Abstract
Background and objectives
In 2011, the U.S. Secretary of Health and Human Services recommended universal screening of newborns for critical congenital heart defects (CCHD), yet few estimates of the number of infants with CCHD likely to be detected through universal screening exist. Our objective was to estimate the number of infants with nonsyndromic CCHD in the United States likely to be detected (true positives) and missed (false negatives) through newborn CCHD screening.
Methods
We developed a simulation model based on estimates of birth prevalence, prenatal diagnosis, late detection, and sensitivity of newborn CCHD screening through pulse oximetry to estimate the number of true positive and false negative nonsyndromic cases of the seven primary and five secondary targets of CCHD screening identified through screening.
Results
We estimated that 875 (95% uncertainty interval [UI]: 705–1,060) U.S. infants with nonsyndromic CCHD, including 470 (95% UI: 360–585) among primary CCHD screening targets, will be detected annually through CCHD newborn screening. An additional 880 (UI: 700–1,080) false negative screenings, including 280 (95% UI: 195–385) among primary screening targets, are expected. We estimated that similar numbers of CCHD (within ~1 case/10,000 live births) would be detected under scenarios comparing “lower” (~19%) and “higher” (~42%) than current prenatal detection prevalences.
Conclusions
A substantial number of nonsyndromic CCHD cases are likely to be detected through universal CCHD screening; however, an equal number of false negative screenings, primarily among secondary targets of screening, are likely to occur. Future efforts should document the true impact of CCHD screening in practice.
Keywords: heart defects, congenital, neonatal screening, Monte Carlo methods
INRODUCTION
Congenital heart defects (CHD) affect ~8/1,000 births and ~25% are considered critical congenital heart defects (CCHD).1 In 2011 the U.S. Secretary of Health and Human Services recommended adding CCHD to the newborn Recommended Uniform Screening Panel.2 Subsequently, screening for CCHD through pulse oximetry, used to supplement standard clinical evaluation and monitoring of newborns, has been implemented in many hospitals.3–5 Screening protocols vary regarding the age of the newborn at screening and the use of pre- and/or post-ductal oxygen saturation measurements.4,6 The American Academy of Pediatrics (AAP), American Heart Association (AHA), and others recommend screening infants at 24–48 hours of life with consideration of both pre- and post-ductal measurements.7
Estimates of the impact of CCHD screening differ.4,8,9 Differences in prenatal diagnosis and “late” detection (i.e., CCHD diagnosis after birth hospital discharge9 or after three days of life8) by CCHD type and geographic location might contribute to the observed variation.8,10–12 Another potential contributor is the sensitivity of the pulse oximetry screening test; although the overall sensitivity is estimated to be 76%,13 it varies considerably by CCHD type, ranging from 36–100%.14
Estimating the number of infants potentially detected (“true positives”) and missed (“false negatives”) through universal CCHD screening must incorporate three key sources of variability: (1) the birth prevalence of the specific CCHD; (2) the prenatal diagnosis prevalence, both across CCHD types and geographic region; and (3) the sensitivity of CCHD screening for different CCHD types. This study incorporated these elements into a simulation model to estimate the number of true positive and false negative CCHD cases in the United States likely to occur through CCHD screening using pulse oximetry.
PATIENTS AND METHODS
We included the seven CCHD considered to be “primary” targets of screening: hypoplastic left heart syndrome (HLHS), pulmonary atresia, dextro-transposition of the great arteries (d-TGA), truncus arteriosus, tricuspid atresia, tetralogy of Fallot (TOF), and total anomalous pulmonary venous return (TAPVR).7 We also included the five “secondary” targets of CCHD screening: coarctation of the aorta (COA), double-outlet right ventricle (DORV), Ebstein anomaly, interrupted aortic arch (IAA), and single ventricle.1 While critical aortic and pulmonary stenoses are also typically considered CCHD, we lacked complete data on lesion severity.15 We combined COA and IAA into one category (COA/IAA) as this was done for one of our data sources.14 To calculate estimates for “all CCHD” by summing across the specific CCHD estimates, we created a “multiple CCHD” category including those cases with multiple CCHD diagnoses, such that no case was counted more than once. We restricted the analysis to infants with CCHD diagnosed before 1 year of life. To better reflect the population of infants eligible for CCHD screening, all analyses were restricted to liveborn infants, and, for consistency across data sources, restricted to infants without chromosomal abnormalities (“nonsyndromic”).
Live Birth Prevalence
We simulated a 2012 birth cohort of infants with nonsyndromic CCHD using data from the Metropolitan Atlanta Congenital Defects Program (MACDP), an active surveillance system for major birth defects in metropolitan Atlanta, Georgia.16 Surveillance is conducted for infants, fetuses, and stillbirths >20 weeks gestation with major birth defects identified before six years of age. Trained abstractors visit birth hospitals, pediatric hospitals, specialty clinics and perinatal offices to identify and abstract clinical and demographic information on potential cases. CHD cases are classified by clinicians with expertise in pediatric cardiology.17 For our analysis, we updated Oster et al.’s (2013)15 analysis to calculate the 2000–2005 live birth prevalence of the 12 selected CCHD types.
