Abstract
Introduction
Laser photocoagulation is the current gold standard treatment for proliferative retinopathy of prematurity (ROP). However, it permanently reduces the visual field and might induce myopia. Vascular endothelial growth factor (VEGF) inhibitors for the treatment of ROP may enable continuing vascularization of the retina, potentially allowing the preservation of the visual field. However, for their use in infants concern remains. This meta-analysis explores the safety of VEGF inhibitors.
Methods
The Ovid Interface was used to perform a systematic review of the literature in the databases PubMed, EMBASE and the Cochrane Library.
Results
This meta-analysis included 24 original reports (including 1.457 eyes) on VEGF inhibitor treatment for ROP. The trials were solely observational except for one randomized and two case-control studies. We estimated a 6-month risk of retreatment per eye of 2.8%, and a 6-month risk of ocular complication without the need of retreatment of 1.6% per eye. Systemic complications were only reported as isolated incidents.
Discussion
VEGF inhibitors seem to be associated with low recurrence rates and ocular complication rates. They may have the benefit of potentially allowing the preservation of visual field and lower rates of myopia. Due to the lack of data, the risk of systemic side effects cannot be assessed.
Introduction
Retinopathy of prematurity (ROP) is one of the major causes of childhood blindness in the industrialized world. It is caused by an abnormal growth of retinal blood vessels [1]. The incidence of ROP is constantly increasing as larger, more mature infants in countries, where expertise in neonatal and ophthalmologic care is nascent, survive to develop ROP and as more immature infants are surviving, which develop ROP despite excellent neonatal care [2]. Laser photocoagulation is the gold standard treatment for ROP. Although laser photocoagulation is successful in many cases, it might reduce the visual field [3] and contribute to the development of myopia [4]. Therefore, an alternative may be useful [5].
Vascular endothelial growth factor (VEGF) is recognized as an important factor in the vascularization of the retina and the development of ROP [1]. Angiogenesis of the retina commences at approximately 17 weeks postmenstrual age. At this stage the metabolic demands of the neural retina outpace the oxygen supplied by the choroid. This physiologic hypoxia causes VEGF secretion stimulating new vessel formation until vascular development is complete just prior to birth. In preterm infants, the sudden increase in oxygen saturation after birth causes a down-regulation of growth factors resulting in a disruption of retinal vascular development. This is followed by a phase in which the attenuated vasculature cannot supply enough oxygen to the developing retina [6]. This hypoxic state leads to a VEGF overexpression inducing pathologic and excessive neovascularization at the avascular junction [7,8].
Anti-VEGF agents are widely used to effectively treat diseases of neovascular origin in adult eyes. In ROP, they may stop or reduce pathologic neovascularization. The biggest advantage of anti-VEGF metabolites is that, in contrast to laser photocoagulation, the retina does not seem to be permanently damaged [9]. However, for the use of anti-VEGF agents in infants concern remains [10,11].
Systemic side effects are of particular interest, as preterm infants with proliferative ROP have a compromised blood-retinal barrier possibly allowing a large amount of VEGF inhibitors to enter the blood stream [12]. Intravitreal bevacizumab and to a lesser extent ranibizumab seem to suppress systemic VEGF and thus systemic side effects cannot be excluded [13–16]. In adults, it is still under debate whether intravitreal injections of VEGF inhibitors increase the risk of thrombotic events [17,18].
Laser photocoagulation remains the standard treatment for ROP. However, laser photocoagulation may destroy large areas of the retina [5]. Therefore an alternative treatment is of interest, especially for preterm infants with zone 1 ROP. Yet, the use of VEGF inhibitors raises issues on ocular and systemic side effects. There is still little evidence on the safety of intravitreal VEGF inhibitors for ROP treatment. This study addresses 7 years of published data on VEGF inhibitors safety in preterm infants. The specific aims of this study were to determine ocular and systemic complications after the use of VEGF inhibitors for the treatment of ROP.
Methods
Search history
From December 27th, 2014, until January 8th, 2015, we used an Ovid Interface to search for the following medical subject headings in the databases PubMed, EMBASE, and The Cochrane Library: “vascular endothelial growth factor” AND “Retinopathy of Prematurity”; “Bevacizumab” AND “Retinopathy of Prematurity”; “Ranibizumab” AND “Retinopathy of Prematurity”; “Aflibercept” AND “Retinopathy of Prematurity”; “Pegaptanib” AND “Retinopathy of Prematurity”. The reference lists of included studies were additionally scanned to identify potentially relevant reports. Two investigators (MD) performed the literature search and study selection.
All trials were included if they reported the use of VEGF inhibitors for retinopathy of prematurity stages 3 or 4. Studies were excluded, if VEGF Inhibitors were used for recurrences after initial treatment with laser photocoagulation or vitrectomy. All animal studies were excluded. Only studies in English language were included. All abstracts and conference proceedings that are not published in peer-reviewed journals were excluded. If VEGF inhibitors were used in combination with other treatment options, they were not included for quantitative analysis. Case reports, small case series (<6 eyes) and trials with selective reporting of cases were reported, but not included in the quantitative analysis.
The primary outcomes are ocular complications requiring retreatment, ocular complication without the need of retreatment and systemic complications. Secondary outcome is refractive error.
Statistical methods
All statistical analyses were performed using MetaXL. We observed pronounced differences regarding the length of follow-up between the individual studies. This may lead to bias, because the risk of experiencing an event (e.g. retreatment, complication) will be a priori higher in a study with e.g. 24 months of follow-up than in a study with e.g. 3 months of follow-up. To minimize bias in the presence of this heterogeneity, we transformed the original endpoint proportions reported by the individual studies to (constant) rates. These rates were pooled using random-effects meta-analysis. Finally, the pooled estimate was transformed into a 6-month proportion of endpoint risk.” In detail, we first calculated the eyetime-at-risk in months for each study, defined as the product of the follow-up duration in months and the number of eyes that did not experience the respective endpoint, plus the product of the number of eyes that experienced the respective endpoint and half of the follow-up duration. This procedure assumes that events occurred on average at the temporal midpoint between baseline and study end. Then, we obtained study-specific endpoint rates by dividing the number of events experienced during follow-up by the eyetime-at-risk in months. For studies without any events during follow-up, we applied a continuity correction and set the number of events to 0.5. Between-study heterogeneity of endpoint risks were assessed using the I-squared statistic and Cochran’s Q. The study-specific endpoint rates were then meta-analyzed using a random effects model (“NumRate” Table function in MetaXL). Finally, to obtain 6-month risks of developing the endpoints of interest, we assumed a constant event rate and hence used the following formula:
, where t is 6 months.
To derive a meta-analysis estimate for the refractive error, we calculated an arithmetic mean weighted by the number of patients in the individual studies.
