Abstract
A novel lumun-lumun sampling methodology was used to obtain a large diversity of micromollusks, including the new species Lienardia totopotens. In turn, from L. totopotens we cultivated a Streptomyces sp. strain that contained new and known spirotetronate polyketides, lobophorins (1–5). The structures were elucidated using spectroscopy, and the compounds were evaluated for cytotoxicity to human cells and activity against Mycobacterium tuberculosis. A structure-activity relationship was discerned, wherein the lack of digitoxose in 1 led to lack of both cytotoxic and antibacterial activity. For compounds 2–5 both activities were in the low μM to mid nM range. Although this likely precludes their direct application in tuberculosis therapy due to possible poor therapeutic index, very slight changes in structure led to widely varying antibacterial:cytotoxicity ratios, providing a possible basis to synthesize more selective derivatives.
Keywords: antibiotics, cone snail, Mycobacterium tuberculosis, Streptomyces
Introduction
There is an urgent need for new antibiotics to combat the rise of resistant strains.1 As an important example, Mycobacterium tuberculosis infects 1/3 of the global population and kills 1.4 million people annually.2 The rate of M. tuberculosis drug resistance is steadily rising, progressing from multiple drug-resistant forms (M-DR) to extremely drug resistant (X-DR) and finally totally drug resistant forms (T-DR).3 Natural products represent a major group of successful anti-tuberculosis drugs, including the first-line therapy, rifampicin. Natural products also continue to provide leads for antimycobacterial therapy,4 indicating that the natural world should still be examined as a source for new anti-tuberculosis drugs.
A problem with natural product-based antibiotic discovery programs is that the rediscovery rate can be high.4 It is straightforward to screen for antibiotic activity, and such screens have been ongoing for about 80 years. Several novel strategies have been suggested to overcome this problem. One approach is to seek novel habitats for bacteria that have not been previously well explored, with the as-yet unproven expectation that new compounds are more likely to be discovered. For this reason, we have been examining bacterial associates of gastropod mollusks, especially from the Family Conidae, which have yielded many novel isolates with novel chemistry.5–9 Many of the bacteria cultivated from gastropods probably reflect the microbiology of the surrounding environment,10 but at least some of these bacteria are long-term associates that produce compounds important to the mollusks.8 Thus, cultivation of mollusk-associated bacteria leads to a library of diverse bacteria, some of which produce new, bioactive natural products.
Symbiotic bacteria from animals provide a good and relatively untapped source of new natural products, but obtaining diverse, novel animals can be time consuming and expensive. We have employed an approach to easily access a large diversity of mollusks, lumun-lumun.11 This method, invented and widely used by fishermen in the central Visayan region of the Philippines, involves placing bundles of nets (lumun-lumun) in the sea for 1–6 months. When the nets are harvested, they are found to contain a large abundance and diversity of micromollusks, which are valuable to collectors. In addition, the obtained micromollusks are often novel species, previously unknown to science.12–14 Conoidean mollusks, the turrids, are related to cone snails and are overrepresented in these nets.15 Turrids are the most speciose of the cone-related mollusks. Ongoing research shows that, like cone snails, each turrid contains a wealth of novel venom peptides that are neuroactive and potentially have other activities.11
