Abstract
IMPORTANCE
Critical congenital heart disease (CCHD) was added to the Recommended Uniform Screening Panel for Newborns in the United States in 2011. Many states have recently adopted or are considering requirements for universal CCHD screening through pulse oximetry in birth hospitals. Limited previous research is directly applicable to the question of how many US infants with CCHD might be identified through screening.
OBJECTIVES
To estimate the proportion of US infants with late detection of CCHD (>3 days after birth) based on existing clinical practice and to investigate factors associated with late detection.
DESIGN, SETTING, AND PARTICIPANTS
Descriptive and multivariable analysis. Data were obtained from a multisite population-based study of birth defects in the United States, the National Birth Defects Prevention Study (NBDPS). We included all live-born infants with estimated dates of delivery from January 1, 1998, through December 31, 2007, and nonsyndromic, clinically verified CCHD conditions potentially detectable through screening via pulse oximetry.
MAIN OUTCOMES AND MEASURES
The main outcome measure was the proportion of infants with late detection of CCHD through echocardiography or at autopsy under the assumption that universal screening at birth hospitals might reduce the number of such late diagnoses. Secondary outcome measures included prevalence ratios for associations between selected demographic and clinical factors and late detection of CCHD.
RESULTS
Of 3746 live-born infants with nonsyndromic CCHD, late detection occurred in 1106 (29.5% [95%CI, 28.1%–31.0%]), including 6 (0.2%) (0.1% –0.4%) first receiving a diagnosis at autopsy more than 3 days after birth. Late detection varied by CCHD type from 9 of 120 infants (7.5%[95%CI, 3.5%–13.8%]) with pulmonary atresia to 497 of 801 (62.0% [58.7%–65.4%]) with coarctation of the aorta. In multivariable analysis, late detection varied significantly by CCHD type and study site, and infants with extracardiac defects were significantly less likely to have late detection of CCHD (adjusted prevalence ratio, 0.58 [95% CI, 0.49–0.69]).
CONCLUSIONS AND RELEVANCE
We estimate that 29.5%of live-born infants with nonsyndromic CCHD in the NBDPS received a diagnosis more than 3 days after birth and therefore might have benefited from routine CCHD screening at birth hospitals. The number of infants in whom CCHD was detected through screening likely varies by several factors, including CCHD type. Additional population-based studies of screening in practice are needed.
Congenital heart defects affect approximately 1%of live births, of which 25%are estimated to be critical and require surgery or catheterization within the first year of life.1 Infants with critical congenital heart defects (also referred to as critical congenital heart disease [CCHD]) who are discharged from birth hospitals without a diagnosis are at risk for cardiovascular collapse and death.1 Newborn screening for CCHD through pulse oximetry can detect some CCHD conditions (eg, those who present with hypoxemia [low blood oxygen saturation] shortly after birth) even in the absence of other physical symptoms and thereby avert late detection.2 Screening is recommended at birth hospitals within 24 to 48 hours of birth.3 Pulse oximetry is a noninvasive test that quantifies hypoxemia. A single reading of less than 90% from a neonate’s hand or foot or the combination of a 90% to 95%single reading and a difference of more than 3% in the readings for the upper and lower extremities is flagged for follow-up.3 In recent clinical studies, pulse oximetry has demonstrated high specificity and moderate sensitivity to detect CCHD and a low false-positive rate.2,4 Critical congenital heart disease was added to the US recommended uniform screening panel for newborns in 2011.5 Legislation to require screening was recently adopted or is under consideration in most states (http://www.aap.org/stateadvocacy).6,7
Previous studies have examined issues related to late CCHD detection (defined for our study as >3 days after birth), although few such studies facilitate direct estimates of the impact that universal screening might have in the United States. For example, several potentially relevant US studies were not population based or lacked sufficient follow-up to identify infants with missed CCHD after discharge from the birth hospital.8–13 Studies from European countries and elsewhere in the world are illuminating, but not directly applicable to the US clinical context.14–27 The most relevant US population-based studies of late detection of CCHD published before the federal recommendation for routine screening through pulse oximetry produced widely varied estimates—ranging from 4.3% to 31.3%—of infants with CCHD who received late diagnoses (Table 1).29–33,35 The substantial variability of those estimates appears to result from differences in case definition, data sources, length of follow-up, study size, and exclusive use of administrative coding to identify CCHD diagnoses. Administrative diagnostic codes may inaccurately classify some heart defects; for example, the severity of aortic or pulmonary stenosis can determine whether such conditions can be detected by screening, although such severity is not distinguished through administrative codes.36,37 Moreover, those studies did not examine late detection in a manner suited to estimate the potential effect of universal screening; for example, some studies examined only missed diagnoses resulting in infant death33,35 or did not examine the full range of CCHD conditions that screening might detect.29–33 At least 2 studies28,34 have examined the population-based effect of newborn CCHD screening in practice: one was a pilot study at 2 hospitals in New York,34 and the other was a statewide study of birth hospitals in New Jersey.28 Both studies reported screening results during a short period and produced very different relative and absolute estimates of late-detected CCHD (25.0% vs 5.9% of newborns with CCHD) (Table 1).
Table 1.
Selected Previous Population-Based Estimates of Late Detection of CCHD Among US Infants
| Source | Study Period |
Cohort, No. of Patients |
No. of Infants With CCHD |
Definition of Late Detection | Infants With Late Detection, No. (%) |
Data Sources and Limitations |
|---|---|---|---|---|---|---|
| Garg et al,28 2013 | 2011 | 72 694 | 51 | True positive CCHD screening results in newborns with unsuspected CCHD | 3 (5.9) | Data from New Jersey statewide POX screening program in birth hospitals; Dx based on clinical case review; postnatal FU period not defined, although <9 mo; late CCHD detection defined as detected through screening; false-negative results NR |
| Peterson et al,29 2013 | 1998–2007 | 2 128 236 | 3603 | Dx after birth hospital discharge | 825 (22.9) | Data from Florida Birth Defects Registry plus statewide inpatient and death records; Dx based on ICD-9-CM codes; 1-y postnatal case ascertainment; not all screening-detectable CCHD examineda |
| Ng and Hokanson,30 2010 | 2002–2006 | 345 572 | NR | Dx after birth hospital discharge | 14 (NC) | Data from Wisconsin statewide hospital and death records; Dx based on ICD-9-CM codes; 2-wk postnatal case ascertainment; not all screening-detectable CCHD examineda |
| Oster et al,31 2013 | 1979–2005 | 1 056 541 | 1295b | Dx after day of birth | 405 (31.3) | Data from Metropolitan Atlanta Congenital Defects Program (including statewide death records); Dx based on ICD-9-CM codes; case ascertainment as long as6yafter birth; not all screening-detectable CCHD examineda; late detection not aligned with current screening recommendationsc |
| Aamir et al,32 2007 | 1999–2004 | 670 245 | 696 | Dx after birth hospital discharge | 47 (6.8) | Data from New Jersey birth certificates and statewide hospital records; Dx based on ICD-9-CM codes; clinical case review for late detection; 1-y postnatal case ascertainment; not all screening-detectable CCHD examineda |
| Chang et al,33 2008 | 1989–2004 | 8 869 336d | NR | Autopsy-confirmed infant death from CCHD with no heart surgery performed | 152 (NC) | Data from California statewide death records; Dx based on ICD-9-CM codes; 1-y postnatal case ascertainment; not all screening-detect-able CCHD examineda; definition of late detection not aligned with current screening recommendationsc |
| Koppel et al,34 2003 | 1998–1999 | 11 296 | 20 | True-positive or false-negative CCHD screening results in asymptomatic newborns | 5 (25.0) | Data from pilot POX screening program in 2 New York state hospitals, New York State Congenital Malformations Registry, and statewide hospital and death records; Dx based on clinical case review; 2-y postnatal case ascertainment |
| Kuehl et al,35 1999 | 1981–1989 | 906 626 | 969 | Dx after infant death | 42 (4.3) | Data from the Baltimore-Washington Infant Study and area death records; Dx based on clinical case review; 1-y postnatal case ascertainment; definition of late detection not aligned with current screening recommendationsc |
Abbreviations: CCHD, critical congenital heart disease; Dx, diagnosis; FU, follow-up; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NC, not calculable; NR, not reported; POX, pulse oximetry.
