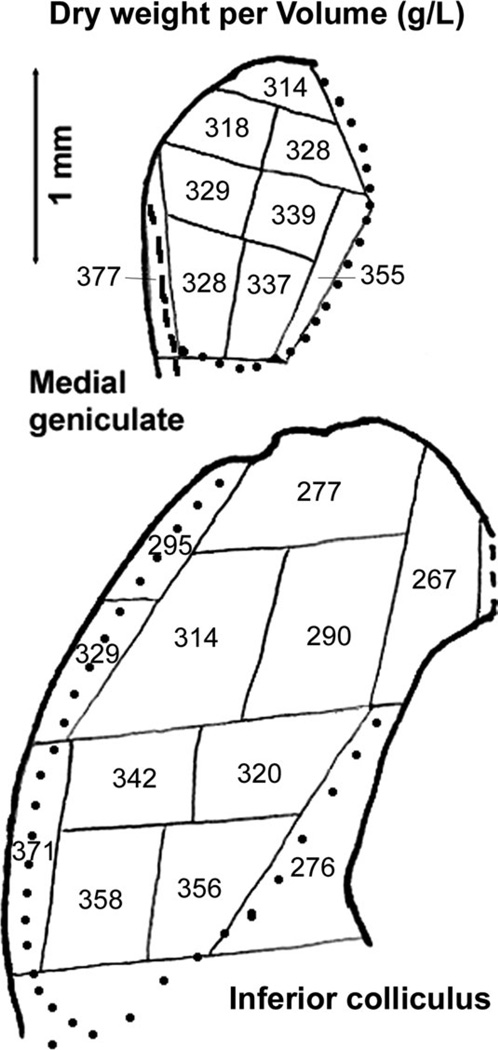
As expected, tissue densities, as dry weight per volume, were highest in fiber tracts containing myelinated axons, especially the trapezoid body (Table I). Lowest values were in auditory cortex layers I–V, inferior colliculus medial part, and cochlear nucleus regions containing high densities of granule cell bodies (AVCN and PVCN granular regions and DCN fusiform soma layer) and terminals (DCN molecular layer; Mugnaini et al., 1980). Within the inferior colliculus, densities tended to be higher ventrally and laterally (Fig. 2), where the lateral lemniscus fibers enter (Oliver et al. 1997, 1999). Within the medial geniculate, densities tended to be slightly higher ventromedially (Fig. 2), in accordance with a report that this medial region is its most heavily myelinated part (Morest, 1964).
Fig. 2.
Average dry weight per volume, in grams per liter, for samples of inferior colliculus and medial geniculate. Data were averaged for all hamsters. The maps and details are the same as in Figure 1, in which regional identifications are shown.
Amino Acid Distributions in Control Hamsters
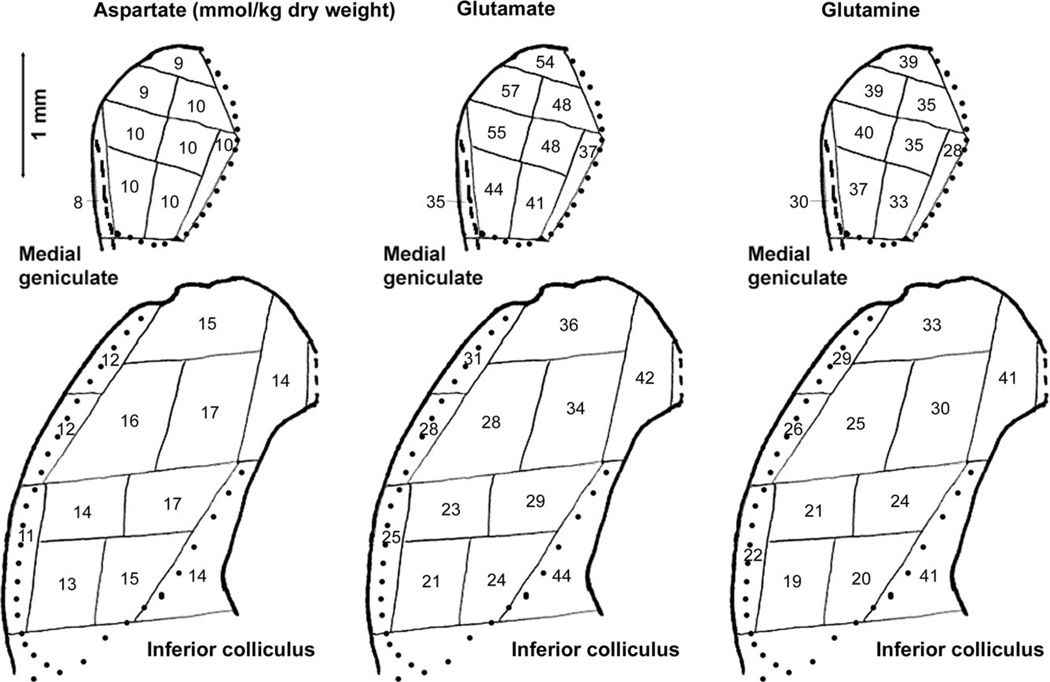
In control hamsters, the highest concentrations of both aspartate and glutamate were measured in layers of the auditory cortex (Table I). Other than this similarity, however, their distributions differed in many ways. Within the cochlear nucleus, aspartate concentrations were relatively low and glutamate concentrations high in regions containing high densities of granule cell bodies and terminals. In the inferior colliculus, glutamate concentrations were higher dorsomedially than ventrolaterally (Fig. 