Abstract
To reduce widespread shortages, attempts are made to use more marginal livers for transplantation. Many of these grafts are discarded for fear of inferior survival rates or biliary complications. Recent advances in organ preservation have shown that ex vivo subnormothermic machine perfusion has the potential to improve preservation and recover marginal livers pre- transplantation. To determine the feasibility in human livers, we assessed the effect of 3 hours of oxygenated subnormothermic machine perfusion (21 °C) on seven livers discarded for transplantation. Biochemical and microscopic assessment revealed minimal injury sustained during perfusion. Improved oxygen uptake (1.30 [1.11–1.94] to 6.74 [4.15–8.16] mL O2/min.kg liver), lactate levels (4.04 [3.70–6.00] to 2.29 [1.20–3.42] mmol/L) and adenosine triphosphate content (45.0 [70.6–87.5] pre-perfusion to 167.5 [151.5–237.2] pmol/mg after perfusion) were observed. Liver function, reflected by urea, albumin and bile production was seen during perfusion. Bile production increased and the composition of bile (bile salts/phospholipid ratio, pH and bicarbonate concentration) became more favorable. In conclusion, ex vivo subnormothermic machine perfusion effectively maintains liver function with minimal injury and sustains or improves various hepatobiliary parameters post-ischemia.
Keywords: Organ preservation, Subnormothermic Machine Perfusion, Liver Transplantation, Donation after Circulatory Death
Introduction
Preserving the viability of the donor liver is essential for successful transplantation. While advances in preservation solutions and immunosuppression have resulted in excellent outcomes following liver transplantation using standard criteria donors (1), transplantation using marginal grafts remains problematic (2,3). Donation after circulatory death (DCD) livers with increased warm ischemic time (WIT), in particular, present a challenge as the incidence of primary nonfunction and biliary complications are increased (4). The increasing use of marginal livers to expand the donor pool demands more sophisticated preservation modalities to prevent or treat the underlying deficiency of these livers.
While conventional static cold storage (SCS) merely slows the deterioration of the donor liver, ex vivo machine perfusion techniques may be useful in sustaining organ viability, improving it, or pre-conditioning the liver for reperfusion. Pre-clinical studies using hypothermic machine perfusion (HMP) at 0–4 °C have shown impressive benefits for preservation (5–8). The first clinical series from 2010 employing HMP in livers donated after brain death (DBD) showed promising initial results and was a large step forward in bringing perfusion techniques to clinical transplantation (9) and a more recent trial showed that hypothermic machine perfusion of DCD livers produced similar results to unperfused DBD livers (10). In contrast, normothermic machine perfusion (NMP) at ±37 °C offers the environment for a fully functional metabolism, which supports the reestablishment of homeostasis and other recovery processes, including an increase in ATP content (11). Additionally, maintaining a metabolically functional organ opens the opportunity of viability testing to assess liver function during perfusion and improve donor liver selection and allocation (12,13). Recently, the feasibility of NMP has been shown in sustaining discarded human DCD livers (14).
While NMP is potentially an improvement over cold perfusion, it still exposes the compromised ischemic and cold-stored liver to a rapid temperature rise. It is our hypothesis that a more gradual rewarming course may relieve this insult by employing a stepwise normalization of temperature and metabolic demand (15,16). Improvement of mitochondrial function in this phase may precondition the liver for normothermic reperfusion (17). Subnormothermic machine perfusion (SNMP) systems have been investigated to assume this intermediate role, benefiting from a lower metabolic demand at subphysiological temperature, while still maintaining sufficient metabolism for viability testing and improvement of graft function (18). In our experience normothermic temperatures required oxygen carriers to achieve sufficient oxygenation (19). Lowering the temperature to 21 °C allowed us to simplify the system by obviating oxygen carriers and allowing ambient air temperature control, which may significantly expedite clinical implementation. This proof-of-concept study, applying SNMP in human livers for the first time, aims to demonstrate controlled re-equilibration of ischemic disturbances through recovery of metabolism and hepatobiliary function.
Materials and methods
Procurement and back-table preparation
Donor livers were obtained from the New England Organ Bank (NEOB) with consent for research from the family after being turned down for clinical transplantation. Extubation of donors after circulatory death was performed by the primary service, which was also responsible for declaration of death 5 minutes after circulatory cessation. The procurement procedure did not begin until after declaration of death. Standard procurement technique includes an in situ flush with University of Wisconsin (UW) solution, intra-abdominal cooling with ice, and an additional back table UW flush. The gallbladder was incised, aspirated of bile, and irrigated with saline. The common bile duct was flushed with UW solution. The relative warm ischemic time for DCD livers is defined as the time between extubation and in situ cold flushing, whereas absolute warm ischemic time begins after circulatory cessation and ends at in situ cold flushing. Donor livers were transported in sterile bags cooled on ice. On arrival at our center and during the priming of the machine perfusion system the donor liver was prepared for connection to the system. The portal vein and hepatic branches of the celiac trunk and/or superior mesenteric artery were dissected free. The portal vein was cannulated distally with a section of tubing (Masterflex 24 L/S, Cole Palmer, Vernon Hills, IL). The aortic segment was opened and the celiac trunk was cannulated at the origin with a vessel cannula (Medtronic, Minneapolis, MN). Other branches of the celiac trunk were tied using 0 silk sutures. The cystic duct was ligated and the gallbladder flushed of residual bile. The common bile duct was cannulated with a vessel cannula, which was then connected to a section of tubing to allow bile collection. All cannulae were secured using 0 silk sutures.
Approval was obtained from the NEOB for the perfusion of discarded human donor livers and this study was declared exempt by the Massachusetts General Hospital (MGH) institutional review board (IRB # 2011P001496).
