Abstract
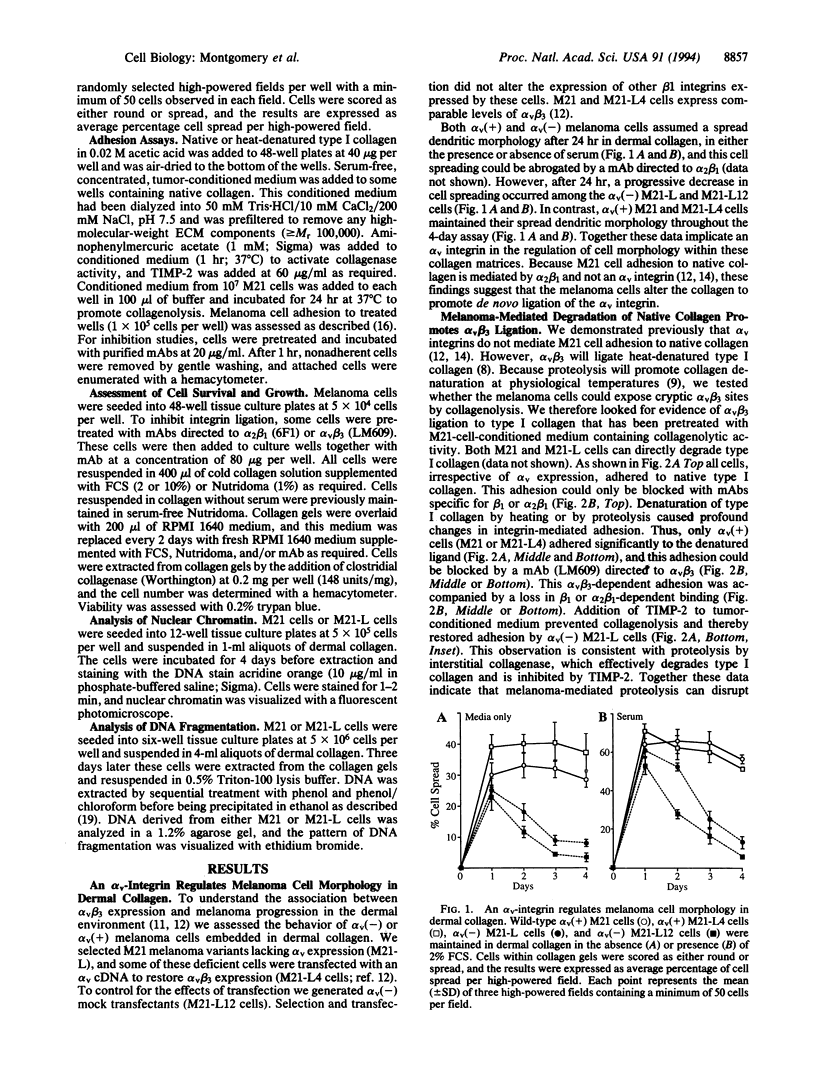
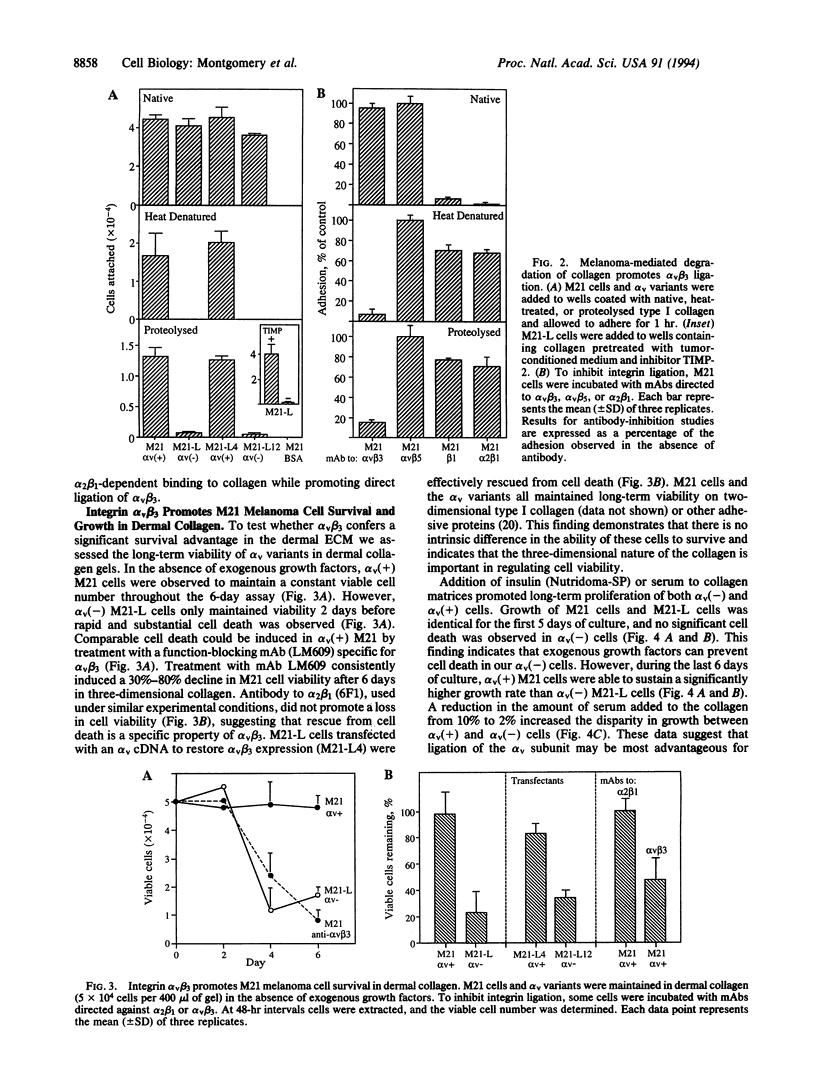
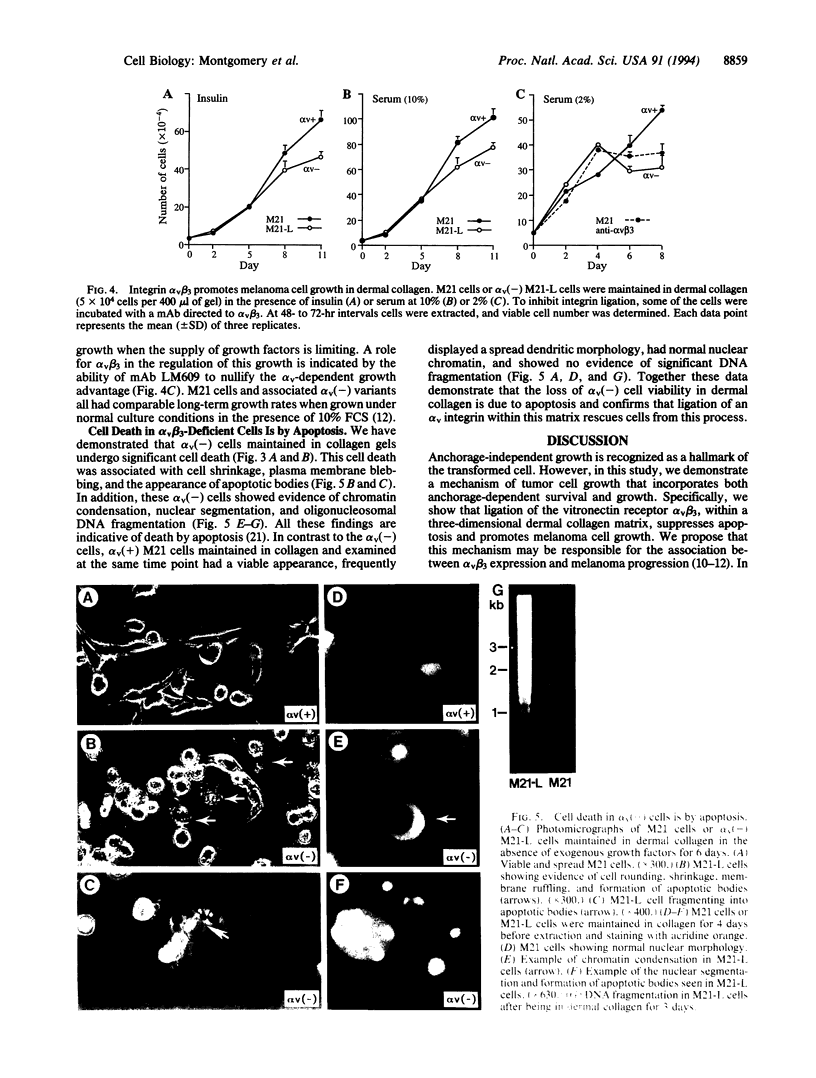
Human melanoma cells required ligation of the integrin alpha v beta 3 to sustain viability and growth in three-dimensional dermal collagen. Variant melanoma cells, lacking the alpha v subunit, progressed rapidly to apoptosis within this matrix, whereas transfection of these cells with an alpha v cDNA restored alpha v beta 3 expression and prevented apoptosis. Furthermore, inhibition of alpha v beta 3 ligation with a monoclonal antibody promoted cell death. Apoptosis of alpha v(-) cells within this matrix could be overcome by the addition of insulin or serum. However, alpha v(+) melanoma cells had a significant growth advantage in the presence of these growth factors. Initial adhesion of the melanoma cells to type I collagen depended on ligation of alpha 2 beta 1, but these cells can degrade this collagen to expose cryptic alpha v beta 3 binding sites. These findings provide evidence that the survival and growth of transformed cells may be regulated by collagen degradation and integrin-dependent anchorage to this proteolysed matrix.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Bianchine P. J., Burd P. R., Metcalfe D. D. IL-3-dependent mast cells attach to plate-bound vitronectin. Demonstration of augmented proliferation in response to signals transduced via cell surface vitronectin receptors. J Immunol. 