Abstract
BACKGROUND
The purpose of this study was to describe the development and early implementation of a national spina bifida (SB) patient registry, the goal of which is to monitor the health status, clinical care, and outcomes of people with SB by collecting and analyzing patient data from comprehensive SB clinics.
METHODS
Using a web-based, SB-specific electronic medical record, 10 SB clinics collected health-related information for patients diagnosed with myelomeningocele, lipomyelomeningocele, fatty filum, or meningocele. This information was compiled and de-identified for transmission to the Centers for Disease Control and Prevention (CDC) for quality control and analysis.
RESULTS
A total of 2070 patients were enrolled from 2009 through 2011: 84.9% were younger than 18 years of age; 1095 were women; 64.2% were non-Hispanic white; 6.5% were non-Hispanic black or African American; and 24.2% were Hispanic or Latino. Myelomeningocele was the most common diagnosis (81.5%).
CONCLUSIONS
The creation of a National Spina Bifida Patient Registry partnership between the CDC and SB clinics has been feasible. Through planned longitudinal data collection and the inclusion of additional clinics, the data generated by the registry will become more robust and representative of the population of patients attending SB clinics in the United States and will allow for the investigation of patient outcomes.
Keywords: spina bifida, registry, quality improvement, spina bifida clinic, research
INTRODUCTION
Advances in medical care and technology have resulted in greater expected longevity for babies born with spina bifida (SB) (Bowman et al., 2001). As the life expectancy of individuals with SB increases, the number of adults in the United States living with SB is growing in spite of a diminishing incidence (Dillon, et al., 2000; Williams, et al., 2005). Meanwhile, knowledge regarding the health status and long-term health outcomes of people with SB is limited. This is significant because people with SB often experience preventable, condition-specific difficulties such as infections, pressure sores, and kidney stones, as well as secondary conditions such as renal failure, all of which detrimentally influence key aspects of their lives (Kinsman and Doehring, 1996). Prior attempts to collect SB-related patient information have used small convenience or clinic samples, which limit generalizability. To gain a better understanding of SB clinics in the United States, in 2005, the Spina Bifida Association (SBA) began a collaboration with the Agency for Healthcare Research and Quality, Centers for Disease Control and Prevention (CDC), Delmarva Foundation for Medical Care (Delmarva), and National Initiative for Children’s Healthcare Quality to survey these clinics. The goal of this effort was to obtain a clearer overall picture of patients attending SB clinics, gain an understanding of clinic operations and services, and elicit information related to care processes and outcomes with the goal of strengthening the quality of clinical care.
Based on this assessment and the experiences of other organizations (such as the Cystic Fibrosis Foundation [Goss et al., 2002]), the SBA Professional Advisory Council proposed the establishment of the National Spina Bifida Patient Registry (NSBPR). The goals of the proposed effort were to provide the infrastructure to support SB clinical research, to promote a systematic approach to describe the SB clinic population, and to document and improve the quality of SB clinical care by pooling data across multiple sites.
The primary focus of the NSBPR has been to describe the patient population attending SB clinics and to detect variations in processes of care that are associated with better health outcomes. This focus requires a clinic-based rather than a population-based SB patient registry (Gliklich and Dreyer, 2012). Although this approach excludes individuals who do not seek care from SB clinics, it provides a sample of patients who do attend these clinics for credible analyses of practices and outcomes. Further, care from an integrated, multidisciplinary, or interdisciplinary SB clinic is recommended best practice (Kaufman et al., 1994). The clinic-based approach is similar to that used by the Cystic Fibrosis Foundation Patient Registry, which has been successful in providing valid data for descriptive reports and hypotheses-driven analyses, and has served as a driver of quality improvement activities in the cystic fibrosis community (Schechter, 2008). The assumption is that data provided from the clinics are reliable and by using the clinic as the unit of analysis, it is possible to obtain a more accurate report of the variety of treatments provided and a clearer picture of the relationship between these treatments and health outcomes. The purpose of this article is to describe the development, implementation, and early success at establishing the NSBPR. We aim to demonstrate the feasibility of launching and maintaining a patient data registry focused on SB clinics.