Frequency of Prenatal Diagnosis
Prenatal diagnosis was estimated using data from the National Birth Defects Prevention Study (NBDPS), a multisite case-control study of risk factors for select major birth defects, including CCHD.18 Cases were identified through birth defects surveillance systems in ten U.S. sites; infants with recognized or strongly suspected single gene disorders or chromosomal abnormalities were excluded. Trained abstractors reviewed medical records of infants/fetuses with CCHD and, to be included in the study, CCHD cases had to be confirmed by echocardiography, cardiac catheterization, surgery, or autopsy.18 Prenatally diagnosed cases were only included if confirmed by autopsy or by a clinician with expertise in pediatric cardiology.19 CCHD type(s) were assigned by physicians with specialized training in clinical genetics or pediatric cardiology.19 We defined prenatal diagnosis as either: 1) a maternal report of a prenatal diagnosis of a CHD (as had been done in a previous analysis10) and/or 2) clinical record of a fetal echocardiography before the date of birth. Then, for each CCHD type, we calculated the 2000–2005 prenatal diagnosis prevalence.
Frequency of Late Detection
NBDPS data were also used to estimate the prevalence of “late” CCHD detection.8 Previously, we categorized infants with echocardiography or autopsy information as having “timely” CCHD detection if their first documented echocardiography was within three days of birth and as having “late” CCHD detection if their first echocardiography (or autopsy) occurred after the third day of life.8 Here, we modified the analysis slightly to be restricted to infants without a prenatal diagnosis (as defined above). We calculated the 2000–2005 late detection prevalence among live-born infants without a prenatal diagnosis.
Sensitivity of Newborn CCHD Screening Through Pulse Oximetry
We obtained CCHD-specific estimates of the sensitivity of screening through pulse oximetry from a review by Prudhoe et al., which used mutually-exclusive CCHD categories but did not provide estimates for Ebstein anomaly or “multiple CCHD”.14 We summed the number of cases reported to be detected through CCHD screening using pulse oximetry and the total number of cases screened, across all secondary screening targets, to obtain an estimate of screening sensitivity for Ebstein anomaly, and across all CCHD, to obtain an estimate for multiple CCHD.
Analysis
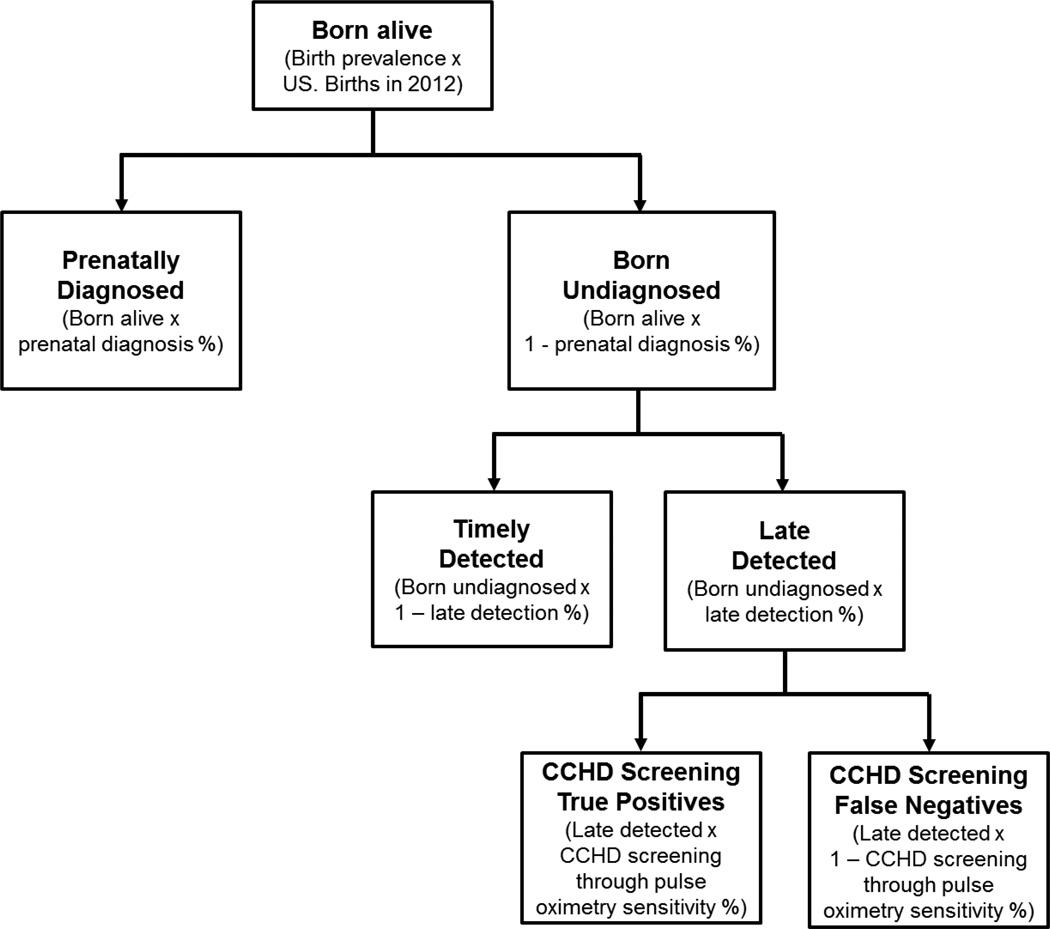
For each CCHD, we estimated the number of true positive and false negative cases resulting from CCHD screening through pulse oximetry (Figure 1). To account for uncertainty in our birth prevalence, prenatal diagnosis, late detection, and CCHD screening sensitivity for each CCHD type, we used normal distributions based on the reported estimated means and standard errors for these parameters (Table 1). However, there were four estimates of CCHD screening sensitivity that were based on exceptionally small numbers (≤ 10 total cases) and, for three of them, there was no sample variance associated with the estimate. For these parameters, we used a uniform distribution based on the lower and upper 95% confidence limits of the Wilson Score exact 95% confidence interval (Table 1). We then used a Monte Carlo simulation approach and drew 10,000 samples from the distributions of each of the parameter estimates, as described above. For each simulation, to avoid negative values, simulated values were truncated with a lower bound at zero cases. We summarized the results of the 10,000 simulations using the mean and a 95% uncertainty interval (UI) defined by the 2.5th and 97.5th percentiles of the distribution of simulated values. Because we had created mutually exclusive CCHD categories throughout the analysis, for each simulation we calculated the sum of each parameter of interest and summarized the results of the simulation using the same statistics (mean, 95% UI) to obtain our estimates for “all CCHD”. To further reflect uncertainty in our estimates, we rounded estimates to the nearest five cases.
