Abstract
The New Zealand endemic bat family Mystacinidae comprises just two Recent species referred to a single genus, Mystacina. The family was once more diverse and widespread, with an additional six extinct taxa recorded from Australia and New Zealand. Here, a new mystacinid is described from the early Miocene (19–16 Ma) St Bathans Fauna of Central Otago, South Island, New Zealand. It is the first pre-Pleistocene record of the modern genus and it extends the evolutionary history of Mystacina back at least 16 million years. Extant Mystacina species occupy old-growth rainforest and are semi-terrestrial with an exceptionally broad omnivorous diet. The majority of the plants inhabited, pollinated, dispersed or eaten by modern Mystacina were well-established in southern New Zealand in the early Miocene, based on the fossil record from sites at or near where the bat fossils are found. Similarly, many of the arthropod prey of living Mystacina are recorded as fossils in the same area. Although none of the Miocene plant and arthropod species is extant, most are closely related to modern taxa, demonstrating potentially long-standing ecological associations with Mystacina.
Introduction
New Zealand’s only native terrestrial mammals are three bat species: the insectivorous Chalinolobus tuberculatus (Forster, 1844) and the omnivorous Mystacina tuberculata Gray, 1843 and M. robusta Dwyer, 1962. Chalinolobus tuberculatus belongs to the cosmopolitan bat family Vespertilionidae. It has close living relatives in Australia and the southwest Pacific (e.g., Chalinolobus gouldii and C. neocaledonicus), and molecular data suggest its ancestor dispersed to New Zealand during the last 2 million years [1]. In contrast, the two Mystacina species belong to the endemic family Mystacinidae and are thought to have had a much longer history in New Zealand, but until now a pre-Pleistocene record for the genus was lacking.
Mystacinidae is one of eight families in the Gondwanan bat superfamily Noctilionoidea, together with five extant South American families (Noctilionidae, Phyllostomidae, Mormoopidae, Thyropteridae, Furipteridae) [2], one in Madagascar (Myzopodidae, whose fossil record includes North Africa) [3] and one extinct family in southern North America (Speonycteridae) [4]. The distribution of Mystacinidae once extended beyond New Zealand, with four Oligo–Miocene taxa known from Australia [5, 6]. Two small, indeterminate taxa are recorded from the early Miocene of New Zealand [7]. The two Recent Mystacina species are well-represented in Quaternary deposits throughout New Zealand [8], the oldest record being 17,340 +/- 140 BP yrs BP (uncalibrated), from Hermit’s Cave, near Charleston, South Island [9]. The threatened M. tuberculata today occupies about a third of its past geographic distribution [10]. The larger M. robusta is critically endangered; it has not been sighted since 1967, on islands off the southern coast of South Island [10, 11], but may possibly still be extant (C. O’Donnell pers. comm. 2013).
Mystacinids, or burrowing bats, are renowned for their peculiar semi-terrestrial habits, spending up to 30% of their foraging time on the forest floor and tree branches [12]. Studies by Riskin et al. [13] of Mystacina tuberculata showed that, unlike the vast majority of bats, it uses a true quadrupedal walking gait when manoeuvring terrestrially, and these habits are reflected in numerous specializations of the skeleton and soft tissues in both Mystacina species (summarized in [14], and see below). Their omnivorous diet of nectar, pollen, fruit, and flying and terrestrial arthropods is among the broadest of any bat [15, 16] and they are one of the few temperate zone bats known to pollinate plants [17–20].
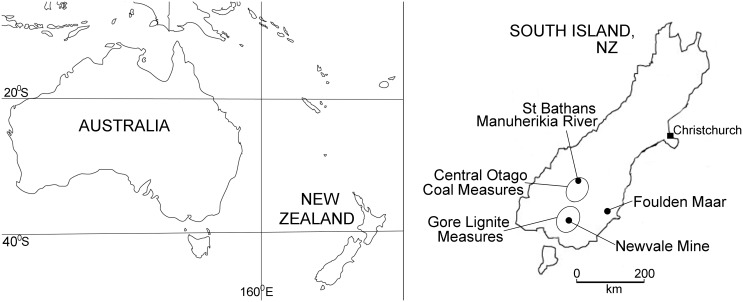
Here, we describe a new species of Mystacina from the early Miocene (19–16 Ma) St Bathans Fauna of Central Otago, South Island (Fig 1). The St Bathans deposit, which consists of near-shore freshwater lacustrine sediments of Miocene Lake Manuherikia, has also produced New Zealand’s oldest frogs, lizards, sphenodontine, land birds (including kiwi, moa and parrots), its only crocodilian, terrestrial turtle and a non-volant mammal [21–29]. We also review the fossil record for plants that are pollinated, dispersed, eaten or used as roosts by modern Mystacina, as well as their arthropod prey, and note potentially deep-rooted ecological associations between New Zealand’s endemic bats, vegetation and arthropod prey.
Fig 1. Map showing the localities of the fossil sites noted in text.
Materials and Methods
Bat taxonomy follows Simmons (2005) [2]. Dental terminology follows Hand [30], Hand et al. [6] and see Fig 2; M refers to upper molar, and P to upper premolar. All necessary permits were obtained for the described study, which complied with all relevant regulations. Fossil specimens are registered in the Canterbury Museum, Christchurch, New Zealand (prefix CM) and the Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand (prefix NMNZ).
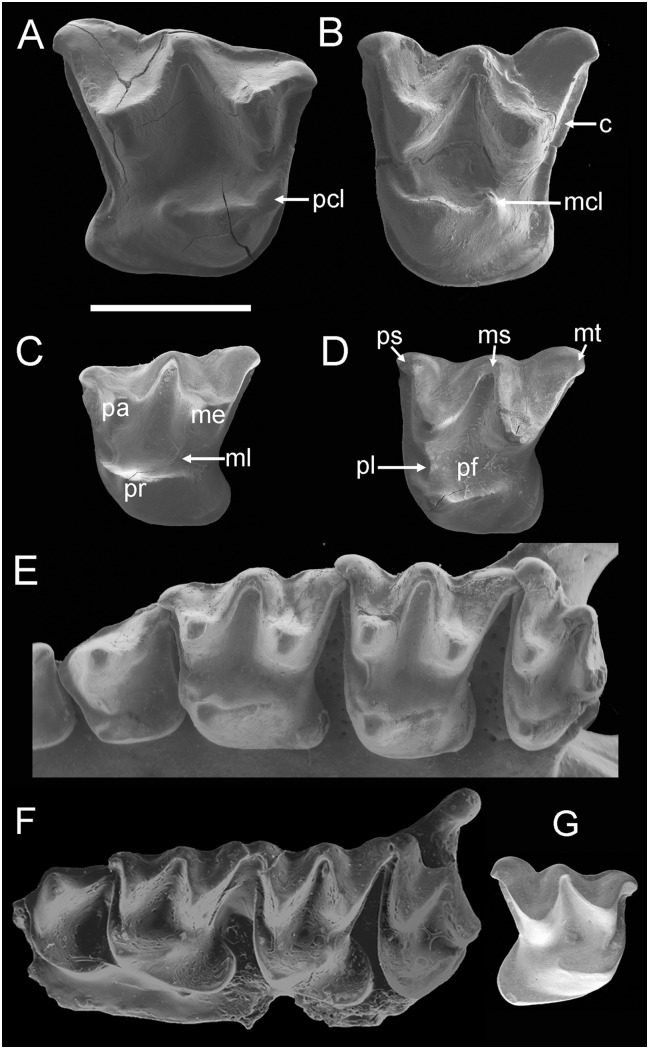
Fig 2. Upper teeth of extinct and extant mystacinid species.
A–B, Mystacina miocenalis sp. nov., St Bathans, Central Otago, New Zealand; Early Miocene. A, holotype, CM2013.18.381, right M1. B, paratype, MNZ S.52355, left M2. C–D, Mystacina tuberculata, Predator Cave, Takaka Hill, Nelson, NZ; Holocene. NMNZ S.32400. C, left M1. D, left M2. E, Mystacina robusta, Exhale Air Cave, Ellis Basin, Mt Arthur, Nelson, NZ; Holocene. NMNZ S.35205, left P4-M3. F, Icarops paradox, Judith’s Horizontalis Site, Riversleigh, Queensland Australia; Early Miocene. QM F30582, left P4-M3. G, Icarops sp., Outasite, Riversleigh; Early Miocene. QM F30586, left M1. Abbreviations: c, cingulum; mcl, metaconule; me, metacone; ml, metaloph; ms, mesostyle; mt, metastyle; pa, paracone; pcl, paraconule; pf, profossa; pl, paraloph; pr, protocone; ps, parastyle. To scale; bar = 2 mm.