METHODS
Site and Patient Selection
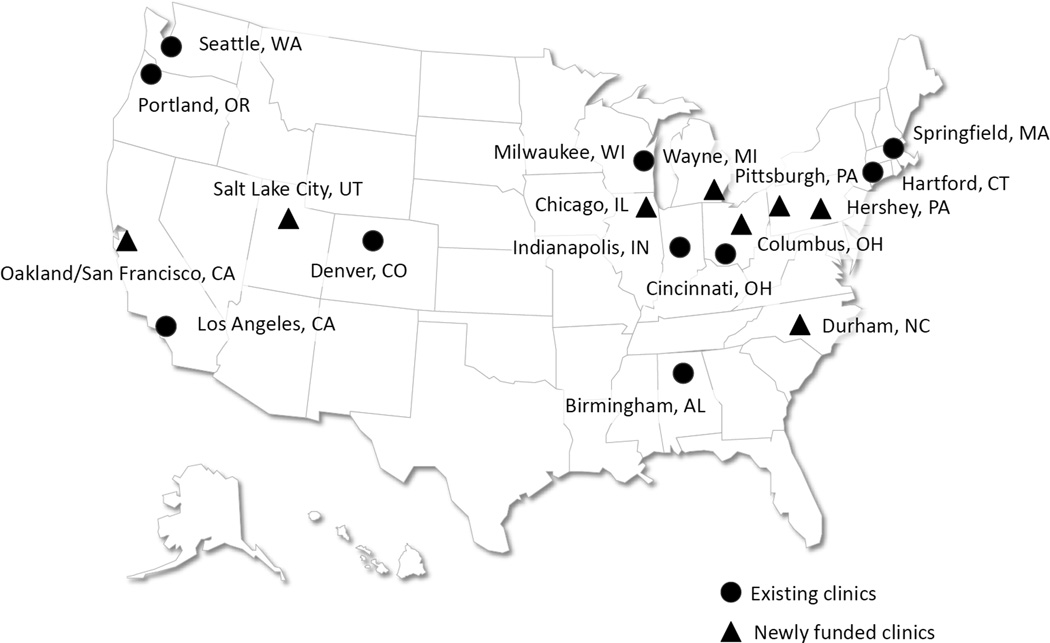
In 2008, the CDC solicited applications from SB clinics to examine the feasibility of using a standardized tool to collect information on patients with SB. Clinics were eligible to apply if they had previously indicated interest in national SB efforts through participation in a 2005 SBA survey, (which was described in the Introduction to this article), were multidisciplinary, and cared for a minimum of 250 patients with SB in the year before applying (to help ensure sufficient enrollment and clinic capacity during the piloting of the registry). Between September 2008 and August 2009, nine SB clinic sites (10 actual clinics; one site included two clinics) were funded to participate in the registry. Figure 1 also indicates newly funded clinics at which data collection had not begun at the time of manuscript development. After receiving institutional review board approval, clinics were expected to contribute longitudinal data on patients having one of four diagnoses, (myelomeningocele, meningocele, lipomyelomeningocele, or fatty filum), and consent and assent (based on youth’s age) to participate. Each enrolled patient would be followed longitudinally for the entire funded period. Clinics began enrollment in 2009 and, within 15 months of the initial funding, all sites were collecting patient data. A descriptive summary of demographic data on those who did not participate is being collected at each site.
Figure 1.
Geographic distribution of multidisciplinary clinics in the National Spina Bifida Registry project, 2011.
SB Registry Development
Selection of core registry variables
A group of SB health care professionals, parents and people affected by SB, and representatives of the SBA, CDC, Agency for Healthcare Research and Quality, and National Initiative for Children’s Healthcare Quality met in Washington, DC, to identify and discuss hundreds of variables considered to be critical to the care and lives of the population with SB. The list was reduced to 20 questions that were considered to be valid indicators of clinical status, feasible to collect, and definable in a uniform way (Schechter, 2008). These questions included basic demographic information; neurosurgery, orthopedic, and urologic procedures; growth measures; and outcomes such as mobility, continence, and pressure sores (See Supplemental Tables 1, 2, and 3, online only).
Data collection tool
Using the hemophilia treatment centers system as a model in which registry variables are embedded in an electronic medical record (EMR) (Versel, 2010), a web-based Spina Bifida-Electronic Medical Record (SB-EMR) was developed to: (1) save time in record keeping and tracking, (2) enhance the quality and availability of data for clinical use and research, (3) facilitate data sharing for studies and collaborations, and (4) improve patient care through the proper comparison of care delivered and outcomes realized across clinical programs. The use of the SB-EMR to collect information from the 20 core registry questions provides a reliable, standardized method for data collection and management for the clinics participating in the registry.
