Abstract
Background
This study’s primary aim was to examine age-specific associations between GABRA2, rule breaking, problematic alcohol use, and substance abuse symptomatology. The secondary aim was to examine the extent to which rule breaking mediates the GABRA2-substance abuse relationship.
Methods
A sample (n = 518) of primarily male (70.9%) and White (88.8%) adolescents from the Michigan Longitudinal Study was assessed from ages 11 to 18. Age-specific effects of GABRA2 on rule breaking, problematic alcohol use, and substance abuse symptomatology were examined using nested path models. The role of rule breaking as a mediator in the association between GABRA2 and substance abuse outcomes was tested using prospective cross-lagged path models.
Results
GABRA2 is significantly (p < .05) associated with rule breaking in mid- to late-adolescence, but not substance abuse symptomatology across adolescence. GABRA2 effects on problematic alcohol use and substance abuse symptomatology operate largely (45.3% and 71.1%, respectively, p < .05) via rule breaking in mid-adolescence.
Conclusions
GABRA2 represents an early risk factor for an externalizing pathway to the development of problematic alcohol and drug use.
Keywords: GABRA2, rule breaking, substance abuse, adolescence, mediation
Introduction
GABRA2 is a gene encoding the α2 subunit of the γ-aminobutyric acid A receptor (GABAA). Research identifies allelic variations in GABRA2 as being associated with alcohol and drug use disorders in adulthood (e.g., Agrawal et al., 2006; Bauer et al., 2007; Edenberg et al., 2004; Enoch, Schwartz, Albaugh, Virkkunen, & Goldman, 2006). A critical next step is to understand mechanistic pathways in the development of these endpoints. Integral to this issue is understanding the mechanisms associated with susceptibility genes and how risk changes across age. GABRA2 studies are largely cross-sectional and focused on adult samples, without reflecting upon mechanisms of risk (e.g., Agrawal et al., 2006; Edenberg et al., 2004). Studies examining adolescents have not demonstrated consistent effects of GABRA2 on alcohol and drug use and abuse (Dick et al., 2006; Matthews, Hoffman, Zezza, Stiffler, & Hill, 2007; Sakai et al., 2010). By taking a developmental approach, this paper examines age-specific relations between GABRA2 and three distinct risk behaviors (rule breaking, problematic alcohol use, substance abuse symptomatology) prospectively across adolescence, and the role of rule breaking as a mediator in the GABRA2-substance abuse relationship.
Variants in GABRA2 have been found to be associated with adult alcohol dependence (e.g., Bauer, et al., 2007; Edenberg, et al., 2004; Enoch, et al., 2006), and extended to drug and nicotine dependence (Cui, Seneviratne, & Li, 2012; Agrawal, et al., 2006). Yet, efforts to replicate findings among younger samples are equivocal (Dick, et al., 2006; Matthews, et al., 2007; Sakai, et al., 2010). This may be due to the relative effect of genetic influences compared to environmental influences during this age. For example, genes may be less relevant to substance use initiation compared to social contexts such as peers during early adolescence, whereas the amount of genetic influences tend to have a stronger impact on substance use disorders in adulthood (Maes et al., 1999). One study demonstrated that at age 14 genes accounted for 18% of the variance in drinking, compared to nearly 50% at age 18 (Rose, Dick, Viken, Pulkkinen, & Kaprio, 2001). This underscores the importance of prospectively examining developmental differences in the impact of candidate genes on problem behaviors associated with alcohol and drug dependence risk.
Cascade models posit that there is a sequential progression from delinquency in childhood, to later riskier and more problematic behaviors, such as illicit drug use (Dodge et al. 2009). Across childhood and adolescence, delinquency is characterized as rule breaking. During this interval, it reflects normative transgressions to authority figures (e.g., lying, stealing). Research generally finds higher levels of rule breaking behaviors among males compared to females (Lahey et al., 2006). Rule breaking begins during early childhood and declines in adulthood (Shaw, Hyde, & Brennan, 2012), while alcohol use tends to increase throughout adolescence, peaking in early adulthood (Kim-Cohen et al., 2003). Research does not generally support the role of problematic alcohol and drug use in childhood as a predictor of rule breaking in adolescence (Windle, 2000). Accordingly, this suggests that rather than focusing on alcohol and drug dependence, there may be utility in examining risk behaviors preceding use in adolescence. Yet, rule breaking is largely overlooked in genetics research. Using a prospective design, a primary aim of this study was to examine age-specific effects of GABRA2 on rule breaking and substance abuse outcomes.
