Abstract
Objective
Use Diffusion Tensor Imaging (DTI) to investigate white matter alterations associated with blast exposure with or without acute symptoms of traumatic brain injury (TBI).
Participants
Forty-five veterans of the recent military conflicts included twenty-three exposed to primary blast without TBI symptoms, six having primary blast mild TBI, and sixteen unexposed to blast.
Design
Cross-sectional case control study.
Main Measures
Neuropsychological testing and DTI metrics that quantified the number of voxel clusters with altered fractional anisotropy (FA) radial diffusivity (RD), and axial diffusivity (AD), regardless of their spatial location.
Results
Significantly lower FA and higher RD was observed in veterans exposed to primary blast with and without mild TBI relative to blast unexposed veterans. Voxel clusters of lower FA were spatially dispersed and heterogeneous across affected individuals.
Conclusion
These results suggest that lack of clear TBI symptoms following primary blast exposure may not accurately reflect the extent of brain injury. If confirmed, our findings would argue for supplementing the established approach of making diagnoses based purely on clinical history and observable acute symptoms with novel neuroimaging-based diagnostic criteria that “look below the surface” for pathology.
Keywords: mild traumatic brain injury (mTBI), diffusion tensor imaging (DTI), primary blast, subconcussive blast exposure, fractional anisotropy, diffusivity, military veterans, white matter
Introduction
Although exposure to explosive forces emanating from bombs and other devices is increasing among civilians and common in veterans of recent military conflicts in Iraq and Afghanistan, relatively little is known about the consequences to the brain.1-8 Animal studies and computer modeling indicate that the blast wave has the potential to induce brain injury by different mechanism(s) than are present in nonpenetrating (closed head) traumatic brain injury (TBI) of more conventional origin, such as impact injury.2;5;7-9 This suggests that secondary injury and recovery processes may also differ. Recent studies have also raised other worrisome possibilities, including subconcussive effects and induction of chronic traumatic encephalopathy (CTE), making study in humans essential.
The severity of TBI is determined primarily by symptoms immediately following the event, such as altered sensorium, loss of consciousness and presence/duration of post traumatic amnesia.10 Most combat-related TBI are classified as mild based on symptoms at the time of injury (e.g., dazed/confused/ “saw stars”, at most a short loss of consciousness or brief period of amnesia).4;6;8;10 Most events involve a combination of primary blast and other forces, often described as “blast plus” or blast-related TBI. 2;5;7;8 Preliminary evidence suggests that early evolution of blast-related mild TBI may differ from other injury mechanisms.1;6 Differences in injury mechanism(s) and/or injury evolution make it essential to determine the effects in the human brain of exposure to primary blast. A case series and two case reports support the vulnerability of white matter (WM) regions to primary blast injury, indicated by small, spatially dispersed areas of abnormally low fractional anisotropy (FA) on diffusion tensor imaging (DTI).11-13
DTI is a type of magnetic resonance imaging (MRI) that is sensitive to the diffusion of water in tissue and able to provide an indirect measure of WM integrity.14;15 Within gray matter water diffuses at similar rates in all directions (isotropic). Within WM microstructural barriers (e.g., membranes, myelin, neurofilaments) cause water to diffuse faster long the longitudinal axis of axons (anisotropic). Using measures such as FA to quantify directionality allows inferences to be made about the integrity of the underlying WM. Higher values of FA reflect greater directionality and suggest healthy WM, while loss of tissue integrity at the microscopic level (e.g., shearing injury, demyelination) will manifest as reduced FA.
Adding to the complexity of the situation, evidence is accumulating that subconcussive blast exposure might injure the brain. 16;17 A recent study in veterans reported a strong association between a history of exposure to blast and reduced first percentile FA that was independent of symptoms at the time of exposure indicative of mild TBI.16 There is also emerging evidence that subconcussive sports-related events are potentially injurious to the brain.18;19 A study comparing young concussion-naïve athletes participating in a contact sport (soccer) and a noncontact sport (swimming) found multiple areas of significantly increased radial and axial diffusivity in the contact group.20 A study of amateur soccer players reported areas of reduced FA in players with higher number of headings, with evidence for a threshold effect.19 Similarly, studies comparing postseason to preseason DTI in young athletes (high school, college) participating in contact sports (ice hockey, football) found multiple areas with significantly altered FA and mean diffusivity postseason in the absence of concussive events during the playing season.21;22 Decrements in neurocognitive and functional imaging measures have also been reported in young athletes following multiple in-game subconcussive hits.23;24 Finally, autopsy-confirmed CTE was reported in a college football player with no reported concussions.25
The purpose of this study was to assess WM integrity in previously deployed veterans with a history of primary blast exposure with and without clinical symptoms of mild TBI at the time of exposure. We hypothesized the effects of primary blast exposure would be detected as reduced WM integrity on DTI and that the magnitude of changes would be associated with the intensity of exposures. Furthermore, we predicted, as with mild TBI due to other types of forces, the compromise to WM integrity would be diffuse and widely dispersed with clear inter-individual spatial heterogeneity. Accordingly, we predicted that cognitive performance deficits would be most prominent for cognitive tasks requiring widely distributed neural systems such as working memory and executive function.
Methods
This study was conducted at the Department of Veterans Affairs (VA) Mid-Atlantic Mental Illness Research, Education and Clinical Center (MIRECC). The welfare of human subjects was protected. Participants provided informed consent to take part in research procedures approved by the Institutional Review Board (IRB) at the W.G. “Bill” Hefner VA Medical Center, Salisbury, North Carolina USA.
