Abstract
Micron- to nanometer-sized ultrasound agents, like encapsulated microbubbles and echogenic liposomes, are being developed for diagnostic imaging and ultrasound mediated drug/gene delivery. This review provides an overview of the current state of the art of the mathematical models of the acoustic behavior of ultrasound contrast microbubbles. We also present a review of the in vitro experimental characterization of the acoustic properties of microbubble based contrast agents undertaken in our laboratory. The hierarchical two-pronged approach of modeling contrast agents we developed is demonstrated for a lipid coated (Sonazoid™) and a polymer shelled (poly D-L-lactic acid) contrast microbubbles. The acoustic and drug release properties of the newly developed echogenic liposomes are discussed for their use as simultaneous imaging and drug/gene delivery agents. Although echogenicity is conclusively demonstrated in experiments, its physical mechanisms remain uncertain. Addressing questions raised here will accelerate further development and eventual clinical approval of these novel technologies.
Keywords: Ultrasound contrast agents, encapsulated microbubbles, liposomes, drug delivery
1. Introduction
In recent years, a number of innovative particulate systems nanoparticles [1], nanoemulsions [2], quantum dots [3], ‘bubbicles’ [4], vesicles and microbubbles are being developed for healthcare applications. They are aimed at the dual purpose of early accurate diagnosis of diseases as contrast enhancing agents for medical imaging and their rapid remediation as delivery vehicles for therapeutic agents. The effectiveness of these agents critically depends on our ability to engineer them using sound physical principles. Here, we will present an overview of the ongoing research in our laboratory on the analysis and characterization of encapsulated microbubbles and acoustically active echogenic liposomes (ELIP) for contrast enhanced ultrasound (CEUS) imaging and drug delivery.
Microbubble based ultrasound contrast agents (UCA) has been approved by the federal Food and Drug Administration (FDA) for echocardiography. Echocardiography is one of the primary tools for diagnosing cardiovascular diseases the leading cause of mortality in the US. However, more than 15% of echocardiographs in the US are suboptimal, i.e. they do not result in definitive diagnosis [5, 6]. UCA can substantially improve the diagnostic abilities of not only echocardiography but also of ultrasound of liver, kidney and other organs [7–9]. On the other hand, of all the particulates, liposomes are a prime candidate for drug delivery because of their structural similarities with biological cells, long circulation times and ability to carry both hydrophobic [10] and hydrophilic [11, 12] drugs. Echogenic liposomes combine these advantages of liposomes with the echogenicity or ultrasound responsiveness of microbubbles, making them an excellent candidate for concurrent ultrasound imaging and drug delivery.
We have been studying the dynamics of these contrast and drug delivery agents through both in vitro experiments and mathematical modeling. Our aim is to develop reliable tools for characterizing their behaviors that can be used to design and develop the next generation contrast and drug delivery agents. This paper presents an overview of the ongoing research on acoustic characterization of several commercial and experimental microbubble based contrast agents and echogenic liposomes. Section 2 presents a broad review of the state of the art of contrast agent applications. In section 3, we briefly discuss the mathematical models to describe the behavior of microbubble based contrast agents including various interfacial rheological models of contrast agent encapsulation. In section 4, we discuss the experimental approaches to characterize the acoustic behaviors of contrast microbubbles. In section 5, we review the experimental results characterizing both the acoustic and drug release properties of echogenic liposomes. Both sections 4 and 5 provide some specific illustrative examples for clarifying key results and their implications. The final section summarizes the findings and discusses the scope of possible future research.
2. Background of contrast agent applications
Diagnostic medical imaging involves various modalities, viz., computed tomography, magnetic resonance imaging, ultrasound imaging etc. Although diagnostic imaging with ultrasound offers a safe, portable, low cost alternative, its applicability is often limited by inferior image quality and lack of spatial resolution in comparison to CT or MRI [7]. However, recent technological advances in the field of ultrasonics coupled with the rapid development of novel ultrasound contrast agents have led the medical community to investigate contrast enhanced ultrasound imaging for diagnosis of various cardiovascular, hepatic, renal, gastrointestinal and pancreatic diseases [7–9].
The poor scattering properties of human blood are a cause of non-definitive ultrasound images. A major breakthrough in this regard was the accidental discovery of the effectiveness of micron sized gas bubbles in enhancing ultrasound image contrast [13]. Due to the presence of the highly compressible gaseous core, these microbubbles can significantly enhance the backscatter of incident ultrasound waves through ‘active scattering’ [14]. For micron-sized bubbles, this active scattering cross-section is often several orders of magnitude higher than the ‘passive scattering’ cross-section (passive scattering is the primary source of scattered echo from tissues and blood). However, the potential for clinical applications of such uncoated microbubbles was severely restricted by their highly unstable nature. The pressure inside a gas bubble is higher than the outside pressure due to the surface tension forces at the air-water interface. This results in their rapid dissolution—in milliseconds for micron sized air bubble at room temperature [15, 16]. To stabilize these gas bubbles against dissolution, microbubbles are encapsulated with a layer of lipid/protein/surfactant/polymer molecules [17]. Most commercially available and experimental contrast agents are 1–10 μm in diameter with a low solubility gas (viz., perfluorocarbons, and sulfur hexafluoride) inside and is stabilized with such an outer coating [17].
Significant effort has been made in the last decade to develop the next generation contrast microbubbles with applications extending beyond the scope of diagnostic imaging. Bubble effects beyond simple backscattering stable and inertial cavitation [18–20], microstreaming [19], radiation force generation [21–23], ultrasound-mediated destruction [24, 25] are being investigated for therapeutic applications like modulation of vascular and cellular permeability [26, 27], thrombolysis [28, 29] and gene delivery [30]. Novel ligand-mediated targeted imaging or molecular imaging are being developed that would allow noninvasive detection of physiological changes in patients at molecular and cellular levels [31–34]. Like liposomes, microbubbles can also be used as drug delivery vehicles for both hydrophobic [10] and hydrophilic [11, 12] molecules, and achieve localized or targeted delivery through ultrasound-mediated destruction and/or other external triggers. Such drug release strategies can be used for treatment of cancer and atherosclerotic plaques [35]. A number of reviews have been published in this field [7, 28, 31, 35–40]. Figure 1(a) shows a schematic representation of an ultrasound contrast microbubble with drug delivering capabilities. Instead of lipids, other materials can be used in the stabilizing encapsulation as mentioned earlier. Hydrophilic drugs can be loaded on the surface, whereas water insoluble drugs can be loaded within the oil layer in between the encapsulation and perfluorocarbon core. Electrostatic and ligand mediated interactions can also be utilized to load drugs outside the microbubbles. Figure 1(b) shows the various drug loading strategies utilized for contrast microbubbles.
Figure 1.
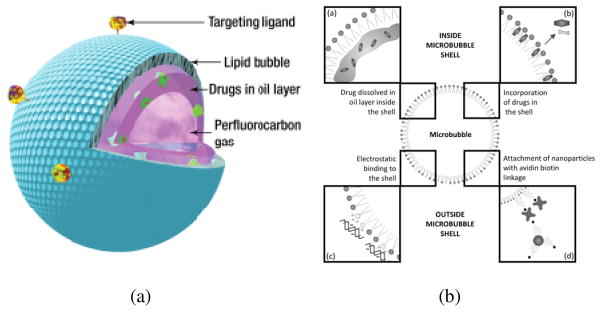
(a) Schematic representation of a contrast microbubble constructed for drug-delivery [Reproduced with permission from [41]]. (b) Drug loading strategies implemented with contrast microbubbles [Reproduced with permission from REF [40]].
In spite of the rapid development of microbubble based ultrasound contrast agents, clinical applications are often limited due to potential safety concerns [18, 42, 43] and lower circulation time [35]. The relatively larger size of microbubbles in comparison to nanometer sized pores observed in the leaky vasculature associated with cancerous tissues [35] and the constraints on the drug payload [44] also reduces their applicability in cancer therapy as drug delivery agents. These limitations motivated researchers to explore the possibility of echogenic liposomes that combine the favorable properties of microbubble based contrast agents and the drug delivering liposomes. Since their discovery in 1965, by A.D. Bangham [45, 46], liposomes have been used extensively as drug delivery vehicles. Liposomes are typically nanometer sized vesicles with a hydrated lipid bilayer encapsulating an aqueous phase. The bilayer membrane is spontaneously formed due to thermodynamic interactions when phospholipids are dispersed in an aqueous phase [47]. Structurally, liposomes are very similar to biological cells. Hence, liposomes offer several favorable properties like longer circulation time in the blood stream, lesser toxicity, and increased uptake by target organs/tissues, making them suitable for use as drug delivery vehicles [48–51]. Currently, there are about 10 liposomal drug formulations approved by the FDA for human use [48, 52]. Liposomes conjugated with targeted ligands can achieve active targeting of intended sites [51]. Several exogenous (e.g., temperature [53], light [54]) and endogenous (e.g., pH [55], enzymes [56, 57], redox [58]) triggers have been used to make stimuli-responsive drug delivery vehicles. Such formulations offer local control over payload release resulting in reduced systemic toxicity. Recently, ultrasound has also been investigated as possible external trigger for releasing liposomal contents [48, 49, 59, 60].
Acoustically responsive liposomes were first reported in 1996 [61] and termed echogenic liposomes (ELIPs). The preparation protocol was later optimized by Huang and coworkers [62–64] through years of research to establish a standardized methodology involving 3 to 5 freeze-thaw cycles and lyophilization in presence of a weak cryoprotectant mannitol. These steps are critical in ensuring the echogenicity (i.e. capability to scatter incident acoustic waves effectively) of these liposomes. It is hypothesized that the freeze-thaw and lyophilization in presence of mannitol creates bilayer defects, which later allow the entrapment of air during reconstitution [65, 66]. Presence of entrapped air makes these liposomes echogenic. Although, echogenicity of these liposomes have been conclusively demonstrated through both in vitro [66, 67] and in vivo [68] experiments, the exact location of entrapped air remains uncertain. Possible explanations are the existence of a gas pocket within the bilayer [52, 63, 69] or presence of a lipid monolayer coated bubble floating within the aqueous compartment [63]. Since, ELIPs retain all the favorable properties of normal liposomes [52], they have also been investigated for simultaneous imaging and ultrasound mediated drug release studies [69–76]. Figure 2 below shows two hypothetical structures of echogenic liposomes. ELIPs can be loaded with both hydrophilic and lipophilic drugs represented in the figure by fluorescent green circles and red boxes respectively. Like microbubbles, liposomes can also be prepared with ligand mediated targeting properties. Another strategy to incorporate favorable properties of microbubbles and liposomes in the same formulation can be conjugation of gas filled microbubbles and liposomes. Several groups have also been investigating such microbubble-liposome conjugates [77–79].
Figure 2.
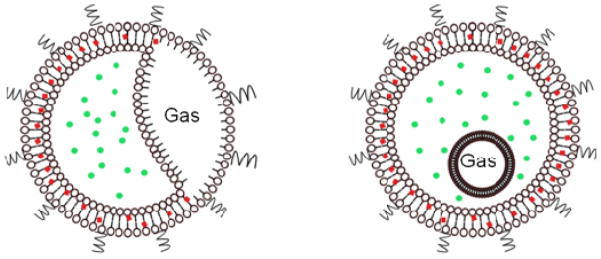
Hypothesized structure of echogenic liposomes (ELIPs).
In light of the above discussion, it can be concluded that microbubbles and liposomes hold great potential for clinical imaging and therapeutic applications. Their realization will depend on understanding of the physical principles behind their behaviors through experiments and mathematical modeling. Following sections will a give an overview of such studies undertaken in our laboratory along with some specific examples that can motivate future research.
3. Bubble dynamics
3.1 Free bubble dynamics
Gas bubbles are an intriguing physical system primarily due to their complex non-linear dynamics. The dynamics of uncoated gas bubbles have been studied extensively both mathematically and experimentally [80, 81]. The bubble dynamics is governed by the Rayleigh-Plesset (RP) equation [82–86]:
| (1) |
where R is the instantaneous radius of the spherical bubble, Ṙ and R̈ are the first and second order time derivatives of the bubble radius, ρ is the density of the surrounding liquid, PG is the pressure of the gas inside the bubble, μ is the liquid viscosity, γ is the gas-liquid surface tension, P0 is the ambient pressure, and pA(t) is the time dependent excitation pressure. Note that the classical RP equation (1) assumes the surrounding liquid to be incompressible. Several modifications of the RP equation, e.g., Keller-Miksis equation [87], Trilling Equation [88], Herring equation [89], and Gilmore equation [90] have been suggested to include liquid compressibility. Prosperetti and Lezzi [91–93] in their pioneering work proved that these equations are essentially members of the same family of differential equations, but it remains difficult to ascertain which equation will give the most accurate numerical results. Brenner and co-workers, suggested that the following form of the RP equation, which also incorporates liquid compressibility, is stable at high Mach numbers [94].
| (2) |
where the last term (c is the speed of sound) is the correction due to compressibility. If the gas inside is assumed to obey a polytropic law and diffusion is neglected, the inside gas pressure can is given by
| (3) |
where R0 the initial bubble radius, PG0 is the initial gas pressure and k is the polytropic exponent. Incorporating (3) in (2), we obtain the following form of compressible RP equation:
| (4) |
3.2 Encapsulated bubble dynamics
3.2.1 A brief review of the existing models for encapsulated microbubbles
Two recent articles [95, 96] present excellent reviews of the topic. The earliest attempt to model dynamics of contrast microbubbles dates back to 1990, where Roy and co-workers modeled the encapsulation as a viscous liquid [97]. de Jong and others modeled Albunex [98–101], the first clinically approved contrast agent, by including ad hoc terms shell friction and elasticity factors in the RP equation which nonetheless represented the correct physics that the encapsulation is a viscoelastic shell. The first rigorous theoretical model was developed by Church [102], where he assumed the encapsulation material to be an incompressible solid with a linear viscoelastic constitutive equation, which effectively represented a Kelvin-Voigt type relation. Hoff et al. modified this model by incorporating a thin shell approximation, and matched the model predictions with the experimental data for Nycomed [103]. Morgan et al. [104] proposed a modified Herring equation with an elastic term derived using Glazman’s approach [105], to describe the encapsulated bubble dynamics. Khismatullin and Nadim [106] introduced compressibility and viscoelasticity in the surrounding liquid, and showed that that they have negligible effects on the dynamics. Allen et al. [107] assumed the encapsulation to be a purely viscous liquid layer, with bulk viscosity parameters to model the encapsulation of an experimental therapeutic microbubble named MRX-552 (ImaRx Therapeutics, Tucson, AZ). Allen and Rashid [108] later proposed another model to predict large amplitude oscillations of polymeric spheres that can be used to model polymer coated microbubbles.