Data Entry and Data Quality Assurance
Both medical record abstraction and patient interview were the sources of patient data. Data were collected via standardized paper forms and entered into the SB-EMR, then reviewed for accuracy and completeness by clinic staff, after which the de-identified data were electronically transmitted to the CDC for further analysis.
To ensure the quality of registry data, systematic procedures were implemented at the SB clinic sites and at the CDC Data Management Center during the registry onset and implementation phase. For example, a ‘Report Functionality’ tool, a single page data summary screen, embedded into the EMR application as part of its build, prevents clinics from transmitting data to the CDC if data elements are missing. In addition to the Report Functionality tool, the compiled dataset goes through an automatic Quality Assurance/Quality Control (QA/QC) system at the CDC. SAS software (version 9.3; SAS Institute Inc., Cary, NC) is used for data management and SAS macros were developed for data QA/QC to identify duplications, discrepancies, outliers, and data entry errors. The automatic system generates site-specific data quality reports that are sent to the corresponding clinics to check and correct the data as necessary. At each clinic site and at the initiation of data collection, confirmation of the information obtained from the medical record is performed for a minimum of 3 months. A minimum of 20% of the records submitted per month are randomly selected and all of the data elements are verified by someone other than the initial abstractor. (Table 1 has more detailed activities of the data quality assurance at clinic sites and the CDC.)
Table 1.
Procedures at the CDC Data Management Center and local sites during different stages of registry development and implementation for the assurance of data quality (modified from Arts et al., 2002)
| CDC Data Management Center | Local clinic sites |
|---|---|
Registry onset
|
Registry onset
|
Implementation
|
Implementation
|
Ongoing
|
Ongoing
|
Future
|
Future Participate in reliability study. |
CDC, Centers for Disease Control and Prevention; SB, spina bifida; PI, XXX; SB-EMR, Spina Bifida-Electronic Medical Record.
RESULTS
A total of 2070 patients were enrolled in the registry from 2009 to 2011. The patient enrollment ranged from 61 to 382 patients per clinic site with six sites each enrolling over 200 patients. The rate of refusal per site ranged from none to 14.6% with an overall refusal rate of 6.5%.
Only a few of the participating clinics provide services to adults and thus the sample predominantly consisted of patients younger than 18 years. In addition, there were more women than men. Although primarily white, the ethnic diversity of the sample does reflect the underlying catchment area populations of the participating clinics. Individuals of Hispanic ethnicity have been reported to have a higher incidence of SB than black or African Americans and the sample reflects that pattern. Myelomeningocele was the predominant diagnosis and all levels of lesion are represented (Table 2).
Table 2.
Patient Demographics
| Variable | No. of patients (%) |
|---|---|
| Age at enrollment (yrs) | |
| Younger than 2 | 349 (16.9) |
| 2 to <5 | 330 (15.9) |
| 5 to <10 | 431 (20.8) |
| 10 to <13 | 245 (11.8) |
| 13 to <18 | 403 (19.5) |
| 18 to <22 | 200 (9.7) |
| 22 or older | 112 (5.4) |
| Sex | |
| Male | 975 (47.1) |
| Female | 1095 (52.9) |
| Race/ethnicity | |
| Non-Hispanic white | 1309 (64.2) |
| Non-Hispanic black | 132 (6.5) |
| Hispanic or Latino | 487 (24.2) |
| Other | 142 (5.2) |
| SB type | |
| Myelomeningocele | 1686 (81.5) |
| Meningocele | 55 (2.7) |
| Lipomyelomeningocele | 279 (13.5) |
| Fatty Filum | 49 (2.4) |
| Level of lesion | |
| Thoracic (flaccid lower extremities) | 295 (14.3) |
| High-lumbar (hip flexion present) | 197 (9.5) |
| Mid-lumbar (knee extension present) | 530 (25.6) |
| Low-lumbar (foot dorsiflexion present) | 370 (17.9) |
| Sacral (foot plantar flexion present) | 678 (32.8) |
Note: Site distribution ranged from 61 (2.9%) to 382 (18.5%).
SB, spina bifida.
There have been challenging issues in the interpretation of particular registry variables (i.e., urinary continence), which have been resolved through extensive discussion both on monthly coordinating committee conference calls and during annual coordinating committee meetings. The methods of collecting height and weight measurements are still under discussion, as variations in measurement practices have been detected across clinics.