Participants
Study eligibility of veterans who served since September 11, 2001 was determined by review of medical records and a screening interview. Exclusion criteria include a pre-deployment history of neuropsychiatric, neuropsychological and/or neurological symptoms including head trauma (other than mild concussion, indicated by not more than momentary loss of consciousness), seizures, strokes, prior neurosurgery, evidence of mental retardation, neurological impairments, substance dependence, Axis I psychiatric disorders; exposure to conditions during or following deployment likely to result in a TBI due to forces other than primary blast (e.g., impact injury); presence of shrapnel, metallic implants, devices or conditions contraindicating neuroimaging. There was one subject with inadequate quality of DTI images from each of the three groups. Thus, data was analyzed from: (i) unexposed (n=16), reported no exposure during deployment to any conditions likely to result in a TBI, (ii) primary blast exposed (n=23), reported exposure only to primary blast forces, and either no symptoms at the time or symptoms that did not meet criteria for mild TBI, (iii) primary blast TBI (n=6), reported exposure only to primary blast forces, symptoms at the time consistent with mild TBI according to established criteria10, and present symptoms consistent with residual mild TBI as determined by an experienced VA clinician.
Clinical Testing
Psychiatric status was determined by administration of the Structured Clinical Interview for DSM-IV Axis I Disorders (SICD-I) by an experienced clinical psychologist. Computer-based neurocognitive testing utilized the following eight tests from the Cambridge Neuropsychological Test Automated Battery (CANTABeclipse v3.0, Cambridge Cognition Ltd, Cambridge UK): Reaction Time (RT, cognitive and motor processing speed), Delayed Matching to Sample (DMS, visual working memory), Spatial Working Memory (SWM, visuospatial working memory), Stockings of Cambridge (SOC, visuospatial working memory and sequence planning), Intra-Extra Dimensional Shift (IED, learning by inferring rules and set-shifting), Cambridge Gambling Task (CGT, decision-making and risk-taking behavior outside a learning context), Affective Go-NoGo (AGN, information processing bias and inhibitory control for positive and negative), and Rapid Visual Information Processing (RVP, visual working memory and sustained attention).
Image acquisition
Images were acquired on a General Electric Signa HDxt 1.5 Tesla scanner with an 8-channel receive coil. All participants underwent DTI with 2×2×6-mm voxel size, 128×128×24 matrix automatically resampled to 1×1×6-mm voxel size, FOV 240×192-mm, flip angle 90°, TR=8,500-ms, TE=107-ms, 1 average, 25 noncollinear directions (diffusion gradients), SENSE factor=1, non-zero b-value=1,000 s/mm2, scanning time=12’30”. All images were visually inspected for the purpose of quality assurance.
Preprocessing of diffusion imaging data
Preprocessing was carried out using the FMRIB Diffusion Tool Box (FDT; FMRIB Centre, Oxford University UK) to remove eddy current distortions caused by the stretching and shearing of diffusion weighted images by gradient coils, and to correct for simple head motion. Fractional anisotropy (FA), the primary measure acquired from the DTI data is a scalar metric describing the WM integrity, was calculated from the orientational coherence of the diffusion compartments within a voxel. The analytic approach was based on skeleton voxels identified by tract-based spatial statistics (TBSS). All subjects in the sample were co-registered using a method that insured WM alignment using an intermediate degrees-of-freedom, nonlinear registration to a 1 × 1 × 1 mm template (FMRIB58_FA) of male and female subjects aged 20–50 years (http://www.fmrib.ox.ac.uk/fsl/). These normalized re-sampled images were averaged to generate a mean FA image. In TBSS, a mean FA skeleton was created as lines and surfaces that pass through the centers of WM tracts in the mean FA image. After thresholding the skeleton to exclude low-FA values (<0.2) indicative of non-WM, each subject's aligned FA image was projected onto the mean FA skeleton. A voxelwise whole-brain approach, was used instead of a region of interest (ROI) approach: computing statistics for each voxel independently.
Significance testing for abnormal FA
Assumptions about the distribution of DTI data (e.g. Gaussianity) were avoided by using permutation testing to make inferences about group differences and associations with clinical regressor variables from a sample-specific probability distribution of means. This non-parametric analysis was conducted using randomise (FMRIB Centre, University of Oxford, UK), an implementation of permutation testing with covariates (clinical regressors) for whole brain voxelwise analyses, to establish a significance level for every skeleton voxel from a distribution generated by 5,000 permutations of the group label. For details about permutation testing with regressors see Kennedy (1995), or Anderson and Robinson (2001).26;27
Clinical regressors
Four covariates were included in the initial whole brain voxelwise analysis of FA: age, pre-military exposure to mild concussion, post traumatic stress disorder (PTSD) diagnosis, and alcohol use score, which was compiled from assessing participants’ amount of alcohol consumption, level of psychological impact, level of physiological impact, and symptoms of withdrawal. Intermediate results showed that the alcohol use score and pre-military mild concussions were poorly correlated with FA (pmin > .9). Therefore, only significant and trend level significant covariates of age and PTSD diagnosis were retained in the whole brain analyses.
Correction for multiple comparisons
The results of permutation testing were followed up with correction for multiple comparisons using threshold free cluster enhancement (TFCE) as an alternative to the overly conservative corrections such as Bonferroni that have poor control of Type II error.28 Rather than requiring the selection of an arbitrary initial clustering threshold for subsequent computation of p-values based on Gaussian Random Field theory (cluster-level-correction), TFCE accounts for “cluster-like local spatial support,” i.e. intensity and extent of the test statistic. Recommended (default) TFCE parameters for cluster height (H=2), cluster extent (E=1), and cluster connectivity C (C=26) were used with randomise.