Exploring genetic pathways to substance abuse may be fruitful since substance abuse often develops gradually and is typically predicted by risk-related behaviors (Dick, et al., 2006). One study demonstrated that impulsivity partially mediated the association between GABRA2 and lifetime alcohol problems in adulthood (Villafuerte, Strumba, Stoltenberg, Zucker, & Burmeister, 2013). There is strong evidence that behavior problems, such as rule breaking, are associated with impulsivity (Burt & Donnellan, 2008). GABRA2 is also associated with conduct disorder (Dick, et al., 2006) reflecting impulsivity and rule breaking behavior; these symptoms in turn, predict rates of later substance dependence (Swendsen et al., 2010). These findings suggest that behaviors associated with impulsivity such as rule breaking may be adolescent manifestations of GABRA2 effects, which may impact the emergence of problematic substance use. Therefore, our secondary aim was to examine rule breaking as a mediator between GABRA2 and problematic alcohol use and substance abuse symptomatology using a cross-lagged mediational path model. We are unaware of studies investigating rule breaking pathways from GABRA2 to substance abuse. It was hypothesized that: 1) those carrying the minor G allele would have higher levels of rule breaking compared to those with the non-risk allele, 2) GABRA2 would have weak direct effects on problematic alcohol use and substance abuse symptomatology, 3) rule breaking would mediate the effects of GABRA2 on problematic alcohol use and substance abuse symptomatology, and 4) problematic alcohol use and substance abuse symptomatology would not predict rule breaking.
Methods
Sample
The sample consisted of adolescents from the Michigan Longitudinal Study (MLS), an ongoing, prospective, multi-wave study (Zucker, Ellis, Fitzgerald, Bingham, & Sander, 1996). The MLS follows a community sample of high-risk families comprised of men convicted of drunk driving who met criteria for an alcohol use disorder (AUD) diagnosis, their son, and their son’s biological mother. A control sample of low-risk families from the same neighborhoods without a substance abuse history was also recruited. Community-identified AUD-diagnosed men and their families were recruited as an intermediate-risk group. Full biological siblings were also included. See Zucker and colleagues (1996) for a full description of the sample.
Procedure
Parents and children completed assessments following initial recruitment (Wave 1, ages 3 to 5) with subsequent assessments occurring every 3 years (e.g., Wave 2, ages 6 to 8). Children were also assessed annually beginning when the child turned 11. For this study, only self-report measures were examined given that adolescents report more problem behaviors compared to collaterals, and the consensus view is that non-reporting is more of a constraint on validity than manufactured reporting (Moffitt, Caspi, Rutter, & Silva, 2001). Families were asked to provide blood or saliva for genotyping. Written informed consent and assent was obtained from the parents and adolescents. The Institutional Review Board at the University of Michigan approved the study.
This sample included 518 children from 304 families. Approximately one third (29.9%) of children came from a single-child family, 186 children (35.9%) had another sibling participate, 144 (27.8%) had two siblings participate, and 33 (6.4%) had three or four siblings participate in the study. Participants came from the following alcohol risk categories: low (37.4%), intermediate (26.2%), and high (36.4%). Due to recruitment strategies (females and non-White families included after the initial wave) the sample was predominantly male (70.9%) and White (88.8%). This is important, as delinquency and substance use rates tend to be higher among males. Participants included in analyses did not differ significantly from those without available genetic or annual data (n = 135) on sex, race, rule breaking, problematic alcohol use, or substance abuse symptomatology at the larger wave-levels (Waves 4 and 5). Accordingly, missing data likely had minimal impact on the results. Given that we were interested in age-related effects, rather than using wave-level data (3-year span), we calculated values reflecting the maximum value of problem behavior specific to a two-year span (e.g., 11–12 years of age) to balance the availability of longitudinal data with age specificity.
Measures
Rule breaking
Rule breaking was assessed using the delinquency subscale of the Youth Self Report (YSR; Achenbach, 1991). Items are rated on a 3-point likert scale (0 = not true to 2 = very true or often true). Sample items included: I destroy things belonging to others, I set fires, and I steal from places other than home. The YSR has been used extensively and had good internal consistency across the larger wave-level assessments (Cronbach’s alpha = 0.88 and 0.87 at Wave 4 and 5, respectively).
Substance use
To assess substance use outcomes more germane to adolescence, rather than including a binary measure of dependence, we examined multiple markers of risk using the Drinking and Drug History form (Zucker, Fitzgerald, & Noll, 1990). Dimensional outcomes increase reliability and power (Kraemer, Noda, & O’Hara, 2004). Problematic alcohol use was assessed with an item reflecting past year maximum alcoholic beverages consumed in 24 hours. Past year substance abuse symptomatology was assessed with the stem, “Have you ever had any of the following things happen because of your [alcohol] [drug] use?” Participants rated problems related to alcohol use (37-items) and a variety of illicit drugs (22-items) such as marijuana, cocaine, and hallucinogens. Sample items included: being absent from school, and experiencing physical or medical problems…because of your alcohol (your drug) use. The internal consistency for problems related to alcohol use and illicit drugs was good (Cronbach’s alpha 0.99 across Waves 4 and 5). The combined number of endorsed drinking- and endorsed drug-related problems was summed to create a substance abuse symptomatology composite, which also had good internal consistency (Cronbach’s alpha = .98 across Waves 4 and 5). This questionnaire has been used extensively in a variety of research and clinical settings (e.g., Buu, Wang, Schroder, Kalaida, Puttler, & Zucker, 2012; Nigg et al., 2006).
