Summary
Background
Ara h 2 and Ara h 6 are moderately homologous and highly potent peanut allergens.
Objective
To identify IgE-binding linear epitopes of Ara h 6, compare them to those of Ara h 2, and to stratify binding based on clinical histories.
Methods
Thirty highly peanut-allergic subjects were stratified by clinical history. Sera were diluted to contain the same amount of anti-peanut IgE. IgE binding to overlapping 20-mer peptides of Ara h 2 and Ara h 6 was assessed using microarrays.
Results
Each subject had a unique IgE-binding fingerprint to peptides; these data were coalesced into epitope binding. IgE from subjects with a history of more severe reactions (n = 19) had a smaller frequency of binding events (BEs) for both Ara h 2 (52 BEs of 152 (19×8epitopes) possible BEs and Ara h 6 (13 BEs of 133 (19×7 epitopes) possible BEs) compared to IgE from those with milder histories (n = 11) (Ara h 2: 47 BEs of 88 (11×8 epitopes) possible BEs, P < 0.01; Ara h 6: 25 BEs of 77 (11×7 epitopes) possible BEs, P < 0.001). Using an unsupervised hierarchal cluster analysis, subjects with similar histories tended to cluster. We have tentatively identified a high-risk pattern of binding to peptides of Ara h 2 and Ara h 6, predominantly in subjects with a history of more severe reactions (OR = 12.6; 95% CI: 2.0–79.5; P < 0.01).
Conclusions and Clinical Relevance
IgE from patients with more severe clinical histories recognize fewer linear epitopes of Ara h 2 and Ara h 6 than do subjects with milder reactions and bind these epitopes in characteristic patterns. Close examination of IgE binding to epitopes of Ara h 2 and Ara h 6 may have prognostic value.
Keywords: allergens, Ara h 2, Ara h 6, IgE, microarrays, peanut, peptides
Introduction
Peanuts are a major cause of severe IgE-mediated food allergy, which affects approximately 1–2% of the population in the United States [1, 2]. Allergy to peanuts rarely resolves and persists in approximately 80% of peanut-allergic patients [3]. Levels of anti-peanut IgE > 14 kU/mL and skin tests of > 5 mm are associated with a positive oral peanut challenge, while binding of IgE to Ara h 2 is associated with clinical reactivity to peanuts. However, there are no in vitro tests that correlate well with the severity of clinical history or responses to food challenges [4–8].
As of 2014, thirteen peanut allergens (Ara h 1–13) have been officially recognized by the International Union of Immunological Societies, Allergen Nomenclature Sub-Committee (http://www.allergen.org/search.php?allergensource=peanut&searchsource=Search). Of these, two 2S albumins, Ara h 2 and Ara h 6, together account for approximately 10% of the peanut proteome and are recognized by IgE from most peanut-allergic children and adults [9, 10]. Ara h 2 and Ara h 6 are moderately homologous in both their primary and secondary structure (4 and 5 disulphide bonds, respectively), are the most potent peanut allergens, and are substantially redundant in their biologic activity [11–21].
Microarray immunoassays with overlapping peptides that bind IgE have been described for a variety of food allergens. The potential utility of these assays has recently been reviewed [6, 22–36]. Peptide-based microarrays have been used to distinguish truly peanut-allergic subjects from those who are sensitized but not clinically allergic [37].
Patients with a more severe clinical history have been reported to have IgE that recognizes a high number of linear epitopes of Ara h 1, 2, and 3 [29, 38]. In support of these findings, IgE from milk-allergic subjects with a higher grade of anaphylaxis following allergen challenge bound a greater number of milk peptides than did IgE from other milk-allergic subjects [32]. In contrast, other studies have demonstrated that epitope diversity correlates better with levels of food-specific IgE than with clinical history. For example, Ayuso et al. found that young age and higher shrimp-specific IgE, but not clinical history, was associated with more epitope diversity for shrimp allergen peptides [31]. Verada et al. [39] reported that epitope recognition for lentil allergen pep-tides, as well as respiratory symptoms, were associated with IgE levels, but did not find an association between epitope recognition and respiratory symptoms independent of IgE levels. Willumsen and colleagues reported that the complexity of the Der p 2-specific IgE repertoire increases with the concentration of Der p 2-spe-cific IgE [40]. Thus, levels of allergen-specific IgE can be an important factor in interpretation of peptide-based microarray assays of IgE binding.
Given the potent effector activity and structural homology of Ara h 2 and Ara h 6, we have developed a microarray with Ara h 2 and Ara h 6 peptides to examine IgE binding to linear epitopes of these proteins. This assay, in which we normalize each serum to equivalent concentrations of peanut-specific IgE to remove the level of peanut-specific IgE as a confounding variable, demonstrates subtleties regarding IgE binding to linear epitopes of these two related proteins and an unexpected correspondence of these findings to the severity of clinical histories. This approach may have clinical utility in identifying subjects at risk for more severe clinical reactions.
Materials and methods
Subjects and their classification
We enrolled 47 subjects, ages 7–70 years, with a strong history of peanut-induced immediate hypersensitivity. These individuals were referred by local allergists, responded to university-wide emails, volunteered at a booth set-up at The Walk for Food Allergy, or were referred by other subjects. We also enrolled one subject with the hyper-IgE and recurrent infection syndrome (HIE) with a total IgE of 19 736 IU/mL. This serum was diluted 1 : 5, had undetectable anti-peanut IgE, and served as an IgE control. Of these 47 subjects enrolled, we identified 30 peanut-allergic subjects who met the following criteria: (i) physician-diagnosed peanut allergy and (ii) peanut-specific IgE ≥ 14 KAU/L (ImmunoCap, Phadia; Uppsala, Sweden) in serum collected within 6 months of this study, a finding that is consistent with 95% confidence of true peanut allergy [5]. Sera from these 30 subjects were used in these studies. These subjects, who were rigorously avoiding peanuts, had experienced their most recent reactions 5 ± 6 (mean ± SD) years (range, 0.5–10 years) before enrolment. All adult patients and the parents or guardians of minors signed informed consent. Minors who were > 6 years of age signed an assent. The University of Colorado Denver Institutional Review Board approved this study. Patients’ reported symptoms after naturally occurring exposure to peanuts were classified into grades of anaphylaxis according to criteria established by the World Health Organization for evaluation of allergic reactions in the context of allergen-specific immunotherapy: grade I, cutaneous symptoms alone (angioedema, rash, hives); grade II, involvement of two organ systems (e.g. lower respiratory and cutaneous or gastrointestinal and lower respiratory); grade III, involvement of three or more organ systems and/or upper respiratory involvement (upper respiratory angioedema, stridor); grade IV, cardiovascular compromise (shock, decreased pulse, syncope) [41].
Proteins
Native Ara h 2 and Ara h 6 were purified as previously described [20].
Peptides for microarrays
A library of 77 peptides consisting of 20 amino acids (AAs) in length overlapping by 17 AAs (offset of 3) and covering the entire sequence of Ara h 2.01 as well as Ara h 6 were synthesized at the same time on solid support (80–90% purity). These peptides were then cleaved, diluted to 250–500 µM in a DMSO-based buffer system, and immobilized on glass slides (JPT Peptide Technologies, Berlin, Germany) [42]. Random samples (5%) of the peptide library were analysed by liquid chromatography/mass spectrometry or matrix-assisted laser desorption/ionization. The PepStar™ peptide microarray platform (JPT Peptide Technologies), which places a proprietary 403 Dalton linker arm (Ttds-linker) at the C-terminus of each peptide as a spacer between the support and the reactive ligand, was used to generate customized peptide microarrays on glass slides as described by Shreffler [38]. Purified native Ara h 2 and native Ara h 6 were also immobilized within each array on the slides. Empty spots served as negative controls. Approximately 40% of the peptides (variable among the sera) had low IgE binding that was indistinguishable from the empty spots. Peptides found in other isoforms of Ara h 2 and Ara h 6 were examined and had binding patterns that were similar to that seen with peptides derived from the parent isoform (data not shown).
Microarray printing
Triplicate spots were printed on APiX Protein Micro-array Slides coated with a proprietary optically clear nitrocellulose (Intuitive Biosciences, Madison, WI, USA). All slides were stored at 4°C and used within 6 months from the date of manufacture.