3). Aspartate concentrations showed a slightly similar tendency, but the most obvious trend was for lower aspartate concentrations in the most lateral parts of the inferior colliculus. Whereas glutamate concentrations in the medial geniculate were almost as high as those in the auditory cortex, aspartate concentrations in the medial geniculate were lower than in all other regions, except fiber tracts. Aspartate concentrations were uniformly distributed in the medial geniculate, whereas glutamate concentrations were higher dorsolaterally than ventromedially (Fig. 3).
Fig. 3.
Average concentrations of aspartate, glutamate, and glutamine (in mmol/kg dry weight) for samples of inferior colliculus and medial geniculate. The maps and details are the same as in Figure 1, in which regional identifications are shown.
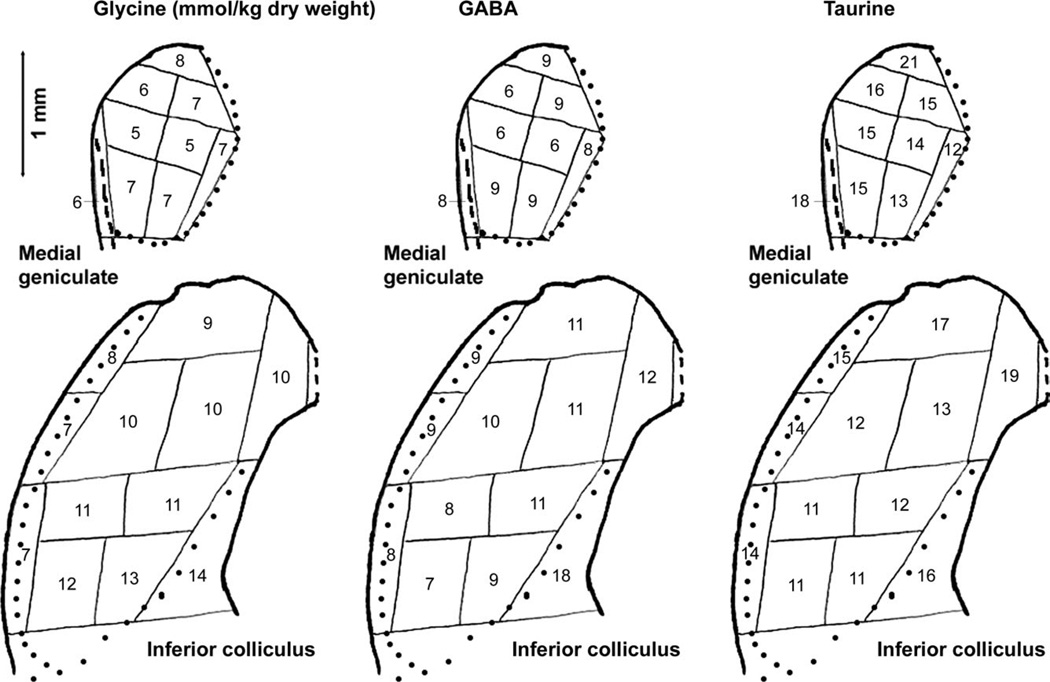
The relative distributions of GABA and glycine concentrations were quite similar within the cochlear nucleus (correlation coefficient = 0.90) and medial geniculate (correlation coefficient = 0.93), but they were somewhat inversely related across auditory regions as a whole. Whereas glycine concentrations reached their highest values in the cochlear nucleus and were relatively low in layers of the auditory cortex, GABA concentrations were relatively low in cochlear nucleus regions and reached their highest values in layers I–IV of the auditory cortex and dorsal and medial portions of the inferior colliculus. Within the inferior colliculus, GABA concentrations were higher dorsomedially than ventrolaterally, whereas glycine concentrations were higher ventrally than dorsally and relatively low most laterally (Fig. 4).
