Abstract
Objective
To perform a systematic review to evaluate the risk of malignancy associated with computed tomography (CT) of the head and/or neck in infants, children, and adolescents.
Data Sources
Pubmed, EMBASE, and the Cochrane Library were assessed from the date of their inception to January 2014. Additionally, manual searches of bibliographies were performed and topic experts were contacted.
Review Methods
Data were obtained from studies measuring or estimating the risks of malignancy associated with radiation from head and/or neck CT in pediatric populations according to an a priori protocol. Two independent evaluators corroborated the extracted data.
Results
There were 16 criterion-meeting studies that included data from n = 858,815 patients. The radiation-related risk of malignancy was estimated using primary patient data for both the exposure and outcome in a minority of studies, with most analyses utilizing mathematical modeling techniques. The data regarding otolaryngology-specific studies were limited and suggested a borderline significant increase in the risk of all combined cancers after facial CT (incidence rate ratio [IRR] = 1.14; 95% CI, 1.01–1.28) and neck/spine CT (IRR = 1.13; 95% CI, 1.00–1.28). Cohort data suggest that 1 excess brain malignancy occurred after 4000 brain CTs (40 mSv per scan) and that the estimated risk in the 10 years following CT exposure was 1 brain tumor per 10,000 patients exposed to a 10 mGy scan at less than 10 years of age.
Conclusion
Detailed understanding of any potential malignancy risk associated with pediatric imaging of the head and neck furthers our ability to engage in rational, shared, informed decision making with families considering CT scan.
Keywords: computed tomography, imaging, radiation, malignancy, pediatric, infant, child, adolescent, systematic review
Introduction
The potential harms of radiation exposure through medical imaging have been featured prominently in the recent lay media. A New York Times Op Ed from January of this year displayed the headline “We Are Giving Ourselves Cancer” and introduced a discussion of computed tomography (CT)-associated exposures and risks with the statement, “We are silently irradiating ourselves to death.”1 Similarly, Newsweek featured an article in April of this year titled “Death Rays” and commented on the potential hazards of both medical imaging and radiation therapy.2 The New York Times piece prompted 6 published response letters to the editor, with authors spanning the gamut from the American College of Radiology, the College of Emergency Physicians, internists, and the George Washington School of Public Health.3 The Newsweek article, less than 1 week old at the time of this writing, has already resulted in a response from the American College of Radiology.4
In the setting of concerns raised among the general public, we may see patients and families who voice apprehension or a desire for further information regarding the potential long-term ramifications of CT scan. Given that radiation effects have been shown to be increased in younger age groups,5–12 parents considering CT for their children may have increased concern. As otolaryngologists, we are not the primary stewards of radiation science, imaging exposure protocols, or long-term prospective cohort studies of patients who have undergone related imaging; nonetheless, we are often the primary interface with patients and families when these studies are ordered. Since radiation physicists, radiologists, and many epidemiologists do not interface directly with patients and their families, the responsibility of weighing the related implications and their complexities typically falls to providers such as ourselves. While we are often well versed in the potential diagnostic yield of these studies, a confident discussion of the potential future malignancy risks after CT exposure in childhood may be more likely to fall outside the standard otolaryngologist’s comfort zone.
Understanding the associated peer-reviewed evidence regarding the risk associated with head and neck CT may prove invaluable to facilitating meaningful, factual, and productive conversations with families of considered children. In fact, although it has not been studied specifically with regard to otolaryngological imaging, management decisions based on established data (ie, evidence-based practice) have been shown to improve patient outcomes spanning the gamut of cardiovascular, respiratory, neurological, and surgical disease.13–17 Systematic reviews provide a proven, rigorous method to demonstrate the current best evidence and available data regarding a specific clinical question.18–20 A systematic review is an analysis of the medical literature that “uses explicit methods to systematically search, critically appraise, and synthesize the world literature on a specific issue.”21 When performed according to the standard rigorous techniques, it minimizes bias, random error, and confounding and is thus more powerful than a traditional narrative review.20,21 Because of this, they top hierarchies of evidence in international systems20,22,23 and are understood to require as much or more effort than the underlying source articles.24,25 Accordingly, the systematic review underlies significant documents such as clinical practice guidelines22,26 and forms the foundation for global collaborative work-groups such as the Cochrane Collaboration. In fact, some systematic reviews are cited hundreds of times annually.20
The objective of the current systematic review was to evaluate the risk of malignancy associated with radiation exposure from CT of the temporal bone, head, and/or neck at a young age. Specifically, we sought to (1) test the null hypothesis that there is no impact of head and neck CT scan on subsequent risk of malignancy and (2) determine the magnitude of any such effect according to the current available best evidence.
Methods
Risk of Malignancy with CT-related Radiation Exposure
A computerized search was performed to identify the risk of malignancy associated with head, temporal bone, and/or neck CT scan in infants, children, and adolescents. Computerized and manual searches were performed to identify all relevant data. A PubMed search of MEDLINE from 1966 through January 2014 was performed. Articles mapping to the medical subject heading tomography, X-ray computed (exploded) were collected into a first group. Articles that mapped to the medical subject headings head and neck neoplasms, brain neoplasms, cranial nerve neoplasms, and soft tissue neoplasms, under subheadings of etiology and epidemiology, along with papers mapping to the medical subject headings neoplasms, radiation-induced (exploded) were collected into a second group. Next, articles that mapped to the exploded medical subject headings child and infant and those that mapped to the text words pediatric and newborn were collected into a third group. Articles from these 3 groups were then cross-referenced and limited to those with human subjects and English language. Case reports as defined by the database indices were excluded. Two independent searches were performed by individuals blinded to the others’ results. In addition, searches with corresponding terms were repeated in EMBASE and the Cochrane Library through January 2014. In accordance with standard systematic review techniques, all journals indexed to these databases were by default included, thus spanning the range of all available impact factors. In general, a computerized search has limitations, particularly if the topic assessed in diagnosis; the sensitivity and specificity of best single term and combinations for high sensitivity MEDLINE searches is just 0.80 and 0.77, respectively.27 Accordingly, a systematic review typically includes a manual search to supplement the computerized inquiry.28
The computerized search strategy yielded 1169 studies. The abstracts were evaluated according to the inclusion and exclusion criteria described in the following. Reference lists from criteria-meeting publications and narrative reviews were manually searched for additional studies, yielding 37 potential articles. In addition, experts in the field were contacted for any additional reports of published or unpublished data. Titles and abstracts for all identified studies were reviewed, and ultimately, 158 full articles were evaluated in detail (Figures 1, 2).