Immunoassay
Slides were placed in a SIMplexTM 64-well device (Intuitive Biosciences) and blocked with PBS containing 0.05% Tween 20 (PBST) and 1% BSA. In preliminary experiments, we determined that the assay had a 10- to 1000-fold signal-to-noise ratio when the sera were diluted to give peanut-specific IgE values of 8–12 kU/L (data not shown). In the experiments reported here, sera were diluted in PBST with 0.1% BSA to give a final concentration of peanut-specific IgE of 9 kU/L (22 ng/ mL), applied to each array, and incubated overnight at 4°C. Slides were washed, incubated with biotinylated murine monoclonal anti-human IgE (1 : 10 000) (Invi-trogen, Carlsbad, CA, USA), washed again, and developed with anti-biotin gold conjugate followed by SilverQuant® Reagents A and B according to the manufacturer’s instructions (Intuitive Biosciences). After incubation with buffer or the serum from our HIE patient (diluted 1 : 5), backgrounds were indistinguishable. Duplicate samples of each serum were assayed twice with three replicates within each array.
Analysis of microarray data
Peptides
The slides were scanned in an APiX Scanner (Intuitive Biosciences). Peptide-specific and background signals were determined. Z-scores were calculated as described by Lin and colleagues [43]. Briefly, the signal (S) for each spot containing a peptide was the log2 transformation of the ratio of the median signal of the spot to the median signal of empty spots within the specific microarray. A z-score for each spot was determined using the equation [43]:
The reported z-scores are the average of those from two independent assays carried out on separate days. Each assay included two arrays with triplicate spots of each peptide, duplicate spots of native Ara h 2 and Ara h 6, and 21 blank spots within each array. Binding to an individual peptide was considered to be positive if the z-score was > 3 [43]. A z-score of > 3 indicates that the signal was significantly (P < 0.003) above the background [43]. For comparison, the data were also analysed by calculating the signal minus the mean of the background (empty spots) plus 3 or plus 4 standard deviations of the background data. In addition, the data were normalized based on relative binding to native Ara h 2 or Ara h 6 (for the appropriate peptides) within each array.
Epitopes
An IgE-binding epitope was defined as a region containing the same 6–13 amino acid sequence that was present in 3–5 contiguous peptides for which the mean z-score was > 3. This is similar to the approach described by Ayuso and colleagues [31].
Immunodominance
IgE-binding epitopes recognized by at least 50% of our peanut-allergic subjects were considered immunodominant. This is similar to previously published criteria [26, 44, 45].
Competition experiments
Microarray assay
A pool of four sera (D64, D70, D80, and D103; normalized by anti-peanut IgE content) was pre-incubated with a preparation containing native Ara h 2 and Ara h 6, then added to the microarray assay. These sera were chosen on the basis of having strong signals in the microarray assay. Subjects D64 and D80 reported clinical reactivity of grade II, whereas subjects D70 and D103 reported reactivity of grades IV and III, respectively (Table 1).
Table 1.
IgE values and grade of reaction
| Subject | Total IgE (IU/ml) | Pnt IgE (kU/L) | Rxn Grade |
|---|---|---|---|
| D19 | 136 | 59 | 2 |
| D44 | 1015 | 65.3 | 3 |
| D48 | 138 | 71.1 | 2 |
| D50 | 538 | 37.8 | 3 |
| D53 | 1185 | 74.7 | 3 |
| D59 | 290 | 13.4 | 3 |
| D60 | 454 | 159 | 4 |
| D61 | 161 | 64 | 1 |
| D63 | 3263 | 78.6 | 3 |
| D64 | 2803 | 591 | 2 |
| D65 | 2421 | 180 | 3 |
| D66 | 186 | 77 | 3 |
| D67 | 568 | 137 | 3 |
| D68 | 212 | 25.2 | 4 |
| D69 | 212 | 22.3 | 4 |
| D70* | 1423 | 500 | 2 |
| D70B* | 245 | 52.7 | 4 |
| D71 | 4939 | 88.4 | 2 |
| D72 | 474 | 14.1 | 1 |
| D74 | 194 | 28.9 | 3 |
| D77 | 505 | 48.7 | 3 |
| D78 | 1325 | 62.6 | 2 |
| D80 | 545 | 164 | 2 |
| D81 | 268 | 67.5 | 3 |
| D82 | 265 | 18.9 | 3 |
| D98 | 80.5 | 16.1 | 4 |
| D103 | 1797 | 787 | 3 |
| D105 | 88.2 | 14 | 2 |
| D107 | 199 | 65.3 | 3 |
| D114 | 117 | 22.4 | 1 |
D70 and D70B are not related.
Cell release assay
Rat basophilic leukaemia (RBL-SX38) cells were grown, labelled with tritium-labelled serotonin (3H-5-HT), sensitized, and triggered with Ara h 2 or Ara h 6 as previously described [15]. For the competition experiments, cells were pre-incubated for 10 min with a 10 000–100 000 molar excess (limited by solubility) of purified 10–20-mer peptides containing dominant epitopes (described in the Results section) before addition of Ara h 2 or Ara h 6, and release of 3H-5-HT into the supernatant was measured as previously described [15].
Statistical analyses
In our initial analysis, we examined the raw signal for the binding of IgE to each peptide, the raw signal for the binding of IgE to each peptide normalized by binding to the parent molecule (Ara h 2 or Ara h 6), and binding of IgE to each peptide based on z-scores ranging from −2 to 28. Use of a validated statistical tool, a threshold of z > 3 for a positive signal, allowed us to analyse the data based on binary binding (yes, z > 3; no, z ≤ 3) to individual peptides and binary binding (yes, z > 3; no, z ≤ 3) to epitopes. We did this binary analysis because we hypothesized that the degree of binding of IgE to a peptide (size of the signal) is not as important as the fact that there is statistically significant binding.
After generating the final microarray data, heat maps and unsupervised hierarchical cluster analyses were performed with R-2.14.1 software for Windows (http://cran.r-project.org/bin/windows/base/) to determine whether patients sorted into clusters based on clinical history. GraphPad Prism 5.0c for the Macintosh (GraphPad, La Jolla, CA, USA) was used to generate graphs and for statistical analysis. The following tests were used: Spearman rank-order correlation coefficients for correlations; Mann-Whitney test for comparisons of the number of binding events; and Fisher’s exact test for comparing frequencies of two possible outcomes. All comparisons were two-tailed. Where multiple comparisons were performed, the Bonferroni correction was used and corrected P-values denoted as pc. P-values of < 0.05 were considered to be significant.
Results
Clinical data
Sera from 30 peanut-allergic individuals were assessed. Serologic data and grades based on reported symptoms for each subject are shown in Table 1 with the subjects listed numerically to allow cross-referencing to other studies using some of the same subjects. Reported symptoms and grades of anaphylaxis for each subject are shown in Table S1. Three subjects (10%) were classified as grade I, 8 (27%) as grade II, 14 (47%) as grade III, and 5 (17%) as grade IV. The total IgE, peanut-specific IgE, the ratio of peanut IgE to total IgE, and the intensity of binding of IgE against Ara h 2 and Ara h 6 had no discernible relationship to the severity of the reported clinical reactions to peanuts (data not shown).
