Abstract
This study was designed to test whether obese adults and adults with metabolic syndrome (MetSyn) exhibit altered hyperemic responses to hypoxia at rest and during forearm exercise when compared with lean controls. We hypothesized blood flow responses due to hypoxia would be lower in young obese subjects (n = 11, 24 ± 2 years, BMI 36 ± 2 kg m−2) and subjects with MetSyn (n = 8, 29 ± 3 years BMI 39 ± 2 kg m−2) when compared with lean adults (n = 13, 29 ± 2 years, BMI 24 ± 1 kg m−2). We measured forearm blood flow (FBF, Doppler Ultrasound) and arterial oxygen saturation (pulse oximetry) during rest and steady-state dynamic forearm exercise (20 contractions/min at 8 and 12 kg) under two conditions: normoxia (0.21 FiO2, ~ 98% SaO2) and hypoxia (~0.10 FiO2, 80% SaO2). Forearm vascular conductance (FVC) was calculated as FBF/mean arterial blood pressure. At rest, the percent change in FVC with hypoxia was greater in adults with MetSyn when compared with lean controls (p = 0.02); obese and lean adult responses were not statistically different. Exercise increased FVC from resting levels in all groups (p < 0.05). Hypoxia caused an additional increase in FVC (p < 0.05) that was not different between groups; responses to hypoxia were heterogeneous within and between groups. Reporting FVC responses as absolute or percent changes led to similar conclusions. These results suggest adults with MetSyn exhibit enhanced hypoxic vasodilation at rest. However, hypoxic responses during exercise in obese adults and adults with MetSyn were not statistically different when compared with lean adults. Individual hypoxic vasodilatory responses were variable, suggesting diversity in vascular control.
Keywords: Blood flow, Functional hyperemia, Prediabetes, Exercise
Introduction
Systemic hypoxia leads to a reduction in arterial oxygen content (Koskolou et al. 1997; Roach et al. 1999). This reduction in the arterial partial pressure of oxygen has been shown to elicit carotid chemoreceptor-mediated increases in sympathetic nerve activity and resultant vasoconstriction (Saito et al. 1988; Stickland et al. 2009). In order to preserve oxygen delivery under hypoxic conditions, an increase in oxygen extraction and/or compensatory peripheral vasodilation occurs (Roach et al. 1999; Koskolou et al. 1997; Calbet et al. 2003). The result is a balance of vasodilatory mediators with global autonomic vasoconstrictor outflow that ultimately determines blood flow (Rowell and Blackmon 1986; Stickland et al. 2009; Dinenno et al. 2003). In healthy lean adults, this results in net hypoxic vasodilation (Wilkins et al. 2006; Wilkins et al. 2008; Casey et al. 2010).
In the face of global rises in human obesity, the impact of obesity on vascular responses to environmental stressors is an important area of research. As a result of hypoxia, skeletal muscle resistance arteries from obese rats (obese Zucker rat) consistently exhibit impaired vasodilation (Frisbee 2001; Goodwill et al. 2008). This dysfunction may be the result of enhanced vasoconstriction (Frisbee 2004) and/or altered vasodilatory mechanisms (Frisbee 2003; Goodwill et al. 2008). However, this animal model of obesity is confounded by dyslipidemia, hyperglycemia, and hypertension—a clustering of cardiovascular disease risk factors more appropriately termed metabolic syndrome (MetSyn) (Cornier et al. 2008; Frisbee and Stepp 2001; Grundy et al. 2004).
Obesity-related disorders shown to increase the risk of developing cardiovascular disease (such as obstructive sleep apnea and Type 2 diabetes) appear to complicate the acute vascular response to systemic hypoxia in humans. Previous research suggests such conditions may lead to reduced (Di Vanna et al. 2007; Remsburg et al. 1999), preserved (Moradkhan et al. 2010), or increased (Weisbrod et al. 2004) forearm vasodilation in response to acute hypoxia at rest. Given the above disease-states are often confounded by obesity, it is interesting that no studies have assessed the effect of obesity alone on hypoxia-mediated vasodilation. It may be that differences between findings are due solely to differing control groups—including lean (Di Vanna et al. 2007; Remsburg et al. 1999) and overweight adults (Weisbrod et al. 2004; Moradkhan et al. 2010).
Previous research suggests forearm vasodilation at rest and during normoxic exercise is maintained with human obesity (Limberg et al. 2010a). However, the addition of a hypoxic stimulus provides an enhanced metabolic signal relative to exercise alone that challenges oxygen delivery (Wilkins et al. 2006; DeLorey et al. 2004) and alters sympathetic tone (Seals et al. 1991). In response to these combined environmental stressors, an imbalance between vasodilatory and vasoconstrictor mechanisms may manifest.
With this information, we designed an experiment to test the hypothesis that changes in resting and exercise-induced blood flow due to hypoxia would be reduced in young obese adults when compared with young lean controls. Given the presence of additional cardiovascular disease risk factors, we hypothesized adults with MetSyn would exhibit further impairments in blood flow responses to hypoxia.
Methods
Subjects
Three groups of young adults (lean, obese, and MetSyn; 18–43 years) participated in the present study to avoid any confounding effects of aging (Casey et al. 2011). All subjects completed a screening process in which physical activity and personal health history were assessed. All subjects were generally healthy non-smokers, were not taking any cardiovascular medications, and were free from overt cardiovascular disease and known sleep apnea, as determined by self-report. All subjects led a sedentary lifestyle and did not participate in regular aerobic exercise (current aerobic exercise was <90 min week−1).
Obese subjects had a body mass index (BMI) ≥30 kg m−2. Adults were characterized as having MetSyn if they met three of the following National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria as modified by the American Diabetes Association: central obesity (waist circumference >88 cm women/>102 cm men), pre-hypertension (resting blood pressure ≥130/≥85 mmHg), hypertriglyceridemia (triglycerides ≥ 150 mg dL−1), hyperglycemia (fasting glucose ≥100 mg dL−1) and/or dyslipidemia (HDL <50 mg dL−1 women/<40 mg dL−1 men) (Grundy et al. 2004).