Figure 1.
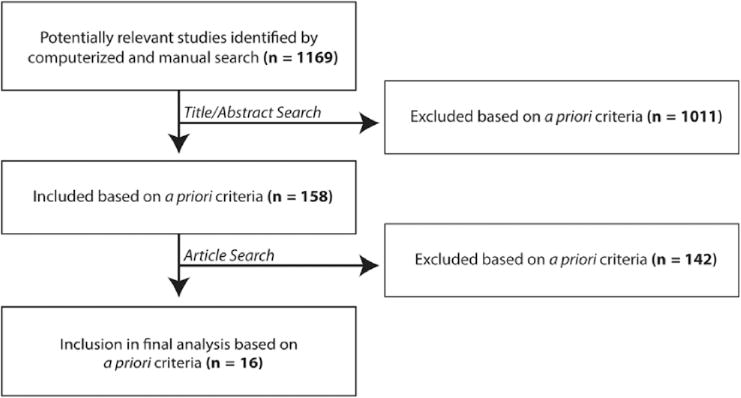
Flow diagram showing the stages of identification of studies in total.
Figure 2.
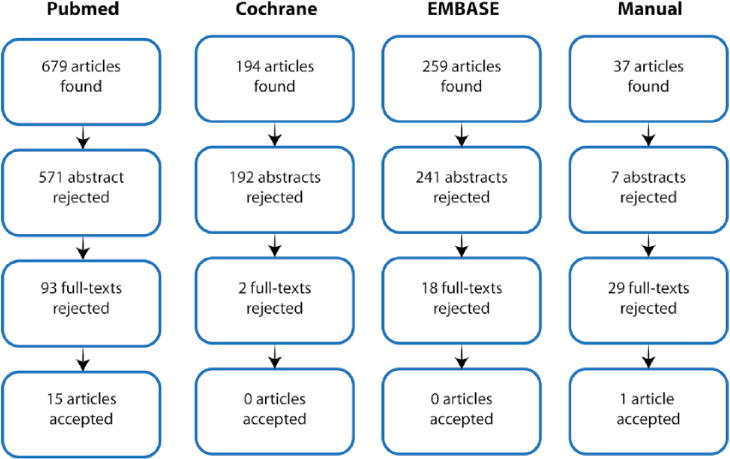
Flow diagram showing the stages of identification of studies by citation source.
Inclusion/Exclusion Criteria
Articles identified by the search strategy described previously were evaluated to identify those that met the following inclusion criteria: (1) patient population predominantly under 21 years of age; (2) CT temporal bone, head, or neck performed for diagnostic purposes; (3) outcome measured in terms of the proportion of those undergoing CT who developed subsequent malignancies or the relative risks of those undergoing CT for subsequent malignancies. Prospective, retrospective, comparative studies, case series, and mathematical modeling analyses were included. Articles were excluded if (a) patients were predominantly over 21 years of age; (b) patient populations had plain film radiography or MRI rather than CT; (c) no quantitative data were presented; (d) cone beam CT for dentistry (which has lower radiation dose than a standard CT) was utilized; (e) hypocycloidal, linear, or X-ray tomography was utilized, rather than standard CT; (f) positron emission tomography was the diagnostic study assessed; (g) the study population was already afflicted with malignancy at the outset; or (h) they were isolated case reports. Case reports were defined according to a standard definition of a “single clinical observation whose principal purpose is to generate hypotheses regarding human disease or provide insight into clinical practice.”29,30 This process yielded 16 studies that met our inclusion criteria.
Data Extraction
Extracted data included (1) the estimated risk of subsequent malignancies associated with CT, (2) counts and types of malignancies/neoplasms identified or predicted after CT, and (3) the P value, confidence interval, or standard error of the mean as reported. Data collection also included multiple potential sources of heterogeneity or bias among studies: (a) the age group investigated, (b) any predictive model utilized, (c) the method of calculating risk, and (d) study design with potential confounders. Two reviewers corroborated extracted data independently using standardized tables.
Quantitative Data Analysis
An a priori plan was developed to determine whether pooling data from the subset with directly measured patient exposures and outcomes were appropriate, but given baseline differences among the two such criteria-meeting studies, as well as the small number of non–mathematical modeling studies meeting criteria, no pooled analysis was performed. Numbers needed to harm were calculated according to standard definitions according to the absolute risk increase.
Results
Study Characteristics
A total of 16 criteria-meeting studies were included.5,8,31–44 One study44 focused on the subset of temporal bone and sinus CTs, but all included data specific to head and neck imaging. There were 2 historical cohort studies (n = 858,815 patients)5,31 and 3 other studies using primary data (n = 875 patients), among which 2 studies used patient data from case series33 and 1 study used data from a prospective patient database.32 Eleven studies mathematically modeled data from published dose and risk rates,8,35,40,42–44 such as those provided in the Biological Effects of Ionizing Radiation (BEIR) reports by the National Academy of Sciences committee.
Highest Level of Evidence: Primary Human Data for Both Exposure and Outcome
Two studies reported primary human data for both patient exposure and outcome (Table 1). Most recently, Mathews et al31 described a historical cohort study of 680,211 children exposed to CT in comparison with 10,939,680 without exposure. The mean time to follow-up was 9.5 years in the exposed group and 17.3 years in the unexposed group. Relevant to otolaryngological imaging, the authors evaluated a cohort with facial CTs and with neck/spine CTs. The incidence rate ratio (IRR) of all cancers combined after facial CT demonstrated a borderline significant elevation in risk 1.14 (95% CI, 1.01–1.28). When specific types of malignancies were considered, the subsequent incidence of thyroid and brain malignancies after facial CT was likewise significantly increased, but the associated confidence intervals approached the null (IRR = 1.53; 95% CI, 1.05–2.22; and IRR = 1.60, 95% CI, 1.04, 2.47, respectively). The risk of leukemia, lymphomas, melanoma, soft tissue malignancies, and other solid cancers showed nonsignificant results.
Table 1.