In 2003, our group proposed, for the first time, an interfacial rheological model for contrast agent encapsulation [109]. We argued that the encapsulation which is typically a few molecule thick most often a monolayer cannot be assumed to be a homogeneous continuum with bulk material properties (viscosity and elasticity) at least in the thickness direction. Clearly, the three orders of magnitude separation of length scale between the overall dimension of the microbubble (micrometer) and the thickness of the encapsulation (nanometer) warrants a proper multi-scale approach; treating them simultaneously would be a prohibitively costly computational task. Therefore interfacial rheology, where the interface is treated as a zero-thickness surface with complex interfacial properties as opposed to bulk rheological properties that effectively represent the thickness averaged material response, is the appropriate approach for modeling the encapsulation. Note that the surface tension used to characterize an air-liquid interface, either pure or contaminated with surfactants, is also an interfacial rheological property [109]. Over the years, we have developed a two-pronged interfacial rheological characterization effort which includes one experiment to determine the characteristic properties of the encapsulation and a second independent experiment that validates the characterization [109–112]. The independent validation distinguishes this effort from other similar modeling studies. It also incorporates a way to improve a model where sophistication is introduced as warranted by the modeling effort as opposed to prescribed in advance. In 2003, we adopted the simplest interfacial rheology Newtonian, i.e., purely viscous with a constant surface tension (γ0) and a dilatational viscosity (κs). We determined these two parameters for a number of contrast agents using attenuation of ultrasound through a contrast agent suspension. However, we obtained an unreasonably large value of surface tension (~0.7–40 N/m) compared to the value (0.072 N/m) of a pure air-water interface [109], whereas one would expect a lower value due to the absorption of the surface-active molecules at the interface. Accordingly in 2005, we developed a new model constant elasticity model including an interfacial dilatational elasticity (Es) [110]. Characterization with this model obtained a more reasonable surface tension value (smaller than the pure air water interface). However, the model performed poorly in validation, i.e., the predicted scattered subharmonic response did not match well with experimental measurement [110]. We attributed the failure to the shortcoming of the linearity constant dilatational elasticity for predicting nonlinear scattering. In 2010, we implemented an exponential strain-softening dilatational elasticity to account for the large amplitude non-linear oscillations in 2010 [111]. The model performed very well in predicting the behaviors of contrast agent Sonazoid [111].
Meanwhile, Marmorttant et al. [113] introduced a linear viscoelastic model with a radius dependent surface tension. The model is equivalent to our constant elasticity model the parameter χ here being the same as Es except that it accounts for rupture and buckling of the encapsulation. The Marmottant model has gained wide acceptability because of its ability to predict several non-linear behaviors of lipid shells e.g. compression only behavior, where the bubbles compresses more than they expand. Doinikov and Dayton, in 2007, proposed a model for lipid shelled microbubbles assuming the shell to be a viscoelastic Maxwell fluid [114]. Tsiglifis et al. [115] implemented three different constitutive laws, viz., Kelvin-Voigt, Mooney-Rivlin, and Skalak models to describe the elastic properties of the encapsulating shell. Stride, in 2008, proposed a model for contrast agent encapsulation by treating it as a homogenous insoluble molecular monolayer with both viscosity and interfacial tension varying with the instantaneous molecular concentration at the interface [116]. Doinikov et al. proposed another model with a non-linear viscosity term in addition to the Kelvin-Voigt elasticity term to better predict non-linear behavior of lipid shells [117]. Marmottant et al. have also proposed a recent modification of their existing model for lipid encapsulation to extend its applicability to solid like encapsulating shells [118]. In an attempt to explain the variation of estimated properties with bubble size, Li et al. [119] have proposed an integration of the nonlinear elasticity of the Marmottant model with the nonlinear viscosity proposed by Doinikov and co-workers to have a ‘nonlinear shell elasticity and viscosity’ model (NSEV).
3.2.2 Mathematical formulation of encapsulated bubble dynamics
The different models to describe dynamics of encapsulated microbubbles discussed in the preceding paragraph are essentially modified versions of the classical Rayleigh-Plesset equation that can be represented in a single framework [120]:
| (5) |
where γ(R) is the effective surface tension and κs(R) is the effective dilatational viscosity. One can linearize Eq. (5) and express it in the form of linear harmonic oscillator:
| (6) |
The linearized equation can be used to obtain the damping coefficient (δ) and the resonance frequency (ω0 = 2πf0). The damping term has three separate contributions one each from liquid viscosity, shell viscosity and acoustic radiation:
| (7) |
The total damping and the resonance frequency are useful for estimating model material parameters of the encapsulation. The contribution due to the thermal damping requires more rigorous treatment [121–123], and hence difficult to include in a simplified form. Moreover, most of the available thermal damping models are developed for linear oscillations with limited validity in the non-linear regime. Hence, thermal damping is either neglected—assuming nearly isothermal or adiabatic oscillations—or included through an additional thermal viscosity term just like the viscous damping due the surrounding liquid. Usually thermal damping is negligible in comparison to the encapsulation damping for contrast agents [124, 125], but one must be aware that such assumptions might not always be valid. The mathematical descriptions of the interfacial rheological models proposed by our group are discussed in the next section.
3.2.3 Interfacial rheological models for UCA encapsulations
(A) Newtonian model (NM) [109, 110]
As mentioned above, here the encapsulation of a contrast microbubble was modeled as a purely viscous interface of infinitesimal thickness:
| (8) |
The resonance frequency is given by
| (9) |
We estimated the properties of an encapsulation by fitting a model prediction to experimentally measured attenuation data. As noted before, for several commercial contrast agents like Sonazoid and Optison this model predicted unrealistically large values for surface tension (~ 0.6–40 N/m) [109, 110] due to the absence of an interfacial elasticity term in the model.
(B) Constant elasticity viscoelastic Model (CEM) [110]
This model assumes a constant dilatational elasticity and viscosity:
| (9) |
where is the fractional change in area from an unstrained or stress free position (radius RE) and γ0 is the reference surface tension at that radius. The equilibrium radius (RE) is given by . This ensures a balance of inside and outside pressure at initial radius. The resonance frequency is given by
| (10) |
Using CEM led to a reasonable value for surface tension lower than the air-water interface.
(C) Viscoelastic model with exponentially varying elasticity (EEM) [111]
The inability of the CEM model to match the experimentally observed subharmonic thresholds as per experimental observations led us to propose nonlinear strain-softening [111]. We proposed two simple non-linear extensions of the constant elasticity (Hooke’s law)—elasticity varying linearly with area fraction i.e. a quadratic elasticity model (QEM), and an exponentially varying elasticity model (EEM). They both performed equally well in predicting the subharmonic response. Since the exponential variation of surface elasticity seems more physical we implemented it for all our subsequent numerical investigations of contrast agent dynamics. The effective surface tension term and viscosity terms of the EEM is given below
| (11) |
where . Enforcing the balance of pressure at initial radius, we have an expression of equilibrium radius given by . The resonance frequency due to EEM is given as
| (12) |
Note that in general one can have γ(R) negative, i.e. the encapsulation is in compressive stress. However, one can also impose that under compression the encapsulation buckles and effectively the surface tension becomes zero [15]. Imposition of such non-negativity leads to compression-only behavior but usually predicts higher subharmonic thresholds [111]. The results shown here are obtained using the constant elasticity and the exponential models without the condition of non-negativity imposed on them.
(D) Marmottant model (MM) [113, 118]
The Marmottant model assumes the surface tension to have three distinct regimes: a buckled state of the encapsulation with zero surface tension below a prescribed buckling radius, an elastic state with linearly varying elasticity similar to the CEM, and a ruptured state with surface tension same as that of the air-water interface above a rupture radius. The effective surface tension and the viscosity terms due to this model are given as
| (13) |
where χ [identical to Es in (9)] is the elastic modulus of the shell, , and Although such an effective surface tension behavior is physically quite realistic, the choice for the different limiting radii remains hard to determine, and typically made so that the results match with experimental observations. The breakup radius is difficult to estimate and is usually considered to be same as the rupture radius. We also assumed γ(R0) to be zero for all the simulations presented in this paper. It ensures a pressure equilibrium at the initial unstrained state. Note that due to the discontinuous variation of effective surface tension with radius, it is difficult to give an expression of the resonance frequency. However, one can derive the expression assuming that the bubble exists completely in the elastic regime:
| (14) |
Recently smoother forms of Marmottant model have been proposed that involves a smoothing near the discontinuities [126, 127]. One such form is given below
| (15) |
where H is the Heaviside step function which can be smoothed by a Peskin cosine function to avoid sharp transitions as shown below
| (16) |
3.2.4 Estimation of model parameters describing encapsulation rheology
Estimation of model parameters remains a difficult problem to date. Standard low frequency techniques for direct measurement of interfacial properties such as Langmuir trough are of limited validity for measuring material properties of contrast agents oscillating at megahertz frequencies. Hence, several different approaches have been utilized to measure material properties using various experiments, e.g., backscattering measurements [128], attenuation measurements [66, 109–111, 129, 130], light scattering experiments [131, 132], high-speed optical observations [124, 133–135], atomic force microscopy [136] and measurements using Fluorescence Recovery After Photobleaching (FRAP) [137], fluorescence lifetime imaging [138].
We use the experimentally obtained attenuation data to determine the unknown model parameters and then validate our model predictions against nonlinear scattering [110–112]. Usually, attenuation experiments are performed at low amplitude excitations. Hence, one can use the linearized version of RP equation to get expressions for both the damping coefficient [See (7)] and the resonance frequency [See (9), (10), (12), (14)]. A least square minimization is used with the error function:
| (17) |
where αmeas(ω) is the experimentally measured attenuation coefficient and α(ω) is the theoretical prediction of attenuation coefficient which can be calculated using the following expression
| (18) |
where e is the base of the natural logarithm, n(R)dR is the number of bubble per unit volume within the radius range (R,R + dR), and total range of bubble radii in the distribution is given by (Rmax, Rmin). A Matlab® (Mathworks, Natick, MA, USA) code is used to obtain the model parameters using the above technique. Hughes et al. [139] and Grishenkov et al. [140] suggested a more stringent test by simultaneously fitting both attenuation and phase velocity for PVA-shelled microbubbles. Due to the ill posed nature of the problem, the fitting process is difficult and also very sensitive to several factors like polydispersity of the suspension, initial guess of parameters, etc. [112]. Also, attenuation data might not always reflect the linearized dynamics. Recent experimental observations have demonstrated the occurrence of non-linear behaviors e.g., compression only behavior [141], shift of resonance frequency [141], subharmonic generation [126], etc., even at very low acoustic excitation pressures of 50 kPa. This may result in inaccurate predictions.
3.2.5 Prediction of encapsulated bubble dynamics and scattering
Once the interfacial rheological properties of the encapsulation corresponding to a specific model have been determined, one can solve Eq. (5) using standard numerical techniques for solving stiff ordinary differential equations. We use the ode15s solver in Matlab® with the initial conditions of R(t = 0) = R0 and Ṙ(t = 0) = 0. The scattered Ps(r,t) and scattering cross-section σs(r,t) are also calculated from the radial dynamics [142]:
| (19) |
We obtain the scattered response spectrum using the fast Fourier Transform (FFT) and integrating the contributions from all the bubbles of different radii ranging from Rmin to Rmax using
| (20) |
The peak values corresponding to different frequencies of interest can be extracted from the FFT, and utilized for model validation purposes as shown in subsequent sections. Note that (20) assumes absence of multiple scattering effects. If undetermined model parameters are estimated by use of experimentally measured radial dynamics, the numerical solution of the RP equation can be directly fitted with data.
4. Characterization of ultrasound contrast microbubbles
This section sketches acoustic characterization techniques for ultrasound contrast microbubbles. Although encapsulated microbubbles are also being developed as targeted drug delivery vehicles with stimuli responsive release properties, a discussion of those studies are omitted here for brevity. Along with the review of existing literature, specific results for both attenuation and scattering data will also be presented as illustrative examples for two different contrast microbubbles viz., Sonzoid® (GE Healthcare, Oslo, Norway)1 with a phospholipid coating and Poly(DL-lactic acid) polymer (PLA) encapsulated microbubbles. The preparation protocol of Sonazoid, a commercially available contrast agent, is unavailable. The PLA microbubbles were developed by Prof. Margaret A. Wheatley at the Biomedical Engineering Department, Drexel University [11, 12, 143–148]. The detailed description of the experimental setups used to study acoustic scattering from and attenuation through a suspension of above mentioned contrast agents can be found in our earlier publications [110, 112, 149], and hence, not discussed here.
4.1 Attenuation and estimation of interfacial rheological properties
Attenuation measures the loss of energy of an acoustic wave as it travels through a medium. It is enhanced in presence of microbubbles. If the attenuation due to the contrast microbubbles is too high, the scattered signal can be lost completely before being received by the transducers. Hence, the earliest standardized measurements of contrast agent efficacy utilized a parameter called STAR (scattering to attenuation cross-section ratio). For a good contrast agent this value should be as high as possible indicating a high backscatter of signal with minimal loss of energy of the scattered wave during transmission. Apart from a measure of contrast agent efficacy, the frequency dependent attenuation measurement can also capture the resonance behavior of a monodisperse bubble population as evident from (18). The peak in attenuation curve indicates the resonance frequency. For a polydisperse suspension the peak will indicate a weighted average resonance frequency. Note that multiple scattering effects can be neglected for attenuation experiments due to low concentration of microbubbles [129]. A linear increase of attenuation with microbubble concentration indicates the validity of this assumption. If the attenuation measurements are acquired at low enough excitation pressure, the dynamics can be described by the linearized bubble dynamics equation.
Figure 3(a) shows the frequency dependent attenuation data acquired experimentally for Sonazoid microbubbles at a concentration of 8.1×104 bubbles/ml solution. It also shows the best fitting obtained with different models for encapsulations using the technique mentioned in Section 3.2.4. Fitting was executed with an average size (1.6 μm) and the total number concentration. Figure 3(b) shows similar experimental data for PLA microbubbles at a concentration of 4.0×104 bubbles/ml of solution and the best fitting curves for various models with the full size distribution; using an average size with PLA resulted in unsatisfactory results owing to the effects of polydispersity as mentioned earlier in Section 3.2.4.
Figure 3.
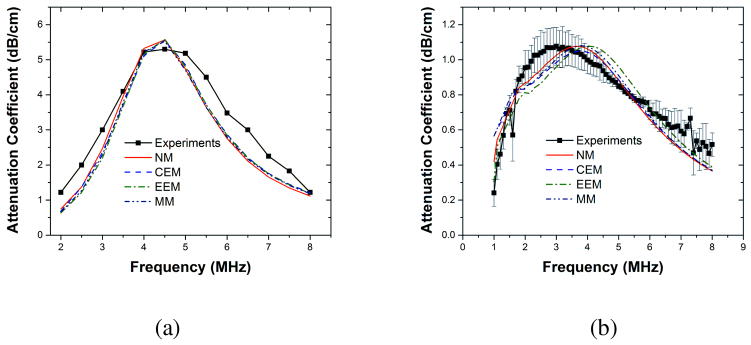
Experimentally measured and fitted attenuation data for (a) Sonazoid (Reproduced with permission from [110] and [111]). (b) PLA microbubbles (Reproduced with permission from [112]). NM: Newtonian Model, CEM: Constant Elasticity Model, EEM: Exponential Elasticity model, MM: Marmottant model.
