Abstract
Purpose
Cisplatin-based combination chemotherapy is considered standard treatment for advanced/recurrent cervical carcinoma; however, the majority of patients do not respond. This study was undertaken to identify the prognostic factors and develop a model predictive of (non-) response to chemotherapy.
Methods
Four-hundred twenty-eight patients with advanced cervical cancer who received a cisplatin-containing combination in three Gynecologic Oncology Group (GOG) protocols (110, 169 and 179) were evaluated for baseline clinical characteristics and multivariate analysis was conducted to identify factors independently prognostic predictive of response using a Logistic regression model. A predictive model was developed and externally validated using an independent GOG protocol (149) data.
Results
Multivariate analysis identified five factors (African-American, performance status [PS] > 0, pelvic disease, prior radiosensitizer and time interval from diagnosis to first recurrence ≤ one year) independently prognostic of poor response. A simple prognostic index was derived based on the total number of risk factors. When patients were classified into three risk groups (low risk: 0–1 factor; mid risk: 2–3 factors; high risk: 4–5 factors), patients with 4–5 risk factors were estimated to have a response rate of only 13%, and median progression-free and overall survival of 2.8 months and 5.5 months, respectively. The accuracy of the index was supported by both internal and external datasets.
Conclusions
A simple index based on five prognostic factors may have utility in clinical practice to identify the women who are not likely to respond to the cisplatin-containing regimens. This subgroup of patients should be considered for non-cisplatin chemotherapy or investigational trials.
Keywords: cervical cancer, cisplatin, tumor response, prognostic factor
INTRODUCTION
In 2008 there will be an estimated 11,070 new cases of invasive cervical cancer in the United States. Although most cases will be cured with treatments based on surgery or radiation therapy, 3870 women will eventually succumb to this disease.1 When cervical cancer is beyond curative treatment with surgery or radiation therapy, the prognosis is poor and palliation is the primary objective.
For over three decades, the Gynecologic Oncology Group (GOG) has studied a number of cytotoxic agents and combinations for the treatment of women with recurrent/metastatic cervical cancer. After the identification of cisplatin as a drug with significant activity, GOG phase III trials focused on the development of cisplatin-containing regimens that might yield superior results compared to single-agent therapy. Unfortunately, the higher response rates associated with combination therapy often did not result in improved survival. Median survival in this patient population remained less than one year, and combination therapy lead to more adverse effects—particularly myelosuppression.2
Most patients with recurrent and/or metastatic cervical cancer do not respond to cisplatin-based chemotherapy and thus experience treatment-related toxicity with no derived benefit. The ability to identify patients a priori who are unlikely to respond to conventional cytotoxic therapy would at least avoid the administration of futile treatment and at best direct these patients into other areas of investigation or even best supportive care. A pooled analysis of three published phase III GOG studies was undertaken in hopes of identifying prognostic factors and developing a model predictive of (non-) response to chemotherapy.