Results
Included studies
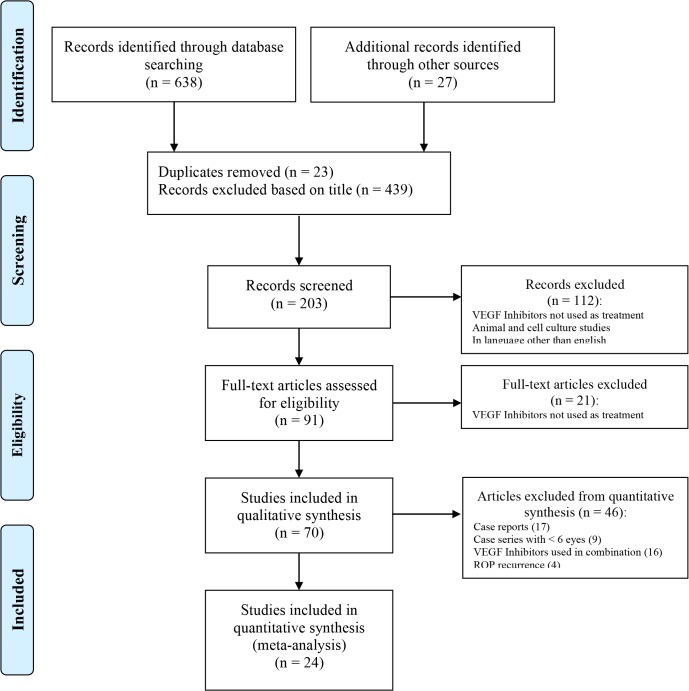
Our search identified 665 records. Of those 70 original papers were found to be relevant for our study. From these 17 were case reports, 9 case series with <6 eyes, 2 case series with ≥6 eyes, 28 retrospective, 11 prospective non-randomized, 2 case-controls and 1 randomized trial. In 4 of these 70 trials, VEGF inhibitors were compared to laser photocoagulation. The other 66 trials were purely observational data.
Case reports (17 trials), case series with less than six eyes (9 trials), trials investigating bevacizumab in combination with other treatment options (16 trials) and trials investigating the use of VEGF inhibitors for recurrences (4 trials) were excluded from quantitative analysis (see Fig 1).
Fig 1. Flow chart of study exclusion.
Adoption of the PRISMA flow diagram. It shows the number of trials identified, included and excluded, and reason for exclusions.
24 studies with 1.457 eyes were included for quantitative analysis 21 (see Tables 1 and 2 and S1 Table). Out of these 1.457 eyes, 1.121 eyes (596 patients) fitted our criteria (no combined treatment, no recurrence). Of these 24 studies, ranibizumab was used in two and aflibercept in one trial.
Table 1. Characteristics of included studies.
| Study | Study Type | Number of eyes | Intervention | Follow-Up (months)** | Ocular complication requiring retreatment | Ocular complication without the need of retreatment |
|---|---|---|---|---|---|---|
| Bancalari, 2014 [19] | retrospective | 24 | Bevacizumab | 12 | 8 (33.3%) | 1 (4.2%) |
| Chen, 2014 [20] | retrospective | 72 | Ranibizumab Bevacizumab | 12 | 0 (0%) | 0 (0%) |
| Chen, 2014 [21] | retrospective | 57* | Bevacizumab w/ or w/o Laser coagulation | 24 | - | - |
| Cheng, 2014 [22] | retrospective | 13 | Bevacizumab | - | 1 (7.7%) | 0 (0%) |
| Geloneck, 2014 [23] | Follow-Up of Mintz-Hittner (38) | 211* | Bevacizumab Laser coagulation | 30 | - | - |
| Harder, 2014 [24] | retrospective | 57 | Bevacizumab | - | 1 (1.8%) | 0 (0%) |
| Henaine-Berra, 2014 [25] | prospective, non-randomized | 46 | Bevacizumab | - | 0 (0%) | 0 (0%) |
| Kuniyoshi, 2014 [26] | retrospective | 8 | Bevacizumab | - | 2 (25%) | 0 (0%) |
| Lepore, 2014 [27] | case-control | 24* | Bevacizumab Laser coagulation | 12 | 0 (0%) | 0 (0%) |
| Moran, 2014 [28] | case-control | 28* | Bevacizumab Laser coagulation | 24 | 3 (21.4%) | 0 (0%) |
| Salman, 2014 [29] | prospective, non-randomized | 26 | Aflibercept | 12 | 1 (3.8%) | 0 (0%) |
| Yetik, 2014 [30] | prospective, non-randomized | 238 | Bevacizumab | - | 11 (4.6%) | 0 (0%) |
| Castellanos, 2013 [31] | prospective, non-randomized | 6 | Ranibizumab | 36 | 0 (0%) | 0 (0%) |
| Harder, 2013 [32] | retrospective | 49* | Bevacizumab Laser coagulation | 36 | 0 (0%) | 0 (0%) |
| Martinez-Castellanos, 2013 [33] | prospective, non-randomized | 18* | Bevacizumab | 60 | 0 (0%) | 0 (0%) |
| Sahin, 2013 [34] | retrospective | 30 | Bevacizumab | - | 2 (6.7%) | 5 (16.7%) |
| Wu, 2013 [35] | retrospective, multi-center | 162 | Bevacizumab | - | 19 (11.7%) | 4 (2.5%) |
| Dani, 2012 [36] | prospective, non-randomized | 7* | Bevacizumab w/ or w/o Laser coagulation | - | 0 (0%) | 0 (0%) |
| Harder, 2012 [37] | prospective, non-randomized | 32* | Bevacizumab Laser coagulation | 12 | - | - |
| Axer-Siegel, 2011 [38] | retrospective | 18* | Bevacizumab w/ or w/o Laser coagulation | - | 1 (10%) | 0 (0%) |
| Harder, 2011 [39] | retrospective | 23 | Bevacizumab | - | 0 (0%) | 0 (0%) |
| Mintz-Hittner, 2011 [40] | prospective, randomized | 286* | Bevacizumab Laser coagulation | 12 | 6 (4.3%) | - |
| Dorta, 2010 [41] | retrospective | 12 | Bevacizumab | - | 0 (0%) | 1 (8.3%) |
| Mintz-Hittner, 2008 [42] | retrospective | 22 | Bevacizumab | - | 0 (0%) | 0 (0%) |
Studies are sorted by date of publication.
*Not all patients included for quantitative analysis, as only selected patient groups fitted our criteria (no combined treatment, no recurrences).