Although many turrids are found in a single net, we carefully first examined a small subset of these turrids to explore their potential for production of novel microbial natural products. In particular, a new species, Lienardia totopotens,12 was found in a lumun-lumun net, dissected, and used to obtain novel venoms. Tissues were obtained, crushed, and plated on microbial media. We previously reported a structurally relatively novel compound from one of these bacterial strains, but unfortunately we could not determine the activity of the compound.7 Here, we report a series of less novel compounds, which are in fact relatively trivial new derivatives of the lobophorin series of metabolites. The antimycobacterial compound, rifampicin, works by inhibiting the DNA-dependent RNA polymerase (DDRP).16 Since it was shown that a related compound also hit DDRP,17 this led us to screen these compounds in assays against human cells and M. tuberculosis. Here, we describe novel compounds in the lobophorin series18–20 with antimycobacterial activity. Very recently, antimycobacterial activity of other lobophorins was described.21
Materials and methods
General experimental procedures
UV spectra were obtained using a Perkin-Elmer Lambda2 UV/vis spectrometer. NMR data were collected using either a Varian (Palo Alto, CA) INOVA 500 (1H 500 MHz, 13C 125 MHz) NMR spectrometer with a 3 mm Nalorac MDBG probe or a Varian INOVA 600 (1H 600 MHz, 13C 150 MHz) NMR spectrometer equipped with a 5 mm 1H[13C,15N] triple resonance cold probe with a z-axis gradient, utilizing residual solvent signals for referencing. High-resolution mass spectra (HRESIMS) were obtained using a Bruker (Billerica, MA) APEXII FTICR mass spectrometer equipped with an actively shielded 9.4 T superconducting magnet (Magnex Scientific Ltd.. Oxford, UK), an external Bruker APOLLO ESI source, and a Synrad (Mukilteo, WA) 50W CO2 CW laser. Supelco (St. Louis, MO) Discover HS (4.6 × 150 mm) and semipreparative (10 × 150 mm) C18 (5 μm) columns were used for analytical and semipreparative HPLC, respectively, as conducted on a Hitachi (Dallas, TX) Elite Lachrom System equipped with a Diode Array L-2455 detector. Antibacterial and cytotoxicity assay plates were evaluated by measuring A580 on a Multiskan FC plate reader (Fisher Scientific, Waltham, MA). Chemicals were sourced from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Waltham, MA) with the exception of the following: fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA), ADC supplement from Remel (Lenexa, KS), and antimycotic/antibacterial supplement from MP Biomedicals (Solon, OH).
Bacterial material
Bacteria were cultivated from L. totopotens obtained by professional collectors near Mactan Island, Cebu, Philippines as previously described. Relevant permission from local and national authorities in the Philippines was obtained prior to beginning this study. Streptomyces sp. 1053U.I.1a.3b was obtained from dissected hepatopancreas tissue, purified into an axenic culture, and stored as a glycerol stock at −80°C. The strain was subsequently recovered from a glycerol stock and used for further chemical analysis. The 16S gene was cloned using primers 8–27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-TACGGYTACCTTGTTACGA CTT-3′) and submitted to GenBank (accession number KF137580).
Fermentation and extraction
The strain Streptomyces sp. 1053U.I.1a.3b was cultured at 30 °C in a New Brunswick BioFlo110 fermenter containing 10 L of the medium ISP2 (0.4% yeast extract, 1% malt extract, 0.4% glucose, 2% NaCl). After 8 days, the broth was centrifuged and the supernatant was extracted with Diaion HP-20 resin for 4 h. The resin was filtered through cheesecloth and washed with H2O to remove salts. The filtered resin was eluted with MeOH to yield a primary extract.
Purification
The primary extract (300 mg) was separated into 4 fractions (Fr1–Fr4) on a C18 column using step-gradient elution of MeOH in H2O (40%, 60%, 70%, 80%). Fr 3 eluted in 70% MeOH and was further purified by C18 HPLC using 68% MeOH in H2O with 0.05% TFA to obtain compounds 2 (1.1 mg), 3 (1.5 mg), 4 (2.0 mg) and 5 (0.9 mg). Fr 4 eluted in 80% MeOH and was further purified by C18 HPLC using 75% MeOH in H2O with 0.05% TFA to obtain compound 1 (1.3 mg).
lobophorin H (1): colorless solid); UV (MeOH) λmax 204, 241, 267 nm; 1H and 13C NMR (see Table 1); HRESIMS m/z 753.4344 [M+H]+ (calcd for C42H61N2O10, 753.4326).
Table 1.
1H and 13C NMR data for compounds 1 and 2.