METHODS
Between June 1990 and September 2002, the GOG conducted, in sequence, four phase III for the treatment of cervical cancer that was beyond curative treatment with either surgery or radiation therapy.3–6 All studies were reviewed and approved by institutional review boards and participating patients provided written informed consent consistent with local, state and federal regulations. In comparison to single-agent cisplatin, GOG protocol 110 investigated the combination of cisplatin plus ifosfamide. The investigational arms of GOG 169 and GOG 179 were cisplatin plus paclitaxel, and cisplatin plus topotecan, respectively. GOG 149 was a phase III trial of cisplatin plus ifosfamide with or without bleomycin. Eligibility for GOG protocols 110, 149, and 169 was limited to patients with squamous cell carcinomas. Acknowledging no apparent difference in the objective response rates in phase II trials between squamous cell carcinomas and adenocarcinomas of the cervix, eligibility for GOG protocol 179 included patients with both histological types. One important difference in the protocol populations was the number of patients who had previously received chemotherapy (concurrent to radiation therapy) as part of their primary therapy. The frequency of prior chemotherapy increased from 25% for GOG 110 to 24% for GOG 169 to 58% for GOG 179.3,5,6
The study population for this retrospective analysis was derived from the database of patients who received a cisplatin-containing combination in these studies. All these combination regimens had a comparable response rate of ~30% (please refer to the previous publications). The study population was split into two subsets. Patients from GOG protocols 110, 169 and 179 were used for model development (training dataset) and patients from GOG protocol 149 were used for model validation (testing dataset). Treatment response by patient characteristics (age, race, performance status, FIGO (International Federation of Gynecology and Obstetrics) stage, histology, grade, site of disease recurrence, prior use of chemotherapy concurrent with radiation, or time to recurrence) was compared using the Pearson chi-square method. Multivariate analysis based on a Logistic model was conducted to identify factors independently prognostic of response. The significant variables identified in this analysis were applied to establish a model and the Hosmer-Lemeshow (HL) statistic was used for testing model goodness-of-fit.7 Given that the five risk factors identified conferred comparable weights and there were no interactions across them, a simple prognostic index was developed by combining the number of risk factors and the population was classified into three groups: low-risk, mid-risk and high-risk. The progression-free survival (PFS) and overall survival (OS) by the risk group were also estimated using the Kaplan-Meier procedure to further validate the predictive index. All the analyses were done using SAS version 9.1 (Cary, NC).
RESULTS
Among the 428 eligible patients who enrolled in GOG protocols 110, 169 and 179 that were included for analysis, the median age was 47 years (range 21–84 years) and 64% of them were Caucasians, 71% had a performance status of 1–2. Clinically, most of them (84%) were recurrent patients and 95% had squamous cell carcinoma. The distribution of the site of disease was 45%, 42% and 13%, respectively for pelvic site, distant site and both. Prior radiosensitizing chemotherapy was used in 36% of patients. The median interval from diagnosis to 1st recurrence was 11.6 months and median interval from 1st protocol chemotherapy was 3.9 months (Table 1).
TABLE 1.
Patient Characteristics
| Training Data (GOG # 110,169,179) N=428 |
Testing Data (GOG # 149) N=280 |
|||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| Age (years) | ||||
| Median (range) | 47.4 (21.0–84.0) | 46.0 (21.0–81.0) | ||
| ≤50 | 252 | (58.9) | 180 | (62.3) |
| > 50 | 176 | (41.1) | 100 | (35.7) |
| Race | ||||
| White | 272 | 63.6 | 200 | 71.4 |
| Black | 109 | 25.5 | 50 | 17.9 |
| Other | 47 | 11.0 | 30 | 10.7 |
| Stage | ||||
| IVB/persistent | 69 | (16.1) | 34 | (21.1) |
| Recurrent | 359 | (83.9) | 246 | (87.9) |
| Histology | ||||
| Squamous | 409 | (95.6) | 279 | (99.6) |
| Other | 19 | (4.4) | 1 | (0.4) |
| Tumor Grade | ||||
| 1 | 17 | (4.0) | 11 | (3.9) |
| 2 | 246 | (57.5) | 180 | (64.3) |
| 3 | 165 | (38.6) | 89 | (31.8) |
| Site of disease | ||||
| Pelvic | 194 | (45.3) | 105 | (37.5) |
| Distant | 178 | (41.6) | 120 | (42.9) |
| Combined | 56 | (13.1) | 55 | (19.6) |
| Prior radiosensitizer | ||||
| Yes | 154 | 36.0 | 92 | 32.1 |
| No | 274 | 64.0 | 188 | 67.9 |
| 1st relapse within 1 year from diagnosis | ||||
| Yes | 203 | (47.4) | 123 | (43.9) |
| No | 225 | (52.6) | 157 | (56.1) |
| Chemotherapy within 4 weeks from 1st recurrence | ||||
| Yes | 218 | (50.9) | 138 | (50.7) |
| No | 210 | (49.1) | 142 | (49.3) |
| Treatment response 1 | ||||
| Yes | 130 | (31.8) | 91 | (33.1) |
| No | 279 | (68.2) | 184 | (66.9) |
Evaluable for 409 patients for training data and 275 patients for testing data.