Screening-detectable CCHD conditions include hypoplastic left heart syndrome, pulmonary atresia, dextrotransposition of the great arteries, truncus arteriosus, tricuspid atresia, tetralogy of Fallot, total anomalous pulmonary venous return, critical aortic stenosis, coarctation of the aorta, double-outlet right ventricle, Ebstein anomaly, interrupted aortic arch, critical pulmonary stenosis, and single ventricle.1
Estimate excluded infants with noncardiac anomalies.
Screening is recommended to occur at birth hospitals within 24 to 48 hours of birth.3
Cohort size reported by California Department of Health for study years (http://www.cdph.ca.gov/data/statistics/Pages/default.aspx).
As CCHD screening is more widely adopted, more precise estimation of its impact may be possible by reviewing actual clinical experiences for many years in multiple geographic areas. Until then, retrospective review of infants’ CCHD diagnostic experiences remains a relevant way to estimate the potential future effect of universal screening. The purpose of this study was to estimate the proportion of US infants with clinically validated, nonsyndromic, screening-detectable CCHD whose condition was detected late, defined as detection more than 3 days after birth, and to investigate clinical and demographic factors associated with late detection.
Methods
Study Population
The National Birth Defects Prevention Study (NBDPS) is an ongoing, multisite, population-based case-control study conducted in 10 states (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey [through 2002], New York, North Carolina [beginning 2003], Texas, and Utah [beginning 2003]) to investigate genetic and environmental risk factors for selected major structural birth defects.38 Population-based ascertainment of infants with birth defects at each study site ranges from entire states (Arkansas, Iowa, New Jersey, and Utah) to selected regions within states (California, Georgia, Massachusetts, New York, North Carolina, and Texas). New York was the only NBDPS site included in this analysis that relied on a combination of active and passive case ascertainment; all other study sites used active case ascertainment. For our purposes, active ascertainment means that trained staff culled multiple medical records to identify and extract pertinent phenotypic information. Infants with recognized or strongly suspected chromosomal abnormalities or single-gene conditions were excluded from the study. The NBDPS reports clinical information abstracted from maternal and infant medical records by birth defects surveillance programs at each study site. Inclusion criteria for congenital heart defects in the NBDPS require that the defects be confirmed by echocardiography, catheterization, surgery, or autopsy findings.39 The NBDPS gathers additional information on demographic characteristics, exposures (eg, nutritional, behavioral, or occupational) and medication use before and during pregnancy through telephone interviews with the mothers. Interviews are conducted in English or Spanish 6 weeks to 24 months after an infant’s estimated date of delivery (EDD). Approximately 63% of mothers of infants with congenital heart defects participated in the telephone interview. The NBDPS was approved by institutional review boards at the Centers for Disease Control and Prevention and all study sites.
In this analysis, we considered all live-born infants with congenital heart defects with an EDD from January 1, 1998, through December 31, 2007, and whose mothers were interviewed for the NBDPS. We excluded all infants born to mothers residing in New Jersey for all years and to mothers residing in Texas with an EDD before June 1998, because those study sites included only a sample of eligible infants with congenital heart defects in the NBDPS. The NBDPS methods for classifying congenital heart defects in infants have been described previously.39,40 Briefly, classification is based on the primary congenital heart defect by a team of clinicians with expertise in pediatric cardiology and clinical genetics.
For this study, we restricted our analysis to infants with CCHD potentially detectable by screening, defined as hypoplastic left heart syndrome, pulmonary atresia, dextrotransposition of the great arteries, truncus arteriosus, tricuspid atresia, tetralogy of Fallot, total anomalous pulmonary venous return, critical aortic stenosis, coarctation of the aorta, double-outlet right ventricle, Ebstein anomaly, interrupted aortic arch, critical pulmonary stenosis, and single ventricle.1 The first 7 conditions usually present with hypoxemia and are classified as primary screening targets.3 Infants with at least 1 screening-detectable CCHD condition were identified through the existing NBDPS heart classification system,39 with 2 exceptions. First, infants with congenital heart defects classified as multiple complex, other associations, unbalanced atrioventricular septal defects with or without outflow tract obstruction, or laterality defects underwent review by one of us (T.R.-C.) with expertise in pediatric cardiology and the NBDPS heart classification system to determine if 1 or more of the screening-detectable CCHD conditions was present. Second, infants with aortic or pulmonary stenosis were included only when the NBDPS clinical classifiers’ comments indicated that the infant underwent valvuloplasty or had critical or severe valve stenosis. Among infants with 1 screening-detectable CCHD, results are presented by individual CCHD type. Infants with more than 1 such condition (eg, coarctation of aorta and double-outlet right ventricle) are reported in a multiple CCHD category.