**The follow-up period is only reported if follow-up was a defined period of time.
Table 2. Characteristics of included studies for refractive error.
| Study | Study Type | Number of eyes | Intervention | Follow-Up (months)* | Refractive Error | Refractive Error |
|---|---|---|---|---|---|---|
| Sphere | Cylinder | |||||
| Chen, 2014 [21] | retrospective | 40 | Bevacizumab | 24 | 0.98 | 2.23 |
| 17 | Bevacizumab with laser coagulation | 24 | -2.4 | 2.23 | ||
| Geloneck, 2014 [23] | Follow-Up of | 110 | Bevacizumab | 30 | -1,02 | |
| Mintz-Hittner (38) | 101 | Laser coagulation | 30 | -6,73 | ||
| Harder, 2013 [32] | retrospective | 23 | Bevacizumab | 12 | 0 | -1 |
| 26 | Laser coagulation | 12 | -5.5 | 1.5 | ||
| Harder, 2012 [37] | prospective, | 12 | Bevacizumab | - | 0.635 | 1.015 |
| non-randomized | 20 | Laser coagulation | - | -5.225 | 1.69 | |
| Axer-Siegel, 2011 [38] | retrospective | 10 | Bevacizumab | - | 0.5 | 0.5 |
| 8 | Bevacizumab with laser coagulation | - | -7.25 | 0 | ||
| Mintz-Hittner, 2011 [40] | prospective, | 140 | Bevacizumab | 12 | 0.99 | - |
| randomized | 146 | Laser coagulation | 12 | 7.01 | - |
Studies are sorted by date of publication.
*The follow-up period is only reported if follow-up was a defined period of time.
All other trials investigated bevacizumab. The dosage for bevacizumab was 0.625mg in twelve, 0.25mg in one, 0.375mg in four, 0.5mg in one, 0.75mg in one and 1.25mg in two trials. The two trials investigating ranibizumab used 0.25mg and the trial investigating aflibercept used 1.0mg.
Ocular complications
Ocular complications could be calculated for 882 eyes. Of these 55 eyes (6.2%) developed an ocular complication requiring retreatment. Using random effects meta-analysis, we estimated the 6-month risk of developing an ocular complication requiring retreatment at 2.8%. The reasons for retreatment were recurrent neovascularization in 32 cases (58.2%), retinal hemorrhage in 10 cases (18.2%), retinal detachment in 9 cases (16.4%), partial retinal detachment in 1 case (1.8%), macular dragging in 2 cases (3.6%) and persistent plus disease in 1 case (1.8%).
Ocular complications without the need of retreatment occurred in 11 (1.2%) out of 882 eyes. The 6-month risk of this endpoint was estimated at 1.6% per eye. The ocular complications were retinal hemorrhage spontaneously regressing in 8 cases (72.7%), cataract in 1 case (9.1%), mild macular traction in 1 case (9.1%) and exotropia in 1 case (9.1%). There was no correlation of ocular complication rate and dosage.
Systemic complications
Systemic complications were investigated in 585 patients. Of these 8 patients (1.4%) reported systemic complications after intravitreal injections. However, in none of the cases the treatment with VEGF inhibitors was considered to be the cause of the complication.
In the only randomized study by Mintz-Hittner [40], five infants died in the bevacizumab group compared to two infants in the laser photocoagulation group. The five patients receiving intravitreal bevacizumab died of asphyxia (two infants, 16.3 and 12.3 weeks after intravitreal injection of bevacizumab or IVB), respiratory failure (two infants, 4.8 and 13 weeks after IVB) and adherence to do-not-resuscitate order (one infant, 0.4 weeks after IVB). The infants of the control group died of sepsis (one infant, 4.8 weeks after laser photocoagulation) and respiratory failure (one infant, 4.2 weeks after laser photocoagulation). In a case-control study, there was a report of an infant, who died after intravitreal injection [27]. In one study, one infant showed delay in growth, pulmonary dysplasia and intraventricular hemorrhage [33]. The vascular activity in the contralateral eye decreased after unilateral injection of bevacizumab in one patient [34].
The trials investigating ranibizumab and aflibercept [20,29,31] did not report any systemic complications.
Studies, not included in quantitative analysis
In the studies, which were not included in this papers quantitative analysis, the most frequent reported ocular complication was retinal detachment requiring treatment after intravitreal injection of VEGF inhibitors [43–50]. There was one case report of spontaneous reattachment [51]. Especially important are the reports of initial regression with delayed recurrent neovascularization after bevacizumab (with or without laser photocoagulation), which occurred up to 19 weeks after intravitreal injection [52–57]. Other serious side effects included acute contraction of proliferative membrane [44,58–59], vascular sheathing [57], choroidal ischemia [60], optic atrophy [61], disc pallor [62], retinal break, RPE rupture [61] and choroidal rupture [63], which all occurred after bevacizumab combined with laser photocoagulation. Less severe side effects were cataract [51,57], increased IOP [51,64] and mild vitreous organization [65].
At the same time there are also many reports of VEGF inhibitor use in ROP without any ocular complications [66–78].
There were 27 trials with 592 patients not included in this papers quantitative analysis investigating systemic complications. In 25 trials no systemic complications were reported [43–44,47,49–50,57,59,62,64–68,74–85]. There was one report of short-term raised liver enzymes after bevacizumab injection [61] and one case report showing reduced vascular activity in the contralateral eye [5].
Discussion
In this meta-analysis, retreatment was required in 2.8% of cases within 6 months and ocular complication rates without the need of retreatment were 1.6% per eye. Systemic complications were only reported as isolated incidents.
Laser treatment has proven useful to reduce progression of ROP. However, visual outcomes especially for zone 1 ROP are poor [86] and progression requiring retreatment occurs in approximately 11–20% of eyes despite adequate peripheral ablation [78,87–91]. Laser photocoagulation is associated with visual field defects, severe myopia, strabismus, glaucoma, cataract formation, corneal edema, and intraocular hemorrhage [86,92–95].
Intravitreal injection of VEGF inhibitors may be a useful alternative, especially for zone 1 ROP, as it allows continuing vascularization of the retina. Thus it may reduce visual field defects.
Further, VEGF inhibitors may reduce the incidence of severe myopia. The mean refractive error was always lower after intravitreal VEGF inhibitors if compared to laser photocoagulation (see Table 2). This difference is speculated to be a result of the minimal or absent development of the anterior segment of the retina after laser photocoagulation [23].
VEGF inhibitors might also be useful in severe cases of ROP for salvage treatment. Injection of VEGF inhibitors may reduce vascularization and thus allow laser treatment [48,73,78,84–85]. It might also improve the outcome after vitrectomy [77,96] and lower recurrences if combined with laser photocoagulation [82].
There are, however, many open questions. The biggest is on safety of VEGF inhibitors for preterm infants. Retreatment rates of ROP (e.g. retinal detachment or neovascularization requiring retreatment) were low in our meta-analysis. Similarly ocular side effects, which did not require retreatment, such as cataract, IOP increase and retinal hemorrhage were low. In the only randomized study comparing both modalities by Mintz-Hittner [40], the recurrence rate was higher in laser photocoagulation (31.7% respectively 5.9%).