Figure 1.
Model estimating the number of U.S. nonsyndromic critical congenital heart defect (CCHD) cases in 2012 estimated to be born alive, prenatally diagnosed, born undiagnosed, timely detected, late detected, and false negatives and true positives of CCHD screening through pulse oximetry, assuming universal implementation of CCHD screening in all states
Notes: CCHD=critical congenital heart defects
Table 1.
Simulation model inputs used to estimate the number of true positive and false negative nonsyndromic critical congenital heart defect (CCHD) cases resulting from CCHD screening using pulse oximetry in 2012 in the United States, assuming universal implementation of CCHD screening in all states
| CCHD type (nonsyndromic cases only) | Live birth prevalence per 10,000 births (SE)a |
Prenatal detection (SE)b |
Late detection (SE)c |
Sensitivity of CCHD screening through pulse oximetry (SE)d |
|---|---|---|---|---|
| Primary targets of CCHD screening | ||||
| Hypoplastic left heart syndrome | 1.66 (0.23) | 56% (3.2%) | 30% (4.4%) | 91% (6.1%) |
| Pulmonary atresia | 0.42 (0.12) | 51% (5.8%) | 11% (5.1%) | 44%–100%e |
| Tetralogy of Fallot | 3.90 (0.36) | 26% (2.1%) | 36% (2.6%) | 39% (9.2%) |
| Total anomalous pulmonary venous return | 0.55 (0.13) | 5% (2.2%) | 39% (4.8%) | 91% (6.1%) |
| Dextro-transposition of the great arteries | 2.17 (0.27) | 28% (2.3%) | 19% (2.4%) | 92% (3.9%) |
| Tricuspid atresia | 0.45 (0.12) | 45% (7.1%) | 19% (7.5%) | 44%–97%e |
| Truncus arteriosus | 0.39 (0.11) | 49% (8.4%) | 39% (11.5%) | 91% (8.7%) |
| Secondary targets of CCHD screening | ||||
| Coarctation of the aorta or interrupted aortic arch | 3.67 (0.34) | 16% (1.6%) | 72% (2.2%) | 36% (6.8%) |
| Double outlet right ventricle | 0.16 (0.07) | 40% (6.4%) | 46% (8.4%) | 57%–100%e |
| Ebstein anomaly | 0.62 (0.14) | 19% (5.4%) | 23% (6.4%) | 48% (6.4%)f |
| Single ventricle complex | 0.75 (0.16) | 55% (6.1%) | 37% (8.8%) | 61%–100%e |
| Multiple CCHD | 0.36 (0.11) | 50% (4.6%) | 28% (5.9%) | 71% (3.2%)f |
| All nonsyndromic CCHDg | - | - | - | - |
Notes: SE=standard error based on a normal distribution
Adapted from an analysis by Oster et al. (2013)15 of CCHD live birth prevalence from 2000–2005 using data from the Metropolitan Atlanta Congenital Defects Program
Adapted from an analysis by Ailes et al. (2013)10 of the frequency of maternal report of prenatal congenital heart defects diagnosis from 2000–2005 and an analysis by Peterson et al. (2014)8 of the prevalence of late CCHD detection using data from the National Birth Defects Prevention Study (NBDPS); defined as the proportion of cases with either a maternal report of a prenatal diagnosis and/or a documented fetal echocardiography occurring before the date of birth
Adapted from an analysis by Peterson et al. (2014)8 of the prevalence of late CCHD detection using data from the NBDPS; defined as the number of cases not prenatally diagnosed that had a documented pediatric echocardiography occurring more than three days after birth
For all defects except Ebstein anomaly and multiple CCHD, this information was derived from the analysis by Prudhoe et al. (2013)14 of the sensitivity of CCHD screening through pulse oximetry
Exact 95% confidence interval used when total was ≤ 10
Calculated as the average of the pulse oximetry sensitivities, among the secondary CCHD screening targets with sensitivities reported by Prudhoe et al. (2013) (for Ebstein anomaly) or as the average of the pulse oximetry sensitivities, among all CCHD screening targets with sensitivities reported by Prudhoe et al. (2013) (for multiple CCHD)
No model inputs were used for the “all nonsyndromic CCHD” category as estimates of the number of cases detected/missed through CCHD screening through pulse oximetry were derived by summing across the CCHD categories for each of the 10,000 simulations, then calculating the mean, median and 95% uncertainty interval across the 10,000 simulations for a given parameter
As a secondary analysis, we repeated the analysis under scenarios of “lower” and “higher” prevalence of prenatal diagnosis than the current estimates. Using NBDPS data, we identified the three sites with the highest and three with the lowest prevalence of prenatal detection. We calculated the prevalence of prenatal diagnosis and late detection within each of these sub-groups and used them as separate inputs into our simulation model, while keeping the same estimates for birth prevalence and CCHD screening sensitivity as in the primary analysis.