Identifying Late CCHD Detection
Based on abstracted medical record information, we identified the first date on which infants with CCHD underwent a diagnostic echocardiography (fetal or postnatal) or autopsy. Because CCHD screening is recommended to occur at 24 to 48 hours after birth,3 we classified CCHD detection as late if the infant did not have abstracted evidence of having received a diagnostic echocardiography prenatally or within 3 days of birth. We conservatively selected 3 days rather than 2 because the NBDPS does not capture time of birth; therefore, a cutoff of 2 days might erroneously identify infants as having late CCHD detection when a diagnosis was made within 48 hours. Every infant who received a first diagnosis at autopsy could reasonably be considered to have late detection of CCHD. However, we excluded infants with a diagnosis at autopsy within 3 days of birth because we aimed to quantify the proportion of infants with CCHD who might benefit from proposed universal screening, and such infants might not have the chance to undergo screening. We also excluded infants who did not have a recorded echocardiography. Such infants were assumed to have incomplete records in the NBDPS because interventions (ie, cardiac catheterization or surgery) are usually preceded by or accompanied by imaging studies. We restricted the analysis to infants with CCHD diagnosis by echocardiography performed within 1 year of age.1
Statistical Analysis
We first assessed the timing of infants’ CCHD diagnosis (prenatal, postnatal, or at autopsy) through descriptive statistics by calculating frequencies and their corresponding 95%Wald CIs. We used exact 95%CIs for cell counts less than 10. We then estimated crude and adjusted prevalence ratios (PRs) and corresponding 95% CIs for late detection based on selected infant and maternal demographic and clinical characteristics in Poisson regression models with robust sandwich error variance.41,42We assessed the following characteristics from information abstracted from birth defects surveillance data: NBDPS study site, the presence of extracardiac defects (ie, major defects in organ systems outside of the heart),39 CCHD type, gestational age at delivery, and EDD year. We assessed the following characteristics from information reported during the NBDPS maternal interview: first-degree family history of congenital heart defects, plurality, and maternal characteristics, including race/ethnicity, age at delivery, education, diabetes mellitus before or during the index pregnancy, prepregnancy body mass index, hypertension before or during the pregnancy, fertility treatments, previous pregnancy losses, and trimester of the first prenatal care visit. The analysis was conducted using commercially available statistical software (SAS, version 9.2; SAS Institute, Inc).
Results
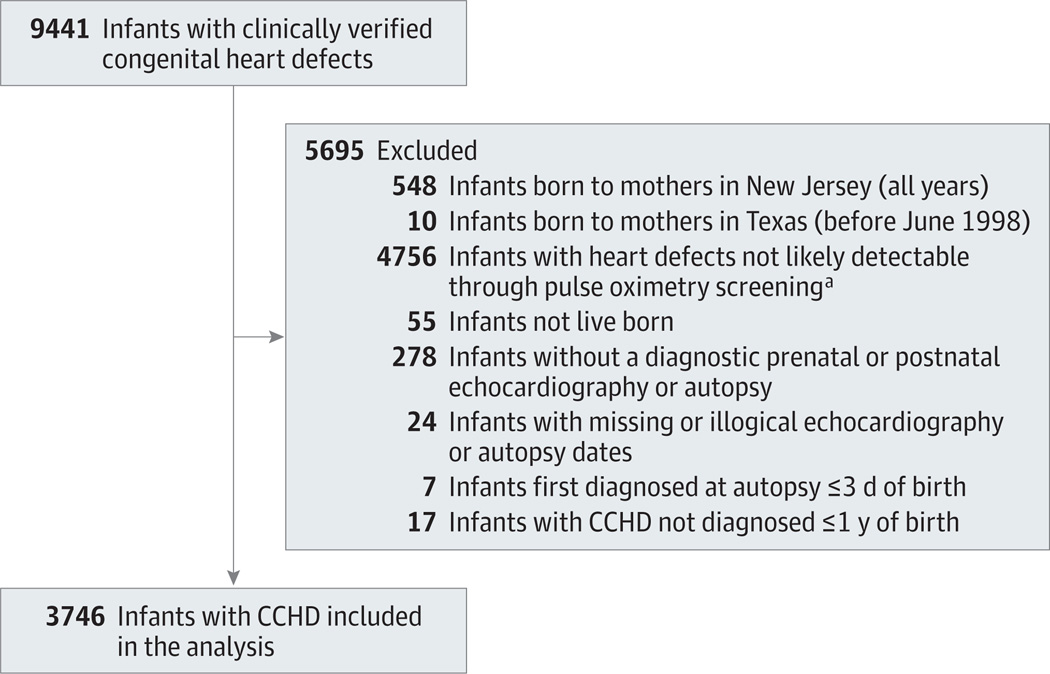
Of 9441 infants with nonsyndromic congenital heart defects and a 1998–2007 EDD whose mothers participated in an NBDPS interview, 3746 were included in the analysis (Figure). Of these, 1106 (29.5% [95% CI, 28.1%–31.0%]) underwent diagnosis through echocardiography more than 3days after birth (Table 2). For 6 infants (0.2% [95% CI, 0.1%–0.4%]), CCHD diagnosis occurred at autopsy more than 3 days after birth (Table 2). Late detection by CCHD type ranged from 9 of 120 infants (7.5%[95%CI, 3.5%–13.8%])with pulmonary atresia to 497 of 801 (62.0%[58.7%–65.4%])with coarctation of the aorta (Table 2). The frequency of late detection varied within CCHD types by the presence or absence of extracardiac defects and by NBDPS study site (Supplement [eFigures 1 and 2]). For 542 infants (14.5% [95% CI, 13.3%–15.6%]), the first echocardiogram documented in the abstracted medical record was prenatal. Among infants with late-detected CCHD diagnosed through echocardiography (n = 1100), the median time from birth to diagnosis was 14 (range, 4–363; interquartile range [IQR], 7–48) days (Table 2). Among the 6 infants who received the initial diagnosis at autopsy more than 3 days after birth (n = 6), the median time from birth to diagnosis was 5 (range, 4–21; IQR, 4–11) days (data not shown).
Figure.
Derivation of Study Sample of Infants With Critical Congenital Heart Disease (CCHD) in the National Birth Defects Prevention Study, 1998–2007
aHypoxemic structural heart defects potentially detectable through pulse oximetry screening at birth hospitals include critical aortic stenosis, coarctation of the aorta, double-outlet right ventricle, dextrotransposition of the great arteries, Ebstein anomaly, hypoplastic left heart syndrome, pulmonary atresia, interrupted aortic arch, critical pulmonary stenosis, single ventricle, truncus arteriosus, total anomalous pulmonary venous return, tetralogy of Fallot, and tricuspid atresia.
Table 2.
