Abstract
Background
Lung cancer patients and their spouses may engage in blame attributions regarding the cancer cause, which may adversely affect their psychological adjustment.
Purpose
To examine whether dyadic adjustment and network support moderate the association between blame and distress in couples affected by lung cancer.
Methods
Patients and their spouses completed questionnaires within 1 month of treatment initiation (baseline) and at 6-month follow-up.
Results
Multilevel modeling of data from 158 couples revealed that, at baseline, dyadic adjustment moderated the association between blame and distress for patients but not spouses (p<.05). Controlling for baseline distress, baseline blame predicted later distress (p<.05) for both patients and spouses regardless of dyadic adjustment. Network support moderated this association at follow-up.
Conclusion
For patients experiencing low dyadic adjustment, blame was associated with increased distress. Not initially but later, network support may protect against low levels but not high levels of blame in patients and spouses.
Keywords: Lung cancer, attributions of blame, couples, distress, dyadic adjustment, social support
INTRODUCTION
Lung cancer is a devastating disease resulting in the death of approximately 157,000 men and women annually, making it the most fatal type of cancer in the United States (1). Because lung cancer patients often suffer from physical and psychiatric comorbidities (2), it is not surprising that they are at high risk for experiencing psychological distress more so than other cancer populations. For instance, a large study of 620 lung cancer patients found that 43% were distressed (3). To successfully cope with the cancer diagnosis and treatment, patients need support from their close others. Most often, patients’ spouses are the primary (4) and the most valued source of support (5); yet, as spouses deal with patients’ deteriorating health, ongoing caregiving demands, and fear of possible loss of their loved one, they tend to be no less distressed than patients themselves (6).
Attributions of Blame, Smoking and Distress in Lung Cancer
As individuals cope with a traumatic event such as cancer, they often attempt to make meaning by finding causal explanations of the event (7). Because at least 80% of lung cancers are linked to a history of poor health behaviors particularly smoking (8), patients may blame themselves for developing cancer, which may contribute to or exacerbate their psychological distress. In fact, a few studies have demonstrated that lung cancer patients are more vulnerable to blaming themselves (9-11) for developing cancer compared to other cancer populations including breast and prostate cancer patients whose illness etiology is less lifestyle dependent (11-13). Nevertheless, it is unclear if lung cancer patients who have a history of smoking are actually more vulnerable to attributions of blame compared to never smokers. For example, Salander (14) conducted qualitative interviews of 16 smokers diagnosed with lung cancer and found that only two attributed the cancer to their smoking. Similarly and importantly, in a sample of a 120 lung cancer patients, even though 70% of patients indicated smoking as a cause of their illness, 81% of them minimized the relevance of smoking as an etiological factor during follow-up interviews (15). In contrast, although less is known about spouses’ tendency to blame the patient for developing cancer, a recent study found that primary caregivers of lung cancer patients (68% of them were spouses) attributed the cancer cause to patients’ smoking behavior—particularly if the patient refused to quit smoking after the cancer diagnosis (16). However, the authors did not examine if caregivers’ own smoking status moderated their tendency to blame the patient. In order to identify couples at risk for blame, it is important to examine the role of patients and spouses’ smoking history in attributions of blame. (For purpose of clarification, from this point forward, blame refers to a patient blaming him or herself and a spouse blaming the patient for developing cancer so that the patient is always the target of blame).
Of note, attributions of blame are problematic because they are directly associated with psychological distress. Historically, there has been some controversy regarding the extent to which self-blame is harmful or possibly beneficial to psychological functioning. Attempting to clarify inconsistent findings, researchers have distinguished between behavioral self-blame (blaming particular behaviors such as smoking) and characterological self-blame (blaming global and stable personality characteristics)(17). Even though some have proposed that behavioral self-blame may be linked to improved adjustment—perhaps not initially but at later follow-up time points—because blaming one’s behavior may facilitate a sense of control, there is a consensus in the cancer literature that both types of blame are associated with poor psychological adjustment (12, 13, 18). Because we are interested in the role of a lifestyle behavior (i.e., smoking), the current research focuses on behavioral blame.
Although spouses may be vulnerable to blame attributions (16, 19), it is not clear if blaming the patient is linked to increased spousal distress. Based on findings in the marital literature, making relationship compromising attributions such as blaming one's partner for unfortunate events is distressing (20). Blaming one's loved one for developing a life-threatening disease may be particularly psychologically taxing because spouses may experience conflicting cognition and emotions (.e.g., anger, guilt, fear, worry, compassion, and affection). Furthermore, based on Weiner's Attribution-Emotion-Action Model of help-giving behavior (21), spouses’ affective responses to the patient's illness may be influenced by their attributions of the cancer cause. This model was preliminarily supported in lung cancer caregivers (19) in that caregivers who blamed the patient for developing cancer were more likely to experience negative affect, which in turn was associated with fewer helping behaviors. Thus, while couples attempt to cope with the stressors associated with the cancer diagnosis and treatment, both may suffer from the undercurrents of blame, which may compromise their psychological adjustment and exacerbate distress.
Moderators of the Blame and Distress Association
There is reason to believe that attributions of blame may affect couples differently depending on their levels of dyadic and social functioning possibly buffering the negative consequences associated with blame. Pinpointing which couples are likely to experience increased distress when engaging in attributions of blame is paramount to the design of programs facilitating cancer adjustment in couples. For example, dyadic adjustment, which is the general functioning of one's committed romantic relationship (i.e., satisfaction, stability, mutuality, affection)(22) has been linked to decreased psychological distress in lung cancer patients and their spouses (6, 23). Similarly, network social support has been identified as a major coping resource (24). Patients whose disease can be linked to a behavioral cause are vulnerable to experience or perceive stigma (9, 25), which may be associated with self-blame, as demonstrated in lung cancer patients (10, 11). Yet, when patients receive high levels of support such as love and acceptance from their social network, attributions of blame may be less distressing. Network support is also an important predictor of adjustment for caregivers of patients with lung cancer (26) and experiencing supportive relationships may alleviate distress associated with blaming one's loved one for developing a life-threatening disease.