Analyses were performed using SAS, v.9.3 (SAS Institute, Cary, NC). MACDP was approved by the Institutional Review Board (IRB) at the Center for Disease Control and Prevention (CDC) and NBDPS by IRBs at the CDC and study sites.
RESULTS
As inputs for our simulation, we estimated that the 2000–2005 live birth prevalence for specific nonsyndromic CCHD ranged from 0.36 (0.11) per 10,000 births for multiple CCHD to 3.90 (0.36) per 10,000 births for TOF (Table 1). Prenatal diagnosis was most frequent for HLHS and single ventricle and lowest for TAPVR and COA/IAA. Late detection (diagnosis at >3 days of birth) was more common for infants with COA/IAA and less frequent for infants with pulmonary atresia and d-TGA.
The simulation estimated that 5,965 infants (95% UI: 5,415–6,515) are born alive with at least one nonsyndromic CCHD annually in the United States, with TOF, COA/IAA, d-TGA and HLHS accounting for ~75% of all liveborn CCHD cases (Table 2). Excluding CCHD cases estimated to be detected prenatally (n=1,800 overall), an estimated 2,410 CCHD (95% UI: 2,150–2,680) would receive a timely diagnosis and 1,755 CCHD cases (95% UI: 1,540–1,980) would be detected “late” (at > 3 days of birth) and be most likely to benefit from CCHD screening through pulse oximetry; infants with COA/IAA accounted for approximately half of late detected cases.
Table 2.
Monte Carlo simulation model estimates of the number of nonsyndromic critical congenital heart defect (CCHD) cases in 2012 in the United States estimated to be born alive, prenatally diagnosed, born undiagnosed, timely detected, late detected, and true positives and false negatives resulting from CCHD screening through pulse oximetry, assuming universal implementation of CCHD screening in all states
|
Results of Monte Carlo simulation using 10,000 iterations Mean (95% uncertainty interval) of the estimated number of CCHD cases in 2012a that would be… |
|||||||
|---|---|---|---|---|---|---|---|
| CCHD type | Born alive | Prenatally diagnosed |
Born undiagnosed | Timely detected | Late detected | CCHD screening through pulse oximetry |
|
| True positives | False negatives | ||||||
| Primary targets | |||||||
| Hypoplastic left heart syndrome | 655 (475–835) | 365 (260–475) | 290 (200–380) | 200 (135–275) | 85 (55–125) | 80 (50–120) | 10 (0–20) |
| Pulmonary atresia | 165 (75–255) | 85 (40–140) | 80 (35–135) | 75 (30–120) | 10 (0–20) | 5 (0–15) | 0 (0–10) |
| Tetralogy of Fallot | 1,540 (1,265–1,815) | 405 (310–500) | 1,135 (920–1,350) | 730 (580–885) | 405 (315–505) | 160 (80–250) | 245 (160–345) |
| Total anomalous pulmonary venous return | 220 (115–320) | 10 (0–25) | 205 (105–305) | 125 (65–190) | 80 (40–125) | 75 (35–115) | 10 (0–20) |
| Dextro-transposition of the great arteries | 860 (655–1,065) | 240 (175–315) | 620 (465–775) | 500 (375–630) | 120 (80–160) | 110 (70–150) | 10 (0–20) |
| Tricuspid atresia | 180 (85–275) | 80 (35–135) | 100 (45–160) | 80 (35–135) | 20 (5–40) | 15 (0–35) | 5 (0–10) |
| Truncus arteriosus | 155 (70–240) | 75 (30–130) | 80 (35–135) | 50 (20–90) | 30 (10–65) | 30 (10–60) | 5 (0–10) |
| Subtotal | 3,770 (3,345–4,190) | 1,260 (1,085–1,445) | 2,510 (2,200–2,815) | 1,760 (1,535–1,995) | 750 (630–880) | 470 (360–585) | 280 (195–385) |
| Secondary targets | |||||||
| Coarctation of the aorta and interrupted aortic arch | 1,450 (1,185–1,720) | 235 (175–300) | 1215 (990–1450) | 345 (265–435) | 870 (705–1050) | 315 (190–455) | 560 (410–730) |
| Double outlet right ventricle | 65 (10–120) | 25 (5–50) | 40 (5–75) | 20 (5–45) | 20 (0–35) | 15 (0–30) | 5 (0–10) |
| Ebstein anomaly | 245 (135–350) | 45 (15–85) | 195 (105–290) | 150 (80–230) | 45 (20–85) | 20 (10–40) | 25 (10–45) |