| No | 1a | 2b | ||
|---|---|---|---|---|
|
| ||||
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | 168.4 C | - | ND |
| 2 | - | 103.1 C | - | ND |
| 3 | - | 204.0 C | - | 200.5 C |
| 4 | - | 51.8 C | - | 53.2 C |
| 5 | 1.89 m | 43.4 CH | 2.11 dd (10.0, 8.5) | 46.1 CH |
| 6 | 1.47 m | 31.5 CH | 1.51 m | 33.2 CH |
| 7 | 1.44 m | 42.2 CH2 | 1.49m, 1.58 m | 43.9 CH2 |
| 8 | 2.07 m | 35.4 CH | 2.20 m | 36.6 CH |
| 9 | 3.40 m | 74.9 CH | 3.40 dd (10.5, 5.1) | 87.2 CH |
| 10 | 1.90 m | 39.4 CH | 2.02 brdd (12.0, 11.0) | 40.7 CH |
| 11 | 6.02 d (10.0) | 128.0 CH | 5.72 brd (10.2) | 127.5 CH |
| 12 | 5.28 m | 125.8 CH | 5.36 ddd (10.0, 5.3, 2.7) | 130.0 CH |
| 13 | 3.39 m | 52.8 CH | 3.78 brd (4.9) | 53.3 CH |
| 14 | - | 136.3 C | - | 139.2 C |
| 15 | 5.04 d (9.4) | 123.9 CH | 5.23 brd (8.5) | 123.6 CH |
| 16 | 2.30m, 2.18 m | 31.8 CH2 | 2.44 ddd (15.2, 9.6, 2.4), 2.20 m | 32.8 CH2 |
| 17 | 4.12 brs | 79.7 CH | 4.24 brs | 81.2 CH |
| 18 | - | 138.6 C | - | 139.3 C |
| 19 | 4.97 d (10.0) | 119.1 CH | 5.25 brd (9.3) | 119.7 CH |
| 20 | 3.48 dd (10.0, 2.5) | 40.4 CH | 3.63 brd (9.3) | 43.8 CH |
| 21 | 5.34 brs | 120.9 CH | 6.66 brs | 155.2 CH |
| 22 | - | 142.5 C | 146.0 C | |
| 23 | 2.55 m | 27.7 CH | 2.84 dd (9.2, 2.7) | 27.2 CH |
| 24 | 2.24 m, 1.75 m | 35.4 CH2 | 2.27 dd (14.5, 7.5), 1.68 m | 36.2 CH2 |
| 25 | 83.6 C | 85.2 C | ||
| 26 | 197.6 C | ND | ||
| 27 | 1.44 s | 15.6 CH3 | 1.47 s | 16.0 CH3 |
| 28 | 0.55 brs | 23.0 CH3 | 0.65 d (6.7) | 23.7 CH3 |
| 29 | 0.92 d (6.8) | 14.0 CH3 | 1.12 d (6.5) | 15.3 CH3 |
| 30 | 1.29 s | 14.4 CH3 | 1.37 s | 15.2 CH3 |
| 31 | 1.33 s | 15.4 CH3 | 1.42 s | 16.0 CH3 |
| 32 | 4.00 d (13.4), 3.93 d (13.4) | 63.8 CH2 | 9.43 s | 196.4 C |
| 33 | 1.14 d (7.0) | 20.4 CH3 | 1.31 d (7.3) | 21.1 CH3 |
| A1 | 4.75 brd (4.4) | 100.3 CH | ||
| A2 | 2.37 dd (14.5, 3.4), 1.72 m | 32.3 CH2 | ||
| A3 | 4.01 dd (6.0, 3.0) | 70.0 CH | ||
| A4 | 3.26 dd (9.6, 3.3) | 74.0 CH | ||
| A5 | 4.10 dq (9.6, 6.3) | 66.5 CH | ||
| A6 | 1.19 d (6.3) | 18.6 CH3 | ||
| B1 | 5.18 brd (3.4) | 93.9 CH | ||
| B2 | 2.09 m, 1.99 ddd (15.0, 3.8, 3.6) | 36.5 CH2 | ||
| B3 | 4.17 dd (6.3, 3.6) | 68.8 CH | ||
| B4 | 3.28 dd (9.9, 3.6) | 84.0 CH | ||
| B5 | 4.04 dq (9.4, 6.6) | 64.4 CH | ||
| B6 | 1.19 d (6.5) | 18.6 CH3 | ||
| C1 | 4.94 brd (9.7) | 101.4 CH | ||
| C2 | 2.04 m, 1.72 m | 39.3 CH2 | ||
| C3 | 4.29 dd (6.0, 3.1) | 64.9 CH | ||
| C4 | 2.84 dd (9.2, 2.7) | 84.3 CH | ||
| C5 | 3.82 m | 70.5 CH | ||
| C6 | 1.22 d (6.5) | 19.1 CH3 | ||
| C7 | 3.69 s | 53.6 CH3 | ||
| D1 | 4.75 d (9.8) | 97.6 CH | 4.52 brd (9.6) | 99.5 CH |
| D2 | 1.90 m, 1.73 m | 37.0 CH2 | 2.71 brd (15.0), 1.79 dd (15.0m 9.4) | 37.0 CH2 |
| D3 | - | 57.0 C | - | 92.9 C |
| D4 | 3.55 m | 54.0 CH | 4.35 brs | 55.8 CH |
| D5 | 3.99 m | 67.9 CH | 3.52 q | 70.7 CH |
| D6 | 1.00 d (6.2) | 17.6 CH3 | 1.13 d (6.5) | 18.1 CH3 |
| D7 | 1.18 s | 24.2 CH3 | 1.52 s | 26.3 CH3 |
| D8 | 158.5 C | - | 160.7 C | |
| D9 | 3.55 s | 52.5 CH3 | 3.68 s | 53.6 CH3 |
in DMSO-d6.