Timing of CCHD Detection via Echocardiography or Autopsy Among 3746 Infants in the National Birth Defects Prevention Study, 1998–2007
| No. of Patients | Timely Detection, No. (%) [95% CI]a |
No. of Patients | Total Late Detection, No. (%) [95% CI]a |
EKG Dx in Late Detection, Time After Birth, Median (Range), db |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Before Birth |
Day | Day | Dx at Autopsy (>Day 3) |
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ≥7 | |||||||
| Single CCHD | |||||||||||||
| Pulmonary atresia | 120 | 25 | 52 | 28 | 6 | 111 (92.5) [87.8–97.2] | 1 | 1 | 0 | 7 | 0 | 9 (7.5) [3.5–13.8]c | 8 (4–205) |
| Tricuspid atresia | 90 | 23 | 37 | 15 | 4 | 79 (87.8) [81.0–94.5] | 1 | 1 | 1 | 8 | 0 | 11 (12.2) [5.5–19.0] | 18 (4–95) |
| Hypoplastic left heart syndrome | 427 | 113 | 143 | 62 | 53 | 371 (86.9) [83.7–90.1] | 16 | 6 | 9 | 23 | 2 | 56 (13.1) [9.9–16.3] | 6 (4–131) |
| Dextrotransposition of the great arteries | 650 | 84 | 282 | 159 | 37 | 562 (86.5) [83.8–89.1] | 9 | 5 | 3 | 70 | 1 | 88 (13.5) [10.9–16.2] | 13 (4–205) |
| Aortic stenosis, critical | 20 | 3 | 7 | 4 | 2 | 16 (80.0) [62.5–97.5] | 0 | 1 | 0 | 3 | 0 | 4 (20.0) [5.7–43.7]c | 9 (5–61) |
| Ebstein anomaly | 90 | 11 | 40 | 18 | 2 | 71 (78.9) [70.5–87.3] | 6 | 0 | 2 | 11 | 0 | 19 (21.1) [12.7–29.5] | 7 (4–129) |
| Single ventricle | 127 | 37 | 35 | 21 | 6 | 99 (78.0) [70.7–85.2] | 5 | 2 | 4 | 17 | 0 | 28 (22.0) [14.8–29.3] | 10 (4–182) |
| Pulmonary stenosis, critical | 101 | 8 | 29 | 30 | 11 | 78 (77.2) [69.1–85.4] | 4 | 4 | 0 | 15 | 0 | 23 (22.8) [14.6–31.0] | 10 (4–215) |
| Interrupted aortic arch | 43 | 5 | 5 | 12 | 9 | 31 (72.1) [58.7–85.5] | 2 | 0 | 0 | 10 | 0 | 12 (27.9) [14.5–41.3] | 10 (4–93) |
| Tetralogy of Fallot | 733 | 94 | 178 | 164 | 93 | 529 (72.2) [68.9–75.4] | 33 | 10 | 10 | 151 | 0 | 204 (27.8) [24.6–31.1] | 23 (4–361) |
| Double-outlet right ventricle | 94 | 14 | 27 | 19 | 5 | 65 (69.1) [59.8–78.5] | 3 | 2 | 1 | 23 | 0 | 29 (30.9) [21.5–40.2] | 17 (4–144) |
| Truncus arteriosus | 68 | 11 | 19 | 9 | 8 | 47 (69.1) [58.1–80.1] | 3 | 2 | 0 | 15 | 1 | 21 (30.9) [19.9–41.9] | 14 (4–89) |
| Total anomalous pulmonary venous return | 190 | 8 | 48 | 44 | 12 | 112 (58.9) [52.0–65.9] | 4 | 3 | 2 | 69 | 0 | 78 (41.1) [34.1–48.1] | 29 (4–330) |
| Coarctation of the aorta | 801 | 61 | 80 | 90 | 73 | 304 (38.0) [34.6–41.3] | 42 | 26 | 27 | 400 | 2 | 497 (62.0) [58.7–65.4] | 15 (4–363) |
| Multiple CCHDd | 192 | 45 | 77 | 32 | 11 | 165 (85.9) [81.0–90.9] | 7 | 3 | 3 | 14 | 0 | 27 (14.1) [9.2–19.0] | 9 (4–120) |
| Total | 3746 | 542 | 1059 | 707 | 332 | 2640 (70.5) [69.0–71.9] | 136 | 66 | 62 | 836 | 6 | 1106 (29.5) [28.1–31.0] | 14 (4–363) |
Abbreviations: CCHD, critical congenital heart disease; Dx, diagnosis; EKG, echocardiography.
Late detection defined as a Dx more than 3 days after birth via echocardiography or autopsy. Percentages have been rounded and might not total 100.
Total includes 1100 infants, excluding 6 who received a first Dx at autopsy (median number of days from birth to autopsy, 5; range: 4–21).
Indicates exact 95%CI.
Indicates more than 1 screening-detectable CCHD.
When we controlled for all demographic and clinical factors under consideration, the prevalence of late detection among infants with CCHD varied significantly by the presence of extracardiac defects, CCHD type, and NBDPS study site (Table 3). The estimated adjusted prevalence of late detection among infants with extracardiac defects was 42% less (adjusted PR, 0.58 [95% CI, 0.49–0.69]) than the adjusted prevalence in infants without extracardiac defects (Table 3). The estimated adjusted prevalence of late detection among infants with Ebstein anomaly, single ventricle, critical pulmonary stenosis, interrupted aortic arch, tetralogy of Fallot, double-outlet right ventricle, truncus arteriosus, total anomalous pulmonary venous return, and coarctation of the aorta were each significantly greater than the adjusted prevalence among infants with hypoplastic left heart syndrome (the reference group). Late detection varied significantly by NBDPS study site, with a 2-fold difference between the sites with the lowest and highest adjusted prevalence of late detection (adjusted PR, 2.09 [95% CI, 1.66–2.63]) (Table 3).
Table 3.