Visualization of results
The significance maps (p < .05; corrected) were superimposed on the normalized group skeleton of the FMRIB58_FA template. The TBSS-fill feature was applied to all significance maps to enhance the visualization of TBSS results as commonly used in similar studies.29-31 Tracts are reported according to standard nomenclature.32
Statistical Analyses of z-score voxel clusters
Preprocessing of DTI data was performed as described earlier. We compared the number of voxels with abnormal DTI metrics with minimal spatial constraints among participants to determine the possible effect of primary blast on small regions of WM. The individual subject and the reference group were registered to the template. Each participant's DTI metric map was compared to images of the mean and standard deviation of the reference group of 16 unexposed control participants. An individual z-score map was created for each participant in the primary blast TBI and the primary blast exposed group that was thresholded at z = −2.0 or below for FA, and z = 2.0 or above for radial diffusivity (RD) and axial diffusivity (AD). Given that the z-statistic is valid only for normally distributed data, the z-map was masked with reference-group values that were normally distributed as assessed by the Lilliefors test.33 Overall, this procedure was designed to identify voxels with values in the extremes of the healthy distribution rather than voxels deemed statistically significantly different than the healthy mean.34;35
Group statistical analyses of z-score voxel clusters
We adopted an approach similar to White et al35 for analyzing spatially heterogeneous changes in DTI metrics among subjects and groups. Summary statistics for the primary blast exposed and primary blast TBI group were computed from the individual subject comparisons to the reference group in the previous step. Group statistics for average number of voxel clusters with abnormal metrics (FA less than z = –2, RD or AD more than z =2) were binned according to size in increments of 25 voxels. Voxel clusters were classified into three sizes: small (25-49), medium (50-74), and large (75-100). Very small voxel clusters (fewer than 25 voxels) and voxel clusters with extremely low cluster counts owing to their large size (exceeding 100 voxels) were excluded from the analyses to minimize spurious results (Type I error). The number of small, medium, and large voxel clusters for each DTI metric was tabulated for each participant as well as the mean and standard deviation for the primary blast TBI, primary blast exposed, and the unexposed control groups. Histogram plots were generated for the number of voxel clusters of each size in the three participant groups. We used repeated measures GLM to quantify differences between the primary blast exposed and primary blast TBI groups compared to the unexposed control group. Repeated measures were counts of small, medium, and large voxel clusters. The GLM included covariates for PTSD diagnosis16;36, diagnosis of alcohol use disorders37, pre-military exposure to mild concussion, and age38 given prior reports of association with FA. However, alcohol use disorder, pre-military exposure to mild concussion, and PTSD diagnosis were poorly correlated with FA in intermediate results (data not shown). Therefore, age was the only covariate retained in our primary DTI analyses. Finally, we examined the spatial distribution of FA voxel clusters by creating a whole brain voxelwise histogram of the number of voxel clusters exceeding 25 voxels (≥ small voxel cluster) in the each of the two affected groups. The histogram map was produced by indicating the ordinal count of the number of subjects for which a given voxel was part of a voxel cluster.
Correspondence between DTI findings and cognitive performance
Principal component analysis (PCA) was conducted on the 9 voxel cluster variables (AD, RD, and FA for small, medium, and large voxel clusters) to reduce multicollinearity among these variables for use in subsequent hierarchical linear regression models. These 9 variables loaded onto 2 components (all variables loaded at .93 or greater) using direct-oblimin rotation to explain 91% of the underlying variance. The first component included all RD and AD variables and the second component included all FA variables. Hierarchical linear regression analyses were conducted to examine how the two DTI component scores related to performance on cognitive testing beyond possibly confounding demographic variables (age, education, race) known to relate to cognitive performance, as well as the number of PTSD and major depression (MDD) symptoms reported during the SCID. Demographic variables were entered as step 1, PTSD and MDD symptoms as step 2, followed by the two DTI component scores as step 3. Neuropsychological tests, selected based on cognitive constructs requiring an anatomically distributed network of regions given the widespread pattern of voxel clusters, included: DMS (percent correct), IED (stages completed, percent pre-shift errors, percent post-shift errors), CGT (quality of decision making and risk taking), SOC (problems completed in minimum moves and average number of moves), RTI (simple reaction time), and SWM total errors.
Results
Clinical Variables
Detailed demographic and clinical characteristics of the three participant groups are provided in Table 1.
Table 1.
Demographic and Clinical Characteristics of Participants*
| Characteristic | Blast mild TBI n = 6 | Blast Exposed n = 23 | Unexposed Control n = 16 | Group Comparison (mild TBI vs. control) | Group Comparison (exposed vs. control) |
|---|---|---|---|---|---|
| Age (years), [SD] | 35.8 [8.7] | 35.8 [7.4] | 37.3 [11.5] | t(20) = 0.28, p < .78 | t(37)=0.49, p = .63 |
| Gender, No. (%) of females | 0 (0) | 5 (21.7) | 4 (25.0) | X2 (1) = 1.8, p < .18 | X2(1) = 0.06, p = .81 |
| Handedness, No. (%) right-handed | 6 (100.0) | 21 (91.3) | 15 (93.8) | X2 (1) = 0.39, p < .53 | X2(1) = 0.08, p < .78 |
| Race, No. (%) of Caucasian subjects | 5 (83.3) | 15 (65.2) | 12 (75.0) | X2 (1) = 0.01, p < .91 | X2(1) = 1.2, p < .27 |
| Education (years), [SD] | 13.8 [.98] | 14.3 [2.2] | 13.7 [2.0] | t(20) = −0.17, p < .87 | t(37) = −0.83, p < .41 |
| Prior exposure to concussion (%) | 0 (0) | 3 (13.0) | 2 (12.5) | X2 (1) = 0.87, p < .35 | X2 (1) = 0.002, p = .96 |
| SCID PTSD symptoms (mean) [SD] | 14.7 [1.4] | 6.4 [5.6] | 3.8 [4.8] | t(20) = −5.4, p < .001 | t(37) = −1.5, p < .14 |
| Diagnosis of PTSD, No. (%) | 6 (100) | 7 (30.0) | 3 (18.8) | X2 (1) = 11.9, p < .001 | X2 (1) = 0.68, p = .41 |
| Diagnosis of Anxiety Disorder, No. (%) | 6 (100) | 9 (39.1) | 4 (25.0) | X2 (1) = 7.1, p = .008 | X2 (1) = 0.85, p = .36 |
| Alcohol Use Disorder, No. (%) | 3 (50) | 9 (39.1) | 1 (6.3) | X2 (1) = 0.23, p = .63 | X2 (1) = 5.4, p = .02 |
| Diagnosis of Drug Dependence, No. (%) | 3 (50) | 6 (26.1) | 3 (18.8) | X2 (1) = 1.2, p = .26 | X2 (1) = 0.29, p = .58 |
Data values represent means except where indicated otherwise.