Genotyping
Genetic variation in GABRA2 is distinguished by numerous single nucleotide polymorphisms (SNPs) that are in linkage disequilibrium (LD), resulting in two common forms of the GABRA2 gene “Ying-Yan” haplotypes. This means that it largely does not matter which SNP is analyzed as any will tag the haplotypes. For this study three SNPs were selected: rs279826, rs279827 and rs279858. All SNPs were in strong linkage disequilibrium (LD; > 0.77) and in a region previously implicated in impulsivity, alcohol, and drug dependence (e.g., Edenberg, et al., 2004; Enoch, et al., 2006). SNPs rs279826 (intron 4) and rs279858 (exon 5, K132K) were genotyped by Taqman (Villafuerte, et al., 2012). SNP rs279827 was included in the Illumina Addiction biology SNP array designed by Hodgkinson and colleagues (2008) using the Illumina GoldenGate platform (Illumina Inc., San Diego, CA). We included duplicates (78 for the array and 12 for the Taqman assay) and no discrepancies were observed. LD between markers was calculated with Haploview (Barrett, Fry, Maller, & Daly, 2005). Sample demographics by SNP are presented in Table 1. All SNPs were in Hardy-Weinberg equilibrium. Findings focus on SNP rs279826 for simplicity and clarity although results were largely consistent across SNPs. Approximately half of the sample carried the heterozygous genotype (AG, n = 261, 50.39%), while a quarter were homozygous for the major allele (AA, n = 133, 25.68%) and the minor allele (GG, n = 124, 23.94%). Genotype data was dichotomized (0 = AA, 1= G-allele carriers) consistent with previous studies (e.g., Pieruchhi-Lagha et al., 2005) and given that most studies found that the minor (but still common) haplotype increases risk (Bauer et al., 2007; Edenberg et al., 2004; Enoch et al., 2006).
Table 1.
Summary of Sample Demographics by GABRA2 SNPs
| Race n (% within SNP) | Sex n (% within SNP) | |||
|---|---|---|---|---|
| Minority | White | Female | Male | |
| rs279826 (n = 518) | ||||
| AA (n = 133, 25.68%) | 11 (2.12) | 122 (23.55) | 47 (9.07) | 86 (16.60) |
| G-allele carriers (n = 385, 74.32%) | 47 (9.07) | 338 (65.25) | 104 (20.08) | 281 (54.25) |
| rs279827 (n = 445) | ||||
| AA (n = 120, 26.97%) | 14 (3.15) | 106 (23.82) | 40 (8.99) | 80 (17.98) |
| G-allele carriers (n = 325, 73.03%) | 33 (7.42) | 292 (65.62) | 86 (19.33) | 239 (53.71) |
| rs279858 (n = 509) | ||||
| AA (n = 161, 31.63%) | 23 (4.52) | 138 (27.11) | 58 (11.39) | 103 (20.24) |
| G-allele carriers (n = 348, 68.37%) | 30 (5.89) | 318 (62.48) | 92 (18.07) | 256 (50.29) |
Data Analysis
A series of nested models were run in Mplus version 7.1 (Muthén & Muthén, 1998–2010) to examine age-specific effects of GABRA2 on rule breaking, problematic alcohol use, and substance abuse symptomatology. Freely estimated paths between GABRA2 predicting problem behaviors at four different time points (i.e., age 11–12, 13–14, 15–16, and 17–18) were estimated first. Then, a nested model was evaluated to assess if GABRA2 equally predicts problem behaviors at different ages by constraining all paths to be equal. The change in chi-square and the Comparative Fit Index (CFI) between the two models was assessed. Research using simulated data demonstrates that a change in CFI greater than −0.01 represents a significant decrement in model fit (Cheung & Rensvold, 2002). In cases demonstrating a significant change in chi-square and CFI, modification indices were examined to identify which paths should be freed. Once age-specific effects of GABRA2 were identified, two cross-lagged mediation path models including rule breaking and each substance use outcome at two time points were tested. Sex (0 = females, 1 = males) and race1 (0 = non-Whites, 1 = Whites) were included as covariates. CFI, Root Mean Square Error of Approximation (RMSEA), and Tucker-Lewis Index (TLI) were used to determine model fit following recommended cutoff values (Hu & Bentler, 1999).