For estimation of properties, we used ρ =1000 kg/m3, μ =0.001 kg/m s, c = 1485 m/s, p0 = 101325 Pa. The values of polytropic constant (k) used were 1.07 for Sonzaoid bubbles and 1.00 for air-filled PLA bubbles. Note that the choice of polytropic exponent is a nontrivial problem. Using an analysis due to Prosperetti [121], which is valid for small amplitude oscillations, we determined the oscillations are nearly adiabatic for Sonazoid bubbles (perfluorocarbon gas inside) and nearly isothermal for PLA microbubbles (air filled). However, such simplifications might not be valid for large amplitude non-linear oscillations, and a more rigorous approach might be required [150, 151]. Table 1 provides the estimated material properties of the encapsulation for both Sonazoid and PLA microbubbles. Note the difference in estimated properties for the two different kinds of encapsulation. The Newtonian model predicts surface tension value for Sonazoid (~0.6 N/m) that are higher than the air-water interfacial tension value 0.072 N/m and physically unrealistic. The predictions improve with incorporation of elasticity in the rheological model. For PLA microbubbles however the Newtonian model predicts low surface tension values. Hence, introduction of elasticity results in prediction of elasticity values (~ 0.05 N/m) that are an order of magnitude smaller than those predicted for the lipid encapsulation. In fact, for this very reason, for PLA bubbles, even the Newtonian model fares very well.
Table 2.
Comparison of previously reported size distribution measurements with ELIPs
| Kopechek et al. | Paul et al. | |
|---|---|---|
| Size corresponding to the largest number density | 65 nm | ~150 nm |
| Range of particle size detected* | 30 nm - 6 μm | 100 nm – 2 μm |
| Polydispersity Index (PDI) | Not Reported | 0.63 – 1.00 |
Kopechek et al. concatenated size distributions from DLS (0– 450 nm) and Coulter Counter (450 nm or above.)
4.2 Nonlinear scattering experiments and model validation
The predictive capabilities of different models are judged by their ability to capture experimentally observed dynamics. We argue that the model validation should be done against experiments other than the one used for model parameter estimation. We have followed such an approach in our lab the model parameters are determined using attenuation and then the full nonlinear RP equation with the estimated property values is numerically solved to calculate the far field scattered pressure. The model predictions are compared against experimental data for both fundamental and subharmonic scattered responses. Since the fundamental response can be matched very accurately even with the linearized version of RP equations [111] the performance of the models is judged by its ability to predict the scattered nonlinear response.
Most of the imaging applications utilize the fundamental response — the response obtained at the frequency of excitation — from contrast microbubbles. However, due to the interference with signals originating from surrounding tissues, they often result in a poor signal to noise ratio (SNR). Due to their nonlinear nature, only contrast microbubbles can generate subharmonic response — response at half the excitation frequency — which can provide better SNR [152, 153]. Hence, subharmonic imaging has been widely studied [154–158]. Subharmonic imaging has also been investigated for high frequency imaging applications [159–162] and noninvasive blood pressure estimation [163–174]. We have been investigating scattered subharmonic response from contrast microbubbles both experimentally and through numerical simulations [110–112, 120, 125, 175]. Figure 4 below shows both the experimentally measured and simulated subharmonic responses from Sonazoid and PLA coated microbubbles. Note that the excitation pressure dependent subharmonic curves show all the typical features where there is no subharmonic response below a threshold pressure followed by a rapid rise beyond threshold and eventual saturation of the response. The simulated responses from various models are obtained by solving the full RP type equation with estimated properties given in Table 1.
Figure 4.
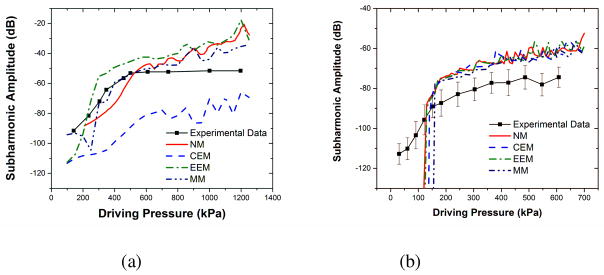
Comparison of experimentally measured and predicted scattered sub-harmonic responses predicted by different interfacial models for (a) Sonazoid microbubbles at 2 MHz excitation (Reproduced with permission from [110] and [111]). (b) PLA microbubbles at 2.25 MHz excitation (Reproduced with permission from [112]).
As seen in the Figure 4, the generation of subharmonic occurs only when a certain threshold excitation is exceeded. Our previous studies [111, 120, 125] have shown that interfacial models with nonlinear elasticity term viz., EEM and MM, can predict subharmonic thresholds accurately. Nonlinear interfacial rheology was found to be required to describe the large amplitude oscillations of contrast microbubbles.
4.3 Radial dynamics of contrast microbubbles
Measuring material properties using attenuation through a bulk suspension of microbubbles have several limitations e.g., polydispersity of microbubbles affects the predictions, linearized dynamics might not be a valid assumption, the material properties might not be the same over the entire range of bubble population etc. Due to these limitations associated with attenuation, experimentally obtained radius-time signatures of microbubble are also used for the estimation of interfacial properties. The radial dynamics of individual microbubble can be captured directly using high speed cameras [135, 176, 177] and streak cameras [104, 178–180] or indirectly e.g., using light scattering measurements [131, 132, 181] and an acoustical camera [182]. Although, direct optical observations of microbubbles offer several advantages like more accurate measurement, isolation of response from individual microbubble, minimal effects of signal attenuation and no requirement of accurate calibration, they often have limited optical resolution, constraints over data collection, and require ultra-fast cameras that are expensive and not easily accessible. Indirect measurements provide an inexpensive, less complicated, real-time alternative with no limitations on the data acquisition. However, they cannot provide the wide-range visual information obtained from direct optical observations. Techniques using radius-time data have been successful in accurately capturing the radial dynamics of several different contrast microbubbles like Quantison® [183], Definity [132, 184], SonoVue [131, 135, 176, 185], BR14 [124, 126, 182] Sonazoid [181], Optison® [181], Targestar [186] etc. The numerical solution of the RP type equation can be fitted with these experimentally measured radius-time curves to obtain the material properties using an error minimization algorithm mentioned earlier. The fitting can be done with just the knowledge of the bubble’s initial radius and the excitation pulse. It has been successfully implemented to estimate model parameters for different encapsulated microbubbles using several different rheological models of encapsulation [117, 119, 124, 131, 132, 176]. Several interesting observations have been made during experimental investigations of the radial dynamics of contrast microbubbles like compression-only behavior [113, 141, 185], existence of a threshold for the onset of oscillations [141, 187], mode vibration of bubbles [188, 189], non-spherical oscillations [189–191], buckling of shells [192] etc. These observations reflect the nonlinearity of the encapsulated bubble dynamics even at low acoustic excitation pressures, which is neglected in fitting the linearized dynamics with experimental attenuation data. This gives a definite advantage to this kind of property estimation technique to assess the applicability and validity of various models. To illustrate this we can consider the compression-only behavior at low excitation pressures, certain phospholipid coated bubbles (e.g., BR14, SonoVue) show asymmetric oscillations, more compression than expansion, about the initial diameter. This behavior has been attributed the buckling of the phospholipid shells [192]. Most models for encapsulated bubble dynamics cannot capture this behavior except Marmottant’s model [113], Doinikov’s nonlinear viscosity model [117], the NSEV model [119] and the exponential elasticity model with non-negative surface tension. Thus, comparison with experimentally observed radius-time curves can also assess the capabilities of various rheological models and can be used for characterization of contrast agents.
As noted before, polydispersity poses a significant challenge for characterization and modeling. As a result, significant efforts have been made to develop single bubble acoustic characterization techniques [193–205]. An alternate approach has been to produce monodisperse bubble suspensions [206–210] and characterize them with standard attenuation and scattering experiments [206, 208, 211, 212]. These efforts are critical for better understanding and improvement of rheological models of microbubble encapsulations.
5. Characterization of echogenic liposomes (ELIP)
Since the first report of echogenic liposomes, many studies have been undertaken for characterization of their behaviors. As noted before, they combine drug bearing capacity of liposomes with ability to respond to ultrasound stimulation. Here we will briefly discuss their preparation, acoustic characterization and drug release studies.
5.1. Preparation protocol of echogenic liposomes
The modified preparation protocol for preparing echogenic liposomes is critical for ensuring their acoustically responsive nature. The updated and detailed methodology proposed by Huang and co-workers can be found in a recent publication [64]. ELIPs can be prepared in a pressurized or a non-pressurized environment. The lipids are mixed in the desired molar ratio in a round bottomed flask and dissolved in an organic solvent e.g., choloroform. The solvent is then evaporated at 40 °C, usually in a rotary evaporator, to obtain a thin film. Residual traces of the solvent are removed by placing the flask under high vacuum overnight. The lipid films are then hydrated with a 0.32M mannitol solution in buffer. The hydrating solution can also contain hydrophilic molecules, which will be encapsulated within the aqueous core of the liposomes. The mutilamellar vesicles, formed after the hydration, are bath sonicated for 10 minutes. The resulting solution of liposomes is frozen at −70 °C for 30 minutes followed by thawing at room temperature. Around 3–5 freeze-thaw cycles have been suggested for echogenic liposome production [64]. The liposomes are again frozen at −70 °C and lyophilized in a freeze-drying apparatus. The lyophilized dry cake thus obtained is stored at 4 °C until further use. For the pressurization technique, the sonicated liposomal solution is collected in a screw-cap vial and pressurized by a gas using a syringe. The pressurized-gas/liposome dispersion is incubated for 30 minutes and then frozen at −78 °C on dry ice for another 30 minutess followed by immediate depressurization. The frozen liposomes are thawed at room temperature to change the temperature from −78 °C to 24 °C within 10 minutes.
The lyophilized cakes of ELIPs, obtained either way, are reconstituted in a phosphate buffered saline (PBS) solution for further investigations. Adding 5% by weight of bovine serum albumin (BSA) to the PBS prevents the aggregation of ELIPs [66] and substantially improves the detection of their acoustical reflectivity. The critical steps in the above-mentioned methodology for echogenicity of liposomes are freeze-thaw cycles and lyophilization in presence of mannitol, a weak cryoprotectant, and the subsequent reconstitution process. It has been proposed that the lipid bilayer develops defects during the freezing steps due to the weak cryo-protection abilities of mannitol [64], thereby exposing the hydrophobic portions of the lipid bilayer. The fluffy dry cake formed after lyophilization also increases the surface area of contact. The air is entrapped through these bilayer defects during the reconstitution phase form gas pockets stabilized by lipid monolayer [65, 66]. The exact location or dimensions of this structure remains unascertained. Moreover, the protocol also does not guarantee that all the liposomes in the suspension will be associated with such a structure [64]. Nevertheless, the echogenicity of the liposomes prepared following this protocol has been conclusively demonstrated through several independent experiments including ones in our laboratory described below. Note that the preparation protocol can be further modified to include different lipid formulations and targeting ligands or to replace the entrapped air with other bioactive gases. Two such novel ELIP formulations with simultaneous imaging and targeted delivering capabilities have been developed by us and will be discussed in a later section.
5.2 Acoustic characterization of echogenic liposomes
Earliest studies of the echogenicity of ELIPs primarily employed a 20 MHz high frequency intravascular ultrasound (IVUS) catheter for both in vitro [61, 62] and in vivo [65, 68, 69, 213] characterization. Subsequently their design was optimized using the same probe as well as videodensitometric analysis [63, 214].
The first comprehensive in vitro characterization of echogenic liposomes was performed by Coussios et al.[215]. They used a 3.5 MHz lightly focused immersion transducer and compared the echogenicity of ELIPs with that of the microbubble based contrast agent Optison®. They reported that the backscattering coefficient of liposomes can be even higher than that of Optison® with the liposomes having higher scattering-to-attenuation ratio (STAR). This demonstrated the potential of ELIPs to be used as ultrasound contrast agents.
Kopechek et al.[66] extended this study to a wider range of frequencies for both attenuation and backscattering experiments using single element immersion transducers. Unlike contrast microbubbles (Figure 1), ELIPs showed no definite peak in broadband attenuation in the range of 3–25 MHz. The attenuation was fitted with the Church’s model for encapsulated bubbles to predict a shear viscosity of 0.30 Pa·s and a shear modulus of 125 MPa. These values are equivalent to a dilatational viscosity κs = 9×10−10 N.s/m and a dilatational elasticity Es = 0.56 N/m 0.56 N/m. They also reported a backscattering coefficient of 0.011–0.023 (cm-str)−1 in the range of 6–30 MHz resulting in a STAR of 8 to 22%, which is comparable with the values for contrast microbubbles.
We have also measured broadband attenuation and pressure dependent scattered response from ELIPs using single element immersion transducers [67]. We found no peak in attenuation for a frequency range of 1–13 MHz. Scattering measurements, conducted at 3.5 MHz and 50–800 kPa, showed a 15–20 dB enhancement over control (i.e., in absence of ELIPs in suspension) at a lipid concentration of 1.67 μg/ml. Although the scattered response showed second-harmonic response, no subharmonic response was observed under these excitation conditions.
Lu et al. [216] followed a similar method to prepare echogenic liposomes with an average size of 1600±200 nm and conducted in vitro acoustic studies. They also found no distinct peak in attenuation, but concluded that the resonance lies in the range 7–11 MHz. Their scattering experiments at 10 MHz excitation showed enhancement of both fundamental and second-harmonic responses. These liposomes were not found to be very robust with an effective operation time of 10 minutes and a destruction threshold of 150 kPa at 2.25 MHz excitation. Authors also detected echogenicity of such liposomes with 25 MHz B-mode pulses. Using a Phillips L12-5 linear array transducer system [217], ELIPs were found to generate robust echoes for both continuous 6–9 MHz fundamental and 4–5 MHz harmonic B-mode pulses. A more recent in vitro study by Radhakrishnan and co-workers [218] evaluated the performance of ELIPs as a blood pool contrast agent using a physiologic flow phantom. ELIPs were found to be stable in physiologic conditions with proper care. Around 14–17 dB enhancement of echogenicity was reported in citrate-phosphate-dextrose whole blood. Echogenicity was reported to be sensitive to abnormalities of red blood cells and rapid cooling below body temperature. Suitability of ELIPs as contrast agents for passive cavitation imaging have also been reported [219].
We have recently reported echogenicity of several modified ELIP formulations [75, 76] thereby demonstrating their potential for development as therapeutic ultrasound contrast agents. The ELIP formulations were tested for echogenicity using an in vitro acoustic setup employing single element focused ultrasound transducers. All the acoustic characterizations were done at 3.5 MHz frequency and at an acoustic excitation pressure of 500 kPa with a 32 cycle sinusoidal pulse. The ELIPs were also imaged using a diagnostic ultrasound scanner (Terason t3200, MedCorp LLC., Tampa, FL, USA). Sax and Kodama [220] have also prepared echogenic liposomes encapsulating perfluoropropane gas to study their stability in vitro and in vivo by varying their lipid compositions. Echogenicity measurements were acquired using a high frequency US imaging system generating B-mode pulses. They reported that increasing the molar ratio of polyethylene glycol (PEG) lipids significantly enhanced the half-life of the liposomes, both in vitro (55 MHz probe) and in vivo (40 MHz probe). However, in contrast to previous reports, cholesterol was shown to reduce stability of the liposomes by increasing membrane permeability and gas leakage.