Fig. 4.
Average concentrations of glycine, GABA, and taurine (in mmol/kg dry weight) for samples of inferior colliculus and medial geniculate. The maps and details are the same as in Figure 1, in which regional identifications are shown.
Taurine concentrations were relatively high in auditory cortex, including a remarkably high concentration, for a fiber tract, in the external capsule deep to auditory cortex, and in cochlear nucleus regions containing high densities of granule cell bodies and terminals (Table II). Concentrations in both the inferior colliculus and medial geniculate were highest in the most dorsal parts (Fig. 4). Unlike glycine and GABA concentrations, taurine concentrations were relatively high in the most lateral part of the inferior colliculus.
The distribution of glutamine concentrations correlated closely with that of glutamate concentrations, including within the medial geniculate and inferior colliculus (Fig. 3). Correlation coefficients, with the fiber tracts (trapezoid body and external capsule) excluded, were 0.90 for cochlear nucleus, 0.99 for inferior colliculus, 0.94 for medial geniculate, 0.87 for auditory cortex, and 0.91 overall.
Although arginine concentrations were relatively low overall, the highest values were in cochlear nucleus regions. Threonine concentrations were highest in auditory cortex, especially layers I–IV, and the medial part of the inferior colliculus. Serine concentrations were relatively low in the inferior colliculus and medial geniculate compared with other regions.
Amino Acid Distributions in Intense-Tone-Exposed Hamsters
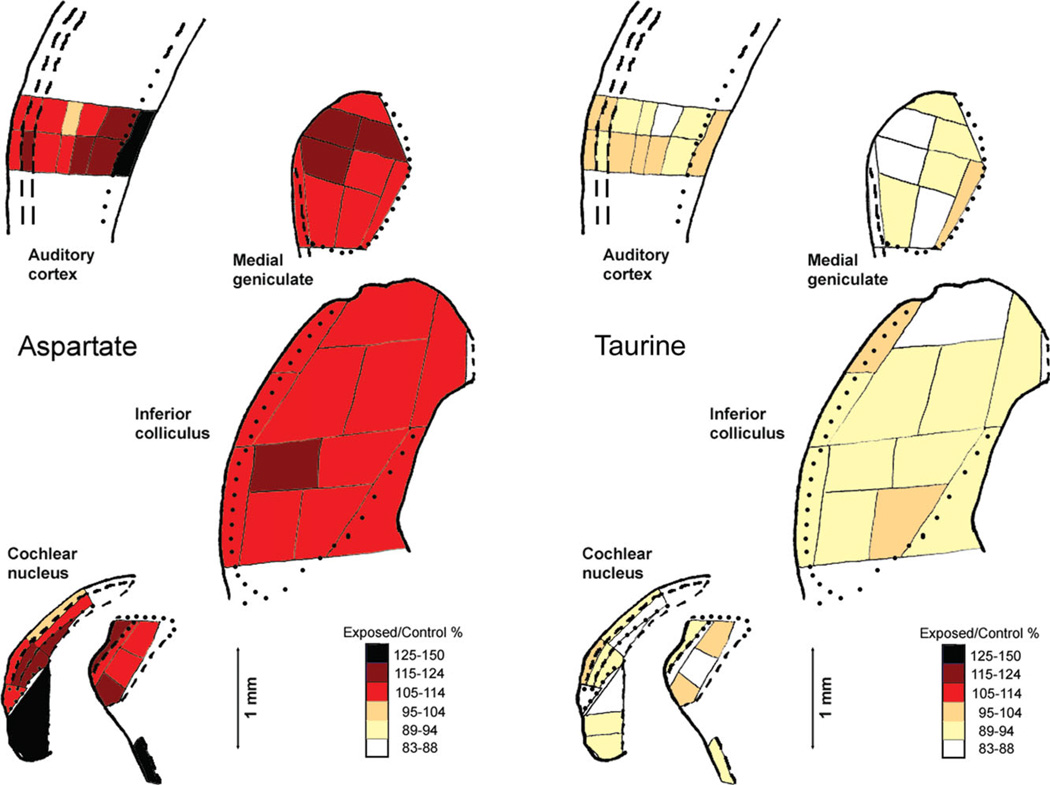
At 20 weeks after intense tone exposure, there were no large differences in amino acid concentrations between the exposed and the unexposed hamsters, but there were some statistically significant small differences between them (Tables I and II). The largest number of statistically significant differences was for aspartate. In each case of a statistically significant difference, the aspartate concentration was higher in exposed than in control hamsters (Table I). These differences occurred in some parts of the ventral cochlear nucleus and the trapezoid body, in most parts of the inferior colliculus and medial geniculate, and in the deeper layers of the auditory cortex and underlying external capsule. Concentrations of glutamate also were usually higher in exposed than in control hamsters, but only the differences