Analysis of Factors Associated With Late Detection of CCHD Among 3746 Infants in the National Birth Defects Prevention Study, 1998–2007a
| Characteristic | No. (%) of Infants | PR (95% CI) | ||
|---|---|---|---|---|
| Total | Late Detectionb | Crude Analysis | Adjusted Analysis | |
| Extracardiac defectsc | ||||
| No | 3110 | 980 (31.5) | 1 [Reference] | 1 [Reference] |
| Yes | 636 | 126 (19.8) | 0.63 (0.53–0.74) | 0.58(0.49–0.69) |
| CCHD type | ||||
| Single CCHD | ||||
| Pulmonary atresia | 120 | 9 (7.5) | 0.57 (0.29–1.12) | 0.73 (0.37–1.43) |
| Tricuspid atresia | 90 | 11 (12.2) | 0.93 (0.51–1.71) | 1.05 (0.56–1.97) |
| Hypoplastic left heart syndrome | 427 | 56 (13.1) | 1 [Reference] | 1 [Reference] |
| Dextrotransposition of the great arteries | 650 | 88 (13.5) | 1.03 (0.76–1.41) | 1.21 (0.87–1.69) |
| Aortic stenosis, critical | 20 | 4 (20.0) | 1.53 (0.61–3.79) | 1.64 (0.46–5.86) |
| Ebstein anomaly | 90 | 19 (21.1) | 1.61 (1.01–2.57) | 1.72(1.02–2.88) |
| Single ventricle | 127 | 28 (22.0) | 1.68 (1.12–2.53) | 1.92(1.26–2.95) |
| Pulmonary stenosis, critical | 101 | 23 (22.8) | 1.74 (1.12–2.68) | 1.94(1.23–3.04) |
| Interrupted aortic arch | 43 | 12 (27.9) | 2.13 (1.24–3.65) | 1.86 (0.98–3.52) |
| Tetralogy of Fallot | 733 | 204 (27.8) | 2.12 (1.62–2.78) | 2.42(1.81–3.24) |
| Double-outlet right ventricle | 94 | 29 (30.9) | 2.35 (1.59–3.47) | 2.90(1.90–4.43) |
| Truncus arteriosus | 68 | 21 (30.9) | 2.35 (1.53–3.62) | 2.60(1.64–4.12) |
| Total anomalous pulmonary venous return | 190 | 78 (41.1) | 3.13 (2.32–4.22) | 3.38(2.44–4.68) |
| Coarctation of the aorta | 801 | 497 (62.0) | 4.73 (3.68–6.08) | 5.26(4.02–6.89) |
| Multiple CCHDd | 192 | 27 (14.1) | 1.07 (0.70–1.64) | 1.40 (0.90–2.17) |
| Estimated year of delivery | ||||
| 1998 | 261 | 81 (31.0) | 1 [Reference] | 1 [Reference] |
| 1999 | 357 | 105 (29.4) | 0.95 (0.74–1.21) | 0.95 (0.75–1.2) |
| 2000 | 351 | 121 (34.5) | 1.11 (0.88–1.40) | 1.07 (0.86–1.34) |
| 2001 | 362 | 109 (30.1) | 0.97 (0.76–1.23) | 1.01 (0.81–1.27) |
| 2002 | 330 | 96 (29.1) | 0.94 (0.73–1.20) | 0.95 (0.75–1.20) |
| 2003 | 352 | 112 (31.8) | 1.03 (0.81–1.30) | 0.96 (0.76–1.22) |
| 2004 | 463 | 131 (28.3) | 0.91 (0.72–1.15) | 0.88 (0.70–1.10) |
| 2005 | 419 | 117 (27.9) | 0.90 (0.71–1.14) | 0.88 (0.70–1.11) |
| 2006 | 444 | 108 (24.3) | 0.78 (0.61–1.00) | 0.71(0.55–0.91) |
| 2007 | 407 | 126 (31.0) | 1.00 (0.79–1.26) | 0.86 (0.68–1.09) |
| Family history of congenital heart defects | ||||
| No | 3613 | 1072 (29.7) | 1 [Reference] | 1 [Reference] |
| Yes | 133 | 34 (25.6) | 0.86 (0.64–1.16) | 0.87 (0.65–1.15) |
| Gestational age, wk | ||||
| <32 (Very preterm) | 138 | 51 (37.0) | 1.26 (1.01–1.58) | 1.20 (0.96–1.50) |
| 32–36 (Preterm) | 557 | 151 (27.1) | 0.93 (0.80–1.07) | 1.04 (0.89–1.22) |
| 37–45 (Full term) | 3020 | 885 (29.3) | 1 [Reference] | 1 [Reference] |
| Unknown/missing | 31 | 19 (61.3) | NC | NC |
| Plurality | ||||
| Singleton | 3509 | 1029 (29.3) | 1 [Reference] | 1 [Reference] |
| Twins or higher-order birth | 229 | 72 (31.4) | 1.07 (0.88–1.31) | 1.03 (0.84–1.27) |
| Unknown/missing | 8 | 5 (62.5) | NC | NC |
| Maternal race/ethnicity | ||||
| Non-Hispanic white | 2285 | 645 (28.2) | 1 [Reference] | 1 [Reference] |
| Non-Hispanic black | 368 | 109 (29.6) | 1.05 (0.88–1.24) | 1.20 (0.99–1.44) |
| Hispanic | 840 | 281 (33.5) | 1.19 (1.06–1.33) | 1.18 (1.00–1.39) |
| Other/unknown | 253 | 71 (28.1) | 0.99 (0.81–1.22) | 1.06 (0.85–1.32) |
| Maternal age at delivery, y | ||||
| ≤24 | 1151 | 356 (30.9) | 1 [Reference] | 1 [Reference] |
| 25–34 | 2014 | 605 (30.0) | 0.97 (0.87–1.08) | 0.96 (0.84–1.08) |
| ≥35 | 581 | 145 (25.0) | 0.81 (0.68–0.95) | 0.87 (0.72–1.05) |
| Maternal education | ||||
| Less than high school graduate | 636 | 204 (32.1) | 1 [Reference] | 1 [Reference] |
| High school graduate or equivalent | 908 | 274 (30.2) | 0.94 (0.81–1.09) | 1.09 (0.92–1.29) |
| College or university, some or graduate | 2132 | 607 (28.5) | 0.89 (0.78–1.01) | 1.08 (0.92–1.28) |
| Unknown/missing | 70 | 21 (30.0) | NC | NC |
| Maternal prepregnancy BMIe | ||||
| <18.5 (Underweight) | 193 | 47 (24.4) | 0.84 (0.65–1.09) | 0.79 (0.61–1.02) |
| 18.5–24.0 (Normal weight) | 1800 | 523 (29.1) | 1 [Reference] | 1 [Reference] |
| 25.0–29.0 (Overweight) | 839 | 257 (30.6) | 1.05 (0.93–1.19) | 1.07 (0.95–1.21) |
| ≥30.0 (Obese) | 733 | 218 (29.7) | 1.02 (0.9–1.17) | 1.02 (0.89–1.18) |
| Unknown/missing | 181 | 61 (33.7) | NC | NC |
| Diabetes mellitus diagnosis before or during index pregnancyf | ||||
| No | 3301 | 977 (29.6) | 1 [Reference] | 1 [Reference] |
| Yes | 420 | 122 (29.0) | 0.98 (0.84–1.15) | 0.91 (0.77–1.08) |
| Unknown/missing | 25 | 7 (28.0) | NC | NC |
| Hypertension at any time | ||||
| No | 3183 | 908 (28.5) | 1 [Reference] | 1 [Reference] |
| Yes | 554 | 195 (35.2) | 1.23 (1.09–1.40) | 1.08 (0.95–1.23) |
| Unknown/missing | 9 | 3 (33.3) | NC | NC |
| Maternal fertility treatments | ||||
| No | 3493 | 1032 (29.5) | 1 [Reference] | 1 [Reference] |
| Yes | 200 | 58 (29.0) | 0.98 (0.79–1.23) | 1.03 (0.83–1.29) |
| Unknown/missing | 53 | 16 (30.2) | NC | NC |
| Previous pregnancy losses | ||||
| None | 2361 | 707 (29.9) | 1 [Reference] | 1 [Reference] |
| 1 | 853 | 241 (28.3) | 0.94 (0.83–1.07) | 0.93 (0.82–1.05) |
| 2 | 324 | 94 (29.0) | 0.97 (0.81–1.16) | 1.04 (0.87–1.24) |
| ≥3 | 189 | 59 (31.2) | 1.04 (0.84–1.30) | 1.06 (0.85–1.31) |
| Unknown/missing | 19 | 5 (26.3) | NC | NC |
| First prenatal care visit | ||||
| First trimester | 3104 | 909 (29.3) | 1 [Reference] | 1 [Reference] |
| Second trimester | 415 | 127 (30.6) | 1.04 (0.90–1.22) | 0.93 (0.80–1.09) |
| Third trimester | 25 | 7 (28.0) | 0.96 (0.51–1.80) | 0.94 (0.48–1.81) |
| Unknown/missing | 202 | 63 (31.2) | NC | NC |
| Study site | ||||
| A | 537 | 117 (21.8) | 1 [Reference] | 1 [Reference] |
| B | 582 | 146 (25.1) | 1.15 (0.93–1.42) | 1.17 (0.95–1.46) |
| C | 460 | 123 (26.7) | 1.23 (0.98–1.53) | 1.18 (0.92–1.52) |
| D | 383 | 105 (27.4) | 1.26 (1.00–1.58) | 1.32(1.05–1.67) |
| E | 359 | 98 (27.3) | 1.25 (0.99–1.58) | 1.40(1.11–1.77) |
| F | 229 | 67 (29.3) | 1.34 (1.04–1.74) | 1.35 (1.04–1.75) |
| G | 329 | 104 (31.6) | 1.45 (1.16–1.82) | 1.40 (1.10–1.79) |
| H | 465 | 174 (37.4) | 1.72 (1.41–2.10) | 1.75 (1.41–2.17) |
| I | 402 | 172 (42.8) | 1.96 (1.61–2.39) | 2.09 (1.66–2.63) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CCHD, critical congenital heart disease; NC, not calculated; PR, prevalence ratio.