Figure 5 shows a comparison of the attenuation measurements by Kopechek et al. with that conducted in our laboratory. The flat nature of the attenuation curves in both set of experiments can be attributed to the highly polydisperse size distribution of ELIPs that can range from nanometer sized to micron sized particles. The larger error bars associated with the attenuation data from both the previous reports can be attributed to the inherent variability in the acoustic properties of ELIPs, possibly again due to their high polydispersity. It should also be mentioned that Kopechek et al. assumed the volume of the gas pocket to be 18% of the entire range of liposomal size to obtain the fitting. Since, no conclusive experiments have validated this assumption, we did not attempt to fit our attenuation data with any model to obtain material parameters. Moreover, most conventional sizing techniques like dynamic light scattering and Coulter counter measurements might not be accurate for such highly polydisperse size distribution. In fact, in spite of following the same protocol, the size distributions reported by Kopechek et al. and our measurements were significantly different [See Table 2]. Detailed studies on exact location of the gas pockets and their dimensions along with more reliable size distribution data will be essential for better understanding the underlying mechanisms of ELIP behavior. Nevertheless, thorough in vitro acoustic studies conclusively demonstrated the echogenic nature of this new variation of liposomes which was an essential step in the validation of the proof of concept.
Figure 5.
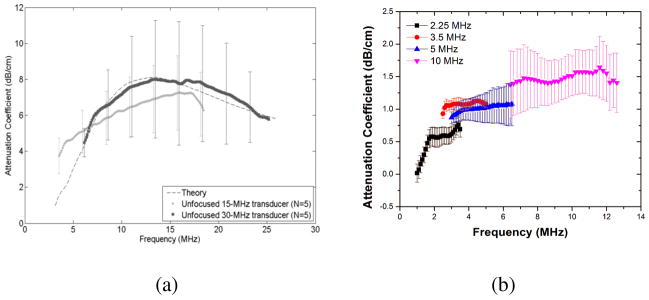
Frequency dependent broadband attenuation measurements form ELIP suspensions reported by (a) Kopechek et al.(Reproduced with permission from [66]) at a lipid concentration of 0.05 mg/ml and (b) Paul et al. (Reproduced with permission from [67]) at a lipid concentration of 10 μg/ml.
Table 1.
Estimated interfacial rheological properties of the encapsulation of Sonzaoid and PLA microbubble for different interfacial models
| Interfacial Model | Sonazoid® | PLA Microbubbles |
|---|---|---|
| Newtonian Model (NM) |
γ = 0.60 ± 0.14 N/m κs = 1.0 ± 0.004 × 10−8 N · s/m |
γ = 0.06 ± 0.03 N/m κs = 6.0 ± 3.5 × 10−9 N · s/m |
| Constant Elasticity Model (CEM) |
γ0 = 0.02 N/m Es = 0.51 ± 0.11 N/m κs = 1.0 ± 0.004 × 10−8 N · s/m |
γ0 = 0.01 ± 0.006 N/m Es = 0.05 ± 0.03 N/m κs = 6.0 ± 3.5 × 10−9 N · s/m |
| Exponential Elasticity Model (EEM) |
γ0 = 0.02 N/m Es = 0.55 ± 0.10 N/m κs = 1.2 ± 0.40 × 10−8 N · s/m α = 1.5 |
γ0 = 0.01 ± 0.006 N/m κs = 6.0 ± 3.5 × 10−9 N · s/m α = 1.5 |
| Marmottant Model (MM) |
γ0 = 0.0 N/m (i.e. Rbuckling = R0) χ = 0.53 ± 0.10 N/m κs = 1.2 ± 0.40 × 10−8 N · s/m Rbreak-up = 1.5Rbuckling |
γ0 = 0.0 N/m (i.e. Rbuckling = R0) χ = 0.06 ± 0.02 N/m κs = 6.0 ± 3.5 × 10−8 N · s/m Rbreak-up = 1.5Rbuckling |
As mentioned earlier, freeze drying in presence of mannitol is critical for echogenicity of liposomes [62, 63, 65]. Although there are conflicting reports of optimal mannitol concentration during preparation [62, 69], the established protocol suggests the use of 320 mM considering both echogenicity and encapsulation efficiency of the liposomes [63]. We have investigated in detail the effects of mannitol concentration on echogenicity [67]; a finite amount of mannitol was found to be essential for acoustic reflectivity of the ELIPs. We also found that liposomes were only echogenic when they were lyophilized in presence of mannitol for different ELIP formulations.
As mentioned above, although these studies have demonstrated the potential of ELIPs as ultrasound contrast agents, there remains important unanswered questions relating to the exact cause of echogenicity. There is a need to determine the exact location and dimension of gas pockets. This problem has eluded researchers since the first report of echogenic liposomes, fueling the skepticism regarding their echogenicity. There have been microscopic pictures that suggests gas pockets [66, 67, 76, 221]. However, the pictures are not as conclusive as one would like them to be so that it can end the decade long debate about these purported gas pockets. In our personal experience, these pictures have been extremely difficult to obtain. Figure 6 shows a transmission electron microscopic (TEM) image of a polymer coated ELIP obtained by our group. A gas pocket similar to that shown in the hypothesized structure presented in Figure 2 can be seen. Even if these gas pockets exist, their dimensions will be too small to have a scattering cross-section large enough to be detected accurately by 1–10 MHz acoustic waves. On the other hand, experimental evidence clearly shows that the echogenicity is only achieved when the modified preparation protocol is followed. We believe that the echogenicity is primarily due to the existence of a smaller fraction of larger liposomes, which will have larger gas pockets. Presence of larger vesicles is indicated by the high polydispersity index of dynamic light scattering measurements with ELIP suspensions. Atomic force microscopic images (AFM), shown in Figure 7, also show the presence of different sized vesicles, even with micron range diameters. However, due to lack of conclusive evidence, existence of separate lipid monolayer coated microbubbles in the suspension, which may be created during the preparation of liposomes, cannot be completely ruled out.
Figure 6.
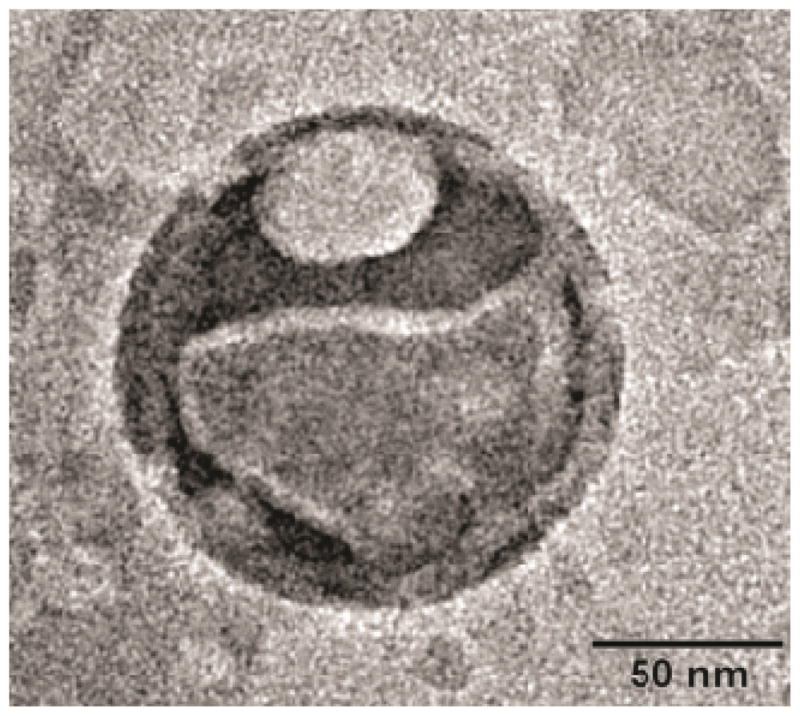
Transmission electron micrographic image of a negatively stained polymer coated ELIP with 1% phophotungstic acid, captured using a JEOL JEM-2100-LaB6 microscope at 200 kV (Reproduced with permission from [76]).
Figure 7.
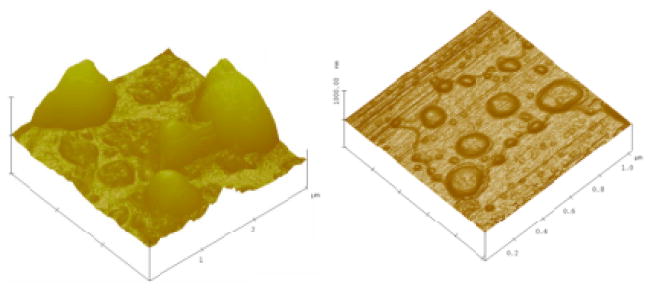
Multimode™ atomic force microscopic images of (a) Conventional ELIPs [reproduced with permission from [67]], and (b) Polymer coated ELIPs [reproduced with permission from [76]].
5.3 Stimuli responsive release characteristics of echogenic liposomes
Since echogenic liposomes retain all favorable properties of normal liposomes, they have been extensively studied as ultrasound triggered drug delivery vehicles [52, 74, 222, 223]. Anti-intercellular adhesion molecule-1 (ICAM-1) [224–227], anti-vascular cell adhesion molecule (VCAM-1), anti-fibrin, anti-fibrinogen and anti-tissue factor conjugated with ELIPs [68, 228] have also been developed to achieve both in vitro and in vivo targeting. ELIPs can be loaded with both hydrophilic and lipophilic molecules [70]. By suitably modifying the preparation protocol, ELIPs have been made to entrap genes [229], fluorescent molecules like calcein [63, 69, 70, 76] and carboxyfluorescein [75] as drug surrogates, antibiotics [230], peroxisomal proliferator-activated receptor agonists [231], a thrombolytic enzyme rt-PA (recombinant tissue-plasminogen activator) [74, 223, 232–234], a vasodilator papverine [70, 235], an anti-diabetic drug rosiglitazone [236, 237] and NF-κB decoy oligonucleotides [222]. By virtue of its preparation protocol, ELIPs can encapsulate a gaseous phase, which is usually air. However, with suitable manipulations of the preparation protocol, ELIPs can also encapsulate bioactive gases like xenon [238] and nitric oxide [239–241]. Note that in all these studies, incorporation of a payload did reduce the echogenicity of ELIPs significantly.
Fluorescent molecules like calcein [77, 242, 243] and carboxyfluorescein [244, 245] are often used as surrogates for hydrophilic drugs for evaluating triggered release from liposomes. Hence, ultrasound triggered release from ELIPs has also been studied by detecting changes in fluorescence due to the release of calcein or carboxyfluorescein. Huang and McDonald [69] used a continuous wave ultrasound pulse at 1 MHz frequency and at an output power of 2 W/cm2, generated using a Sonitron ultrasound system, for a duration of 10s. Depending on the number of excitation cycles, 30–60% release of contents was reported with no mention of passive release in absence of ultrasound. Huang et al. [63] in a later study used a similar ultrasound system to excite calcein loaded ELIPs with 1 MHz continuous wave ultrasound at 8 W/cm2 output power for a duration of 10s. The passive and ultrasound triggered release (over 10s) from air containing ELIPs was both around 10% indicating negligible effects from ultrasound excitation. The release improved to about 30% with argon or perfluorocarbon encapsulated ELIPs. Note that none of these studies reported the calibration techniques for determining the actual output power. Kopechek et al. [246] did a detailed calibration of Sonitron systems to show that presence of standing waves can play a critical role in the above mentioned in vitro studies—the pressure field can be corrupted due to the constructive and destructive interference. A more detailed study of ultrasound mediated release of calcein was performed by Kopechek and co-workers using color Doppler ultrasound [70]. A CL15-7 linear array transducer was used to generate 6 MHz ultrasound pulses at 2 MPa peak-negative pressure and a PRF of 150 Hz. Although 47.5±33 % release of calcein was reported with ultrasound, no release was observed for the lipophilic drug papaverine in the same study. However, a later study by the same group concluded that the results might be erroneous due to effects of gas bubbles on fluorescence measurements [237]. The same study, which used 6 MHz color Doppler ultrasound pulses (1250 Hz PRF and 0.17 W/cm2 calibrated output power) from CL15-7 transducer, reported no ultrasound mediated release of calcein and rosiglitazone, even after detection of both inertial and stable cavitation. Smith et al. [74] have also shown therapeutically relevant release of rt-PA from ELIPs using color Doppler ultrasound. Other studies have demonstrated thrombolytic efficacy of rt-PA loaded ELIPs [223, 232, 247] Buchanan et al. [222] had studied ultrasound mediated release of oligonucleotides (ODN) using a Sonitron 1000 system to generate 1 MHz continuous wave ultrasound at a peak negative pressure of 0.26 MPa for a duration of 60s. Around 42% release of ODN from ELIPs was reported compared to around 18% release from non-echogenic liposomes. However, it is not clear if their measurements are also susceptible to changes caused by the presence of gas bubble as mentioned earlier.
It is evident from the preceding discussions that ultrasound mediated release of liposomal contents is often uncertain and susceptible to several other factors that can critically affect the release efficiency. Moreover, the release is not always optimal, ranging from 20–50%. This motivated us to pursue the development of echogenic liposomes with dual release triggers—a combination of a different exogenous or endogenous trigger with ultrasound—to achieve considerable higher amount of contents release. To date we have developed two such ELIP formulations: a substrate lipopeptide conjugated ELIP formulation that can be triggered (or cleaved) by the extracellular enzyme matrix metalloproteinase-9 (MMP-9) [75] and a polymer coated redox triggered ELIP formulation capable of cytosolic drug delivery [76]. MMP-9 is overexpressed in atherosclerotic diseases and in metastatic cancers [248–253]. We have also developed an ELIP formulation with pH tunable echogenicity [unpublished work].
For our drug release studies, we used a single element flat faced ultrasound transducer that has been carefully calibrated to determine accurately the output energy of the ultrasound pulse. The transducer was excited with a 3 MHz continuous wave ultrasound pulse. The output pressure and duration of excitation was chosen for optimal release of contents under static conditions [See Table 3]. For a set of positive control experiments, we also utilized a 22.5 kHz sonic dismembrator at 4 W output setting.
Table 3.
Ultrasound parameters used in the release studies with two different ELIP formulations with dual triggers.
| Type of echogenic liposome | Ultrasound Parameters |
|---|---|
| MMP-9 cleavable ELIPs | Frequency: 3 MHz Duty cycle: 100% Peak negative output pressure: 3 MPa Duration of exposure: 3 min |
| Redox triggered ELIPs | Frequency: 3 MHz Duty cycle: 100% Peak negative output pressure: 0.53 MPa Duration of exposure: 2 min |
For the MMP-9 cleavable ELIPs, carboxyfluorescein was encapsulated to quantify the release of liposomal contents by employing a self-quenching strategy. Figure 8(a) shows ultrasound enhanced recombinant MMP-9 triggered release of contents from the ELIPs. About 50–60% release was observed with recombinant MMP-9 enzymes whereas a 30–50% release was observed with conditioned cell culture media from cancer cells secreting MMP-9. This release was further enhanced by 10–15% and 20–30% for recombinant enzyme and conditioned media respectively by the application of ultrasound. Note that negligible release (4%) was seen when only ultrasound was used to trigger release. Also, the simultaneous application of ultrasound and enzymatic trigger showed a reduction in release, possibly due to localized increase of temperature during ultrasonic excitation that reduced the activity of the recombinant enzymes (bulk temperature was maintained constant by using an ice bath). It should also be mentioned that we utilized the non-lyophilized version of the liposomes for our release studies. Hence, we do not expect any interference in our fluorescence measurements due to presence of gas bubbles.
Figure 8.