Goals of Current Research
In summary, the purpose of this study was to examine associations between smoking history, attributions of blame, and distress in patients and their spouses facing lung cancer. We also examined whether dyadic adjustment and network support moderated the association between blame and distress. We hypothesized that:
Patients and spouses’ smoking history is associated with their tendency to engage in attributions of blame.
Dyadic adjustment and network support moderate the association between blame and distress such that those with low levels of dyadic adjustment and network support experience increased distress when engaging in attributions of blame.
Because previous work has revealed social role differences (patient vs. spouse) in the associations between relationship processes and psychosocial adjustment (27-31), we tested whether these buffering associations are further moderated by social role. Additionally, we sought to examine whether these associations might change over time as couples adjust; thus, we examined these associations both within 1 month of treatment initiation (baseline) and at 6-month follow-up. Lastly, because we were interested in the long-term effects of attributions of blame, we performed prospective analyses examining baseline levels of blame and its moderators predicting 6-month follow-up distress while controlling for baseline distress.
METHODS
Participants
The current data are part of a longitudinal study of the psychological and relationship functioning of couples coping with lung cancer (6, 30). Data were collected at baseline (within 1 month of treatment initiation) and 3 and 6 months later. Only data collected at baseline and 6 months are analyzed in this study because we were interested in the long-term effects of blame on adjustment.
Patients were eligible if they: 1) were initiating treatment for lung cancer; 2) had a physician-rated Eastern Cooperative Oncology Group performance status score ≤2 meaning, at minimum, patients were up more than 50% of waking hours and ambulatory and capable of all self-care but unable to carry out any work activities (32); 3) were able to provide informed consent; 4) could speak, read, and understand English; 5) were age 18 years or older; and 6) had a partner (spouse or significant other) with whom they had lived for at least 1 year.
We obtained consent from 270 patients and their spouses; 158 (59%) of these couples returned the baseline questionnaire; there were an additional 11 couples in which only the patient returned a survey and nine couples in which only the spouse returned a survey. Detailed recruitment information was previously described (6). Prior to mailing the 6-month questionnaires, six patients died and 12 couples withdrew so that only 140 sets of surveys were mailed, of which 108 (68% of the original 158) were returned. Importantly, t test analyses found that patients and spouses who did not complete the follow-up surveys did not significantly differ from those who did in their scores for baseline distress, dyadic adjustment, attributions of blame, and network support.
Procedure
Prior to data collection, this study was approved by The University of Texas MD Anderson Cancer's Center's Institutional Review Board. Research staff approached patients and their spouses during weekly appointments at the Thoracic Clinic, screened them for eligibility, and obtained their informed consent. Patients and spouses separately completed paper-pencil questionnaires and returned them in individually sealed postage-paid envelopes. Follow-up surveys were mailed 3 and 6 months later to couples who had completed the baseline questionnaires. Participants received $10 gift cards for survey completion (up to $60 total per couple).
Measures
Demographic/medical factors
At baseline, patients and spouses provided demographic information including age, sex, level of education, race/ethnicity, marital status, and employment status. Patients were asked questions about their disease, including time since diagnosis, treatments, and disease stage.
Smoking history
Patients and spouses were asked about their current tobacco smoking status with an one-item question categorizing participants as never smokers, former smokers (quit date ≥ 6 months), recent quitters (quit date ≤ 6 months), or current smokers.
Blame
We used the Glinder and Compas one-item measure of behavioral blame (i.e., “How much do you blame yourself for the kinds of things you did, that is, for any behaviors that may have led to your cancer?”) that has been widely used in the cancer literature to assess behavioral blame (13). We modified the item for spouses to ask the extent to which they blamed the patient's behaviors leading to his/her cancer. Participants responded using a 4-point Likert-type scale (1=not at all; 4=completely).
Dyadic adjustment
Patients and spouses completed the 32-item Dyadic Adjustment Scale, which assesses four components of marital adjustment: consensus, satisfaction, cohesion, and affectionate expression (22). Marital distress (poor dyadic adjustment) is defined by cutoff scores of ≤ 97 (33). Alpha coefficients for patients’ and spouses’ scores were .91 and .90, respectively.
Network support
Network support was assessed with the Medical Outcomes Study-Social Support Scale (34). This 20-item instrument measures perceived available support from one's social network using four subscales of functional support (emotional/informational, tangible, affectionate, and positive social interaction) and a summed total score. We reported on the total score. Alpha coefficients for the total score for patients and spouses’ scores were .97 and .95, respectively.
Psychological distress
Patients and spouses completed the well-validated Brief Symptom Inventory (35), a 53-item self-report measure of psychological functioning over the past week in nine symptom dimensions. It also yields a global severity index. A score ≥63 on the global severity index or on two of the Brief Symptom Inventory's nine primary dimensions defines “caseness” for distress and indicates a need for further clinical evaluation. For this report, we used the global severity index raw scores.
Data Analysis Strategy
We calculated descriptive statistics (e.g., means, standard deviations, correlations) for each of the major study variables and performed paired t tests to determine whether mean scores differed for patients and spouses. Additionally, we performed paired t tests to examine whether participants’ baseline scores differed from the 6-month follow-up scores. We used separate analysis of variance (ANOVA) for patients and spouses to test the associations between smoking history and blame. Because none of the assessed demographic variables (i.e., age, sex, race/ethnicity, level of education, employment status) and patients’ medical factors (i.e., time since diagnosis, Eastern Cooperative Oncology Group performance status, stage of disease, treatment initiation and type of treatment) were associated (with significance defined as p<.05) with the outcome variable (i.e., distress), we did not include them as control variables in our main analyses.