As noted, the sample included siblings from the same nuclear family. Ignoring nested multilevel data structure can bias results. Therefore, an intercept only model estimated family clustering. Results indicated that a significant amount of variance in outcomes was accounted for by family clustering. Thus, multilevel models were estimated.
A prospective design establishes temporal precedence between the mediator and outcome, which is key for testing mediation (Kraemer, Kiernan, Essex, & Kupfer, 2008). In Mplus it is not possible to control for clustering while using resampling approaches. Therefore, indirect effects when controlling for cluster-effects were compared to bias-corrected bootstrap confidence intervals. Maximum likelihood estimation with robust standard errors was used to accommodate non-normality in substance use outcomes. The full information maximum likelihood estimation was used to handle missing values.
Results
Table 2 presents descriptive statistics for study variables. Rule breaking, problematic alcohol use, and substance abuse symptomatology significantly (p < .001) increased over time, except for the interval between rule breaking behavior at age 15–16 to age 17–18. GABRA2 had few significant associations with other study variables. There was evidence for stability effects and significant associations between rule breaking, problematic alcohol use, and substance abuse symptomatology, except for substance abuse symptomatology at age 11–12.
Table 2.
Means, Standard Deviations, and Correlations for Study Variables
| Correlations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| 1. GABRA2 (0 = AA, 1 = G-allele) | 0.74 | 0.44 | -- | |||||||||||||
| 2. Race (0 = female, 1 = male) | 0.89 | 0.32 | −0.05 | -- | ||||||||||||
| 3. Sex (0 = non-White, 1 = White) | 0.71 | 0.45 | 0.08 | 0.11 | -- | |||||||||||
|
| ||||||||||||||||
| Rule Breaking | ||||||||||||||||
| 4. Age 11–12 | 1.98 | 1.96 | 0.02 | −0.09 | 0.04 | -- | ||||||||||
| 5. Age 13–14 | 2.89 | 2.38 | 0.15 | −0.00 | 0.11 | 0.49 | -- | |||||||||
| 6. Age 15–16 | 3.66 | 2.72 | 0.13 | −0.04 | 0.04 | 0.38 | 0.59 | -- | ||||||||
| 7. Age 16–18 | 3.88 | 2.74 | 0.05 | −0.04 | 0.04 | 0.42 | 0.57 | 0.64 | -- | |||||||
|
| ||||||||||||||||
| Problem Alc Use | ||||||||||||||||
| 8. Age 11–12 | 0.05 | 0.36 | 0.03 | 0.04 | −0.05 | 0.15 | 0.36 | 0.12 | 0.23 | -- | ||||||
| 9. Age 13–14 | 0.65 | 2.16 | 0.00 | 0.06 | 0.02 | 0.28 | 0.37 | 0.24 | 0.32 | 0.41 | -- | |||||
| 10. Age 15–16 | 2.93 | 5.58 | 0.00 | 0.05 | −0.02 | 0.37 | 0.40 | 0.48 | 0.34 | 0.13 | 0.40 | -- | ||||
| 11. Age 17–18 | 6.24 | 7.54 | 0.09 | −0.02 | 0.15 | 0.32 | 0.38 | 0.44 | 0.46 | 0.18 | 0.30 | 0.56 | -- | |||
|
| ||||||||||||||||
| SA Symptom | ||||||||||||||||
| 12. Age 11–12 | 0.01 | 0.14 | −0.01 | 0.03 | 0.06 | 0.11 | 0.16 | 0.14 | 0.13 | −0.01 | 0.20 | 0.09 | 0.07 | -- | ||
| 13. Age 13–14 | 0.27 | 1.07 | −0.02 | 0.07 | −0.04 | 0.22 | 0.37 | 0.15 | 0.27 | 0.47 | 0.70 | 0.31 | 0.32 | 0.02 | -- | |
| 14. Age 15–16 | 1.58 | 4.01 | 0.00 | 0.02 | −0.09 | 0.18 | 0.27 | 0.45 | 0.32 | 0.03 | 0.27 | 0.65 | 0.35 | 0.03 | 0.21 | -- |
| 15. Age 17–18 | 2.72 | 4.23 | 0.04 | −0.01 | 0.11 | 0.19 | 0.32 | 0.44 | 0.51 | −0.04 | 0.19 | 0.42 | 0.65 | −0.05 | 0.23 | 0.37 |
Note. SD = standard deviation; Alc = Alcohol; SA symptom = substance abuse symptomatology; bold values represent significant (p < .05) effects.