Identification of IgE-binding fingerprints to peptides of Ara h 2 and Ara h 6 for each individual
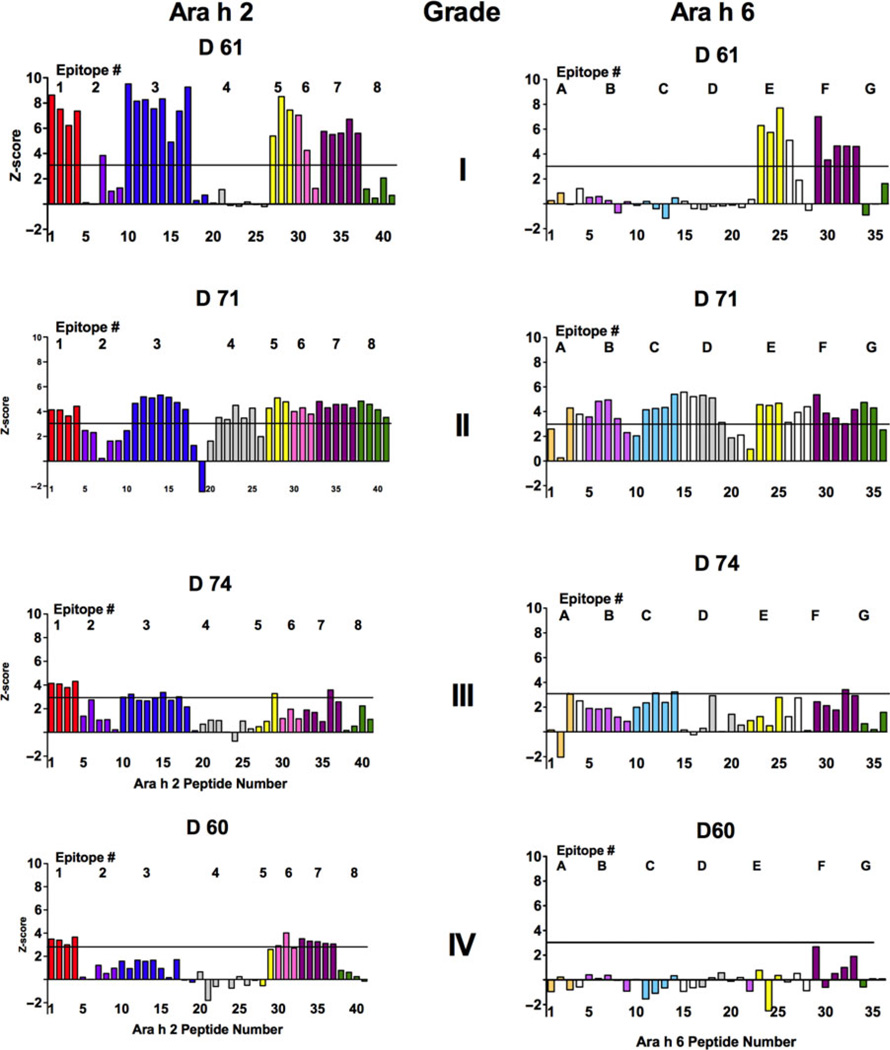
For each of the 30 sera, we determined the intensity of binding to the individual peptides, intact native Ara h 2 and Ara h 6, and blank spots (all on the array). Then, we calculated z-scores. Each subject had a distinctive IgE-binding configuration: a fingerprint for the peptides of Ara h 2 and Ara h 6. Z-score data from 4 subjects, one with each grade of anaphylaxis, are shown in Fig. 1. The threshold for positive binding, a z-score > 3, is shown as a horizontal line on each graph. Colour-coding in this and subsequent figures and tables denotes peptides containing specific epitopes.
Fig. 1.
Microarray analysis of IgE binding to overlapping 20-mer peptides of Ara h 2, left, and Ara h 6, right. Sera are from 4 of 30 representative patients with varying histories regarding the severity of reactivity. From top to bottom: D61, grade I (less severe); D71, grade II; D74 grade III; and D60, grade IV (most severe). Linear peptide regions that bind peanut-specific IgE are colour-coded (see Fig. 2). The Y axis is a calculated z-score (see Methods). The established cut-off score of z = 3 is shown as a horizontal line.
Data for these 4 subjects are again presented in Fig. S1A (Ara h 2) and Fig. S1B (Ara h 6), in which the data are expressed as z-scores (Fig. S1A and S1B; graphs A, E, I, and M), as mean values minus the mean of the background +3 SD of the background (Fig. S1A and S1B; graphs B, F, J, and N), or as mean values minus the mean of the background +4 SD of the background (Fig. S1A and S1B; graphs C, G, K, and 0). Data are also presented after further normalization for binding to native Ara h 2 or native Ara h 6 for the Ara h 2 and Ara h 6 peptides, respectively (Fig. S1A and S1B; graphs D, H, L, and P). Regardless of the method of expressing the data, the patterns are consistent.
Binding values for each of the 30 sera for each of 41 peptides of Ara h 2 and for native Ara h 2 are shown in Tables S2A–F; binding values for 36 peptides of Ara h 6 and for native Ara h 6 are shown in Tables S3A–F. Even after adjusting the sera to contain the same amounts of peanut-specific IgE before each assay (see Materials and methods), there is considerable variability in the maximal binding to peptides with different sera. This variability was not reduced by further adjusting the binding based on that to native Ara h 2 or native Ara h 6 (data not shown).
Hierarchical analyses of IgE binding to peptides
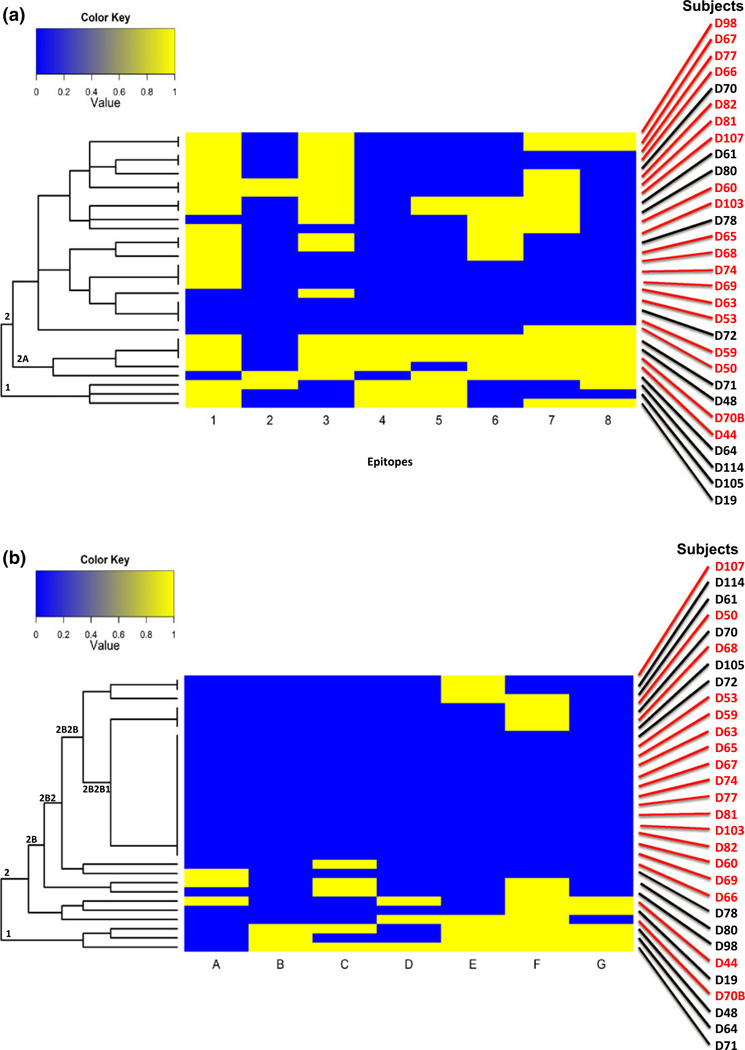
To analyse the binding of IgE to these peptides, we generated group-peptide plots for binding to Ara h 2 (Fig. S2A) and Ara h 6 (Fig. S2B) and performed an unsupervised hierarchal cluster analysis for the 30 subjects using the intensity of IgE binding (z-scores) to each peptide to Ara h 2 (Fig. S3A) and Ara h 6 (Fig. S3B). Patients with relatively severe clinical histories (shown in red numbers) did not significantly cluster into specific groups although, at the bottom of the heat map for Ara h 6, there appeared to be clustering of data from subjects with more severe clinical histories (Fig. S3B).
Analysis based on a binary (yes or no) binding to peptides
We therefore re-examined our data based on positive or negative binding (binary; yes, z > 3 or no, z < 3) to the peptides of Ara h 2 (Fig. S4A) and Ara h 6 (Fig. S4B). We also performed unsupervised cluster analyses for Ara h 2 (Fig. S5A) and Ara h 6 (Fig. S5B). Using this dichotomous approach for IgE binding to peptides, there was a suggestion that subjects clustered into patterns, but we did not find any statistically significant relationships.