Four-hundred nine patients had tumor response data available (19 patients had missing data). Overall treatment response was 32% (95% CI (confidence interval): 27–36%). Tumor response rates by patient characteristics are shown in Table 2. Multivariate analysis identified five factors significantly associated with tumor response (Table 3). African-American patients (OR (odds ratio): 0.49, 95% CI: 0.28–0.83), patients with PS > 0 (OR: 0.60, 95% CI: 0.38–0.94), pelvic disease (OR: 0.58, 95% CI: 0.38–0.90), prior radiosensitizing chemotherapy (OR: 0.52, 95% CI: 0.32–0.85), and experiencing 1st recurrence within one year from diagnosis (OR: 0.61, 95% CI: 0.39–0.95) were more likely to have a poor response.
TABLE 2.
Treatment Response Rate by Patient Characteristics (n=409)
| No. patients | Response (%) | P value | |
|---|---|---|---|
| Age group (years) | 0.175 | ||
| ≤50 | 240 | 29.2 | |
| > 50 | 169 | 35.5 | |
| Race | 0.029 | ||
| White | 258 | 36.7 | |
| Black | 107 | 21.5 | |
| Other | 44 | 34.1 | |
| Performance Status | 0.023 | ||
| 0 | 126 | 41.3 | |
| 1 | 110 | 27.3 | |
| 2 | 173 | 27.8 | |
| Stage | 0.848 | ||
| IVB/persistent | 65 | 30.8 | |
| Recurrent | 344 | 32.0 | |
| Histology | 0.054 | ||
| Squamous | 391 | 32.7 | |
| Other | 18 | 11.1 | |
| Tumor grade | 0.944 | ||
| 1 | 16 | 31.3 | |
| 2 | 231 | 32.5 | |
| 3 | 162 | 30.9 | |
| Site of disease | 0.021 | ||
| Pelvic | 183 | 28.4 | |
| Distant | 173 | 38.7 | |
| Combined | 53 | 20.8 | |
| Prior radiosensitizer | 0.008 | ||
| Yes | 141 | 23.4 | |
| No | 268 | 36.2 | |
| 1st Recurrence within one year since diagnosis | 0.007 | ||
| Yes | 191 | 25.1 | |
| No | 218 | 37.6 | |
| Chemotherapy within 4 weeks since 1st recurrence | 0.689 | ||
| Yes | 208 | 32.7 | |
| No | 201 | 30.9 | |
| Total | 409 | 31.8 |
Pearson chi-square method used to compare the difference of proportions by the group.
TABLE 3.
Multivariate Analysis of Prognostic Factors of Treatment Response
| OR | 95% CI | P value | |
|---|---|---|---|
| Race | |||
| Black vs. Non-black | 0.49 | 0.28–0.83 | 0.008 |
| GOG performance | |||
| 1 or 2 vs. 0 | 0.60 | 0.38–0.94 | 0.027 |
| Site of disease | |||
| Pelvic vs. Non-pelvic | 0.58 | 0.38–0.90 | 0.015 |
| Radiosensitizer | |||
| Yes vs. No | 0.52 | 0.32–0.85 | 0.009 |
| 1st Recurrence within one year since diagnosis | |||
| Yes vs. No | 0.61 | 0.39–0.95 | 0.027 |
Odds ratio (OR) in favor of treatment response estimated from Logistic regression model, adjusted for covariates.