| Single ventricle complex | 295 (175–415) | 160 (90–240) | 135 (75–205) | 85 (40–140) | 50 (20–85) | 40 (15–75) | 10 (0–25) |
| Subtotal | 2,050 (1,740–2,370) | 465 (360–575) | 1,585 (1,325–1,845) | 600 (480–730) | 985 (810–1,165) | 390 (260–535) | 595 (445–765) |
| Multiple CCHD | 140 (60–225) | 70 (30–120) | 70 (30–115) | 50 (20–85) | 20 (5–35) | 15 (5–25) | 5 (0–10) |
| All nonsyndromic CCHDb | 5,965 (5,415–6,515) | 1,800 (1,580–2,020) | 4,165 (3,765–4,580) | 2,410 (2,150–2,680) | 1,755 (1,540–1,980) | 875 (705–1,060) | 880 (700–1,080) |
Notes: The mean number born alive should equal the mean number prenatally diagnosed plus the mean number born undetected and the mean number born undiagnosed should equal the mean number timely detected plus the mean number late detected, but any differences in the total are due to rounding all estimates to the nearest fives cases
Calculated using the number of live births in 2012 (3,952,937)26
No model inputs were used for the “all nonsyndromic CCHD” category as estimates of the number of cases detected/missed through CCHD screening through pulse oximetry were derived by summing across the CCHD categories for each of the 10,000 simulations, then calculating the mean, median and 95% uncertainty interval across the 10,000 simulations for a given parameter
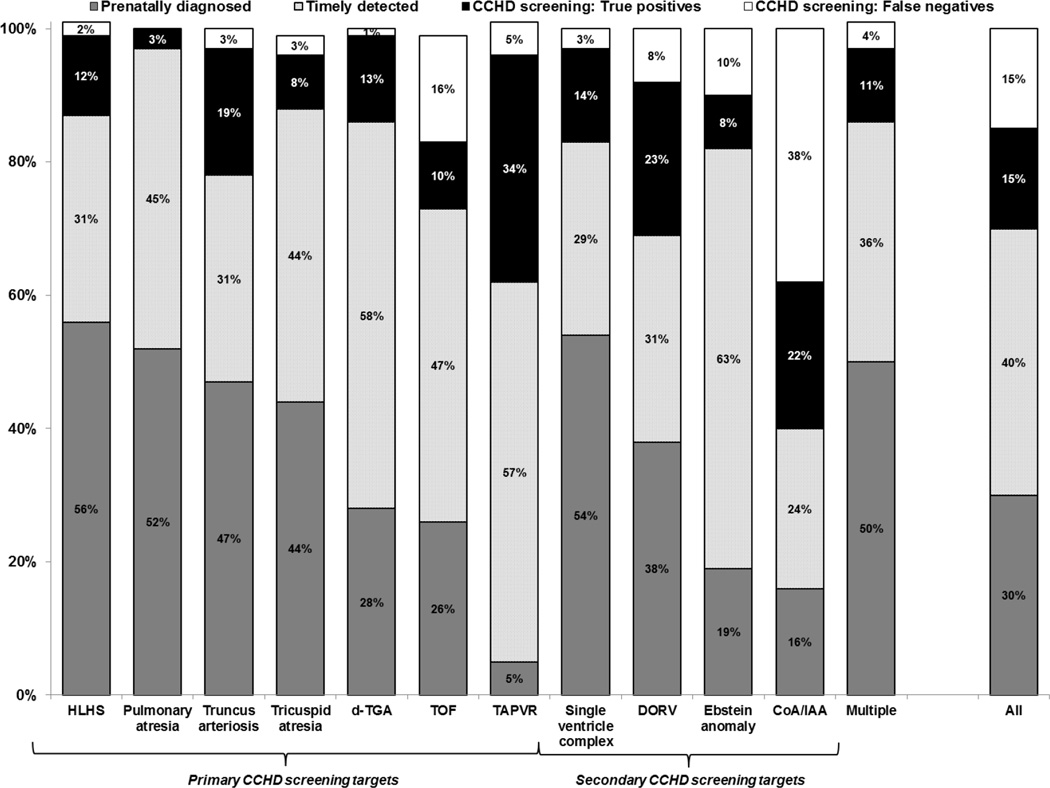
After accounting for the estimates of CCHD screening sensitivity using pulse oximetry, we estimated that 875 (95% UI: 705–1,060) infants with CCHD in the U.S., including 470 (95% UI: 360–585) among primary CCHD screening targets alone, would be detected using CCHD screening through pulse oximetry (true positives) each year, corresponding to about 15% of all CCHD cases (Figure 2). An additional 880 (95% UI: 700–1,080; 280 [95% UI: 195–385] among primary screening targets alone) would be missed (false negatives), corresponding to about 15% of all CCHD cases (Figure 2). COA/IAA and TOF cases were the main contributors to both of these estimates. CCHD screening through pulse oximetry appears to offer the greatest benefit for infants with TAPVR, DORV, and COA/IAA; 20–30% of cases of each of these defects were estimated to be detected through CCHD screening using pulse oximetry (Figure 2).
Figure 2.
Proportion of U.S. nonsyndromic critical congenital heart defect (CCHD) cases in 2012 estimated to be prenatally diagnosed, timely detected, and true positive, and false negative of CCHD screening through pulse oximetry, by CCHD type, assuming universal implementation of CCHD screening in all states
Notes: CCHD=critical congenital heart defects; HLHS=hypoplastic left heart syndrome; d-TGA=dextro-transposition of the great arteries; TOF=tetralogy of Fallot; TAPVR=total anomalous pulmonary venous return; DORV=double outlet right ventricle; COA/IAA=coarctation of the aorta/interrupted aortic arch; Multiple=Multiple critical congenital heart defects
In our secondary analysis, under a scenario assuming “low” prenatal detection across the United States (19% across all CCHD types; eTable), we estimated that approximately 1,105 (95% UI: 885–1,350) true positive and 1,020 (95% UI: 805–1,260) false negative CCHD cases would result from CCHD screening using pulse oximetry, corresponding to approximately 2.80 and 2.58 cases per 10,000 live births annually (Table 3). Comparatively, in a scenario assuming “high” prenatal detection (42% across all CCHD types; eTable), we estimated that approximately 740 (95% UI: 575–925) true positive and an additional 785 (95% UI: 610–975) false negative CCHD cases would result from CCHD screening using pulse oximetry, corresponding to approximately 1.87 and 1.99 cases per 10,000 live births annually (Table 3).