Voxelwise whole brain analyses
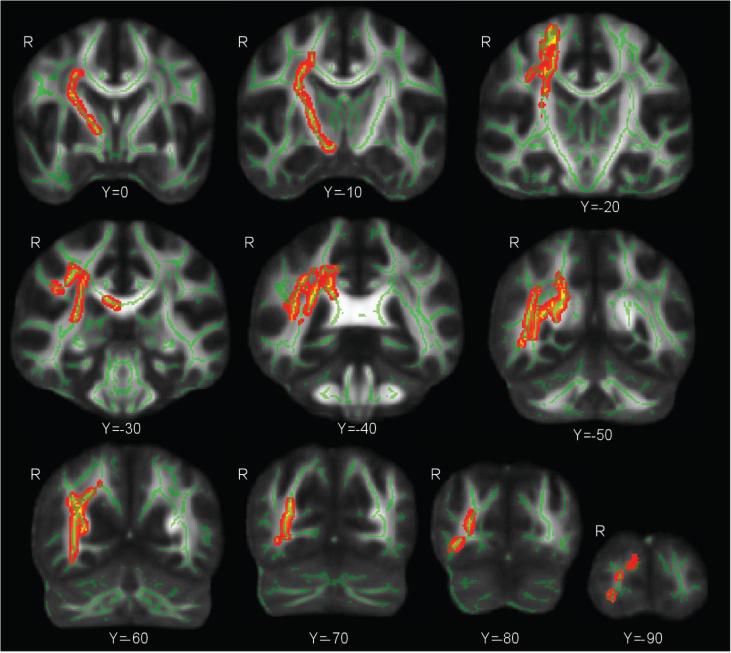
A whole brain voxelwise comparison of FA between the blast exposed and the blast unexposed control group was covaried for age and diagnosis of PTSD assessed with the SCID (Figure 1). These results show significantly lower FA in the primary blast exposed group compared to the unexposed group in diffuse cortical and subcortical tracts in the right hemisphere including the forceps major, superior and inferior longitudinal fasciculus, anterior thalamic radiations, inferior fronto-occipital fasciculus, and the corticospinal tract (p < .05; corrected). The left hemisphere did not show differences in FA between the primary blast exposed group and the unexposed control group (p < .05; corrected). At a reduced significance level (p < .15; corrected) the left hemisphere showed group-differences in FA that were closer in magnitude and extent to the finding in the right hemisphere. The inconsistent findings between the left and right hemisphere highlighted spatial heterogeneity among primary blast exposed individuals further motivating an alternate approach for assessing WM injury independent of spatial location. There were no significant differences with this technique comparing the primary mild TBI group to the unexposed group or to the primary blast-exposed group.
Figure1.
Whole brain voxelwise comparison of FA between the blast exposed group and the blast unexposed control group was covaried for age and PTSD diagnosis. These results show significantly lower FA in the blast exposed group compared to the blast unexposed group in diffuse cortical and subcortical tracts based in the right hemisphere including the forceps major, superior and inferior longitudinal fasciculus, anterior thalamic radiations, inferior fronto-occipital fasciculus, and the corticospinal tract (p < .05; corrected).
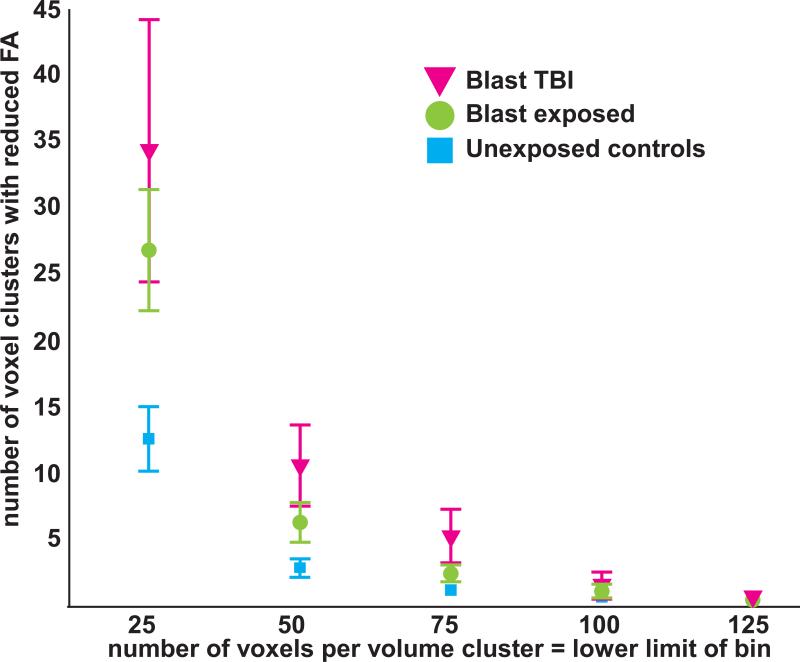
Spatially independent assessment of FA reductions
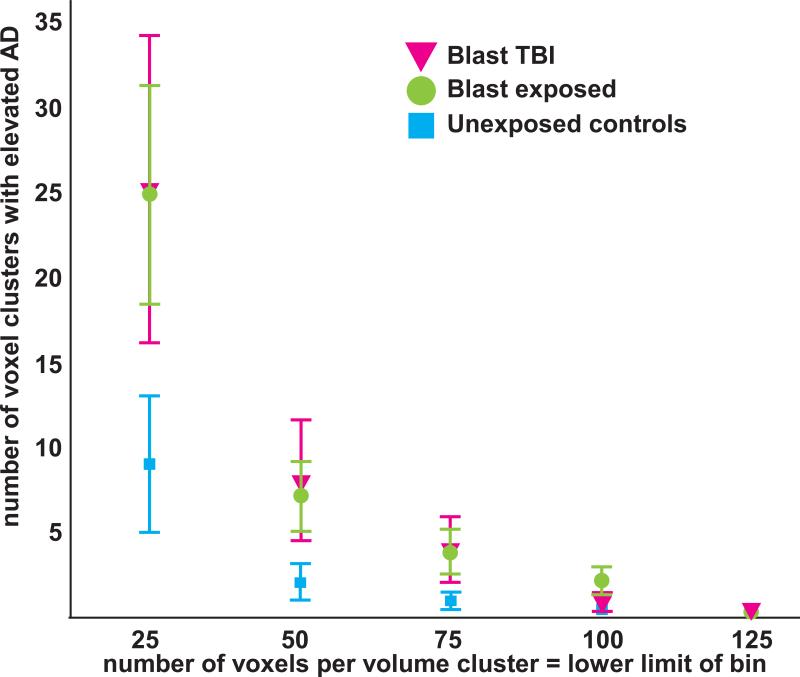
The magnitude of WM injury as measured by FA that was independent of spatial location is shown for the primary blast TBI, primary blast exposed, and the unexposed control groups in Figure 2. There was a significant main effect of group on the number of small, medium, and large voxel clusters (repeated measures) [F(2,42)=4.4, p<.02]. The unexposed control group had significantly fewer voxel clusters than the primary blast TBI group (p = .009) and the primary blast exposed group (p = .036); the primary blast TBI and primary blast exposed groups were not significantly different (p =.2). Thus, based on the number of voxel clusters, the primary blast exposed group resembled the primary blast TBI group when considered in relation to the unexposed control group (Figure 2). Age was significantly correlated with number of voxel clusters [F(1,41)=7.3, p= .01]. Also as expected, there was a main effect of volume size on the number of voxel clusters [F(2,82)=19.4, p< .0001] with significantly fewer large voxel clusters than medium voxel clusters, and fewer medium voxel clusters the small voxel clusters. Finally, there was an interaction of group * volume size [F(4,82)=3.8, p =.007] where the small voxel clusters had greater between-group differences than the medium and larger volume sizes (Figure 2).