in caudal PVCN and dorsal and ventral inferior colliculus regions were statistically significant. Concentrations of GABA were slightly higher in exposed hamsters in the inferior colliculus dorsal and lateral parts. Concentrations of taurine were consistently lower in exposed than in control hamsters, and the differences in caudal PVCN and overlying granular region, dorsal and ventral inferior colliculus regions, and ventral medial geniculate region were statistically significant. Although the differences between exposed and control hamsters for aspartate and taurine were not large, they were remarkably consistent across and within regions, with aspartate increased in exposed hamsters and taurine decreased (Fig. 5).
Fig. 5.
Ratios of average exposed-hamster concentrations to average control-hamster concentrations, coded as percentages, for aspartate and taurine in auditory brain regions. The maps and details are the same as in Figure 1, in which regional identifications are shown. The ratio for taurine in the PVCN granular region (70%) was outside the range of the codes, but an additional code was not added to accommodate this one sample. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Volumes of Cochlear Nucleus Regions in Control and Intense-Tone-Exposed Hamsters
Our measurements of volumes of cochlear nucleus regions showed no difference in any region between control and intense-tone-exposed hamsters (Table III). Volumes in intense-tone-exposed hamsters were not lower than those in control hamsters.
TABLE III.
Volumes of Cochlear Nucleus Regions Of Control And Intense-Tone-Exposed Hamsters*
| Region | Control | Exposed |
|---|---|---|
| AVCN | 0.289 ± 0.035 | 0.299 ± 0.013 |
| PVCN | 0.136 ± 0.012 | 0.155 ± 0.008 |
| IN | 0.049 ± 0.008 | 0.053 ± 0.009 |
| Granular regions | 0.135 ± 0.004 | 0.136 ± 0.009 |
| DCN deep layer | 0.045 ± 0.009 | 0.051 ± 0.005 |
| DCN fusiform | 0.086 ± 0.008 | 0.072 ± 0.002 |
| DCN molecular | 0.110 ± 0.010 | 0.105 ± 0.004 |
| Acoustic striae | 0.028 ± 0.005 | 0.025 ± 0.003 |
Data are in cubic millimeters, presented as mean ± SEM, for four control and six intense-tone-exposed hamsters.
AVCN, anteroventral cochlear nucleus; DCN, dorsal cochlear nucleus; fusiform, fusiform soma layer; IN, interstitial nucleus (auditory nerve root); molecular, molecular layer; PVCN, posteroventral cochlear nucleus.
There were no statistically significant differences between control and exposed hamsters.