Ara h 2 and Ara h 6 share homologous linear epitopes
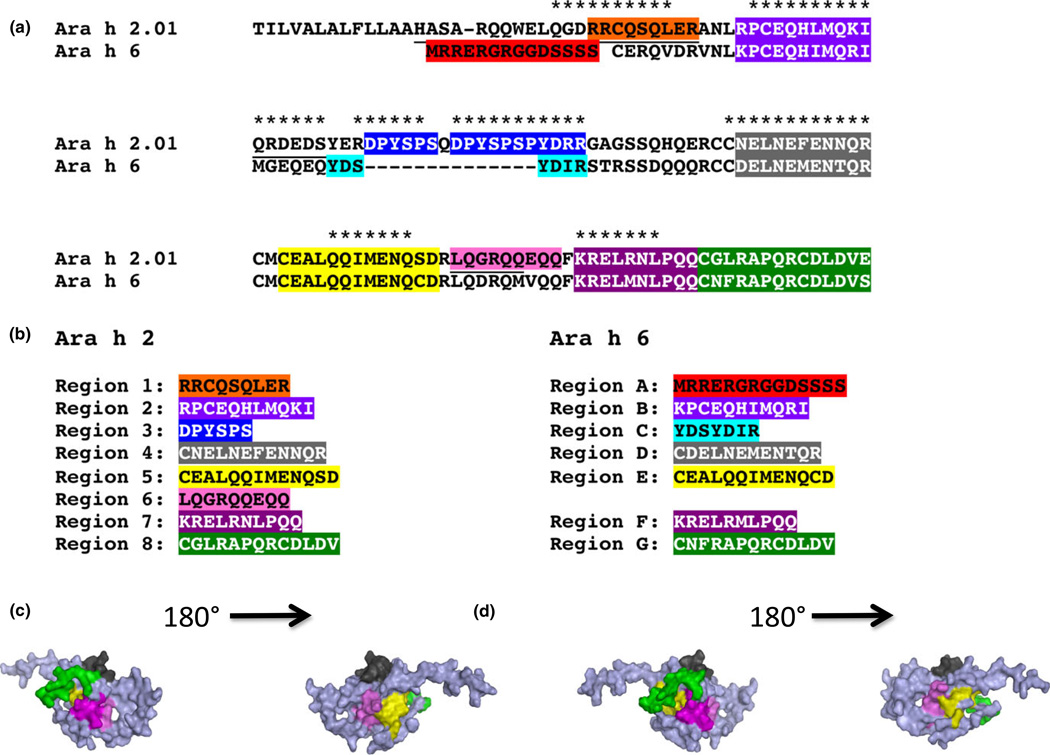
As stated in Materials and Methods, an IgE-binding epi-tope was defined as a region containing the same 6–13 amino acid sequence that was present in 3–5 contiguous peptides for which the mean z-score was > 3. For example, in Fig. 1, the binding pattern for serum D61 for Ara h 2 shows that there is binding to epitopes 1, 3, 5, 6, and 7 (see Fig. 2). These were determined to be epitopes because the average z-score for binding to peptides that contain a specific epitope (noted by different colours) was > 3. Overall, when all 30 sera were examined, eight IgE-binding epitopes were identified for Ara h 2 (Fig. 2a), which is consistent with published data [38, 46]. These epitopes of Ara h 2 are notated here as epitopes 1–8 (Fig. 2b). Seven novel IgE-binding epitopes were identified for Ara h 6 (Fig. 2a); these are notated as epitopes A-G (Fig. 2b). Three-dimensional images of these epitopes in the context of the intact molecule are shown in Fig. 2c and Fig. 2d for Ara h 2 and Ara h 6, respectively.
Fig. 2.
Amino acid alignment is shown for Ara h 2 and Ara h 6 (a). Different colours denote epitopes for Ara h 2 and Ara h 6; these are listed (b). The asterisks (*) in A, denote linear sequences for Ara h 2 that have been previously identified [38, 46]. A colour-coded 3-D image is shown (c).
As shown in Fig. 2a, two epitopes of Ara h 6 (A and C) are unique in that they have no significant homol-ogy to Ara h 2, whereas 5 epitopes (B, D, E, F, and G) are highly homologous (70–93%) to epitopes of Ara h 2 (2, 4, 5, 7, and 8, respectively). Epitope 6 in Ara h 2 shares homology to a corresponding sequence in Ara h 6 (67%). However, given a lack of significant IgE binding (mean z-score < 3), this region in Ara h 6 was not identified as an IgE-binding epitope.
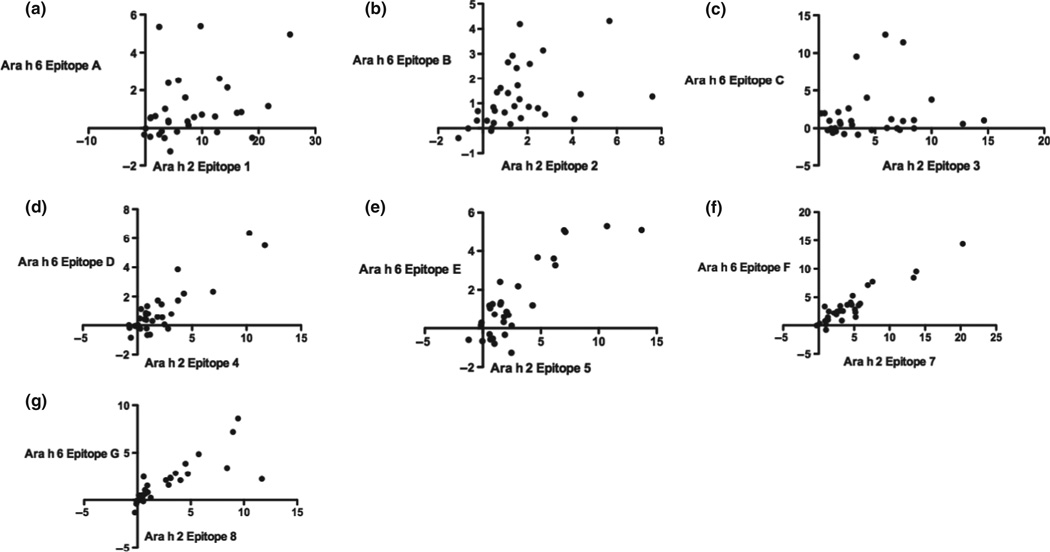
Spearman rank correlation coefficients of IgE binding revealed a modest correlation for binding to epitope 1 of Ara h 2 and epitope A of Ara h 6 (Fig. 3a; homology = 0%; r = 0.37, P = 0.04, pc = ns); a strong correlation for binding to epitope 2 of Ara h 2 and epitope B of Ara h 6 (Fig. 3b; homology = 81%; r = 0.55, pc = 0.01); no significant correlation for binding to epitopes 3 of Ara h 2 and epitope C of Ara h 6 (Fig. 3c; homology = 0%; r = 0.21, pc = ns); and strong correlations for binding to epitope 4 of Ara h 2 and epitope D of Ara h 6 (Fig. 3d; homology = 70%; r = 0.64, pc < 0.01), epitope 5 of Ara h 2 and epitope E of Ara h 6 (Fig. 3e; homology = 92%; r = 0.65, pc < 0.01), epitope 7 of Ara h 2 and epitope F of Ara h 6 (Fig. 3f; homology = 83%; r = 0.82, pc < 0.001), and epitope 8 of Ara h 2 and epitope G of Ara h 6 (Fig. 3g; homology = 85%; r = 0.90, pc < 0.001).
Fig. 3.
Correlation of IgE binding to linear epitopes. Spearman correlation analysis was performed with z-scores for each homologous pair of epitopes of Ara h 2 and Ara h 6 (horizontal and vertical axes, respectively): epitope 1 of Ara h 2 and epitope A of Ara h 6 (a, r = 0.37, P = 0.04, pc = ns); epitope 2 of Ara h 2 and epitope B of Ara h 6 (b, r = 0.55, pc = 0.01); epitope 3 of Ara h 2 and epitope C of Ara h 6 (c, r = 0.21, pc = ns); epitope 4 of Ara h 2 and epitope D of Ara h 6 (d, r = 0.64, pc < 0.01); epitope 5 of Ara h 2 and epitope E of Ara h 6 (e, r = 0.65, pc < 0.01); epitope 7 of Ara h 2 and epitope F of Ara h 6 (f, r = 0.82, pc < 0.001); and epitope 8 of Ara h 2 and epitope G of Ara h 6 (g, r = 0.90, pc < 0.001).