To examine the association between attributions of blame and distress, whether this association is moderated by dyadic adjustment and network support, and whether these associations differed for patients and spouses, we regressed participants’ distress on the three-way interaction between blame, dyadic adjustment or network support, and social role (i.e., patient or spouse). Given the dyadic nature of our data, we used a multilevel modeling technique in which the couple was the unit of analysis (Actor Partner Interdependence Model – APIM)(36), using the PROC MIXED procedure in SAS (37). As opposed to the general linear model, multilevel modeling allows testing of nonindependent data without introducing bias to the probability estimates. Additionally, instead of using list-wise deletion for cases with missing data, PROC MIXED uses a likelihood-based estimation method for missing data so that attrition is less of a concern for the prospective analyses (38). Separate multilevel models were conducted to examine the moderating associations of dyadic adjustment and network support. The predictor variables were centered at their grand mean (36); effect coding was used for social role (patient=1 and partner=−1), and effect sizes were calculated using the formula r=[t2/(t2+df)]1/2 (39) for significant effects. In case of significant interactions, we used simple slope analyses as outlined by Preacher et al. (40), which allows determining at which level of the moderator (i.e., network support, dyadic adjustment) the focal variable (i.e., blame) is associated with the outcome (i.e., distress). Otherwise, the model was reduced to examine two-way interactions and main effects. We repeated these analyses for the 6-month follow-up. For the prospective analyses, we tested baseline predictors of follow-up distress while controlling for baseline distress.
RESULTS
Descriptive Results
At baseline, 85.4% of patients reported a history of smoking with15 patients (9.8%) identifying themselves as current smokers, 38 (26.8%) as recent quitters, and 72 (48.8%) as former smokers; only 24 (14.6%) identified themselves as never smokers. In the spouse sample, 53.7% reported a history of smoking with 28 (19.1%) identifying themselves as current smokers, 4 (2.5%) as recent quitters, and 47 (32.1%) as former smokers; 70 (46.3%) identified themselves as never smokers. Regarding distress, 34.6% of patients and 36.4% of spouses met the Brief Symptom Inventory “caseness” criterion for distress (6), and 10.9% of patients and 14.1% of spouses could be classified as “maritally distressed.” Importantly, 46.7% of patients indicated that they blamed themselves either “very much” or “completely” for behaviors that may have caused their cancer. In contrast, 89.9% of spouses blamed the patient's behavior either “not at all” or only “somewhat.” Table 1 summarizes baseline demographic and medical characteristics of the sample.
Table 1.
Baseline Demographic and Medical Characteristics of Lung Cancer Patients and their Spouses
| Characteristic | Patients (N=169) | Spouses (N=167) |
|---|---|---|
| Men (%) | 63.7 | 32.9 |
| White (%) | 89.3 | 91.0 |
| Hispanic or Latino (%) | 4.8 | 2.4 |
| Age (mean ± standard deviation) (range), years | 62.9±10.1 (30.3-86.6) | 60.5±11.1 (30.6-86.4) |
| College ≥2 yrs (%) | 61.3 | 57.5 |
| Employment status (%) | ||
| Full-time | 33.9 | 37.5 |
| Part-time | 5.4 | 7.5 |
| Unemployed | 10.1 | 7.5 |
| Retired | 50.6 | 47.5 |
| Married (%) | 95.8 | |
| Length of marriage (mean ± standard deviation), years | 25.57 ± 13.02 | |
| Disease stage at initial diagnosis: (%) | ||
| I | 16.3 | |
| II | 14.5 | |
| III | 32.5 | |
| IV | 36.7 | |
| Time since diagnosis (mean ± standard deviation), months | 2.3 ± 1.7 | |
| Had initiated treatment (%) | ||
| Yes | 57.1 | |
| Type of treatment* (%) | ||
| Chemotherapy | 55.8 | |
| Radiotherapy | 24.2 | |
| Chemoradiotherapy | 3.2 | |
| Surgery | 16.8 |
Note:
percentages of those who had initiated treatment at baseline assessment.
Table 2 shows the correlations, means, and standard deviations for major study variables by social role (i.e., patient or spouse). As expected, blame was associated with distress for patients as well as for spouses at baseline. Interestingly, at baseline, the correlation between blame and distress was stronger for spouses (r=.32, p<.0001) than for patients (r=.16, p<.05), even though patients were significantly more likely to engage in attributions of blame (t158=6.40, p<.0001, paired t test). Patients also reported receiving more total network support (t158=6.05, p<.0001, paired t test) than did spouses, but network support was associated with distress at baseline (r=−.27, p<.0001) and follow-up (r=−.35, p<.0001) for spouses but not for patients. Spouses who reported more network support experienced less distress. The global severity scores on the Brief Symptom Inventory (indicating distress) and the dyadic adjustment scores did not differ between patients and their spouses. In previously published analyses, we reported differences on specific subscales of the Brief Symptom Inventory and Dyadic Adjustment Scale spouses had lower scores on somatization and paranoid ideation on the Brief Symptom Inventory compared to patients, as well as lower scores for satisfaction on the dyadic adjustment scale (6). Lastly, patients and spouses’ mean scores for the study variables at baseline did not significantly differ from the means at the 6-month follow-up assessment except for patients’ self-blame scores (t(95)=2.32, p<.05); patients reported significantly more blame at baseline than at follow-up.
Table 2.