Figure 2.
There was a significant main effect of group [F(2,42)=4.4, p<.02] on the number of small (25-50 voxels), medium (50-75 voxels), and large (75-100 voxels) voxel clusters, which were defined by low fractional anisotropy (FA) values (z < −2). The unexposed control group had significantly fewer voxel clusters than the blast TBI group (p = .009) and the blast exposed group (p = .036). Based on the number of voxel clusters, the blast exposed group resembled the blast TBI group when considered in relation to the unexposed control group
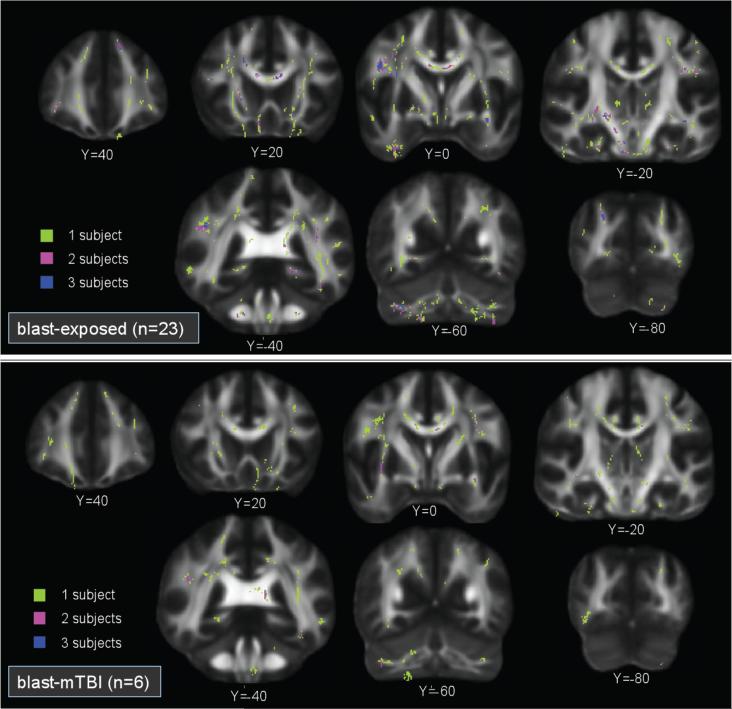
Spatial distribution of FA reductions
The histogram map of voxel clusters larger than 25 voxels (Figure 3) demonstrates that voxel clusters in both the primary blast TBI and primary blast exposed group were distributed heterogeneously both within and across subjects, which was consistent with our second hypothesis. The histogram map shows that for the preponderance of locations (voxels), there were voxel clusters present in only 1 participant, a few locations where voxels clusters were present in 2 or 3 participants, and rare cases of locations where voxel clusters were present in 4-7 participants.
Figure 3.
A whole brain voxelwise histogram of voxel clusters over 25 voxels in size demonstrates that voxel clusters in blast exposed (top panel) and blast TBI (lower panel) groups were distributed heterogeneously both within and across subjects. The voxelwise histogram overlay shows that for most locations (voxels), there were voxel clusters present in only 1 participant (lime-green). There were a few locations where voxel clusters were present in 2 (violet) or 3 (dark blue) participants. Only rarely were locations present in 4-7 subjects (data not shown).