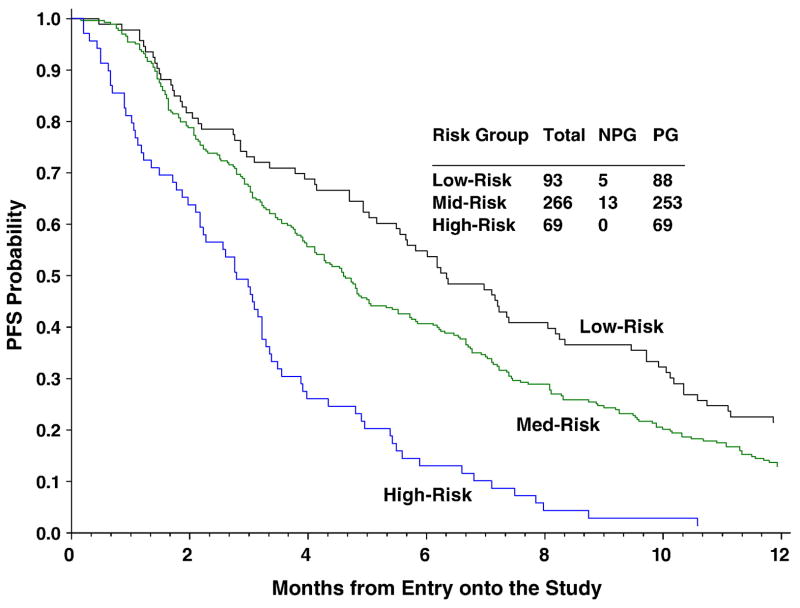
A prognostic model for tumor response based on the five factors (race, PS, site of disease, prior radiosensitizer and interval between diagnosis and 1st recurrence) was established. Given the similar weights and no interactions across these factors, an index based on the total number of risk factors was derived. The internal validity of this prognostic model was satisfied (P=0.624 for HL test) (Figure 1). When patients were classified into three risk groups (low-risk: 0–1 factor, mid-risk: 2–3 factors and high-risk: 4–5 factors), patients with 4–5 of risk factors were estimated to have a response rate of only 13%, a median PFS of 2.79 months and a median OS of 5.49 months (Figures 2 and 3). This subgroup of patients consist ~16% of the study population. The prognostic index was also evaluated using GOG 149 data that had comparable patient characteristics and was not used for model development (external validity). The results further support the accuracy of the model. Both internal and external validation results are summarized in Table 4.
Fig. 1.
Predicted and observed response rate by number of risk factors.
FIGURE 2.
Kaplan-Meier Estimate of Progression-free Survival (PFS) by Number of Risk Factors: Low-risk = 0–1 risk factors; Mid-risk = 2–3 risk factors; High-risk = 4–5 risk factors.
FIGURE 3.
Kaplan-Meier Estimate of Overall Survival (OS) by Number of Risk Factors: Low-risk = 0–1 risk factors; Mid-risk = 2–3 risk factors; High-risk = 4–5 risk factors.
TABLE 4.
Validation of Prognostic Model
| Internal Validation (GOG 110, 169 and 179) | External Validation (GOG 149) | |||||||
|---|---|---|---|---|---|---|---|---|
| Response Rate (%) | Median Survival (months) | Response Rate (%) | Median Survival (months) | |||||
| Estimated | Observed | PFS | OS | Predicted | Observed | PFS | OS | |
| Low-Risk | 50.6 | 47.3 | 6.34 | 11.10 | 50.9 | 42.6 | 6.87 | 11.93 |
| Mid-Risk | 29.4 | 31.4 | 4.60 | 9.17 | 29.0 | 29.4 | 4.44 | 7.59 |
| High-Risk | 13.0 | 9.8 | 2.79 | 5.49 | 13.5 | 14.3 | 3.38 | 5.58 |
Low risk: 0–1 risk factor; mid risk: 2–3 risk factors; and high risk: 4–5 risk factors.