Correlations, means and standard deviations, and paired t tests results for major study variables for patients and spouses
| Variable | Mean ± Standard Deviation (Range) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | Patients | Spouses | |||||||||
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | t+ | ||
| 1. Global Severityb | .15 | −.39*** | .32*** | −.27*** | .72*** | −.17 | .07 | −.23* | .41±.37 (0-2) | .45 ±.71 (0-2) | −.95 |
| 2. Dyadic Adjustmentb | −.29*** | .50*** | −.23** | .47*** | −.40*** | .61*** | −.17 | .46*** | 12.40± 18.10 (53-149) | 118.40 ± 18.11 (59-152) | .94 |
| 3. Blameb | .16* | −.09 | .17* | −.17 | .17 | −.94** | 5.7*** | −.22* | 2.47 ± 1.21 (1-4) | 1.76 ± .91 (1-4) | 6.40*** |
| 4. Supportb | −.15 | .36*** | −.06 | − .02 | −.37*** | .30** | −.07 | .67*** | 4.59 ± .54 (2-5) | 4.08 ± .89 (1-5) | 6.05*** |
| 5. Global Severity6mo | .64*** | −.19 | .19 | −.20* | .24* | −.31** | .16 | −.35*** | .46 ± 51 (0-4) | .39 ± 40 (0-2) | 1.33 |
| 6. Dyadic Adjustment6mo | −.16 | .64*** | .06 | .24* | −.24* | .25* | −.32*** | .40*** | 120.65 ±14.79 (72-156) | 118.97 ±19.93 (54-148) | .42 |
| 7. Blame6mo | −.02 | −.01 | .61*** | −.13 | −.03 | .00 | .40*** | −.06 | 1.18 ± .18 (1-4) | .94 ± .14 (1-4) | 1.46 |
| 8. Network Support6mo | −.16 | .23* | −.11 | .27** | −.22* | .26* | −.11 | .05 | 4.52 ± .66 (1-5) | 4.05 ±.92 (1-5) | 3.56*** |
Notes: Patient correlations are on lower diagonal, spouse correlations on upper diagonal, and correlations between the patients’ and spouses’ scores are on the diagonal in bold font.
p<.05.
p<.01.
p<.001.
Paired t test examined differences in patient and spouse scores.
Baseline.
6-month follow-up.
Smoking History and Attributions of Blame
ANOVA for patients revealed that patients’ smoking history reported at baseline was significantly associated with attributions of blame (F(3,157)=17.97, p<.0001) at baseline and at 6-month follow-up (F(3,90)=.6.77, p<.001). Post hoc comparisons using Tukey adjustments revealed that current smokers were more likely to blame themselves than were former smokers (p<.05) and never smokers (p<.0001). Attributions of blame did not significantly differ between current smokers and recent quitters or between recent quitters and former smokers. Patients’ smoking history was also associated with spouses’ tendency to blame the patient at baseline (F(3,154)=10.98, p<.0001) and at 6-month follow-up (F(3,91)=2.90, p<.05). Post hoc analyses at baseline revealed that spouses were more likely to blame patients if the patients were current smokers or recent quitters compared with former or never smokers (p<.01). Additionally, spouses’ personal smoking history reported at baseline was associated with blame (F(3,158)=3.24, p<.05); spouses who had never smoked were more likely to blame the patient than were spouses who were current smokers (p<.05). Spouses’ smoking history was not associated with blame at 6-month follow-up. Least squares means for attributions of blame by patients’ smoking history at baseline are presented in Table 3.
Table 3.
Least squares means for lung cancer patients' and spouses' attributions of blame at baseline and 6-month follow-up by patients' smoking history at baseline.
| Baseline | 6-Month Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients' Blame (N=160) | Spouses' Blame (N=150) | Patients' Blame (N=95) | Spouses' Blame (N=95) | |||||
| Patients' smoking history | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation |
| Current smoker | 3.31 | .26 | 2.47 | .74 | 2.25 | .40 | 2.00 | .82 |
| Recent quitter | 2.95 | .16 | 2.11 | 1.04 | 2.83 | .23 | 1.79 | .98 |
| Former smoker | 2.46 | .12 | 1.68 | .88 | 2.29 | .16 | 1.66 | 1.00 |
| Never smoker | 1.21 | .22 | 1.05 | .21 | 1.08 | .31 | 1.00 | .00 |
| Total mean | 2.49 | 1.21 | 1.77 | .93 | 2.26 | 1.24 | 1.62 | .94 |
Note: For recent quitters, quit date ≤6 months. For former smokers, quit date ≥6 months. Range on blame scale: 1 to 4, with higher scores indicating greater blame.