Comparative Fossil and Subfossil Material
Mystacinidae: Mystacina robusta NMNZ S.35205, Exhale Air Cave, Ellis Basin, Mt Arthur, Nelson, New Zealand; M. tuberculata NMNZ S.32400-parts 1–20, Predator Cave, Takaka Hill, Nelson, NZ; Mystacinidae indet. 1 & 2, NMNZ S.41867, S.42215, S.44260, S.51739, S.51742, S.52083, S.52401, S.52402, S.52920, Manuherikia River section, Bannockburn Formation, Home Hills Station, St Bathans, Central Otago, New Zealand; Icarops aenae Queensland Museum QM F30573–4 and I. paradox QM F30580–2, Riversleigh World Heritage Area, Queensland, Australia. A list of modern comparative specimens is given in Appendix.
Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix "http://zoobank.org/". The LSID for this publication is: urn:lsid:zoobank.org:pub: D40335E8-A564-4E5B-A863-0D593C83DC10. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
Systematic Paleontology
Mammalia Linnaeus, 1758
Chiroptera Blumenbach, 1779
Noctilionoidea Gray, 1821
Mystacinidae Dobson, 1875
Mystacina Gray, 1843
Type Species: Mystacina tuberculata Gray, 1843
Additional Species: Mystacina robusta Dwyer, 1962; Mystacina miocenalis sp. nov.
Mystacina miocenalis sp. nov. Hand, Lee, Worthy & Archer urn:lsid:zoobank.org:act: BFA97159-09A1-4170-AD24-6DBA2269B485 (Fig 2)
Holotype: CM2013.18.381, right M1.
Etymology: The species name is derived from the Miocene age of this species and Latin alis, belonging or pertaining to.
Type Locality and Age: In the lower part of a clay layer enveloping stromatolites, Site FF1 [31], at 44.90359°S, 169.85840°E, Manuherikia River, Central Otago, South Island, New Zealand. The localities are registered in the New Zealand Fossil Record File (NZ FRF) system administered by the Geoscience Society of New Zealand and GNS Science as H41/f058 (stromatolites) and H41/f059 (clay draping stromatolites). Early Miocene (Altonian local stage) 19–16 Ma; St Bathans Fauna [22].
Paratype: NMNZ S.52355, left M2. Locality HH1a, Manuherikia River section, Bannockburn Formation, Home Hills Station, St Bathans 44.907944° S, 169.858222°E. NZ FRF number H41/f088. Early Miocene (Altonian) 19–16 Ma; St Bathans Fauna.
Species Diagnosis: Mystacina miocenalis (Fig 2A and 2B) differs from all other mystacinids in its larger size and from Quaternary Mystacina species (Fig 2C and 2E) in having M1 and M2 with a distinct and continuous anterior, lingual and posterior cingulum. It differs from M. tuberculata in its M1 lacking a metaloph and its M2 with hypocone shelf (heel) directed more posteriorly than posterolingually and shallower protofossa, and differs from M. robusta in its M2 being square (rather than transversely wide).
Mystacina miocenalis differs from Icarops species and mystacinid indet. [6] (Fig 2F and 2G) in M1-2 having a less developed (smaller) hypocone shelf and paraconule, and M1-2 being nearly square, rather than transversely developed (wider than long). It differs additionally from I. paradox in M2 having only a faint metaloph (that does not close the protofossa) and from I. aenae in M1 having a wider paracingulum.
Description: CM2013.18.381, the holotype of Mystacina miocenalis, represents a right M1 (Fig 2A). This tooth is just longer than it is wide. The metacone is larger and taller than the paracone, which is approximately the same height as the protocone. The apex of the protocone is located posterolingual to the paracone apex. The ectoloph is W-shaped with the centrocrista reaching the buccal margin of the tooth. On the buccal margin, a shallow ectoflexus occurs between the mesostyle and metastyle. The preparacrista is shorter than the postparacrista and premetacrista, which in turn are shorter than the postmetacrista. The curved preparacrista merges with the short, anteriorly directed parastyle in an obtuse angle. The paracone is low, and the paracone basin is very shallow. A discontinuous buccal cingulum occurs between the flattened buccal flanks of the parastyle, mesostyle and metastyle. The pre-and postparacrista meet at an angle of nearly 90° which is slightly greater than that formed between pre-and postmetacristae (80°) and postparacrista and premetacrista (70°). The posteriorly opening protofossa is longer than broad and is relatively deep. The base of the paracone flank (lingual crest) bears a wear facette and it is unclear whether a paraloph was present or not. Even if a paraloph were present, it did not extend to meet the preprotocrista. A swelling (?paraconule) is developed independently in the preprotocrista. No metaloph is evident. The preprotocrista continues buccally as the paracingulum to the base of the parastyle. The posterobuccally directed postprotocrista ends well short of the metacone base, terminating in a small metaconule. A narrow posterior cingulum extends from the metastyle to the posterolingual cingulum surrounding the small heel, with which it is continuous. The lingual cingulum continues to a point anterior to the base of the paracone. The heel is short, narrow and directed posteriorly. It lacks a hypocone and there is no lingual notch separating it from the protocone base. The heel lacks a basin but its wide but not tall cingulum forms a conspicuous shelf posterolingually. The tooth has three roots; the protocone root is very long and broad, the metacone root large and posteriorly inclined, and the paracone root straighter and smaller.
NMNZ S.52355 is a left M2 (Fig 2B). It is described in so far as it differs from M1. M2 is just wider than long. The metacone is clearly taller than both the paracone and protocone. In lingual view, the paracone has a much deeper basin than in M1. On the buccal margin two ectoflexae occur, one between the parastyle and mesostyle and the other between the mesostyle and metastyle. The preparacrista, postparacrista, premetacrista and postmetacrista are of increasing length. The preparacrista is relatively much longer than in M1 and meets the parastyle at an angle of approximately 90°. The pre-and postparacristae, pre-and postmetacristae and postparacrista and premetacrista meet at angles of approximately 55–60°. A mesostylar shelf extends from the parastyle to metastyle. A discontinuous, poorly developed buccal cingulum occurs between the buccal flanks of the parastyle, mesostyle and metastyle. A well-developed paraloph extends posterolingually from the base of the paracone but does not reach the tip of the protocone. A paraconule is not evident. A very faint metaloph extends lingually from the base of the metacone to meet the posterobuccally directed postprotocrista at the metaconule, thereby closing the protofossa. The hypocone shelf (heel) is shorter but broader than in M1.
Measurements of the new mystacinid specimens are given in Table 1.
Table 1. Measurements (mm) of upper teeth (P4-M2) and postcranial remains (humerus and radius) of St Bathans Early Miocene mystacinids (bold) compared with summary statistics for those elements in New Zealand Quaternary Mystacina species and Australian Oligo–Miocene Icarops species.
| Taxon | P4L | P4W | M1L | M1W | M2L | M2W | HPW | HDW | RPW | |
|---|---|---|---|---|---|---|---|---|---|---|
| † Mystacina miocenalis | 2.97 | 2.8 | 2.78 | 2.94 | ||||||
| †Mystacinid indet. 1 | 1.55 | 1.75 | 4.35 | 2.15 | 2.65 | |||||
| †Mystacinid indet. 2 | 1.25 | 1.45 | 2.7 | |||||||
| Mystacina tuberculata | min. | 1.18 | 1.19 | 1.65 | 1.45 | 1.65 | 1.6 | 3.76 | 3.1 | 2.46 |
| max. | 1.34 | 1.48 | 1.9 | 1.7 | 1.85 | 1.85 | 4.19 | 3.65 | 2.8 | |
| Mystacina robusta (E) | min. | 1.69 | 1.81 | 1.9 | 1.9 | 1.9 | 2.0 | 4.65 | 3.76 | 3.04 |
| max. | 2.37 | 2.25 | 2.2 | 2.4 | 4.83 | 4.3 | 3.22 | |||
| M. robusta (Waitomo) | min. | 2.1 | 2.0 | 2.1 | 2.2 | 4.4 | 4.1 | 2.84 | ||
| max. | 2.5 | 2.6 | 2.5 | 2.6 | 4.8 | 4.5 | 3.33 | |||
| † Icarops paradox | min. | 0.9 | 1.1 | 1.3 | 1.5 | 1.3 | 1.7 | |||
| max. | 1.1 | 1.2 | 1.5 | 1.6 | 1.5 | 1.8 | ||||
| † Icarops aenae | min. | 1.9 | 1.9 | 3.15 | ||||||
| max. | 2.0 | 2.1 |
Measurements of New Zealand Quaternary Mystacina species from Worthy et al. [32], Worthy and Scofield [33] and this study; those of Australian Oligo–Miocene Icarops species are from Hand et al. [6, 14]. Mystacina robusta (E) is from Stewart Island area [33]; M. robusta (Waitomo) is from Waitomo and Hawkes Bay, North Island, where this species is largest [32]. Abbreviations: D, distal; H, humerus; L, length; P, proximal; P4, posterior upper premolar; M1, first upper molar; M2, second upper molar; max., largest specimen in sample; min., smallest specimen in sample; R, radius; W, width;
†, extinct.