Ex vivo Subnormothermic Machine Perfusion
The subnormothermic perfusion system consisted of two independent circulations for portal and arterial perfusion, each including a roller pump (Masterflex L/S, Cole Palmer), hollow-fiber oxygenator (Affinity NT, Medtronic, Minneapolis, MN), and a bubble trap (Radnoti, Monrovia, CA) (Figure 1). Sensors allowed for continuous measurement of pressure on the inflow vessels. The livers were perfused with phenol-red Williams’ medium E (Sigma-Aldrich, St. Louis, MO, USA), supplemented with insulin (5 U/L Humulin R; Eli Lilly & Co, Indianapolis, IN, USA), penicillin/streptomycin (40,000 U/L / 40,000 μg/L) (Gibco/Invitrogen, Camarillo, CA, USA), and 10 mg/L hydrocortisone (Solu-Cortef, Pharmacia & Upjohn, Kalamazoo, MI). The complete composition can be found in Table S1. Additional sodium bicarbonate (8.4%) was added to correct a drop in pH after introducing the liver to the system. The solution was oxygenated and buffered with a carbogen mixture of 95%O2 / 5%CO2, achieving maximum partial oxygen pressure of >700mmHg and undepleted oxygen outflow (>200 mmHg).
Figure 1. Subnormothermic machine perfusion system.
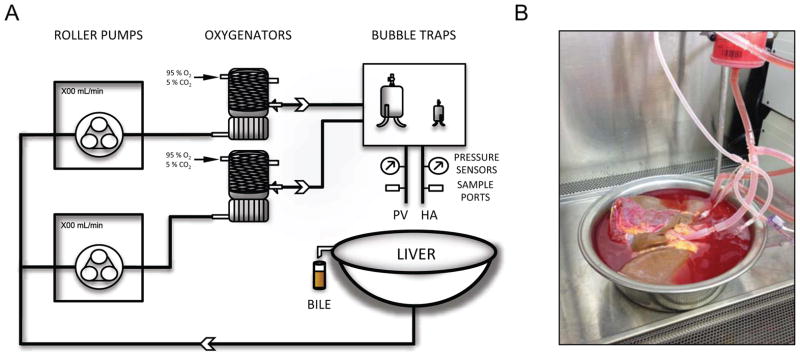
Schematic representation (A) and actual photo (B) of a liver in the system.
Immediately before connection to the machine perfusion system the donor liver was flushed with 2 L of cold 0.9% NaCl solution through the portal vein and 1 L through the artery. Prior to and following machine perfusion the liver was weighed. The liver was gradually warmed to room temperature (21 °C) over the first hour of perfusion. Donor livers were all perfused for 3 hours, a duration chosen following our animal studies that demonstrate full reconstitution of tissue ATP content before 3 hours of perfusion (18). Flow was regulated manually to achieve a target pressure of 4–7 mmHg over the portal vein and 50–80 mmHg on the artery. Resistance was calculated throughout perfusion as the quotient of pressure and flow.
Hepatocellular and biliary function and injury
Perfusate samples were taken frequently in the first half hour and every half hour thereafter. To assess tissue damage, alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) were analyzed. ALT was analyzed using an Infinity liquid stable reagent (Cellomics/Thermo Electron, Pittsburgh, PA, USA). LDH activity was quantified using a colorimetric assay based on the conversion on NAD+ to NADH (BioVision, Inc, Milpitas, CA, USA). Urea and albumin production were measured to assess metabolic and synthetic function. Urea was assayed enzymatically (Cell Biolabs, San Diego, CA), and albumin was detected using a BCG-based assay (QuantiChrom, BioAssay Systems, Hayward, CA). Samples were drawn from the portal venous and arterial inflow and from the caval outflow for half-hourly blood gas analysis (BGA)(Rapidpoint 500, Siemens). BGA included electrolyte (Na+, K+, Ca2+, Cl−), lactate and glucose concentrations, and acid/base physiology (pH, pO2, pCO2, HCO3-, base excess). Oxygen uptake rate (OUR) was calculated by subtracting the oxygen outflow from the combined inflow through the portal vein and hepatic artery (OUR= (pvO2IN +haO2IN) − O2OUT). The oxygen concentration in each was derived using Henry’s law, CdO2 = aO2 x PO2, where CdO2 is the concentration of dissolved oxygen, aO2 is the solubility coefficient for oxygen (0.00314 mL O2/ mmHg O2 / dL) and P02 is the partial oxygen pressure. pvO2IN/haO2IN and O2OUT were calculated by multiplying the O2 concentration by the flow rate, and finally OUR was divided by the weight of the liver.
Wedge biopsies were taken for analysis of tissue ATP content. Prior to homogenization of the tissue, biopsies were pulverized in liquid nitrogen. Pulverized tissue was subsequently analyzed for ATP content using a luminescence-based cell viability assay (BioVision). ATP content was normalized to protein content, measured spectrophotometrically after the reaction with Coomassie dye.
Bile was collected throughout and quantified at 1-hour intervals. Alkaline phosphatase (ALP) was measured fluorometrically in the perfusate as a marker of biliary and hepatocellular injury (BioVision). Phospholipids (lecithin, lysolecithin, sphingomyelin) were analyzed in bile using the choline oxidase – DAOS method and measured calorimetrically (Wako Diagnostics, Richmond, VA). Total bile acids in bile were measured colorimetrically using a commercially available enzymatic kit (BioQuant, San Diego, CA). Bile samples were collected under mineral oil to prevent atmospheric equilibration and allow evaluation of bicarbonate and pH of the bile sample.
Histology
Liver parenchyma biopsies were fixed in 10% formalin and transferred to 70% ethylalcohol until processing for light microscopy. After paraffin embedding, samples were sectioned and stained with hematoxylin and eosin (H&E). Samples were assessed by a blinded transplantation pathologist (RNS).