in CD3OD-d4
lobophorin I (2): colorless solid); UV (MeOH) λmax 204, 241, 267 nm; 1H and 13C NMR (see Table 1); HRESIMS m/z 1185.5962 [M+H]+ (calcd for C61H89N2O21, 1185.5958).
Bacillus subtilis assay
B. subtilis ATCC 6633 was plated on Mueller-Hinton agar media. Compounds and controls were added to paper disks at varying concentrations, and zones of inhibition were detected by eye.
M. tuberculosis inhibition
The assay was performed as previously described,22 with the exception that 4 wells per compound were used for each dilution for MIC determination. Rifampicin and DMSO diluent only served as positive and negative controls, respectively.
Cytotoxicity assay
Cytotoxicity testing was performed using CEM-TART lymphoblastoid cells23 cultured in RPMI 1640 with 20% FBS and antibiotic/antimycotic supplement at 37°C with 5% CO2 in moisture saturated atmosphere in 96 well culture clusters. Test compounds dissolved in 1 μL DMSO, negative controls (DMSO alone) or positive controls (doxorubicin in DMSO) were added in triplicate wells together with 50,000 cells in 200 μL of fresh media and incubated for 72 hours. Each well then received 11 μL of 5 mg/ml MTT (Sigma St. Louis, MO) followed by 2 hours incubation after which the formazan precipitate was pelleted by centrifugation. The spent media was aspirated and the formazan crystals solubilized in DMSO. Absorbance at 580nm was quantified on a Multiskan FC (Fisher Scientific, Waltham, MA) plate reader and inhibition determined as described in the antimycobacterial assay procedure.
Results
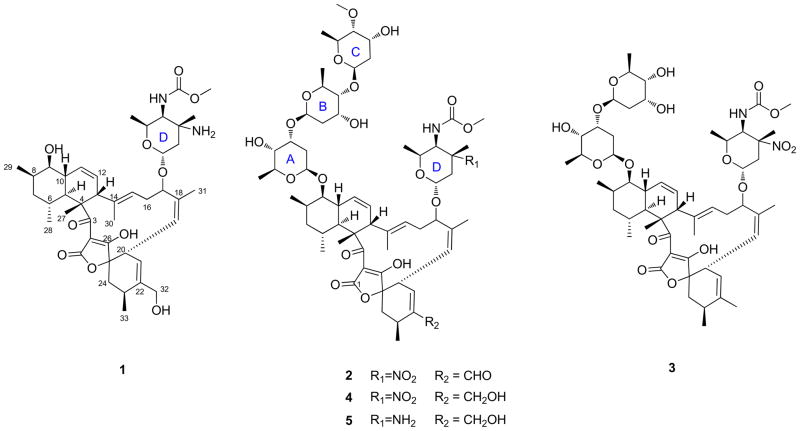
Strain Streptomyces sp. 1053U.I.1a.3b was cultivated from dissected hepatopancreas tissue tissues of L. totopotens, a new species of conoidean mollusk, which was found in a lumun-lumun net in the Philippines. Crude extracts of 1053U.I.1a.3b was active in an antibacterial assay against B. subtilis. HPLC analysis showed that the strain produced a series of related families of metabolites, which were further purified by bioassay-guided fractionation to yield compounds 1–5 (Figure 1). Using NMR and mass spectrometry, three compounds were shown to be identical to the previously reported lobophorins F (3),20 B (4),18 and C (5),19 while the remaining two compounds had not been previously described and were named lobophorin H (1) and I (2).