Results
As in other mystacinids [6], the St Bathans molars described here exhibit the following combination of features: M1–2 with a low protocone, some hypocone shelf development but hypocone absent, metaconule present, large mesostyle, central crests of ectoloph not parallel, paracingulum continuous with preprotocrista, postprotocrista terminates before reaching metacone (and does not continue as metacingulum), and a conspicuously long protocone root; M2 wider than M1; parastyles increasing in size and more lingually directed from M1 to M2.
Unlike Icarops species and as in Mystacina species, the M1-2 hypocone shelf (heel) is only moderately developed and the apex of the protocone is located posterolingually to the paracone rather than directly lingual to that cusp. On M1 the paracone basin is shallow and there is no ectoflexus between the parastyle and mesostyle; on M2 the parastyle is shorter and less hooked (less lingually directed) than in Icarops species. In M. tuberculata and M. robusta, an anteriorly directed paraloph on M1 extends to a conspicuous paraconule or swelling on the preprotocrista. In the new species, this feature is possibly absent on M1 (see Description) but present on M2. The presence of an M1-2 paraloph and metaloph may be intraspecifically variable in M. miocenalis, and possibly other mystacinid taxa: for example, in a sample of M. tuberculata from Predator Cave, Nelson (NMNZ S.32400) that we examined, a metaloph on M2 is variably present irrespective of wear.
Mystacina miocenalis shares features with both M. tuberculata and M. robusta (see Diagnosis above). No obvious features exclude it from ancestry of either, or both, Quaternary species. The evolutionary relationship between Quaternary M. tuberculata and the larger M. robusta is not anagenetic, with the two species occurring sympatrically for as long as records exist (c. 17,500 yrs BP (uncalibrated) at Hermit’s Cave, South Island) [9]. A gap in the New Zealand fossil bat record from then until the 19–16 Ma St Bathans deposit precludes further insight.
Because the Miocene Mystacina miocenalis represents a species larger than M. robusta, exceeding even the largest northern specimens of this latitudinally variable taxon [32] as well as M. tuberculata (Table 1), it would appear that the Mystacina lineage did not increase in size over time (contravening Cope’s Rule). The molars of M. miocenalis are almost twice the size of those of small individuals of M. tuberculata (e.g. from Codfish Island) [33] and up to 36% larger than those of southern M. robusta (from Stewart Island area) [33]. In comparison, a size difference of between 11–34% for tooth measurements of the sympatric species M. robusta and M. tuberculata in southern New Zealand was found by Worthy and Scofield [33].
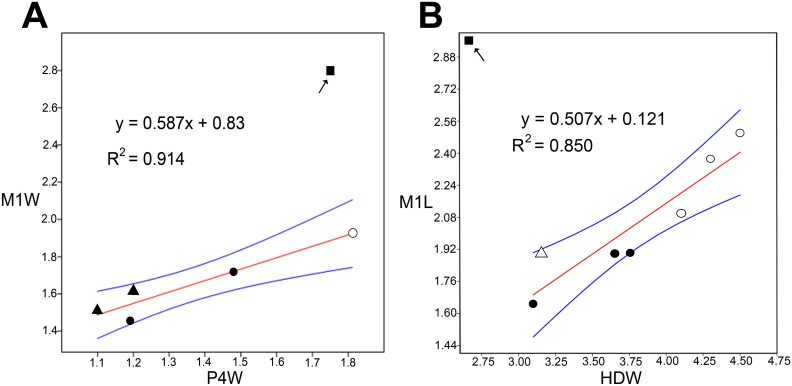
Hand et al. [7] referred previously recovered mystacinid specimens from the St Bathans fossil deposit to Mystacinidae indet 1 & 2. These specimens consist of an upper canine, a lower canine, two posterior upper premolars, two distal humeri and two proximal radii. Although their familial identity is clear, they currently cannot be referred confidently to either Mystacina or Icarops. It is possible that these specimens may be referred ultimately to Mystacina miocenalis, but they are smaller than the same elements in M. robusta (and mostly even smaller than in M. tuberculata; Table 1), and simple linear regression analyses confirm that they are unlikely to belong to M. miocenalis (Fig 3).
Fig 3. Simple linear regression plots (OLS) with 95% confidence limits (blue lines) of dental and postcranial measurements of mystacinids (Table 1).
A, Posterior upper premolar width (P4W) against first upper molar width (M1W). B, Distal humerus width (HDW) against first upper molar length (M1L). Square in each graph indicates M1 of Mystacina miocenalis plotted against value for the largest specimens of P4 and HD (respectively) for mystacinids previously recovered from St Bathans [7]. Mystacina tuberculata (filled circle), M. robusta (open circle), Icarops paradox (filled triangle), I. aenae (open triangle).
To estimate body mass in extinct bats, Gunnell et al. [34] developed a set of algorithms based on dental, skeletal and weight measurements in 1,160 extant bats representing eight families (but not including Mystacinidae). Using the proxy of upper first molar (M1) area [34; Table 1], the estimated weight for M. miocenalis is 39.2 g, suggesting a relatively large bat (compared with the median value of 13.8 g for 905 extant bat species [35, 36]). The body mass estimate for M. tuberculata using this equation [34] and M1 data from Table 1 is 10.6–14.5 g, compared with its measured average weight of 13.6 g [sd 1.3; 16]. Live weights for M. robusta are not known, but values extrapolated from forearm measurements of M. tuberculata range from 15 g [16] to 25–35 g [12, 37] for M. robusta, compared with 16.3–30.3 g calculated here based on M1 area [34] from data in Table 1.
Worthy et al. [32] and Worthy & Scofield [33] found that overall size in M. robusta increases markedly with decreasing latitude (contravening Bergmann’s Rule), while that of M. tuberculata does not, with northern populations slightly smaller than southern ones in South Island. Those trends result in character (size) displacement between Mystacina tuberculata and M. robusta being greatest at lower latitudes indicating increased niche separation. Worthy et al. [32] suggested that the unusual clinal variation found in M. robusta (smaller size in colder climes) may be due to roosting behaviour and hibernation physiology, with smaller bats being better adapted to re-warming after hibernation in higher latitudes than are larger ones. A large size difference, probably greater than that found between the Quaternary Mystacina species, also appears to separate Mystacina miocenalis and the smaller mystacinid(s) in early Miocene St Bathans, which at that time had a warmer climate than today, with the estimated mean annual temperature of 18°C for southern New Zealand exceeding that of northern-most New Zealand today [38, 39]. The presence of the two smaller mystacinids in the warmer-than-now early Miocene precludes explaining the large size of M. miocenalis as an effect of a decreasing temperature gradient over time and thus it is not a corollary of the latitudinal trend in Quaternary New Zealand.
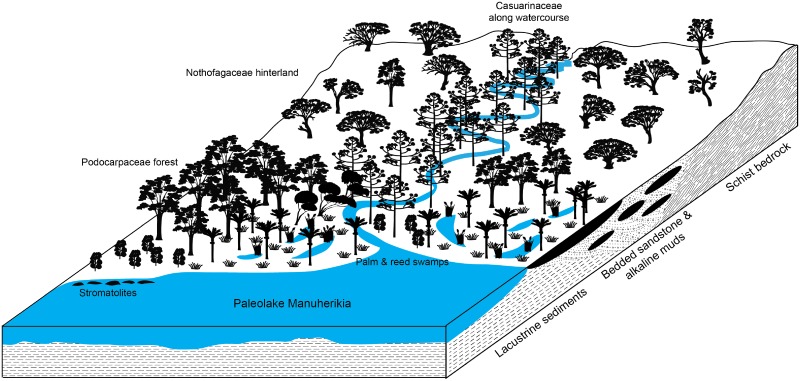
Although no plant fossils are present in the sediment immediately surrounding the Mystacina fossils, palynofloras were obtained from the same horizon (upper part of clay draping stromatolite-encrusted boulders; H41/f061) and from several other samples slightly higher in the section (H41/f100; H41/f101; H41/f102) (Table 2). The palynoflora in the samples associated with stromatolites was dominated by the lacustrine algae Pediastrum and Botryococcus [40]. It also contained several ferns, including the tree fern Cyathea and a range of lake margin and forest species which likely represent the rainforest habitat occupied by early Miocene Mystacina. All samples contain a range of conifers, including an araucarian (Agathis (or perhaps extinct Araucaria)) and several podocarps including the swamp-forest Dacrycarpus (kahikatea), Dacrydium (rimu), Phyllocladus (celery pine), several Podocarpus (totara) species, as well as the now locally extinct Microcachrys (creeping strawberry pine) and Lagarostrobos franklinii (Huon pine). At least one species of palm was present in the vegetation, as were species of Metrosideros (Myrtaceae). The pollen count is overwhelmingly dominated by the high pollen producers Casuarinaceae (sheoaks) and several species of Nothofagaceae (southern beech); although Casuarinaceae and the Brassospora group of southern beeches are no longer present in the New Zealand flora, the Fuscospora beeches and Myrtaceae are still major components of the modern vegetation [41].