Adjusted results from a Poisson regression model with robust error variance that included all listed variables and excluded infants with at least 1missing value for any included variable. Boldface indicates statistically significant (P < .05).
Defined as a diagnosis more than 3 days after birth via echocardiography or autopsy.
Chromosomal abnormalities, single-gene disorders, and birth defects with known etiology are excluded from the National Birth Defects Prevention Study.
Multiple CCHD refers to more than 1 screening-detectable CCHD.
Calculated from self-reported height and weight.
Includes types 1 and 2 and gestational.
Discussion
Based on data from the NBDPS, we estimated that the diagnosis of nonsyndromic CCHD occurred more than 3 days after birth in 29.5%of infants, including fewer than 1%with the initial diagnosis at autopsy. These infants, therefore, might have benefited from universal screening through pulse oximetry at their birth hospitals. Infants with extracardiac defects were significantly less likely to have late detection, and late detection varied by CCHD type and NBDPS study site.
Our study focused explicitly on the potential effect of new US recommendations for CCHD screening using multisite data and examined the diagnostic experience of infants with CCHD during the entire first year of life. Our estimate is similar to that of a retrospective study at an NBDPS contributing site— metropolitan Atlanta—that estimated that 31.3%of infants with CCHD did not receive a diagnosis on their day of birth.31 Other retrospective US studies with substantially lower estimated proportions of infants with late detection of CCHD (ie, 4%–7%)32,35 examined fewer CCHD types than our study or identified late detection of CCHD only through the occurrence of death.33,35 However, estimates from most previous studies of late CCHD detection28–30,32–35 (Table 1) appear to have included infants with genetic disorders, whereas our study excluded such infants. Most previous studies29,30,32,35 used exclusively administrative coding to identify CCHD diagnoses, which might inaccurately classify heart defects or fail to capture whether a defect such as aortic stenosis or pulmonary stenosis is critical.36,37 Previous studies of late CCHD detection also used different data sources—such as hospital admission records with or without accompanying statewide death records— to identify infants with late detection of CCHD. One previous study30 ascertained infants with late detection of CCHD less than 1 month after birth. Finally, previous studies were limited to 2 hospitals,34 a single metropolitan area,31,35 or a single state.28–30,32,33
In our study, the prevalence of late detection varied widely (from 7.5% to 62.0%) by CCHD type. Evidence suggests that the sensitivity of CCHD screening through pulse oximetry also may vary substantially by CCHD type—a proxy for the presence of hypoxemia. A recent meta-analysis2 reported that pulse oximetry conducted at least 24 hours after birth was 78% sensitive to detect CCHD overall. However, a review of 13 screening studies43 (with 258 809 infants undergoing screening, of whom 256 were ultimately diagnosed as having CCHD) from 1998 through 2009 reported sensitivities ranging from 36% (95% CI, 24%–50%) for coarctation of the aorta and interrupted aortic arch (18 of 50 infants) to 100% (95% CI, 44%–100%) for single ventricle (6 of 6 infants), double-outlet right ventricle (5 of 5 infants), and pulmonary atresia with intact septum (3 of 3 infants). Screening-detectable CCHD constitutes a heterogeneous group of rare congenital heart defects, and the numbers of infants included in these CCHD defect–specific estimates are very small. The high rate of late detection among infants with coarctation of the aorta (62.0%) in our study influenced our overall estimate of 29.5% late detection; excluding these infants would result in an overall estimate of late detection in 609 of 2945 (20.7% [95% CI, 19.2%–22.2%]). Nonhypoxemic cases of coarctation of the aorta (ie, not detectable through screening) likely contributed to our estimated prevalence of late detection for that condition. Unfortunately, we were unable to ascertain lesion severity.
Infants with extracardiac defects were less likely to have late detection of CCHD in our study. Infants with birth defects affecting multiple organ systems may receive additional medical attention prenatally or at birth, which might explain why late detection was significantly lower among such infants. The proportion of infants in our study with nonsyndromic extracardiac defects (17.0% [95% CI, 15.8%- 18.2%]) was similar to those of other population-based studies of infants and children with congenital heart defects.44,45 However, because the NBDPS excludes infants with genetic syndromes, our study might have estimated a higher proportion of late detection than actually exists in the population. Our results also indicated that late CCHD detection varied significantly among the 9 NBDPS study sites included in this analysis. This variation may reflect, in part, nonuniformity in neonatal clinical practice, which cannot be addressed using existing birth defects surveillance data in the NBDPS. In addition, the NBDPS sites draw from different populations in terms of socioeconomic status, urbanicity, and geographic region; thus, inference about the underlying meaning of the observed study site variability would require further investigation.
We found no significant temporal trend in terms of increasing or decreasing prevalence of late CCHD detection during the study period (Table 2). Recent studies46–49 have reported inconsistent findings about whether race/ethnicity is associated with outcomes such as mortality and hospital readmission among infants with congenital heart defects, although no significant racial/ethnic associations were observed in this analysis. We found no significant association between the timing of the first prenatal care visit and timely CCHD detection; however, this variable is a limited indicator of the experience of prenatal care.