DISCUSSION
Potter and colleagues reviewed their results with cisplatin chemotherapy for the treatment of 74 women with recurrent cervical cancer.8 Although the location of disease did not affect OS, patients with isolated chest metastasis did have a 53% complete response rate compared to no complete responses for patients with localized pelvic recurrences. Patient age, clinical stage, lesion size, and duration from primary treatment to recurrence were of no significance with respect to response to chemotherapy or survival.8 In a larger multivariate analysis of 190 patients equally divided among platinum-based or non-platinum-based regimens (14 protocols overall), Brader et al. noted that age (younger patients) and site of disease (pelvic) were significant determinants of poor response. Tumor stage or grade, performance status, race, socioeconomic status, prior therapy, and time to recurrence were not significant predictors of response.9 Our results are comparable in that pelvic recurrences were consistently unfavorable. The substantially larger size of our study population, strict eligibility criteria for protocol entry, and standardization of protocol treatment may explain in part why other factors were of significance in our study but not in these retrospective series. Of interest is the fact that each independent prognostic factor used in the development of the model was of approximately equal weight, and thus no single factor was given greater significance than another. The external validation of the model further substantiates the potential utility of these assessments in future protocol design.
Primary, untreated cervical cancer is quite chemo-sensitive, as evidenced by the impressive response rates reported with neoadjuvant chemotherapy.10 There are a number of reasons why women with recurrent cervical cancer fare poorly when treated with chemotherapy, including: 1) prior radiation therapy limits bone marrow function; 2) adequate drug distribution is limited for recurrences in previously-irradiated tissues; 3) some patients may have renal dysfunction secondary to ureteral obstruction, thus limiting or precluding the administration of some drugs such as cisplatin.11,12 It is also quite likely that tumors resistant to the effects of ionizing radiation are also resistant to the effects of classical cytotoxic agents such as cisplatin.
Both GOG protocols 169 and 179 incorporated patient-reported quality of life using validated instruments among the study endpoints. Despite greater adverse effects associated with combination therapy, neither study discerned any appreciable decrement in quality of life as compared to single-agent cisplatin.10,13 Although reassuring, these findings cannot be construed as evidence that cisplatin-containing chemotherapy “does no harm.” (Conversely, the fact that objective responses did not translate into improvements in quality of life scores does not mean that chemotherapy does no good). Even limited toxicity, in the face of non-response to treatment or disease progression, is unacceptable. It is therefore tempting to conclude that high-risk patients—as defined by the prognostic model—should be spared the toxicity of ineffective treatment and instead be considered for non-cisplatin chemotherapy or investigational trials. It was estimated that the high-risk patients accounted for approximately 16% of the entire study population; therefore, excluding these patients will have a minor impact on future accrual to phase III studies. However, it is a clinical and ethical imperative to avoid adverse effects from predictably ineffective treatments. By excluding these patients, there may be an even better opportunity to realize superiority for a particular regimen by limiting eligibility to potential responders.