IgE binds to more linear epitopes of Ara h 2 than to those of Ara h 6
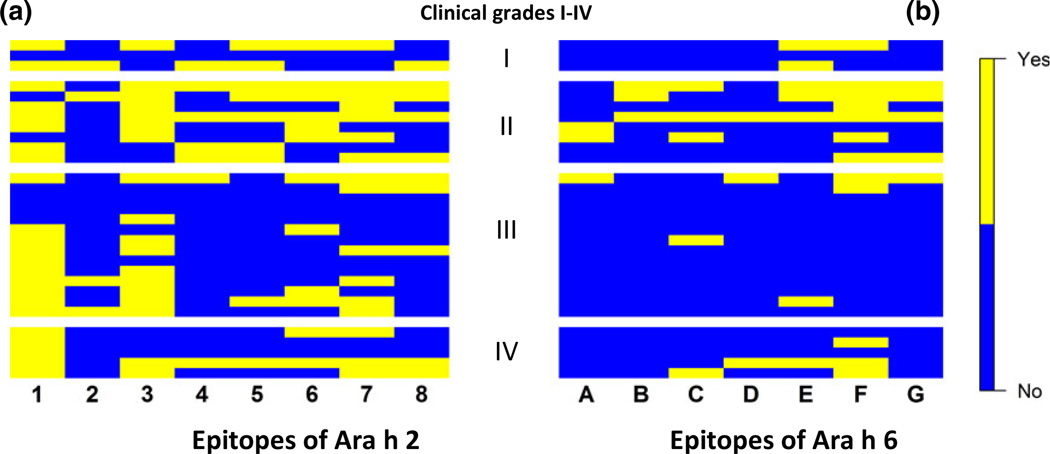
Linear epitopes are not able to cross-link IgE-FcεRI complexes [47, 48] and yet may be part of larger conformational complexes. We reasoned that the presence or absence of binding (yes or no) beyond a set threshold (z-score > 3) would be more informative than the relative intensities of binding to specific epitopes. Accordingly, we next examined our data based on positive or negative binding to linear epitopes rather than binding to peptides. We then generated group-epitope heat maps for Ara h 2 (Fig. 4a) and for Ara h 6 (Fig. 4b). Viewing the binding of IgE to linear epitopes in this way, we found that, regardless of clinical history, subjects’ IgE had more frequent positive binding (z > 3) to linear epitopes of Ara h 2 (Table 2A) [median = 3 epitopes/serum (E/S)] than to the linear epitopes of Ara h 6 (Table 2B) (median = 1 E/S; P < 0.001). In Tables 2A and B, a ‘yes’ or ‘Y’ corresponds to a yellow box; the absence of ‘Y’ denotes lack of significant binding and corresponds to a blue box in Figs 4 and 5.
Fig. 4.
Heat maps of binary (yes or no; z ≤ 3/z > 3) binding of IgE to epitopes for Ara h 2 (a) and Ara h 6 (b) for all 30 sera sorted according to grades of reaction based on clinical histories, ‘yes’ is denoted by yellow and ‘no’ is denoted by blue.
Table 2.
A Binary binding of IgE to epitopes of (A) Ara h 2 (B) Ara h 6
| Epitope |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom | Serum | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| (A) | |||||||||
| Grade I | D61 | Y | Y | Y | Y | Y | |||
| D72 | |||||||||
| D114 | Y | Y | Y | Y | Y | ||||
| Grade II | D48 | Y | Y | Y | Y | Y | Y | Y | |
| D64 | Y | Y | Y | Y | Y | Y | |||
| D70 | Y | Y | Y | ||||||
| D71 | Y | Y | Y | Y | Y | Y | Y | ||
| D78 | Y | Y | Y | ||||||
| D80 | Y | Y | Y | ||||||
| D105 | Y | Y | Y | ||||||
| D19 | Y | Y | Y | Y | Y | ||||
| Grade III | D44 | Y | Y | Y | Y | Y | Y | ||
| D50 | Y | Y | |||||||
| D53 | |||||||||
| D59 | |||||||||
| D63 | Y | ||||||||
| D65 | Y | Y | |||||||
| D66 | Y | Y | |||||||
| D67 | Y | Y | Y | Y | |||||
| D74 | Y | ||||||||
| D77 | Y | Y | |||||||
| D81 | Y | Y | Y | Y | |||||
| D103 | Y | Y | Y | ||||||
| D107 | Y | Y | Y | Y | Y | ||||
| D82 | Y | Y | Y | Y | |||||
| Grade IV | D60 | Y | Y | Y | |||||
| D68 | Y | ||||||||
| D69 | Y | ||||||||
| D70B | Y | Y | Y | Y | Y | Y | Y | ||
| D98 | Y | Y | Y | Y | |||||
| Epitope |
||||||||
|---|---|---|---|---|---|---|---|---|
| Symptom | Serum | A | B | C | D | E | F | G |
| (B) | ||||||||
| Grade I | D61 | Y | Y | |||||
| D72 | ||||||||
| D114 | Y | |||||||
| Grade II | D48 | Y | Y | Y | Y | Y | ||
| D64 | Y | Y | Y | Y | ||||
| D70 | Y | |||||||
| D71 | Y | Y | Y | Y | Y | Y | ||
| D78 | Y | |||||||
| D80 | Y | Y | Y | |||||
| D105 | ||||||||
| D19 | Y | Y | ||||||
| Grade III | D44 | Y | Y | Y | Y | |||
| D50 | Y | |||||||
| D53 | ||||||||
| D59 | ||||||||
| D63 | ||||||||
| D65 | ||||||||
| D66 | Y | |||||||
| D67 | ||||||||
| D74 | ||||||||
| D77 | ||||||||
| D81 | ||||||||
| D103 | ||||||||
| D107 | Y | |||||||
| D82 | ||||||||
| Grade IV | D60 | |||||||
| D68 | Y | |||||||
| D69 | ||||||||
| D70B | Y | Y | Y | |||||
| D98 | Y | Y | ||||||
Fig. 5.
Unsupervised cluster analysis of the data from Fig. 4 for Ara h 2 (a) and Ara h 6 (b). ‘yes’ is denoted by yellow; ‘no’ is denoted in blue. Sera from subjects with a history of grade I or II reactivity are in black. Those with a history of grade III or IV reactivity are in red.
Because 30 sera could potentially bind to each of 8 epitopes of Ara h 2 and 7 epitopes of Ara h 6, we determined that there were, respectively, 240 and 210 potential binding events (BEs) for Ara h 2 and Ara h 6. For all 30 sera, there were 99 BEs for Ara h 2 (Table 2A) compared to 38 for Ara h 6 (Table 2B) (P < 0.0001). For Ara h 2, epitopes 1 (z > 3 for 23 sera), 3 (z > 3 for 18 sera), and 7 (z > 3 for 17 sera) are immunodominant. For Ara h 6, only epitope F (z > 3 for 15 sera) was immunodominant.
The frequency of IgE binding to linear epitopes of Ara h 2 and Ara h 6 corresponds to clinical histories
Characteristic patterns emerged when subjects’ IgE binding to linear epitopes was examined in the context of their histories of reaction to peanut exposure. For Ara h 2 (Table 2A), we found 52 BEs (34%) of 152 (19×8 epitopes) possible BEs for the 19 subjects with more severe histories (grades III + IV) compared to 47 BEs (53%) of 88 (11×8 epitopes) possible BEs (53%) for the 11 subjects with less severe histories (grades I + II) (P < 0.01). For Ara h 6 (Table 2B), we found only 16 BEs (12%) of 133 (19×7 epitopes) possible BEs for the 19 subjects with grades III + IV compared to 25 BEs (32%) of 77 (11×7 epitopes) possible BEs for the 11 subjects with histories of grade I + II reactivity (P < 0.001). When specific epitopes are examined individually, IgE from subjects with less severe histories (grades I and II) bind epitope 5 of Ara h 2 more frequently (7 of 11 sera) than does IgE from subjects with more severe histories (2 of 19 sera) (P = 0.004, pc = 0.03) (Table 2A). A similar trend was seen with binding to epitope G of Ara h 6 (Table 2B), but this did not achieve statistical significance after correction for multiple comparisons. IgE from 4 of 11 patients with less severe histories (grades I and II) bind epitope G of Ara h 6 compared to 1 of 19 subjects with more severe histories (grades III and IV) but this was not statistically significant (P = 0.047, pc = 0.33). Thus, IgE from patients with relatively more severe clinical histories recognize fewer linear epitopes of both Ara h 2 and Ara h 6 when the epitopes are viewed as a whole.