Figure 1.
Structures of lobophorin compounds 1–5.
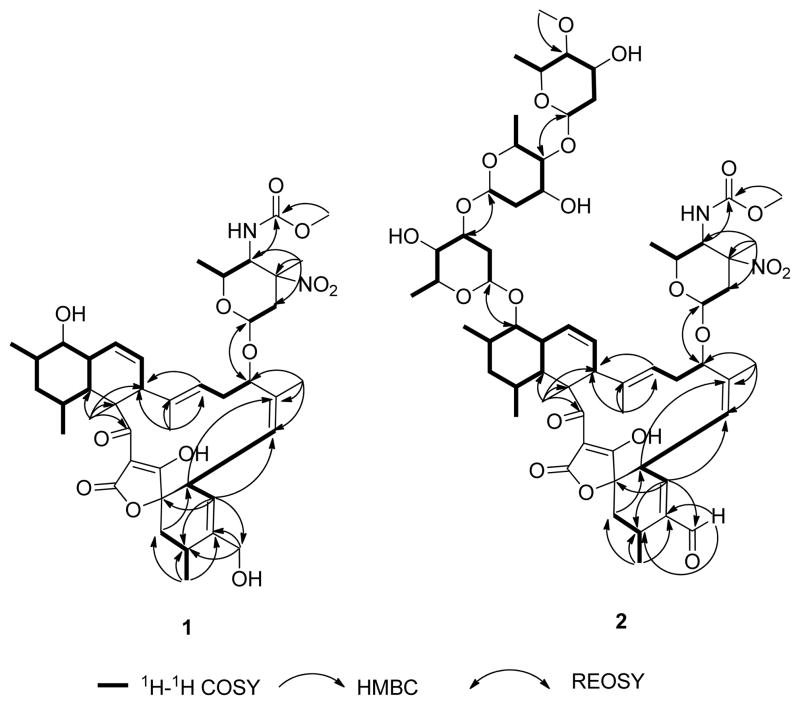
The molecular formula of 1 was established as C42H60N2O10 by high-resolution electrospray ionization mass spectrometry (HRESIMS) (m/z 753.4344 for [M+H]+). 1D and 2D NMR data supported a lack of three sugar units (A-C) in comparison to that of 5 (Table 1). Indeed, only one hemiacetal group (δH 4.75, δC 96.7) was observed and assigned as the anomeric carbon of amino sugar unit D. The carbon chemical shift of C-9 at 74.9 ppm indicated a free secondary alcohol group instead of an O-glucosidic bond. On the basis of these data, as well as HMBC and ROESY, (Figure 2), the structure of 1 was identified as the aglycon of 5.
Figure 2.
Key HMBC, 1H-1H COSY and ROESY correlations for compounds 1 and 2.
The molecular formula of 2 was determined as C61H88N2O21 by HRESIMS (m/z 1185.5962 for [M+H]+) and 1H and 13C NMR data (Table 1). The NMR data of 2 were largely identical to previously reported lobophorin C (4). In fact, the only major difference was the presence of an aldehyde residue (δH 9.43 and δC 196.4) in place of a CH2OH group. The C-21 and C-22 13C resonances of 2 were shifted 34.3 ppm and 3.5 ppm downfield in comparison to those of 4, indicating conjugation between the aldehyde and double bond C-21–C-22. The HMBC correlations from H-32 to C-32 and from H-21 to C-32 confirmed the partial structure of the conjugated aldehyde group. The planar structure of 2 was further confirmed by 2D NMR (Figure 2).
Compounds 1–5 were tested for their inhibitory activity toward the growth of M. tuberculosis H37Ra. All compounds except 1 showed strong inhibitory activity to M. tuberculosis with MIC values ranging from 1.3 to 7.8 μM (Table 2). In order to examine the potential to develop lobophorins to treat tuberculosis, we also tested the cytotoxicity of these compounds against human CEM-TART cancer cell line. Unfortunately, all the antimycobacterial active compounds also showed strong cytotoxicity with potencies similar to those of their antitmycobacterial activities (Table 2).
Table 2.
Antimycobacterial activity (MIC90) and CEM-TART cytotoxicity (LD50) for compounds 1–5.