This study has a number of limitations. The NBDPS does not explicitly seek information on the initial diagnosis of congenital heart defects, but instead a diagnosis by specific means (echocardiography, autopsy, catheterization, or surgery). Therefore, we may have overestimated the proportion of infants with late CCHD detection owing to missing information on initial diagnoses. However, echocardiography is recommended to diagnose CCHD, even if an infant receives a definitive diagnosis through other means.3,50 Missing or erroneous examination information might vary by NBDPS site because ascertainment of follow-up records (ie, outpatient echocardiography) is not standardized. Another limitation is that we restricted our analysis to infants in the NBDPS whose mothers were interviewed. Because infants of noninterviewed mothers did not undergo classification by NBDPS clinicians, we were unable to compare the 2 groups. A related limitation is that many of the factors we assessed were based on mothers’ self-reported demographic and clinical information (ie, timing of entry into prenatal care, diabetes mellitus status, and prepregnancy body mass index).
Our study has 3 notable strengths that distinguish it from previous US studies. First, we used data compiled from multiple population-based birth defects surveillance programs that included infants with clinically validated CCHD diagnoses.51 Second, because we used abstracted medical records to identify and classify infants according to CCHD type, we likely have achieved greater clinical accuracy than previous studies that relied exclusively on administrative data to classify CCHD diagnoses. Third, we used clinical definitions of CCHD and timely detection that are directly pertinent to new US federal recommendations for universal newborn screening for CCHD through pulse oximetry.
Conclusions
We estimate that 29.5% of live-born infants with nonsyndromic CCHD in the NBDPS received the diagnosis more than 3 days after birth. The proportions of infants with late CCHD detection varied substantially by CCHD type, from 7.5% (pulmonary atresia) to 62.0% (coarctation of the aorta). These results suggest that many infants with CCHD might benefit from screening through pulse oximetry before birth hospital discharge. Whether such infants are detected through screening is likely to vary by a number of factors, including CCHD type and the presence of extracardiac defects. Additional population-based studies of universal screening in practice are needed.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by cooperative agreements under PA 96043, PA 02081 and FOA DD09-001 from the Centers for Disease Control and Prevention (CDC).
Role of the Sponsor: The funding source designed the protocol for the original data collection and approved authors’ protocol for this study. The funding source had no role in the design and conduct of the study; analysis or interpretation of the data; and preparation of the manuscript. The CDC and the California Department of Public Health reviewed the manuscript and approved submission for publication.
We thank the participating families, staff, and scientists who contribute to the National Birth Defects Prevention Study. We thank the Maternal, Child, and Adolescent Health Program of the California Department of Public Health for providing data on study subjects for the National Birth Defects Prevention Study.
Footnotes
Author Contributions: Drs Peterson and Ailes contributed equally to this study. Drs Peterson and Ailes had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Peterson, Ailes, Gilboa.
Acquisition of data: Peterson.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Peterson.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ailes, Gilboa.
Administrative, technical, or material support: All authors.
Study supervision: Gilboa.
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the California Department of Public Health.
REFERENCES
- 1.Mahle WT, Newburger JW, Matherne GP, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 2.Thangaratinam S, Daniels J, Ewer AK, Zamora J, Khan KS. Accuracy of pulse oximetry in screening for congenital heart disease in asymptomatic newborns: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F176–F180. doi: 10.1136/adc.2006.107656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5):e1259–e1267. doi: 10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 4.Ewer AK. Review of pulse oximetry screening for critical congenital heart defects in newborn infants. Curr Opin Cardiol. 2013;28(2):92–96. doi: 10.1097/HCO.0b013e32835d7e42. [DOI] [PubMed] [Google Scholar]
- 5.Discretionary Advisory Committee on Heritable Disorders in Newborns and Children. [Accessed November 1, 2011];HHS Secretary adopts recommendation to add critical congenital heart disease to the recommended uniform screening panel. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/index.html. Published 2011.
- 6.Centers for Disease Control and Prevention. Rapid implementation of pulse oximetry newborn screening to detect critical congenital heart defects: New Jersey, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(15):292–294. [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Assessment of current practices and feasibility of routine screening for critical congenital heart defects: Georgia, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(15):288–291. [PMC free article] [PubMed] [Google Scholar]
- 8.Hoke TR, Donohue PK, Bawa PK, et al. Oxygen saturation as a screening test for critical congenital heart disease: a preliminary study. Pediatr Cardiol. 2002;23(4):403–409. doi: 10.1007/s00246-002-1482-8. [DOI] [PubMed] [Google Scholar]
- 9.Reich JD, Miller S, Brogdon B, et al. The use of pulse oximetry to detect congenital heart disease. J Pediatr. 2003;142(3):268–272. doi: 10.1067/mpd.2003.87. [DOI] [PubMed] [Google Scholar]
- 10.Reich JD, Connolly B, Bradley G, et al. The reliability of a single pulse oximetry reading as a screening test for congenital heart disease in otherwise asymptomatic newborn infants. Pediatr Cardiol. 2008;29(5):885–889. doi: 10.1007/s00246-008-9214-3. [DOI] [PubMed] [Google Scholar]
- 11.Schultz AH, Localio AR, Clark BJ, Ravishankar C, Videon N, Kimmel SE. Epidemiologic features of the presentation of critical congenital heart disease: implications for screening. Pediatrics. 2008;121(4):751–757. doi: 10.1542/peds.2007-0421. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw EA, Cuzzi S, Kiernan SC, Nagel N, Becker JA, Martin GR. Feasibility of implementing pulse oximetry screening for congenital heart disease in a community hospital. J Perinatol. 2012;32(9):710–715. doi: 10.1038/jp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh W. Evaluation of pulse oximetry screening in Middle Tennessee: cases for consideration before universal screening. J Perinatol. 2011;31(2):125–129. doi: 10.1038/jp.2010.70. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Harb M, Hey E, Wren C. Death in infancy from unrecognised congenital heart disease. Arch Dis Child. 1994;71(1):3–7. doi: 10.1136/adc.71.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wren C, Richmond S, Donaldson L. Presentation of congenital heart disease in infancy: implications for routine examination. Arch Dis Child Fetal Neonatal Ed. 1999;80(1):F49–F53. doi: 10.1136/fn.80.1.f49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakr AF, Habib HS. Combining pulse oximetry and clinical examination in screening for congenital heart disease. Pediatr Cardiol. 2005;26(6):832–835. doi: 10.1007/s00246-005-0981-9. [DOI] [PubMed] [Google Scholar]