DISCUSSION
Comparisons With Quantitative Amino Acid Distributions in Other Mammals
The distributions of amino acids in control hamster cochlear nucleus resemble those in cats (Godfrey et al., 1977) and rats (Godfrey et al., 2000) in that, for example, aspartate concentrations are lower, whereas glutamate concentrations, when expressed per dry weight, are higher in granular regions and the DCN molecular layer than elsewhere, and GABA and glycine concentrations are higher in the DCN than in the ventral cochlear nucleus. As in rats (Godfrey et al., 2000), taurine concentrations in hamsters are higher in granular regions and the DCN molecular layer than elsewhere in the cochlear nucleus, and the distribution of glutamine correlates closely with that of glutamate. The distributions of amino acids in control hamster inferior colliculus have similarities to those previously reported for cat inferior colliculus (Adams and Wenthold, 1979): aspartate concentrations are lower in the lateral than in the central part, glutamate concentrations higher dorsally than ventrally, glycine concentrations higher ventrally than dorsally, and taurine concentrations higher peripherally than centrally.
Comparisons With Previous Measurements for Hamster Cochlear Nucleus
The cochlear nucleus data for control hamsters in this study are generally comparable to those in our previous study with shorter survival times after monaural intense tone exposure (Godfrey et al., 2008) in terms of which regions have relatively high or low concentrations of particular amino acids. Correlation coefficients for comparisons between the averages in the two studies across cochlear nucleus regions range from 0.79 to 0.97 for aspartate, glutamate, GABA, glycine, taurine, serine, and threonine. However, the correlation coefficient was only 0.53 for glutamine. Lack of correlation for arginine probably reflects the very small variation of its low concentrations among regions. Differences between the studies likely reflect differences among hamsters as well as technical differences.
The small decreases in taurine concentrations 5 months after intense tone exposure, as reported here, resemble the findings for the DCN at 2 days and 1 month after exposure (Godfrey et al., 2008). The small increases in aspartate concentrations 5 months after exposure, on the other hand, were not found in the DCN at 1 month after exposure, although glutamate concentrations showed some tendency toward increases in exposed hamsters at both times. Decreases in threonine and serine concentrations in the DCN at 1 month after exposure were not found at 5 months, other than a small tendency for decreased serine concentrations.
Comparisons With Other Chemical Measurements for the Central Auditory System After Intense-Tone Exposure
Decreases in glycine receptor subunit expression and glycine receptor binding have been reported for DCN fusiform cells 4 months after exposure of rats to intense octave-band noise (Wang et al., 2009). We did not find any statistically significant changes in glycine concentrations in central auditory regions 5 months after bilateral intense tone exposure, but there were possible small decreases in average glycine concentration in most cochlear nucleus regions, especially those containing high densities of granule cells, including the DCN fusiform soma layer. These might suggest a slight decrease in inhibitory glycinergic synaptic activity that could result in increased neuronal excitability. The report of an increased expression of mRNA for glycine receptor subunit α1, after an initial decrease, in the guinea pig whole ipsilateral cochlear nucleus 4 weeks after monaural acoustic trauma (Dong et al., 2010), appears to disagree with these findings, but it was previously found that expression of this mRNA does not correlate well with expression of the glycine receptor α1 protein (Wang et al., 2009). The same limitation may apply to increased expression of mRNA for GABAA receptor subunit α1 in guinea pig ipsilateral whole cochlear nucleus 4 weeks after monaural acoustic trauma (Dong et al., 2010). No change in mRNA for glutamic acid decarboxylase (GAD), the enzyme responsible for synthesis of GABA, was found in that same study, and we found no change in GABA concentration in any cochlear nucleus region 5 months after bilateral acoustic trauma.
Evidence for increased expression of mRNA for glutamate receptor subunits, especially N-methyl-D-aspartate (NMDA) subunit 1, in guinea pig ipsilateral whole cochlear nucleus 4 weeks after monaural acoustic trauma (Dong et al., 2010), together with our evidence for increased aspartate and glutamate concentrations in many parts of the hamster cochlear nucleus 5 months after bilateral acoustic trauma, may suggest that acoustic trauma induces chronically increased excitatory neurotransmission.