Risk of Malignancy after CT Scan: Studies with Primary Data for Both Patient Exposure and Outcome.
| Author, Year |
Study Design (sample size) |
Estimated Risk of Subsequent Malignancy Associated with CT |
Counts and Types of Malignancy/ Neoplasm Identified or Predicted |
Method of Risk Calculation |
Age at the Time of CT |
Additional Comments |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mathews, 201331 | Historical cohort study, n = 10,939,680, among which 680,21 1 were exposed to CT |
|
|
Estimated IRRs by Poisson regression | 0–19 years at the start of the study | Electronic Medicare records of Australians 0–19 years old between 1985 and 2005 The cohort was followed through 2007 by electronic linkage to the Australian Cancer Database and the National Death Index |
||||||||
| Incidence rate ratiosa for type of cancer
|
Number of malignancies reported in CT-exposed subjects
|
|||||||||||||
| B | T | L | A | B | T | L | A | |||||||
|
|
|
|||||||||||||
| Brain CT | 2.44 (2.12–2.81) | 1.33 (1.13–1.57) | 1.16 (0.99–1.37) | 1.23 (1.18–1.29) | Brain CT | 210 | 155 | 149 | 1964 | |||||
| Facial bones CT | 1.60 (1.04–2.47) | 1.53 (1.05–2.22) | 1.07 (0.70–1.62) | 1.14 (1.01–1.28) | Facial bones CT | 21 | 28 | 22 | 280 | |||||
| Spine/neck CT | 1.19 (0.69–2.05) | 1.78 (1.24–2.58) | 1.31 (0.85–2.04) | 1.13 (1.00–1.28) | Spine/neck CT | 13 | 29 | 20 | 252 | |||||
|
|
|
|||||||||||||
| Pearce, 20125 | Historical cohort study, n = 178,604 for leukemia analysis, n = 176,587 for brain tumor analysis |
|
Leukemia: 74 of 178,604 patients Brain tumors: 1 35 of 176,587 patients |
Poisson relative risk models fitted by maximum likelihood | <22 years | Sites scanned: 64% head, 9% abdomen, 7% chest After 2001,5–10 head CTs in children < 15 yrs result in accumulation of about 50 mGy red bone marrow dose and 2–3 head CTs results in about a 60 mGy cumulative brain dose |
||||||||
| Cumulative exposure Relative risk (95% CI) Reference0020b: <5 mGy | ||||||||||||||
|
| ||||||||||||||
| Leukemia | >30 mGy (mean 51.1 mGy): 3.18(1.46–6.94) | |||||||||||||
| Brain tumors | Estimate 1:50–74 mGy (mean 60.4 mGy): 2.82 (1.33–6.03) Estimate 2: >50 mGy (mean 104.2 mGy): 3.32 (1.84–6.42) |
|||||||||||||
|
| ||||||||||||||
Abbreviations: IRR, incidence rate ratios; B, brain; T, thyroid; L, leukemia; A, all cancer.
For exposed versus unexposed by site of CT scan and type of cancer, based on a 1-year lag period, stratified for age, sex, and year of birth.
The reference category to which the higher exposures were compared was <5 mGy.
Among children who underwent neck/spine CT, there was a borderline increase in risk of all cancers (IRR = 1.13; 95% CI, 1.00–1.28), and the incidence of thyroid malignancy was significantly elevated (IRR = 1.78; 95% CI, 1.24–2.58). There was no significant increase in risk in leukemia, lymphomas, brain malignancies, melanoma, soft tissue, and other solid cancers. As this was a larger study not limited to head and neck imaging, absolute excess risks and estimated radiation doses specific to facial or neck/spine CTs were not reported.
These specific measures were, however, reported in the authors’ analysis of brain malignancies, which was a greater focus of the study. The IRR of all cancers combined after exposure to brain CT was significantly elevated (1.23; 95% CI, 1.18–1.29), and the absolute excess incidence rate for brain cancer was reported as 2.97 (95% CI, 2.28–3.66) per 100,000 person-years. The authors estimated that if all excess brain cancers after a brain CT were considered attributable to the imaging alone, then 1 brain malignancy would occur after approximately 4000 brain CTs (estimated average brain dose 40 mSv per scan). For brain and for all cancers combined, IRRs were highest for CT exposures in the youngest children (<5 years of age) and decreased with increasing age at first exposure (P < .001). The IRR also increased by 0.16 (95% CI, 0.13–0.19) with each additional CT scan (P < .001 for trend).
Similarly, Pearce et al5 conducted a historical cohort study, including an evaluation of the incidence of brain tumors after head CT (n = 176,587) and leukemia after CT (n = 178,604). They studied patients who were first exposed to CT before 22 years of age and tracked their cancer incidence and mortality over 5 to 23 years. The relative risk (Poisson) of brain tumors for patients receiving >50 mGy was 3.32 (95% CI, 1.84–6.42) compared with those receiving less than 5 mGy of radiation. The estimated excess risk of brain tumors among patients 0 to 20 years of age at exposure was 0.32 cases per 10,000 exposed children. The estimated risk in the 10 years following the exposure was reported as 1 brain tumor per 10,000 patients exposed to a 10 mGy scan at less than 10 years of age. The relative risk after brain CT also varied with age.
With regard to leukemia, the authors concluded that the relative risk (Poisson) for patients receiving cumulative doses of at least 30 mGy was 3.18 (95% CI, 1.46–6.94) compared with those receiving less than 5 mGy of radiation. The associated excess risk was 0.83 cases per 10,000 exposed children. The estimated risk in the 10 years following the exposure was reported as 1 leukemia per 10,000 patients exposed to a 10 mGy scan at less than 10 years of age. For leukemia, the dose response did not vary between age at exposure, time since exposure, sex, or other covariates.
Estimates of Risk Using a Combination of Patient Data and Calculated Projections
Three studies32–34 employed patient data and mathematical modeling in estimating the risk of malignancies associated with CT (Table 2). The studies collected information on the amount of radiation absorbed by pediatric patients and extrapolated theoretical risks of cancer induction using previously published data from the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), the BIER reports from the National Academy of Sciences committee, or the ImPACT CT dosimetry spreadsheets (St George’s healthcare NHS Trust, London). Muchow et al32 compared the radiation from cervical spine CT versus plain film radiographs and estimated that the excess relative risk of thyroid cancer was 13% (range, 10%–66%) for males and 25% (range, 8.0%–116.0%) for females. King et al33 estimated that the lifetime attributable risk (LAR) of excess cases of cancer was highest in the youngest age groups and also varied significantly between institutions in this bi-institutional study. Lastly, Fletcher et al34 calculated that for 2 infant patients (<1 year of age) with skin ionizing radiation doses of 63 mGy, such doses might be associated with as high as a 1 in 300 chance of causing malignant disease.
Table 2.