Table 3.
Monte Carlo simulation model estimates of number of nonsyndromic critical congenital heart defect (CCHD) cases in 2012 in the United States estimated to be prenatally diagnosed, timely detected, and true positives and false negatives resulting from CCHD screening through pulse oximetry, assuming universal implementation of CCHD screening in all states, in the primary analysis and under scenarios of low and high prenatal diagnosis prevalence
|
Results of Monte Carlo simulation using 10,000 iterations Mean (95% uncertainty interval) of the estimated number of CCHD cases in 2012a,b that would be… |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | CCHD screening through pulse oximetry | |||||||||||||
| Born alive | Prenatally diagnosed | Timely detected | True positives | False negatives | ||||||||||
| N | Per 10,000 births |
N | (%) | Per 10,000 births |
N | (%) | Per 10,000 births |
N | (%) | Per 10,000 births |
N | (%) | Per 10,000 births |
|
| Primary | 5,965 | 15.09 | 1,800 (1,580–2,020) |
(30%) | 4.55 | 2,410 (2,150–2,680) |
(40%) | 6.10 | 875 (705–1,060) |
(15%) | 2.21 | 880 (700–1,080) |
(15%) | 2.23 |
|
If prenatal diagnosis were universally… | ||||||||||||||
| "Low" (~19%)c |
5,970 | 15.10 | 1,095 (895–1,310) |
(18%) | 2.77 | 2,750 (2,415–3,100) |
(46%) | 6.96 | 1,105 (885–1,350) |
(19%) | 2.80 | 1,020 (805–1,260) |
(17%) | 2.58 |
| "High" (~ 42%)c |
5,965 | 15.09 | 2,455 (2,155–2,785) |
(41%) | 6.21 | 1,985 (1,720–2,270) |
(33%) | 5.02 | 740 (575–925) |
(12%) | 1.87 | 785 (610–975) |
(13%) | 1.99 |
Notes: Differences in the number born alive are due to rounding
Calculated using the number of live births in 2012 (3,952,937)26
Model inputs were for each specific CCHD type and these estimates for all CCHD combined were derived by summing across the CCHD categories for each of the 10,000 simulations, then calculating the mean and 95% uncertainty interval across the 10,000 simulations for a given parameter
See Methods and eTable
DISCUSSION
We estimated that ~900 infants with nonsyndromic CCHD could be detected annually in the United States through universal implementation of CCHD screening using pulse oximetry. The majority of these infants would be those with CCHD less likely to be detected prenatally or clinically at birth, such as COA/IAA, TOF, or d-TGA. These estimates were reassuring in that very few cases (0%–16% overall) of defects considered to be “primary” targets of CCHD screening were estimated to be false negatives. However, due to high birth prevalence, low prenatal detection rates, and limited CCHD screening sensitivity,14 an estimated ~900 infants with nonsyndromic CCHD, primarily those with COA/IAA, or TOF, were likely to be false negatives resulting from CCHD screening through pulse oximetry, suggesting that the sensitivity of CCHD screening in practice for all primary and secondary targets combined may be closer to 50% than the previous estimate of 76%.13 In our secondary analysis, despite the large difference in the proportion of infants with nonsyndromic CCHD identified prenatally under scenarios of “low” compared to “high” prenatal diagnosis prevalence (19% vs. 42%), the subsequent large difference in the number of infants with undiagnosed CCHD at birth was greatly diminished by “timely” diagnosis. Thus, the number of true positive nonsyndromic CCHD cases estimated to be identified by CCHD screening was relatively similar across the two prenatal screening scenarios (within ~1 case per 10,000 live births), even though the relative difference was about 50%.
Our estimate of the number of infants with CCHD likely to be detected through screening (875 [95% UI: 705–1,060]) is similar to the 1,189 estimate from a recent cost effectiveness analysis.20 Differences may be attributable to the inclusion of infants with genetic syndromes in the cost effectiveness analysis or their use of overall estimates of prenatal diagnosis, late detection, and screening sensitivity for all CCHD combined rather than each specific CCHD type. Our estimate differs from that suggested by a report describing the first nine months of CCHD screening in New Jersey, the first state to mandate and implement state-wide CCHD screening, which found that three infants with CCHD were detected through screening alone.4 If extrapolated to the annual U.S. birth population, the New Jersey experience equates to approximately 220 CCHD cases,1 much lower than our estimate. One potential reason for this discrepancy may be differences in prenatal diagnosis prevalence. Of the 55 infants with CCHD identified in the NJ study, 48 (87%) were not reported as having a failed screen for a number of potential reasons, including having a prenatal diagnosis.4
In our analysis, a large proportion of nonsyndromic CCHD cases estimated to be both missed and detected through CCHD screening were infants with COA/IAA. As COA can have varying degrees of severity that may confer varying levels of hypoxia, it is possible that the more severe cases of coarctation are more likely to be detected prenatally or possibly identified through CCHD screening but less severe ones may be missed. Furthermore, unlike some previous studies of CCHD screening sensitivity,13,14 our CCHD definition allowed for diagnosis of CCHD within one year, rather than 28 days, of life, also potentially leading us to include less severe cases of COA. However, we were unable to examine the impact of severity of these lesions on the likelihood of being detected prenatally or through CCHD screening as information on severity was not available in our data sources.