Decreased expression of GAD in the inferior colliculus of rats up to 30 days after binaural acoustic trauma has been found by Western blotting, although no change in optical density of GAD immunoreactivity was detectable (Abbott et al., 1999; Milbrandt et al., 2000). More recently, no change was found in expression of mRNA for GAD in either inferior colliculus of guinea pigs 4 weeks after monaural acoustic trauma (Dong et al., 2010). Although the Western blot results suggest that there may be less GABA synthesized in the inferior colliculus 30 days after intense tone exposure, we measured somewhat increased GABA concentrations in the ventral and lateral parts of the inferior colliculus of hamsters 5 months after intense tone exposure. Somewhat increased GABAA receptor binding was found in the inferior colliculus, especially in its dorsal cortex, 30 days after binaural acoustic trauma (Milbrandt et al., 2000). At 4 weeks after monaural acoustic trauma in guinea pigs, expression of mRNA for GABAA receptor subunit α1 was decreased in the contralateral inferior colliculus but somewhat increased ipsilaterally (Dong et al., 2010). Thus, although there is evidence for changes in the GABA neurotransmitter system in the inferior colliculus at 1 month or more after acoustic trauma, there are inconsistencies among the results.
Lack of evidence in our study for glycine concentration changes in the inferior colliculus 5 months after bilateral acoustic trauma in hamsters is consistent with absence of changes in expression of mRNA for glycine receptor subunit α1 in either colliculus 4 weeks after monaural acoustic trauma in guinea pigs, although bilateral decreases were found at shorter survival times (Dong et al., 2010). Our finding of increased concentrations of aspartate and glutamate in most parts of the inferior colliculus 5 months after binaural acoustic trauma in hamsters was not matched by any increased expression of mRNA for glutamate receptor subunits in guinea pigs 4 weeks after monaural acoustic trauma (Dong et al., 2010).
The difference between our results and those of others concerning volume decreases in the ventral cochlear nucleus after acoustic trauma does not relate to post-exposure time, because such decreases should increase over time and have been reported to do so (Feng et al., 2011) and because our postexposure time was longer than those of the other studies. It could relate to different effects in our hamsters compared with mice (Feng et al., 2011) and rats (Kraus et al., 2011). Also, technical differences among the studies might contribute to the different findings, including the type of sound exposure and how volume measurements were made. The measurements for mice were made in just one subregion of the PVCN, whereas the measurements for rats and hamsters were made for the entire PVCN and AVCN. The measurements for rats were made by comparing areas of ipsilateral and contralateral cochlear nuclei in sections at 180 µm intervals after monaural sound exposure, whereas measurements for mice and hamsters were made as traced area times intervening distance in sections at 30-µm and 40–60-µm intervals, respectively, after binaural sound exposure.
Functional Considerations in Relation to Tinnitus
There is evidence that the increased neural activities in the DCN, inferior colliculus, and auditory cortex after acoustic trauma, which may be related to tinnitus, result from decreased inhibitory synaptic activity in the central auditory system (Roberts et al., 2010; Wang et al., 2011). Although the hamsters in our study were not tested behaviorally for tinnitus, there is previous evidence that hamsters similarly treated show behavioral evidence of tinnitus (Heffner and Harrington, 2002; Kaltenbach et al., 2004). It has been previously reported that the same acoustic trauma can produce more severe effects in some individual animals than in others (Kaltenbach et al., 2000; Wang et al., 2009; Dehmel et al., 2012), and it is possible that some of our exposed hamsters would have shown behavioral evidence of tinnitus, whereas others might not. However, the relative variation of amino acid concentrations among exposed hamsters, as expressed by the standard errors of the means in Tables I and II, were generally not larger than those among control hamsters.
Our results suggest that both increased excitatory and decreased inhibitory synaptic activity may be involved in the long-term effects of acoustic trauma and that the chemical changes underlying increased central auditory spontaneous activity may be somewhat indirect. Although there is no strong evidence for a neurotransmitter function of aspartate, it is metabolically closely related to the excitatory neurotransmitter glutamate, through the activity of aspartate aminotransferase (Ross et al., 1995). Thus, the widespread increased aspartate concentrations 5 months after intense tone exposure could indirectly contribute to increased excitatory neurotransmission by serving as a reservoir for synthesis of glutamate.