A definite disadvantage of VEGF inhibitors is the possibility of systemic side effects. In our meta-analysis there are only few reported cases. Systemic side effects are however difficult to assess, as infants with ROP develop neurologic and other developmental disorders more often than other infants [96]. In fact in only selected studies [28,33,50] pediatric assessment and/or an MRI brain scan were performed specifically to evaluate any abnormalities. No systemic complications were found in these cases. However the number needed to definitely determine an increase in mortality after bevacizumab injection compared to laser treatment for ROP is estimated at 2800 infants [40].
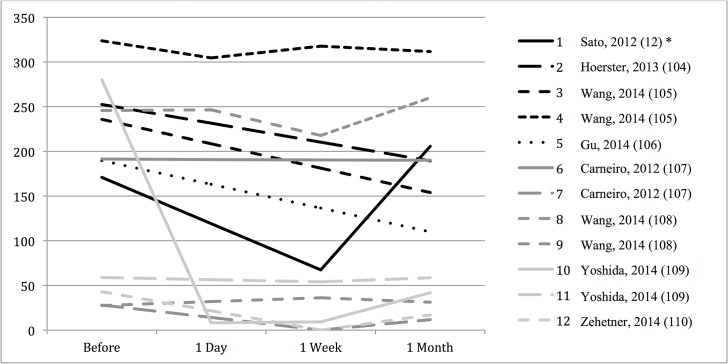
In adults, systemic complications of intravitreal VEGF inhibitors are still under debate. Some studies showed an increase in mortality, while others did not (see Table 3). After intravitreal injection, VEGF inhibitors are found in the systemic circulation and systemic VEGF levels decrease [97–103]. Therefore concern remains about systemic toxicity of intravitreal VEGF inhibitors in infants (see Fig 2).
Table 3. Adverse events after anti-VEGF treatment in adults in relative risk.
| Study | Study Type | VEGF inhibitor | Disorder | Deaths | Thromboembolic adverse events | Non-ocular hemorrhage |
|---|---|---|---|---|---|---|
| Thulliez, 2014 [18] | Meta-analysis | Ranibizumab | DME RVO AMD | - | 1.14 | - |
| Solomon, 2014 [104] | Meta-analysis | Pegaptanib | AMD | 1.12 | 0.63** | - |
| Meta-analysis | Ranibizumab | AMD | 0.82 | 1.14 | 1.64 | |
| Meta-analysis*** | Bevacizumab vs Ranibizumab | AMD | 1.28 (1.12) | 1.07 (1.13) | - | |
| Cheng, 2012 [105] | Meta-analysis | Ranibizumab Bevacizumab Pegaptanib | AMD DME RVO | 0.68* | 0.87 | - |
| RESTORE 2014 [106] | Trial | Ranibizumab | DME | 0.69 | 1.23 | |
| CATT, 2012 [107] | Trial | Bevacizumab vs Ranibizumab | AMD | 1.7 | 1.11 | - |
| ABC, 2010 [108] | Trial | Bevacizumab | AMD | 1.02 | 1.02 | - |
| FOCUS, 2008 [109] | Trial | Ranibizumab | AMD | 1.87 | 0.86** | - |
| Marina, 2006 [110] | Trial | Ranibizumab | AMD | 0.91 | 1.48 | 1.68 |
Studies are sorted by date of publication.
*Only vascular deaths were reported.
**Only cardiovascular events were reported.
***Events reported after 1 year (after 2 years).
Fig 2. Systemic VEGF suppression after VEGF inhibitors.
Research papers investigating systemic levels of VEGF after intravitreal injection of VEGF inhibitors over a defined period of time. *1 = infants with retinopathy of prematurity, unilateral injection of bevacizumab (14); 2 = infants with retinopathy of prematurity, unilateral injection of ranibizumab (105); 3 = patients with age-related macular degeneration (AMD), unilateral injection of bevacizumab (106); 4 = patients with AMD, bilateral injection of bevacizumab (106); 5 = patients with AMD, unilateral injection of ranibizumab (107); 6 = patients with AMD, unilateral injection of bevacizumab (108); 7 = patients with AMD, unilateral injection of ranibizumab (108); 8 = patients with AMD, unilateral injection of aflibercept (109); 9 = patients with AMD, unilateral injection of ranibizumab (109); 10 = patients with AMD, unilateral injection of ranibizumab (110); 11 = patients with AMD, unilateral injection of aflibercept (110); 12 = patients with AMD, unilateral injection of ranibizumab (111). ** y-axis in pg/ml.
Another possible disadvantage of anti-VEGF treatment are the reports of delayed recurrences, which occurred up to 19 weeks after injection. Thus, VEGF inhibitors require extensive, long-term follow-up [46].
To reduce complications, the lowest sufficient dosage should be used to treat ROP. At the moment, the recommended bevacizumab dosage for ROP infants is 0.625mg, which is half the adult dosage. However, the size-adjusted dose would be 0.4mg, which may be sufficient [111]. In our meta-analysis, there was no correlation of ocular complication rate and dosage. There is not enough evidence to support a recommended dosage.
In the trials using ranibizumab [20,31] no ocular and no systemic complications were reported. In the trial investigating aflibercept [29] one eye required retreatment, but no systemic complications were reported. Further research is needed on the comparison of safety and efficacy between bevacizumab, ranibizumab and aflibercept.
This is a meta-analysis of mostly observational data. Therefore, our data need to be interpreted in the sense that most of the evidence underlying our estimates stems from non-randomized trials. So far, there is only one randomized study by Mintz-Hittner [40]. Given that preterm infants are an especially vulnerable study group, this might not change in the near future. This paper explores the complications with the currently available literature.
There is a large heterogeneity in the studies included in this meta-analysis (see Table 1). Therefore we did not simply pool results, but used a random effects analysis to correct for the heterogeneity in the event rates. These differences in reported complication rates are probably not due to differences in the respective population but due to differences in reporting criteria. The endpoints studied in this meta-analysis were rarely clearly defined in the original papers and thus the criteria for reporting complications may differ greatly between studies.
The authors have no financial disclosure.
The data published so far suggest that intravitreal injection of VEGF inhibitors are a safe alternative for ROP treatment. The risk of systemic side effects cannot be fully assessed.
Conclusion
VEGF inhibitors seem to be associated with low recurrence rates and ocular complication rates. They may have the benefit of potentially allowing the preservation of visual field and lower rates of myopia. However concern remains, especially about possible systemic complications. There is a dire need for larger and randomized trials on the safety of VEGF Inhibitors for the treatment of ROP.