The observation that African-American patients were more likely to have a poor response to chemotherapy merits further comment. Using state registry data, Brookfield and associates showed that African-American women with cervical carcinoma had a worse prognosis; however, multivariate analysis did not identify race as an independent predictor of poor outcome. Racial disparities in outcome could be explained by later stage at presentation and under-treatment.14
A worse outcome has been reported for African-American patients with breast cancer,15 squamous cell cancers of the anus16 and head and neck,17 endometrial cancer,18 and even for pediatric patients undergoing treatment for Hodgkin’s lymphoma.19 In their analysis of 19,457 adult cancer patients treated on Southwest Oncology Group (SWOG) phase III trials, Albain and colleagues determined that African-American patients with early-stage premenopausal and postmenopausal breast cancer, advanced-stage ovarian cancer, and advanced-stage prostate cancer had increased mortality. There was no association between race and survival for lung cancer, colon cancer, lymphoma, leukemia, or multiple myeloma. Despite enrollment on phase III SWOG trials with uniform stage, treatment, and follow-up. African-American patients with gender-specific cancers had worse survival than white patients.20
In contrast, other investigators have found no apparent association between race and outcome for prostate cancer21 or breast cancer.22 In an analysis of patients undergoing treatment for lung cancer, Farjah and colleagues found that black patients were less likely to undergo surgical resection than white patients; however, racial differences in receiving optimal therapy did not affect outcome. The authors concluded that perceptions about cancer treatment and limited access to care might have a more dominant role in perpetuating racial disparities than previously recognized.23 Using GOG data, Farley et al. showed that there was no difference in clinical outcome between black and white women with advanced stage epithelial ovarian cancer when they received similar treatment as clinical trials participants. In fact, black patients appeared to experience less severe gastrointestinal toxicity or leukopenia compared with whites when treated with platinum-based chemotherapy.24
Difficulties in deciphering conflicting data pertaining to race and outcome have been discussed by Brewster.25 Among patients meeting predefined eligibility criteria and receiving identical protocol chemotherapy with standard response and toxicity assessments, differences in outcome cannot be explained on the basis of treatment. Instead, the worse outcome for African-American patients in our study could be due to clinical factors that were not assessed in the database such as nutritional status or smoking behaviors that could potentially influence treatment outcome. Alternatively, it may be that African-American women have biologically worse cervical cancers. It would be important to study functional alterations in DNA base-excision repair and mismatch repair pathways,26 the functional status of apoptotic pathways,27 or biomarkers of cisplatin resistance such as Eme1 in African-American women with cervical cancer.28 It cannot be over-emphasized that the predictive model defines high-risk patients by the accumulation of independent prognostic factors; black women would not be excluded from trials involving cisplatin-based chemotherapy on the basis of race alone.
We advise caution pending further study, and certainly before applying this model to patient management outside the context of clinical trials. The model was derived from a retrospective multivariate analysis of platinum-based regimens, and thus the appropriateness of extrapolating these data to non-platinum-containing regimens, or cisplatin in combination with biologic agents, must be questioned. It is intended that this model will be prospectively evaluated in the next GOG phase III trial for this patient population.
Supplementary Material
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517).
The following institutions participated in this study: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Emory University Clinic, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke’s Medical Center, SUNY Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Women’s Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Case Western Reserve University, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Ellis Fischel Cancer Center, University of Arkansas Medical Center, and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Moore DH. Chemotherapy for advanced, recurrent, and metastatic cervical cancer. J Natl Compr Canc Netw. 2008;6:53–7. doi: 10.6004/jnccn.2008.0006. [DOI] [PubMed] [Google Scholar]