1992 Dec 1;149(11):3665–3671. [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B. M., Matsuura N., Takada Y., Zetter B. R., Hemler M. E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991 Mar 29;251(5001):1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990 Dec;111(6 Pt 1):2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Cheresh D. A. Structure, function and biological properties of integrin alpha v beta 3 on human melanoma cells. Cancer Metastasis Rev. 1991 May;10(1):3–10. doi: 10.1007/BF00046839. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Davis G. E. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A., Yean T. D., Lu H. S., Ting J., Langley K. E. Inhibition of autoproteolytic activation of interstitial procollagenase by recombinant metalloproteinase inhibitor MI/TIMP-2. J Biol Chem. 1991 Feb 25;266(6):3893–3899. [PubMed] [Google Scholar]
- Felding-Habermann B., Mueller B. M., Romerdahl C. A., Cheresh D. A. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992 Jun;89(6):2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg D., Gehlsen K. R., Turner D. C., Ahlén K., Zijenah L. S., Barnes M. J., Rubin K. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell--collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J. 1992 Nov;11(11):3865–3873. doi: 10.1002/j.1460-2075.1992.tb05479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Marx J. Cell death studies yield cancer clues. Science. 1993 Feb 5;259(5096):760–761. doi: 10.1126/science.8430327. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguell J. J., Hardy C. L. Restorative effect of IL-3 on adherence of cloned hemopoietic progenitor cell to stromal cell. Exp Hematol. 1993 Jan;21(1):55–60. [PubMed] [Google Scholar]