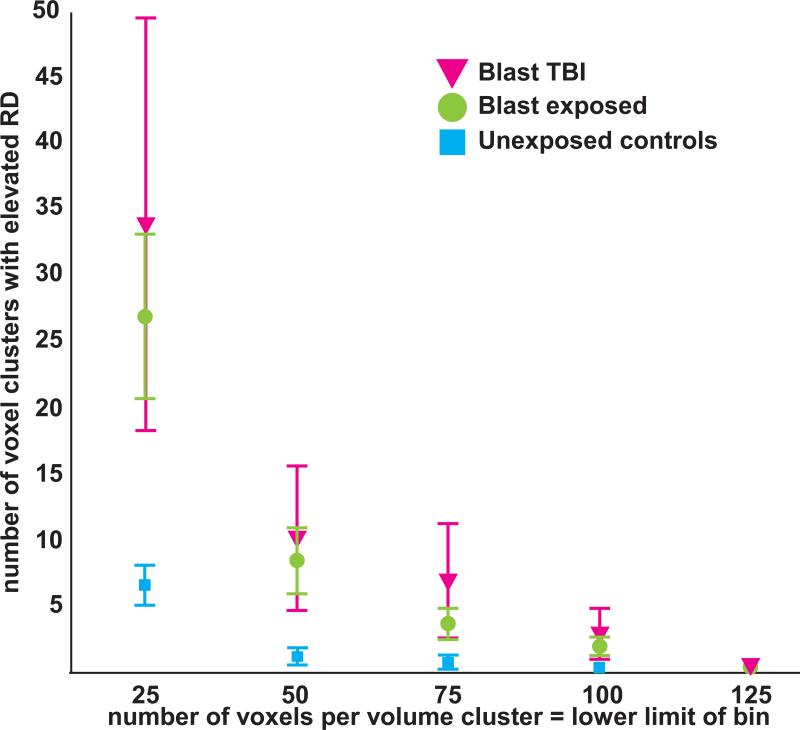
Spatial distribution of radial and axial diffusivity elevations
The magnitude of WM injury, measured as elevations in RD that were independent of spatial location, is shown for primary blast TBI, primary blast exposed, and the unexposed control groups in Figure 4. There was a significant main effect of group on the number of small, medium, and large RD voxel clusters (repeated measures) [F(2,42)=3.7, p=.034]. The unexposed control group had significantly fewer RD voxel clusters than the primary blast TBI group (p = .03) and the primary blast exposed group (p = .025), with no significant difference between the primary blast TBI and primary blast exposed groups (p = .5). Age was not significantly correlated with number of voxel clusters [F(1,41)=.005, p = .9]. There was no main effect of size on the number of RD voxel clusters [F(2,82)=2.2, p = .12]. Finally, there was an interaction of group * volume size [F(4,82)=4.2, p =.004] where small voxel clusters had greater between-group differences than the medium and large voxel clusters (Figure 4).
Figure 4.
There was a significant main effect of group [F(2,42)=3.7, p=.034] on the number of small (25-50 voxel), medium (50-75 voxel), and large (75-100 voxel) voxel clusters, which were defined by high radial diffusivity (RD) (z >2). The blast unexposed control group had significantly fewer voxel clusters than the blast TBI group (p = .03) and the blast exposed group (p = .025). Based on the number of voxel clusters, the blast exposed group resembled the blast TBI group when considered in relation to the blast unexposed control group.
The magnitude of WM injury, measured as elevations in AD that were independent of spatial location, is shown for the primary blast TBI, primary blast exposed, and the unexposed control groups in Figure 5. There was a non-significant main effect of group on the number of small, medium, and large AD voxel clusters (repeated measures) [F(2,42)=2.0, p=.15]. Age was not significantly correlated with number of voxel clusters [F(1,41)=2.4, p= .13]. There was a main effect of size on the number of AD voxel clusters [F(2,82)=8.7, p< .0001] with significantly fewer large than medium voxel clusters, and fewer medium voxel clusters than small.
Figure 5.
There was a non-significant main effect of group [F(2,42)=2.0, p=.15] on the number of small (25-50 voxel), medium (50-75 voxel), and large (75-100 voxel) voxel clusters, which were defined by voxels with high axial diffusivity (AD) values (z >2).
Neuropsychological measures
Hierarchical linear regression models including age, race, education, PTSD symptoms, MDD symptoms, and DTI metric components significantly predicted the IED shift number of stages completed [F(7,37)=2.4, p<.04] and post-shift percent errors [F(7,37)=2.8, p<.02]; simple reaction time [F(7,37)=5.8, p<.001]; as well as the number of errors on the SWM test [F(7,37)=2.3, p<.05]. The addition of DTI metric components significantly improved the IED post-shift percent errors (p<.05) and simple reaction time (p<.05), but not the SWM (p<0.17) or IED stages complete (p<.08) models. At the individual level, the FA component was a significant predictor of simple reaction time (Beta=−.340, p<.02) and SWM errors (Beta=−.343, p<.04), each in the expected direction. The diffusivity component was a significant predictor of IED post-shift percent error (Beta=.348, p<.02) in the expected direction.
DISCUSSION
The major finding of this study is that altered WM metrics were present in veterans with a remote (>1 year) history of exposure to primary blast forces both with (primary blast TBI) and without (primary blast exposed) self-report of symptoms at the time of the event consistent with criteria for mild TBI. A spatially diffuse distribution of abnormal voxel clusters was present in both groups consistent with the anatomically heterogeneous and individually variable nature of injury identified in both imaging and neuropathological studies of mild TBI.39;40 The analytic approach allowing for heterogeneity in the location of altered metrics (z-score analysis) was more sensitive than the conventional whole brain voxelwise method (TBSS) that relies on consistency in location. Although the TBSS analysis suggested WM vulnerability was limited to the right parietal lobe in the primary blast-exposed group and left parietal lobe in the primary blast TBI group, the z-score voxel cluster analysis demonstrated that the WM vulnerability was far more extensive. Diagnosis of PTSD was not correlated with WM injury using either the spatially independent or conventional whole brain analysis methods. The z-score approach did not indicate heightened vulnerability of any specific WM locations.