Hierarchical analyses of IgE binding to linear epitopes
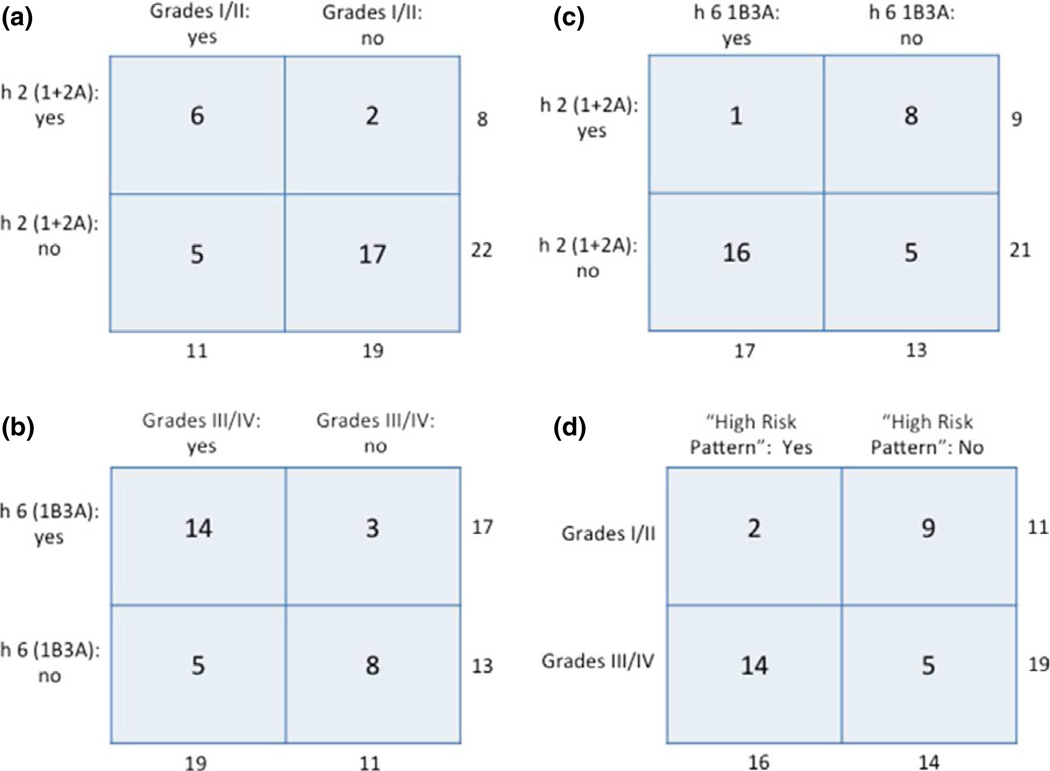
We also performed an unsupervised hierarchical cluster analysis (Fig. 5). For Ara h 2 (Fig. 5a), 6 of 8 subjects who clustered together into nodes 1 and 2A had a history of grade I or II severity whereas, of the 22 remaining subjects who did not cluster to these nodes, 5 had grades I or II severity (Figs 5a and 6a; P = 0.028). For Ara h 6 (Fig. 5b), the largest single node, 2B2B1, contained 17 subjects, 14 of which had a history of grade III or IV severity. Of the 13 subjects who did not cluster into this node, 5 had severity grades of III or IV (Figs 5b and 6b; P = 0.02). Thus, subjects with milder symptoms (grades I and II) clustered into nodes 1 and 2A of Ara h 2 node, while those with more severe symptoms (grades III and IV) clustered into node 2B2B1 of Ara h 6. Moreover, of the 17 individuals who clustered to node 2B2B1 of Ara h 6, only one subject, D105, clustered to either node 1 or 2A of Ara h 2, as compared with 16 who did not (Figs 5a, b, and 6c; P < 0.01).
Fig. 6.
Contingency plots of key data from the unsupervised cluster analysis shown in Fig. 5. Totals for each horizontal and vertical set of numbers are shown. For all plots, n = 30. (a) Frequency of subjects with a history of grades I or II severity cluster in either node 1 or 2A of Ara h 2 (P = 0.03); (b) frequency of subjects with a history of grades III or IV severity cluster in node 2B2B1 of Ara h 6 (P = 0.02); (c) frequency of subjects who cluster in node 2B2B1 of Ara h 6 and do not cluster in either node 1 or 2A of Ara h 2 (P < 0.01); and (d) frequency of subjects having the high-risk pattern [h 6 (2B2B1), yes and h 2 (1 + 2A), no] (P < 0.01).
Node 2B2B1 of Ara h 6 is characterized by lack of significant (z ≤ 3) binding to all epitopes of Ara h 6 or to only epitope F and is populated predominantly by individuals having a history of grades III or IV severity (Figs 5b and 6b). Taken together, subjects who cluster into this node do not cluster into nodes 1 and 2A of Ara h 2, which are characterized by significant (z > 3) binding to epitope 4 or to epitopes 2 and 5 of Ara h 2 and are predominantly populated by those having a history of grade I or II severity (Figs 5a, 6a). Thus, we have tentatively identified a high-risk pattern in which subjects with allergies having high grades of severity cluster to node IB3B of Ara h 6 but not to nodes 1 or 2A of Ara h 2. As shown in Fig. 6d, 16 subjects had this high-risk pattern; of these, 14 had a history of grades III or IV severity, whereas only two had a history of grade I or II severity. Moreover, of the 14 subjects who did not demonstrate this high-risk pattern, nine had grade I or II severity (OR = 12.6; 95% CI = 2.0–79.5; P < 0.01).
Competition experiments with linear epitopes
To determine whether the binding of IgE to peptides in the microarray was specific, a pool of 4 sera, D64, D70, D80, and D103, normalized by anti-peanut IgE content, was pre-incubated with native Ara h 2 or native Ara h 6. This resulted in > 90% inhibition of binding to their respective peptides (data not shown).
We also considered that the identified linear epitopes of Ara h 2 and Ara h 6 might effectively block IgE cross-linking of FcεRI and resultant activation of mast cells. In that, Albrecht and colleagues [48] have shown that peptides of Ara h 2 were unable to block cross-linking of IgE and cell activation by Ara h 2, and we performed similar experiments only with Ara h 6. In separate assays, we sensitized RBL SX-38 cells with IgE from each of three patients (D19, D64, and, D70) with a history of mild reactions (grade I or II) and evidence of predominant binding to epitopes E, F, and G of Ara h 6. We pre-incubated these sensitized cells with 10 000 molar excess of epitope E (limited by solubility) plus a 100 000 molar excess of epitopes F and G and then added Ara h 6 as described in Materials and Methods. We were unable to detect a significant inhibitory effect (data not shown).
Discussion
In this study, we used peptide microarrays to examine IgE binding to linear peptides and then to epitopes of the highly potent peanut allergens, Ara h 2 and Ara h 6, for 30 subjects with strong histories and serologic evidence of clinically important peanut allergy. The eight epitopes identified for Ara h 2, that are similar to those previously reported, include the epitopes that have been recently shown to be responsible for cross-reactivity with Ara h 1 and Ara h 3 [38, 46, 49]. Seven epitopes were newly identified for Ara h 6 (Fig. 2); six of these showed positive correlations for IgE binding to homologous epitopes of Ara h 2 (P < 0.001) (Fig. 3). Unexpectedly, although it was not true for all sera, the intensity of binding to peptides of Ara h 2 was generally greater than the intensity of binding to peptides of Ara h 6 (Figs 3, S1, S2, S3, S4, and S5).
Given that binding of IgE to denatured proteins (e.g. on immunoblots) does not reflect the importance of IgE to cell activation following allergen-IgE cross-linking (discussed in Zhuang and Dreskin, [21]), we hypothesized that the absolute or relative extent of binding of IgE to peptides may not accurately reflect the importance of that peptide’s contribution to the clinically important phenomenon of allergen-IgE cross-linking. For this reason, we focused on a dichotomous model in which a specific serum either did or did not have positive binding to a specific linear epitope. We defined a positive signal for an epitope if > 50% of the peptides containing the putative epitope gave z-score of > 3 (see Materials and Methods).
Overall, individual IgE samples bound to linear epitopes of Ara h 2 more frequently than to epitopes of Ara h 6 (P < 0.002) (Fig. 4; Table 2). This was unexpected in that there is significant homology between IgE-binding linear epitopes of these two molecules, as well as significant correlation of IgE binding (Fig. 2 and Fig. 3). We interpret this as evidence that conformational epitopes may be more important for Ara h 6 than for Ara h 2. We hypothesize that Ara h 6 is more tightly folded than Ara h 2 and that this leads to conformational IgE-binding epitopes that are less cross-reactive with the linear epitopes that may contribute to the conformational epitopes. This also explains the observation that the intensity of IgE binding to the peptides that contain the linear epitopes is also less for Ara h 6 than for Ara h 2. This is compatible with the observation that Ara h 6 has 5 disulphide bonds compared with 4 disulphide bonds for Ara h 2 and with our experience that proper folding of recombi-nant Ara h 6 is particularly important to preserve aller-genic activity [21].