Table 2. List of palynomorphs recorded from the St Bathans Mystacina fossil locality (H41/f061), and from three other sites (H41/f100, H41/f101, H41/f102) stratigraphically higher in the same section.
| ST BATHANS FOSSIL LOCALITIES | ||||
|---|---|---|---|---|
| PALYNOMORPHS | H41/f061 | H41/f100 | H41/f101 | H41/f102 |
| Botryococcus freshwater alga | x | x | x | |
| Pediastrum freshwater alga | x | x | x | |
| monolete spores | x | x | ||
| Cyathidites Cyatheaceae (Cyathea-type) | x | x | x | |
| Foveotriletes lacunosus Lycopodiaceae | x | |||
| Gleichenia circinata Gleicheniaceae | x | |||
| Ischyosporites Filicopsida | x | |||
| Latrobosporites marginis Lycopodiaceae | x | |||
| Lycopodium fastigiatum Lycopodiaceae | x | |||
| ?Lygodium Schizaeaceae | x | |||
| Peromonolites vellosus Pteridaceae | x | |||
| Polypodiaceoisporites Pteridaceae | x | x | ||
| Polypodiisporites minimus Polypodiaceae, Davalliaceae | x | x | x | |
| Polypodiisporites radiatus Polypodiaceae | x | |||
| CONIFER POLLEN | ||||
| Araucariacites australis Araucariaceae (Araucaria, Agathis) | x | x | x | |
| Dacrycarpites australiensis Podocarpaceae (Dacrycarpus) | x | x | x | |
| Dacrydiumites praecupressinoides Podocarpaceae (Dacrydium) | x | x | x | |
| ?Libocedrus Cupressaceae | x | |||
| Microalatidites paleogenicus Podocarpaceae (Phyllocladus) | x | x | x | |
| Phyllocladidites mawsonii Podocarpaceae (Lagarostrobos franklinii) | x | |||
| Podocarpidites | x | x | x | x |
| Podocarpidites puteus Podocarpaceae (Podocarpus/Prumnopitys) | x | x | x | |
| Podosporites brevisaccatus Podocarpaceae (Microcachrys) | x | x | ||
| Podosporites parvus Podocarpaceae (Microcachrys) | x | x | ||
| ANGIOSPERM POLLEN | ||||
| Arecipites Arecaceae | x | |||
| Arecipites otagoensis Arecaceae | x | x | ||
| Chenopodiaceae | ||||
| cf. Dracophyllum Ericales | x | x | ||
| Haloragacidites amolosus Haloragaceae | x | |||
| Haloragacidites harrisii Casuarinaceae, Myricaceae | x | x | x | x |
| Haloragacidites myriophylloides Haloragaceae | x | |||
| Liliacidites | x | x | ||
| Liliacidites aviemorensis Liliaceae | x | |||
| Lymingtonia cenozoica Nyctaginaceae | x | |||
| Malvaceae | x | |||
| Malvacipollis subtilis Euphorbiaceae or Malvaceae | x | |||
| Milfordia homeopunctata Restionaceae, Flagellariaceae | x | x | x | |
| Myrtaceidites mesonesus Myrtaceae (Metrosideros) | x | x | x | x |
| Myrtaceidites parvus Myrtaceae (Leptospermum-type) | x | x | ||
| Nothofagidites asperus Nothofagaceae (Nothofagus subgenus Lophozonia) | x | x | x | |
| Nothofagidites cranwelliae Nothofagaceae (Nothofagus subgenus Brassospora) | x | x | x | |
| Nothofagidites lachlaniae Nothofagaceae (Nothofagus subgenus Fuscospora) | x | x | x | x |
| Nothofagidites spinosus Nothofagaceae (Nothofagus subgenus Brassospora) | x | x | x | |
| Nyssapollenites endobalteus Euphorbiaceae | x | |||
| Palaeocoprosmadites zelandiae Rubiaceae (Coprosma) | x | |||
| Ptychotriporines | x | x | ||
| Rhoipites alveolatus? Euphorbiaceae | x | |||
| Rhoipites hekelii | x | |||
| Rhoipites rhomboidaliformis | x | x | ||
| Tetracolporites | x | |||
| ?Tricolpites delicatulus | x | x | ||
| Triptyches | x | x | ||
| Tubulifloridites antipodica Asteraceae | x | x | ||
| Tubulifloridites simplis Asteraceae | x | x | ||
| Typha Typhaceae | x | |||
Discussion
The new large mystacinid from St Bathans, Central Otago is referred to Mystacina and provides the first pre-Pleistocene record for New Zealand’s endemic bat genus. The St Bathans sediments are estimated to be early Miocene in age on the basis of palynological data that constrain the age of the Bannockburn Formation to the local Altonian Stage, or 19–16 Ma [43–46] (Fig 1). The discovery extends the evolutionary history of Mystacina back at least 16 million years, a longevity consistent with that for other bat genera, which range from around 40 to less than 2 million years [47]. The presence of a species of Mystacina in the St Bathans Fauna also suggests separation of New Zealand and Australian mystacinid lineages since at least the early Miocene. It confirms that Mystacina has had a long presence in New Zealand, in common with other genera of terrestrial vertebrates in the St Bathans Fauna including frogs (e.g. Leiopelma), lizards (Hoplodactylus, Oligosoma) and some birds (Aptornis, Pelecanoides, Aegotheles) [25, 28, 29, 48].
The family Mystacinidae itself is an ancient lineage that molecular data indicate diverged from other bats c.51–41 Ma [49–52]. It is one of eight families in the diverse and speciose superfamily Noctilionoidea, whose geographic origins are unclear, but which probably represents a Southern Hemisphere group [51]. Gunnell et al. [3] suggested that noctilionoids originated in Eastern Gondwana with a subsequent dispersal south into Australia (mystacinids) and then westward to South America via Antarctica (this lineage leading to the five Neotropical noctilionoid families). The presence of what may be plesiomorphic mystacinids (species of Icarops) in the Australian fossil record (as argued by Hand et al. [5, 6, 14]) also suggests that Australia was the source of New Zealand’s mystacinids. The oldest fossil record for mystacinids is from c. 26 Ma Etadunna Formation of Lake Palankarinna, South Australia [6, 53]. The divergence of mystacinids from ancestral noctilionoids post-dates separation of Zealandia from the rest of Gondwana, beginning about 81 Ma, with its last connection to Australia, via the Lord Howe Rise, severed at about 52 Ma [54].
Like its congeners, Mystacina miocenalis was probably semi-terrestrial. All mystacinids for which postcranial elements are known exhibit skeletal specializations for semi-terrestrial locomotion [14], including smaller mystacinids of unclear generic identity also recovered from the St Bathans deposit [7] and Oligo–Miocene species of Icarops from Australia [6]. These specializations include derived features of the distal humerus such as development of the lateral supraepicondylar groove and lateral inclination of the humeroradial articulation: study of the myology of M. tuberculata indicates that these features are functionally correlated with terrestrial locomotion in mystacinids [14]. Mystacina tuberculata’s terrestrial habits are reflected in numerous specializations of the wing, foot, leg, spine and pectoral and pelvic girdles [12, 55–57]. When moving on its wrists and backward facing feet, its wings are furled tightly in a protective sheath-like portion of the plagiopatagium [12]. Reduced pro- and uropatagia enable free movement of fore and hindlimbs respectively [58, 59], while secondary talons on the thumb and toe claws increase grip on the substrate, as does a system of adhesive grooves in the soles of its feet [60].
Mystacina tuberculata populations are restricted today to extensive areas of undisturbed old-growth, temperate, closed evergreen forest types dominated by Podocarpus, Dacrydium, Agathis and species of Nothofagus [16, 61] with large trees (>1 m girth and >25 m high) available for well-insulated colonial roosts, abundant epiphytes and deep leaf-litter: the species also occurs in low numbers in areas adjacent to undamaged old-growth forest [10, 62]. The habitat preferences of M. robusta are not well known, but it is presumed to have had similar requirements to M. tuberculata [10, 16]. All of the tall forest conifers (Agathis, Dacrydium, Dacrycarpus, Podocarpus, Prumnopitys) and most of the angiosperms (Nothofagus, Metrosideros, Weinmannia) in which extant Mystacina is known to roost have a long history in New Zealand, dating back at least to the Oligocene to early Miocene (e.g. Gore Lignite Measures, Newvale Mine, Southland [63]; Foulden Maar, Otago [40, 41]) (Fig 1). Fig 4 provides a schematic reconstruction of the mixed Nothofagaceae—Podocarpaceae forest habitat that would have been occupied by Mystacina, based on the palynofloras recorded in Table 2.
Fig 4. Schematic reconstruction of the forest habitat on the shores of paleolake Manuherikia, South Island, New Zealand in the early Miocene.