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| MIC90 (μM) against M. tuberculosis | >100 | 2.6 | 7.8 | 1.3 | 1.4 |
| IC50 (μM) cytotoxicity | >100 | 8.6 | 0.3 | 1.6 | 1.7 |
Discussion
Lobophorins belong to a family of bacterial spirotetronates, which comprise a large class of antibiotics and antitumor agents. In addition to lobophorins,18–20 the family includes antlermicins,24,25 chlorothricin,26 kijanimicin,27 decatromicins,28 saccharocarcins,29 tetrocarcins,30 and versipelostatins.31 The mechanism of action of tetrocarcin A was previously studied. It was shown that tetrocarcin A inhibited DDRP at nanomolar concentrations, but this was likely not the major mechanism of action.17 This finding inspired us to screen lobophorins against M. tuberculosis, although the mechanism is probably not due to DDRP inhibition and requires testing. We found that the lobophorins are relatively potent: compounds 4 and 5 were about 4 x less potent than rifampicin control. Many spirotetronates are known to be toxic to mammalian cells, including the known compounds in the lobophorin series. Here, we show that all antimycobacterial compounds also exhibit toxicity to human cells, limiting their potential utility.
Some interesting structure-activity relationships were observed, which might be useful in designing more selective antimycobacterial agents. Derivative 1 lacks the three digitoxose sugar moieties, and concomitantly also lacks activity against M. tuberculosis and human cells. Loss of one of the digitoxose units, as in compound 3, led to a >25-fold selective toxicity to human vs. bacterial cells. Compounds 4 and 5 have nearly equal potency to both cell types. By contrast, new compound 2, which contains an α,β-unsaturated aldehyde, provided a >3-fold selectivity for antitubercular activity vs. cytotoxicity. This finding suggests that simple modifications of the lobophorin scaffold may provide derivatives that are selective antimicrobial agents. Recently, biosynthetic gene clusters for lobophorins and its polyketide relatives have been described, providing a platform for engineering to produce desired derivatives.32–34 Interestingly, elucidation of the glycosylation steps shows definitively that compounds similar to 1 are precursors to other compounds in the series.
The lumun-lumun collection method provides rapid access to diverse micromollusks, including many new species. This method was also shown to be of utility in providing rapid access to diverse, novel, and bioactive venom peptides that might find therapeutic application.11 Here, and in a recent report on structurally novel compounds, we show that this method also provides a third axis of biological and chemical diversity. Cultivation of bacteria associated with these mollusks affords novel compounds, some of which may have therapeutic potential. Further work is required to distinguish true symbionts from casual associates and to determine the diversity of unique natural products that might be found in this novel habitat.
Supplementary Material
Acknowledgments
Collection, cultivation, isolation, and chemical characterization was funded by ICBG grant U01TW008163 from Fogarty (NIH). We thank the government of the Philippines and the community of Mactan Island for permission to conduct this study. Bioassay work was funded by ICBG grant U01T006671 from Fogarty (NIH). CEM-TART cells were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH - Drs. Herbert Chen, Terence Boyle, Michael Malim, Bryan Cullen, and H. Kim Lyerly.
References
- 1.Lewis K. Platforms for antibiotic discovery. Nat Rev Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 2.Tuberculosis Fact Sheet No104. 2013 (Accessed at http://www.who.int/mediacentre/factsheets/fs104/en/.)