Female subjects were not pregnant and were studied during the early follicular phase (days 1–5) of the menstrual cycle to avoid any confounding effects of changes in female hormone levels (Owen 1975; Charkoudian 2001; Williams et al. 2001). Oral contraception was allowed (Lean n = 3, Obese n = 3) and women on contraception were studied during the placebo phase. Subjects were instructed to refrain from exercise, NSAIDS, alcohol, and caffeine for 24 h prior to the study day. Written informed consent was obtained from all subjects. All procedures were approved by the Institutional Review Board at the University of Wisconsin—Madison and conformed to the standards set by the Declaration of Helsinki.
Measurements
Weight and height measurements were obtained, and body mass index was calculated (BMI, kg m−2). A waist circumference was collected and used for confirmation of central obesity. Plasma levels of HDL, triglycerides, and glucose were measured using venous blood collected after an overnight fast. Forearm volume was determined using water displacement.
Electronic sphygmomanometer (Datex-Ohmeda; Helsinki, Finland) on the upper arm (dominant, resting limb) at rest and during steady-state exercise was used as a primary measure of blood pressure. In addition, beat-to-beat changes in blood pressure were measured using finger photoplethysmography (Finapres; Netherlands) to confirm responses to exercise and/or hypoxia. Heart rate was monitored continuously by a three-lead ECG (Datex-Ohmeda; Helsinki, Finland).
Blood flow
Forearm blood flow (FBF; artery diameter, blood velocity) was measured using Doppler ultrasound (Vivid 7, General Electric; Milwaukee, WI). A 12-MHz linear array probe was placed approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle with a probe insonation angle <60° and the sample volume adjusted to cover the width of the artery using identical methods as described previously (Limberg et al. 2010a, b). The ultrasound probe operator continuously adjusted the probe position to maintain a fixed insonation angle, compensating for subjects’ movements during exercise.
Forearm exercise model
Each subject was supine with the non-dominant arm extended to the side (approximately 90°) at heart level. Dynamic and rhythmic, non-dominant forearm exercise required subjects to squeeze and release two handles together 4–5 cm to raise and lower a weight over a pulley at a rate of 20 times/min (1 s contraction: 2 s relaxation) (Limberg et al. 2010a, b; Schrage et al. 2004; Wilkins et al. 2006). Forearm exercise was completed at absolute workloads (8 and 12 kg) that corresponded with approximately 20 and 30% of forearm maximal voluntary contraction (MVC). MVC of the non-dominant arm was determined as the average of the two highest measurements from five consistent trials using a hand dynamometer (LaFayette Instruments; LaFayette, IN, USA). Given maximal workloads have been shown to be reduced under hypoxic conditions, exercise at absolute intensities was chosen as an appropriate method to analyze hemodynamic responses to hypoxia (Richardson et al. 1999). In addition, exercise at absolute intensities has been shown to require the same absolute oxygen consumption (Donato et al. 2006) and typical activities of daily living are done at absolute workloads (i.e. household chores, etc.).
Hypoxic exercise model
Subjects completed exercise under two conditions: normoxia (0.21 FiO2, ~98% SaO2) and hypoxia (~0.10 FiO2, 80% SaO2). Subjects were instrumented with a nose clip and mouthpiece. Subjects breathed through a low-resistance two-way non-rebreathing valve (model 2400, Hans Rudolph) for both normoxic and hypoxic trials. This system allowed for maintenance of constant dead space ventilation independent of study condition.
During normoxic trials, subjects breathed room air. During hypoxic trials, the level of inspired oxygen was titrated to achieve an arterial oxygen saturation of 80% as assessed by pulse oximetry on the dominant hand (Datex-Ohmeda; Helsinki, Finland) and head (Nellcor, N-595; Pleaston, CA, USA). Two separate gas tanks [(9% O2 + 91% N2]; [5% CO2 + 21% O2 + 74% N2)] were mixed using a blender (air-oxygen mixer, Puritan-Bennett; Los Angeles, CA, USA) in attempt to maintain normoxic CO2 levels (~5.5%, isocapnic hypoxia) while achieving desired SaO2 (80%). Data were transferred to and analyzed on a metabolic cart (Medgraphics, Ultima PFX; St. Paul, MN, USA).
Venous blood gasses
A 5-cm, 20-gauge venous catheter was inserted into the antecubital vein in the non-dominant, exercising arm with the subject supine. The catheter was continuously flushed with saline at 1 mL min−1 and was used to take blood samples throughout the study. Samples (2–4 mL) of venous blood were drawn anaerobically in heparinized syringes over 10–20 s at rest and after 3 min of exercise. All samples were placed on ice and were analyzed within 2 h of collection. Samples were collected and analyzed in duplicate and used for measurements of venous PO2 (PvO2), PCO2 (PvCO2), and pH with a blood-gas analyzer calibrated with tonometered blood (ABL500, Radiometer). All results were temperature-corrected.
Study protocol
A total of four trials (8 and 12 kg exercise during normoxia and hypoxia) were randomized between subjects with a 10-min normoxic rest period after each trial. Within each trial, steady-state ventilation at the desired SaO2 was maintained for an average of 4 min prior to the collection of quiet resting measures. After quiet rest, subjects completed 3.5 min of dynamic exercise.
Data acquisition and analysis
Forearm blood flow
Blood flow was determined as the product of mean blood velocity (cm s−1) and brachial artery cross sectional area (π radius2 with radius measured in cm) and was reported in ml min−1. Artery diameters were obtained from video images taken at rest and 3 min of exercise. Except during intermittent artery diameter measurements, arterial blood velocity was continuously assessed throughout rest and each exercise workload; diameter measurements typically resulted in loss of pulse wave signal for 15 s. Pulse-wave velocity was measured beat-to-beat and analyzed as an average of the last 30 s of rest and steady-state exercise to reduce contraction-to-contraction-induced variability in blood flow (Limberg et al. 2010a, b).
To determine vessel cross sectional area, artery diameter was taken as the median of five measurements in late diastole, at rest and during steady-state exercise (between muscle contractions). Arterial diameter was measured on B-mode images in a longitudinal section of the artery running perpendicular to the ultrasound beam and was identified by strong wall signals (measuring the distance between proximal and distal wall intima-media interface). All measurements were made off-line by a well-trained operator (Limberg et al. 2010a, b).