Possibly correlated with its semi-terrestrial capabilities, extant Mystacina tuberculata is an omnivorous and opportunistic feeder [12, 15–17, 64] that consumes moths and other flying insects and terrestrial arthropods, as well as gleaning wood and moss fragments, eating fruit and sipping nectar and perhaps incidentally ingesting flower fragments from several plant groups (see below). The new fossil species M. miocenalis probably had a similarly broad diet, based on strikingly similar molar morphology to that of M. tuberculata and M. robusta and little to suggest any different specialization. These similarities include features typically found in insectivorous as well as omnivorous bats [65] including unspecialised dilambdodonty with a relatively wide buccal shelf, a deep protofossa and prominent protocone. Differences observed in M. miocenalis, such as better developed para- and metaconules (producing more complex blade systems) and a continuous, thickened cingulum (better protecting the gums), may indicate inclusion of harder and perhaps more diverse foods in the diet, as has been proposed for other large extinct bats [e.g., 66]. In contrast, more conspicuous differences between Mystacina and Icarops species in molar morphology, such as much larger hypocone shelves (heels) and longer postmetacristae, have been interpreted to indicate greater reliance on insectivory by some Australian mystacinids [6].
Plants for which Mystacina may have been an important pollinator also have long fossil records in New Zealand. Today, Mystacina tuberculata is New Zealand’s only native mammalian pollinator for plants including the liane kiekie (Freycinetia banksii) (Pandanaceae), perching lilies (Collospermum hastatum and C. microspermum) (Asteliaceae), rata and pohutukawa (Metrosideros spp.) and rewarewa (Knightia excelsa) (Proteaceae) [10, 16], with pollen occurring in its guano, stomach and fur [10, 12, 15–17, 19, 20]. Of these Mystacina-pollinated plants, Metrosideros has a long fossil record in New Zealand (as Myrtaceidites mesonesus) extending back to the Oligocene to early Miocene in Southland and Central Otago [42, 44, 67]. Leaf fossils and pollen both confirm a long record of Proteaceae that were much more diverse in New Zealand in the past (e.g. Newvale Mine and Foulden Maar [68]). Knightia has probably been present since the Miocene and K. excelsa since the Pliocene [69].
In modern New Zealand forests, Mystacina appears to be one of the few native pollinators of the unusual obligate root parasite, Dactylanthus taylorii (wood rose) [70]. In general, most flowers visited by bats and non-volant mammals are dull-coloured and odorous, producing copious amounts of nectar and pollen, whereas diurnal birds usually visit brightly coloured (often red) and odourless flowers ([71]; but see [72]). Dactylanthus taylorii is the only fully holoparasitic plant in the New Zealand flora and the sole New Zealand representative of the family Balanophoraceae which is widespread in the tropics and subtropics. It is characterized by ground-flowering, sweetly scented, pollen-filled inflorescences that are extremely attractive to Mystacina [64, 70]. Dactylanthus taylorii has distinctive periporate pollen grains and a well-constrained fossil history in New Zealand. Fossil pollen grains of Parsonsidites multiporus Mildenhall & Crosbie [73] are assigned with confidence to Dactylanthus [42] and are recorded back to the Waiauan local stage (12.7–11.0 Ma), and with less certainty back to the Altonian (18.7–15.9 Ma) in the southern South Island (Gore Lignite Measures: D.C. Mildenhall pers. observation). Although Dactylanthus taylorii is currently restricted to the North Island and perhaps the northern South Island [74], pollen grains of this species were found in an early Pleistocene warm interglacial fossil forest site near Colac Bay, on the shore of Foveaux Strait (D.C. Mildenhall, pers. observation), confirming suggestions that during past periods of warmer climate it was able to migrate much further south [75].
Fruits eaten by modern Mystacina include those of kiekie, perching lilies, and hinau (Elaeocarpus dentatus: Elaeocarpaceae) [12]. Mystacina also disperses the seeds of kiekie and perching lilies among others [10, 16, 17]). Daniel [12] reported that Mystacina ate the succulent bracts of kiekie in autumn and winter, as well as berries of two species of Collospermum and part of the exocarp and mesocarp of Elaeocarpus dentatus [17]. Freycinetia (as the pollen type Lateropora glabra [67]) has a long fossil record in New Zealand, dating back at least to the late Oligocene in Southland [67] and possibly the late Eocene [76]. The epiphytic lily Collospermum is closely related to, and possibly nested within, the genus Astelia [77]: Astelia pollen ranges back to the late Eocene and well-preserved Astelia leaves occur in earliest Miocene sediments at Foulden Maar [78] (Fig 1).
As well as eating plant material, Mystacina is noted for consuming large quantities of both flying and terrestrial arthropods. On average, Mystacina tuberculata individuals consume 5–7 g of arthropods per night or 36–50% of pre-feeding body mass [12, 16, 79]. Arkins et al. [15] and Lloyd [16] noted that most of the identifiable arthropod taxa observed in the droppings of Mystacina were from four insect orders: Coleoptera (Scarabeidae, Curculionidae, Carabidae and Chrysomelidae), Lepidoptera, Diptera (Tipulidae, Muscidae and Psychodidae) and Orthoptera. Spiders were also present, as were Myriapoda and less commonly Neuroptera, Acarina and Hymenoptera (Formicidae) [15]. A high proportion of these families of native arthropods eaten by Mystacina have been discovered recently as fossils in early Miocene deposits at Foulden Maar, geographically close to St Bathans [80, 81]. Foulden Maar is a paleolake deposit in the Waipiata Volcanic Field of earliest Miocene age that was surrounded by a dense, evergreen rainforest [40] (Fig 1). It preserves New Zealand’s first described pre-Quaternary terrestrial arthropod fauna, the majority of which are ground-dwelling, forest litter and wood-living taxa with low dispersal ability. The fauna to date includes representatives of the coleopteran families Curculionidae and Chrysomelidae, the dipteran family Tipulidae, hymenopterans (Formicidae), and a variety of spiders (Araneae) [81], all of which are included in the prey of living Mystacina.
Within the superfamily Noctilionoidea, nectarivory and bat-plant pollination systems appear to have developed several times: independently at least twice in the Neotropical family Phyllostomidae [82–85] and presumably once in Mystacinidae. Flower-visiting bats are generally found in the tropics and subtropics [86], although some may migrate seasonally into temperate regions [18]. In temperate regions, in contrast, bats visiting flowers are rare, seasonal or absent and mammalian pollinators are more typically non-volant (e.g. rodents, primates and marsupials) [71, 72]. Perhaps for this reason, the importance of bat pollination in modern New Zealand’s temperate ecosystems by the semi-terrestrial Mystacina has been significantly underestimated, as noted by Pattemore and Wilcove [19] and Cummings et al. [20].
The presence of at least two, and possibly three, mystacinid species in the 19–16 Ma St Bathans deposit of Central Otago signals that members of the family had arrived and diversified in New Zealand by the earliest Miocene. Identification of one of these as a species of the modern genus Mystacina provides further evidence that the landmass of New Zealand has remained large enough to maintain long-term survival of indigenous vertebrate lineages. Accumulating data indicate that even at maximum marine transgression in the late Oligocene–earliest Miocene, emergent New Zealand was at least equivalent in size to modern New Caledonia [87]. The St Bathans mystacinids provide one of the longest fossil records for an endemic lineage of island bats globally [7]. The only other “island” record of similar length is the recently reported, as yet undescribed Miocene hipposiderids from Madagascar [88], another microcontinent rifted from the supercontinent Gondwana.
In summary, description of a new species of Mystacina from the 19–16 Ma St Bathans deposit of Central Otago indicates separation of the New Zealand Mystacina and Australian Icarops lineages since the early Miocene. The Mystacina fossils are contemporaneous with, or slightly younger than diverse plant and invertebrate fossils that occur in other early Miocene sites near or at the St Bathans bat locality. Many of the plants, particularly the forest dominants, and terrestrial arthropods are remarkably similar to those in the modern endemic New Zealand biota, and suggest remarkably long-term ecological associations with Mystacina in regards to its colonial roosts, arthropod prey, and pollination and seed dispersal services for forest plants. In tropical ecosystems, many bat species provide pollination and seed dispersal services, but in temperate New Zealand only one currently fills this role. Nevertheless, the fossil record suggests that this could represent a deep-rooted, mutualistic relationship between a semi-terrestrial bat lineage and the forest ecosystems in which it has evolved for at least 16 million years.
Acknowledgments
We thank landowners Ann and Euan Johnstone for graciously allowing us access to the bat-bearing fossil deposits and many colleagues for helping in the St Bathans excavations. We also gratefully acknowledge the following for access to and loan of comparative material in their care: R. Coory and G. Stone, Museum of New Zealand Te Papa Tongarewa; S. Ingleby and T. Ennis, Australian Museum, Sydney; E. Westwig and N. Duncan, American Museum of Natural History, New York; H. Kafka, National Museum of Natural History, Smithsonian Institution, Washington D.C.; K. Krohmann, Naturmuseum Senckenberg, Frankfurt; and J.-M. Pons, Muséum National d'Histoire Naturelle, Paris. We thank N.B. Simmons, W.G. Lee, J.G. Conran, T. Myers, A. Ravel and an anonymous reviewer for constructive criticism of the drafts of the manuscript, and T. Reichgelt for preparing Fig 4.