- 3.Klopper M, Warren RM, Hayes C, Gey van Pittius NC, Streicher EM, Müller B, Sirgel FA, Chabula-Nxiweni M, Hoosain E, Coetzee G, David van Helden P, Victor TC, Trollip AP. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2013;19:449–455. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12:388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, Antemano RR, Hughen RW, Tianero MD, Peraud O, Haygood MG, Concepcion GP, Olivera BM, Light A, Schmidt EW. Pulicatins A-E, neuroactive thiazoline metabolites from cone snail-associated bacteria. J Nat Prod. 2010;73:1922–1926. doi: 10.1021/np100588c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Z, Reilly CA, Antemano R, Hughen RW, Marett L, Concepcion GP, Haygood MG, Olivera BM, Light A, Schmidt EW. Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria. J Med Chem. 2011;54:3746–3755. doi: 10.1021/jm101621u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Z, Flores M, Forteza I, Henriksen NM, Concepcion GP, Rosenber G, Haygood MG, Olivera BM, Light AR, Cheatham TE, Schmidt EW. Totopotensamides, polyketide-cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp. J Nat Prod. 2012;75:644–649. doi: 10.1021/np200886x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Z, Torres JP, Ammon MA, Marett L, Teichert RW, Reilly CA, Kwan JC, Hughen RW, Flores M, Tianero MD, Peraud O, Cox JE, Light AR, Villaraza AJ, Haygood MG, Concepcion GP, Olivera BM, Schmidt EW. A bacterial source for mollusk pyrone polyketides. Chem Biol. 2013;20:73–81. doi: 10.1016/j.chembiol.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Marett L, Hughen RW, Flores M, Forteza I, Ammon MA, Concepcion GP, Espino S, Olivera BM, Rosenberg G, Haygood MG, Light AR, Schmidt EW. Neuroactive diol and acyloin metabolites from cone snail-associated bacteria. Bioorg Med Chem Lett. 2013 doi: 10.1016/j.bmcl.2013.06.088. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peraud O, Biggs JS, Hughen RW, Light AR, Concepcion GP, Olivera BM, Schmidt EW. Microhabitats within venomous cone snails contain diverse actinobacteria. Appl Environ Microbiol. 2009;75:6820–6826. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seronay RA, Fedosov AE, Astilla MA, Watkins M, Saguil N, Heralde FM, Tagaro S, Poppe GT, Aliño PM, Oliverio M, Kantor YI, Concepcion GP, Olivera BM. Accessing novel conoidean venoms: Biodiverse lumun-lumun marine communities, an untapped biological and toxinological resource. Toxicon. 2010;56:1257–1266. doi: 10.1016/j.toxicon.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg G, Stahlschmidt P. A new species of Lienardia (Gastropoda: Conoidea) from the Philippines and the Spratly Islands. Proc Acad Nat Sci Philadephia. 2011;161:105–115. doi: 10.1635/053.161.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilburn RN, Fedosov AE, Olivera BM. Revision of the genus (Gastropoda: Conoidea: Turridae) with the description of six new species. Zootaxa. 2012;244:1–58. [PMC free article] [PubMed] [Google Scholar]
- 14.Fedosov A, Watkins M, Heralde FM, 3rd, Corneli PS, Concepcion GP, Olivera BM. Phylogeny of the genus Turris: correlating molecular data with radular anatomy and shell morphology. Mol Phylogenet Evol. 2011;59:263–270. doi: 10.1016/j.ympev.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivera BM, Watkins M, Bandyopadhyay P, Imperial JS, de la Cotera EP, Aguilar MB, Vera EL, Concepcion GP, Lluisma A. Adaptive radiation of venomous marine snail lineages and the accelerated evolution of venom peptide genes. Ann N Y Acad Sci. 2012;1267:61–70. doi: 10.1111/j.1749-6632.2012.06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White RJ, Lancini GC, Silvestri LG. Mechanism of action of rifampin on Mycobacterium smegmatis. J Bacteriol. 1971;108:737–741. doi: 10.1128/jb.108.2.737-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamaoki T, Tomita F, Kawamura F, Saito H. Mechanism of action of tetrocarcin A. Agric Biol Chem. 1983;47:59–65. [Google Scholar]
- 18.Jiang ZD, Jensen PR, Fenical W. Lobophorins A and B, new antiinflammatory macrolides produced by a tropical marine bacterium. Bioorg Med Chem Lett. 1999;9:2003–2006. doi: 10.1016/s0960-894x(99)00337-6. [DOI] [PubMed] [Google Scholar]