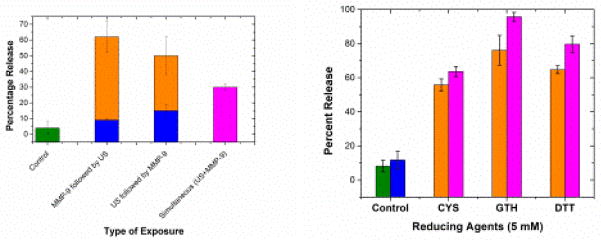
(a) Ultrasound (US) enhanced recombinant MMP-9 triggered release of contents from MMP-9 cleavable ELIPs. (b) Ultrasound (US) enhanced redox triggered release of contents from polymer coated disulfide cross-linked ELIPs [Reproduced with permission from [76]]. In both the figures, the olive, blue, orange, and magenta colored bars represents the passive release in absence of any trigger, release due to US only, release due to other triggers (MMP-9 or redox) only, and the release with simultaneous application of US and the other trigger respectively.
The polymer coated ELIPs had a disulfide cross-linkage that is stable in mildly oxidizing environment but unstable in presence of reducing agents. Typically cytosolic concentration of reducing agents is higher than that in plasma and extracellular matrix [254]. Hence, this disulfide crosslinker has been effective for cytosolic drug delivery [255–257] using the reversible disulfide thiol conversion [254]. In order to achieve active internalization of the ELIPs in cancer cells, we incorporated a folate conjugated lipid in the ELIPs. A CoCl2 quenching strategy was implemented to quantify release of calcein from these polymer coated ELIPs in the presence of both redox and ultrasound triggers. Negligible release (less than 5%) was observed in our control samples i.e., both without ultrasound and reducing agents. At a very low concentration of glutathione (5 μM, corresponds to its concentration in extracellular matrix), the release was also less than 5%, but it increased significantly with increasing reducing agent concentration. We were able to obtain up to 90% release with just reducing agents at 10 mM concentrations (typical in cell cytoplasm). As with the MMP-9 cleavable ELIPs, there was no release with just the application of 3 MHz ultrasound. However, about 8–20% enhancement over redox triggered release was observed with simultaneous application of 5 mM redox and ultrasound. Figure 8(b) shows the release of liposomal contents from these liposomes with dual triggers – ultrasound and reducing agents. Different reducing agents were used for comparison, which includes dithiothreitol (DTT), cysteine (CYS) and glutathione GSH).
5. Summary and scope for future research
Encapsulated microbubbles and echogenic liposomes are being rapidly developed for several of diagnostic imaging applications as well as targeted drug delivery for treatment of cardiovascular diseases and several types of cancer. This review, although provides more details on recent research performed by our group, was intended to provide a broad overview of the progress in this field of research and motivate future research. Several clinical and therapeutic applications of both encapsulated microbubble based ultrasound contrast agents and echogenic liposomes were discussed to familiarize the reader with developments in this field over the past few decades.
We present a review of the various modeling strategies proposed till date for encapsulated microbubbles along with a detailed discussion of the newly proposed interfacial models. Several different experimental techniques for characterization of encapsulated microbubble dynamics are then discussed. Estimation of the rheological properties of the encapsulation by various models remains a difficult task. Various experimental strategies employed for this are also discussed briefly with a detailed overview of the hierarchical approach used in our lab. The predictive capabilities of the different interfacial models are discussed with specific examples of Sonzoid and Poly(DL-lactic acid) polymer (PLA) encapsulated microbubbles by comparing experimentally measured subharmonic responses with model predictions. The interfacial models with nonlinear elasticity and/or viscosity are found to be better equipped to capture complex encapsulated bubble dynamics. Some relevant analytical results for subharmonic thresholds are also presented for better understanding of model predictions.
We also present a comprehensive review of the characterization of the acoustic properties and stimuli responsive release properties of echogenic liposomes. Echogenic liposomes are often found to behave differently from conventional microbubble based contrast agents. A critical analysis of the various hypotheses for their echogenicity is also presented to initiate further research interests.
As evident from the preceding discussion, the recent developments in interfacial models have significantly improved our understanding of encapsulated bubble dynamics, and equipped us with powerful predictive tools of contrast agent behavior. However, none of the models enjoys unambiguous validity and each comes with a set of strengths and weaknesses. Moreover, emerging experimental techniques are reporting several new and interesting contrast agent behaviors. Therefore, further experiment driven model improvement is required to improve their reliability and widen their scope of applicability. Clinical applications of contrast agents involve polydisperse bubble population at fairly high concentrations. Model predictions are critically dependent on the bubble size distributions. Therefore, more reliable and accurate size measurements techniques are required especially those which can handle highly polydisperse systems. Increasing levels of sophistications and complexity can be introduced into modeling e.g. multiple scattering [258], presence of blood vessels [259–261], non-spherical bubble oscillations [188, 262], ultrasound mediated bubble destruction, and effects of drug loading and targeting ligands on bubble dynamics [263, 264]. Experimental characterization of encapsulated bubble dynamics also have several potential areas for further development e.g., devising sophisticated experimental techniques for correct estimation of shell viscoelastic properties, accounting for polydispersity of bubble suspensions during experiments, characterization of nonlinear behavior at low acoustic pressure, characterization of bubble-wall interactions, determination of the thresholds for subharmonic generation, characterization of rupture, break-up, dissolution dynamics of encapsulated bubbles etc.
Acoustic measurements with echogenic liposomes have conclusively demonstrated their potential for applications as ultrasound contrast agents. However, the exact mechanisms for their echogenicity are not completely understood primarily due to the uncertainty regarding the exact location of the gas pockets. As is obvious from the review above, due to such lack of understanding, unlike microbubble contrast agents, there has not been much progress in modeling behaviors of echogenic liposomes. The only model [66], as mentioned above, estimated the gas volume to be certain percentage of the entire liposomal population, and computed attenuation. Only an accurate knowledge of the locations and dimensions of the gas pockets will enable us to develop improved mathematical models of their acoustic behaviors. The high polydispersity of ELIPs also pose an important hurdle for better mathematical characterization and hence, requires sophisticated size measurement techniques that can handle such wide range of particle sizes in the same suspension. Considering the experimental results for stimuli responsive release from ELIPs, it can be safely concluded that ELIPs can be potentially used for simultaneous imaging and therapeutic applications. If implemented successfully, such contrast agents can provide powerful treatment strategies for several cardiovascular diseases and cancer. However, there also is a need for detailed parametric study of ultrasound mediated release form ELIPs in vitro using clinically relevant ultrasound pulses to ascertain the optimal excitation conditions. It will be also be beneficial to detect the role of cavitation associated with such release. Such studies will improve our understanding of the physical mechanisms, and pave the way for clinical translation of these technologies.
Acknowledgments
This research was supported by NIH grants 1R01 CA 113746, 1R01 CA 132034, NSF grant DMR 1005011 to SM and DMR-1005283, CBET 1033256, CBET 1205322 to KS.
Footnotes
Development suspended in USA and EU. It is currently approved for use in Japan.
Contributor Information
Shirshendu Paul, Department of Mechanical Engineering, University of Delaware, Newark DE 19716, USA.
Rahul Nahire, Department of Pharmaceutical Sciences, North Dakota State University, Fargo ND 58108, USA.
Sanku Mallik, Department of Pharmaceutical Sciences, North Dakota State University, Fargo ND 58108, USA.
Kausik Sarkar, Department of Mechanical and Aerospace Engineering, The George Washington University, Washington, DC 20052, USA.
References
- 1.Liu J, et al. Nanoparticles as image enhancing agents for ultrasonography. Physics in Medicine and Biology. 2006 May 7;51:2179–2189. doi: 10.1088/0031-9155/51/9/004. [DOI] [PubMed] [Google Scholar]
- 2.Gao Z, et al. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008 Aug;48:260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhyner MN, et al. Quantum dots and multifunctional nanoparticles: new contrast agents for tumor imaging. Nanomedicine. 2006 Aug;1:209–217. doi: 10.2217/17435889.1.2.209. [DOI] [PubMed] [Google Scholar]
- 4.Phillips D, et al. Acoustic backscatter properties of the particle/bubble ultrasound contrast agent. Ultrasonics. 1998 Jul;36:883–892. doi: 10.1016/s0041-624x(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 5.Waggoner AD, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: Recommendations of the American Society of Echocardiography Council on Cardiac Sonography. Journal of the American Society of Echocardiography. 2001;14:417–420. doi: 10.1067/mje.2001.113817. [DOI] [PubMed] [Google Scholar]
- 6.Mulvagh SL, et al. Contrast echocardiography: Current and future applications. Journal of the American Society of Echocardiography. 2000 Apr;13:331–342. doi: 10.1067/mje.2000.105462. [DOI] [PubMed] [Google Scholar]
- 7.Klibanov AL. Ultrasound contrast agents: Development of the field and current status. Contrast Agents Ii. 2002;222:73–106. [Google Scholar]
- 8.Postema M, Gilja OH. Contrast-enhanced and targeted ultrasound. World Journal of Gastroenterology. 2011 Jan 7;17:28–41. doi: 10.3748/wjg.v17.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JB, et al. Contrast-enhanced Ultrasound Imaging: State of the Art. Journal of Medical Ultrasound. 2005;13:109–126. [Google Scholar]
- 10.Phillips LC, et al. Localized ultrasound enhances delivery of rapamycin from microbubbles to prevent smooth muscle proliferation. Journal of Controlled Release. 2011 Aug 25;154:42–49. doi: 10.1016/j.jconrel.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbrey JR, et al. Development and optimization of a doxorubicin loaded poly(lactic acid) contrast agent for ultrasound directed drug delivery. Journal of Controlled Release. 2010 Apr 2;143:38–44. doi: 10.1016/j.jconrel.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenbrey JR, et al. Delivery of Encapsulated Doxorubicin by Ultrasound-Mediated Size Reduction of Drug-Loaded Polymer Contrast Agents. IEEE Transactions on Biomedical Engineering. 2010 Jan;57:24–28. doi: 10.1109/TBME.2009.2030497. [DOI] [PubMed] [Google Scholar]
- 13.Gramiak R, Shah PM. Echocardiography of the aortic root. Investigatory Radiology. 1968;3:356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Hilgenfeldt S, et al. Response of bubbles to diagnostic ultrasound: a unifying theoretical approach. European Physical Journal B. 1998 Jul;4:247–255. [Google Scholar]
- 15.Katiyar A, et al. Effects of Encapsulation Elasticity on the stability of an Encapsulated Microbubble. Journal of Colloid and Interface Science. 2009;336:519–525. doi: 10.1016/j.jcis.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar K, et al. Growth and dissolution of an encapsulated contrast microbubble. Ultrasound in Medicine and Biology. 2009;35:1385–1396. doi: 10.1016/j.ultrasmedbio.2009.04.010. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postema M, Schmitz G. Bubble dynamics involved in ultrasonic imaging. Expert Review of Molecular Diagnostics. 2006 May;6:493–502. doi: 10.1586/14737159.6.3.493. [DOI] [PubMed] [Google Scholar]
- 18.Miller DL, et al. Bioeffects considerations for diagnostic ultrasound contrast agents. Journal of Ultrasound in Medicine. 2008 Apr;27:611–632. doi: 10.7863/jum.2008.27.4.611. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Advanced Drug Delivery Reviews. 2008 Jun 30;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Coussios CC, Roy RA. Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annual Review of Fluid Mechanics. 2008;40:395–420. [Google Scholar]
- 21.Dayton P, et al. Acoustic radiation force in vivo: A mechanism to assist targeting of microbubbles. Ultrasound in Medicine and Biology. 1999 Oct;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 22.Dayton PA, et al. The magnitude of radiation force on ultrasound contrast agents. Journal of the Acoustical Society of America. 2002 Nov;112:2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 23.Dayton PA, et al. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 1997 Nov;44:1264–1277. [Google Scholar]
- 24.Casciaro S, et al. Experimental investigations of nonlinearities and destruction mechanisms of an experimental phospholipid-based ultrasound contrast agent. Investigative Radiology. 2007 Feb;42:95–104. doi: 10.1097/01.rli.0000251576.68097.d1. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee D, et al. Ultrasound-mediated destruction of contrast microbubbles used for medical imaging and drug delivery. Physics of Fluids. 2005 Oct;17:100603. [Google Scholar]
- 26.Ward M, et al. Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. Journal of the Acoustical Society of America. 1999 May;105:2951–2957. doi: 10.1121/1.426908. [DOI] [PubMed] [Google Scholar]
- 27.Ward M, et al. Experimental study of the effects of Optison (R) concentration on sonoporation in vitro. Ultrasound in Medicine and Biology. 2000 Sep;26:1169–1175. doi: 10.1016/s0301-5629(00)00260-x. [DOI] [PubMed] [Google Scholar]
- 28.Unger EC, et al. Therapeutic applications of lipid-coated microbubbles. Advanced Drug Delivery Reviews. 2004 May 7;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Xie F, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound in Medicine and Biology. 2005 Jul;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Taniyama Y, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002 Mar 12;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 31.Bull JL. The application of microbubbles for targeted drug delivery. Expert Opinion on Drug Delivery. 2007 Sep;4:475–493. doi: 10.1517/17425247.4.5.475. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen JP, Lindner JR. Molecular and cellular imaging with targeted contrast ultrasound. Proceedings of the Ieee. 2005 Apr;93:809–818. [Google Scholar]
- 33.Klibanov AL. Microbubble contrast agents - Targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Investigative Radiology. 2006 Mar;41:354–362. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- 34.Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. Journal of Nuclear Cardiology. 2004 Mar-Apr;11:215–221. doi: 10.1016/j.nuclcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara K, et al. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annual Review of Biomedical Engineering. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 36.Bekeredjian R, et al. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. Journal of the American College of Cardiology. 2005 Feb 1;45:329–335. doi: 10.1016/j.jacc.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 37.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Advanced Drug Delivery Reviews. 2008 Jun 30;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nature Reviews Drug Discovery. 2004 Jun;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 39.Martin KH, Dayton PA. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2013. Current status and prospects for microbubbles in ultrasound theranostics. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lentacker I, et al. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter. 2009;5:2161–2170. [Google Scholar]