Data Availability
All relevant data are within the paper.
Funding Statement
This research has been supported by Australian Research Council grants DP120100486 and DP130100197 and a Marsden Grant from the Royal Society of New Zealand.
Appendix
List of comparative bat material used in this study
Institutional abbreviations: AM, Australian Museum, Sydney; AMNH, American Museum of Natural History, New York; AR, Vertebrate Palaeontology Collection, University of New South Wales, Sydney; CM, Canterbury Museum, Christchurch; FMNH, Field Museum of Natural History, Chicago; MNHN, Muséum national d'Histoire naturelle, Paris; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington; QM, Queensland Museum, Brisbane, Australia; SMF, Naturmuseum Senckenberg, Frankfurt; USNM, National Museum Natural History, Smithsonian Institution, Washington D. C.
Fossil and subfossil specimens: Mystacinidae: Mystacina robusta NMNZ S.35205, Exhale Air Cave, Ellis Basin, Mt Arthur, Nelson, New Zealand; M. tuberculata NMNZ S.32400-parts 1–20, Predator Cave, Takaka Hill, Nelson, NZ; Mystacinidae indet. 1 & 2, NMNZ S.41867, S.42215, S.44260, S.51739, S.51742, S.52083, S.52401, S.52402, S.52920, Manuherikia River section, Bannockburn Formation, Home Hills Station, St Bathans, Central Otago, New Zealand; Icarops aenae QM F30573–4 and I. paradox QM F30580–2, Riversleigh World Heritage Area, Queensland, Australia.
Modern comparative specimens: Craseonycteridae: Craseonycteris thonglongyai SMF 54513. Emballonuridae: Emballonura atrata FMNH 176360; Saccolaimus saccolaimus AM M3491; Saccopteryx bilineata AMNH M267842; Taphozous georgianus AR20502. Furipteridae: Furipteris horrens AMNH M267213. Hipposideridae: Anthops ornatus AM M5831; Aselliscus tricuspidatus SMF 24745; Hipposideros bicolor AM M9231; Hipposideros diadema AR5194; Rhinonicteris aurantia AR20501. Megadermatidae: Lavia frons AR20507; Macroderma gigas AR20505. Miniopteridae: Miniopterus orianae AR1868; M. australis AM M5556. Molossidae: Cheiromeles parvidens AMNH M241942; Chaerephon plicatus AMNH M107932; Cynomops planirostris AMNH M234455; Eumops auripendulus AMNH M248212; Molossus molossus AMNH M267243; Mops brachypterus AMNH M241062; Mormopterus beccarii AM M8509; Mormopterus planiceps AR20503; Myopterus daubentonii AMNH M48855; Promops nasutus AMNH M184648; Tadarida australis AM M7910. Mormoopidae: Mormoops blainvillei AMNH M238144; Pteronotus davyi AMNH M204961. Mystacinidae: Mystacina tuberculata NMNZ LM1231. Myzopodidae: Myzopoda aurita USNM 448932; MNHN 1907.618. Natalidae: Natalus stramineus USNM 362103. Noctilionidae: Noctilio albiventris USNM 390592. Nycteridae: Nycteris capensis AM M6071; Nycteris javanica AMNH M103269. Phyllostomidae: Anoura geoffroyi AMNH M264935; Artibeus jamaicensis AR21504; Desmodus rotundus AMNH M248941; Glossophaga soricina AMNH M230206; Micronycteris hirsuta AMNH M267860; Phyllostomus hastatus AMNH M267901; Tonatia saurophila AMNH M267912; Trachops cirrhosus AMNH M266081. Pteropodidae: Balionycteris maculata AMNH M233970; Nyctimene robinsoni AR17520; Pteropus scapulatus AR4764; Rousettus amplexicaudatus USNM 78616. Rhinolophidae: Rhinolophus megaphyllus AR1655. Rhinopomatidae: Rhinopoma hardwickei AR21506. Thyropteridae: Thyroptera tricolor AMNH M266356. Vespertilionidae: Antrozous pallidus USNM 564036; Chalinolobus morio AR1653; Eptesicus fuscus AMNH M139513; Falsistrellus tasmaniensis AM M3360; Kerivoula papillosa AMNH M247576; Myotis macropus AM M5453; Nyctophilus gouldi AM M12548; Scoteanax ruepelli AM M5163; Scotorepens orion AM M5163; Vespadelus vulturnus AM M11413.
References
- 1. O'Donnell CFJ (2005) New Zealand long-tailed bat In: King CM, editor. The Handbook of New Zealand Mammals 2nd edition Melbourne: Oxford University Press; pp. 98–109. [Google Scholar]
- 2. Simmons NB (2005) Order Chiroptera In: Wilson DE, Reeder DM, editors. Mammal Species of the World: A Taxonomic and Geographic Reference. Washington D.C.: Smithsonian Institution Press; pp. 312–529. [Google Scholar]
- 3. Gunnell GF, Simmons NB, Seiffert ER (2014) New Myzopodidae (Chiroptera) from the Late Paleogene of Egypt: emended family diagnosis and biogeographic origins of Noctilionoidea. PLoS ONE 9(2): e86712 10.1371/journal.pone.0086712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Czaplewski NJ, Morgan GS (2012) New basal noctilionoid bats (Mammalia: Chiroptera) from the Oligocene of subtropical North America In: Gunnell GF, Simmons NB, editors. Evolutionary History of Bats. Boston: Cambridge University Press; pp. 162–209 [Google Scholar]
- 5. Hand SJ, Murray PF, Megirian D, Archer M, Godthelp H (1998) Mystacinid bats (Microchiroptera) from the Australian Tertiary. Journal of Paleontology 72: 538–545. [Google Scholar]
- 6. Hand SJ, Archer M, Godthelp H ( 2005) Australian Oligo-Miocene mystacinids (Microchiroptera): upper dentition, new taxa and divergence of New Zealand species. Geobios 38: 339–352. [Google Scholar]
- 7. Hand SJ, Worthy TH, Archer M, Worthy JP, Tennyson AJD, Scofield RP (2013) Miocene mystacinids (Chiroptera: Noctilionoidea) indicate a long history for endemic bats in New Zealand. Journal of Vertebrate Paleontology 33: 1442–1448. [Google Scholar]
- 8. Worthy TH, Holdaway RN (2002) The Lost World of the Moa: Prehistoric Life of New Zealand Indiana: Indiana University Press; Xxxiii + 718 pp. [Google Scholar]
- 9. Worthy TH, Holdaway RN (1994) Scraps from an owl’s table—predator activity as a significant taphonomic process recognised from New Zealand Quaternary deposits. Alcheringa 18: 229–245. [Google Scholar]
- 10. Lloyd BD (2005) Lesser short-tailed bat In: King CM, editor. The Handbook of New Zealand Mammals 2nd edition Melbourne: Oxford University Press; pp. 111–126. [Google Scholar]
- 11. O’Donnell CFJ (1999) Search for Pekapeka (Bats) on Putauhina Island, Southern Titi Islands, 6–9 November 1999. Christchurch, New Zealand: Department of Conservation. [Google Scholar]
- 12. Daniel MJ (1979) The New Zealand short-tailed bat, Mystacina tuberculata; a review of present knowledge. New Zealand Journal of Zoology 6: 357–370. [Google Scholar]
- 13. Riskin DK, Parsons S, Schutt WA, Carter GG, Hermanson JW (2006) Terrestrial locomotion of the New Zealand short-tailed bat Mystacina tuberculata and the common vampire bat Desmodus rotundus . Journal of Experimental Biology 209: 1725–1736. [DOI] [PubMed] [Google Scholar]
- 14. Hand SJ, Weisbecker V, Beck RMD, Archer M, Godthelp H, Tennyson AJD et al. (2009) Bats that walk: a new evolutionary hypothesis for the terrestrial behaviour of New Zealand’s endemic mystacinids. BMC Evolutionary Biology 9: 169 ( 13 p.). 10.1186/1471-2148-9-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arkins AM, Winnington AP, Anderson S, Clout MN (1999) Diet and nectarivorous foraging behaviour of the short-tailed bat (Mystacina tuberculata). Journal of Zoology (London) 247: 183–187. [Google Scholar]
- 16. Lloyd BD (2001) Advances in New Zealand mammalogy 1990–2000: short-tailed bats. Journal of the Royal Society of New Zealand 31: 59–81. [Google Scholar]
- 17. Daniel MJ (1976) Feeding by the short-tailed bat Mystacina tuberculata on fruit and possibly nectar. New Zealand Journal of Zoology 3: 391–398. [Google Scholar]
- 18. Rojas-Martinez A, Valiente-Banuet A, Arizmendi MC, Alcantara-Eguren A, Arita HT (1999) Distribution of the long-nosed bat (Leptonycteris curasoae) in North America: does a generalized migration pattern really exist? Journal of Biogeography 26: 1065–1077. [Google Scholar]