A commercial interface unit (Multigon Industries, Yonkers, NY, USA) processed the angle-corrected, intensity-weighted Doppler audio information from the GE Vivid ultrasound system into a flow velocity signal that was sampled in real time with signal-processing software (PowerLab, ADinstruments, Colorado Springs, CO, USA). All hemodynamic data were digitized, stored on a computer at 400 Hz, and analyzed off-line using PowerLab; post-processing using PowerLab's Chart application package yielded mean blood velocities.
Ventilation
Inspiratory and expiratory flow rates, as well as inspired and expired gases were sampled at the mouth (Med-Graphics, Ultima PFX; St. Paul, MN, USA). All signals were displayed on a chart recorder (MedGraphics Breeze Suite; St. Paul, MN, USA) and sampled at 75 Hz. Data were analyzed as an average of the last 30 s of rest and steady-state exercise in a subset of participants (Lean n = 9, Obese n = 9, MetSyn n = 6) due to data storage issues.
Data analysis and statistics
All resting measures are reported as an average of two trials during a given condition (normoxia, hypoxia). Given higher blood pressures in adults with MetSyn, blood flow measurements were normalized for mean arterial blood pressure and are reported as vascular conductance (FVC; mL min−1 100 mmHg−1). At rest, FVC responses to hypoxia were analyzed as a percent change from normoxic levels to account for individual variability (Remsburg et al. 1999; Moradkhan et al. 2010). During exercise, the main dependent variable was a change in FVC due to hypoxia. To account for altered baseline FVC with hypoxia, the change due to hypoxia was calculated by subtracting resting FVC during normoxia from FVC values obtained during steady-state hypoxic exercise (Casey et al. 2010; Wilkins et al. 2008). The primary analysis tested whether the change in FVC due to hypoxia was lower in obese adults and adults with metabolic syndrome when compared with results from lean controls at rest and during exercise. Additionally, to assess vascular responsiveness to hypoxia, the slopes of the relationship between FVC and arterial oxygen saturation were determined (Remsburg et al. 1999; Reichmuth et al. 2009).
Statistical analysis was done using SAS (Version 9.2). Subject characteristics were compared via unpaired Student's t test. Hemodynamic variables were analyzed using a proc-mixed repeated measures approach to determine the significance of the fixed effect of group (Lean, Obese, MetSyn), gas (Normoxia, Hypoxia), and/or exercise intensity (Rest, 8 kg, 12 kg) on parameters of interest. Bonferroni post hoc comparisons were performed when significant effects were observed. All data are presented as mean ± standard error, and significance was determined a priori at p < 0.05.
Results
Subject characteristics
Eight adults with MetSyn, eleven obese and thirteen lean adults completed the study (84% White non-Hispanic, 13% White Hispanic, 3% African American). Subject characteristics are summarized in Table 1. There were no significant differences between groups in regard to age (p > 0.05). Glucose, triglycerides, and HDL were not significantly different between lean and obese adults (p > 0.05). Subjects in the obese and MetSyn groups were clinically obese, displaying significantly higher weight, body mass index (BMI), and waist circumference (p < 0.05) when compared to lean adults. Obese and MetSyn subjects also had greater forearm volume (p < 0.05). Adults with MetSyn exhibited greater fasting glucose, greater triglycerides, and lower HDL when compared with lean and obese adults (p < 0.05).
Table 1.
Subject demographics
| Lean | Obese | MetSyn | |
|---|---|---|---|
| Sex (M/F) | 9/4 | 6/5 | 7/1 |
| Age (years) | 29 ± 2 | 24 ± 2 | 29 ± 3 |
| Height (cm) | 176 ± 2 | 175 ± 3 | 181 ± 3 |
| Weight (kg) | 74 ± 3 | 111 ± 8* | 127 ± 8* |
| BMI (kg/m2) | 24 ± 1 | 36 ± 2* | 39 ± 3* |
| Waist (cm) | 80 ± 3 | 109 ± 6* | 121 ± 5* |
| FAV (mL) | 1011 ± 50 | 1391 ± 104* | 1546 ± 86* |
| MVC (kg) | 41 ± 2 | 40 ± 4 | 47 ± 2 |
| Glucose (mg/dL) | 76 ± 4 | 72 ± 3 | 88 ± 4† |
| Triglyceride (mg/dL) | 104 ± 16 | 96 ± 12 | 230 ± 40*† |
| HDL (mg/dL) | 63 ± 5 | 58 ± 6 | 38 ± 7* |
| Blood pressure (mmHg) | 92 ± 1 | 92 ± 2 | 101 ± 2*† |
Lean n = 13, Obese n = 11, MetSyn n = 8 unless otherwise noted. Data are presented as Mean ± SE
BMI body mass index, FAV forearm volume, MVC maximal voluntary contraction
p < 0.05 versus lean
p < 0.05 versus obese. Waist (lean n = 12, obese n = 10, MetSyn n = 7), Glucose (obese n = 10)
Systemic responses
Results are summarized in Table 2. Blood pressure was greater in adults with MetSyn when compared with lean controls (p < 0.05) and values increased with exercise in all groups (p < 0.05). In response to hypoxia, increases in heart rate (~ 15 beat/min, p < 0.05) were not different between groups. Oxygen saturation and end-tidal carbon dioxide levels both decreased with hypoxia (p < 0.05) and were not significantly different between groups. Minute ventilation increased with hypoxia in all groups (p < 0.05) although absolute values were greater in adults with MetSyn when compared with both lean and obese adults (p < 0.05).
Table 2.