- 41.Blomley MJK, et al. Microbubble contrast agents: a new era in ultrasound. BMJ. 322:1222–1225. doi: 10.1136/bmj.322.7296.1222. 2001-05-19 00:00:00 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Main ML, et al. Ultrasound contrast agents: balancing safety versus efficacy. Expert Opinion on Drug Safety. 2009;8:49–56. doi: 10.1517/14740330802658581. [DOI] [PubMed] [Google Scholar]
- 43.Haar G. Safety and bio-effects of ultrasound contrast agents. Medical & Biological Engineering & Computing. 2009 Aug 01;47:893–900. doi: 10.1007/s11517-009-0507-3. [DOI] [PubMed] [Google Scholar]
- 44.Klibanov AL, et al. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: A tool for targeted drug delivery. Journal of Controlled Release. 2010 Nov 20;148:13–17. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bangham A. The 1st Description of Liposomes - a Citation Classic Commentary on Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids by Bangham, A.D., Standish, M.M., and Watkins, J.C. Current Contents/Life Sciences. 1989 Mar 27;:14–14. [Google Scholar]
- 46.Bangham AD, et al. Diffusion of Univalent Ions across Lamellae of Swollen Phospholipids. Journal of Molecular Biology. 1965;13:238. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 47.Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16:307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 48.Lian T, Ho RJY. Trends and developments in liposome drug delivery systems. Journal of Pharmaceutical Sciences. 2001 Jun;90:667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 49.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. 2005 Feb;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 50.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in Lipid Research. 2003 Nov;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 51.Ding N, et al. Folate receptor-targeted fluorescent paramagnetic bimodal liposomes for tumor imaging. International Journal of Nanomedicine. 2011;6:2513–2520. doi: 10.2147/IJN.S23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang SL. Liposomes in ultrasonic drug and gene delivery. Advanced Drug Delivery Reviews. 2008 Jun 30;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Turner DC, et al. Near-infrared image-guided delivery and controlled release using optimized thermosensitive liposomes. Pharm Res. 2012 Aug;29:2092–103. doi: 10.1007/s11095-012-0738-0. [DOI] [PubMed] [Google Scholar]
- 54.Leung SJ, et al. Wavelength-Selective Light-Induced Release from Plasmon Resonant Liposomes. Adv Funct Mater. 2011 Mar 20;21:1113–1121. doi: 10.1002/adfm.201002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu FQ, et al. pH Triggered Doxorubicin Delivery of PEGylated Glycolipid Conjugate Micelles for Tumor Targeting Therapy. Molecular Pharmaceutics. 2012 Sep;9:2469–2478. doi: 10.1021/mp300002v. [DOI] [PubMed] [Google Scholar]
- 56.Banerjee J, et al. Release of liposomal contents by cell-secreted matrix metalloproteinase-9. Bioconjug Chem. 2009 Jul;20:1332–9. doi: 10.1021/bc9000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarkar N, et al. Matrix metalloproteinase-assisted triggered release of liposomal contents. Bioconjugate Chemistry. 2008 Jan;19:57–64. doi: 10.1021/bc070081p. [DOI] [PubMed] [Google Scholar]
- 58.Ong W, et al. Redox-triggered contents release from liposomes. J Am Chem Soc. 2008 Nov 5;130:14739–44. doi: 10.1021/ja8050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, et al. Nanoparticles in medicine: Therapeutic applications and developments. Clinical Pharmacology & Therapeutics. 2008 May;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 60.Schroeder A, et al. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chemistry and Physics of Lipids. 2009 Nov;162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 61.AlkanOnyuksel H, et al. Development of inherently echogenic liposomes as an ultrasonic contrast agent. Journal of Pharmaceutical Sciences. 1996 May;85:486–490. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 62.Huang SL, et al. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. Journal of Pharmaceutical Sciences. 2001 Dec;90:1917–1926. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- 63.Huang SL, et al. A method to co-encapsulate gas and drugs in liposomes for ultrasound-controlled drug delivery. Ultrasound in medicine & biology. 2008 Aug;34:1272–80. doi: 10.1016/j.ultrasmedbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang S-L. Ultrasound-Responsive Liposomes. In: Weissig V, editor. Liposomes. Vol. 605. Humana Press; 2010. pp. 113–128. [DOI] [PubMed] [Google Scholar]
- 65.Huang SL, et al. Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents. Ultrasound in Medicine and Biology. 2002 Mar;28:339–348. doi: 10.1016/s0301-5629(01)00512-9. [DOI] [PubMed] [Google Scholar]
- 66.Kopechek JA, et al. Acoustic characterization of echogenic liposomes: Frequency-dependent attenuation and backscatter. Journal of the Acoustical Society of America. 2011 Nov;130:3472–3481. doi: 10.1121/1.3626124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paul S, et al. In vitro measurement of attenuation and nonlinear scattering from echogenic liposomes. Ultrasonics. 2012 Sep;52:962–969. doi: 10.1016/j.ultras.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamilton AJ, et al. Intravascular ultrasound molecular Imaging of atheroma components in vivo. Journal of the American College of Cardiology. 2004 Feb 4;43:453–460. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 69.Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochimica Et Biophysica Acta-Biomembranes. 2004 Oct 11;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Kopechek JA, et al. Ultrasound-mediated release of hydrophilic and lipophilic agents from echogenic liposomes. Journal of Ultrasound in Medicine. 2008 Nov;27:1597–1606. doi: 10.7863/jum.2008.27.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang SL, et al. Liposomes as ultrasound imaging contrast agents and as ultrasound-sensitive drug delivery agents. Cellular & Molecular Biology Letters. 2002;7:233–235. [PubMed] [Google Scholar]
- 72.Tiukinhoy-Laing S, et al. Ultrasound-facilitated clot lysis using tPA-loaded echogenic liposomes. Circulation. 2005 Oct 25;112:U696–U696. [Google Scholar]
- 73.Laing ST, et al. Ultrasound-mediated delivery of echogenic immunoliposomes to porcine vascular smooth muscle cells in vivo. Journal of Liposome Research. 2010 Jun;20:160–167. doi: 10.3109/08982100903218918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith DAB, et al. Ultrasound-Triggered Release of Recombinant Tissue-Type Plasminogen Activator from Echogenic Liposomes. Ultrasound in Medicine and Biology. 2010 Jan;36:145–157. doi: 10.1016/j.ultrasmedbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nahire R, et al. Ultrasound Enhanced Matrix Metalloproteinase-9 Triggered Release of Contents from Echogenic Liposomes. Molecular Pharmaceutics. 2012 Sep 04;9:2554–2564. doi: 10.1021/mp300165s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nahire R, et al. Polymer-Coated Echogenic Lipid Nanoparticles with Dual Release Triggers. Biomacromolecules. 2013 Mar;14:841–853. doi: 10.1021/bm301894z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klibanov AL, et al. Ultrasound-triggered release of materials entrapped in microbubble–liposome constructs: A tool for targeted drug delivery. Journal of Controlled Release. 2010;148:13–17. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geers B, et al. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. Journal of Controlled Release. 2011;152:249–256. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 79.Kheirolomoom A, et al. Acoustically-active microbubbles conjugated to liposomes: Characterization of a proposed drug delivery vehicle. Journal of Controlled Release. 2007 Apr 23;118:275–284. doi: 10.1016/j.jconrel.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng ZC, Leal LG. NONLINEAR BUBBLE DYNAMICS. Annual Review of Fluid Mechanics. 1997;29:201–243. [Google Scholar]
- 81.Plesset MS, Prosperetti A. Bubble Dynamics and Cavitation. Annual Review of Fluid Mechanics. 1977 Jan 01;9:145–185. [Google Scholar]
- 82.Rayleigh L. On the pressure development in a liquid during the collapse of a spherical cavity. Philosophical Magazine. 1917;32(S8):94–98. [Google Scholar]
- 83.Plesset M. The dynamics of cavitation bubbles. ASME Journal of Applied Mechanics. 1949;16:277–282. [Google Scholar]
- 84.Noltingk BE. Cavitation produced by Ultrasonics. Proceedings of the Physical Society Section B. 1950;63:674–685. [Google Scholar]
- 85.Neppiras EA. Cavitation Produced by Ultrasonics: Theoretical Conditions for the Onset of Cavitation. Proceedings of the Physical Society Section B. 1951;64:1032–1038. [Google Scholar]
- 86.Poritsky H. The collapse or growth of a spherical bubble or cavity in a viscous fluid. 1952. pp. 813–821. [Google Scholar]
- 87.Keller JB, Miksis M. BUBBLE OSCILLATIONS OF LARGE AMPLITUDE. Journal of the Acoustical Society of America. 1980;68:628–633. [Google Scholar]
- 88.Trilling L. The collapse and rebound of a gas bubble. Journal of Applied Physics. 1952;23:14–17. [Google Scholar]
- 89.Herring C. Theory of the Pulsations of the Gas Bubble Produced by an Underwater Explosion. Vol. 236. Washington: 1941. [Google Scholar]
- 90.Gilmore FR. California Institute of Technology. Hydrodynamics Laboratory; Pasadena, CA 26–4: 1952. The Growth Or Collapse of a Spherical Bubble in a Viscous Compressible Liquid. [Google Scholar]
- 91.Lezzi A, Prosperetti A. Bubble Dynamics in a Compressible Liquid .2. 2nd-Order Theory. Journal of Fluid Mechanics. 1987 Dec;185:289–321. [Google Scholar]
- 92.Prosperetti A, Lezzi A. Bubble Dynamics in a Compressible Liquid .1. 1st-Order Theory. Journal of Fluid Mechanics. 1986 Jul;168:457–478. [Google Scholar]
- 93.Prosperetti A. The Equation of Bubble Dynamics in a Compressible Liquid. Physics of Fluids. 1987 Nov;30:3626–3628. [Google Scholar]
- 94.Brenner MP, et al. Single-bubble sonoluminescence. Reviews of Modern Physics. 2002 Apr;74:425–484. [Google Scholar]
- 95.Doinikov AA, Bouakaz A. Review of shell models for contrast agent microbubbles. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2011;58:981–993. doi: 10.1109/TUFFC.2011.1899. [DOI] [PubMed] [Google Scholar]
- 96.Faez T, et al. 20 years of ultrasound contrast agent modeling. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2013;60:7–20. doi: 10.1109/TUFFC.2013.2533. [DOI] [PubMed] [Google Scholar]
- 97.Roy RA, et al. Cavitation produced by short pulses of ultrasound. Frontiers of Nonlinear Acoustics: Proceedings of 12th International Symposium of Nonlinear Acoustics; London. 1990. pp. 476–481. [Google Scholar]
- 98.deJong N, et al. Higher Harmonics of Vibrating Gas-Filled Microspheres .2. Measurements. Ultrasonics. 1994 Nov;32:455–459. [Google Scholar]
- 99.deJong N, et al. Higher Harmonics of Vibrating Gas-Filled Microspheres .1. Simulations. Ultrasonics. 1994 Nov;32:447–453. [Google Scholar]
- 100.deJong N, Hoff L. Ultrasound Scattering Properties of Albunex Microspheres. Ultrasonics. 1993 May;31:175–181. doi: 10.1016/0041-624x(93)90004-j. [DOI] [PubMed] [Google Scholar]
- 101.deJong N, et al. Absorption and Scatter of Encapsulated Gas Filled Microspheres - Theoretical Considerations and Some Measurements. Ultrasonics. 1992 Mar;30:95–103. doi: 10.1016/0041-624x(92)90041-j. [DOI] [PubMed] [Google Scholar]
- 102.Church CC. The Effects of an Elastic Solid-Surface Layer on the Radial Pulsations of Gas-Bubbles. Journal of the Acoustical Society of America. 1995 Mar;97:1510–1521. [Google Scholar]
- 103.Hoff L, et al. Oscillations of polymeric microbubbles: Effect of the encapsulating shell. Journal of the Acoustical Society of America. 2000 Apr;107:2272–2280. doi: 10.1121/1.428557. [DOI] [PubMed] [Google Scholar]
- 104.Morgan KE, et al. Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2000 Nov;47:1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- 105.Glazman RE. Effects of Adsorbed Films on Gas Bubble Radial Oscillations. Journal of the Acoustical Society of America. 1983;74:980–986. [Google Scholar]
- 106.Khismatullin DB, Nadim A. Radial oscillations of encapsulated microbubbles in viscoelastic liquids. Physics of Fluids. 2002 Oct;14:3534–3557. [Google Scholar]
- 107.Allen JS, et al. Dynamics of therapeutic ultrasound contrast agents. Ultrasound in Medicine and Biology. 2002 Jun;28:805–816. doi: 10.1016/s0301-5629(02)00522-7. [DOI] [PubMed] [Google Scholar]
- 108.Allen JS, Rashid MM. Dynamics of a hyperelastic gas-filled spherical shell in a viscous fluid. Journal of Applied Mechanics-Transactions of the Asme. 2004 Mar;71:195–200. [Google Scholar]
- 109.Chatterjee D, Sarkar K. A Newtonian rheological model for the interface of microbubble contrast agents. Ultrasound in Medicine and Biology. 2003 Dec;29:1749–1757. doi: 10.1016/s0301-5629(03)01051-2. [DOI] [PubMed] [Google Scholar]
- 110.Sarkar K, et al. Characterization of ultrasound contrast microbubbles using in vitro experiments and viscous and viscoelastic interface models for encapsulation. Journal of the Acoustical Society of America. 2005 Jul;118:539–550. doi: 10.1121/1.1923367. [DOI] [PubMed] [Google Scholar]
- 111.Paul S, et al. Material characterization of the encapsulation of an ultrasound contrast microbubble and its subharmonic response: Strain-softening interfacial elasticity model. Journal of the Acoustical Society of America. 2010 Jun;127:3846–3857. doi: 10.1121/1.3418685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paul S, et al. Determination of the Interfacial Rheological Properties of a Poly(DL-lactic acid)-Encapsulated Contrast Agent Using In Vitro Attenuation and Scattering. Ultrasound in medicine & biology. 2013 doi: 10.1016/j.ultrasmedbio.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marmottant P, et al. A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture. Journal of the Acoustical Society of America. 2005 Dec;118:3499–3505. [Google Scholar]
- 114.Doinikov AA, Dayton PA. Maxwell rheological model for lipid-shelled ultrasound microbubble contrast agents. Journal of the Acoustical Society of America. 2007 Jun;121:3331–3340. doi: 10.1121/1.2722233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsiglifis K, Pelekasis NA. Nonlinear radial oscillations of encapsulated microbubbles subject to ultrasound: The effect of membrane constitutive law. Journal of the Acoustical Society of America. 2008 Jun;123:4059–4070. doi: 10.1121/1.2909553. [DOI] [PubMed] [Google Scholar]
- 116.Stride E. The influence of surface adsorption on microbubble dynamics. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2008 Jun 28;366:2103–2115. doi: 10.1098/rsta.2008.0001. [DOI] [PubMed] [Google Scholar]
- 117.Doinikov AA, et al. Modeling of nonlinear viscous stress in encapsulating shells of lipid-coated contrast agent microbubbles. Ultrasonics. 2009 Feb;49:269–275. doi: 10.1016/j.ultras.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marmottant P, et al. Buckling resistance of solid shell bubbles under ultrasound. Journal of the Acoustical Society of America. 2011 Mar;129:1231–1239. doi: 10.1121/1.3543943. [DOI] [PubMed] [Google Scholar]