- 19. Pattemore DE, Wilcove DS (2012) Invasive rats and recent colonist birds partially compensate for the loss of endemic New Zealand pollinators. Proceedings of the Royal Society of London, Series B 279: 1597–1605. 10.1098/rspb.2011.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cummings G, Anderson S, Dennis T, Toth C, Parsons S (2014) Competition for pollination by the lesser short-tailed bat and its influence on the flowering phenology of some New Zealand endemics. Journal of Zoology 293: 281–288. [Google Scholar]
- 21. Worthy TH, Tennyson AJD, Archer M, Musser AM, Hand SJ, Jones C et al. (2006) Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proceedings of the National Academy of Sciences USA 103: 19419–19423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Worthy TH, Tennyson AJD, Jones C, McNamara JA, Douglas BJ (2007) Miocene waterfowl and other birds from Central Otago, New Zealand. Journal of Systematic Palaeontology 5: 1–39. [Google Scholar]
- 23. Jones MEH, Tennyson AJD, Worthy JP, Evans SE, Worthy TH (2009) A sphenodontine (Rhynchocephalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon). Proceedings of the Royal Society of London B 276: 1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tennyson AJD, Worthy TH, Jones CM, Scofield RP, Hand SJ (2010) Moa’s Ark: Miocene fossils reveal the great antiquity of moa (Aves: Dinornithiformes) in Zealandia. Records of the Australian Museum 62: 105–114. [Google Scholar]
- 25. Lee MSY, Hutchinson MN, Worthy TH, Archer M, Tennyson AJD, Worthy JP et al. (2009) Miocene skinks and geckos reveal long-term conservatism of New Zealand's lizard fauna. Biology Letters 10.1098/rsbl.2009.0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Worthy TH, Tennyson AJD, Scofield RP (2011) An Early Miocene diversity of parrots (Aves, Strigopidae, Nestorinae) from New Zealand. Journal of Vertebrate Paleontology 31: 1102–1116. [Google Scholar]
- 27. Schwarzhans W, Scofield RP, Tennyson AJD, Worthy JP, Worthy TH (2012) Fish remains, mostly otoliths, from the non-marine Early Miocene of Otago, New Zealand. Acta Palaeontologica Polonica 57: 319–350. [Google Scholar]
- 28. Worthy TH, Tennyson AJD, Scofield RP, Hand SJ (2013a) Early Miocene fossil frogs (Anura: Leiopelmatidae) from New Zealand. Journal of the Royal Society of New Zealand 4: 211–230. [Google Scholar]
- 29. Worthy TH, Worthy JP, Tennyson AJD, Salisbury SW, Hand SJ, Scofield RP (2013b) Miocene fossils show that kiwi (Apteryx, Apterygidae) are probably not phyletic dwarves In: Göhlich UB, Kroh A, editors. Paleornithological Research 2013—Proceedings of the 8th International Meeting of the Society of Avian Paleontology and Evolution. Vienna: Natural History Museum Vienna; pp. 63–80. 10.1371/journal.pone.0082000 [DOI] [Google Scholar]
- 30. Hand SJ (1990) First Tertiary molossid (Microchiroptera: Molossidae) from Australia: its phylogenetic and biogeographic implications. Memoirs of the Queensland Museum 28: 175–192. [Google Scholar]
- 31. Lindqvist J (1994) Lacustrine stromatolites and oncoids: Manuherikia Group (Miocene), New Zealand In: Bertrand-Safati J, Monty C, editors. Phanerozoic Stromatolites II. Netherlands: Kluwer Academic Publishers; pp. 227–254 [Google Scholar]
- 32. Worthy TH, Daniel MJ, Hill JE (1996) An analysis of skeletal size variation in Mystacina robusta Dwyer, 1962 (Chiroptera: Mystacinidae). New Zealand Journal of Zoology 23: 99–110. [Google Scholar]
- 33. Worthy TH, Scofield RP (2004) Skeletal and dental variation within and between Mystacina species in southern New Zealand. New Zealand Journal of Zoology 31: 351–361. [Google Scholar]
- 34. Gunnell GF, Worsham SR, Seiffert ER, Simons EL (2009) Vampyravus orientalis Schlosser (Chiroptera) from the Early Oligocene of Egypt—body mass, humeral morphology and affinities. Acta Chiropterologica 11: 271–278. [Google Scholar]
- 35. Smith FA, Lyons SK, Morgan EK, Jones KE, Kaufman DM, Dayan T et al. (2003) Body mass of Late Quaternary mammals. Ecological archives E084-094. Ecology 84: 3403. [Google Scholar]
- 36. Giannini NP, Gunnell GF, Habersetzer J, Simmons NB (2012) Early evolution of body size In: Gunnell GF, Simmons NB, editors. Evolutionary History of Bats. Boston: Cambridge University Press; pp. 530–555 [Google Scholar]
- 37. Daniel MJ (1990) Lesser short-tailed bat In: King CM, editor. The Handbook of New Zealand Mammals. Auckland: Oxford University Press; pp. 123–130 [Google Scholar]
- 38. Pole M (2014) The Miocene climate in New Zealand: estimates from paleobotanical data. Palaeontologia Electronica 17: 2; 27A; 79 pp. [Google Scholar]
- 39. Reichgelt T, Kennedy EM, Conran JG, Mildenhall DC, Lee DE (2015) The early Miocene paleolake Manuherikia: vegetation heterogeneity and warm-temperate to subtropical climate in southern New Zealand. Journal of Paleolimnology 10.1007/s10933-015-9827-5 [DOI] [Google Scholar]
- 40. Mildenhall DC, Kennedy EM, Lee DE, Kaulfuss U, Bannister JM, Fox B et al. (2014) Palynology of the early Miocene Foulden Maar, Otago, New Zealand: Diversity following destruction. Review of Palaeobotany and Palynology 204: 27–42. [Google Scholar]
- 41. Lee DE, Conran JG, Lindqvist JK, Bannister JM, Mildenhall DC (2012) New Zealand Eocene, Oligocene and Miocene macrofossil and pollen records and modern plant distributions in the Southern Hemisphere. The Botanical Review 78: 235–260. [Google Scholar]
- 42. Raine JI, Mildenhall DC, Kennedy EM (2011) New Zealand fossil spores and pollen: an illustrated catalogue 4th edition GNS Science Miscellaneous Series 4 http://data.gns.cri.nz/sporepollen/index.htm [Google Scholar]
- 43. Mildenhall DC (1989) Summary of the age and paleoecology of the Miocene Manuherikia Group, Central Otago, New Zealand. Journal of the Royal Society of New Zealand 19: 19–29. [Google Scholar]
- 44. Mildenhall DC, Pocknall DT, (1989) Miocene-Pleistocene spores and pollen from Central Otago, South Island, New Zealand. New Zealand Geological Survey Palaeontological Bulletin 59: 1–128. [Google Scholar]
- 45. Pole M, Douglas BJ (1998) A quantitative palynostratigraphy of the Miocene Manuherikia Group, New Zealand. Journal of the Royal Society of New Zealand 28: 405–420. [Google Scholar]
- 46. Pole M, Douglas BJ, Mason G (2003) The terrestrial Miocene biota of southern New Zealand. Journal of the Royal Society of New Zealand 33: 415–426. [Google Scholar]
- 47. Eiting TP, Gunnell GF (2009) Global completeness of the bat fossil record. Journal of Mammalian Evolution 16: 151–173. [Google Scholar]
- 48. Worthy TH, Worthy JP, Archer M, Hand SJ, Scofield RP, Marshall BA et al. (2011) A decade on, what the St Bathans Fauna reveals about the Early Miocene terrestrial biota of Zealandia In: Litchfield NJ, Clark K, editors. Abstract volume, Geosciences 2011 Conference, Nelson, New Zealand: Geoscience Society of New Zealand Miscellaneous Publication; 130A: 120. [Google Scholar]
- 49. Kennedy M, Paterson AM, Morales JC, Parsons S, Winnington AP, Spencer HG (1999) The long and short of it: branch lengths and the problem of placing the New Zealand short-tailed bat, Mystacina . Molecular Phylogenetics and Evolution 13: 405–416. [DOI] [PubMed] [Google Scholar]
- 50. Van Den Bussche RA, Hoofer SR (2000) Further evidence for inclusion of the New Zealand short-tailed bat (Mystacina tuberculata) within Noctilionoidea. Journal of Mammalogy 81: 865–874. [Google Scholar]
- 51. Teeling EC, Springer MS, Madsen O, Bates P, O'Brien SJ, Murphy WJ (2005) A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307: 580–584. [DOI] [PubMed] [Google Scholar]
- 52. Miller-Butterworth CM, Murphy WJ, O'Brien SJ, Jacobs DS, Springer MS, Teeling EC (2007) A family matter: conclusive resolution of the taxonomic position of the long-fingered bats, Miniopterus . Molecular and Biological Evolution 24: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 53. Archer M (1978) Australia’s oldest bat, a possible rhinolophid. Proceedings of the Royal Society of Queensland 89: 23. [Google Scholar]
