Abstract
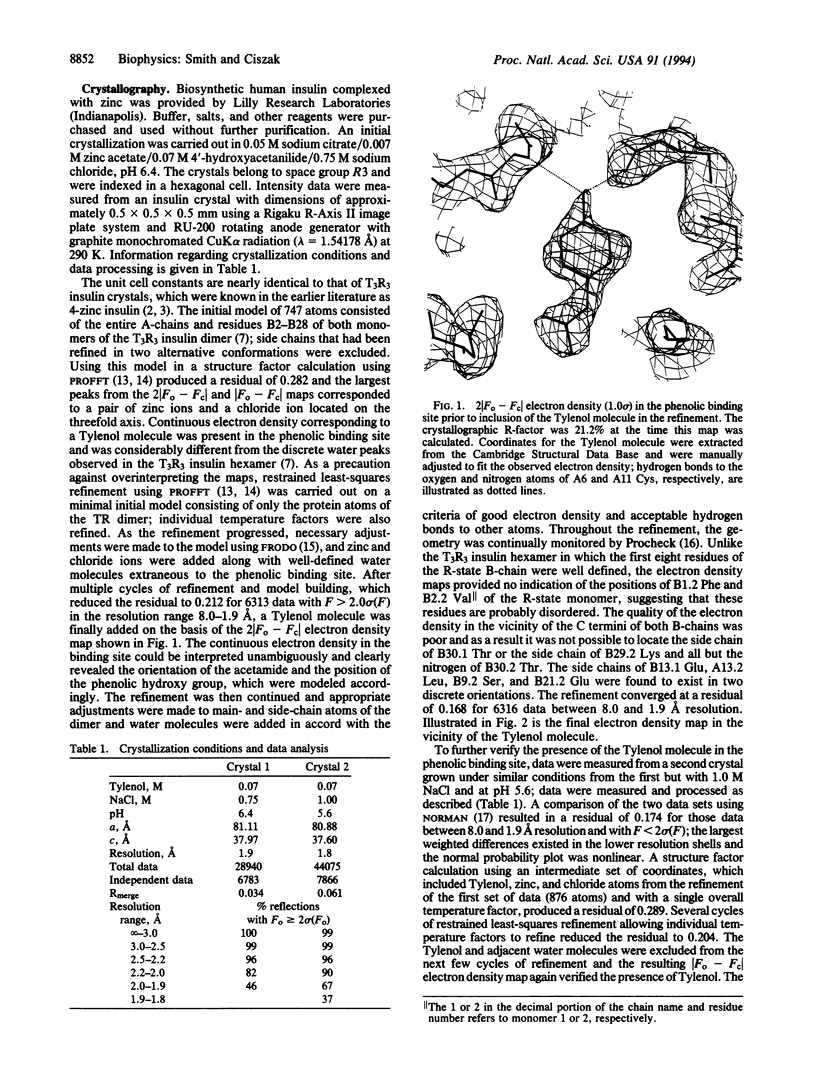
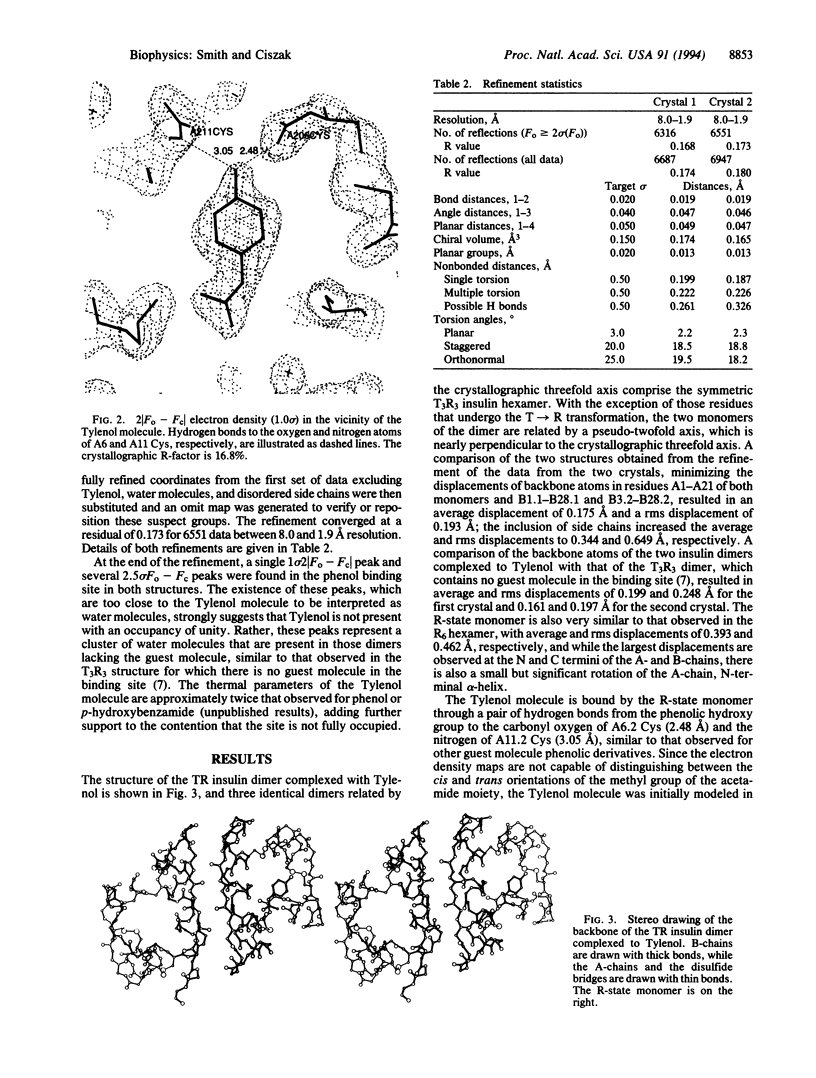
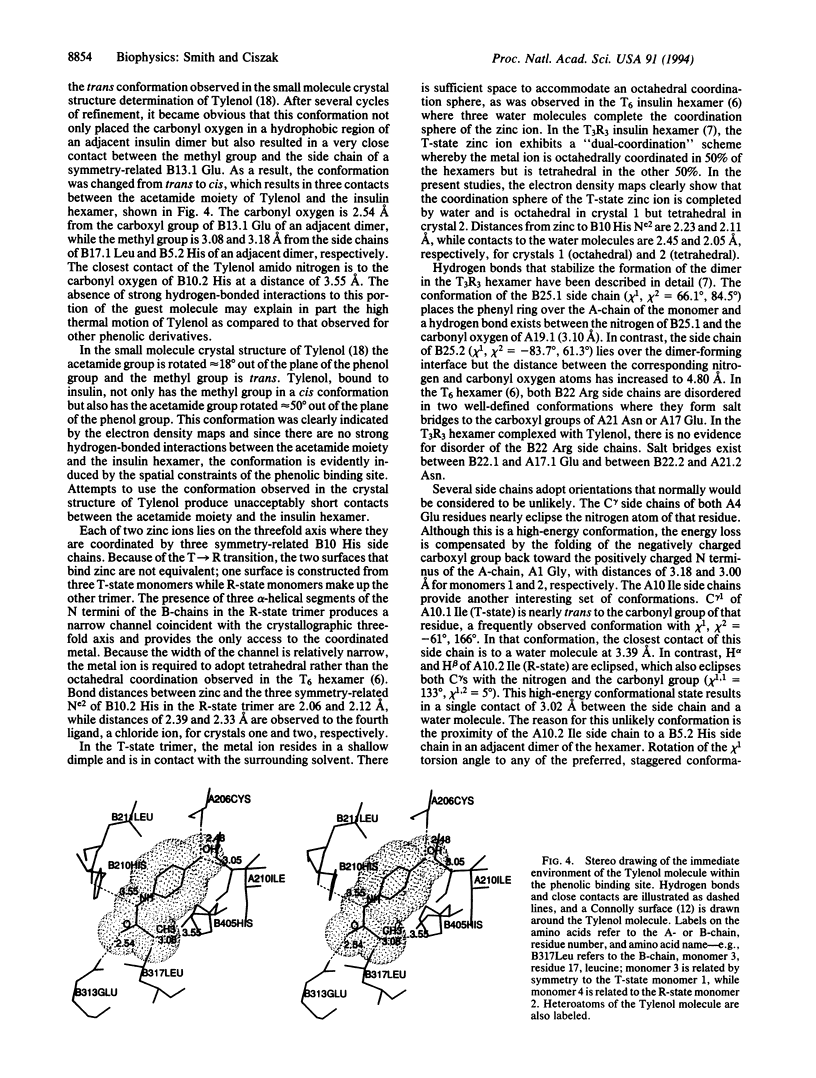
X-ray crystallographic studies have been carried out on human insulin crystals grown in the presence of 4'-hydroxyacetanilide (Tylenol) and show that this nontoxic phenolic derivative can induce the T-->R transition, producing a T3R3 hexamer. Two different crystals, grown under different conditions, are rhombohedral, space group R3, with cell constants a = 81.11, c = 37.97 and a = 80.88, c = 37.60 A. The T3R3 hexamer is symmetric, resulting from the presence of a crystallographic threefold axis, and the asymmetric unit consists of a TR dimer. Data to a resolution of 1.9 A were measured on a crystal from each of the two crystallizations and the structures have been refined to residuals of 0.168 and 0.173. The guest molecule is bound by the R-state monomer through the formation of two hydrogen bonds from the hydroxy group of Tylenol to the carbonyl oxygen and the nitrogen of A6 Cys and A11 Cys, respectively. Due