Systemic responses to exercise and hypoxia
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 8 (kg) | 12 (kg) | Rest | 8 (kg) | 12 (kg) | |
| Diameter (cm) | ||||||
| Lean | 0.43 ± 0.02 | 0.45 ± 0.02a | 0.46 ± 0.01a | 0.43 ± 0.02 | 0.46 ± 0.02a | 0.47 ± 0.02a |
| Obese | 0.44 ± 0.02 | 0.47 ± 0.02a | 0.48 ± 0.02a | 0.44 ± 0.02 | 0.48 ± 0.02a | 0.48 ± 0.02a |
| MetSyn* | 0.47 ± 0.02 | 0.49 ± 0.02a | 0.49 ± 0.02a | 0.48 ± 0.01 | 0.49 ± 0.01a | 0.50 ± 0.01a |
| Heart rate (beat/min)‡ | ||||||
| Lean | 67 ± 2 | 73 ± 2a | 73 ± 3a | 84 ± 3 | 88 ± 4a | 87 ± 2a |
| Obese* | 62 ± 2 | 68 ± 3a | 69 ± 2a | 79 ± 3 | 81 ± 3a | 82 ± 3a |
| MetSyn† | 71 ± 3 | 74 ± 4a | 76 ± 4a | 86 ± 5 | 89 ± 4a | 88 ± 5a |
| Blood pressure (mmHg) | ||||||
| Lean | 92 ± 1 | 95 ± 1a | 97 ± 1a,b | 94 ± 2 | 95 ± 2a | 99 ± 1a,b |
| Obese | 92 ± 2 | 96 ± 2a | 100 ± 2a,b | 94 ± 2 | 97 ± 2a | 99 ± 2a,b |
| MetSyn*,† | 101 ± 2 | 104 ± 2a | 107 ± 2a,b | 100 ± 1 | 104 ± 1a | 108 ± 2a,b |
| SpO2 (%)‡ | ||||||
| Lean | 99 ± 0.2 | 99 ± 0.3 | 97 ± 1.9 | 80 ± 1.0 | 78 ± 1.2 | 80 ± 0.6 |
| Obese | 98 ± 0.3 | 98 ± 0.3 | 98 ± 0.4 | 80 ± 0.7 | 79 ± 1.2 | 80 ± 1.0 |
| MetSyn | 97 ± 0.6 | 97 ± 0.4 | 97 ± 0.5 | 79 ± 0.6 | 80 ± 0.5 | 81 ± 1.5 |
| Ventilation (BTPS) (L/min)* | ||||||
| Lean (n = 9) | 10.1 ± 0.4 | 10.4 ± 0.8 | 11.6 ± 0.9 | 16.4 ± 3.4 | 18.2 ± 3.9 | 17.0 ± 3.9 |
| Obese (n = 9) | 10.8 ± 0.6 | 11.4 ± 0.6 | 12.4 ± 0.9 | 13.1 ± 1.0 | 14.9 ± 1.2 | 15.8 ± 1.4 |
| MetSyn (n = 6)*,† | 13.4 ± 0.8 | 14.7 ± 0.8 | 15.2 ± 0.8 | 16.6 ± 1.1 | 18.1 ± 1.4 | 18.9 ± 1.2 |
| Tidal volume (L) | ||||||
| Lean (n = 9) | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Obese (n = 9) | 1.0 ± 0.2 | 1.1 ± 0.4 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 |
| MetSyn (n = 6) | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| Breathing frequency (br/min) | ||||||
| Lean (n = 9) | 15.9 ± 1.5 | 14.8 ± 2.1 | 15.4 ± 1.5 | 17.1 ± 1.7 | 19.1 ± 2.0 | 17.9 ± 1.9 |
| Obese (n = 9) | 15.2 ± 1.3 | 17.7 ± 1.4 | 17.6 ± 1.2 | 15.5 ± 1.4 | 17.3 ± 1.3 | 15.4 ± 1.4 |
| MetSyn (n = 6) | 17.8 ± 2.3 | 19.1 ± 2.2 | 20.1 ± 1.6 | 15.2 ± 1.6 | 18.1 ± 1.6 | 20.2 ± 1.8 |
| End-tidal CO2 (%)‡ | ||||||
| Lean (n = 9) | 5.7 ± 0.2 | 5.9 ± 0.3 | 5.8 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.3 | 5.4 ± 0.2 |
| Obese (n = 9) | 5.8 ± 0.1 | 5.8 ± 0.1 | 5.9 ± 0.1 | 5.3 ± 0.2 | 5.1 ± 0.2 | 5.2 ± 0.2 |
| MetSyn (n = 6) | 5.6 ± 0.3 | 5.5 ± 0.3 | 5.8 ± 0.3 | 5.0 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 |
Lean n = 13, Obese n = 11, MetSyn n = 8 unless otherwise noted. Data are presented as Mean ± SE
Main effect of group:
p < 0.05 versus lean
p < 0.05 versus obese
Main effect of exercise:
p < 0.05 versus rest
p < 0.05 versus 8 kg
Main effect of hypoxia
Blood flow responses to exercise
Mean blood flow and vascular conductance responses to rest and dynamic exercise under normoxic/hypoxic conditions are summarized in Table 3. There was a main effect of exercise intensity on FVC. Steady-state FVC at rest and during exercise was greater in obese adults and adults with MetSyn when compared to lean controls (p < 0.05). Reporting results as FBF provided comparable results (Table 3). This increase may be due to larger forearm sizes, given when data were expressed relative to forearm volume, group responses were not significantly different (data not shown).
Table 3.