Attributions of Blame, Distress, and Dyadic Adjustment
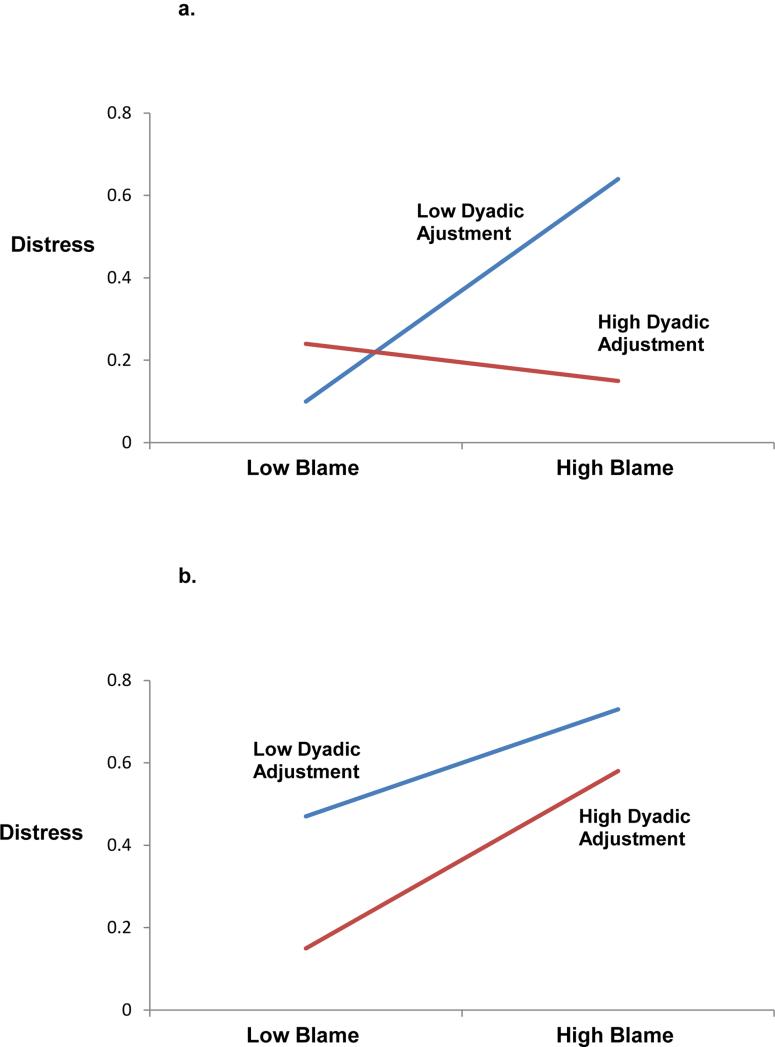
As hypothesized, multilevel model analyses revealed a significant three-way interaction of blame, dyadic adjustment, and social role at baseline (F(1,263)=6.49, p=.01). Table 4 represents this finding. Simple slope analyses revealed that dyadic adjustment buffers the association between attributions of blame and distress for patients but not for spouses. More specifically, for patients reporting high levels of dyadic adjustment, blame was not associated with distress (z=.52, p=.61), but for patients experiencing low levels of dyadic adjustment, blame was associated with increased distress (z=2.72, p=.01). In contrast, for spouses, blame was associated with increased distress regardless of the level of dyadic adjustment (z=2.03, p=.05). Figure 1 illustrates this finding decomposed by social role. Because the three-way interaction was significant, we did not examine lower order terms (41).
Table 4.
Multilevel analysis results examining dyadic adjustment at baseline (p<.05) and network support at 6-month follow-up (p<.05) as moderators of the blame and distress association in couples affected by lung cancer
| Baseline Distress (N=158 Couples) | 6-Month Follow-Up Distress (N=108 Couples) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Variables | B | Standard Error | t | r | Follow-Up Variables | B | Standard Error | t | r |
| Intercept | 0.49 | 0.03 | Intercept | .41 | .04 | 11.11 | |||
| Blame | 0.12 | 0.03 | 3.54*** | .21 | Blame | .03 | .03 | 1.16 | |
| Dyadic adjustment | −0.01 | 0.00 | −4.17*** | .24 | Network support | −0.10 | 0.03 | −3.11** | .26 |
| Social role | −0.11 | 0.04 | −2.69** | .19 | Blame × network support | −0.10 | .03 | 2.30* | .20 |
| Blame × dyadic adjustment | 0.00 | 0.00 | 1.11 | ||||||
| Blame × social role | −0.06 | 0.04 | −1.59 | ||||||
| Dyadic adjustment × social role | 0.00 | 0.00 | 1.80 | ||||||
| Blame × dyadic adjustment × social role | −0.01 | 0.00 | −2.55* | .16 | |||||
Notes:
p<.05.
p<.01. Social Role (patient=1, spouse=-1). Effect size r=[t2/(t2+df)]1/2. B, raw coefficient;
Fig. 1.
A multilevel model analysis reveals a three-way interaction (p<.05) depicting baseline distress as a function of blame and dyadic adjustment in patients (a) and spouses (b). Scores are plotted at mean ±1 standard deviation. Higher scores indicate greater distress.
At 6-month follow-up, the three-way interaction was not significant. We reduced the model to examine two-way interactions and main effects. The only significant main effect was that of dyadic adjustment (t(183) =−3.95, p<.0001); couples with higher dyadic adjustment reported decreased distress. Similarly, prospective analyses did not reveal significant three-way or two-way interactions. However, when we controlled for baseline distress (t(177)=11.86, p<.0001), there was a significant main effect of baseline attributions of blame (t(171)=2.31, p<.05) predicting later distress.
Attributions of Blame, Distress, and Network Support
Multilevel model analyses did not reveal a significant interaction of blame, network support, and social role at baseline. A reduced model yielded a marginally significant interaction between attributions of blame and network support on baseline distress (t(292)=−1.62, p=.10). Simple slope analyses revealed that, consistent with our hypothesis, for those with high levels of support, blame was not associated with distress (z=1.59, p=.11); whereas for those with low levels of support, higher levels of blame were associated with increased distress (z=3.65, p<.001).