Supporting Information
(TIF)
Level of evidence as recommended by the Oxford Centre for Evidence-based Medicine. *Observational Studies.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Hartnett ME. Vascular Endothelial Growth Factor Antagonist Therapy for Retinopathy of Prematurity. Clin Perinatol. 2014. December;41(4):925–943. 10.1016/j.clp.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Zin A; International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005. May;115(5):e518–25. Epub 2005 Apr 1. [DOI] [PubMed] [Google Scholar]
- 3. McLoone E, O'Keefe M, McLoone S, Lanigan B. Effect of diode laser retinal ablative therapy for threshold retinopathy of prematurity on the visual field: results of goldmann perimetry at a mean age of 11 years. J Pediatr Ophthalmol Strabismus. 2007. May-Jun;44(3):170–3. [DOI] [PubMed] [Google Scholar]
- 4. Shah PK, Ramakrishnan M, Sadat B, Bachu S, Narendran V, Kalpana N. Long term refractive and structural outcome following laser treatment for zone 1 aggressive posterior retinopathy of prematurity. Oman J Ophthalmol. 2014. September;7(3):116–9. 10.4103/0974-620X.142592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karaca C, Oner AO, Mirza E, Polat OA, Sahiner M. Bilateral effect of unilateral bevacizumab injection in retinopathy of prematurity. JAMA Ophthalmol. 2013. August;131(8):1099–101. 10.1001/jamaophthalmol.2013.400 [DOI] [PubMed] [Google Scholar]
- 6. Smith LE, Hard AL, Hellström A. The biology of retinopathy of prematurity: how knowledge of pathogenesis guides treatment. Clin Perinatol. 2013. June;40(2):201–14. 10.1016/j.clp.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Velez-Montoya R, Clapp C, Rivera JC, Garcia-Aguirre G, Morales-Cantón V, Fromow-Guerra J, et al. Intraocular and systemic levels of vascular endothelial growth factor in advanced cases of retinopathy of prematurity. Clin Ophthalmol. 2010. September 7;4:947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu H, Zhang W, Xu Z, Caldwell RW, Caldwell RB, Brooks SE. Hyperoxia causes regression of vitreous neovascularization by downregulating VEGF/VEGFR2 pathway. Invest Ophthalmol Vis Sci. 2013. February 1;54(2):918–31. 10.1167/iovs.12-11291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mintz-Hittner HA. Treatment of retinopathy of prematurity with vascular endothelial growth factor inhibitors. Early Hum Dev. 2012. December;88(12):937–41. 10.1016/j.earlhumdev.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 10. Malik MA, Shukla S, Azad SV, Kaur J. Vascular endothelial growth factor (VEGF-634G/C) polymorphism and retinopathy of prematurity: a meta-analysis. Saudi J Ophthalmol. 2014. October;28(4):299–303. 10.1016/j.sjopt.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace DK, Wu KY. Current and future trends in treatment of severe retinopathy of prematurity. Clin Perinatol. 2013. June;40(2):297–310. 10.1016/j.clp.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 12. Hård AL, Hellström A. On safety, pharmacokinetics and dosage of bevacizumab in ROP treatment—a review. Acta Paediatr. 2011. December;100(12):1523–7. 10.1111/j.1651-2227.2011.02445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoerster R, Muether P, Dahlke C, Mehler K, Oberthür A, Kirchhof B, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol. 2013. February;91(1):e74–5. 10.1111/j.1755-3768.2012.02469.x [DOI] [PubMed] [Google Scholar]
- 14. Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012. February;153(2):327–333.e1 10.1016/j.ajo.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, et al. Serum Levels of Vascular Endothelial Growth Factor and Related Factors After Intravitreous Bevacizumab Injection for Retinopathy of Prematurity. JAMA Ophthalmol. 2015 Jan 8. [DOI] [PubMed]
- 16.Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of Bevacizumab and its Effects on Serum VEGF and IGF-1 in Infants with Retinopathy of Prematurity (ROP). Invest Ophthalmol Vis Sci. 2015 Jan 22. [DOI] [PubMed]
- 17.Schmid MK, Bachmann LM, Fäs L, Kessels AG, Job OM, Thiel MA. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: a trade-off analysis. Br J Ophthalmol. 2014 Jun 11. pii: bjophthalmol-2014-305149. [DOI] [PubMed]
- 18. Thulliez M, Angoulvant D, Le Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014. November;132(11):1317–26. 10.1001/jamaophthalmol.2014.2333 [DOI] [PubMed] [Google Scholar]
- 19. Bancalari M A, Schade Y R, Peña Z R, Pavez P N. Intravitreal bevacizumab as single drug therapy for retinopathy of prematurity in 12 patients. Arch Argent Pediatr. 2014. April;112(2):160–3. 10.1590/S0325-00752014000200009 [DOI] [PubMed] [Google Scholar]
- 20.Chen SN, Lian I, Hwang YC, Chen YH, Chang YC, Lee KH, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: Comparison Between Ranibizumab and Bevacizumab. Retina. 2014 Dec 1. [Epub ahead of print]. [DOI] [PubMed]
- 21. Chen YH, Chen SN, Lien RI, Shih CP, Chao AN, Chen KJ, et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye (Lond). 2014. September;28(9):1080–6; quiz 1087. 10.1038/eye.2014.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng HC, Lee SM, Hsieh YT, Lin PK. Efficacy of intravitreal injection of anti-vascular endothelial growth factor agents for stage 4 retinopathy of prematurity. Retina. 2014 Oct 13. [Epub ahead of print] [DOI] [PubMed]
- 23. Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. ; BEAT-ROP Cooperative Group. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014. November;132(11):1327–33. 10.1001/jamaophthalmol.2014.2772 [DOI] [PubMed] [Google Scholar]
- 24. Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal low-dosage bevacizumab for retinopathy of prematurity. Acta Ophthalmol. 2014. September;92(6):577–81. 10.1111/aos.12266 [DOI] [PubMed] [Google Scholar]
- 25. Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, Martinez-Castellanos MA. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J AAPOS. 2014. April;18(2):120–3. 10.1016/j.jaapos.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 26. Kuniyoshi K, Sugioka K, Sakuramoto H, Kusaka S, Wada N, Shimomura Y. Intravitreal injection of bevacizumab for retinopathy of prematurity. Jpn J Ophthalmol. 2014. May;58(3):237–43. 10.1007/s10384-014-0310-z [DOI] [PubMed] [Google Scholar]
- 27. Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal Bevacizumab versus Laser Treatment in Type 1 Retinopathy of Prematurity: Report on Fluorescein Angiographic Findings. Ophthalmology. 2014. November;121(11):2212–9. 10.1016/j.ophtha.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 28. Moran S, O'Keefe M, Hartnett C, Lanigan B, Murphy J, Donoghue V. Bevacizumab versus diode laser in stage 3 posterior retinopathy of prematurity. Acta Ophthalmol. 2014. September;92(6):e496–7. 10.1111/aos.12339 [DOI] [PubMed] [Google Scholar]