- 119.Li Q, et al. Modeling complicated rheological behaviors in encapsulating shells of lipid-coated microbubbles accounting for nonlinear changes of both shell viscosity and elasticity. Physics in Medicine and Biology. 2013 Feb 21;58:985–998. doi: 10.1088/0031-9155/58/4/985. [DOI] [PubMed] [Google Scholar]
- 120.Katiyar A, Sarkar K. Excitation threshold for subharmonic generation from contrast microbubbles. Journal of the Acoustical Society of America. 2011 Nov;130:3137–3147. doi: 10.1121/1.3641455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prosperetti A. Thermal effects and damping mechanisms in the forced radial oscillations of gas bubbles in liquids. The Journal of the Acoustical Society of America. 1977;61:17–27. doi: 10.1121/1.3474220. [DOI] [PubMed] [Google Scholar]
- 122.Ainslie MA, Leighton TG. Review of scattering and extinction cross-sections, damping factors, and resonance frequencies of a spherical gas bubble. The Journal of the Acoustical Society of America. 2011;130:3184–3208. doi: 10.1121/1.3628321. [DOI] [PubMed] [Google Scholar]
- 123.Prosperetti A. The thermal behaviour of oscillating gas bubbles. Journal of Fluid Mechanics. 1991;222:587–616. [Google Scholar]
- 124.van der Meer SM, et al. Microbubble spectroscopy of ultrasound contrast agents. Journal of the Acoustical Society of America. 2007 Jan;121:648–656. doi: 10.1121/1.2390673. [DOI] [PubMed] [Google Scholar]
- 125.Katiyar A, Sarkar K. Effects of encapsulation damping on the excitation threshold for subharmonic generation from contrast microbubbles. Journal of the Acoustical Society of America. 2012;132:3576–3585. doi: 10.1121/1.4757099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sijl J, et al. Subharmonic behavior of phospholipid-coated ultrasound contrast agent microbubbles. The Journal of the Acoustical Society of America. 2010;128:3239–3252. doi: 10.1121/1.3493443. [DOI] [PubMed] [Google Scholar]
- 127.Prosperetti A. A general derivation of the subharmonic threshold for non-linear bubble oscillations. The Journal of the Acoustical Society of America. 2013;133:3719–3726. doi: 10.1121/1.4802742. [DOI] [PubMed] [Google Scholar]
- 128.Chang PH, et al. Second-Harmonic Imaging and Harmonic Doppler Measurements with Albunex(R) Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 1995 Nov;42:1020–1027. [Google Scholar]
- 129.Hoff L. Acoustic Characterization of Contrast Agents for Medical Ultrasound Imaging. Norwell: Kluwer Academic; 2001. [Google Scholar]
- 130.Gorce JM, et al. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents - A study of SonoVue (TM) Investigative Radiology. 2000 Nov;35:661–671. doi: 10.1097/00004424-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 131.Tu J, et al. Estimating the shell parameters of SonoVue (R) microbubbles using light scattering. Journal of the Acoustical Society of America. 2009 Dec;126:2954–2962. doi: 10.1121/1.3242346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tu J, et al. Microbubble Sizing and Shell Characterization Using Flow Cytometry. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2011 May;58:955–963. doi: 10.1109/TUFFC.2011.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morgan K, et al. The effect of the phase of transmission on contrast agent echoes. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 1998 Jul;45:872–875. doi: 10.1109/58.710539. [DOI] [PubMed] [Google Scholar]
- 134.Dayton PA, et al. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 1999 Jan;46:220–232. doi: 10.1109/58.741536. [DOI] [PubMed] [Google Scholar]
- 135.de Jong N, et al. Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera. Ultrasound in Medicine and Biology. 2000 Mar;26:487–492. doi: 10.1016/s0301-5629(99)00159-3. [DOI] [PubMed] [Google Scholar]
- 136.Sboros V, et al. Nanointerrogation of ultrasonic contrast agent microbubbles using atomic force microscopy. Ultrasound in medicine & biology. 2006;32:579–585. doi: 10.1016/j.ultrasmedbio.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 137.Kooiman K, et al. Lipid distribution and viscosity of coated microbubbles. Ultrasonics Symposium (IUS), 2010 IEEE; 2010. pp. 900–903. [Google Scholar]
- 138.Hosny NA, et al. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proceedings of the National Academy of Sciences; June 4, 2013; 2013. pp. 9225–9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hughes MS, et al. Broadband time-domain reflectometry measurement of attenuation and phase velocity in highly attenuating suspensions with application to the ultrasound contrast medium Albunex (R) Journal of the Acoustical Society of America. 2000 Aug;108:813–820. doi: 10.1121/1.429614. [DOI] [PubMed] [Google Scholar]
- 140.Grishenkov D, et al. Characterization of Acoustic Properties of Pva-Shelled Ultrasound Contrast Agents: Linear Properties (Part I) Ultrasound in Medicine and Biology. 2009 Jul;35:1127–1138. doi: 10.1016/j.ultrasmedbio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 141.Overvelde M, et al. Nonlinear Shell Behavior of Phospholipid-Coated Microbubbles. Ultrasound in Medicine and Biology. 2010 Dec;36:2080–2092. doi: 10.1016/j.ultrasmedbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 142.Brennen CE. Cavitation and Bubble Dynamics. Oxford University Press; 1995. [Google Scholar]
- 143.Eisenbrey JR, et al. Effect of molecular weight and end capping on poly(lactic-co-glycolic acid) ultrasound contrast agents. Polymer Engineering and Science. 2008 Sep;48:1785–1792. [Google Scholar]
- 144.El-Sherif DM, Wheatley MA. Development of a novel method for synthesis of a polymeric ultrasound contrast agent. Journal of Biomedical Materials Research Part A. 2003 Aug 1;66A:347–355. doi: 10.1002/jbm.a.10586. [DOI] [PubMed] [Google Scholar]
- 145.Forsberg F, et al. Effect of shell type on the in vivo backscatter from polymer-encapsulated microbubbles. Ultrasound in Medicine and Biology. 2004 Oct;30:1281–1287. doi: 10.1016/j.ultrasmedbio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 146.Sirsi SR, et al. Formulation of polylactide-co-glycolic acid nanospheres for encapsulation and sustained release of poly(ethylene imine)-poly(ethylene glycol) copolymers complexed to oligonucleotides. Journal of nanobiotechnology. 2009;7:1. doi: 10.1186/1477-3155-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wheatley MA, et al. Comparison of in vitro and in vivo acoustic response of a novel 50:50 PLGA contrast agent. Ultrasonics. 2006 Nov;44:360–367. doi: 10.1016/j.ultras.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 148.El-Sherif D. PhD. Drexel University; Philadelphia: 2003. Development of novel PLGA contrast agents for use as ultrasound targeted drug delivery vehicles. [Google Scholar]
- 149.Shi WT, Forsberg F. Ultrasonic characterization of the nonlinear properties of contrast microbubbles. Ultrasound in Medicine and Biology. 2000 Jan;26:93–104. doi: 10.1016/s0301-5629(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 150.Preston AT, et al. A reduced-order model of diffusive effects on the dynamics of bubbles. Physics of Fluids. 2007 Dec;19 [Google Scholar]
- 151.Nigmatulin RI, et al. Dynamics, Heat and Mass-Transfer of Vapor-Gas Bubbles in a Liquid. International Journal of Heat and Mass Transfer. 1981;24:1033–1044. [Google Scholar]
- 152.Shankar PM, et al. Advantages of subharmonic over second harmonic backscatter for contrast-to-tissue echo enhancement. Ultrasound in Medicine and Biology. 1998 Mar;24:395–399. doi: 10.1016/s0301-5629(97)00262-7. [DOI] [PubMed] [Google Scholar]
- 153.Forsberg F, et al. Subharmonic imaging of contrast agents. Ultrasonics. 2000 Mar;38:93–98. doi: 10.1016/s0041-624x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 154.Bhagavatheeshwaran G, et al. Subharmonic signal generation from contrast agents in simulated neovessels. Ultrasound in Medicine and Biology. 2004 Feb;30:199–203. doi: 10.1016/j.ultrasmedbio.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 155.Krishna PD, et al. Subharmonic generation from ultrasonic contrast agents. Physics in Medicine and Biology. 1999 Mar;44:681–694. doi: 10.1088/0031-9155/44/3/004. [DOI] [PubMed] [Google Scholar]
- 156.Shankar PM, et al. Subharmonic backscattering from ultrasound contrast agents. Journal of the Acoustical Society of America. 1999 Oct;106:2104–2110. doi: 10.1121/1.428142. [DOI] [PubMed] [Google Scholar]
- 157.Shi WT, et al. Subharmonic imaging with microbubble contrast agents: Initial results. Ultrasonic Imaging. 1999 Apr;21:79–94. doi: 10.1177/016173469902100201. [DOI] [PubMed] [Google Scholar]
- 158.Faez T, et al. Characterizing the Subharmonic Response of Phospholipid-Coated Microbubbles for Carotid Imaging. Ultrasound in Medicine and Biology. 2011 Jun;37:958–970. doi: 10.1016/j.ultrasmedbio.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 159.Goertz DE, et al. Subharmonic contrast intravascular ultrasound for vasa vasorum imaging. Ultrasound in Medicine and Biology. 2007 Dec;33:1859–1872. doi: 10.1016/j.ultrasmedbio.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 160.Frijlink ME, et al. Intravascular ultrasound tissue harmonic imaging: A simulation study. Ultrasonics. 2006 Dec 22;44:E185–E188. doi: 10.1016/j.ultras.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 161.Goertz DE, et al. Contrast harmonic intravascular ultrasound - A feasibility study for vasa vasorum imaging. Investigative Radiology. 2006 Aug;41:631–638. doi: 10.1097/01.rli.0000229773.11715.da. [DOI] [PubMed] [Google Scholar]
- 162.Shekhar H, Doyley MM. Improving the sensitivity of high-frequency subharmonic imaging with coded excitation: A feasibility study. Medical Physics. 2012 Apr;39:2049–2060. doi: 10.1118/1.3694101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi WT, et al. Pressure dependence of subharmonic signals from contrast microbubbles. Ultrasound in Medicine and Biology. 1999 Feb;25:275–283. doi: 10.1016/s0301-5629(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 164.Shi WT, et al. Noninvasive pressure estimation with US microbubble contrast agents. Radiology. 1999 Nov;213P:101–101. [Google Scholar]
- 165.Adam D, et al. On the relationship between encapsulated ultrasound contrast agent and pressure. Ultrasound in Medicine and Biology. 2005 May;31:673–686. doi: 10.1016/j.ultrasmedbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 166.Leodore L, et al. Subharmonic contrast microbubble signals for Noninvasive pressure estimation: an in vitro study. Circulation. 2007 Oct 16;116:646–646. [Google Scholar]
- 167.Leodore L, et al. In Vitro Pressure Estimation Obtained from Subharmonic Contrast Microbubble Signals. IEEE Ultrasonics Symposium; 2007. pp. 2207–2210. [Google Scholar]
- 168.Leodore LM, et al. Implementation of Noninvasive Subharmonic Pressure Estimation on a Commercial Ultrasound Scanner. Circulation. 2008 Oct 28;118:S1039–S1039. [Google Scholar]
- 169.Frinking PJA, et al. Subharmonic scattering of phospholipid-shell microbubbles at low acoustic pressure amplitudes. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2010;57:1762. doi: 10.1109/tuffc.2010.1614. [DOI] [PubMed] [Google Scholar]
- 170.Dave JK, et al. Noninvasive Estimation of Dynamic Pressures In Vitro and In Vivo Using the Subharmonic Response From Microbubbles. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2011 Oct;58:2056–2066. doi: 10.1109/TUFFC.2011.2056. [DOI] [PubMed] [Google Scholar]
- 171.Dave JK, et al. Investigating the Efficacy of Subharmonic Aided Pressure Estimation for Portal Vein Pressures and Portal Hypertension Monitoring. Ultrasound in Medicine and Biology. 2012 Oct;38:1784–1798. doi: 10.1016/j.ultrasmedbio.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dave JK, et al. Subharmonic microbubble emissions for noninvasively tracking right ventricular pressures. American Journal of Physiology-Heart and Circulatory Physiology. 2012 Jul 1;303:H126–H132. doi: 10.1152/ajpheart.00560.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dave JK, et al. Noninvasive LV Pressure Estimation Using Subharmonic Emissions From Microbubbles. Jacc-Cardiovascular Imaging. 2012 Jan;5:87–92. doi: 10.1016/j.jcmg.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Halldorsdottir VG, et al. Subharmonic Contrast Microbubble Signals for Noninvasive Pressure Estimation under Static and Dynamic Flow Conditions. Ultrasonic Imaging. 2011 Jul;33:153–164. doi: 10.1177/016173461103300301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katiyar A, et al. Modeling subharmonic response from contrast microbubbles as a function of ambient static pressure. Journal of the Acoustical Society of America. 2011 Apr;129:2325–2335. doi: 10.1121/1.3552884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chetty K, et al. High-speed optical observations and simulation results of SonoVue microbubbles at low-pressure insonation. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2008;55:1333–1342. doi: 10.1109/TUFFC.2008.796. [DOI] [PubMed] [Google Scholar]
- 177.Chin CT, et al. Brandaris 128: A digital 25 million frames per second camera with 128 highly sensitive frames. Review of Scientific Instruments. 2003 Dec;74:5026–5034. [Google Scholar]
- 178.Sun Y, et al. High-frequency dynamics of ultrasound contrast agents. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2005 Nov;52:1981–1991. doi: 10.1109/tuffc.2005.1561667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Caskey CF, et al. Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall. Journal of the Acoustical Society of America. 2007 Aug;122:1191–1200. doi: 10.1121/1.2747204. [DOI] [PubMed] [Google Scholar]
- 180.Sun Y, et al. Observation of contrast agent response to chirp insonation with a simultaneous optical-acoustical system. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2006 Jun;53:1130–1137. doi: 10.1109/tuffc.2006.1642511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guan JF, Matula TJ. Using light scattering to measure the response of individual ultrasound contrast microbubbles subjected to pulsed ultrasound in vitro. Journal of the Acoustical Society of America. 2004 Nov;116:2832–2842. doi: 10.1121/1.1795334. [DOI] [PubMed] [Google Scholar]
- 182.Renaud G, et al. An “acoustical camera” for in vitro characterization of contrast agent microbubble vibrations. Applied Physics Letters. 2012 Mar 5;100 [Google Scholar]
- 183.Postema M, et al. Ultrasound-induced encapsulated microbubble phenomena. Ultrasound in medicine & biology. 2004;30:827–840. doi: 10.1016/j.ultrasmedbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 184.Hsu MJ, et al. Characterization of individual ultrasound microbubble dynamics with a light-scattering system. Journal of Biomedical Optics. 2011 Jun;16 doi: 10.1117/1.3583575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Jong N, et al. “Compression-only” behavior of phospholipid-coated contrast bubbles. Ultrasound in Medicine and Biology. 2007 Apr;33:653–656. doi: 10.1016/j.ultrasmedbio.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 186.Vos HJ, et al. Orthogonal Observations of Vibrating Microbubbles. Ultrasonics Symposium, 2007. IEEE; 2007. pp. 765–768. [Google Scholar]
- 187.Emmer M, et al. The onset of microbubble vibration. Ultrasound in Medicine and Biology. 2007 Jun;33:941–949. doi: 10.1016/j.ultrasmedbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 188.Dollet B, et al. Nonspherical Oscillations of Ultrasound Contrast Agent Microbubbles. Ultrasound in medicine & biology. 2008;34:1465–1473. doi: 10.1016/j.ultrasmedbio.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 189.de Jong N, et al. Ultrasonic characterization of ultrasound contrast agents. Medical & Biological Engineering & Computing. 2009 Aug;47:861–873. doi: 10.1007/s11517-009-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vos HJ, et al. Nonspherical vibrations of microbubbles in contact with a wall - A pilot study at low mechanical index. Ultrasound in Medicine and Biology. 2008 Apr;34:685–688. doi: 10.1016/j.ultrasmedbio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 191.Zhao SK, et al. Asymmetric oscillation of adherent targeted ultrasound contrast agents. Applied Physics Letters. 2005 Sep 26;87 doi: 10.1063/1.2061872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Versluis M. Nonlinear behavior of ultrasound contrast agent microbubbles and why shell buckling matters. [Google Scholar]