Risk of Malignancy after CT Scan: Studies with Primary Data for Patient Exposure and Calculated Predicted Outcome.
| Author, Year | Study Design (sample size) | Estimated Risk of Subsequent Malignancy Associated with CT | Counts and Types of Malignancy/Neoplasm Identified or Predicted | Method of Risk Calculation | Age at the Time of CT | Additional Comments | ||
|---|---|---|---|---|---|---|---|---|
| Muchow, 201232 | Retrospective evaluation of data from a prospective database (n = 393 with CT; n = 224 with plain radiograph) | The median ERR for 1 cervical spine CT in males was 13.0% (range, 10%–66%) and females was 25.0% (range, 8.0%–1 16.0%), P < .001 when compared with plain radiographs Young (0–6 years) males and females have the highest ERR for 1 CT scan, 21.5% and 45.6%, respectively, compared with the older 2 groups. |
Estimated absolute risk of thyroid cancer: after CT increased from 5.2 to 5.87/100,000 (0.0052% → 0.00587%) for males and from 15.2 to 19.0/100,000 (0.0152% → 0.019%) for females | Total thyroid radiation dose from CT was calculated via the Monte Carlo technique using the ImPACT CT dosimetry spreadsheets | < 17 years | Cervical spine multidirectional CT in trauma patients | ||
| King, 200933 | Retrospective case series of randomly sampled patients (n = 480) |
|
LAR of excess deaths from exposure per 100,000 persons after 1 head CT Solid tumors: 7.0–16.7 Leukemia: 0.9–1.9 |
LAR estimates based on BEIRVII Effective dose calculated from ImPACT CT dosimetry spreadsheets |
0–14 years | All children had undergone at least 1 head CT without contrast in a trauma center or regional children’s hospital | ||
| Excess cases per 100,000 persons after 1 head CT
|
||||||||
| Age | Solid Tumors | Leukemia | ||||||
|
| ||||||||
| 0–3 yrs | 19.5–35.6 | 1.7–2.7 | ||||||
| 4–9 yrs | 17.0–32.8 | 1.5–2.5 | ||||||
| 10–14 yrs | 13.7–29.0 | 1.2–2.2 | ||||||
|
| ||||||||
| Fletcher, 198634 | Estimates based on calculating effective doses for imaged infants (n = 2) | Brain CT: Based on an estimated skin dose of 62 mGy, CT has a calculated 1 in 300 chance of causing malignant disease | No additional estimates reported | Radiation dose calculated with data from UNSCEAR | 1 year of age (infants born in 1982 and imaged in 1983) | The true risk may be considerably lower because the assumed dose to other parts of the body was as high as to the skull | ||
Abbreviations: ERR, estimated relative risk; LAR, lifetime attributable risk; BEIR, Biological Effects of Ionizing Radiation reports by the National Academy of Sciences committee; ImPACT CT dosimetry spreadsheet, tool for calculating patient estimated doses from CT scans, making use of the National Radiological Protection Board Monte Carlo dose data sets (St George’s Healthcare NHS Trust, London, UK); UNSCEAR, United Nations Scientific Committee on the Effects of Atomic Radiation.
Estimates of Risk from Mathematical Models
Eleven studies used various mathematical models to estimate both effective exposure and cancer risks associated with CT radiation (Table 3). Four studies focused on estimating the risk of thyroid cancer after CT scan. Mazonakis et al44 was the only study to specifically delineate ear-related imaging; the lifetime attributable risk for thyroid cancer induction in “inner ear” CT was 4 to 8 per 1,000,000. The authors also calculated estimates for the LAR of thyroid cancer after brain and neck spiral CTs; these studies were associated with 36 to 65 and 114 to 390 episodes per 1,000,000 patients, respectively. Mahajan et al35 estimated that the lifetime attributable risk of thyroid malignancy when exposed to parathyroid 4-dimensional CT (range, 0–15 years of age) was higher: 30 to 583 per 100,000 exposed (highest in female infants). Jimenez et al41 calculated the excess relative risk of thyroid cancer after head CT to be 0.03 at 1 year of age and 0.02 at 5 years of age. Lastly, Schonfeld et al37 estimated the mean excess lifetime thyroid cancer risk after head and C-spine CTs for females and males at 0, 1, 5, and 10 years of age to be from 0 (95% CI, 0–1) to 33 (95% CI, 7–102) per 10,000, predicting the highest risks for the youngest, female patients.
Table 3.
Risk of Malignancy after CT Scan: Studies Calculating Both Estimated Patient Exposure and Risks of Malignant Outcome.
| Author, Year |
Study Design | Estimated Risk of Subsequent Malignancy Associated with CT |
Counts and Types of Neoplasm Identified or Predicted |
Method of Risk Calculation |
Age at the Time of CT |
Additional Comments |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mahajan, 201235 | Mathematical model |
|
Thyroid cancer was the sole malignancy assessed | LAR estimates based on BEIRVII Effective dose calculated from ImPACT CT dosimetry spreadsheets |
Pediatric subset analysis of 0–20 years | 4-dimensional parathyroid CT (4DCT) is a means to localize parathyroid adenoma Calculated effective dose: 4DCT(I0.4 mSv) |
||||
| Age at exposure (yrs) | LAR of thyroid malignancy: incidence per 100,000 persons exposed to CT | |||||||||
|
| ||||||||||
| Male | Female | |||||||||
|
| ||||||||||
| 0 | 106 | 583 | ||||||||
| 5 | 70 | 385 | ||||||||
| 10 | 46 | 253 | ||||||||
| 15 | 30 | 164 | ||||||||
| 20 | 19 | 104 | ||||||||
|
| ||||||||||
| Koral, 201236 | Mathematical estimates |
|
No specific malignancies reported | Radiation doses referenced to adult head phantom; effective doses calculated using ImPACT spreadsheet; risk factors from BIERVII applied to effective doses per age group | Average age of first neuroimaging study was 1.89 years (1 day–18.65 years) | Study estimates risk associated with biannual surveillance scans of children with hydrocephalus | ||||
| CT type | ||||||||||
|
| ||||||||||
| Low dose (average effective dose of 1.1 mSv) | High dose (average effective dose of 2.5 mSv) | |||||||||
|
| ||||||||||
| Number of excess lifetime fatal malignancies | 1 per 230 patients | 1 per 97 patients | ||||||||
| Assumptions: | 2 head CTs per year until 20 years of age | |||||||||
|
| ||||||||||
| Schonfeld, 201137 | Mathematical model |
|
Thyroid cancer was the sole malignancy assessed | Doses estimated from existing organ dose database;45 risks projected through a modified version of model recommended by BIERVII | 0 years, 1 year, 5 years, 10 years | |||||
| Mean lifetime thyroid cancer risk per 10,000 scans (95% CI)
|
||||||||||
| Sex and age (yrs) | Head CT | C-spine CT | ||||||||