Also unexpectedly, IgE from subjects with histories of relatively severe allergic responses to peanut exposure recognized a significantly more limited breadth of linear epitopes of Ara h 2 and Ara h 6 than did those from subjects who reported less severe responses to peanuts (Table 2). This result is contrary to several reports of more binding of IgE to linear epitopes of allergens with IgE from patients with severe histories [26, 29, 32, 38]. However, it is compatible with reports of a positive relationship between the amount of IgE binding to linear epitopes and allergen-specific IgE concentrations in the sera and no relationship to clinical history [31, 39, 40]. The most likely explanations of this finding are that, in contrast to most previous reports of IgE binding to peptides in microarrays [26, 29, 31, 32, 38, 39, 50], we adjusted our sera to the same content of anti-peanut IgE to avoid bias due to differences in peanut-specific IgE levels and used a dichotomous model (discussed above) to determine statistically significant binding. Normalization based on peanut-specific IgE levels removes the confounding variable of differences in peanut-specific IgE among the samples. For example, without this normalization, sera with very high IgE levels may have statistically significant, but biologically unimportant, binding to minor epitopes. Of note, we found that further normalization of our data based on binding of IgE to native Ara h 2 and/or Ara h 6 within each microarray assay did not substantially change the final data (Fig. S1).
These observations that point towards the importance of conformational as opposed to linear epitopes are in line with recent observations that conformational epitopes rather than linear epitopes are critical for binding of IgE and for activation of IgE-sensitized basophils and/ or mast cells [48, 51, 52]. As reported by Albrecht and colleagues [48], peptides of Ara h 2 were unable to block cross-linking of IgE and cell activation by Ara h 2. In our hands, peptides containing epitopes of Ara h 6 were unable to block IgE-mediated activation of sensitized RBL SX-38 cells by Ara h 6 (data not shown). Because Ara h 2 and Ara h 6 are stable to digestion, it is likely that conformational epitopes are important with regard not only to immediate reactivity (as is the experimental set-up for these studies), but also following ingestion and exposure to the digestive track. We propose that conformational epitopes are important for all IgE cross-linking activity by Ara h 2 and Ara h 6 and are most important for patients with relatively severe reactions. These epitopes likely have substantially greater affinity for IgE binding than do linear epitopes.
Finally, in an unsupervised hierarchical cluster analysis, we have tentatively identified a novel high-risk pattern in which patients with high grades of severity cluster to node 2B2B1 of Ara h 6 (Fig. 5b) but not to nodes 1 or 2A of Ara h 2 (Figs 5a and 6d). Of note, node 2B2B1 of Ara h 6 is characterized by either binding only to epitope F (the only epitope of Ara h 6 that met our criteria of immunodominance) or no detectable binding above our predetermined threshold (z > 3) to any IgE-binding linear epitopes of Ara h 6 (Fig. 5b).
Weaknesses of this study include the use of historical clinical data rather than data obtained by double-blind placebo-controlled food challenge and the modest number of patients. Based on the data presented here, a post hoc power analysis (not shown) reveals that the analysis of the ‘high-risk pattern’ required only 24 subjects to have 90% power. Another issue is that the ‘high-risk pattern’ is associated with lower binding to Ara h 6. This can only be assessed in the context of control sera that have high levels of binding to both Ara h 2 and Ara h 6. Even given these limitations, our approach has yielded dramatic findings that may have significant clinical utility. However, this final observation needs to be repeated with an independent cohort.
In conclusion, subjects with a history of relatively severe clinical reactions have characteristic patterns of binding to linear epitopes of Ara h 2 and Ara h 6, which may be useful in predicting clinical reactions to future exposure to peanuts. The overall approach of focusing on IgE-binding epitopes of the most potent allergens identified in functional assays may also be useful for other IgE-mediated food allergies.
Supplementary Material
Acknowledgements
We thank Dr. Yonghua Zhuang for purified native Ara h 2 and Ara h 6; Dr. Bill Patterson for assistance in analysis of the peptide arrays; Darcy G. Schlichting, our study coordinator, and Drs. Allen Adinoff, Karen Andrews, Dan Atkins, Sandy Avner, Alan Bock, Leon Greos, John James, Grant Olson, Jerry Koepke, Jeffery Rumbyrt, and Catherine Van Kerckhove for referring patients; and our study subjects. We also thank Dr. Wayne Shreffler and Dr. Lawrence Eckler for sharing their experience in producing peptide microarrays and Dr. Rafeul Alam for critical reading of the manuscript.
Funding
This study was supported by grants R01-AI052164, a supplemental ARRA grant, and R01-AI099029 from the National Institute of Allergy and Infectious Diseases to Dr. Dreskin and divisional funds. This study was also supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Author contributions
Dr. Dreskin and Dr. Otsu conceived and designed the experiments. Dr. Otsu did the microarray assays. Grants to Dr. Dreskin and funds raised by Dr. Dreskin funded the project. Dr. Guo performed the heat map analysis and assisted in statistical analysis. All three authors contributed to analysis of the data and to writing the manuscript.
Conflict of interest
Drs. Dreskin and Otsu have a patent pending based on methodology described in this manuscript. Dr. Guo does not have any conflict of interests to disclose regarding this manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Demographics, clinical history, and grade of reaction.
Table S2A. Ara h 2. Intensities for sera #1–5.
Table S2B. Ara h 2. Intensities for sera #6–10.
Table S2C. Ara h 2. Intensities for sera #11–15.
Table S2D. Ara h 2. Intensities for sera #16–20.
Table S2E. Ara h 2. Intensities for sera #21–25.
Table S2F. Ara h 2. Intensities for sera #26–30.
Table S3A. Ara h 6. Intensities for sera #1–5.
Table S3B. Ara h 6: Intensities for sera #6–10.
Table S3C. Ara h 6: Intensities for sera #11–15.
Table S3D. Ara h 6: Intensities for sera #16–20.
Table S3E. Ara h 6: Intensities for sera #21–25.
Table S3F. Ara h 6: Intensities for sera #26–30.
Figure S1A. Further analysis of IgE binding to overlapping 20-mer peptides of Ara h 2.
Figure S1B. Further analysis of IgE binding to overlapping 20-mer peptides of Ara h 6.
Figure S2. Heat maps of IgE-binding to individual peptides (z-scores) for peptides of Ara h 2 (a) and Ara h 6(b).
Figure S3A. Unsupervised cluster analysis of the data from Figure S2 for Ara h 2.
Figure S3B. Unsupervised cluster analysis of the data from Figure S2 for Ara h 6.
Figure S4. Heat maps of binary (yes or no; z ≤ 3/z > 3) binding of IgE to peptides of Ara h 2 (a) and Ara h 6 (b).
Figure S5A. Unsupervised cluster analysis of the data from Figure S4 for Ara h 2.
Figure S5B. Unsupervised cluster analysis of the data from Figure S4 for Ara h 6.