Nested Model Analyses
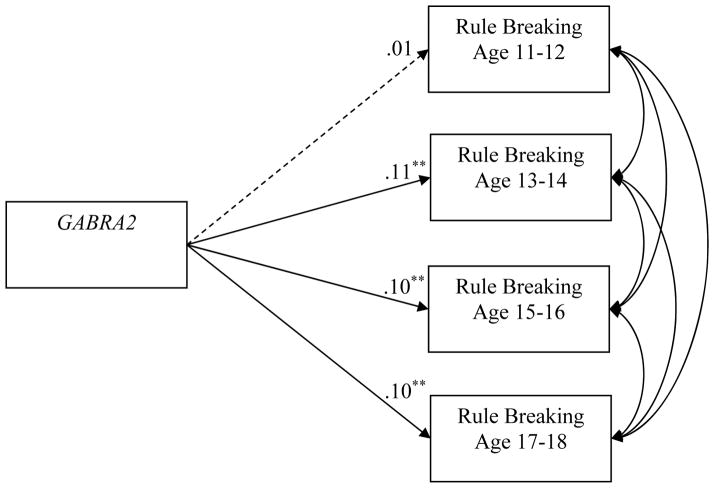
G-allele carriers had high rates (p < .01) of rule breaking at ages 13–14 and 15–16 compared to those with the AA genotype. A scaling correction to better approximate chi-square under non-normality was employed as recommended (Satorra, 2000). Constraining all paths to be equal resulted in a significant change in chi-square (χ2Δ = 8.20 (3), p < .05) and CFI (ΔCFI = −0.02) indicating a decrement in model fit. Modification indices suggested that rule breaking at age 11–12 should be freely estimated. After freeing this path, a significant (p < .01) effect from GABRA2 to rule breaking was observed at ages 13–14, 15–16, and 17–18, but not for rule breaking at age 11–12 (p = .94; see Figure 1). This indicates that G-allele carriers have higher rates of rule breaking compared to those with the AA genotype, this association is not significant in early adolescence, and it is undifferentiated in mid- to late-adolescence. GABRA2 did not predict substance use outcomes across adolescence.
Figure 1.
Nested chi-square model. Note: Path to rule breaking age 11–12 is freely estimated and the other paths are constrained to be equal. Values represent standardized path coefficients. Dashed line represents a non-significant path; ** = p < .01.
Cross-Lagged Mediation Models
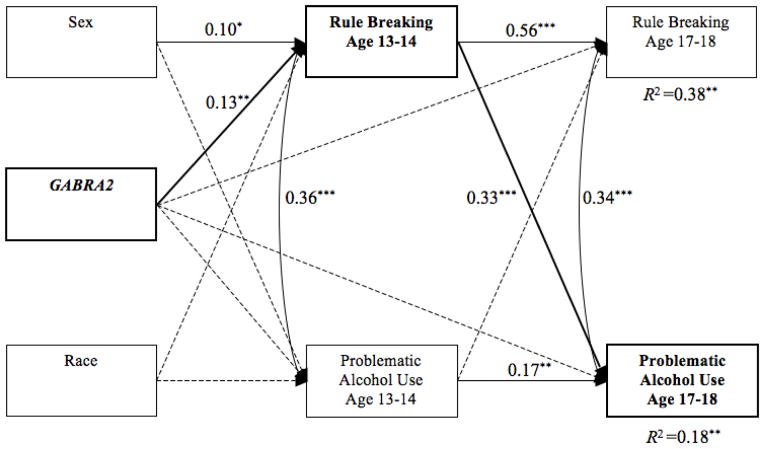
Two separate cross-lagged mediation path models were tested for each outcome. We tested the youngest age (13–14) of rule breaking that was significant in the nested model along with the oldest time period assessed (age 17–18) in order to examine the effect of early indicators of risk on more distal outcomes. The problematic alcohol use model provided a good fit to the data and accounted for 37.8% and 18.1% of the variance in rule breaking and problematic alcohol use at age 17–18, respectively (see Figure 2). Males and G-allele carriers reported more rule breaking at age 13–14. As expected, rule breaking at age 13–14 prospectively predicted problematic alcohol use at age 17–18 above and beyond previous rates of use; however, problematic alcohol use did not predict rule breaking. The indirect effect from GABRA2 to problematic alcohol use was significant (estimate = 0.69, p = .02; 95% bias-corrected bootstrap confidence interval (BCBCI) = 0.22 to 1.37) and 45.3% of the total effect of GABRA2 on problematic alcohol use operated through rule breaking.
Figure 2.
Cross-lagged model for problematic alcohol use. Note. Model fit: RMSEA = .019, CFI = .996, TLI = .985. Values represent standardized path coefficients. Dashed lines represent non-significant paths (* = p < .05, ** = p < .01, *** = p < .001). Bold lines represent a significant mediated path.