to steric constraints of the phenolic binding site, the acetamide group of Tylenol is rotated approximately 50 degrees out of the plane of the phenyl group and the methyl group is cis; no hydrogen bonds exist between the acetamide group and the hexamer. Although the zinc ion, which is bound to the R-state trimer, has tetrahedral coordination in both structures, the T-state zinc is observed to have octahedral coordination in one structure but tetrahedral coordination in the other. The side chain of A10 Ile in the R-state monomer adopts a high-energy conformation as a result of close contact to a residue in an adjacent dimer and may explain in part the differences between therapeutic preparations of beef insulin, for which A10 is a Val residue, and human insulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., Reynolds C. D. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 6;319(1195):369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- Bentley G., Dodson E., Dodson G., Hodgkin D., Mercola D. Structure of insulin in 4-zinc insulin. Nature. 1976 May 13;261(5556):166–168. doi: 10.1038/261166a0. [DOI] [PubMed] [Google Scholar]
- Ciszak E., Smith G. D. Crystallographic evidence for dual coordination around zinc in the T3R3 human insulin hexamer. Biochemistry. 1994 Feb 15;33(6):1512–1517. doi: 10.1021/bi00172a030. [DOI] [PubMed] [Google Scholar]
- Kaarsholm N. C., Ko H. C., Dunn M. F. Comparison of solution structural flexibility and zinc binding domains for insulin, proinsulin, and miniproinsulin. Biochemistry. 1989 May 16;28(10):4427–4435. doi: 10.1021/bi00436a046. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Dodson G. G. Structure of a rhombohedral R6 insulin/phenol complex. Proteins. 1992 Nov;14(3):401–408. doi: 10.1002/prot.340140309. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Swenson D. C., Dodson E. J., Dodson G. G., Reynolds C. D. Structural stability in the 4-zinc human insulin hexamer. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7093–7097. doi: 10.1073/pnas.81.22.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]