- 29. Salman AG, Said AM. Structural, Visual and Refractive Outcomes of Intravitreal Aflibercept Injection in High-Risk Prethreshold Type 1 Retinopathy of Prematurity. Ophthalmic Res. 2014. November 29;53(1):15–20. 10.1159/000364809 [DOI] [PubMed] [Google Scholar]
- 30.Yetik H, Gunay M, Sirop S, Salihoglu Z. Intravitreal bevacizumab monotherapy for type-1 prethreshold, threshold, and aggressive posterior retinopathy of prematurity—27 month follow-up results from Turkey. Graefes Arch Clin Exp Ophthalmol. 2014 Dec 14. [DOI] [PubMed]
- 31. Castellanos MA, Schwartz S, García-Aguirre G, Quiroz-Mercado H. Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol. 2013. July;97(7):816–9. 10.1136/bjophthalmol-2012-302276 [DOI] [PubMed] [Google Scholar]
- 32. Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B, Jonas JB. Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol. 2013. June;155(6):1119–1124.e1. 10.1016/j.ajo.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 33. Martínez-Castellanos MA, Schwartz S, Hernández-Rojas ML, Kon-Jara VA, García-Aguirre G, Guerrero-Naranjo JL, et al. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013. February;33(2):329–38. 10.1097/IAE.0b013e318275394a [DOI] [PubMed] [Google Scholar]
- 34. Şahin A, Şahin M, Cingü AK, Çınar Y, Türkcü FM, Yüksel H, et al. Intravitreal bevacizumab monotherapy for retinopathy of prematurity. Pediatr Int. 2013. October;55(5):599–603. 10.1111/ped.12124 [DOI] [PubMed] [Google Scholar]
- 35. Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in taiwan. Am J Ophthalmol. 2013. January;155(1):150–158.e1. 10.1016/j.ajo.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 36. Dani C, Frosini S, Fortunato P, Bertini G, Pratesi S, Pollazzi L, et al. Intravitreal bevacizumab for retinopathy of prematurity as first line or rescue therapy with focal laser treatment. A case series. J Matern Fetal Neonatal Med. 2012. November;25(11):2194–7. 10.3109/14767058.2012.684109 [DOI] [PubMed] [Google Scholar]
- 37. Harder BC, von Baltz S, Schlichtenbrede FC, Jonas JB. Early refractive outcome after intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol. 2012. June;130(6):800–1. 10.1001/archophthalmol.2012.1 [DOI] [PubMed] [Google Scholar]
- 38. Axer-Siegel R, Snir M, Ron Y, Friling R, Sirota L, Weinberger D. Intravitreal bevacizumab as supplemental treatment or monotherapy for severe retinopathy of prematurity. Retina. 2011. Jul-Aug;31(7):1239–47. 10.1097/IAE.0b013e31820d4000 [DOI] [PubMed] [Google Scholar]
- 39. Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal bevacizumab for retinopathy of prematurity. J Ocul Pharmacol Ther. 2011. December;27(6):623–7. 10.1089/jop.2011.0060 [DOI] [PubMed] [Google Scholar]
- 40. Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011. February 17;364(7):603–15. 10.1056/NEJMoa1007374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dorta P, Kychenthal A. Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina. 2010. April;30(4 Suppl):S24–31. 10.1097/IAE.0b013e3181ca1457 [DOI] [PubMed] [Google Scholar]
- 42. Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008. June;28(6):831–8. 10.1097/IAE.0b013e318177f934 [DOI] [PubMed] [Google Scholar]
- 43. Kong L, Mintz-Hittner HA, Penland RL, Kretzer FL, Chévez-Barrios P. Intravitreous bevacizumab as anti-vascular endothelial growth factor therapy for retinopathy of prematurity: a morphologic study. Arch Ophthalmol. 2008. August;126(8):1161–3. 10.1001/archophthalmol.2008.1 [DOI] [PubMed] [Google Scholar]
- 44. Lee BJ, Kim JH, Heo H, Yu YS. Delayed onset atypical vitreoretinal traction band formation after an intravitreal injection of bevacizumab in stage 3 retinopathy of prematurity. Eye (Lond). 2012. July;26(7):903–9; quiz 910. 10.1038/eye.2012.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ittiara S, Blair MP, Shapiro MJ, Lichtenstein SJ. Exudative retinopathy and detachment: a late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J AAPOS. 2013. June;17(3):323–5. 10.1016/j.jaapos.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 46. Patel RD, Blair MP, Shapiro MJ, Lichtenstein SJ. Significant treatment failure with intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol. 2012. June;130(6):801–2. 10.1001/archophthalmol.2011.1802 [DOI] [PubMed] [Google Scholar]
- 47. Spandau U, Tomic Z, Ewald U, Larsson E, Akerblom H, Holmström G. Time to consider a new treatment protocol for aggressive posterior retinopathy of prematurity? Acta Ophthalmol. 2013. March;91(2):170–5. 10.1111/j.1755-3768.2011.02351.x [DOI] [PubMed] [Google Scholar]
- 48. Suk KK, Berrocal AM, Murray TG, Rich R, Major JC, Hess D, et al. Retinal detachment despite aggressive management of aggressive posterior retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2010. December 22;47 Online:e1–4. [DOI] [PubMed] [Google Scholar]
- 49. Choovuthayakorn J, Ubonrat K. Intravitreal bevacizumab injection in advanced retinopathy of prematurity. J Med Assoc Thai. 2012. April;95 Suppl 4:S70–5. [PubMed] [Google Scholar]
- 50.Araz-Ersan B, Kir N, Tuncer S, Aydinoglu-Candan O, Yildiz-Inec D, Akdogan B, et al. Preliminary Anatomical and Neurodevelopmental Outcomes of Intravitreal Bevacizumab As Adjunctive Treatment for Retinopathy of Prematurity. Curr Eye Res. 2014 Jul 15:1–7. [DOI] [PubMed]
- 51. Minami T, Kuniyoshi K, Kusaka S, Sugioka K, Sakuramoto H, Sakamoto M, et al. Intravitreal injection of bevacizumab for retinopathy of prematurity in an infant with peters anomaly. Case Rep Ophthalmol. 2014. October 2;5(3):318–24. 10.1159/000368298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoang QV, Kiernan DF, Chau FY, Shapiro MJ, Blair MP. Fluorescein angiography of recurrent retinopathy of prematurity after initial intravitreous bevacizumab treatment. Arch Ophthalmol. 2010. August;128(8):1080–1. 10.1001/archophthalmol.2010.145 [DOI] [PubMed] [Google Scholar]
- 53. Yaz Y, Erol N, Gursoy H, Basmak H, Bilgec MD. A rare association of intravitreal bevacizumab injection with double ridge formation in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2014. October 8;51 Online:e66–8. 10.3928/01913913-20140930-01 [DOI] [PubMed] [Google Scholar]