- 54. Gaina C, Müller DR, Royer J- Y, Stock J, Hardebeck J, Symonds P (1998) The tectonic history of the Tasman Sea: a puzzle with 13 pieces. Journal of Geophysical Research 103: 12413–12433. [Google Scholar]
- 55. Dobson GE (1884) On some peculiarities in the geographical distribution and in the habits of certain mammals inhabiting continental and oceanic islands. Annals and Magazine of Natural History 1884, 14 (5th ser.): 153–159. [Google Scholar]
- 56. Miller GS (1907) The families and genera of bats. Bulletin of the United States National Museum 57: 1–282. [Google Scholar]
- 57. Walton DW, Walton GM (1970) Post-cranial osteology of bats In: Slaughter BH, Walton DW, editors. About Bats: a Chiropteran Symposium. Dallas: Southern Methodist University Press; pp. 93–126. [Google Scholar]
- 58. Dwyer PD (1960) New Zealand bats. Tuatara 8: 61–71. [Google Scholar]
- 59. Dwyer PD (1962) Studies on the two New Zealand bats. Zoology Publications from Victoria University of Wellington 28: 1–28. [Google Scholar]
- 60. Carter GG, Riskin DK (2006) Mystacina tuberculata. Mammalian Species 790: 1–8. [Google Scholar]
- 61. Sedgeley JA (2003) Roost site selection and roosting behaviour in lesser short-tailed bats (Mystacina tuberculata) in comparison with long-tailed bats (Chalinolobus tuberculatus) in Nothofagus forest, Fiordland. New Zealand Journal of Zoology 30: 227–241. [Google Scholar]
- 62. O’Donnell CFJ (2009) The ecology and conservation of New Zealand bats In: Fleming TH, Racey PA, editors. Island Bats. Evolution, Ecology and Conservation. Chicago: University of Chicago Press; pp. 460–495. [Google Scholar]
- 63. Jordan GJ, Carpenter RJ, Bannister JM, Lee DE, Mildenhall DC, Hill RS (2011) High conifer diversity in Oligo-Miocene New Zealand. Australian Systematic Botany 24: 121–136. [Google Scholar]
- 64. McCartney J, Stringer IAN, Potter MA (2007) Feeding activity in captive New Zealand lesser short-tailed bats (Mystacina tuberculata). New Zealand Journal of Zoology 34: 227–238. [Google Scholar]
- 65. Freeman PW (1988) Frugivorous and animalivorous bats (Microchiroptera): dental and cranial adaptations. Biological Journal of the Linnean Society 33: 249–272. [Google Scholar]
- 66. Ravel A, Adaci M, Bensalah M, Mahboubi M, Mebrouk F, Essid EM et al. (2014) New philisids (Mammalia, Chiroptera) from the Early–Middle Eocene of Algeria and Tunisia: new insight into the phylogeny, palaeobiogeography and palaeoecology of the Philisidae. Journal of Systematic Palaeontology 10.1080/14772019.2014.941422 [DOI] [Google Scholar]
- 67. Pocknall DT, Mildenhall DC (1984) Late Oligocene–early Miocene spores and pollen from Southland, New Zealand. New Zealand Geological Survey Paleontological Bulletin 51: 66 pp. [Google Scholar]
- 68. Carpenter RJ, Bannister JM, Lee DE, Jordan GJ (2012) Proteaceae leaf fossils from the Oligo-Miocene of New Zealand: new species and evidence of biome and trait conservatism. Australian Systematic Botany 25: 375–389. [Google Scholar]
- 69. Pole M (2007) Plant-macrofossil assemblages during Pliocene uplift, South Island, New Zealand. Australian Journal of Botany 55: 118–142. [Google Scholar]
- 70. Ecroyd CE (1995) Dactylanthus and bats: the link between two unique endangered New Zealand species and the role of the community in their survival In: Saunders AD, Craig JL, Mattiske EM, editors. Nature Conservation 4: the Role of Networks. Chipping Norton, New South Wales: Surrey Beatty and Sons; pp. 78–87 [Google Scholar]
- 71. Sussman RW, Raven PH (1978) Pollination by lemurs and marsupials: an archaic coevolutionary system. Science 200: 731–736. [DOI] [PubMed] [Google Scholar]
- 72. Carthew SM RL Goldingay RL (1997) Non-flying mammals as pollinators. Trends in Ecology and Evolution 12: 104–108. [DOI] [PubMed] [Google Scholar]
- 73. Mildenhall DC, Crosbie YM (1980) Some porate pollen from the upper Tertiary of New Zealand. New Zealand Journal of Geology and Geophysics 22: 499–508. [Google Scholar]
- 74. Macphail MK, Mildenhall DC (1980) Dactylanthus taylori: in North-West Nelson, New Zealand? New Zealand Journal of Botany 18: 148–152. [Google Scholar]
- 75. Wood JR, Wilmshurst JM, Worthy TH, Holzapfel AS, Cooper A (2012) A lost link between a flightless parrot and a parasitic plant and the potential role of coprolites in conservation paleobiology. Conservation Biology 26: 1091–1099. 10.1111/j.1523-1739.2012.01931.x [DOI] [PubMed] [Google Scholar]
- 76. Pocknall DT, Turnbull IM (1989) Palaeoenvironmental and stratigraphic significance of palynomorphs from Upper Eocene (Kaiatan) Beaumont Measures and Orauea Mudstone, Waiau Basin, western Southland, New Zealand. New Zealand Journal of Geology and Geophysics 32: 371–378. [Google Scholar]
- 77. Birch J, Keeley SC, Morden CM (2012) Molecular phylogeny and dating of the Asteliaceae (Asparagales): Astelia s.l. evolution provides insight into the Oligocene history of New Zealand. Molecular Phylogenetics and Evolution 65: 102–115. 10.1016/j.ympev.2012.05.031 [DOI] [PubMed] [Google Scholar]
- 78. Maciunas E, Conran JG, Bannister JM, Paull R, Lee DE (2011) Miocene Astelia (Asparagales: Asteliaceae) macrofossils from southern New Zealand. Australian Systematic Botany 24: 19–31. [Google Scholar]
- 79. Lloyd BD, McQueen SM (2002) Measuring mortality in short-tailed bats (Mystacina tuberculata) as they return from foraging after an aerial 1080 possum control operation. New Zealand Journal of Ecology 26: 53–59. [Google Scholar]
- 80. Kaulfuss U, Harris AC, Lee DE (2010) A new fossil termite (Isoptera, Stolotermitidae, Stolotermes) from the early Miocene of Otago, New Zealand. Acta Geologica Sinica 84: 705–709. [Google Scholar]
- 81. Kaulfuss U, Lee DE, Barratt BIP, Leschen RAB, Larivière M-C, Dlussky GM et al. (2014) A diverse fossil terrestrial arthropod fauna from New Zealand: evidence from the early Miocene Foulden Maar fossil lagerstätte . Lethaia 10.1111/let.12106 [DOI] [Google Scholar]
- 82. Datzmann T, von Helversen O, Mayer F (2010) Evolution of nectarivory in phyllostomid bats (Phyllostomidae Gray, 1825, Chiroptera: Mammalia). BMC Evolutionary Biology 10: 165 14 pp. 10.1186/1471-2148-10-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rojas D, Vale A, Ferrero V, Navarro L (2011) When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Molecular Ecology 20: 2217–2228. 10.1111/j.1365-294X.2011.05082.x [DOI] [PubMed] [Google Scholar]
- 84. Monteiro LR, Nogueira MR (2011) Evolutionary patterns and processes in the radiation of phyllostomid bats. BMC Evolutionary Biology 11: 137 (23 p.). 10.1186/1471-2148-11-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dávalos LM, Cirranello AL, Geisler JH, Simmons NB (2012) Understanding phylogenetic incongruence: lessons from phyllostomid bats. Biological Reviews 87: 991–1024. 10.1111/j.1469-185X.2012.00240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fleming TH, Geiselman C, Kress WJ (2009) The evolution of bat pollination: a phylogenetic perspective. Annals of Botany (London) 104: 1017–1043. 10.1093/aob/mcp197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee DE, Lindqvist JK, Beu AG, Robinson JH, Ayress MA, Morgans HEG et al. (2014) Geological setting and diverse fauna of a Late Oligocene rocky shore ecosystem, Cosy Dell, Southland. New Zealand Journal of Geology and Geophysics 57: 195–208. [Google Scholar]
- 88. Samonds KE, Gunnell GF, Simmons NB (2014) Filling the Cenozoic gap: Miocene bats from Nosy Makamby, Madagascar. Journal of Vertebrate Paleontology, Program and Abstracts, 2014: 220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.