|
| ||||||||||
| Female, 0 | 3(1–9) | 33 (7–102) | ||||||||
| Female, 1 | 1 (0–2) | 17 (4–50) | ||||||||
| Female, 5 | 0(0–1) | 8 (2–24) | ||||||||
| Female, 10 | 0(0–1) | 6 (1–17) | ||||||||
| Male,0 | 1 (0–2) | 6 (1–19) | ||||||||
| Male, 1 | 0 (0–0) | 3 (1–9) | ||||||||
| Male, 5 | 0 (0–0) | 1 (0–4) | ||||||||
| Male, 10 | 0 (0–0) | 1 (0–3) | ||||||||
|
| ||||||||||
| Pflugbeil 201138 | Mathematical model |
|
Model uses estimates from epidemiological data and reported risk profiles, primarily from Germany | 0–14 years | Reports both their own calculated data (Tables 3, 4) and a review of risk estimates extrapolated from other publications | |||||
| Age at exposure (yrs) | Brain tumors | Meningiomas | Leukemias | |||||||
|
| ||||||||||
| Induced cases per 100,000 children per year | 0.20 | 0.08 | 0.1–0.2 | |||||||
| Induced cases per 1000 CTs | 0.24a | 0.10a | 0.12–0.24 | |||||||
|
| ||||||||||
| Feng, 201039 | Mathematical model |
|
|
MDCT studies on phantom; LAR estimated using BEIR VII Report | Phantom modeled 5-year-olds | 64-slice MDCT scanner | ||||
| LAR of cancer from head CT, % | LAR from head CT,% | |||||||||
|
|
|
|||||||||
| 5-year-old boys | 0.015 | Other solid | ||||||||
| 5-year-old girls | 0.036 | Thyroid | organ | |||||||
|
|
|
|||||||||
| Boys | 0.003 | 0.010 | ||||||||
| Girls | 0.015 | 0.015 | ||||||||
|
| ||||||||||
| Berrington, 200940 | Mathematical model |
|
Projected number of future malignancies related to CT scans performed in 2007 Head: 18.7 m scans − 4000 (1100–8700) cases C-spine: 1000 (300–2300) cases |
LAR estimates based on BEIR VII and a Monte Carlo simulation Age/sex distribution for CT type based on data from a national commercial insurance database |
3 years, 15 years | Estimated total malignancy risk associated with CTs performed in the US in 2007 evaluated Head CTs (33% of scans) and C-spine CTs (3% of scans) included in the analysis |
||||
| Mean lifetime cancer risk per 10,000
|
||||||||||
| Age (yrs) | Head CT | C-spine CT | ||||||||
|
| ||||||||||
| 3 | 8–9 | 10–70 | ||||||||
| 15 | 4–5 | 10–50 | ||||||||
|
| ||||||||||
| Jimenez, 200841 | Mathematical model |
|
Thyroid cancer was the only malignancy assessed | Excess relative risks per gray estimated based on Sadetzki et al, 200646 | Phantoms modeled 1 -year-old and 5-year-old | Estimates radiation dose during evaluation for c-spine injuries | ||||
| Excess relative risk of thyroid cancer
| ||||||||||
| Age (yrs) | Head CT | C-spine CT | ||||||||
|
| ||||||||||
| 1 | 0.03 | 2 | ||||||||
| 5 | 0.02 | 0.7 | ||||||||
|
| ||||||||||
| Stein, 200842 | Mathematical model utilizing data from meta-analysis of published dose and risk rates |
|
Markov model simulation to estimate the risk of a single head CT | 1–20 years | Data obtained from prior publications regarding head CT radiation dose/risk and modeling only | |||||
| LAR of radiation induced malignancy, % by age of exposure
|
Data obtained from multiple sources in the literature46–48
|
|||||||||
| Age (yrs) | Tumor | Fatality | Malignancy | Rl | 95% CI | |||||
|
|
|
|||||||||
| 1 | 0.22 | 0.070 | Thyroid carcinoma | 0.466 | 0.400–0.531 | |||||
| 2 | 0.15 | 0.060 | ||||||||
| 5 | 0.12 | 0.050 | Meningioma | 0.345 | 0.273–0.417 | |||||
| 10 | 0.08 | 0.033 | ||||||||
| 15 | 0.05 | 0.020 | Glioma | 0.189 | 0.109–0.270 | |||||
| 20 | 0.04 | 0.015 | ||||||||
|
|
|
|||||||||
| Chodick, 200743 | Mathematical model |
|
LAR estimated using the method described by Brenner et al, 2001;15 CT usage data obtained from the largest health management organization in Israel |
< 18 years | In Maccabi, Israel, 17,686 pediatric CT scans were performed per year Data were reported for head CT and body CT |
|||||
| Projected annual number of pediatric CT related lifetime cancer deaths: head, face, or neck CT
|
||||||||||
| Age (yrs) | Male | Female | ||||||||
|
| ||||||||||
| <3 | 0.78 | 0.48 | ||||||||
| 4–6 | 0.51 | 0.30 | ||||||||
| 7–9 | 0.48 | 0.34 | ||||||||
| 10–12 | 0.36 | 0.32 | ||||||||
| 13–15 | 0.25 | 0.20 | ||||||||
| 16–18 | 0.23 | 0.17 | ||||||||
|
| ||||||||||
| Mazonakis, 200744 | Mathematical model |
|
Thyroid cancer was the sole malignancy assessed | LAR estimates based on risk report from International Commission on Radiologic Protection (and National Council on Radiation Protection and Measurements), mean calculated doses for phantoms, and Monte Carlo simulation | Phantoms modeled newborn infant, 1-year-old, 5-year-old, 10-year-old, 15-year-old | Thyroid exposure to scattered radiation from head CT is associated with a low but not negligible risk of cancer induction of 4–65 per million patients; including the thyroid (neck) increases the risk to 114–390/million Effective mAs per study: brain (90–320), sinus (40–100), inner ear (40–120), neck 40–150) |
||||
| LAR for thyroid cancer per 1,000,000 persons after 1 CT
| ||||||||||
| Brain, sequential | 16–21 | |||||||||
| Brain, spiral | 36–65 | |||||||||
| Sinuses, spiral | 20–36 | |||||||||
| Inner ear, sequential | 5–8 | |||||||||
| Inner ear, spiral | 4–7 | |||||||||
| Neck, spiral | 114–390 | |||||||||
|
| ||||||||||
| Brenner, 20018 | Mathematical model | Estimates for children < 1 Syears of age, annually: ~600,000 abdominal and head CT examinations in US; accounts for ~4% of all CTs, but results in ~20% of the total potential cancer mortality from CT radiation ~500 cancer deaths attributable to CT radiation ~ 170 cancer deaths attributable to head CT radiation Age- and gender-specific data are presented in Figure 3. |
Brain cancers constitute the bulk of the estimated malignant lesions Approximately 10% arise from thyroid Estimated leukemia rates are also elevated |
LAR estimated using BEIRV, tissue-V, tissue-specific radiation-carcinogenic sensitivities from the International Commission on Radiological Protection | Pediatric subsets modeled at 0, 1,5, 15 years | |||||
Abbreviations: LAR, lifetime attributable risk; RI, relative incidence; BEIR, Biological Effects of Ionizing Radiation reports by the National Academy of Sciences committee; ImPACT CT dosimetry spreadsheet, tool for calculating patient estimated doses from CT scans, making use of the National Radiological Protection Board Monte Carlo dose datasets (St. George’s Healthcare NHS Trust, London, UK); UNSCEAR, United Nations Scientific Committee on the Effects of Atomic Radiation; MDCT, multidetector row computed tomography.