- 193.Sijl J, et al. Acoustic characterization of single ultrasound contrast agent microbubbles. Journal of the Acoustical Society of America. 2008 Dec;124:4091–4097. doi: 10.1121/1.2997437. [DOI] [PubMed] [Google Scholar]
- 194.Sboros V, et al. Absolute measurement of ultrasonic backscatter from single microbubbles. Ultrasound in medicine & biology. 2005;31:1063–1072. doi: 10.1016/j.ultrasmedbio.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 195.Sboros V, et al. Acoustic Rayleigh scattering at individual micron-sized bubbles. Applied Physics Letters. 2007 Mar 19;90 [Google Scholar]
- 196.Klibanov AL, et al. Detection of Individual Microbubbles of Ultrasound Contrast Agents: Imaging of Free-Floating and Targeted Bubbles. Investigative Radiology. 2004;39:187–195. doi: 10.1097/01.rli.0000115926.96796.75. [DOI] [PubMed] [Google Scholar]
- 197.Klibanov AL, et al. Detection of Individual Microbubbles of an Ultrasound Contrast Agent: Fundamental and Pulse Inversion Imaging. Acad Radiol. 2002;9:S279–S281. doi: 10.1016/s1076-6332(03)80203-9. [DOI] [PubMed] [Google Scholar]
- 198.Sboros V. A review of single microbubble acoustics. 2010. pp. 710–714. [Google Scholar]
- 199.Thomas DH, et al. Acoustic detection of microbubble resonance. Applied Physics Letters. 2009;94:243902–3. [Google Scholar]
- 200.Sijl J, et al. Combined optical and acoustical detection of single microbubble dynamics. The Journal of the Acoustical Society of America. 2011;130:3271–3281. doi: 10.1121/1.3626155. [DOI] [PubMed] [Google Scholar]
- 201.Thomas DH, et al. Single Microbubble Response Using Pulse Sequences: Initial Results. Ultrasound in medicine & biology. 2009;35:112–119. doi: 10.1016/j.ultrasmedbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 202.Guidi F, et al. Microbubble characterization through acoustically induced deflation. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2010;57:193–202. doi: 10.1109/TUFFC.2010.1398. [DOI] [PubMed] [Google Scholar]
- 203.Chitnis PV, et al. Influence of Shell Properties on High-Frequency Ultrasound Imaging and Drug Delivery Using Polymer-Shelled Microbubbles. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2013 Jan;60:53–64. doi: 10.1109/TUFFC.2013.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chitnis PV, et al. Rupture threshold characterization of polymer-shelled ultrasound contrast agents subjected to static overpressure. Journal of Applied Physics. 2011 Apr 15;109 doi: 10.1063/1.3565062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ketterling JA, et al. Excitation of polymer-shelled contrast agents with high-frequency ultrasound. Journal of the Acoustical Society of America. 2007 Jan;121:El48–El53. doi: 10.1121/1.2401270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gong Y. 3529049 PhD. Boston University; United States -- Massachusetts: 2013. Acoustic characterization of ultrasound contrast agents with lipid-coated monodisperse microbubble. [Google Scholar]
- 207.Pancholi KP, et al. Novel methods for preparing phospholipid coated microbubbles. European Biophysics Journal with Biophysics Letters. 2008 Apr;37:515–520. doi: 10.1007/s00249-007-0211-x. [DOI] [PubMed] [Google Scholar]
- 208.Stride E, Edirisinghe M. Novel preparation techniques for controlling microbubble uniformity: a comparison. Medical & Biological Engineering & Computing. 2009 Aug 01;47:883–892. doi: 10.1007/s11517-009-0490-8. [DOI] [PubMed] [Google Scholar]
- 209.Talu E, et al. Maintaining Monodispersity in a Microbubble Population Formed by Flow-Focusing. Langmuir. 2008 Mar 01;24:1745–1749. doi: 10.1021/la703065v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hettiarachchi K, et al. Formulation of Monodisperse Contrast Agents in Microfluidic Systems for Ultrasonic Imaging. Microtechnologies in Medicine and Biology, 2006 International Conference on; 2006. pp. 230–232. [Google Scholar]
- 211.Gong Y, et al. Relationship between size and frequency dependent attenuation of monodisperse populations of lipid coated microbubbles. Bubble Science, Engineering & Technology. 2010;2:41–47. [Google Scholar]
- 212.Gong Y, et al. Pressure-dependent resonance frequency for lipid-coated microbubbles at low acoustic pressures. Ultrasonics Symposium (IUS), 2010 IEEE; 2010. pp. 1932–1935. [Google Scholar]
- 213.Demos SM, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. Journal of the American College of Cardiology. 1999 Mar 1;33:867–875. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- 214.Buchanan KD, et al. Echogenic liposome compositions for increased retention of ultrasound reflectivity at physiologic temperature. Journal of Pharmaceutical Sciences. 2008 Jun;97:2242–2249. doi: 10.1002/jps.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Coussios CC, et al. In vitro characterization of liposomes and Optison (R) by acoustic scattering at 3.5 MHz. Ultrasound in Medicine and Biology. 2004 Feb;30:181–190. doi: 10.1016/j.ultrasmedbio.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lu SC, et al. Echogenic Liposomes in High-Frequency Ultrasound Imaging. Ultrasonics Symposium 2007. IEEE. 2007:2203–2206. [Google Scholar]
- 217.Smith DAB, et al. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound in Medicine and Biology. 2007 May;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 218.Radhakrishnan K, et al. Stability of Echogenic Liposomes as a Blood Pool Ultrasound Contrast Agent in a Physiologic Flow Phantom. Ultrasound in Medicine and Biology. 2012 Nov;38:1970–1981. doi: 10.1016/j.ultrasmedbio.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Haworth KJ, et al. Passive imaging with pulsed ultrasound insonations. The Journal of the Acoustical Society of America. 2012;132:544–553. doi: 10.1121/1.4728230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sax N, Kodama T. Optimization of Acoustic Liposomes for Improved In Vitro and In Vivo Stability. Pharmaceutical Research. 2013 Jan;30:218–224. doi: 10.1007/s11095-012-0864-8. [DOI] [PubMed] [Google Scholar]
- 221.Laing ST, McPherson DD. Cardiovascular therapeutic uses of targeted ultrasound contrast agents. Cardiovascular Research. 2009 Sep 1;83:626–635. doi: 10.1093/cvr/cvp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Buchanan KD, et al. Encapsulation of NF-kappa B decoy oligonucleotides within echogenic liposomes and ultrasound-triggered release. Journal of Controlled Release. 2010 Jan 25;141:193–198. doi: 10.1016/j.jconrel.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shaw GJ, et al. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thrombosis Research. 2009 Jul;124:306–310. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Herbst SM, et al. Delivery of Stem Cells to Porcine Arterial Wall with Echogenic Liposomes Conjugated to Antibodies against CD34 and Intercellular Adhesion Molecule-1. Molecular Pharmaceutics. 2010 Jan-Feb;7:3–11. doi: 10.1021/mp900116r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hamilton A, et al. A Physiologic flow chamber model to define intravascular ultrasound enhancement of fibrin using echogenic liposomes. Investigative Radiology. 2002 Apr;37:215–221. doi: 10.1097/00004424-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 226.Demos SM, et al. In vitro targeting of antibody-conjugated echogenic liposomes for site-specific ultrasonic image enhancement. Journal of Pharmaceutical Sciences. 1997 Feb;86:167–171. doi: 10.1021/js9603515. [DOI] [PubMed] [Google Scholar]
- 227.Kim H, et al. In Vivo Volumetric Intravascular Ultrasound Visualization of Early/Inflammatory Arterial Atheroma Using Targeted Echogenic Immunoliposomes. Investigative Radiology. 2010;45:685–691. doi: 10.1097/RLI.0b013e3181ee5bdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hamilton A, et al. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes - Studies in a new experimental model. Circulation. 2002 Jun 11;105:2772–2778. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- 229.Tiukinhoy SD, et al. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Investigative Radiology. 2000 Dec;35:732–738. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 230.Tiukinhoy SD, et al. Novel echogenic drug-immunoliposomes for drug delivery. Investigative Radiology. 2004 Feb;39:104–110. doi: 10.1097/01.rli.0000111207.92580.44. [DOI] [PubMed] [Google Scholar]
- 231.Huang SL, et al. Multi-functional echogenic liposomes for image-guided and ultrasound-controlled PPAR agonist delivery. Journal of the American College of Cardiology. 2007 Mar 6;49:365a–365a. [Google Scholar]
- 232.Laing ST, et al. Ultrasound-Enhanced Thrombolytic Effect of Tissue Plasminogen Activator-Loaded Echogenic Liposomes in an In Vivo Rabbit Aorta Thrombus Model-Brief Report. Arteriosclerosis Thrombosis and Vascular Biology. 2011 Jun;31:1357–1359. doi: 10.1161/ATVBAHA.111.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tiukinhoy-Laing SD, et al. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. Journal of Drug Targeting. 2007;15:109–114. doi: 10.1080/10611860601140673. [DOI] [PubMed] [Google Scholar]
- 234.Tiukinhoy-Laing SD, et al. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thrombosis Research. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kee P, et al. Synthesis and acoustic characterization of a novel ultrasound controlled drug delivery system based on echogenic liposomes. Journal of the American College of Cardiology. 2007 Mar 6;49:120a–120a. [Google Scholar]
- 236.Moody MR, et al. Bioactive Gas/Drug Co-encapsulation and Release Improve Attenuation of Intimal Hyperplasmia Following Acute Arterial Injury. Circulation. 2008:S573–S573. [Google Scholar]
- 237.Kopechek JA, et al. The impact of bubbles on measurement of drug release from echogenic liposomes. Ultrasonics Sonochemistry. 2013;20:1121–1130. doi: 10.1016/j.ultsonch.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Britton GL, et al. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation. 2010 Oct 19;122:1578–87. doi: 10.1161/CIRCULATIONAHA.109.879338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Huang SL, et al. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. Journal of the American College of Cardiology. 2009 Aug 11;54:652–9. doi: 10.1016/j.jacc.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Huang SL, et al. Nitric oxide loaded echogenic liposomes inhibit intimal hyperplasia in an acute arterial injury model. Circulation. 2007 Oct 16;116:294–294. [Google Scholar]
- 241.Britton G, et al. Nitric Oxide loaded Echogenic Liposomes for Ultrasound controlled Nitric Oxide Delivery and Regulation of Artery Diameter. Stroke. 2009 Apr;40:E119–E120. [Google Scholar]
- 242.Evjen TJ, et al. Distearoylphosphatidylethanolamine-based liposomes for ultrasound-mediated drug delivery. Eur J Pharm Biopharm. 2010 Aug;75:327–33. doi: 10.1016/j.ejpb.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 243.Lin HY, Thomas JL. Factors affecting responsivity of unilamellar liposomes to 20 kHz ultrasound. Langmuir: the ACS journal of surfaces and colloids. 2004 Jul 20;20:6100–6. doi: 10.1021/la049866z. [DOI] [PubMed] [Google Scholar]
- 244.Sarkar N, et al. Matrix metalloproteinase-assisted triggered release of liposomal contents. Bioconjugate Chemistry. 2007;19:57–64. doi: 10.1021/bc070081p. [DOI] [PubMed] [Google Scholar]
- 245.Chandra B, et al. Formulation of photocleavable liposomes and the mechanism of their content release. Org Biomol Chem. 2006;4:1730–1740. doi: 10.1039/b518359f. [DOI] [PubMed] [Google Scholar]
- 246.Kopechek JA, et al. Calibration of the 1-Mhz Sonitron Ultrasound System. Ultrasound in Medicine and Biology. 2010 Oct;36:1762–1766. doi: 10.1016/j.ultrasmedbio.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Laing S, et al. Doppler Ultrasound Enhances the Thrombolytic Activity of Tissue Plasminogen Activator-Loaded Echogenic Liposomes In Vivo. Circulation. 2008 Oct 28;118:S643–S643. [Google Scholar]
- 248.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochimica Et Biophysica Acta-Reviews on Cancer. 2012 Jan;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 249.Pytliak M, et al. Matrix metalloproteinases and their role in oncogenesis: a review. Onkologie. 2012;35:49–53. doi: 10.1159/000336304. [DOI] [PubMed] [Google Scholar]
- 250.Bloomston M, et al. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002 Aug;9:668–74. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 251.Duffy MJ, McCarthy K. Matrix metalloproteinases in cancer: prognostic markers and targets for therapy (review) Int J Oncol. 1998 Jun;12:1343–8. doi: 10.3892/ijo.12.6.1343. [DOI] [PubMed] [Google Scholar]
- 252.Jones CB, et al. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003 Oct 1;59:812–23. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 253.Hobeika MJ, et al. Matrix metalloproteinases in peripheral vascular disease. Journal of Vascular Surgery. 2007;45:849–857. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 254.West KR, Otto S. Reversible covalent chemistry in drug delivery. Curr Drug Discov Technol. 2005 Sep;2:123–60. doi: 10.2174/1570163054866882. [DOI] [PubMed] [Google Scholar]
- 255.Goldenbogen B, et al. Reduction-sensitive liposomes from a multifunctional lipid conjugate and natural phospholipids: reduction and release kinetics and cellular uptake. Langmuir. 2011 Sep 6;27:10820–9. doi: 10.1021/la201160y. [DOI] [PubMed] [Google Scholar]
- 256.Cho H, et al. Redox-sensitive polymeric nanoparticles for drug delivery. Chem Commun (Camb) 2012 May 10; doi: 10.1039/c2cc31463k. [DOI] [PubMed] [Google Scholar]
- 257.Wen H, et al. Engineered redox-responsive PEG detachment mechanism in PEGylated nano-graphene oxide for intracellular drug delivery. Small. 2012 Mar 12;8:760–9. doi: 10.1002/smll.201101613. [DOI] [PubMed] [Google Scholar]
- 258.Stride E, Saffari N. Investigating the significance of multiple scattering in ultrasound contrast agent particle populations. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:2332–2345. doi: 10.1109/tuffc.2005.1563278. [DOI] [PubMed] [Google Scholar]
- 259.Qamar A, et al. Dynamics of micro-bubble sonication inside a phantom vessel. Applied Physics Letters. 2013;102:013702–5. doi: 10.1063/1.4773909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Garbin V, et al. Changes in microbubble dynamics near a boundary revealed by combined optical micromanipulation and high-speed imaging. Applied Physics Letters. 2007;90:114103–3. [Google Scholar]
- 261.Doinikov AA, et al. Acoustic scattering from a contrast agent microbubble near an elastic wall of finite thickness. Phys Med Biol. 2011;56:6951–6967. doi: 10.1088/0031-9155/56/21/012. [DOI] [PubMed] [Google Scholar]
- 262.Loughran J, et al. Modeling non-spherical oscillations and stability of acoustically driven shelled microbubbles. The Journal of the Acoustical Society of America. 2012;131:4349–4357. doi: 10.1121/1.4707479. [DOI] [PubMed] [Google Scholar]
- 263.Pauzin MC, et al. Development of a finite element model of ultrasound contrast agent. 2007. pp. 1989–1992. [Google Scholar]
- 264.Maul TM, et al. Optimization of ultrasound contrast agents with computational models to improve selection of ligands and binding strength. Biotechnol Bioeng. 2010;107:854–864. doi: 10.1002/bit.22857. [DOI] [PMC free article] [PubMed] [Google Scholar]