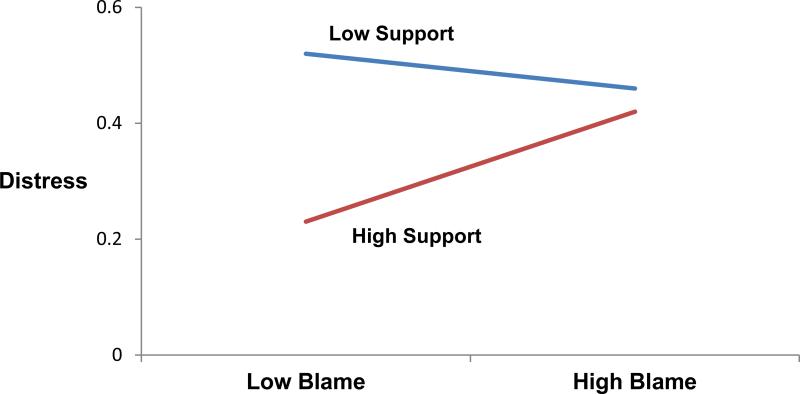
At 6-month follow-up, the interaction was significant (F(91,123)=5.30, p<.05), but the direction of the interaction did not indicate a buffering effect of network support. Table 4 presents this finding. Simple slope analysis revealed that high levels of support were associated with decreased distress for those reporting low (z=2.44, p<.05) but not high (z=.65, p=.51) levels of blame. Figure 2 further illustrates this finding. When we controlled for baseline distress (t(166)=11.36, p<.0001), prospective analyses showed that the interaction between baseline network support and blame predicting follow-up distress was marginally significant (t(169)=1.90, p=.06). Similar to the results of the 6-month simple slope analyses, high levels of support at baseline were marginally associated with decreased follow-up distress if blame was low (z=1.70, p=.09) but not high (z=−.89, p=.38).
Fig. 2.
A multilevel model analysis reveals an interaction at 6-month follow-up (p<.05) depicting distress as a function of blame and network social support at mean ±1 standard deviation. Higher scores indicate greater distress.
DISCUSSION
This prospective dyadic study revealed that attributions of blame appear to be quite common in lung cancer patients. As hypothesized, lung cancer patients and their spouses were more likely to make blame attributions if the patient had a smoking history supporting existing literature (19). Additionally, spouses who never smoked were more likely to blame the patient compared to those with a personal smoking history. Consistent with previous work (9-11), we demonstrated that attributions of blame at treatment initiation are harmful because they are associated with increased initial distress in patients and even more so in spouses. Our hypothesis regarding moderators (i.e., dyadic adjustment, network support) of the association between blame and distress was partially supported. For patients, dyadic adjustment buffered against the harmful association of high levels of blame such that only those patients with poor dyadic adjustment experienced increased distress when blaming themselves for developing their disease. There was no evidence for dyadic adjustment protecting against distress in spouses who engaged in high levels of blame attributions. Prospectively, there was no buffering effect for patients or spouses. In fact, initial blame predicted later distress above and beyond initial distress emphasizing the lingering harmful effects of blame in couples. Our findings also suggested that network support may buffer against increased distress associated with high levels of blame in both patients and spouses. However, the interaction between blame and network support was only marginally significant. Further, at the follow-up time point, network support was beneficial to distress outcomes for couples reporting low levels of attributions of blame but not for those reporting high levels.
Our findings not only support the blame attribution literature, but also extend it in several important ways. For one, we identified that both patient and spousal smoking history is associated with attributions of blame. Second, even though blame was more prevalent among patients than spouses, blame was more strongly associated with increased distress in spouses than patients. Third, we extended previous knowledge by examining moderator variables of the blame and distress association. Our findings related to dyadic adjustment were promising for patients and pointed to the benefits associated with satisfying relationships. Because of smoking, patients may experience guilt and shame (10, 42) for engaging in an addictive, unhealthy behavior causing cancer, resources such as affection, acceptance, and positive interactions offered by spouses and possibly their social networks appear to reduce distress associated with blame. It is possible that in well-functioning marriages, couples have discussions about the cancer cause in a factual, non-accusatory manner, as opposed to displaying derogatory or punitive behaviors. As such, spouses may actually help patients cope with mood disturbances associated with self-blame (18) by helping them cognitively process their cancer in a healthy way. Unfortunately, there was no evidence that spouses with high dyadic adjustment reap a similar benefit of alleviating distress linked to blame.
Because attributions of blame are associated with increased distress in both patients and spouses, this research highlights targets for clinical attention and underscores the necessity for a couple-oriented approach for psychosocial care. Regarding the design of future dyadic interventions to reduce distress in couples affected by lung cancer, targeting patients who currently smoke or have recently quit appears to be particularly fruitful as they tend to be most vulnerable to self-blame. Further, intervening early in the treatment trajectory may prevent a snowballing effect of distress, as initial distress and blame were strong predictors of later distress. Programs incorporating cognitive-behavioral therapy may be most helpful for reducing distress, as they can teach patients and spouses how to restructure cognitions involving blame and to effectively utilize their marriage and social network to cope with such distressing attributions (19). Additionally, based on the current evidence of a buffering effect of well-adjusted relationships, programs enhancing marital functioning through teaching open and supportive communication may also be beneficial. For instance, communication patterns of couples with low marital functioning tend to be characterized by mutual avoidance of concerns and demand-withdrawal communication behaviors, which are associated with increased distress in couples coping with cancer (27, 43, 44). Thus, rather than holding back distressing thoughts and emotions, teaching couples how to mutually engage and openly exchange thoughts, concerns, and feelings with the goal of providing and receiving support may be particularly effective components of such programs. Importantly, because studies have shown that patient and partner distress levels are related (30, 45), the effectiveness of interventions should be optimized by including both patients and spouses (6, 30, 46).