While this analysis focused on CCHD cases likely to be detected and missed through universal CCHD screening, infants with non-CCHD conditions are likely to result in “false positive” screens. In a meta-analysis of 13 studies of CCHD screening through pulse oximetry, Thangaratinam et al. (2012) estimated the false positive rate to be 0.14% (95% CI: 0.06–0.33), which dropped to 0.05% (95% CI: 0.02–0.12) when screening was conducted at >24 hours after birth, the timeframe recommended by the AAP, AHA, and others.7,13 While infants with false positive screens do not have CCHD, they may have other clinically relevant conditions that contributed to their failed screening, including pneumonia and sepsis.4,21
Our analysis was subject to additional limitations. We restricted our estimates of birth prevalence, prenatal diagnosis, and late detection to 2000–2005 as we were only able to obtain maternal report of prenatal diagnosis through 2005 in the NBDPS.10 Our birth prevalence estimates were restricted to only the five central counties of metropolitan Atlanta and our prenatal diagnosis and late detection estimates were derived from a study conducted in 10 states, thus our estimates may not be reflective of the entire United States. Despite potential improvements in prenatal diagnosis over time22 and differing definitions of prenatal diagnosis, our range of prenatal diagnosis estimates from 2000–2005 NBDPS data were consistent with those from a study using national 2006–2012 Society of Thoracic Surgeons data23, and our “high” prenatal diagnosis estimates were similar to a recent analysis of Massachusetts data.22 Our definition of late detected CCHD was based on the timing of the first documented echocardiography confirming the defect, not necessarily the first time echocardiography was ever performed, thus some infants may have been misclassified. However, our overall estimate of late detection is similar to that of a study of a cohort of Florida births, in which the authors defined late detection as diagnosis after birth hospitalization.9 Additionally, it is possible that some infants that we classified as having “timely” diagnosis could still have benefited from screening; thus, our estimates may be altered if screening is performed earlier. An additional limitation is that we relied on published estimates of CCHD-specific screening sensitivity that included studies with screening algorithms different from that recommended by the AAP, AHA, and others and we were unable to assess the impact of these differences, such as the age at screening, in our study. It is possible that classification of specific defects differed across our other data sources (MACDP, NBDPS, Prudhoe et al.). Finally, our estimates of the number of infants potentially detected through CCHD screening only apply to the subset of infants, estimated to be approximately 88%,15 with CCHD not associated with a genetic syndrome.
In the absence of national implementation and data collection on CCHD screening, our analysis used modeling approaches to estimate the potential impact of screening and had several strengths making it a valuable contribution to the literature. We used data from a population-based active surveillance system to estimate CCHD prevalence and a population-based case-control study to estimate prenatal diagnosis and late detection. To better account for uncertainty, we used a range of estimates for our model parameters (typically the mean and standard error). Additionally, estimates from our secondary analysis allow public health professionals and policy makers to consider the prevalence of prenatal diagnosis in their communities when estimating the likely impact of CCHD screening.
CONCLUSIONS
Based on our model, nearly 900 infants per year with nonsyndromic CCHD are likely to be detected through universal CCHD screening in the United States; however, an equal number are likely to be missed. While many infants with CCHD will likely be identified through screening, there will still be many false negatives, suggesting that the general practitioner should not rely on CCHD screening alone to rule out a CCHD.25 Our analysis also suggests that increases in prenatal diagnosis of CCHD are unlikely to substantially impact the number of infants detected through CCHD screening. Future efforts should focus on documenting the true impact of CCHD screening in practice, and linking CCHD screening data with birth defects surveillance data,24 in order to identify the outcome of infants with false negative screening results.
Supplementary Material
What’s Known on This Subject
Newborn screening for critical congenital heart defects (CCHD) has been implemented in many hospitals, yet there is uncertainty about the number of infants with CCHD that might be detected through universal implementation of newborn CCHD screening in the United States.
What This Study Adds
We estimated that ~875 infants with CCHD might be detected, and ~880 missed, annually through universal CCHD screening in the United States. Increases in prenatal diagnosis are unlikely to substantially impact the number of infants detected through CCHD screening.
Acknowledgments
Funding source: This work was partially supported through cooperative agreements under PA 96043, PA 02081 and FOA DD09-001 from the Centers for Disease Control and Prevention.
Abbreviations
- AAP
American Academy of Pediatrics
- AHA
American Heart Association
- COA
coarctation of the aorta
- CCHD
critical congenital heart defects
- d-TGA
dextro-transposition of the great arteries
- DORV
double-outlet right ventricle
- HLHS
hypoplastic left heart syndrome
- IAA
interrupted aortic arch
- MACDP
Metropolitan Atlanta Congenital Defects Program
- NBDPS
National Birth Defects Prevention Study
- TOF
tetralogy of Fallot
- TAPVR
total anomalous pulmonary venous return
- UI
uncertainty interval
Footnotes
Financial Disclosure: All authors (ECA, SMG, MAH, MEO) have no financial relationships relevant to this article to disclose.