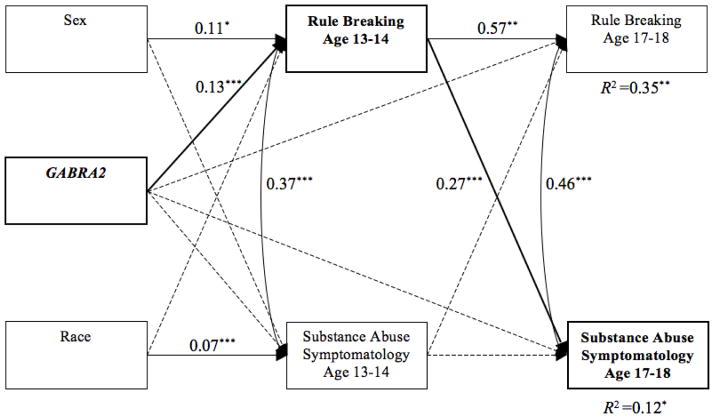
The substance abuse symptomatology model provided a good fit to the data and accounted for 34.7% and 11.7% of the variance in rule breaking and substance abuse symptomatology at age 17–18, respectively (see Figure 3). Males and G-allele carriers reported more rule breaking at age 13–14, and White adolescents reported more substance abuse symptomatology at age 13–14. As expected, rule breaking at age 13–14 prospectively predicted substance abuse symptomatology at age 17–18 above and beyond prior rates of substance abuse, but substance abuse symptomatology did not predict rule breaking. The indirect effect from GABRA2 to substance abuse symptomatology was significant (estimate = 0.34, p = .03; 95% BCBCI = 0.09 to 0.76) and 71.1% of the total effect of GABRA2 on substance abuse symptomatology operated through rule breaking.
Figure 3.
Cross-lagged model for substance abuse symptomatology. Note. Model fit: RMSEA = .022, CFI = .995, TLI = .979. Values represent standardized path coefficients. Dashed lines represent non-significant paths (* = p < .05, ** = p < .01, *** = p < .001). Bold lines represent a significant mediated path.
Discussion
Substance abuse has a complex etiology and considerable individual differences in susceptibility. Advances in genetics research have identified GABRA2 as predicting alcohol and drug dependence in adulthood (e.g., Covault, et al., 2004; Edenberg, et al., 2004; Enoch, et al., 2006); yet, findings are mixed in younger samples (Dick, et al., 2006; Matthews, et al., 2007; Sakai, et al., 2010). This may be due to the relative importance of genetic factors in the etiology of substance dependence across development (Rhee & Waldman, 2002). Weak direct effects of GABRA2 on alcohol and drug dependence in adolescence highlight the need to understand developmental pathways to psychopathology. This study examined age-specific effects of GABRA2 across adolescence on one nonspecific risk behavior and two substance use-specific behaviors in a predominantly White male sample. Rule breaking as a mediator in the relation between GABRA2 and problematic alcohol use and substance abuse symptomatology was also examined. Four study hypotheses were tested: 1) those carrying the minor allele would have higher levels of rule breaking, 2) GABRA2 would have weak direct effects on substance use outcomes, 3) rule breaking would mediate the effects of GABRA2 on substance use outcomes, and 4) substance use would not predict rule breaking. All hypotheses were supported. Namely, G-allele carriers reported higher levels of rule breaking in mid-to late-adolescence, while GABRA2 did not predict substance use outcomes across adolescence. Rule breaking in mid-adolescence mediated the association between GABRA2 on substance use outcomes in late-adolescence. Lastly, substance use did not predict rule breaking.
Age-Specific Effects of GABRA2
GABRA2 had a stronger impact on rule breaking in mid- to late-adolescence versus early adolescence. Namely, G-allele carriers endorsed higher rates of rule breaking compared to those homozygous for the A allele. This is consistent with work demonstrating associations between GABRA2 and problem behaviors (Dick et al., 2006). Animal research demonstrates that GABRA2 receptors are expressed primarily in the amygdala and areas activated from the striatum, such as the substantia nigra (Schwarzer et al., 2001), which is associated with individual differences in impulsivity and reward (Brody, Chen, & Beach, 2013). It is likely that G-allele carriers are more impulsive or find rule breaking more rewarding. It may be that during earlier developmental periods GABRA2 has a more pronounced effect on precursors to behavioral problems such as difficult temperament (e.g., disinhibition; Edenberg et al., 2004).
GABRA2 did not predict problematic alcohol use or substance abuse symptomatology across adolescence. Previous research suggests that genetic effects on substance use outcomes increase as individuals age, with some reporting a peak effect at ages 30 to 33 (Kendler, Gardner, & Dick, 2011). Conversely, the effects of genetic risk in adolescence may be non-specific and impact more general problem behaviors (Moffitt et al., 2001). Genetic risk for problematic alcohol and drug use may become more relevant in early to mid-adulthood during typical onset of abuse and dependence (Kendler, et al., 2011). Effects at these ages can be studied in the MLS when the sample gets older.