- 54. Chen W, Binenbaum G, Karp K, Baumritter A, Pearson DJ, Maguire AM, et al. Late recurrence of retinopathy of prematurity after treatment with both intravitreal bevacizumab and laser. J AAPOS. 2014. August;18(4):402–4. 10.1016/j.jaapos.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehta S, Hubbard GB 3rd. Delayed recurrent neovascularization and persistent avascular retina following intravitreal bevacizumab for retinopathy of prematurity. Retin Cases Brief Rep. 2013. Summer;7(3):206–9. 10.1097/ICB.0b013e318285238e [DOI] [PubMed] [Google Scholar]
- 56. Jang SY, Choi KS, Lee SJ. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J AAPOS. 2010. October;14(5):457–9. 10.1016/j.jaapos.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 57. Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012. August;130(8):1000–6. [DOI] [PubMed] [Google Scholar]
- 58. Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, Valtierra-Santiago CI, Avila-Gómez CD. Paradoxical vascular-fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye (Lond). 2010. May;24(5):931–3. [DOI] [PubMed] [Google Scholar]
- 59. Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008. July;246(7):1061–3. 10.1007/s00417-008-0786-7 [DOI] [PubMed] [Google Scholar]
- 60. Chhablani J, Rani PK, Balakrishnan D, Jalali S. Unusual adverse choroidal reaction to intravitreal bevacizumab in aggressive posterior retinopathy of prematurity: the Indian Twin Cities ROP screening (ITCROPS) data base report number 7. Semin Ophthalmol. 2014. July;29(4):222–5. 10.3109/08820538.2013.835842 [DOI] [PubMed] [Google Scholar]
- 61. Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5. Arch Dis Child Fetal Neonatal Ed. 2013. July;98(4):F327–33. 10.1136/archdischild-2012-302365 [DOI] [PubMed] [Google Scholar]
- 62. Nazari H, Modarres M, Parvaresh MM, Ghasemi Falavarjani K. Intravitreal bevacizumab in combination with laser therapy for the treatment of severe retinopathy of prematurity (ROP) associated with vitreous or retinal hemorrhage. Graefes Arch Clin Exp Ophthalmol. 2010. December;248(12):1713–8. 10.1007/s00417-010-1430-x [DOI] [PubMed] [Google Scholar]
- 63. Atchaneeyasakul LO, Trinavarat A. Choroidal ruptures after adjuvant intravitreal injection of bevacizumab for aggressive posterior retinopathy of prematurity. J Perinatol. 2010. July;30(7):497–9. 10.1038/jp.2009.166 [DOI] [PubMed] [Google Scholar]
- 64. Kusaka S, Shima C, Wada K, Arahori H, Shimojyo H, Sato T, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008. November;92(11):1450–5. 10.1136/bjo.2008.140657 [DOI] [PubMed] [Google Scholar]
- 65. Kim J, Kim SJ, Chang YS, Park WS. Combined intravitreal bevacizumab injection and zone I sparing laser photocoagulation in patients with zone I retinopathy of prematurity. Retina. 2014. January;34(1):77–82. 10.1097/IAE.0b013e318296e26d [DOI] [PubMed] [Google Scholar]
- 66. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007. November;245(11):1727–30. [DOI] [PubMed] [Google Scholar]
- 67. Lee JY, Chae JB, Yang SJ, Yoon YH, Kim JG. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010. September;248(9):1257–62. 10.1007/s00417-010-1375-0 [DOI] [PubMed] [Google Scholar]
- 68. Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007. Jan-Feb;55(1):75–6. [DOI] [PubMed] [Google Scholar]
- 69. Flavahan PW, Lavy TE, Wykes W. Intravitreal bevacizumab for the treatment of retinopathy of prematurity: a case report. Scott Med J. 2013. August;58(3):130–2. 10.1177/0036933013496933 [DOI] [PubMed] [Google Scholar]
- 70. de Klerk TA, Park DY, Biswas S. Prolonged follow-up period following intravitreal bevacizumab injection for stage 3+ retinopathy of prematurity. Eye (Lond). 2013. October;27(10):1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mota A, Carneiro A, Breda J, Rosas V, Magalhães A, Silva R, et al. Combination of intravitreal ranibizumab and laser photocoagulation for aggressive posterior retinopathy of prematurity. Case Rep Ophthalmol. 2012. January;3(1):136–41. 10.1159/000338623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Travassos A, Teixeira S, Ferreira P, Regadas I, Travassos AS, Esperancinha FE, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007. May-Jun;38(3):233–7. [DOI] [PubMed] [Google Scholar]
- 73. Goldman DR, Baumal CR. Dramatic regression of persistent tunica vasculosa lentis associated with retinopathy of prematurity following treatment with intravitreal bevacizumab. J Pediatr Ophthalmol Strabismus. 2013. June 4;50 Online:e27–9. 10.3928/01913913-20130528-03 [DOI] [PubMed] [Google Scholar]
- 74. Wutthiworawong B, Thitiratsanont U, Saovaprut C, Subhangkasen I, Geyuraphun B, Ampornprut A, et al. Combine intravitreal bevacizumab injection with laser treatment for aggressive posterior retinopathy of prematurity (AP-ROP). J Med Assoc Thai. 2011. August;94 Suppl 3:S15–21. [PubMed] [Google Scholar]
- 75. Ahmed AE, Channa R, Durrani J, Ali A, Ahmad K. Early experience with intravitreal bevacizumab combined with laser treatment for retinopathy of prematurity. Middle East Afr J Ophthalmol. 2010. July;17(3):264–7. 10.4103/0974-9233.65500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nonobe NI, Kachi S, Kondo M, Takai Y, Takemoto K, Nakayama A, et al. Concentration of vascular endothelial growth factor in aqueous humor of eyes with advanced retinopathy of prematurity before and after intravitreal injection of bevacizumab. Retina. 2009. May;29(5):579–85. 10.1097/IAE.0b013e3181a3b848 [DOI] [PubMed] [Google Scholar]
- 77. Xu Y, Zhang Q, Kang X, Zhu Y, Li J, Chen Y, et al. Early vitreoretinal surgery on vascularly active stage 4 retinopathy of prematurity through the preoperative intravitreal bevacizumab injection. Acta Ophthalmol. 2013. June;91(4):e304–10. 10.1111/aos.12055 [DOI] [PubMed] [Google Scholar]
- 78. Ehmann D, Greve M. Intravitreal bevacizumab for exudative retinal detachment post laser therapy for retinopathy of prematurity. Can J Ophthalmol. 2014. April;49(2):228–31. 10.1016/j.jcjo.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 79. Law JC, Recchia FM, Morrison DG, Donahue SP, Estes RL. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS. 2010. February;14(1):6–10. 10.1016/j.jaapos.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 80. Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, Salazar-Teran N, Chan RV. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina. 2008. March;28(3 Suppl):S19–25. 10.1097/IAE.0b013e318159ec6b [DOI] [PubMed] [Google Scholar]
- 81. Roohipoor R, Ghasemi H, Ghassemi F, Karkhaneh R, Riazi-Esfahani M, Nili-Ahmadabadi M. Intravitreal bevacizumab in retinopathy of prematurity: an interventional case series. Graefes Arch Clin Exp Ophthalmol. 2011. September;249(9):1295–301. 10.1007/s00417-011-1678-9 [DOI] [PubMed] [Google Scholar]