- 17.Rosati E, Chitano G, Dipaola L, De Felice C, Latini G. Indications and limitations for a neonatal pulse oximetry screening of critical congenital heart disease. J Perinat Med. 2005;33(5):455–457. doi: 10.1515/JPM.2005.080. [DOI] [PubMed] [Google Scholar]
- 18.Arlettaz R, Bauschatz AS, Mönkhoff M, Essers B, Bauersfeld U. The contribution of pulse oximetry to the early detection of congenital heart disease in newborns. Eur J Pediatr. 2006;165(2):94–98. doi: 10.1007/s00431-005-0006-y. [DOI] [PubMed] [Google Scholar]
- 19.Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92(9):1298–1302. doi: 10.1136/hrt.2005.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellander M, Sunnegårdh J. Failure to diagnose critical heart malformations in newborns before discharge: an increasing problem? Acta Paediatr. 2006;95(4):407–413. doi: 10.1080/08035250500541910. [DOI] [PubMed] [Google Scholar]
- 21.Ruangritnamchai C, Bunjapamai W, Pongpanich B. Pulse oximetry screening for clinically unrecognized critical congenital heart disease in the newborns. Images Paediatr Cardiol. 2007;9(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- 22.Meberg A, Brügmann-Pieper S, Due R, Jr, et al. First day of life pulse oximetry screening to detect congenital heart defects. J Pediatr. 2008;152(6):761–765. doi: 10.1016/j.jpeds.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 23.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F33–F35. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 24.de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meberg A, Andreassen A, Brunvand L, et al. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr. 2009;98(4):682–686. doi: 10.1111/j.1651-2227.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 26.Ewer AK, Middleton LJ, Furmston AT, et al. PulseOx Study Group. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378(9793):785–794. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 27.Turska Kmieć A, Borszewska Kornacka MK, Błaż W, Kawalec W, Zuk M. Early screening for critical congenital heart defects in asymptomatic newborns in Mazovia province: experience of the POLKARD pulse oximetry programme 2006–2008 in Poland. Kardiol Pol. 2012;70(4):370–376. [PubMed] [Google Scholar]
- 28.Garg LF, Van Naarden Braun K, Knapp MM, et al. Results from the New Jersey Statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2):e314–e323. doi: 10.1542/peds.2013-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson C, Dawson A, Grosse SD, et al. Hospitalizations, costs, and mortality among infants with critical congenital heart disease: how important is timely detection? Birth Defects Res A Clin Mol Teratol. 2013;97(10):664–672. doi: 10.1002/bdra.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng B, Hokanson J. Missed congenital heart disease in neonates. Congenit Heart Dis. 2010;5(3):292–296. doi: 10.1111/j.1747-0803.2010.00418.x. [DOI] [PubMed] [Google Scholar]
- 31.Oster ME, Lee KA, Honein MA, Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aamir T, Kruse L, Ezeakudo O. Delayed diagnosis of critical congenital cardiovascular malformations (CCVM) and pulse oximetry screening of newborns. Acta Paediatr. 2007;96(8):1146–1149. doi: 10.1111/j.1651-2227.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 33.Chang RK, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 2008;162(10):969–974. doi: 10.1001/archpedi.162.10.969. [DOI] [PubMed] [Google Scholar]
- 34.Koppel RI, Druschel CM, Carter T, et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111(3):451–455. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 35.Kuehl KS, Loffredo CA, Ferencz C. Failure to diagnose congenital heart disease in infancy. Pediatrics. 1999;103(4, pt 1):743–747. doi: 10.1542/peds.103.4.743. [DOI] [PubMed] [Google Scholar]
- 36.Frohnert BK, Lussky RC, Alms MA, Mendelsohn NJ, Symonik DM, Falken MC. Validity of hospital discharge data for identifying infants with cardiac defects. J Perinatol. 2005;25(11):737–742. doi: 10.1038/sj.jp.7211382. [DOI] [PubMed] [Google Scholar]
- 37.Strickland MJ, Riehle-Colarusso TJ, Jacobs JP, et al. The importance of nomenclature for congenital cardiac disease: implications for research and evaluation. Cardiol Young. 2008;18(suppl 2):92–100. doi: 10.1017/S1047951108002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A National Birth Defects Prevention Study. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA National Birth Defects Prevention Study. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 41.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Tan CS, Chia KS. A practical guide for multivariate analysis of dichotomous outcomes. Ann Acad Med Singapore. 2009;38(8):714–719. [PubMed] [Google Scholar]
- 43.Prudhoe S, Abu-Harb M, Richmond S, Wren C. Neonatal screening for critical cardiovascular anomalies using pulse oximetry. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F346–F350. doi: 10.1136/archdischild-2012-302045. [DOI] [PubMed] [Google Scholar]
- 44.Lurie IW, Kappetein AP, Loffredo CA, Ferencz C. Non-cardiac malformations in individuals with outflow tract defects of the heart: the Baltimore-Washington Infant Study (1981–1989) Am J Med Genet. 1995;59(1):76–84. doi: 10.1002/ajmg.1320590116. [DOI] [PubMed] [Google Scholar]
- 45.Oyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. National time trends in congenital heart defects, Denmark, 1977–2005. Am Heart J. 2009;157(3):467.e1–473.e1. doi: 10.1016/j.ahj.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Ingaramo OA, Khemani RG, Markovitz BP, Epstein D. Effect of race on the timing of the Glenn and Fontan procedures for single-ventricle congenital heart disease. Pediatr Crit Care Med. 2012;13(2):174–177. doi: 10.1097/PCC.0b013e3182231862. [DOI] [PubMed] [Google Scholar]
- 47.Nembhard WN, Salemi JL, Ethen MK, Fixler DE, Dimaggio A, Canfield MA. Racial/ethnic disparities in risk of early childhood mortality among children with congenital heart defects. Pediatrics. 2011;127(5):e1128–e1138. doi: 10.1542/peds.2010-2702. [DOI] [PubMed] [Google Scholar]
- 48.Kogon B, Jain A, Oster M, Woodall K, Kanter K, Kirshbom P. Risk factors associated with readmission after pediatric cardiothoracic surgery. Ann Thorac Surg. 2012;94(3):865–873. doi: 10.1016/j.athoracsur.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Fixler DE, Nembhard WN, Xu P, Ethen MK, Canfield MA. Effect of acculturation and distance from cardiac center on congenital heart disease mortality. Pediatrics. 2012;129(6):1118–1124. doi: 10.1542/peds.2011-3114. [DOI] [PubMed] [Google Scholar]
- 50.Mahle WT, Martin GR, Beekman RH, III, Morrow WR Section on Cardiology and Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129(1):190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 51.Olney RS, Botto L. Newborn screening for critical congenital heart disease: essential public health roles for birth defects monitoring programs. Birth Defects Res A Clin Mol Teratol. 2012;94(12):965–969. doi: 10.1002/bdra.23103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.