Similarly, although there is no strong evidence for taurine as an inhibitory neurotransmitter, there is evidence for taurine effects on inhibitory neurotransmission, by acting as an agonist at GABA and glycine receptors (Albrecht and Schousboe, 2005). Thus, the widespread decreased taurine concentrations 5 months after intense tone exposure could indirectly contribute to decreased inhibitory neurotransmission at glycinergic and GABAergic synapses. There is evidence that administration of taurine can decrease tinnitus in rats (Brozoski et al., 2010), and taurine is considered to be important in recovery from neural injury (Gupta et al., 2006).
One common characteristic of the changes in amino acid concentrations found in our study and most neurotransmitter-related changes found in other studies is that these chemical changes after acoustic trauma are not large. However, this does not rule out such changes as underlying tinnitus, because the estimated intensity of tinnitus sounds is not great (Henry et al., 2005).
ACKNOWLEDGMENTS
We are grateful to Hee Joo (Stacy) Suh for assistance with data calculations.
Contract grant sponsor: National Institutes of Health; Contract grant number: 1R01DC009097; Contract grant sponsor: University of Toledo Foundation.
REFERENCES
- 1.Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience. 1999;93:1375–1381. doi: 10.1016/s0306-4522(99)00300-0. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC, Wenthold RJ. Distribution of putative amino acid transmitters, choline acetyltransferase and glutamate decarboxylase in the inferior colliculus. Neuroscience. 1979;4:1947–1951. doi: 10.1016/0306-4522(79)90067-8. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- 4.Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brozoski TJ, Caspary DM, Bauer CA, Richardson BD. The effect of supplemental dietary taurine on tinnitus and auditory discrimination in an animal model. Hear Res. 2010;270:71–80. doi: 10.1016/j.heares.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus—possible basis for tinnitus-related hyperactivity? J Neurosci. 2012;32:1660–1671. doi: 10.1523/JNEUROSCI.4608-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guineapig auditory brainstem. Eur J Neurosci. 2010;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Bendiske J, Morest DK. Degeneration in the ventral cochlear nucleus after severe noise damage in mice. J Neurosci Res. 2011;90:831–841. doi: 10.1002/jnr.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuentes-Santamaría V, Cantos R, Alvarado JC, García-Atarés N, López DE. Morphologic and neurochemical abnormalities in the auditory brainstem of the genetically epilepsy-prone hamster (GPG/Vall) Epilepsia. 2005;46:1027–1045. doi: 10.1111/j.1528-1167.2005.68104.x. [DOI] [PubMed] [Google Scholar]
- 10.Games KD, Winer JA. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res. 1988;34:1–26. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey DA, Matschinsky FM. Approach to three-dimensional mapping of quantitative histochemical measurements applied to studies of the cochlear nucleus. J Histochem Cytochem. 1976;24:697–712. doi: 10.1177/24.6.781128. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey DA, Carter JA, Berger SJ, Lowry OH, Matschinsky FM. Quantitative histochemical mapping of candidate transmitter amino acids in cat cochlear nucleus. J Histochem Cytochem. 1977;25:417–431. doi: 10.1177/25.6.18539. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey DA, Farms WB, Godfrey TG, Mikesell NL, Liu J. Amino acid concentrations in rat cochlear nucleus and superior olive. Hear Res. 2000;150:189–205. doi: 10.1016/s0378-5955(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey DA, Mikesell NL, Godfrey TG, Fulcomer AB, Kong W, Godfrey MA, Kaltenbach JA, Zhang J. Effects of high-intensity sound exposure on neurotransmitter chemistry in the central auditory system. Semin Hear. 2008;29:259–269. [Google Scholar]
- 15.Gupta RC, Seki Y, Yosida J. Role of taurine in spinal cord injury. Curr Neurovasc Res. 2006;3:225–235. doi: 10.2174/156720206778018776. [DOI] [PubMed] [Google Scholar]
- 16.Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170:83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 17.Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48:1204–1235. doi: 10.1044/1092-4388(2005/084). [DOI] [PubMed] [Google Scholar]