Genetic Pathways
Findings demonstrate that the pathway from GABRA2 to substance abuse operates largely via rule breaking. G-allele carriers endorsed greater levels of rule breaking behaviors in mid-adolescence, which in turn predicted problematic alcohol and drug use in late adolescence. This is consistent with work indicating that impulsivity partially mediates the association between GABRA2 and alcohol abuse in adulthood (Villafuerte, et al. 2013). It is likely that G-allele carriers are less likely to “mature out” of rule breaking and engage in increasingly risky behaviors associated with greater rewards such as alcohol and illicit drug use eventually resulting in problematic use. Clinically, detection of early risk factors and pathways to the abuse endpoint provides information regarding who may be especially susceptible and benefit the most from prevention efforts. The externalizing pathway posits a developmental trajectory to substance use disorders that begins with a genetically driven underlying liability for behavioral disinhibition, which emerges as difficult temperament in infancy, followed by rule breaking behavior, and the eventual onset of problematic alcohol and drug use in later adolescence and early adulthood (Zucker, 2006). Findings provide evidence that carrying the GABRA2 G-allele may represent this early genetic risk for an externalizing pathway to the development of alcohol and drug abuse.
Limitations and Future Directions
Although this work provides a greater understanding of genetic pathways to substance abuse, several limitations should be noted. Our study was predominantly comprised of White males. Rates of delinquency and substance abuse tend to be higher among males (Lahey et al., 2006). Accordingly, our findings may not generalize to mixed-sex or primarily female samples. A future direction is to examine whether sex moderates this pathway using moderated mediation models. Rates of problem behaviors also vary across race and ethnicity (Esbensen, 2010). We did not have an adequate number of racial minorities to test these differences. This sample was enriched for alcoholism, limiting generalizability of results. Study variables were based on youth self-report. Although research suggests that adolescent-report of behavior problems is more accurate compared to collaterals (Moffitt, et al., 2001), this may increase shared-method variance.
Nevertheless, this work provides evidence for the utility of a genetically-informed developmental perspective of GABRA2 effects on substance abuse. Understanding the role of GABRA2 as a risk factor for substance use disorders warrants prospective designs that assess behavior more germane to youth and more proximal to genes. Future work should test whether this externalizing pathway predicts nicotine dependence. Investigating additional mediating mechanisms between GABRA2 and substance dependence is another important future direction. For example, research demonstrates that a positive subjective effect of alcohol is likely enhanced in individuals with the GABRA2 variant (Arias et al., 2013). Other work suggests that GABRA2 impacts temperamental factors such as disinhibition (Edenberg et al., 2004). Given that difficult temperament typically precedes rule breaking, temperament is likely to be a mediator of GABRA2 in childhood. Future work involving reward-processing areas as potential mediators, such as differential activation in the striatum and the nucleus acumbens, also seems warranted given prior work (Sieghart & Sperk, 2002).
Key points.
GABRA2 variants indirectly predict the development of alcohol and drug dependence in adulthood.
There is weak support for the role of GABRA2 on alcohol and drug dependence in adolescence.
GABRA2 may represent an early risk factor for an externalizing pathway to the development of alcohol and substance use problems.
Cross-lagged path models support the role of rule breaking as a mediator in the association between GABRA2 and substance abuse outcomes.
GABRA2 is relevant in younger developmental periods, but its impact on substance abuse outcomes operates largely via rule breaking behavior.
Acknowledgments
This research was supported by grants R01 DA17732 (RAZ, MMH, JKZ), R37 AA07065 (RAZ) and T32 07477 (RAZ, KB).
We thank all the families that participated in the Michigan Longitudinal Study and their commitment to the project throughout the years.
Footnotes
Given a largely White sample, analyses were also conducted on White participants only. Findings were comparable and frequency genotypes did not differ by race (χ2 (1) = 1.54, p = .21). Accordingly, results include consideration of the full sample.
Conflict of interest: No conflicts declared.