In an evaluation of data quality assurance procedures, quality of data transmitted before and after implementation of the Report Functionality tool in October 2010 was compared. Before the Report Functionality tool was available, missing values were as high as 15%. After the tool was activated in the EMR, missing values in core data elements (n = 1464 patients) declined to <0.05% and data accuracy appeared to improve (Soe et al., 2011). In further efforts to improve data quality, the automatic QA/QC system was implemented in 2011 followed by an evaluation of the procedures. In December 31, 2011, the CDC received cumulative data on 2070 registry participants from the original 10 sites. Data quality warnings were identified in two patients (0.10%) from demographics data and 35 patients (1.69%) from interventions and outcomes data (Liu et al., 2012).
DISCUSSION
The greatest challenges in researching a rare condition is the lack of information regarding the course of the disease and the effectiveness of treatments on a sufficient amount of individuals to allow for meaningful interpretations. The small numbers of patients and lack of patient diversity found in single clinics or in small groups of clinics can lead to bias in reporting the status of these conditions (Morgan et al., 1999). Thus, research relying on participant selection from single or small groups of clinics can be problematic. Based on the successful experiences of groups focusing on rare conditions such as cystic fibrosis (Morgan et al., 1999), Pompe disease (Byrne et al., 2011), and growth hormone deficiency (Pugeat, 2004), the CDC in collaboration with the SBA has designed a registry to collect prospective and longitudinal information on patients from multiple SB clinics across the United States. In the first years of the registry, a process was developed to collect valid data, and, as of 2011, 10 clinics have submitted data on more than 2000 patients. Condition-specific registries, such as this one, allow for observational studies that collect data to follow patterns in diagnoses, treatments, and outcomes over time in existing practice settings, affording the opportunity for robust analysis of pertinent issues (Gliklich and Dreyer, 2010).
Experience thus far has demonstrated that a network of SB clinics can be accessed to systematically document health care and health outcomes experiences. Building a partnership between the CDC and SB clinics in the United States will ensure that key stakeholders with an interest in optimal outcomes for individuals affected by SB have an influential voice in the pragmatic implementation of the registry. The clinics have worked together with the CDC to manage the operation of the registry, helping to guide analysis and interpretation of the data. Well-designed patient registries are powerful tools for understanding condition progression and management (Schechter, 2008). The goal of the NSBPR is to monitor systematically the course of SB, with the expectation that the variations in care and subsequent patient outcomes will suggest modifiable practices that are most successful.
Study Limitations and Future Direction
Transition from pediatric to adult care is an important issue to this and other populations with chronic complex conditions. Although not addressed in the early stages of the development and implementation of this registry, the NSBPR should heighten awareness of the issue, as registry participants “age out” of their clinics and of the registry data collection system. Transition is a future interest of the registry and at least one site is piloting transition-specific items for future use in the registry.
Current practice of data abstraction and entry redundancy can also affect the long-term sustainability of the NSBPR. At this time, there is no automatic link between legacy medical record systems of participating SB clinics and the SB EMR software, which requires redundant entries of patient data into both the hospital medical record and the SB-EMR. Although in most cases it takes only a short time (3–5 minutes per patient) for a staff member to enter registry data annually, total resource use for this purpose is not clear. Use of the SB-EMR application can enhance care coordination and the maintenance of SB-specific information to result in a more comprehensive and efficient approach to care provision and follow-up in the multidisciplinary SB clinic setting. Considering the data entry redundancy and its potential adverse impact on the sustainability of the NSBPR, as well as the value of the SB-EMR, to clinic functions and care coordination, the SBA is funding a study at three clinics participating in the registry to explore the feasibility of automatically transferring data from a legacy EMR into the SB-EMR and to demonstrate the utility of the SBEMR application to facilitate and improve the logistics of managing SB clinic functions. Results from this study are anticipated in late 2012.
A registry that focuses on patients of SB clinics might not be representative of the entire SB population, because the percentage of the SB population that attends SB clinics is not known. There are a variety of reasons for people with SB to not attend a clinic, including the unavailability of age-appropriate services for adults, a lack of funding for travel and time away from work, geographic remoteness, and a family’s wish to use specialists of their own choosing and scheduling. Patients in good health might place a lower priority on attending an SB clinic for these or other reasons. Because the health status of those not attending SB clinics is an area of importance, efforts to identify and collect data on patients not attending SB clinics will be included in future research.