Statistical analysis
Data were analyzed using Prism 5.0a for Mac OS X (GraphPad software, Inc., La Jolla, CA). Data was analyzed for normality using the Kolmogorov-Smirnov normality test. After confirming normality (α =0.05), repeated measures ANOVA with Tukey post-tests were used to analyze time-course parameters. Correlation between two continuous variables was analyzed by simple linear regression, using a t-test to determine whether the regression coefficient differed from 0. Statistical difference between high and low ATP groups were analyzed using a Mann–Whitney U test for total perfusate release. Results are presented as median (upper – lower quartile). A p-value <0.05 was considered significant.
Results
Donor and preservation parameters
Seven consecutively perfused discarded human livers were included in this study, 5 of which were DCD, with an average relative WIT of 28 (23–34) minutes (Table 1). Livers were discarded on the basis of prolonged WIT, donor age > 45 years in DCD donors, high macrovesicular steatosis, and inability to allocate. As minimal dissection of the vasculature was performed on procurement, an average of 70 (63–87) minutes was needed to prepare the liver for perfusion, resulting in a mean cold ischemic time of 685 (473–871) minutes.
Table 1.
Donor and graft characteristics
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|
| Donation type (DCD/DBD) | DCD | DCD | DCD | DCD | DBD | DBD | DCD | |
| Primary reason for discard | WIT > 30 min | DCD & Pre- operative LFTs | DCD & Donor age | DCD & Donor age | Donor age | Steatosis | DCD & Donor age | |
| Gender | male | male | male | female | male | male | male | |
| Age (yrs) | 50 | 25 | 50 | 62 | 75 | 24 | 51 | |
| BMI | 24.6 | 26.7 | 25.8 | 30.18 | 26 | 52.9 | 26.5 | |
| Cause of | Anoxia | Anoxia | Head trauma | Resp. failure | Head trauma | Anoxia | Anoxia | |
| Mechanism death of death | Cardio- vascular | Asphyxiation | Blunt injury | Natural causes | Blunt injury | Cardio- vascular | Cardio- vascular | |
| Ischemia | relative WIT | 54 min | 23 min | 28 min | 34 min | N/A | N/A | 20 min |
| absolute WIT | 10 min | 11 min | 10 min | 18 min | N/A | N/A | 11 min | |
| CIT | 1157 min | 1002 min | 296 min | 740 min | 685 min | 420 min | 525 min | |
| Macrosteatosis | < 10% | < 10% | < 10% | < 10% | 20–30% | 80% | < 10% | |
DCD, donation after circulatory death; DBD, donation after brain death; WIT, warm ischemia time; CIT, cold ischemia time; LFT, liver function test
Perfusion and metabolic parameters during SNMP
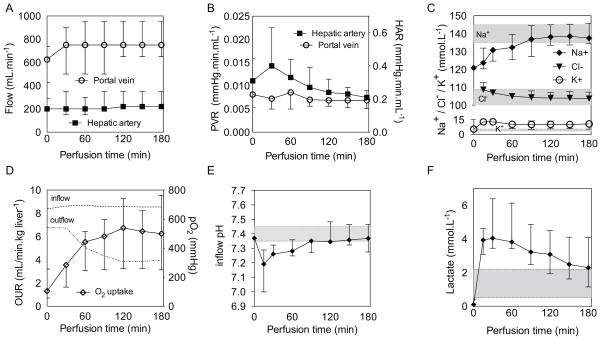
Once connected to the perfusion system the donor livers warmed up gradually, reaching ambient room air temperature of 20.8 ± 1.0 °C after approximately 60 minutes. Pressures of 5.8 (4.8–6.2) mmHg on the portal vein and 56 (47–61) mmHg on the artery were maintained by adjusting the flow rates, resulting in an average flow rate of 767 (683–833) mL/min and 206 ± (195–311) mL/min, respectively (Figure 2a). Portal resistance was constant throughout perfusion, while the arterial resistance increased in the first 30 minutes and began to decrease (Figure 2b). No significant weight change (−0.89 [−5.3– −2.1]) was observed between the beginning and end of perfusion (p=0.9). Sodium and chloride ion concentrations were in the normal range after 90 and 30 minutes respectively and ended at 137.5 (135.2–143.6) mmol/L and 103.7 (101.2–106.5) mmol/L, while the potassium ion concentration increased initially from a baseline of 5.36 to 12.9 (11.7–12.8) mmol/L and subsequently decreased slightly, and then remained steady and ended at 10.7 (7.25–12.7) mmol/L (Figure 2c).
Figure 2. Perfusion and metabolic dynamics.
Development of arterial and portal flow (A) and hepatic arterial (HAR) and portal venous resistance (PVR) (B) during SNMP. Perfusate sodium, potassium and chloride concentrations during SNMP (C). Oxygen uptake rate (OUR) and partial oxygen pressure in the inflow and outflow perfusate (D). pH of the inflow perfusate (E). Lactate concentration in the perfusate during SNMP (F) Shaded areas represent normal electrolyte ranges in blood. Data presented as median and interquartile range (IQR).
Oxygen uptake was immediate and continued to increase during the first 2 hours and plateaued thereafter (1.30 [1.11–1.94] to 6.74 [4.15–8.16] mL O2/min.kg liver−1; p<0.0001)(Figure 2d). Partial oxygen outflow pressure did not fall below 200 mmHg. In all cases an initial drop in pH was observed after 15 minutes, but overall pH returned to within the normal reference range by the second hour (Figure 2e). Bicarbonate levels increased from 25.4 (24.75–26.04) mmol/L to 37.0 (33.3–39.5) mmol/L. Glucose increased during the first 90 minutes and plateaued (final of 341.3 (270.5–490.5) mg/dL). Lactate levels decreased to near normal levels (2.29 [1.20–3.43] mmol/L) after peaking at 30 minutes (4.04 [3.70–5.99] mmol/L)(Figure 2f).