References
- Achenbach TM. Manual for the Youth Self-Report for ages 11–18. Burlington, VT: University of Vermonth Department of Psychiatry; 1991. [Google Scholar]
- Agrawal A, Edenberg HJ, Foroud T, Beirut LJ, Dunne G, Hinrichs AL, Dick DM. Association of GABRA2 with drug dependence n the Collaborative Study of the Genetics of Alcoholism sample. Behavior Genetics. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- Arias AJ, Covault J, Feinn R, Pond T, Yang B, Ge W, Kranzler HR. A GABRA2 variant is associated with increased stimulation and ‘high’ following alcohol administration. Alcohol and Alcoholism. 2014;49:1–9. doi: 10.1093/alcalc/agt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelerneter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in Project MATCH subjects. Alcoholism: Clinical and Experimental Research. 2007;31:1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y, Beach SR. Differential susceptibility to prevention: GABAergic, dopaminergic, and multilocus effects. Journal of Child Psychology and Psychiatry. 2013;54:863–871. doi: 10.1111/jcpp.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Donnellan MB. Personality correlates of aggressive and non-aggressive antisocial behavior. Personality and Individual Differences. 2008;44:53–63. [Google Scholar]
- Buu A, Wang W, Schroder SA, Kalaida NL, Puttler LI, Zucker RA. Developmental emergence of alcohol use disorder symptoms and their potential as early indicators for progression to alcohol dependence in a high risk sample: A longitudinal study from childhood to early adulthood. Journal of Abnormal Psychology. 2012;121:897–908. doi: 10.1037/a0024926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GW, Rensvold RB. Evaulating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling. 2002;9(2):233–255. [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Cui W, Seneviratne C, Gu J, Li MD. Genetics of GABAergic signaling in nicotine and alcohol dependence. Human Genetics. 2012;131:843–855. doi: 10.1007/s00439-011-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE. A dynamic cascade model of the development of substance-use onset. Monographs of the Society for Research in Child Development. 2009;74:1–134. doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen F. Youth violence: Sex and race differences in offending, victimization, and gang membership. Philadelphia: Temple University Press; 2010. [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Goldman D. Addictions biology: Haplotype-based analysis for 130 candidate genes on a single array. Alcohol and Alcoholism. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine. 2011;41:1507–1516. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenlie diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron and Kenny and MacArthur approaches. Health Psychology. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Noda A, O’Hara R. Categorical versus dimesional approaches to diagnosis: Methodological challenges. Journal of Psychiatric Research. 2004;38:17–25. doi: 10.1016/s0022-3956(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Waldman ID, Rodgers JL, D’Onofrio BM, Pedlow S, Keenan K. Testing descriptive hypotheses regarding sex differences in the development of conduct problems and delinquency. Journal of Abnormal Child Psychology. 2006;34:737–755. doi: 10.1007/s10802-006-9064-5. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodward CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Eaves LJ. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: The Virginia Twin Study of Adoelscent Behavioral Development. Journal of Studies on Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: Within-family association and linkage analyses. Journal of Studies on Alcohol and Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the Dunedin Longitudinal Study. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Muthén B, Muthén L. MPlus Version 6.1. Los Angeles: Muthén & Muthén; 1998–2010. [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illict drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128(3):490–529. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcoholism: Clinical and Experimental Research. 2001;25:1594–1604. [PubMed] [Google Scholar]
- Sakai JT, Stallings MC, Crowley TJ, Gelhorn HL, McQueen MB, Ehringer MA. Test of association between GABRA2 (SNP rs279871) and adolescent conduct/alcohol use disorders utilizing a sample of clinic referred youth with serious substance and conduct problems, controls and available first degree relatives. Drug and Alcohol Dependence. 2010;106:199–203. doi: 10.1016/j.drugalcdep.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A. Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In: Heijmans RDH, Pollock DSG, Satorra A, editors. Innovations in multivariate statistical analysis. A Festschrift for Heinz Neudecker. London: Kluwer Academic Publishers; 2000. pp. 233–247. [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. Journal of Comparative Neurology. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, Brennan LM. Early predictors of boys’ antisocial trajectories. Development and Psychopathology. 2012;24:871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and functioning of GABAA receptor subtypes. Current Topics in Medicinal Chemistry. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, Kessler RC. Mental disorders as risk factors for substance use, abuse and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–1128. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Yau WY, Majczenko K, Zubieta JK, Burmeister M. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Molecular Psychiatry. 2012;17:511–519. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Strumba V, Stoltenberg SF, Zucker RA, Burmeister M. Impulsiveness mediates the association between GABRA2 SNPs and lifetime alcohol problems. Genes, Brain, and Behavior. 2013;12:525–531. doi: 10.1111/gbb.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M. A latent growth curve model of delinquent activity among adolescents. Applied Developmental Science. 2000;4:193–207. [Google Scholar]
- Zucker RA. Alcohol use and the alcohol use disorders: A developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen D, editors. Developmental psychopathology, Vol 3: Risk, disorder, and adaptation. 2. Hoboken, NJ: Wiley; 2006. pp. 620–656. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, Sander K. Other evidence for at least two alcoholisms II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology. 1996;8:831–848. [Google Scholar]
- Zucker RA, Fitzgerald HE, Noll RB. Drinking and drug history. East Lansing: Michigan State University; 1990. Unpublished questionnaire. [Google Scholar]