- 19.Wei RB, Xi T, Li J, Wang P, Li FC, Lin YC, Qin S. Lobophorin C and D, new kijanimicin derivatives from a marine sponge-associated actinomycetal strain AZS17. Mar Drugs. 2011;9:359–368. doi: 10.3390/md9030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu S, Li S, Chen Y, Tian X, Zhang H, Zhang G, Zhang W, Yang X, Zhang S, Ju J, Zhang C. Lobophorins E and F, new spirotetronate antibiotics from a South China Sea-derived Streptomyces sp SCSIO 01127. J Antibiot. 2011;64:711–716. doi: 10.1038/ja.2011.78. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Wang J, Guo H, Hou W, Yang N, Ren B, Liu M, Dai H, Liu X, Song F, Zhang L. Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp MS100061. Appl Microbiol Biotechnol. 2013;97:3885–3892. doi: 10.1007/s00253-012-4681-0. [DOI] [PubMed] [Google Scholar]
- 22.Koch M, Bugni TS, Sondossi M, Ireland CM, Barrows LR. Exocarpic acid inhibits mycolic acid biosynthesis in Mycobacterium tuberculosis. Planta Med. 2010;76:1678–1682. doi: 10.1055/s-0030-1249939. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Boyle TJ, Malim MH, Cullen BR, Lyerly HK. Derivation of a biologically contained replication system for human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992;89:7678–7682. doi: 10.1073/pnas.89.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobinata K, Uramoto M, Mizuno T, Isono K. Antlermicins B and C, new members of the antlermicin family. J Antibiot. 1980;33:772–775. doi: 10.7164/antibiotics.33.772. [DOI] [PubMed] [Google Scholar]
- 25.Kobinata K, Uramoto M, Mizuno T, Isono K. A new antibiotic, antlermicin A. J Antibiot. 1980;33:244–246. doi: 10.7164/antibiotics.33.244. [DOI] [PubMed] [Google Scholar]
- 26.Muntwyler R, Keller-Schierlein W. Metabolic products of microorganisms. 107 Structure of chlorothricin, a new type of macrolide antibiotic. Helv Chim Acta. 1972;55:2071–94. doi: 10.1002/hlca.19720550627. [DOI] [PubMed] [Google Scholar]
- 27.Waitz JA, Horan AC, Kalyanpur M, Lee BK, Loebenberg D, Marquez JA, Miller G, Patel MG. Kijanimicin (Sch 25663), a novel antibiotic produced by Actinomadura kijaniata SCC 1256. Fermentation, isolation, characterization and biological properties. J Antibiot. 1981;34:1101–1106. doi: 10.7164/antibiotics.34.1101. [DOI] [PubMed] [Google Scholar]
- 28.Momose I, Hirosawa S, Nakamura H, Naganawa H, Iinuma H, Ikeda D, Takeuchi T. Decatromicins A and B, new antibiotics produced by Actinomadura sp MK73-NF4 II Structure determination. J Antibiot. 1999;52:787–96. doi: 10.7164/antibiotics.52.787. [DOI] [PubMed] [Google Scholar]
- 29.Hegde VR, Patel MG, Das PR, Pramanik B, Puar MS. A family of novel macrocyclic lactones, the saccharocarcins produced by Saccharothrix aerocolonigenes subsp antibiotica II Physico-chemical properties and structure determination. J Antibiot. 1997;50:126–134. doi: 10.7164/antibiotics.50.126. [DOI] [PubMed] [Google Scholar]
- 30.Tamaoki T, Kasai M, Shirahata K, Ohkubo S, Morimoto M, Mineura K, Ishii S, Tomita F. Tetrocarcins, novel antitumor antibiotics. II Isolation, characterization and antitumor activity. J Antibiot. 1980;33:946–950. doi: 10.7164/antibiotics.33.946. [DOI] [PubMed] [Google Scholar]
- 31.Park HR, Chijiwa S, Furihata K, Hayakawa Y, Shin-Ya K. Relative and absolute configuration of versipelostatin, a down-regulator of molecular chaperone GRP78 expression. Org Lett. 2007;9:1457–1460. doi: 10.1021/ol070042t. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Xiao J, Zhu Y, Zhang G, Yang C, Zhang H, Ma L, Zhang C. Dissecting glycosylation steps in lobophorin biosynthesis implies an iterative glycosyltransferase. Org Lett. 2013;15:1374–1377. doi: 10.1021/ol400342e. [DOI] [PubMed] [Google Scholar]
- 33.Ding W, Lei C, He Q, Zhang Q, Bi Y, Liu W. Insights into bacterial 6-methylsalicylic acid synthase and its engineering to orsellinic acid synthase for spirotetronate generation. Chem Biol. 2010;17:495–503. doi: 10.1016/j.chembiol.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Xiao J, Zhang Q, Zhu Y, Li S, Zhang G, Zhang H, Saurav K, Zhang C. Characterization of the sugar-O-methyltransferase LobS1 in lobophorin biosynthesis. Appl Microbiol Biotechnol. 2013 doi: 10.1007/s00253-013-5083-7. epub. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.