CONCLUSION
The NSBPR was designed and created through a partnership between the CDC and the SBA along with a core group of 10 SB clinics to monitor clinical care and the health status of patients attending comprehensive SB clinics. The goals of the registry are to describe the demographic and condition-relevant characteristics of the SB population, provide a platform for clinical and epidemiologic research, and to allow comparisons of clinical care and outcomes among clinics for benchmarking purposes and quality improvement. Having demonstrated the feasibility of the effort through successful recruitment and data collection, the current plan is to expand the registry into additional clinics and enroll more patients into the longitudinal database. Further, interested researchers and clinicians will be recruited to participate and use the data for analyses and initiatives that will allow the realization of our goals.
Supplementary Material
ACKNOWLEDGMENTS
The development of the National Spina Bifida Patient Registry has been successful due to the contributions of all the members of the NSBPR Coordinating Committee, 2008–2011: William Walker, Seattle Children’s Hospital; Kathryn Smith, Children’s Hospital, Los Angeles; Kurt Freeman, Oregon Health and Science University; Pamela Wilson, Children’s Hospital Colorado; Kathleen Sawin, Children’s Hospital of Wisconsin; Jeffrey Thomson, Connecticut Children’s Medical Center; Heidi Castillo, Cincinnati Children’s Hospital Medical Center; Timothy Brei, Riley Hospital for Children; David Joseph, Children’s Hospital of Alabama; and The Spina Bifida Association.
The National Spina Bifida Patient Registry is funded by the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia, grant #1UO1DDD000744.01.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Additional Supporting Information may be found in the online version of this article.
The authors have no conflicts of interest to report.
REFERENCES
- Arts DG, De Keizer NF, Scheffer GJ. Defining and improving data quality in medical registries: a literature review, case study, and generic framework. J Am Med Inform Assoc. 2002;9:600–611. doi: 10.1197/jamia.M1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34:114–120. doi: 10.1159/000056005. [DOI] [PubMed] [Google Scholar]
- Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab. 2011;103:1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Dillon CM, Davis BE, Duguay S, et al. Longevity of patients born with myelomeningocele. Eur J Pediatr Surg. 2000;10(Suppl 1):33–34. doi: 10.1055/s-2008-1072412. [DOI] [PubMed] [Google Scholar]
- Gliklich RE, Dreyer NA, editors. Registries for evaluating patient outcomes: a user’s guide. 2nd ed. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Sep, (Prepared by Outcome DEcIDE Center [Outcome Sciences, Inc. d/b/a Outcome] under Contract No. HHSA29020050035I TO3.) AHRQ Publication No. 10-EHC049. [PubMed] [Google Scholar]
- Goss CH, Mayer-Hamblett N, Kronmal RA, Ramsey BW. The cystic fibrosis therapeutics development network (CF TDN): a paradigm of a clinical trials network for genetic and orphan diseases. Adv Drug Deliv Rev. 2002;54:1505–1528. doi: 10.1016/s0169-409x(02)00163-1. [DOI] [PubMed] [Google Scholar]
- Kaufman BA, Terbrock A, Winters N, et al. Disbanding a multidisciplinary clinic: effects on health care of myelomeningocele patients. Pediatr Neurosurg. 1994;21:36–44. doi: 10.1159/000120812. [DOI] [PubMed] [Google Scholar]
- Kinsman SL, Doehring MC. The cost of preventable conditions in adults with spina bifida. Eur J Pediatr Surg. 1996;6(Suppl 1):17–20. doi: 10.1055/s-2008-1071031. [DOI] [PubMed] [Google Scholar]
- Liu T, Valdez R, Ward B, et al. Poster session presented at the Second World Congress on Spina Bifida Research and Care. Las Vegas, Nevada: 2012. Mar, National spina bifida registry (NSBR): development of a data management and quality control system. [Google Scholar]
- Morgan WJ, Butler SM, Johnson CA, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. Canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Pugeat M. Advances in growth hormone therapy: a new registry tool. Horm Res. 2004;62(Suppl 4):2–7. doi: 10.1159/000080902. [DOI] [PubMed] [Google Scholar]
- Schechter MS. Patient registry analyses: seize the data, but caveat lector. J Pediatr. 2008;153:733–735. doi: 10.1016/j.jpeds.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Soe M, Thibadeau J, Ward E. Poster session presented at the American Public Health Association meeting. Washington, DC: 2011. Oct, ‘Report Functionality’ tool: a single summary screen for real-time data validation. [Google Scholar]
- Versel N. What’s in the data? [Accessed: October 4, 2012];Hemaware. 2010 Available at: http://www.hemaware.org/print/448. [Google Scholar]
- Williams LJ, Rasmussen SA, Flores A, et al. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116:580–586. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.