- 3.Omura GA, Blessing JA, Vaccarello L, et al. A randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–71. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:1832–7. doi: 10.1200/JCO.2002.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–9. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 6.Long HJ, Bundy BN, Grendys EC, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626–33. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. John Wiley & Sons; 2000. [Google Scholar]
- 8.Potter ME, Hatch KD, Potter MY, et al. Factors affecting the response of recurrent squamous cell carcinoma of the cervix to cisplatin. Cancer. 1989;63:1283–6. doi: 10.1002/1097-0142(19890401)63:7<1283::aid-cncr2820630709>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Brader KR, Morris M, Levenback C, et al. Chemotherapy for cervical carcinoma: Factors determining response and implications for clinical trial design. J Clin Oncol. 1998;16:1879–84. doi: 10.1200/JCO.1998.16.5.1879. [DOI] [PubMed] [Google Scholar]
- 10.Moore DH. Neoadjuvant chemotherapy for cervical cancer. Expert Opin Pharmacother. 2003;4:859–67. doi: 10.1517/14656566.4.6.859. [DOI] [PubMed] [Google Scholar]
- 11.Omura GA. Current status of chemotherapy for cancer of the cervix. Oncology. 1992;6:27–32. [PubMed] [Google Scholar]
- 12.Thigpen T, Vance R, Khansur T, et al. The role of ifosfamide and systemic therapy in the management of carcinoma of the cervix. Semin Oncol. 1996;23:56–64. [PubMed] [Google Scholar]
- 13.Monk BJ, Huang HQ, Cella D, et al. Quality of life outcomes from a randomized phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4617–25. doi: 10.1200/JCO.2005.10.522. [DOI] [PubMed] [Google Scholar]
- 14.Brookfield KF, Cheung MC, Lucci J, Fleming LE, Koniaris LG. Disparities in survival among women with invasive cervical cancer: a problem of access to care. Cancer. 2009;115:166–78. doi: 10.1002/cncr.24007. [DOI] [PubMed] [Google Scholar]
- 15.Balmanoukian A, Zhang Z, Jeter S, Slater S, Armstrong DK, Emens LA, et al. African American women who receive primary anthracycline- and taxane-based chemotherapy for triple-negative breast cancer suffer worse outcomes compared with white women. J Clin Oncol. 2009;27:e35–7. doi: 10.1200/JCO.2008.21.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Bentrem DJ, Rock CE, Stewart AK, Ko CY, Halverson A. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: analysis of patients from the National Cancer Data Base. Dis Colon Rectum. 2009;52:624–31. doi: 10.1007/DCR.0b013e31819eb7f0. [DOI] [PubMed] [Google Scholar]
- 17.Settle K, Taylor R, Wolf J, Kwok Y, Cullen K, Carter K, et al. Race impact outcome in stage III/IV squamous cell carcinomas of the head and neck after concurrent chemoradiation therapy. Cancer. 2009;115:1744–52. doi: 10.1002/cncr.24168. [DOI] [PubMed] [Google Scholar]
- 18.Wright JD, Fiorelli J, Schiff PB, Burke WM, Kansler AL, Cohen CJ, Herzog TJ. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115:1276–85. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]
- 19.Metzger ML, Castellino SM, Hudson MM, Rai SN, Kaste SC, Krasin MJ, Kun LE, Pui CH, Howard SC. Effect of race on the outcome of pediatric patients with Hodgkins’s lymphoma. J Clin Oncol. 2008;26:1282–8. doi: 10.1200/JCO.2007.14.0699. [DOI] [PubMed] [Google Scholar]
- 20.Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resnick MJ, Canter DJ, Guzzo TJ, Brucker BM, Bergey M, Sonnad SS, Wein AJ, Malkowicz SB. Does race affect postoperative outcomes in patients with low-risk prostate cancer who undergo radical prostatectomy? Urology. 2009;73:620–3. doi: 10.1016/j.urology.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 22.Chu QD, Smith MH, Williams M, Panu L, Johnson LW, Shi R, Li BD, Glass J. Race/ethnicity has no effect on outcome for breast cancer patients treated at an academic center with a public hospital. Cancer Epidemiol Biomarkers Prev. 2009;18:2157–61. doi: 10.1158/1055-9965.EPI-09-0232. [DOI] [PubMed] [Google Scholar]
- 23.Farjah F, Wood DE, Yanez ND, Vaughan TL, Symons RG, Krishnadasan B, Flum DR. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–8. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell LG. Race does not impact outcome for advanced ovarian cancer patients treated with cisplatin/paclitaxel: an analysis of Gynecologic Oncology Group trials. Cancer. 2009 Jun 17; doi: 10.1002/cncr.24482. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brewster WR. The complexity of race in the disparate outcome and treatment of minority patients. Gynecol Oncol. 2008;111:161–2. doi: 10.1016/j.ygyno.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Fraser M, Abedini MR, Bai T, Tsang BK. Regulation of apoptosis-inducing factor-mediated, cisplatin-induced apoptosis by Akt. Br J Cancer. 2008;98:803–8. doi: 10.1038/sj.bjc.6604223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomoda Y, Katsura M, Okajima M, Hosoya N, Kohno N, Miyagawa K. Functional evidence for Eme1 as a marker of cisplatin resistance. Int J Cancer. 2009;124:2997–3001. doi: 10.1002/ijc.24268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.