Liver function and injury during SNMP
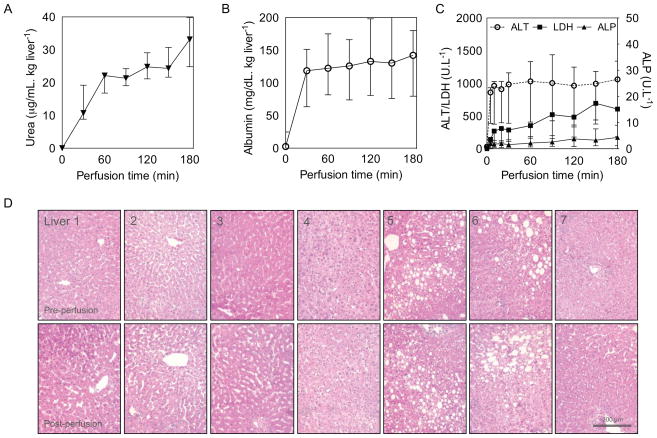
Urea production from amino acids in the hepatic urea cycle was constant and totaled 33.12 (27.01–36.79) μg/mL.kg liver−1 at the end of perfusion(Figure 3a). Total albumin secreted into the perfusate was 142.2 (175.0–107.4) mg/dL.kg liver−1, with a peak output in the first 30 minutes, followed by a moderate rate of secretion thereafter (189.7 [151.8–293.4] mg/kg liver.h−1) (Figure 3b). ALT, LDH and ALP were measured as markers of hepatic and biliary injury (Figure 3c). Substantial ALT release was limited to the first 20 minutes of perfusion and did not increase significantly thereafter, ending at 1062.1 (750.61–1062.4) U/L. Similarly, only a moderate increase in LDH and ALP was observed. ALP increased mildly during perfusion and most steeply to 2.46 (0.78–4.2) U/L in the first hour.
Figure 3. Hepatic function and injury.
Urea production as a biochemical marker of clearance (A), albumin as a synthetic marker (B) and alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) as markers of injury (C) and representative H&E photomicrographs of liver biopsies of the 7 perfused livers, before and after perfusion (D). Data presented as median and IQR.
Histology
Liver biopsies stained with H&E did not show any difference between pre- and post-perfusion histology (Figure 3d). Notably, normal hepatocyte morphology was preserved and no evidence of injury to the sinusoidal endothelium was seen after SNMP. Pre-perfusion biopsies were consistent with donor characteristics including mild to severe steatosis in livers 5 & 6.
Liver energy status (ATP)
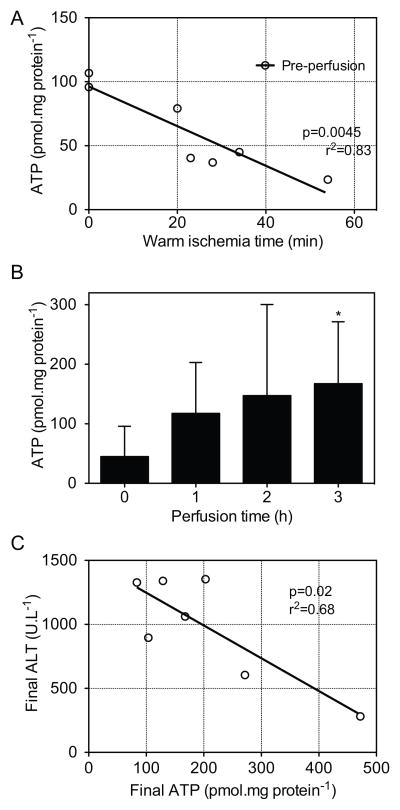
Tissue ATP content at the end of cold storage correlated negatively with relative WIT (p=0.0045, r2=0.83; Figure 4a). ATP increased significantly from 45.0 (70.6–87.5) pmol/mg protein to 167.5 (151.5–237.2) pmol/mg protein (Figure 4b). By the end of machine perfusion a 3.7-fold increase in ATP was observed overall (p=0.022). ATP content was correlated to various non-invasive, donor or real-time parameters to determine whether these could be used as surrogates for invasive ATP analysis. ATP recovery was variable between livers (Figure S1); the tissue ATP content increased to >200 pmol/mg in three of the seven donor livers (Table 2). These livers had significantly higher oxygen uptake (p=0.009) than the other donor livers. Additionally, mean LDH, ALP, ALT, lactate, and relative WIT were lower in these high ATP livers compared to the low ATP livers, although this difference was not statistically significant. ATP at the end of perfusion correlated negatively with final ALT values (p=0.02, r2=0.68; Figure 4c)
Figure 4. Adenosine triphosphate.
Scatter plot of tissue ATP content at the end of cold storage (pre-perfusion) correlated to relative warm ischemic time (A), tissue ATP content per hour of perfusion (B) and ATP content at the end of perfusion correlated to ALT release (C). Data presented as median and IQR. * indicates significantly higher than baseline.
Table 2.
Comparison between high and low recovered ATP grafts
| High ATP* | Low ATP* | p-value | |
|---|---|---|---|
| n=3 | n=4 | ||
| Donor age (years) | 51 (38–63) | 50 (44–53) | 0.59 |
| Oxygen uptake (mL.kg liver−1) | 1204 (1129–1282)* | 564.5 (404–760)* | 0.009 |
| Mean arterial resistance (mmHg.min.mL−1) | 0.42 (0.29–0.44) | 0.26 (0.21–0.31) | 0.63 |
| Mean portal resistance (x10−3 mmHg.min.mL−1) | 5.7 (5.4–7.5) | 8.2 (6.9–9. 9) | 0.86 |
| Peak lactate (mmol/L) | 3.95 (3.70–4.00) | 6.10 (5.06–6.66) | 0.4 |
| ALT release (U/L) | 604.7 (443.2–979.2) | 1194.9 (1020.7–1330.9) | 0.62 |
| ALP release (U/L) | 1.32 (1.25–5.16) | 5.12 (3.38–7.18) | 0.86 |
| LDH release (U/L) | 190.0 (173.3–535.0) | 660 (526.7–1018) | 0.4 |
| Urea output (μg/mL. kg liver−1) | 33.12 (31.2–47.5) | 29.3 (23.7–35.3) | 0.62 |
| Albumin output (mg/dL. kg liver−1) | 135.5 (94.1–138.9) | 175.0 (147.3–190.0) | 0.23 |
| relative WIT (min) | 20 (20–22) | 31 (31–44) | 0.28 |
| CIT (hours) | 11.4 (10.1–14.1) | 9.7 (6.5–14.1) | 0.86 |
High (>200 pmol/mg protein) and low (<200 pmol/mg protein) ATP content post-perfusion; AUC= area under the curve.