Hyperemic responses to exercise and hypoxia
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 8 (kg) | 12 (kg) | Rest | 8 (kg) | 12 (kg) | |
| Blood flow (FBF, mL/min)‡ | ||||||
| Lean | 75 ± 8 | 290 ± 24a | 415 ± 39a,b | 77 ± 11 | 355 ± 29a | 437 ± 36a,b |
| Obese* | 106 ± 13 | 410 ± 24a | 510 ± 29a,b | 117 ± 15 | 467 ± 39a | 590 ± 58a,b |
| MetSyn* | 114 ± 16 | 419 ± 38a | 541 ± 28a,b | 179 ± 21 | 484 ± 31a | 564 ± 27a,b |
| Conductance (FVC, mL/min*100 mmHg)‡ | ||||||
| Lean | 82 ± 9 | 306 ± 24a | 424 ± 36a,b | 82 ± 11 | 374 ± 31a | 443 ± 37a,b |
| Obese* | 119 ± 17 | 429 ± 28a | 513 ± 28a,b | 127 ± 18 | 484 ± 40a | 605 ± 68a,b |
| MetSyn* | 115 ± 16 | 407 ± 40a | 504 ± 25a,b | 179 ± 22 | 463 ± 29a | 526 ± 29a,b |
Lean n = 13, Obese n = 11, MetSyn n = 8. Data are presented as Mean ± SE
Main effect of group:
p < 0.05 versus Lean
Main effect of exercise:
p < 0.05 versus Rest
p < 0.05 versus 8 kg
Main effect of hypoxia
Blood flow responses to hypoxia
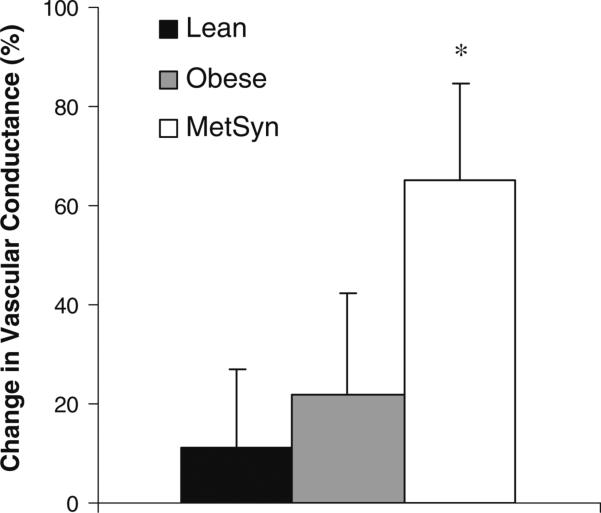
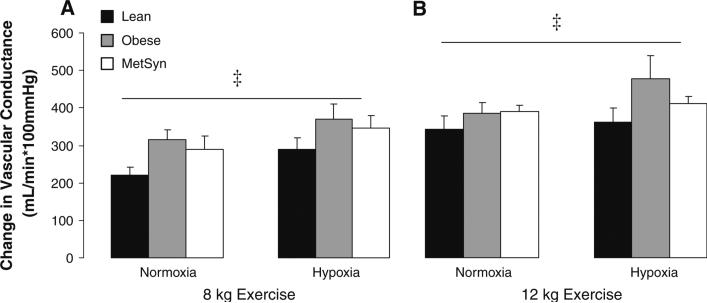
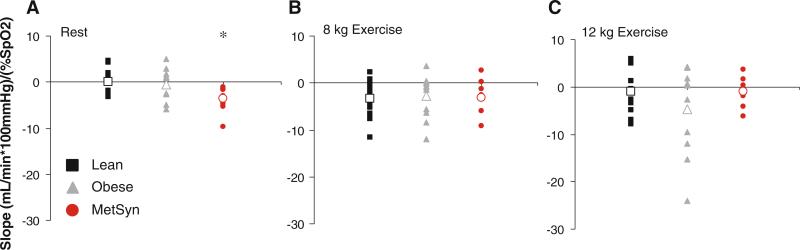
The changes due to hypoxia in resting (percent change) and exercise (absolute change from normoxic rest) FVC are presented in figure form (Figs. 1, 2, 3). At rest, adults with MetSyn exhibited enhanced hypoxic dilation when compared with lean controls (Lean vs. MetSyn p = 0.02); responses between obese and lean adults were not significantly different (Lean vs. Obese = 0.07) (Fig. 1). During exercise, a hypoxia-mediated vasodilatory response was observed that was not group-specific (p < 0.05, main effect of hypoxia; Fig. 2). Taken together, these findings are contrary to our hypothesis. It is important to note analyzing the effect of hypoxia on FVC between groups as absolute values (Table 3, p = 0.47; interaction of group × gas), change from normoxic rest (Fig. 2, p = 0.47; interaction of group × gas), percent change from normoxic steady-state (data not shown, p = 0.20), and the slope of the relationship between FVC and arterial oxygen saturation (Fig. 3, p = 0.09) provided similar conclusions during exercise.
Fig. 1.
Effect of hypoxia on resting vascular conductance. Percent change in vascular conductance from normoxic rest [(hypoxic rest) – (normoxic rest)]/normoxic rest × 100%. *p < 0.05 versus lean
Fig. 2.
Effect of exercise and hypoxia on vascular conductance. Change in vascular conductance due to exercise/hypoxia (FVC exercise – FVC normoxia rest). a Vascular conductance due to exercise/hypoxia during 8 kg exercise. b Vascular conductance due to exercise/hypoxia during 12 kg exercise. Main effect of exercise: p < 0.05 8 kg versus 12 kg exercise. Main effect of hypoxia: ‡p < 0.05 Hypoxia versus normoxia
Fig. 3.
Individual stimulus–response relationships between oxygen saturation and vascular conductance. To assess vascular responsiveness to hypoxia, the slopes of the relationship between vascular conductance (mL min−1 100 mmHg−1) and SaO2 (%) are presented. Negative values represent net hypoxia-mediated vasodilation; positive values represent vasoconstriction. a Individual slopes of the relationship between vascular conductance and SaO2 at rest. b Slopes at steady-state 8 kg exercise. c Slopes at steady-state 12 kg exercise. Group averages in open symbols. *p < 0.05 versus lean
Venous blood gasses
Results are summarized in Table 4. The partial pressure of oxygen in venous blood during hypoxic exercise decreased compared with normoxic trials (~6 Torr, p < 0.05). The partial pressure of carbon dioxide in venous blood decreased (~3 Torr) and venous pH increased (~0.03 units) with hypoxia. These responses were not significantly different between groups. There was a main effect of exercise on PvO2, PvCO2, and venous pH that was not group-specific.
Table 4.