Calculated from the reported data.
Six other studies provided mathematical estimates for the LAR of all malignancies associated with CT. Brenner et al8 in 2001 multiplied age-dependent lifetime cancer mortality risk (per unit dose) utilizing estimated age-dependent organ doses (published data from the BEIR V report), concluding that among the 600,000 children younger than 15 years undergoing CT annually in the United States, approximately 500 (0.083%) might ultimately die from malignancy to the exams’ radiation (Figure 3). Combining Brenner et al’s8 estimates of lifetime risk of radiation-induced fatal cancer with other data from the literature, Stein et al42 calculated that the lifetime risk of radiation-induced cancer tumor and fatality is highest for the youngest ages of exposure (0.22% tumor and 0.07% fatality for a single head CT at 1 year of age). More recently, Berrington et al40 estimated mean lifetime cancer risk is 0.04% to 0.09% per head CT, utilizing BEIR VII data and age- and sex-specific scan frequencies. Pflugbeil et al38 reported an induced incidence of 0.24 brain tumors per 1000 CT scans in children annually, using epidemiological data and studies of neoplasm induction after radiotherapy to model risk. Chodick et al43 applied Brenner et al’s8 method to estimate cancer mortality attributable to CT examinations in patients under 18 years of age and projected the annual number of excess lifetime cancer deaths attributable to pediatric CT to be highest in the youngest children (0.48 for girls, 0.78 for boys), declining with age. Feng et al39 estimated the LAR of cancer from head CT in 5-year-olds to be 0.015% in boys and 0.0366% in girls.
Figure 3.
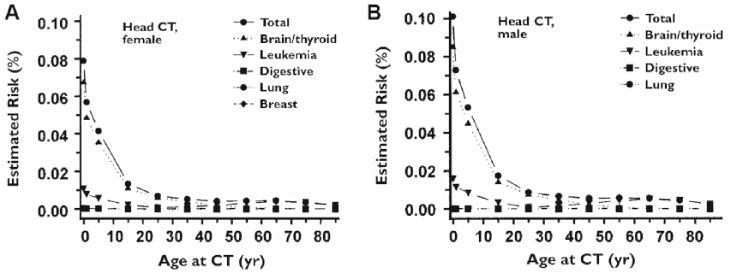
Estimated lifetime CT-attributable cancer mortality risks as a function of age and gender. Risk increases exponentially with decreased age, particularly among the youngest age group. Reprinted from: Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289–296, with permission from the American Journal of Roentgenology.
The remaining study specifically examined the impact of repeated CTs; Koral et al36 evaluated the impact of 2 head CTs per year from the time of diagnosis of hydrocephalus to 20 years of age and reported excess lifetime fatal malignancies of 1 in 230 patients for low dose CTs and 1 in 97 patients for high dose CTs.
Discussion
The data from this systematic review suggest that the null hypothesis is disproved; they imply that there is an impact of head and neck CT scan on subsequent risk of malignancy. Data regarding the magnitude of the effect of otolarygological imaging (ie, temporal bone, sinus, neck) is limited, although reports provide more detailed descriptions of head/brain CT.
In brief, the strongest data came from 2 human cohort studies,5,31 both of which reported data from head/brain CTs and 1 of which reported results after facial and neck/spine CT. Reported incidence rate ratios suggested a significant increase in the incidence of thyroid malignancy after neck/spine CT and significant but near borderline increases in the risk of all cancers after either facial or neck/spine CT. Results for brain cancers after brain CT were reported in more detail in both studies; 1 estimated that if every excess brain cancer after brain CT was attributable only to the imaging itself, then approximately 1 in 4000 brain CTs in children would be followed by a malignancy (mean estimated brain dose 40 mSv per scan).31 The additional cohort study5 estimated that the risk in the 10 years following CT was 1 brain tumor per 10,000 patients exposed to a 10 mGy scan at less than 10 years of age.
Additional reports contained less directly clinically applicable data in that their results were based on calculated projections and mathematical modeling. While these publications constitute the bulk of the reports, the data are more theoretical in nature and were thus less emphasized. One study based on mathematical modeling evaluated “inner ear” CT and calculated a lifetime risk for thyroid cancer of 4 to 8 per 1,000,000.44 The same study also analyzed sinus CT with a predicted risk of 20 to 36 thyroid malignancies per 1,000,000. Another group reported on the lifetime attributable risk of thyroid cancer mathematically modeled after 4-dimensional parathyroid CT and demonstrated risks ranging from 1.9 to 58.3 per 1,000,000 cases, depending on gender and age.35 Another report also suggest an elevated risk of thyroid malignancy after C-spine CT.32
Overall, temporal bone, sinus, and neck CTs are anticipated to confer a lower radiation dose than brain CTs.44 Accordingly, facial and neck/spine CTs were associated with a lower incidence rate ratio for brain malignancy than brain CTs in a large historical cohort study.31 Given that the risk of malignancy increases with increased radiation exposure, it may be inferred that risks with temporal bone, sinus, and neck studies are less than those in the presented studies of brain imaging, although the direct data to support the absolute risk increase of otolaryngological images are limited.