This study has a few limitations. First, the sample had an attrition rate of 32%. Yet, this rate is similar to other studies targeting couples facing advanced cancers and on active treatment (47). Moreover, comparison analyses revealed no difference in baseline characteristics between the participants who were retained and the ones who were not. Second, statistical power was reduced at the follow-up analyses, which may explain the failure to replicate the significant baseline interaction involving dyadic adjustment at the later time point. However, we did use a statistical procedure that maximized the use of all available data. Third, we used a one-item measure to assess general behavioral blame. Despite the brevity of the measure, it is the most frequently used blame measure in the health literature. In the same vein, we did not assess blame specific to smoking tobacco and consequently included all patients in our primary analyses, even never smokers. To this end, we only identified one and the most obvious risk factor of blame, tobacco smoking behavior. Other behaviors (e.g., smoking marijuana, delaying seeking medical attention, alcohol consumption) as well as psychosocial factors may be related to blame. Consequently, future research is needed to develop a more fine-tuned instrument for assessing blame in cancer patients and their spouses and to identify other risk factors for blame. Future research also needs to examine the role of second-hand smoking and blame, particularly in couples in which the spouse smoked and the patient did not. Fourth, regarding the measurement of smoking history, we used a brief self-report measure. As with all self-report assessment, social desirability in responses is a concern; hence, the inclusion of a biomarker of nicotine use would have been optimal. We attempted to reduce any potential social desirability bias by communicating confidentially of responses and instructing patients and spouses to complete and mail their questionnaires individually. Likely, this bias was minimized because approximately 85% of patients self-reported a smoking history, which is what would be expected for a lung cancer sample. Finally, as with all survey data, no causal inferences can be made. Based on prospective analyses, we can be fairly confident regarding the direction of the blame and distress association; however, a bidirectional association is certainly possible.
In summary, patients and to a lesser degree spouses coping with lung cancer are at risk of engaging in attributions of blame for developing cancer, which is related to increased long-term distress. This risk is elevated in patients who are current smokers or had recently quit. The study contributes to attribution research in the cancer literature because it not only examined an understudied population but also included spouses, highlighting the dyadic nature of the cancer coping process. The procedure we employed for data analysis enabled us to examine couple-level data within the same model and test for differential associations for patients and their spouses. This study's prospective design allowed us to confirm the direction of blame and its association with distress over a 6-month period. Additionally, the study revealed preliminary evidence that dyadic adjustment and network support may buffer against this association, particularly in patients. Future intervention research is encouraged to examine cognitive restructuring and enhancing relationship functioning as targets of couple-focused interventions to facilitate patients and spouses’ coping with lung cancer.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
Contributor Information
Kathrin Milbury, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center, Houston, TX
Hoda Badr, Department of Oncological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY
Cindy L. Carmack, Departments of Behavioral Science & Palliative Care and Rehabilitation Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX
REFERENCES
- 1.Howlader N, Noone AM, M. K, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2008. [Google Scholar]
- 2.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 3.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Coyne JC, Fiske V. Couples coping with chronic and catastrophic illness. In: Akamatsu TJ, Stephens MAP, Hobfoll SE, Crowther JH, editors. Family Health Psychology. 129-149. Hemisphere; Washington, DC: 1992. [Google Scholar]
- 5.Neuling SJ, Winefield HR. Social support and recovery after surgery for breast cancer: frequency and correlates of supportive behaviours by family, friends and surgeon. Soc Sci Med. 1988;27:385–392. doi: 10.1016/0277-9536(88)90273-0. [DOI] [PubMed] [Google Scholar]
- 6.Carmack Taylor CL, Badr H, Lee JH, et al. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36:129–140. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci LM, Cartmel B, Turkman YE, et al. Causal attribution among cancer survivors of the 10 most common cancers. J Psychosoc Oncol. 2011;29:121–140. doi: 10.1080/07347332.2010.548445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 9.Gulyn LM, Youssef F. Attribution of blame for breast and lung cancers in women. J Psychosoc Oncol. 2010;28:291–301. doi: 10.1080/07347331003689052. [DOI] [PubMed] [Google Scholar]
- 10.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ. 2004;328:1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Else-Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009;24:949–964. doi: 10.1080/08870440802074664. [DOI] [PubMed] [Google Scholar]
- 12.Bennett KK, Compas BE, Beckjord E, Glinder JG. Self-blame and distress among women with newly diagnosed breast cancer. J Behav Med. 2005;28:313–323. doi: 10.1007/s10865-005-9000-0. [DOI] [PubMed] [Google Scholar]
- 13.Glinder JG, Compas BE. Self-blame attributions in women with newly diagnosed breast cancer: a prospective study of psychological adjustment. Health Psychol. 1999;18:475–481. doi: 10.1037//0278-6133.18.5.475. [DOI] [PubMed] [Google Scholar]
- 14.Salander P. Attributions of lung cancer: my own illness is hardly caused by smoking. Psychooncology. 2007;16:587–592. doi: 10.1002/pon.1121. [DOI] [PubMed] [Google Scholar]