Venous blood-gas responses to exercise and hypoxia
| Normoxia |
Hypoxia |
|||||
|---|---|---|---|---|---|---|
| Rest | 8 (kg) | 12 (kg) | Rest | 8 (kg) | 12 (kg) | |
| PvO2 (Torr)‡ | ||||||
| Lean | 36 ± 2 | 28 ± 2a | 28 ± 2a | 28 ± 1 | 23 ± 2a | 22 ± 1a |
| Obese | 39 ± 3 | 28 ± 2a | 29 ± 3a | 32 ± 1 | 21 ± 1a | 23 ± 2a |
| MetSyn | 43 ± 4 | 27 ± 3a | 27 ± 2a | 32 ± 2 | 23 ± 1a | 23 ± 1a |
| PvCO2 (Torr)‡ | ||||||
| Lean | 41 ± 2 | 51 ± 3a | 47 ± 4a | 40 ± 2 | 45 ± 3a | 46 ± 4a |
| Obese | 38 ± 2 | 44 ± 4a | 45 ± 2a | 33 ± 2 | 43 ± 3a | 47 ± 2a |
| MetSyn | 40 ± 3 | 46 ± 4a | 52 ± 3a | 35 ± 2 | 42 ± 3a | 48 ± 3a |
| Venous pH (units)‡ | ||||||
| Lean | 7.38 ± 0.01 | 7.32 ± 0.01a | 7.30 ± 0.01a | 7.40 ± 0.01 | 7.36 ± 0.01a | 7.38 ± 0.05a |
| Obese | 7.38 ± 0.01 | 7.35 ± 0.00a | 7.33 ± 0.01a | 7.42 ± 0.01 | 7.37 ± 0.01a | 7.35 ± 0.01a |
| MetSyn† | 7.37 ± 0.01 | 7.32 ± 0.01a | 7.29 ± 0.01a | 7.40 ± 0.01 | 7.35 ± 0.01a | 7.32 ± 0.01a |
Lean n = 13, Obese n = 11, MetSyn n = 8. Data are presented as Mean ± SE
Main effect of group:
p < 0.05 versus obese
Main effect of exercise:
p < 0.05 versus rest
Main effect of hypoxia
Discussion
The current study tested the hypothesis that hypoxia-mediated changes in blood flow at rest and during forearm exercise would be reduced in obese adults and adults with MetSyn. Novel findings from this study indicate that hypoxia-mediated vasodilation is (1) enhanced in adults with MetSyn at rest and (2) preserved in young obese adults and adults with MetSyn during moderate-intensity forearm exercise when compared with lean controls.
This is the first reported study to assess vascular responses to hypoxia at rest and during forearm exercise in healthy obese adults and adults with MetSyn. Given the majority of cardiovascular disease-states are confounded by obesity and aging, the inclusion of young lean and obese subjects in addition to a study group at increased risk of developing cardiovascular disease (i.e. MetSyn) are important strengths of the current study. In this way, we were able to assess whether obesity alone has a significant effect on normal hypoxic-vasodilation, or rather when it presents with other cardiovascular disease risk factors.
Normoxic vasodilation
Animal models of obesity, likely a more appropriate model of MetSyn, are known to exhibit reduced nitric oxide bioavailability (Frisbee and Stepp 2001), heightened sympathetic vasoconstrictor tone (Stepp and Frisbee 2002; Frisbee 2004), elevated levels of vasoconstrictor thromboxane (Goodwill et al. 2008), and elevated mediators of inflammation and oxidative stress (Frisbee et al. 2002; Xiang et al. 2008). Each of these factors alone may contribute to impaired functional vasodilation with normoxic exercise. In contrast, results from the current study show steady-state hyperemia during normoxic forearm exercise is not blunted. These findings confirm those shown previously by our lab in young obese humans (Limberg et al. 2010a) and extend these results to MetSyn. Thus, any impairment(s) in vascular function shown to exist in other models of obesity and MetSyn at rest are not seen when total blood flow to the human forearm is assessed during moderate-intensity exercise. This interesting finding may the result of impaired flow distribution, altered metabolic need, or an increased reliance on compensatory vascular control mechanisms in these human populations.
Hypoxic vasodilation at rest
Homeostatic perturbations—such as hypoxia—provide a physiological stressor that could potentially unmask compensatory mechanisms. Along these lines, isolated vessels from obese rats (obese Zucker rat) are known to rely on alternate vascular control mechanisms that, under conditions of hypoxia, lead to impaired vessel dilation (Frisbee 2001; Goodwill et al. 2008). In contrast to animal research, human adults with MetSyn in the current study exhibited enhanced hypoxic-vasodilatory responses when compared with lean controls at rest (Fig. 1).
Previous research suggests obesity-related disorders may lead to reduced (Di Vanna et al. 2007; Remsburg et al. 1999), preserved (Moradkhan et al. 2010), or increased (Weisbrod et al. 2004) forearm vasodilation in response to acute hypoxia at rest. Using overweight controls, Moradkhan et al. (2010) concluded hypoxic dilation was maintained in adults with sleep apnea. In contrast, Weisbrod et al. (2004) reported greater hypoxia-mediated vasodilation in Type 2 diabetics when compared with lean controls. In this context, the current study confirms previous findings, unifies conflicting literature, and extends results to adults with MetSyn. Central to the current study design, results are presented from pertinent control groups for proper comparisons; as a result, it appears obese adults and adults with MetSyn exhibit similar hypoxic dilation, with greater hypoxia-mediated vasodilation in adults with MetSyn when compared with lean controls at rest.
Adults with MetSyn are known to exhibit heightened sympathetic nerve activity that is well correlated with combined obesity and hypertension (Lambert et al. 2007). Although contrary to our hypothesis, enhanced hypoxic vasodilation at rest (Fig. 1) may be achieved by offsetting greater sympathetic constriction with metabolic dilation. It is also possible that adults with MetSyn exhibit a blunted sympathetic vasoconstrictor response to hypoxia at rest. Consistent with this concept, healthy older adults are known to exhibit heightened sympathetic nerve activity combined with blunted adrenergic responsiveness to sympathetic activation at rest (Dinenno et al. 2001, 2002, 2006). If this is the case in MetSyn, blunted adrenergic responsiveness may explain the increase in conductance at rest; however, future studies will be necessary to elucidate potential mechanisms.