The age-specific impact of radiation is well documented5–12; for example, the estimated attributable risk of death from cancer from a single head CT is highest for the youngest patients5,6,31 and rapidly declines after 20 years of age.6 The highest risks were observed in children less than 5 years of age and appear to be exponentially higher among infants less than 1 year of age (Figure 3).5,6,8 This age dependence may be due to the dose relative to body size as well as inherent age-specific risks per unit dose.6,10,12 Moreover, children have a longer life expectancy than adults, and so the window of time for malignancies to appear is larger.6,9,10 These data suggest that when possible, there may be some attenuation in risk by waiting until children are in an older age group to obtain a study; more specifically, it may be beneficial to wait to obtain a scan until the information gleaned will affect management decisions, although this concern must clearly be weighed against potential harms from delays in diagnosis.
We next present an expository discussion of radiation effects according to expressed interest. Radiation effects are of 2 types: cumulative and stochastic.49 Cumulative effects (also known as deterministic effects) occur with a high dose in a short period. In an extreme circumstance, such as with radiation therapy, it may be associated with tissue necrosis or hair loss. These effects are expected based on a threshold dose and are typically not seen with radiological interventions, except perhaps in the form of skin changes after very long interventional procedures. Stochastic effects, in contrast, occur in low doses over a long period of time. In fact, the effects may be seen over a wide range of doses, and the severity of the effect is not clearly related to a threshold dose. CT scans, chest X-rays, and mammograms, for example, may be done at long intervals over a period of many years and over time, the radiation for these serial studies accumulates, although it may cause less immediately noticeable changes. There may be unseen effects, and it is not always clear that the effects are strictly additive. In order to understand the difference between the 2 types, consider the following analogy from nature: A beach may be eroded quickly with sudden clearly defined force, such as after a hurricane (ie, cumulative), or it may be eroded slowly by the repetitive impact of a range of varying and less powerful waves (ie, stochastic).
Increasing our understanding of the data regarding radiation-related risks associated with pediatric CT of the head and neck furthers our ability to engage in meaningful conversation with families when these diagnostic studies are considered; such discourse may occur with increased frequency now that such risks have become headline topics in nonmedical forums. The potential benefits from the diagnostic yield of CT as an integral part of otolaryngological care are certainly also of grave importance and have been addressed in other related manuscripts. Combining an evidence-based understanding of potential risks with knowledge of the potential diagnostic yield of such imaging will help improve caregivers’ ability to make shared, informed decisions when considering CT scan of the head and neck in infants, children, and adolescents.
Acknowledgments
We would like to thank Dr Laura Vitale Romo, MD (Department of Radiology, Harvard Medical School) for input on imaging characteristics and definitions to faciliate decisions regarding criteria-meeting studies. JJS would also like to thank Thomas Y. Lin and Creating Healthcare Excellence through Education and Research for support during the preparation of this manuscript.
Footnotes
Author Contributions
Jenny X. Chen, analysis, interpretation, drafts, final approval; Bart Kachniarz, analysis, interpretation, drafts, final approval; Sapideh Gilani, radiation risk analysis, drafting, final approval; Jennifer J. Shin, concept, design, analysis, interpretation, drafts, final approval.
Disclosures
Competing interests: Jennifer J. Shin receives royalties from the publication of 2 books: Evidence-based Otolaryngology (Springer International), Otolaryngology Prep and Practice (Plural Publishiing).
Sponsorships: None.
Funding source: None.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1.Redberg RF, Smith-Bindman R. We are giving ourselves cancer. New York Times. 2014 Jan 30; [Google Scholar]
- 2.Ericson J. Death rays. Newsweek. 2014 [Google Scholar]
- 3.Ellenbogen PH, Gerardi M, Lee SI, Khipple S, Wen L, McCally M. The uses and abuses of CT scans: To the editor, in response to “We are giving ourselves cancer.”. New York Times. 2014 Feb 6; [Google Scholar]
- 4.Ellenbogen PH. Letter to the editor: in response to “Death rays.”. Newsweek. 2014 [Google Scholar]
- 5.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. New Eng J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 7.Krille L, Zeeb H, Jahnen A, et al. Computed tomographies and cancer risk in children: a literature overview of CT practices, risk estimations and an epidemiologic cohort study proposal. Radiat Environ Biophys. 2012;51:103–111. doi: 10.1007/s00411-012-0405-1. [DOI] [PubMed] [Google Scholar]
- 8.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roent. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Radiation risks and pediatric computed tomography. http://www.cancer.gov/cancertopics/causes/radiation/radiation-risks-pediatric-CT.
- 10.Brody AS, Frush DP, Huda W, Brent RL. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 11.Hall EJ. Lessons we have learned from our children: cancer risks from diagnostic radiology. Ped Radiol. 2002;32:700–706. doi: 10.1007/s00247-002-0774-8. [DOI] [PubMed] [Google Scholar]
- 12.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950–1990. Rad Res. 1996;146:1–27. [PubMed] [Google Scholar]
- 13.Forbes SS, Stephen WJ, Harper WL, et al. Implementation of evidence-based practices for surgical site infection prophylaxis: results of a pre- and postintervention study. J Am Coll Surg. 2008;207:336–341. doi: 10.1016/j.jamcollsurg.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 14.To T, Cicutto L, Degani N, McLimont S, Beyene J. Can a community evidence-based asthma care program improve clinical outcomes?: a longitudinal study. Med Care. 2008;46:1257–1266. doi: 10.1097/MLR.0b013e31817d6990. [DOI] [PubMed] [Google Scholar]
- 15.Shin JJ, Randolph GW, Rauch SD. Evidence-based medicine in otolaryngology, part 1: the multiple faces of evidence-based medicine. Otolaryngol Head Neck Surg. 2010;142:637–646. doi: 10.1016/j.otohns.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 17.Briffa T, Hickling S, Knuiman M, et al. Long term survival after evidence based treatment of acute myocardial infarction and revascularisation: follow-up of population based Perth MONICA cohort, 1984–2005. BMJ. 2009;338:b36. doi: 10.1136/bmj.b36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowther M, Lim W, Crowther MA. Systematic review and meta-analysis methodology. Blood. 2010;116:3140–3146. doi: 10.1182/blood-2010-05-280883. [DOI] [PubMed] [Google Scholar]
- 19.Shin JJ, Hartnick CJ. Introduction to evidence based medicine. In: Shin JJ, Hartnick CJ, Randolph GW, editors. Evidence-based Otolaryngology. New York: Springer; 2008. [Google Scholar]
- 20.Uthman OA, Okwundu CI, Wiysonge CS, Young T, Clarke A. Citation classics in systematic reviews and meta-analyses: who wrote the top 100 most cited articles? PLoS One. 2013;8:e78517. doi: 10.1371/journal.pone.0078517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence Based Medicine: How to Practice and Teach EBM. 1. London: Churchill Livingstone; 1997. [Google Scholar]
- 22.Haynes RB. Of studies, syntheses, synopses, summaries, and systems: the “5S” evolution of information services for evidence-based health care decisions. ACP J Club. 2006;145:A8. [PubMed] [Google Scholar]
- 23.National-Health-and-Medical-Research-Council. NHMRC evidence hierarchy: designations of levels of evidence according to type of research question. 2012 http://www.health.qld.gov.au/healthpact/docs/gen-docs/lvl-of-evidence.pdf.