- 82. Autrata R, Krejcírová I, Senková K, Holoušová M, Doležel Z, Borek I. Intravitreal pegaptanib combined with diode laser therapy for stage 3+ retinopathy of prematurity in zone I and posterior zone II. Eur J Ophthalmol. 2012. Sep-Oct;22(5):687–94. 10.5301/ejo.5000166 [DOI] [PubMed] [Google Scholar]
- 83. Wu WC, Yeh PT, Chen SN, Yang CM, Lai CC, Kuo HK. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in taiwan. Ophthalmology. 2011. January;118(1):176–83. 10.1016/j.ophtha.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 84. Lalwani GA, Berrocal AM, Murray TG, Buch M, Cardone S, Hess D, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008. March;28(3 Suppl):S13–8. 10.1097/IAE.0b013e3181644ad2 [DOI] [PubMed] [Google Scholar]
- 85.Altinsoy HI, Mutlu FM, Güngör R, Sarici SU. Combination of Laser Photocoagulation and Intravitreal Bevacizumab in Aggressive Posterior Retinopathy of Prematurity. Ophthalmic Surg Lasers Imaging. 2010 Mar 9:1–5 [DOI] [PubMed]
- 86. Mutlu FM, Sarici SU. Treatment of retinopathy of prematurity: a review of conventional and promising new therapeutic options. Int J Ophthalmol. 2013. April 18;6(2):228–36. 10.3980/j.issn.2222-3959.2013.02.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gunn DJ, Cartwright DW, Gole GA. Prevalence and outcomes of laser treatment of aggressive posterior retinopathy of prematurity. Clin Experiment Ophthalmol. 2014. July;42(5):459–65. 10.1111/ceo.12280 [DOI] [PubMed] [Google Scholar]
- 88. Banach MJ, Ferrone PJ, Trese MT. A comparison of dense versus less dense diode laser photocoagulation patterns for threshold retinopathy of prematurity. Ophthalmology. 2000. February;107(2):324–7; discussion 328. [DOI] [PubMed] [Google Scholar]
- 89. Rezai KA, Eliott D, Ferrone PJ, Kim RW. Near confluent laser photocoagulation for the treatment of threshold retinopathy of prematurity. Arch Ophthalmol. 2005. May;123(5):621–6. [DOI] [PubMed] [Google Scholar]
- 90. Fallaha N, Lynn MJ, Aaberg TM Jr, Lambert SR. Clinical outcome of confluent laser photoablation for retinopathy of prematurity. J AAPOS. 2002. April;6(2):81–5. [DOI] [PubMed] [Google Scholar]
- 91. Gonzalez VH, Giuliari GP, Banda RM, Guel DA, Wingard M. Confluent laser photocoagulation for the treatment of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2010. Mar-Apr;47(2):81–5; quiz 86–7. 10.3928/01913913-20100308-05 [DOI] [PubMed] [Google Scholar]
- 92. Wu WC, Lin RI, Shih CP, Wank NK, Chen YP, Chao AN, et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119(9):1907–1916. 10.1016/j.ophtha.2012.02.040 [DOI] [PubMed] [Google Scholar]
- 93. Al-Otaibi AG, Aldrees SS, Mousa AA. Long term visual outcomes in laser treated threshold retinopathy of prematurity in Central Saudi Arabia. Saudi J Ophthalmol. 2012. July;26(3):299–303. 10.1016/j.sjopt.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trese MT, Capone A Jr, Drenser K. Macugen in Retinopathy of Prematurity. EBM Reviews—Cochrane Central Register of Controlled Trials. IOVS. Vol.47, pp.ARVO E-abstract 2330, 2006.
- 95. Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003. December;121(12):1684–94. [DOI] [PubMed] [Google Scholar]
- 96. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012. December 27;367(26):2515–26. 10.1056/NEJMra1208129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hoerster R, Muether P, Dahlke C, Mehler K, Oberthür A, Kirchhof B, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol. 2013. February;91(1):e74–5. 10.1111/j.1755-3768.2012.02469.x [DOI] [PubMed] [Google Scholar]
- 98. Wang D, Choi KS, Lee SJ. Serum concentration of vascular endothelial growth factor after bilateral intravitreal injection of bevacizumab. Korean J Ophthalmol. 2014. February;28(1):32–8. 10.3341/kjo.2014.28.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gu X, Yu X, Dai H. Intravitreal injection of ranibizumab for treatment of age-related macular degeneration: effects on serum VEGF concentration. Curr Eye Res. 2014. May;39(5):518–21. 10.3109/02713683.2013.848899 [DOI] [PubMed] [Google Scholar]
- 100. Carneiro AM, Costa R, Falcão MS, Barthelmes D, Mendonça LS, Fonseca SL, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol. 2012. February;90(1):e25–30. 10.1111/j.1755-3768.2011.02240.x [DOI] [PubMed] [Google Scholar]
- 101. Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014. October;158(4):738–744.e1. 10.1016/j.ajo.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 102. Yoshida I, Shiba T, Taniguchi H, Takahashi M, Murano T, Hiruta N, et al. Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2014. September;252(9):1483–9. 10.1007/s00417-014-2717-0 [DOI] [PubMed] [Google Scholar]
- 103.Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R, et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol. 2014 Dec 8. [DOI] [PubMed]
- 104. Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014. August 29;8:CD005139 10.1002/14651858.CD005139.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cheng JW, Cheng SW, Lu GC, Wei RL. Effect of intravitreal anti-vascular endothelial growth factor therapy on the risk of arterial thromboembolic events: a meta-analysis. PLoS One. 2012;7(7):e41325 10.1371/journal.pone.0041325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. ; RESTORE Extension Study Group. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014. May;121(5):1045–53. 10.1016/j.ophtha.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 107. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012. July;119(7):1388–98. 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, et al. ; ABC Trial Investigators. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010. June 9;340:c2459 10.1136/bmj.c2459 [DOI] [PubMed] [Google Scholar]
- 109. Antoszyk AN, Tuomi L, Chung CY, Singh A; FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008. May;145(5):862–74. 10.1016/j.ajo.2007.12.029 [DOI] [PubMed] [Google Scholar]
- 110. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. ; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006. October 5;355(14):1419–31. [DOI] [PubMed] [Google Scholar]
- 111. Spandau U. What is the optimal dosage for intravitreal bevacizumab for retinopathy of prematurity? Acta Ophthalmol. 2013. March;91(2):e154 10.1111/j.1755-3768.2012.02552.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Level of evidence as recommended by the Oxford Centre for Evidence-based Medicine. *Observational Studies.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.