- 15.Faller H, Schilling S, Lang H. Causal attribution and adaptation among lung cancer patients. J Psychosom Res. 1995;39:619–627. doi: 10.1016/0022-3999(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 16.Lobchuk MM, Murdoch T, McClement SE, McPherson C. A dyadic affair: who is to blame for causing and controlling the patient's lung cancer? Cancer Nurs. 2008;31:435–443. doi: 10.1097/01.NCC.0000339253.68324.19. [DOI] [PubMed] [Google Scholar]
- 17.Janoff-Bulman R. Characterological versus behavioral self-blame: Inquries into depression and rape. Journal of Personality and Social Psychology. 1979;37:1798–1809. doi: 10.1037//0022-3514.37.10.1798. [DOI] [PubMed] [Google Scholar]
- 18.Friedman LC, Romero C, Elledge R, et al. Attribution of blame, self-forgiving attitude and psychological adjustment in women with breast cancer. J Behav Med. 2007;30:351–357. doi: 10.1007/s10865-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 19.Lobchuk MM, McClement SE, McPherson C, Cheang M. Does blaming the patient with lung cancer affect the helping behavior of primary caregivers? Oncol Nurs Forum. 2008;35:681–689. doi: 10.1188/08.ONF.681-689. [DOI] [PubMed] [Google Scholar]
- 20.Fincham FD, Bradbury TN. Marital Satisfaction, Depression, and Attributions - a Longitudinal Analysis. Journal of Personality and Social Psychology. 1993;64:442–452. doi: 10.1037//0022-3514.64.3.442. [DOI] [PubMed] [Google Scholar]
- 21.Weiner B. A Cognitive (Attribution)-Emotion-Action Model of Motivated Behavior: An Analysis of Judgements of Help-Giving. Journal of Personality and Social Psychology. 1980;39:186–200. [Google Scholar]
- 22.Spanier GB. Measuring Dyadic Adjustment - New Scales for Assessing Quality of Marriage and Similar Dyads. Journal of Marriage and the Family. 1976;38:15–28. [Google Scholar]
- 23.Badr H, Acitelli LK, Taylor CL. Does talking about their relationship affect couples' marital and psychological adjustment to lung cancer? J Cancer Surviv. 2008;2:53–64. doi: 10.1007/s11764-008-0044-3. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus RS, Folkman S. Stress, appraisal and coping. New York: 1984. [Google Scholar]
- 25.Lebel S, Devins GM. Stigma in cancer patients whose behavior may have contributed to their disease. Future Oncology. 2008;4:717–733. doi: 10.2217/14796694.4.5.717. [DOI] [PubMed] [Google Scholar]
- 26.Ryan PJ, Howell V, Jones J, Hardy EJ. Lung cancer, caring for the caregivers. A qualitative study of providing pro-active social support targeted to the carers of patients with lung cancer. Palliat Med. 2008;22:233–238. doi: 10.1177/0269216307087145. [DOI] [PubMed] [Google Scholar]
- 27.Manne S, Badr H, Zaider T, Nelson C, Kissane D. Cancer-related communication, relationship intimacy, and psychological distress among couples coping with localized prostate cancer. J Cancer Surviv. 2010;4:74–85. doi: 10.1007/s11764-009-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badr H, Carmack Taylor CL. Sexual dysfunction and spousal communication in couples coping with prostate cancer. Psychooncology. 2009;18:735–746. doi: 10.1002/pon.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badr H, Carmack CL, Kashy DA, Cristofanilli M, Revenson TA. Dyadic coping in metastatic breast cancer. Health Psychol. 2010;29:169–180. doi: 10.1037/a0018165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badr H, Carmack Taylor CL. Effects of relationship maintenance on psychological distress and dyadic adjustment among couples coping with lung cancer. Health Psychol. 2008;27:616–627. doi: 10.1037/0278-6133.27.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milbury K, Badr H. Sexual problems, communication patterns, and depressive symptoms in couples coping with metastatic breast cancer. Psychooncology. 2012 doi: 10.1002/pon.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 33.Jacobson NS, Schmaling KB, Holtzworthmunroe A. Component Analysis of Behavioral Marital-Therapy - 2-Year Follow-up and Prediction of Relapse. Journal of Marital and Family Therapy. 1987;13:187–195. [Google Scholar]
- 34.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 35.Derogatis LR, Brief Symptom Inventory. Administration, Scoring, and Procedures Manual. 3rd Ed. National Computer Systems; Minneapolis, MN: 1993. [Google Scholar]
- 36.Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. Guilford Press; New York: 2006. [Google Scholar]
- 37.Campbell L, Kashy DA. Estimating Actor, Partner, and Interaction Effects for Dyadic Data Using PROC MIXED and HLM: A User–Friendly Guide. Personal Relationships. 2002;9:327–342. [Google Scholar]
- 38.Wolfer R, Sang C. Comparing the SAS GLM and MIXED Procedures for Repeated Measures.. Proceedings of the Twentieth Annual SAS Users Group Conference; Cary, NC. Gary, NC. 1995. [Google Scholar]
- 39.Snijders T, Bosker R. Multilevel analysis. Oaks; Thousand Oaks, CA: 1999. [Google Scholar]
- 40.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- 41.Hays WL. Statistics. 4th. Ed. Holt, Rinehart, and Winston, Inc.; Orlando, FL: 1988. [Google Scholar]
- 42.LoConte NK, Else-Quest NM, Eickhoff J, Hyde J, Schiller JH. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clin Lung Cancer. 2008;9:171–178. doi: 10.3816/CLC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 43.Manne SL, Ostroff JS, Norton TR, et al. Cancer-related relationship communication in couples coping with early stage breast cancer. Psychooncology. 2006;15:234–247. doi: 10.1002/pon.941. [DOI] [PubMed] [Google Scholar]
- 44.Manne S, Sherman M, Ross S, et al. Couples' support-related communication, psychological distress, and relationship satisfaction among women with early stage breast cancer. J Consult Clin Psychol. 2004;72:660–670. doi: 10.1037/0022-006X.72.4.660. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y, Kashy DA, Wellisch DK, et al. Quality of life of couples dealing with cancer: dyadic and individual adjustment among breast and prostate cancer survivors and their spousal caregivers. Ann Behav Med. 2008;35:230–238. doi: 10.1007/s12160-008-9026-y. [DOI] [PubMed] [Google Scholar]
- 46.Manne SB,H. Intimacy processes and psychological distress among couples coping with head and neck or lung cancers. Psychooncology. 2010;19:941–954. doi: 10.1002/pon.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manne S. Couples Coping with Cancer - Research Issues and Recent Findings. Journal of Clinical Psychology in Medical Settings. 1994;1:317–330. doi: 10.1007/BF01991076. [DOI] [PubMed] [Google Scholar]