References
- 1.Sicherer SH, Munoz-Furlong A, God-bold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Venter C, Arshad SH. Epidemiology of food allergy. Pediatr Clin North Am. 2011;58:327–349. ix. doi: 10.1016/j.pcl.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. quiz 820. [DOI] [PubMed] [Google Scholar]
- 4.Wainstein BK, Yee A, Jelley D, Ziegler M, Ziegler JB. Combining skin prick, immediate skin application and spe-cific-IgE testing in the diagnosis of peanut allergy in children. Pediatr Allergy Immunol. 2007;18:231–239. doi: 10.1111/j.1399-3038.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 5.Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–151. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Sampson HA. The role of immu-noglobulin E-binding epitopes in the characterization of food allergy. Curr Opin Allergy Clin Immunol. 2009;9:357–363. doi: 10.1097/ACI.0b013e32832d05ba. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaou N, Poorafshar M, Murray C, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. doi: 10.1016/j.jaci.2009.10.008. e191–113. [DOI] [PubMed] [Google Scholar]
- 8.Dang TD, Tang M, Choo S, et al. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129:1056–1063. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Flinterman AE, van Hoffen E, den Hartog Jager CF, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy. 2007;37:1221–1228. doi: 10.1111/j.1365-2222.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 10.Koid AE, Chapman MD, Hamilton RG, et al. Ara h 6 Complements Ara h 2 as an Important Marker for IgE Reactivity to Peanut. J Agric Food Chem. 2013;62:206–213. doi: 10.1021/jf4022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara hi, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004;34:583–590. doi: 10.1111/j.1365-2222.2004.1923.x. [DOI] [PubMed] [Google Scholar]
- 12.Palmer GW, Dibbern DA, Burks AW, et al. Comparative Potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin Immunol. 2005;115:301–312. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann K, Schweimer K, Reese G, et al. Structure and stability of 2S albumin-type peanut allergens: implications for the severity of peanut allergic reactions. Biochem J. 2006;395:463–472. doi: 10.1042/BJ20051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott RA, Porterfield HS, El-Mezayan R, et al. Contribution of Ara h 2 to peanut-specific immunoglobulin E-mediated, cell activation. Clin Exp Allergy. 2007;37:752–763. doi: 10.1111/j.1365-2222.2007.02701.x. [DOI] [PubMed] [Google Scholar]
- 15.Porterfield HS, Murray KS, Schlichting DG, et al. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39:1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanc F, Adel-Patient K, Drumare MF, Pary E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39:1277–1285. doi: 10.1111/j.1365-2222.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- 17.Krause S, Latendorf T, Schmidt H, et al. Peanut varieties with reduced Ara h 1 content indicating no reduced aller-genicity. Mol Nutr Food Res. 2010;54:381–387. doi: 10.1002/mnfr.200900072. [DOI] [PubMed] [Google Scholar]
- 18.Kulis M, Chen X, Lew J, et al. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. 2012;42:326–336. doi: 10.1111/j.1365-2222.2011.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Zhuang Y, Wang Q, et al. Analysis of the Effector Activity of Ara h 2 and Ara h 6 by Selective Depletion from a Crude Peanut Extract. J Immunol Methods. 2011;372:65–70. doi: 10.1016/j.jim.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Wang Q, El-Mezayen R, Zhuang Y, Dreskin SC. Ara h 2 and Ara h 6 have similar allergenic activity and are substantially redundant. Int Arch Allergy Immunol. 2013;160:251–258. doi: 10.1159/000341642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang Y, Dreskin SC. Redefining the major peanut allergens. Immunol Res. 2013;55:125–134. doi: 10.1007/s12026-012-8355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatchatee P, Jarvinen KM, Bardina L, Vila L, Beyer K, Sampson HA. Identification of IgE and IgG binding epitopes on beta- and kappa-casein in cow’s milk allergic patients. Clin Exp Allergy. 2001;31:1256–1262. doi: 10.1046/j.1365-2222.2001.01167.x. [DOI] [PubMed] [Google Scholar]
- 23.Chatchatee P, Jarvinen KM, Bardina L, Beyer K, Sampson HA. Identification of IgE- and IgG-binding epitopes on alpha(sl)-casein: differences in patients with persistent and transient cow’s milk allergy. J Allergy Clin Immunol. 2001;107:379–383. doi: 10.1067/mai.2001.112372. [DOI] [PubMed] [Google Scholar]
- 24.Busse PJ, Jarvinen KM, Vila L, Beyer K, Sampson HA. Identification of sequential IgE-binding epitopes on bovine alpha(s2)-casein in cow’s milk allergic patients. Int Arch Allergy Immunol. 2002;129:93–96. doi: 10.1159/000065178. [DOI] [PubMed] [Google Scholar]
- 25.Jarvinen KM, Chatchatee P, Bardina L, Beyer K, Sampson HA. IgE and IgG binding epitopes on alpha-lactalbumin and beta-lactoglobulin in cow’s milk allergy. Int Arch Allergy Immunol. 2001;126:111–118. doi: 10.1159/000049501. [DOI] [PubMed] [Google Scholar]
- 26.Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immu-noassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004;113:776–782. doi: 10.1016/j.jaci.2003.12.588. [DOI] [PubMed] [Google Scholar]
- 27.Beyer K, Jarvinen KM, Bardina L, et al. IgE-binding peptides coupled to a commercial matrix as a diagnostic instrument for persistent cow’s milk allergy. J Allergy Clin Immunol. 2005;116:704–705. doi: 10.1016/j.jaci.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Untersmayr E, Szalai K, Riemer AB, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol. 2006;43:1454–1461. doi: 10.1016/j.molimm.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 29.Flinterman AE, Knol EF, Lencer DA, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121:737–743. e710. doi: 10.1016/j.jaci.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 30.Cerecedo I, Zamora J, Shreffler WG, et al. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J Allergy Clin Immunol. 2008;122:589–594. doi: 10.1016/j.jaci.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Ayuso R, Sanchez-Garcia S, Lin J, et al. Greater epitope recognition of shrimp allergens by children than by adults suggests that shrimp sensitization decreases with age. J Allergy Clin Immunol. 2010;125:1286–1293. e1283. doi: 10.1016/j.jaci.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Lin J, Bardina L, et al. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J Allergy Clin Immunol. 2010;125:695–702. doi: 10.1016/j.jaci.2009.12.017. 702 e691-702 e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vereda A, van Hage M, Ahlstedt S, et al. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127:603–607. doi: 10.1016/j.jaci.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Shreffler WG. Microarrayed recombi-nant allergens for diagnostic testing. J Allergy Clin Immunol. 2011;127:843–849. doi: 10.1016/j.jaci.2011.02.011. quiz 850-841. [DOI] [PubMed] [Google Scholar]
- 35.Sanz ML, Blazquez AB, Garcia BE. Microarray of allergenic component-based diagnosis in food allergy. Curr Opin Allergy Clin Immunol. 2011;11:204–209. doi: 10.1097/ACI.0b013e3283466fe4. [DOI] [PubMed] [Google Scholar]
- 36.Fiocchi A, Nowak-Wegrzyn A. The fascinating world of molecular diagnosis in the management of food allergy: nondum matura est. Curr Opin Allergy Clin Immunol. 2011;11:200–203. doi: 10.1097/ACI.0b013e32834694ae. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Bruni FM, Fu Z, et al. A bioin-formatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129:1321–1328. e1325. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol. 2005;116:893–899. doi: 10.1016/j.jaci.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Vereda A, Andreae DA, Lin J, et al. Identification of IgE sequential epitopes of lentil (Len c 1) by means of peptide microarray immunoassay. J Allergy Clin Immunol. 2010;126:596–601. e591. doi: 10.1016/j.jaci.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willumsen N, Holm J, Christensen LH, Wurtzen PA, Lund K. The complexity of allergic patients’ IgE repertoire correlates with serum concentration of allergen-specific IgE. Clin Exp Allergy. 2012;42:1227–1236. doi: 10.1111/j.1365-2222.2012.04009.x. [DOI] [PubMed] [Google Scholar]
- 41.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010;125:569–574. doi: 10.1016/j.jaci.2009.10.060. 574 e561-574 e567. [DOI] [PubMed] [Google Scholar]
- 42.Panse S, Dong L, Burian A, et al. Profiling of generic anti-phosphopeptide antibodies and kinases with peptide microarrays using radioactive and fluorescence-based assays. Mol Divers. 2004;8:291–299. doi: 10.1023/b:modi.0000036240.39384.eb. [DOI] [PubMed] [Google Scholar]
- 43.Lin J, Bardina L, Shreffler WG, et al. Development of a novel peptide micro-array for large-scale epitope mapping of food allergens. J Allergy Clin Immunol. 2009;124:315–322. doi: 10.1016/j.jaci.2009.05.024. 322 e311-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA. Recombinant peanut allergen Ara h I expression and IgE binding in patients with peanut hypersensitivity. J Clin Invest. 1995;96:1715–1721. doi: 10.1172/JCI118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke MC, Kilburn SA, Hourihane JO, Dean KR, Warner JO, Dean TP. Serologi-cal characteristics of peanut allergy. Clin Exp Allergy. 1998;28:1251–1257. doi: 10.1046/j.1365-2222.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 46.Stanley JS, King N, Burks AW, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997;342:244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 47.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 48.Albrecht M, Kuhne Y, Ballmer-Weber BK, et al. Relevance of IgE binding to short peptides for the allergenic activity of food allergens. J Allergy Clin Immunol. 2009;124:328–336. doi: 10.1016/j.jaci.2009.05.031. 336 e321-326. [DOI] [PubMed] [Google Scholar]
- 49.Bublin M, Kostadinova M, Radauer C, et al. IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. J Allergy Clin Immunol. 2013;132:118–124. doi: 10.1016/j.jaci.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto N, Okochi M, Matsushima M, et al. Peptide array-based analysis of the specific IgE and IgG4 in cow’s milk allergens and its use in allergy evaluation. Peptides. 2009;30:1840–1847. doi: 10.1016/j.peptides.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Starkl P, Felix F, Krishnamurthy D, et al. An unfolded variant of the major peanut allergen Ara h 2 with decreased anaphylactic potential. Clin Exp Allergy. 2012;42:1801–1812. doi: 10.1111/cea.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apostolovic D, Luykx D, Warmenho-ven H, et al. Reduction and alkylation of peanut allergen isoforms Ara h 2 and Ara h 6; characterization of intermediate- and end products. Biochim Biophys Acta. 2013;1834:2832–2842. doi: 10.1016/j.bbapap.2013.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.