The presence of voxel clusters with abnormal DTI metrics in both the primary blast exposed and primary blast TBI groups is open to multiple interpretations. The challenges of obtaining accurate self-report of symptoms at a considerable time after the occurrence of combat-related events is well recognized. Thus, it is possible that the primary blast exposed group included individuals who had experienced a more severe exposure than their memory of symptoms would indicate. Another possible explanation for relatively similar levels of WM compromise in the primary blast-exposed and the primary blast TBI groups could be the focus of established criteria for mild TBI on altered sensorium. It stands to reason that the level and nature of symptoms experienced relates to the level of disruption of brain regions supporting specific functions. For instance, sufficient injury to the reticular activating system (RAS) can produce altered consciousness41, but a similar degree of injury to a cortical region might not produce overt symptoms. The possibility that primary blast forces may induce injury without causing clear alteration in consciousness is supported by studies reporting evidence for subconcussive effects of low level blast exposures and blunt force impacts in sports. The possibility that acute symptoms following exposure to primary blast may not accurately reflect injury state is also supported by a study in an urban trauma center reporting that almost one third (10/32) of patients presenting with blast-induced head injury (none due to terrorist activity) received a delayed diagnosis of mild TBI following development of altered mental status and/or new onset neuroimaging findings later in the hospital course.1
The widely distributed areas of loss of WM integrity is striking, and consistent with previous findings in mild TBI due to other injury mechanisms, confirming our hypothesis.39;40 Both non-blast and blast injury lead to changes at the cellular level via several biochemical pathways including free radical generation, disruption of calcium homeostasis, and release of inflammatory mediators.2;5;8;39 Earlier models of impact TBI emphasizing focal or multi-focal injury evolving from a peri-contusional process have been supplanted with a model that also includes diffuse mechanical forces of injury that may trigger non-contusion cell death cascades.42;43 The current hypotheses for primary blast-induced neurotrauma include mechanical forces, vasospasm, production of nitric oxide synthase, and glial activation as well as immune-mediated and/or glutamate-mediated cell damage that results in apoptosis and necrosis of glial cells responsible for maintaining homeostasis, formation of myelin, and support of neurons.2;5;8 Our results appear to be consistent with loss of WM integrity that may be proximally explained by factors associated with the magnitude of the mechanical forces, but its diffuse pattern is also consistent with evidence of injury from downstream cascade of neurochemical and neurotoxic processes.42;43
Our result showed significant elevation of both RD and AD in the primary blast exposed and primary blast TBI groups compared to the control group. While it is tempting to interpret increased RD as suggestive of myelin injury and increased AD as suggestive of axonal injury44, such conclusions must be made with caution.45;46 Simulations46 show that a change in RD can cause a fictitious change in AD and vice versa particularly in voxels characterized by crossing fibers. Other confounding factors include partial volume effects from the low anisotropy, characteristic of gray matter, and when the diffusion ellipsoid is oblate, indicating the principal eigenvector has a large cone of uncertainty.47 Given that a typical DTI imaging voxel contains thousands of fibers48, researchers are generally discouraged from interpreting changes in AD and RD on the basis of the underlying tissue structure.46
The neuropsychological effects of subconcussive blast are not well known. We observed a relationship between WM compromise (FA voxel clusters) and tests of simple reaction time and set shifting (an executive function subserved by widely distributed neural systems in the dorsolateral prefrontal, parietal, orbitofrontal, and anterior cingulate cortices) that was present even when controlling for other possibly confounding variables (age, race, education, PTSD and MDD symptoms). However, DTI metrics were not related to performance on several other cognitive tests, including other tests of executive functioning such as decision making, planning, and organization. Similarly, two recent studies have also reported altered DTI metrics associated with presence of functional deficits (sleep disturbances or executive dysfunction) in patients with mild TBI.49;50 Prior evidence of cognitive performance changes associated with subconcussive blast exposure is largely missing from the literature. Reports of pre-post season WM alteration from subconcussive sports injuries have shown decrements in visual motor speed and reaction time21, whereas other studies of sports-related subconcussive exposure have not reported cognitive changes.20;22 Deficits related to WM compromise detected by DTI may manifest later in life when the effects of aging erode the benefits of neural redundancy and compensatory processes.51;52
Caveats and Limitations
A few limitations deserve mention. The sample size of the primary blast TBI group is small. When exposures occur in a battlefield situation, it can be extremely difficult to confidently identify cases of only primary blast injury. Moreover, isolated primary blast injury without secondary or tertiary injury is uncommon. Similarly the number of exposures, particularly those occurring in rapid succession, are difficult to document. Battlefield accounts suggest that repetitive exposure in rapid succession is commonplace, and the dose response of repetitive blast exposure in humans is unknown. Without baseline measurements, it is not possible to infer a causal relationship between blast exposure, alterations in WM integrity, and performance on neuropsychological tests with complete certainty. The self-report and retrospective nature of TBI assessment and diagnosis also limits the certainty of group assignment. Finally, the resolution of the DTI scan was limited with a large spacing between slices (z-dimension of 6-mm) relative to the in-plane resolution (2-mm).
Conclusion
Blast exposed veterans not reporting acute symptoms of TBI display WM abnormalities comparable to cases of primary blast related mild TBI. Our results are consistent with recent reports of WM abnormalities from repetitive subconcussive events in elite sports participants, and suggest the DTI z-score analytic method may be quite sensitive to the diffuse and heterogeneous injuries characteristic of such events. The lack of clear TBI symptoms following blast exposure may lead to the erroneous assumption that there has been little or no effect on the central nervous system. Such injuries may produce subtle cognitive deficits or later manifestation of symptoms. Our results must be considered preliminary and necessitate replication using larger sample sizes to evaluate the clinical utility of DTI z-score analysis. If confirmed, our findings would argue for supplementing the established approach for making diagnoses based purely on clinical history and observable acute symptoms of mild TBI with novel imaging based diagnostic criteria that “look below the surface” for pathology.14;15;44
Supplementary Material
Acknowledgments
Sources of Funding:
This research was supported by a grant from the Department of Defense, Joint Improvised Explosive Device Defeat Organization (51467EGJDO), the Department of Veterans Health Affairs Rehabilitation Research and Development (RX000389-01), and with resources of the Mid-Atlantic Mental Illness Research, Education, and Clinical Center, and W.G. Hefner Veterans Affairs Medical Center
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimers:
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the Department of Defense, or the US government.