area under curve
Biliary function
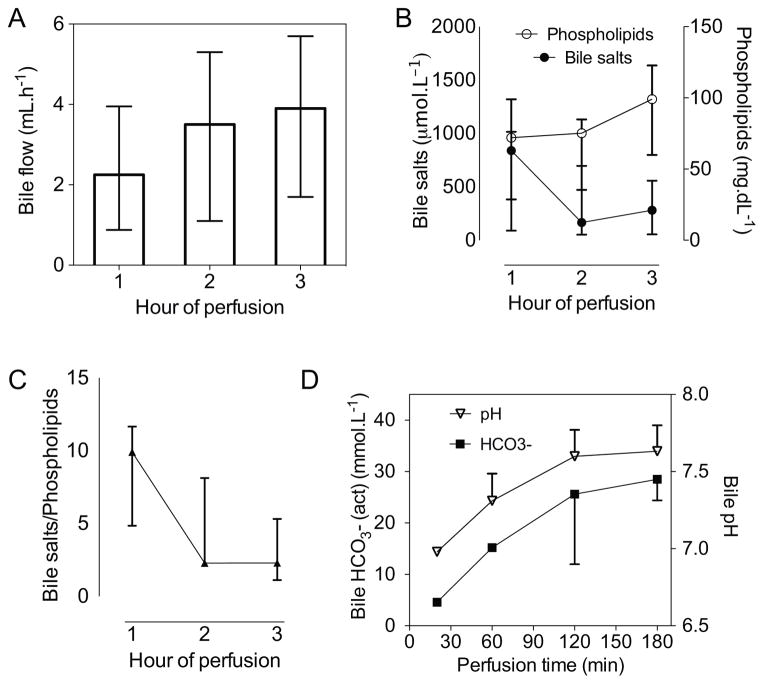
Bile flow generally started within the first 30 minutes of perfusion. Total bile production in the first hour was 2.25 (0.9–2.5) mL, which increased to 3.9 (2.4–5.1) mL in the final hour (Figure 5a). Absence of an enterohepatic circulation of bile salts resulted in a decrease in biliary bile salt concentration from the first hour (Figure 5b). Total phospholipid concentration increased throughout perfusion, which resulted in a lower and less toxic bile salt to phospholipid ratio (Figure 5c). The secretion of cytoprotective bicarbonate increased favorably, which also raised the pH from 6.9 early in perfusion to a more alkaline pH of 7.6 at the end of perfusion (Figure 5d).
Figure 5. Biliary parameters.
Bile flow measured for every hour of perfusion (A), the concentration of bile salts and phospholipids in the bile (B), as well as the bile salt to phospholipid ratio (C), and the bicarbonate concentration and pH of bile during perfusion (D). Data presented as median and IQR.
Discussion
In this preliminary study we demonstrate that human livers can be supported ex vivo by subnormothermic machine perfusion (SNMP) at room temperature. During this ex vivo period the liver is metabolically active, demonstrating synthetic and clearance function, while incurring minimal additional injury. Furthermore, this study shows that subnormothermic machine perfusion can improve various functional and biochemical parameters after a period of extensive warm and cold ischemia, including a significant improvement in oxygen uptake, bile production and tissue ATP content.
SNMP likely operates through a number of cooperative mechanisms. Restoration of mitochondrial respiratory function and reversal of energy deficits have been shown to be pivotal in restoring liver viability (20,21). Mitochondria play a key role in I/R injury through their role in reactive oxygen species (ROS) formation and the induction of apoptosis (22,23). In this work, particular attention has been paid to improvement of the energy balance of the liver. Impairment of oxidative phosphorylation during warm hypoxia leads to a rapid dephosphorylation of ATP, which is only exacerbated during hypothermic preservation (18,24). As a result the ischemic liver is poorly prepared for warm reperfusion and the oxidative and inflammatory stress that follows. Improvement of mitochondrial condition, reflected here by augmentation of tissue ATP content, an increasing consumption of oxygen, and decreasing lactate levels during SNMP, may result in the improved tolerance to oxidative stress seen on reperfusion in animal studies (25). Recovered levels of ATP appear to be a good indicator of mitochondrial respiratory function, as we see that high ATP content at the end of SNMP is correlated to higher oxygen uptake and lower lactate levels during perfusion. Moreover, the ability of the liver to recover ATP seems to depend on the severity of the injury sustained during ischemia. Livers with a poor recovery of ATP were subjected to a longer WIT and showed worse LFTs during SNMP. Conversely, clinical studies have shown that pre-implantation ATP content correlates to liver failure (26) and post-reperfusion ATP recovery to post-transplant function (27). In various animal studies we have demonstrated that ATP content correlates to the number viable cells (28,29) and we have found that post-perfusion ATP content is highly suggestive of outcome in transplant models (18,30).
Additional mechanisms of action may include the normalization of ion and metabolite balances through equilibration with the perfusion solution, which produces a more adequate environment for warm reperfusion. Microcirculatory obstructions, originating from either microthrombi or cellular edema, may be of underappreciated importance and prevent adequate tissue perfusion. Improving sinusoidal conductance prior to transplantation increases the rate of graft recovery (31).