Combined effect of exercise and hypoxia
The lack of statistical significance between group responses to hypoxia during exercise may be due, at least in part, to inter-individual variability. Figure 3 displays the markedly heterogeneous FVC responses to hypoxia in the current study. In a recent editorial, Charkoudian (2010) emphasized the variability in responses may be key to understanding integrative function. In support of this idea, obese patients with obstructive sleep apnea (OSA) have been shown to exhibit inter-individual variability in hyperemic responses to hypoxia (Remsburg et al. 1999, 2009). Remsburg, et al. (1999) observed two distinct patterns of response in the patient group—one-half of adults behaved similarly to controls by reducing resistance to flow with hypoxia, whereas the remaining patients increased resistance. These vasodilatory responses were unable to be explained by differences in weight, BMI, age, or hypertension when analyzed post hoc (Remsburg et al. 1999). Similarly, post hoc comparisons on current data failed to disclose differences in age, waist circumference, MVC, glucose, or triglyceride levels on vasodilatory responses across workloads (data not shown).
Given the variability in the hypoxic exercise responses, it appears there is no uniform pattern for hypoxia-mediated vasodilation within or between groups, despite strictly controlled exercise and hypoxic conditions. Whereas our design did not directly test the roles of sympathetic constriction versus metabolic dilation, we speculate these differential hypoxic responses depict the presence and balance of various redundant vascular control mechanisms (Frisbee 2004). For example, healthy older adults were recently shown to maintain hypoxic vasodilation during low-intensity forearm exercise while at the same time exhibiting blunted nitric oxide signaling; this impairment went on to negatively impact hypoxic-vasodilation at a higher workload (Casey et al. 2011). Future studies in obesity and MetSyn are necessary to elucidate the presence and importance of redundancy in vascular control and the role individual differences play during hypoxic exercise.
Experimental considerations and limitations
This study is novel in that no studies have reported vasodilatory responses to hypoxia in obesity alone or combined with cardiovascular disease risk factors (i.e. MetSyn) in young adult humans. In the context of the current study design, results are independent of potentially complicated and confounding interactions between aging and cardiovascular disease risk. In addition, this is the first study to assess responses to a more severe hypoxic-stimulus (80% SaO2) and higher exercise intensity (12 kg, ~30% MVC). However, some study limitations are worth noting.
It is important to acknowledge that obese adults and adults with MetSyn exhibit greater FVC compared with lean controls independent of hypoxia (Table 3). This difference may be due, in part, to the larger forearm size in these groups (Table 1; p < 0.01 Lean vs. Obese, p < 0.01 Lean vs. MetSyn). Although forearm composition was not measured in the current study, the difference in size is likely due to an increase in adipose tissue rather than skin or lean mass (Blaak et al. 1994). The overall contribution of adipose tissue to total forearm blood flow is relatively small (Blaak et al. 1994) and hypoxia-induced blood flow responses have been shown to be confined to the working muscle (Heinonen et al. 2010). In addition, analyzing hypoxia data as absolute or relative (to forearm volume) changes provides similar conclusions. Thus, differences in forearm size do not appear to impact hypoxic responses in obesity and MetSyn.
Given the invasive nature of collecting arterial blood gasses, such measures were not included in the current study design. This limitation does not allow for the assessment of forearm oxygen consumption. We made the assumption that absolute workloads correlate to similar oxygen consumption between groups (Donato et al. 2006). However, this is an important hypothesis that needs to be tested in future obesity studies. It may be that higher arterial blood flows in obese and MetSyn adults are necessary to maintain normal oxygen consumption or correlate to higher absolute metabolic need.
Sympathetic nerve activity was not directly measured in the current study. Given increases in heart rate were not significantly different between groups in response to hypoxia and exercise (Table 2), we speculate a similar change in autonomic nerve activity occurred between groups. Research suggests changes in plasma catechola-mines in response to a hypoxic stimulus are similar in obesity-related disease states when compared with healthy controls (Weisbrod et al. 2004). Along these lines, it is unlikely altered carotid chemoreceptor sensitivity has an impact on current findings, as research suggests responsiveness to hypoxic stimuli is maintained with obesity when sympathetic nerve activity is assessed (Narkiewicz et al. 1999). Thus, it is unlikely altered sympathetic nerve activity could explain our results.
By mixing separate gas tanks, we attempted to maintain levels of carbon dioxide between trials (isocapnic hypoxia); however, a potential limitation of the present study is the slightly lower (0.5–0.7%) end-tidal carbon dioxide (CO2) levels that occurred during hypoxic trials. Nevertheless, differences in CO2 levels were not physiologically significant nor were responses significantly different between groups (Table 2). Therefore, we feel this is a minor limitation and these differences do not impact our conclusions regarding hypoxic vasodilation in obesity or MetSyn.
Conclusions
Contrary to our hypothesis, adults with MetSyn appear to exhibit enhanced vasodilatory responses to hypoxia at rest. Additionally, our results demonstrate young obese adults and adults with MetSyn exhibit preserved hypoxia-mediated vasodilation during moderate-intensity forearm exercise when compared with lean controls. Interestingly, there appears to be large individual heterogeneity in vascular responses to hypoxia. Future studies are necessary to elucidate the presence and importance of redundant vascular control mechanisms and the role individual differences play in blood flow control during hypoxic exercise in human obesity and MetSyn.
Acknowledgments
We are grateful to the subjects for their participation. In addition, we extend many thanks to John Harrell, Kathleen Grabowski, Adam Kiefer, Patrick Meyer, Caitlin Zillner, and Lee Linstroth for technical assistance. This study was supported by grants from the American Federation on Aging Research #A08235 (WGS), American Heart Association pre-doctoral awards #0815622G (JKL) and #10PRE3870000 (JKL) and American Heart Association post-doctoral award #0825858G (GMB).
Footnotes
Conflict of interest There are no potential conflicts of interest.
Contributor Information
Jacqueline K. Limberg, Department of Kinesiology, School of Education, University of Wisconsin, 1149 Natatorium, Madison, WI 53706, USA
Trent D. Evans, Department of Kinesiology, School of Education, University of Wisconsin, 1149 Natatorium, Madison, WI 53706, USA
Gregory M. Blain, E.A. 4488, Activité Physique, Muscle, Santé, Faculté des Sciences du Sport, Université de Lille 2, Ronchin, France The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA.