Conflicts of Interest: All authors (ECA, SMG, MAH, MEO) have no conflicts of interest to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor’s Statement Page
Elizabeth C. Ailes: Dr. Ailes conceptualized and designed the study, acquired the data, carried out the analysis and interpretation of the data, drafted the initial manuscript, reviewed and revised the manuscript, approved the final manuscript as submitted, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Suzanne M. Gilboa, Margaret A. Honein, and Matthew E. Oster: Drs. Gilboa, Honein, and Oster conceptualized and designed the study, reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Calculation: three infants identified in nine months is equivalent to four infants identified in 12 months; 4 infants identified through screening/73,000 births in New Jersey x ~ 4,000,000 births in the U.S. = 219/year in the U.S.
REFERENCES
- 1.Mahle WT, Newburger JW, Matherne GP, et al. Role of pulse oximetry in examining newborns for congenital heart disease: A scientific statement from the AHA and AAP. Pediatrics. 2009;124(2):823–836. doi: 10.1542/peds.2009-1397. [DOI] [PubMed] [Google Scholar]
- 2.Secretary's Advisory Committe on Heritable Disorders in Newborns and Children. HHS Secretary adopts recommendation to add critical congenital heart disease to the Recommended Uniform Screening Panel. September 21, 2011. Available from: http://www.HRSA.Gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf. [Google Scholar]
- 3.Centers for Disease Control Prevention. Assessment of current practices and feasibility of routine screening for critical congenital heart defects � Georgia, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(15):288–291. [PMC free article] [PubMed] [Google Scholar]
- 4.Garg LF, Van Naarden Braun K, Knapp MM, et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2):e314–e323. doi: 10.1542/peds.2013-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed May, 14, 2014];American Academy of Pediatrics Division of State Government Affairs. CCHD screening map. http://www.Cqstatetrack.Com/texis/viewrpt/main.Html?Event=4f7f371574&run=y. [Google Scholar]
- 6.Walsh W. Evaluation of pulse oximetry screening in middle Tennessee: Cases for consideration before universal screening. J Perinatol. 2011;31(2):125–129. doi: 10.1038/jp.2010.70. [DOI] [PubMed] [Google Scholar]
- 7.Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5):e1259–e1267. doi: 10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 8.Peterson C, Ailes E, Riehle-Colarusso T, et al. Late detection of critical congenital heart disease among us infants: Estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168(4):361–370. doi: 10.1001/jamapediatrics.2013.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson C, Dawson A, Grosse SD, et al. Hospitalizations, costs, and mortality among infants with critical congenital heart disease: How important is timely detection? Birth Defects Res A Clin Mol Teratol. 2013;97(10):664–672. doi: 10.1002/bdra.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ailes EC, Gilboa SM, Riehle-Colarusso T, et al. Prenatal diagnosis of nonsyndromic congenital heart defects. Prenat Diagn. 2013;34(3):214–222. doi: 10.1002/pd.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. The Journal of Pediatrics. 2009;155(1):26–31. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: A population based study. Ultrasound Obstet Gynecol. 2012;40(4):418–425. doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 13.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Accuracy of pulse oximetry in screening for congenital heart disease in asymptomatic newborns: A systematic review. Lancet. 2012;92(3):F176–F180. doi: 10.1136/adc.2006.107656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prudhoe S, Abu-Harb M, Richmond S, Wren C. Neonatal screening for critical cardiovascular anomalies using pulse oximetry. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F346–F350. doi: 10.1136/archdischild-2012-302045. [DOI] [PubMed] [Google Scholar]
- 15.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79(2):65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 17.Riehle-Colarusso T, Strickland MJ, Reller MD, et al. Improving the quality of surveillance data on congenital heart defects in the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2007;79(11):743–753. doi: 10.1002/bdra.20412. [DOI] [PubMed] [Google Scholar]
- 18.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A National Birth Defects Prevention Study. Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–727. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 20.Peterson C, Grosse S, Oster M, Olney R, Cassell C. Cost-effectiveness of routine screening for critical congenital heart disease in us newborns. Pediatrics. 2013;132(3):e595–e603. doi: 10.1542/peds.2013-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewer AK, Middleton LJ, Furmston AT, et al. Pulse oximetry screening for congenital heart defects in newborn infants (pulseox): A test accuracy study. Lancet. 2011;378(9793):785–794. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 22.Liberman RF, Getz KD, Lin AE, et al. Delayed diagnosis of critical congenital heart defects: Trends and associated factors. Pediatrics. 2014;134(2):e373–e381. doi: 10.1542/peds.2013-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quartermain M, Pasquali SP, Hill K, et al. National variation in prenatal diagnosis of congenital heart disease by state and lesion type: An analysis of the society of thoracic surgeons congenital heart surgery database. Paper presented at: American College of Cardiology.2014. [Google Scholar]
- 24.Olney RS, Botto LD. Newborn screening for critical congenital heart disease: Essential public health roles for birth defects monitoring programs. Birth Defects Res A Clin Mol Teratol. 2012;94(12):965–969. doi: 10.1002/bdra.23103. [DOI] [PubMed] [Google Scholar]
- 25.Oster ME, Colarusso T, Glidewell J. Screening for Critical Congenital Heart Disease: A Matter of Sensitivity. Pediatr Cardiol. 2013;34:203–204. doi: 10.1007/s00246-012-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton B, Martin J, Ventura S. Births: Preliminary data for 2012. National Vital Statistics Reports. 2013;68(3) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.