Discussions concerning the optimum phase during the ex vivo period for the application of machine perfusion are ongoing (32). Various groups have considered long perfusion times, aiming to replace static cold preservation entirely and have shown success (33–35). It has been shown in a porcine model that even a short period of cold preservation prior to warm perfusion has adverse effects on function and injury (24,36) of the liver, suggesting that perfusion may benefit from immediate initiation on retrieval. Warm perfusion that spans the transport period has been approached with rightful trepidation, namely considering the risk of equipment failure. Moreover, resource and personnel demands of long-term machine perfusion would make implementation of preservation-spanning perfusions arduous. However, new devices are gaining support and may play a role in the near future (37). Post-procurement machine perfusion, particularly in the case of DCD livers, offers a recovery phase prior to cold preservation that may improve tolerance to extended cold ischemia (38). In this work and previous studies we employ SNMP with the aim of improving the final quality of the liver pre-implantation and, importantly, to allow for the most representative assessment of liver viability. We have previously shown that ATP can be recovered to baseline levels after three hours of perfusion, a duration that is compatible with the logistics of transplantation (18). Learning from clinical experience, various parameters in perfusion can be considered as indicators of liver function or injury and include standard LFTs, bile production, and other hepatic synthetic functions (39,40). Detailed analyses of metabolism can be used to provide accurate criteria for liver viability. By applying dynamic discriminate analyses, Perk et al. have identified metabolites measured during perfusion that discriminate between warm ischemic and fresh livers with a very high specificity (12). Moreover, using easily measured metabolites like glucose, urea, and lactate, indices for transplantation can be constructed that predict transplant success with equally high specificity (13).
Although the importance of preserving and assessing hepatocellular viability is indisputable, progress towards expanding the current donor pool cannot occur without advances in biliary preservation. Bile duct complications remain a limiting factor in the use of DCD livers, with particularly high incidences of non-anastomotic biliary strictures (41). With ischemia as the primary risk factor for biliary complications, SNMP may provide an elegant solution, minimizing further injury and providing a platform for treatment. Secondly, bile salt toxicity has been proposed as a factor in bile duct injury. A compositional change in bile salt to phospholipid ratio has been associated with bile duct injury in clinical studies (42,43). Moreover, a shift to dominantly toxic, hydrophobic bile salts may play an important role (44). It may prove beneficial to supplement the depletion of bile salts observed in this and other work with protective hydrophilic bile salts (45). SNMP and other warm machine perfusion systems have already proven advantageous in reducing markers of biliary injury during preservation and restoring normal biliary physiology (14,46). In this work we confirm machine perfusion’s ability to rectify various compositional disturbances in bile. Moreover, SNMP results in a less toxic and more protective composition of bile through changes in bile salt, phospholipid, and bicarbonate concentrations (47,48).
The precise constituents and setting for optimal machine perfusion of the liver remains uncertain. While simplicity and minimal risk have driven the development of hypothermic systems, normothermic machine perfusion has sparked interest in the prospect of an ex vivo organ with close to normal physiology that opens up the opportunity for intervention, recovery, and viability testing (14,49). The complexity and associated risk have delayed clinical implementation and have even veered groups away. In developing this SNMP system, emphasis has been laid on simplicity while maintaining the goal of developing an effective system for liver recovery and assessment. SNMP at room temperature offers a technical advantage by obviating the need for temperature control, and the reduced metabolic state in turn obviated the need for oxygen carriers in our system (18,50). With regards to efficacy, SNMP has already shown excellent results in both hepatic and biliary preservation.
The effect of SNMP on post-transplant outcome is not determined in this study, as it was not tested in a transplantation or oxygenated whole blood reperfusion model. Future controlled studies that include post-perfusion evaluation are required to determine whether the improved parameters observed in this work translate to the better post-transplant outcome that we have observed in animal models. In this feasibility study we demonstrate that SNMP effectively supports the human liver ex vivo with minimal injury, stable if not improved metabolic activity, and normalized physiological disturbances post-ischemia. Moreover, SNMP provides an environment for restoring the hepatic energy status, reflecting a recovery of mitochondrial function. This work is the first demonstration of the capacity of SNMP to sustain human livers and supports previous experimental work that suggest a pivotal role of machine perfusion in expanding the use of currently discarded livers for transplantation.
Supplementary Material
Acknowledgments
We would like to gratefully acknowledge the New England Organ Bank and the surgical procurement teams for supporting this work through the supply of discarded livers and commendable coordination. We are also grateful to the perfusionists at MGH for their support. Funding from the National Institutes of Health (R00DK080942, R01DK096075, R01EB008678), and the Shriners Hospitals for Children are gratefully acknowledged.