David F. Pegelow, Department of Kinesiology, School of Education, University of Wisconsin, 1149 Natatorium, Madison, WI 53706, USA The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA; Department of Pediatrics, University of Wisconsin Hospital and Clinics, Madison, WI, USA.
Jessica R. Danielson, Department of Kinesiology, School of Education, University of Wisconsin, 1149 Natatorium, Madison, WI 53706, USA
Marlowe W. Eldridge, Department of Kinesiology, School of Education, University of Wisconsin, 1149 Natatorium, Madison, WI 53706, USA The John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA; Department of Pediatrics, University of Wisconsin Hospital and Clinics, Madison, WI, USA.
Lester T. Proctor, Department of Anesthesiology, University of Wisconsin Hospital and Clinics, Madison, WI, USA
Joshua J. Sebranek, Department of Anesthesiology, University of Wisconsin Hospital and Clinics, Madison, WI, USA
William G. Schrage, Department of Kinesiology, School of Education, University of Wisconsin, 1149 Natatorium, Madison, WI 53706, USA
References
- Blaak EE, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. Total forearm blood flow as an indicator of skeletal muscle blood flow: effect of subcutaneous adipose tissue blood flow. Clin Sci (Lond) 1994;87:559–566. doi: 10.1042/cs0870559. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol. 2010;588:373–385. doi: 10.1113/jphysiol.2009.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol. 2011;589(Pt 6):1477–1488. doi: 10.1113/jphysiol.2010.203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N. Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin Auton Res. 2001;11:295–301. doi: 10.1007/BF02332974. [DOI] [PubMed] [Google Scholar]
- Charkoudian N. Heterogeneity in human cardiovascular function contributes to a deeper understanding of integrative mechanisms. J Appl Physiol. 2010;108:473–474. doi: 10.1152/japplphysiol.01385.2009. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH. The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol. 2004;89:293–302. doi: 10.1113/expphysiol.2003.026864. [DOI] [PubMed] [Google Scholar]
- Di Vanna A, Braga AM, Laterza MC, Ueno LM, Rondon MU, Barretto AC, Middlekauff HR, Negrao CE. Blunted muscle vaso-dilatation during chemoreceptor stimulation in patients with heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H846–H852. doi: 10.1152/ajpheart.00156.2007. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation. 2006;13:329–341. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm post-junctional a-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt alpha-adrenergic vasoconstriction in the human forearm. J Physiol. 2003;549:985–994. doi: 10.1113/jphysiol.2003.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson R. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Frisbee JC. Impaired dilation of skeletal muscle microvessels to reduced oxygen tension in diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2001;281:H1568–H1574. doi: 10.1152/ajpheart.2001.281.4.H1568. [DOI] [PubMed] [Google Scholar]
- Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1124–R1134. doi: 10.1152/ajpregu.00239.2003. [DOI] [PubMed] [Google Scholar]
- Frisbee JC. Enhanced arteriolar alpha-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 2004;97:764–772. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2001;281:H1304–H1311. doi: 10.1152/ajpheart.2001.281.3.H1304. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;283:H2160–H2168. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- Goodwill AG, James ME, Frisbee JC. Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension. Am J Physiol Heart Circ Physiol. 2008;295:H1522–H1528. doi: 10.1152/ajpheart.00596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol. 2010;299:R72–R79. doi: 10.1152/ajpregu.00056.2010. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, Calbet JA, Radegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol. 1997;272:H2655–H2663. doi: 10.1152/ajpheart.1997.272.6.H2655. [DOI] [PubMed] [Google Scholar]
- Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol. 2010a;108:349–355. doi: 10.1152/japplphysiol.00551.2009. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol. 2010b;109:1360–1368. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradkhan R, Spitnale B, McQuillan P, Hogeman C, Gray KS, Leuenberger UA. Hypoxia-induced vasodilation and effects of regional phentolamine in awake patients with sleep apnea. J Appl Physiol. 2010;108:1234–1240. doi: 10.1152/japplphysiol.90855.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Kato M, Pesek CA, Somers VK. Human obesity is characterized by a selective potentiation of central chemoreflex sensitivity. Hypertension. 1999;33:1153–1158. doi: 10.1161/01.hyp.33.5.1153. [DOI] [PubMed] [Google Scholar]
- Owen JA. Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28:333–338. doi: 10.1093/ajcn/28.4.333. [DOI] [PubMed] [Google Scholar]
- Reichmuth KJ, Dopp JM, Barczi SR, Skatrud JB, Wojdyla P, Hayes D, Jr, Morgan BJ. Impaired vascular regulation in patients with obstructive sleep apnea: effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 2009;180:1143–1150. doi: 10.1164/rccm.200903-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsburg S, Launois SH, Weiss JW. Patients with obstructive sleep apnea have an abnormal peripheral vascular response to hypoxia. J Appl Physiol. 1999;87:1148–1153. doi: 10.1152/jappl.1999.87.3.1148. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Blackmon JR. Lack of sympathetic vasoconstriction in hypoxemic humans at rest. Am J Physiol. 1986;251:H562–H570. doi: 10.1152/ajpheart.1986.251.3.H562. [DOI] [PubMed] [Google Scholar]
- Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol. 1988;65:1548–1552. doi: 10.1152/jappl.1988.65.4.1548. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduce forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Johnson DG, Fregosi RF. Hypoxia potentiates exercise-induced sympathetic neural activation in humans. J Appl Physiol. 1991;71:1032–1040. doi: 10.1152/jappl.1991.71.3.1032. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;282:H816–H820. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Smith CA, Soriano BJ, Dempsey JA. Sympathetic restraint of muscle blood flow during hypoxic exercise. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1538–R1546. doi: 10.1152/ajpregu.90918.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod CJ, Eastwood PR, O'Driscoll G, Walsh JH, Best M, Halliwill JR, Green DJ. Vasomotor responses to hypoxia in type 2 diabetes. Diabetes. 2004;53:2073–2078. doi: 10.2337/diabetes.53.8.2073. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol. 2006;101:1343–1350. doi: 10.1152/japplphysiol.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol. 2008;586:1195–1205. doi: 10.1113/jphysiol.2007.144113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komsesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]