- 24.Rosenfeld RM. Meta-analysis. ORL J Otorhinolaryngol Relat Spec. 2004;66:186–195. doi: 10.1159/000079876. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld RM. How to systematically review the medical literature. Otolaryngol Head Neck Surg. 1996;115:53–63. doi: 10.1016/S0194-5998(96)70137-7. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld RM, Shiffman RN. Clinical practice guidelines: a manual for developing evidence-based guidelines to facilitate performance measurement and quality improvement. Otolaryngol Head Neck Surg. 2006;135(suppl 4):S1–28. doi: 10.1016/S0194-5998(06)03094-4. [DOI] [PubMed] [Google Scholar]
- 27.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence Based Medicine: How to Practice and Teach EBM. 2. London: Churchill Livingstone; 2000. [Google Scholar]
- 28.Hopewell S, Clarke M, Lefebvre C, Scherer R. Handsearching versus electronic searching to identify reports of randomized trials. Cochrane Database Syst Rev. 2007;2:MR000001. doi: 10.1002/14651858.MR000001.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallin JI, Ognibene FP. Principles and Practice of Clinical Research. 3. Amsterdam: Elsevier/AP; 2012. [Google Scholar]
- 30.Posada de la Paz M, Groft SC. Rare Diseases Epidemiology. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 31.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ (Clinical research ed.) 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muchow RD, Egan KR, Peppler WW, Anderson PA. Theoretical increase of thyroid cancer induction from cervical spine multi-detector computed tomography in pediatric trauma patients. J Trauma Acute Care Surg. 2012;72:403–409. doi: 10.1097/TA.0b013e31823a4bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King MA, Kanal KM, Relyea-Chew A, Bittles M, Vavilala MS, Hollingworth W. Radiation exposure from pediatric head CT: a bi-institutional study. Ped Radiology. 2009;39:1059–1065. doi: 10.1007/s00247-009-1327-1. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EW, Baum JD, Draper G. The risk of diagnostic radiation of the newborn. British J Radiology. 1986;59:165–170. doi: 10.1259/0007-1285-59-698-165. [DOI] [PubMed] [Google Scholar]
- 35.Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg. 2012;36:1335–1339. doi: 10.1007/s00268-011-1365-3. [DOI] [PubMed] [Google Scholar]
- 36.Koral K, Blackburn T, Bailey AA, Koral KM, Anderson J. Strengthening the argument for rapid brain MR imaging: estimation of reduction in lifetime attributable risk of developing fatal cancer in children with shunted hydrocephalus by instituting a rapid brain MR imaging protocol in lieu of Head CT. Amer J Neurorad. 2012;33:1851–1854. doi: 10.3174/ajnr.A3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncology (Royal College of Radiologists) 2011;23:244–250. doi: 10.1016/j.clon.2011.01.159. [DOI] [PubMed] [Google Scholar]
- 38.Pflugbeil S, Pflugbeil C, Schmitz-Feuerhake I. Risk estimates for meningiomas and other late effects after diagnostic X-ray exposure of the skull. Rad Protection Dosimetry. 2011;147:305–309. doi: 10.1093/rpd/ncr344. [DOI] [PubMed] [Google Scholar]
- 39.Feng S-T, Law MW-M, Huang B, et al. Radiation dose and cancer risk from pediatric CT examinations on 64-slice CT: a phantom study. Eur J Radiol. 2010;76:e19–23. doi: 10.1016/j.ejrad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Berrington de González A, Mahesh M, Kim K-P, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Int Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jimenez RR, Deguzman MA, Shiran S, Karrellas A, Lorenzo RL. CT versus plain radiographs for evaluation of c-spine injury in young children: do benefits outweigh risks? Ped Radiology. 2008;38:635–644. doi: 10.1007/s00247-007-0728-2. [DOI] [PubMed] [Google Scholar]
- 42.Stein SC, Hurst RW, Sonnad SS. Meta-analysis of cranial CT scans in children. A mathematical model to predict radiation-induced tumors. Ped Neurosurgery. 2008;44:448–457. doi: 10.1159/000172967. [DOI] [PubMed] [Google Scholar]
- 43.Chodick G, Ronckers CM, Shalev V, Ron E. Excess lifetime cancer mortality risk attributable to radiation exposure from computed tomography examinations in children. Israel Med Assoc J: IMAJ. 2007;9:584–587. [PubMed] [Google Scholar]
- 44.Mazonakis M, Tzedakis A, Damilakis J, Gourtsoyiannis N. Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiology. 2007;17:1352–1357. doi: 10.1007/s00330-006-0417-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee C, Lodwick D, Williams JL, Bolch WE. Hybrid computational phantoms of the 15-year male and female adolescent: applications to CT organ dosimetry for patients of variable morphometry. Med Phys. 2008;35(6):2366–2382. doi: 10.1118/1.2912178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadetzki S, Chetrit A, Lubina A, Stovall M, Novikov I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tineacapitis. J Clin Endocrinol Metab. 2006;91(12):4798–4804. doi: 10.1210/jc.2006-0743. [DOI] [PubMed] [Google Scholar]
- 47.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 48.Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D. Radiation-induced meningioma: a descriptive study of 253 cases. J Neurosurg. 2002;97(5):1078–1082. doi: 10.3171/jns.2002.97.5.1078. [DOI] [PubMed] [Google Scholar]
- 49.Coley BD. Caffey’s Pediatric Diagnostic Imaging. Philadelphia: Elsevier Saunders; 2013. [Google Scholar]