Abbreviations
- DCD
donation after circulatory death
- WIT
warm ischemia time
- SCS
static cold storage
- HMP
hypothermic machine perfusion
- NMP
normothermic machine perfusion
- ATP
adenosine triphosphate
- SNMP
subnormothermic machine perfusion
- NEOB
New England Organ Bank
- UW
University of Wisconsin
- PV
portal vein
- HA
hepatic artery
- ALT
alanine aminotransferase
- LDH
lactate dehydrogenase
- BGA
blood gas analysis
- ALP
alkaline phosphatase
- H&E
hematoxylin and eosin
- OUR
oxygen uptake rate
- I/R
ischemia/reperfusion
- ROS
reactive oxygen species
- LFT
liver function test
- PVR
portal vein resistance
- HAR
hepatic artery resistance
- CIT
cold ischemia time
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Drs. Izamis, Uygun and Yarmush are inventors on a pending patent that is relevant to this study: WO/2011/002926 and Drs. Berendsen, Izamis, Uygun and Yarmush are inventors on a pending patent that is relevant to this study: WO/2011/35223.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232(4):490. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: A matched case-control study. Ann Surg. 2007 Dec;246(6):940–6. doi: 10.1097/SLA.0b013e31815c2a3f. discussion 946–8. [DOI] [PubMed] [Google Scholar]
- 3.Moore DE, Feurer ID, Speroff T, Gorden DL, Wright JK, Chari RS, Pinson CW. Impact of donor, technical, and recipient risk factors on survival and quality of life after liver transplantation. Arch Surg. 2005 Mar;140(3):273–7. doi: 10.1001/archsurg.140.3.273. [DOI] [PubMed] [Google Scholar]
- 4.de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, et al. Liver transplantation using donation after cardiac death donors: Long-term follow-up from a single center. Am J Transplant. 2009 Apr;9(4):773–81. doi: 10.1111/j.1600-6143.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- 5.de Rougemont O, Breitenstein S, Leskosek B, Weber A, Graf R, Clavien PA, Dutkowski P. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009 Nov;250(5):674–83. doi: 10.1097/SLA.0b013e3181bcb1ee. [DOI] [PubMed] [Google Scholar]
- 6.Bessems M, Doorschodt BM, van Marle J, Vreeling H, Meijer AJ, van Gulik TM. Improved machine perfusion preservation of the non-heart-beating donor rat liver using polysol: A new machine perfusion preservation solution. Liver Transpl. 2005 Nov;11(11):1379–88. doi: 10.1002/lt.20502. [DOI] [PubMed] [Google Scholar]
- 7.Monbaliu M, Liu L, Libbrecht L, De Vos DV, Vekemans V, Debbaut D, et al. Preserving morphology and evaluating quality of liver grafts by hypothermic machine perfusion, a proof of concept study using discarded human livers. Liver Transpl. 2012 Sep 17; doi: 10.1002/lt.23550. [DOI] [PubMed] [Google Scholar]
- 8.Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 2013 Jun 29; doi: 10.1016/j.jhep.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant. 2010 Feb;10(2):372–81. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 10.Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2013 Nov 29; doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Berendsen T, Kim K, Soto-Gutiérrez A, Bertheium F, Yarmush ML, Hertl M. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J Surg Res. 2011 Oct 24; doi: 10.1016/j.jss.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perk S, Izamis ML, Tolboom H, Uygun B, Berthiaume F, Yarmush ML, Uygun K. A metabolic index of ischemic injury for perfusion-recovery of cadaveric rat livers. PLoS One. 2011;6(12):e28518. doi: 10.1371/journal.pone.0028518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perk S, Izamis ML, Tolboom H, Uygun B, Yarmush ML, Uygun K. A fitness index for transplantation of machine-perfused cadaveric rat livers. BMC Res Notes. 2012;5:325. doi: 10.1186/1756-0500-5-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MW, Gouw AS, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013 Mar 6; doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 15.Minor T, Efferz P, Fox M, Wohlschlaeger J, Lüer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013 Apr 25; doi: 10.1111/ajt.12235. [DOI] [PubMed] [Google Scholar]
- 16.Shigeta T, Matsuno N, Obara H, Kanazawa H, Tanaka H, Fukuda A, et al. Impact of rewarming preservation by continuous machine perfusion: Improved post-transplant recovery in pigs. Transplant Proc. 2013 Jun;45(5):1684–9. doi: 10.1016/j.transproceed.2013.01.098. [DOI] [PubMed] [Google Scholar]
- 17.Vajdová K, Graf R, Clavien PA. ATP-supplies in the cold-preserved liver: A long-neglected factor of organ viability. Hepatology. 2002 Dec;36(6):1543–52. doi: 10.1053/jhep.2002.37189. [DOI] [PubMed] [Google Scholar]
- 18.Berendsen TA, Bruinsma BG, Lee J, D'Andrea V, Liu Q, Izamis ML, et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res. 2012;1(1):6. doi: 10.1186/2047-1440-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolboom H, Pouw R, Uygun K, Tanimura Y, Izamis ML, Berthiaume F, Yarmush ML. A model for normothermic preservation of the rat liver. Tissue Eng. 2007 Aug;13(8):2143–51. doi: 10.1089/ten.2007.0101. [DOI] [PubMed] [Google Scholar]
- 20.Lüer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010 Sep;23(9):944–50. doi: 10.1111/j.1432-2277.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SJ, Churchill TA, Winslet MC, Fuller BJ. Energy metabolism following prolonged hepatic cold preservation: Benefits of interrupted hypoxia on the adenine nucleotide pool in rat liver. Cryobiology. 1999 Sep;39(2):130–7. doi: 10.1006/cryo.1999.2191. [DOI] [PubMed] [Google Scholar]
- 22.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004 Aug 13;279(33):34682–90. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 23.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 24.Reddy SP, Bhattacharjya S, Maniakin N, Greenwood J, Guerreiro D, Hughes D, et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004 May 15;77(9):1328–32. doi: 10.1097/01.tp.0000119206.63326.56. [DOI] [PubMed] [Google Scholar]
- 25.Ferrigno A, Rizzo V, Boncompagni E, Bianchi A, Gringeri E, Neri D, et al. Machine perfusion at 20°C reduces preservation damage to livers from non-heart beating donors. Cryobiology. 2011 Apr;62(2):152–8. doi: 10.1016/j.cryobiol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Lanir A, Jenkins RL, Caldwell C, Lee RG, Khettry U, Clouse ME. Hepatic transplantation survival: Correlation with adenine nucleotide level in donor liver. Hepatology. 1988;8(3):471–5. doi: 10.1002/hep.1840080306. [DOI] [PubMed] [Google Scholar]