- 18.Hill DW, Walters FH, Wilson TD, Stuart JD. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979;51:1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- 19.Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- 21.Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, Gross J, Morest DK, Potashner SJ. Quantitative study of degeneration and new growth of axons and synaptic endings in the chinchilla cochlear nucleus after acoustic overstimulation. J Neurosci Res. 2004;77:829–842. doi: 10.1002/jnr.20211. [DOI] [PubMed] [Google Scholar]
- 24.Kraus KS, Ding D, Jiang H, Lobarinas E, Sun W, Salvi RJ. Relationship between noise-induced hearing-loss, persistent tinnitus and growth-associated protein-43 expression in the rat cochlear nucleus: does synaptic plasticity in ventral cochlear nucleus suppress tinnitus? Neuroscience. 2011;194:309–325. doi: 10.1016/j.neuroscience.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loftus WC, Malmierca MS, Bishop DC, Oliver DL. The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience. 2008;154:196–205. doi: 10.1016/j.neuroscience.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry OH, Passonneau JV. A flexible system of enzymatic analysis. New York: Academic Press; 1972. [Google Scholar]
- 27.Meleca RJ, Kaltenbach JA, Falzarano PR. Changes in the tonotopic map of the dorsal cochlear nucleus in hamsters with hair cell loss and radial nerve bundle degeneration. Brain Res. 1997;750:201–213. doi: 10.1016/s0006-8993(96)01354-6. [DOI] [PubMed] [Google Scholar]
- 28.Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 29.Møller AR. Hearing; its physiology and pathophysiology. New York: Academic Press; 2000. [Google Scholar]
- 30.Morest DK. The neuronal architecture of the medial geniculate body of the cat. J Anat. 1964;98:611–630. [PMC free article] [PubMed] [Google Scholar]
- 31.Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. San Diego: Academic Press; 2001. [Google Scholar]
- 32.Mugnaini E, Warr WB, Osen KK. Distribution and light microscopic features of granule cells in the cochlear nuclei of cat, rat, and mouse. J Comp Neurol. 1980;191:581–606. doi: 10.1002/cne.901910406. [DOI] [PubMed] [Google Scholar]
- 33.Mulders WH, Robertson D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience. 2011;192:753–760. doi: 10.1016/j.neuroscience.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 34.Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of the cat. J Comp Neurol. 1997;382:215–229. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Oliver DL, Ostapoff E-M, Beckius GE. Direct innervations of identified tectothalamic neurons in the inferior colliculus by axons from the cochlear nucleus. Neuroscience. 1999;93:643–658. doi: 10.1016/s0306-4522(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- 37.Ravizza RJ, Straw RB, Long PD. Laminar origin of efferent projections from auditory cortex in the golden Syrian hamster. Brain Res. 1976;114:497–500. doi: 10.1016/0006-8993(76)90971-9. [DOI] [PubMed] [Google Scholar]
- 38.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross CD, Parli JA, Godfrey DA. Amino acid concentrations and selected enzyme activities in rat auditory, olfactory, and visual systems. Neurochem Res. 1995;20:1483–1490. doi: 10.1007/BF00970598. [DOI] [PubMed] [Google Scholar]
- 40.Ryan AF, Axelsson GA, Woolf NK. Central auditory metabolic activity induced by intense noise exposure. Hear Res. 1992;61:24–30. doi: 10.1016/0378-5955(92)90032-i. [DOI] [PubMed] [Google Scholar]
- 41.Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 42.Vogler DP, Robertson D, Mulders WHAM. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci. 2011;31:6639–6645. doi: 10.1523/JNEUROSCI.6538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res. 2011;279:111–117. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winer JA, Kelly JB, Larue DT. Neural architecture of the rat medial geniculate body. Hear Res. 1999;130:19–41. doi: 10.1016/s0378-5955(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhang JS, Kaltenbach JA, Wang J, Kim SA. Fos-like immunoreactivity in auditory and nonauditory brain structures of hamsters previously exposed to intense sound. Exp Brain Res. 2003;153:655–660. doi: 10.1007/s00221-003-1612-4. [DOI] [PubMed] [Google Scholar]