Reference List
- 1.Bochicchio GV, Lumpkins K, O'Connor J, et al. Blast injury in a civilian trauma setting is associated with a delay in diagnosis of traumatic brain injury. Am Surg. 2008;74:267–270. [PubMed] [Google Scholar]
- 2.Cernak I, Noble-Haeusslein L. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Huang W. Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj. 2011;25:641–650. doi: 10.3109/02699052.2011.580313. [DOI] [PubMed] [Google Scholar]
- 4.MacGregor AJ, Dougherty AL, Galarneau MR. Injury-specific correlates of combat-related traumatic brain injury in Operation Iraqi Freedom. J Head Trauma Rehabil. 2011;26:312–318. doi: 10.1097/HTR.0b013e3181e94404. [DOI] [PubMed] [Google Scholar]
- 5.Bass CR, Panzer MB, Rafaels KA, Wood G, Shridharani J, Capehart B. Brain injuries from blast. Ann Biomed Eng. 2012;40:185–202. doi: 10.1007/s10439-011-0424-0. [DOI] [PubMed] [Google Scholar]
- 6.Sams R, LaBrie DW, Norris J, Schauer J, Frantz E. IED blast postconcussive syncope and autonomic dysregulation. Mil Med. 2012;177:48–51. doi: 10.7205/milmed-d-11-00214. [DOI] [PubMed] [Google Scholar]
- 7.Yeh DD, Schecter WP. Primary blast injuries - An updated concise review. World J Surg. 2012;36:966–972. doi: 10.1007/s00268-012-1500-9. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth JL, Grimes J, Ling GSF. Pathophysiology of battlefield associated traumatic brain injury. Pathophysiology. 2013;20:23–30. doi: 10.1016/j.pathophys.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Ganpule S, Alai A, Plougonven E, Chandra N. Mechanics of blast loading on the head models in the study of traumatic brain injury using experimental and computational approaches. Biomech Model Mechanobiol. 2013 doi: 10.1007/s10237-012-0421-8. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Management of Concussion-mTBI Working Group VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46:CP1–CP68. [PubMed] [Google Scholar]
- 11.MacDonald C, Johnson A, Cooper D, et al. Cerebellar white matter abnormalities following primary blast injury in US military personnel. PLoS one. 2013;8:e55823. doi: 10.1371/journal.pone.0055823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen A, Zhang Y, Zhan W, et al. Radiology corner. Case 41. Arcuate fasciculus damage seen on DTI in a blast-exposed soldier with mild traumatic brain injury (mTBI) with associated conduction aphasia. Mil Med. 2009;174:v–vi. [PubMed] [Google Scholar]
- 13.Hayes JP, Morey RA, Tupler LA. A case of frontal neuropsychological and neuroimaging signs following multiple primary-blast exposure. Neurocase. 2012;18:258–269. doi: 10.1080/13554794.2011.588181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 15.Le Bihan D, Johansen-Berg H. Diffusion MRI at 25: exploring brain tissue structure and function. Neuroimage. 2012;61:324–341. doi: 10.1016/j.neuroimage.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J Head Trauma Rehabil. 2013;28:1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- 17.Jensen KB, Kaptchuk TJ, Kirsch I, et al. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dashnaw ML, Petraglia AL, Bailes JE. An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg Focus. 2012;33:E5. doi: 10.3171/2012.10.FOCUS12284. [DOI] [PubMed] [Google Scholar]
- 19.Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2012;30:171–180. doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koerte IK, Kaufmann D, Hartl E, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus. 2012;33:E3. doi: 10.3171/2012.10.FOCUS12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breedlove EL, Robinson M, Talavage TM, et al. Biomechanical correlates of symptomatic and asymtomatic neurophysiological impairment in high school football. J Biomech. 2012;45:1265–1272. doi: 10.1016/j.jbiomech.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 24.Talavage TM, Nauman EA, Breedlove EL, et al. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma. 2013 doi: 10.1089/neu.2010.1512. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy PE. Randomization tests in econometrics. J Bus Econ Stat. 1995;13:85–94. [Google Scholar]
- 27.Anderson MJ, Robinson J. Permutation tests for linear models. Aust N Z J Stat. 2001;43:75–88. [Google Scholar]
- 28.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 29.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messe A, Caplain S, Paradot G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp. 2011;32:999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuang L, Wen W, Zhu W, et al. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 32.Mori S, Wakana W, Nagae-Poetscher LM, Van Zijl PCM. MRI atlas of human white matter. First ed. Elsevier; Amsterdam: 2005. [Google Scholar]
- 33.Lilliefors HW. On Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;62:399–402. [Google Scholar]
- 34.Jorge RE, Acion L, White T, et al. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiat. 2012;169:1284–1291. doi: 10.1176/appi.ajp.2012.12050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White T, Ehrlich S, Ho B-C, et al. Spatial characteristics of white matter abnormalities in schizophrenia. Schizophr Bull. 2013 doi: 10.1093/schbul/sbs106. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuff N, Zhang Y, Zhan W, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: A MRI study. Neuroimage. 2011;54:S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuity. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage2004. 21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6:108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- 40.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blyth BJ, Bazarian JJ. Traumatic alterations in consciousness: traumatic brain injury. Emerg Med Clin N Am. 2010;28:571–594. doi: 10.1016/j.emc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buki A, Povlishock JT. All roads lead to diconnection?--Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148:181–193. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- 43.Farkas O, Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- 44.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage2002. 17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 45.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med2009. 61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 47.Jones DK. Determining and visualizing uncertainty in estimates of fiber orientation from diffusion tensor MRI. Magn Reson Med. 2003;49:7–12. doi: 10.1002/mrm.10331. [DOI] [PubMed] [Google Scholar]
- 48.Wedeen VJ, Rosene DL, Wang R, et al. The geometric structure of brain fiber pathways. Science. 2012;335:1628–1634. doi: 10.1126/science.1215280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fakhran S, Yaeger K, Alhilali L. Symptomatic white matter changes in mild traumatic brain injury resemble pathololgic features of early Alzheimer dementia. Radiology. 2013;269:249–257. doi: 10.1148/radiol.13122343. [DOI] [PubMed] [Google Scholar]
- 50.Sorg SF, Delano-Wood L, Luc N, et al. White matter integrity in veterans with mild traumatic brain injury: Associations with executive function and loss of consciousness. J Head Trauma Rehabil. 2013 doi: 10.1097/HTR.0b013e31828a1aa4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartres-Faz D, Arenaza-Urquijo EM. Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr. 2011;24:340–357. doi: 10.1007/s10548-011-0195-9. [DOI] [PubMed] [Google Scholar]
- 52.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11:1103–1112. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.