- 27.Kamiike W, Burdelski M, Steinhoff G, Ringe B, Lauchart W, Pichlmayr R. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988 Jan;45(1):138–43. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Usta OB, He X, Kim Y, Ozer S, Bruinsma BG, Lee J, et al. Supercooling as a viable non-freezing cell preservation method of rat hepatocytes. PLoS One. 2013 Jul 16;8(7):e69334. doi: 10.1371/journal.pone.0069334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berendsen TA, Izamis ML, Xu H, Liu Q, Hertl M, Berthiaume F, et al. Hepatocyte viability and adenosine triphosphate content decrease linearly over time during conventional cold storage of rat liver grafts. Transplant Proc. 2011 Jun;43(5):1484–8. doi: 10.1016/j.transproceed.2010.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruinsma BG, Berendsen TA, Izamis ML, Yarmush ML, Uygun K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int J Artif Organs. 2013;36(11):775–80. doi: 10.5301/ijao.5000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minor T, Tolba R, Neumann S, Schulz S, Sitzia M. Fibrinolysis in organ procurement for transplantation after cardiocirculatory compromise. Thromb Haemost. 2003 Aug;90(2):361–2. [PubMed] [Google Scholar]
- 32.Koetting M, Minor T. Donation after cardiac death: Dynamic graft reconditioning during or after ischemic preservation? Artif Organs. 2011 Jun;35(6):565–71. doi: 10.1111/j.1525-1594.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 33.St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heartbeating donor livers with normothermic machine perfusion. Br J Surg. 2002 May;89(5):609–16. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 34.Butler AJ, Rees MA, Wight DGD, Casey ND, Alexander G, White DJG, Friend PJ. Successful extracorporeal porcine liver perfusion for 72 hr1. Transplantation. 2002;73(8):1212. doi: 10.1097/00007890-200204270-00005. [DOI] [PubMed] [Google Scholar]
- 35.Bessems M, Doorschodt BM, Dinant S, de Graaf W, van Gulik TM. Machine perfusion preservation of the pig liver using a new preservation solution, polysol. Transplant Proc. 2006 Jun;38(5):1238–42. doi: 10.1016/j.transproceed.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 36.Reddy S, Greenwood J, Maniakin N, Bhattacharjya S, Zilvetti M, Brockmann J, et al. Non-heart-beating donor porcine livers: The adverse effect of cooling. Liver Transpl. 2005 Jan;11(1):35–8. doi: 10.1002/lt.20287. [DOI] [PubMed] [Google Scholar]
- 37.van der Plaats A, Maathuis MH, 'T Hart NA, Bellekom AA, Hofker HS, van der Houwen EB, et al. The groningen hypothermic liver perfusion pump: Functional evaluation of a new machine perfusion system. Ann Biomed Eng. 2006 Dec;34(12):1924–34. doi: 10.1007/s10439-006-9207-4. [DOI] [PubMed] [Google Scholar]
- 38.Miyagi S, Iwane T, Akamatsu Y, Nakamura A, Sato A, Satomi S. The significance of preserving the energy status and microcirculation in liver grafts from non-heart-beating donor. Cell Transplant. 2008;17(1–2):173–8. doi: 10.3727/000000008783906874. [DOI] [PubMed] [Google Scholar]
- 39.Friend PJ, Imber C, St Peter S, Lopez I, Butler AJ, Rees MA. Normothermic perfusion of the isolated liver. Transplant Proc. 2001;33(7–8):3436–8. doi: 10.1016/s0041-1345(01)02481-2. [DOI] [PubMed] [Google Scholar]
- 40.Vajdová K, Smreková R, Mislanová C, Kukan M, Lutterová M. Cold-preservation-induced sensitivity of rat hepatocyte function to rewarming injury and its prevention by short-term reperfusion. Hepatology. 2000 Aug;32(2):289–96. doi: 10.1053/jhep.2000.8895. [DOI] [PubMed] [Google Scholar]
- 41.Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: A review. Dig Surg. 2008;25(4):245–57. doi: 10.1159/000144653. [DOI] [PubMed] [Google Scholar]
- 42.Geuken E, Visser D, Kuipers F, Blokzijl H, Leuvenink HG, de Jong KP, et al. Rapid increase of bile salt secretion is associated with bile duct injury after human liver transplantation. J Hepatol. 2004 Dec;41(6):1017–25. doi: 10.1016/j.jhep.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Buis CI, Geuken E, Visser DS, Kuipers F, Haagsma EB, Verkade HJ, Porte RJ. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009 Jan;50(1):69–79. doi: 10.1016/j.jhep.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 44.Hertl M, Harvey PR, Swanson PE, West DD, Howard TK, Shenoy S, Strasberg SM. Evidence of preservation injury to bile ducts by bile salts in the pig and its prevention by infusions of hydrophilic bile salts. Hepatology. 1995 Apr;21(4):1130–7. [PubMed] [Google Scholar]
- 45.Imber CJ, St Peter SD, de Cenarruzabeitia IL, Lemonde H, Rees M, Butler A, et al. Optimisation of bile production during normothermic preservation of porcine livers. Am J Transplant. 2002 Aug;2(7):593–9. doi: 10.1034/j.1600-6143.2002.20703.x. [DOI] [PubMed] [Google Scholar]
- 46.Vairetti M, Ferrigno A, Rizzo V, Boncompagni E, Carraro A, Gringeri E, et al. Correlation between the liver temperature employed during machine perfusion and reperfusion damage: Role of ca2+ Liver Transpl. 2008 Apr;14(4):494–503. doi: 10.1002/lt.21421. [DOI] [PubMed] [Google Scholar]
- 47.Hoekstra H, Porte RJ, Tian Y, Jochum W, Stieger B, Moritz W, et al. Bile salt toxicity aggravates cold ischemic injury of bile ducts after liver transplantation in mdr2+/− mice. Hepatology. 2006 May;43(5):1022–31. doi: 10.1002/hep.21169. [DOI] [PubMed] [Google Scholar]
- 48.Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, Beuers U. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012 Jan;55(1):173–83. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 49.Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, et al. Normothermic perfusion: A new paradigm for organ preservation. Ann Surg. 2009 Jul;250(1):1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 50.Tolboom H, Izamis ML, Sharma N, Milwid JM, Uygun B, Berthiaume F, et al. Subnormothermic machine perfusion at both 20°C and 30°C recovers ischemic rat livers for successful transplantation. J Surg Res. 2011 Mar 29; doi: 10.1016/j.jss.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.