- Pauli B. U., Knudson W. Tumor invasion: a consequence of destructive and compositional matrix alterations. Hum Pathol. 1988 Jun;19(6):628–639. doi: 10.1016/s0046-8177(88)80168-0. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Bodmer W. F. Genetics and biochemistry of collagen binding-triggered glandular differentiation in a human colon carcinoma cell line. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5561–5565. doi: 10.1073/pnas.85.15.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M. K., García I., López-Rivas A. Insulin-like growth factor-I inhibits apoptosis in IL-3-dependent hemopoietic cells. J Immunol. 1992 Jul 15;149(2):535–540. [PubMed] [Google Scholar]
- Sanders L. C., Felding-Habermann B., Mueller B. M., Cheresh D. A. Role of alpha V integrins and vitronectin in human melanoma cell growth. Cold Spring Harb Symp Quant Biol. 1992;57:233–240. doi: 10.1101/sqb.1992.057.01.028. [DOI] [PubMed] [Google Scholar]
- Southgate K. M., Davies M., Booth R. F., Newby A. C. Involvement of extracellular-matrix-degrading metalloproteinases in rabbit aortic smooth-muscle cell proliferation. Biochem J. 1992 Nov 15;288(Pt 1):93–99. doi: 10.1042/bj2880093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staatz W. D., Fok K. F., Zutter M. M., Adams S. P., Rodriguez B. A., Santoro S. A. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991 Apr 25;266(12):7363–7367. [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]




