Summary
To generate a mouse model of spontaneous epidermal depigmentation, parental h3TA2 mice, expressing both a human-derived, tyrosinase-reactive T cell receptor on T cells and the matching HLA-A2 transgene, were crossed to keratin 14-promoter driven, stem cell factor transgenic (K14-SCF) mice with intra-epidermal melanocytes. In resulting Vitesse mice, spontaneous skin depigmentation precedes symmetrical and sharply demarcated patches of graying hair. Whereas the SCF transgene alone dictates a greater retinoic acid receptor-related orphan receptor gamma (RORγt)+ T cell compartment, these cells displayed markedly increased IL-17 expression within Vitesse mice. Similar to patient skin, regulatory T cells were less abundant compared to K14-SCF mice, with the exception of gradually appearing patches of repigmenting skin. The subtle repigmentation observed likely reflects resilient melanocytes that co-exist with skin-infiltrating, melanocyte-reactive T cells. Similar repigmenting lesions were found in a different TCR transgenic model of vitiligo developed on an SCF transgenic background, supporting a role for SCF in repigmentation.
Keywords: vitiligo, mouse model, T cell receptor, IL-17, stem cell factor, pigmentation
Introduction
Vitiligo is a pigmentary skin disorder characterized by progressive loss of melanocytes (Le Poole et al., 1993a). Depigmenting lesions are often symmetrically distributed (Taieb and Picardo, 2009). Vitiligo develops in 0.5% of the global population (Abu Tahir et al., 2010), and the uneven skin tone is psychologically stressful to patients (Parsad et al., 2003).
Skin depigmentation is mediated by melanocyte-reactive T cells (van den Wijngaard et al., 2001). Thus, proposed vitiligo therapies should preferably counter autoimmune responses. Unfortunately, current treatments offer limited efficacy (Buggiani et al., 2012).
Limited availability of mouse models has been an obstacle for studying vitiligo and developing new treatment opportunities. Here we describe a model combining epidermal pigmentation and melanocyte-reactive T cells into mice displaying spontaneous epidermal depigmentation. T-cell mediated autoimmune responses towards epidermal melanocytes are reproduced using a transgenic T cell receptor (TCR) derived from human melanocyte and melanoma reactive, CD8- lymphocytes (Das et al., 2001; Mehrotra et al., 2012; Nishimura et al., 1999). The TCR consists of Vα4 and Vβ12 subunits and recognizes tyrosinase. This high-affinity TCR is functional on CD8+, CD4+ and double negative T cells. Expressed together with the HLA-A2 transgene, resulting h3TA2 mice develop progressive depigmentation of the pelage (Mehrotra et al., 2012).
Epidermal pigmentation is introduced by the K14-SCF transgene. Membrane-bound stem cell factor (SCF) expressed by keratinocytes serves to retain epidermal melanocytes, resulting in skin pigmentation and limited infiltration by mast cells (Kunisada et al., 1998). Some keratinocyte membrane bound SCF may be proteolytically released, and affect (auto)immunity (Carter et al., 2008; Longley et al., 1997). This process may affect antigen presenting cell function as shown in systemic lupus erythromatosus (SLE) (Kang et al., 2012). There, SCF-exposed antigen presenting cells can drive Th17 responses, involved in autoimmune disorders (Harrington et al., 2005; Stockinger et al., 2011, Yi et al., 2012).
Likewise in vitiligo, skin-infiltrating Th17 were observed (Wang et al., 2011). Adoptive transfer of melanocyte reactive Th17 cells into C57BL/6 mice resulted in rapid depigmentation (Muranski et al., 2008). Moreover, depigmentation in mice that cannot produce IFN-γ correlates with increased expression of IL-17 (Chatterjee et al, 2014). Several lines of evidence support a central role for IFN-γ producing T cell in vitiligo development (Gregg et al., 2010, Harris et al., 2012). However, the functional contribution of IL-17 producing T cells to depigmentation has yet to be fully appreciated. Appropriate mouse models can help overcome this limitation. Our increased understanding of immune processes in vitiligo also calls for suitable models to test immunotherapeutics (Abu Tahir et al., 2010).
Regulatory T cell responses are important to keep autoimmune responses in check. For several autoimmune diseases, including vitiligo, a dysfunctional regulatory T cell compartment has been reported (Klarquist et al, 2010; Dwivedi et al, 2013). This has prompted studies into restoring the regulatory to IL-17 producing T cell balance as a means to avert autoimmune tissue damage, which may be of use to vitiligo as well (Yang et al, 2011; Kong et al, 2012). A suitable in vivo disease model to study the role of T cell subsets in depigmentation can shed further light on this process.
Thus, K14-SCF and h3TA2 mice were crossed to create triple transgenic offspring expressing epidermal melanocytes, melanocyte reactive T cells, and relevant MHC. We followed vitiligo development and documented depigmentation, studied melanocyte loss, localized HLA-A2 expression and identified relevant skin-infiltrating T cells, quantified antigen-specific IFN-γ and IL-17 responses and the role of SCF, and measured the abundance of an SCF-driven APC subset. The current mouse model provides a tool to study the role of T cell subsets in vitiligo, while offering a useful means of testing therapeutics for this disease.
Materials and Methods
Mice
C57BL/6 and HLA-A2 transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Epidermally pigmented K14-SCF transgenic mice that express membrane-bound SCF in the epidermis and primary lymphoid organs were described earlier (Kunisada et al., 1998; Misfeldt and Grimm, 1994). FH mice express a CD8 dependent transgenic T cell receptor raised against mouse tyrosinase peptide FMDGTMSQV presented in the context of HLA-A2D; the T cell receptor was isolated from T cells generated in albino mice (Gregg et al., 2010). The TCR from FH mice recognize the same peptide presented by mouse melanocytes and T cells from h3T mice that express the tyrosinase-reactive T cell receptor TIL1383i, consisting of Vα4 and Vβ12 subunits and originating from CD4+, HLA-A2 restricted, melanoma-infiltrating human T cells (Nishimura et al, 1999). This T cell receptor is known as TIL1383I reactive with human tyrosinase peptide YMDGTMSQV, is coreceptor independent and functional when expressed by CD4+, CD8 and double negative T cells, as well as Treg (Nishimura et al., 1999, Brusko et al., 2010, Mehrotra et al., 2012). The h3TA2 mice bear both the human tyrosinase-reactive TCR and HLA-A2 transgenes (Mehrotra et al., 2012). The h3TA2 and K14-SCF strains were crossed to generate triple transgenic Vitesse mice, for ‘vitiligo’ and ‘speed’. Mice were kept according to Institutional Animal Care and Use Committee (IACUC) regulations, and experiments were performed in accordance with the EU Directive 2010/63/EU. For genotyping, tail snip DNA was screened for Vβ12 and HLA-A2 transgenes by PCR. Expression of the SCF transgene is associated with pigmentation of the skin and was visually evaluated as needed. Mice were maintained as heterozygotes as animals homozygous for the SCF transgene display limited fertility.
Quantifying depigmentation
Depigmentation was documented at 5 week age intervals using a flatbed scanner (Hewlett-Packard Company, Palo Alto, CA) and Adobe Software (Adobe Systems, Inc., San Jose, CA). Depigmentation was calculated as described (Denman et al., 2008). Briefly, anesthetized mice were placed on a flatbed scanner and resulting images were subjected to image analysis using Adobe Photoshop. Depigmentation was calculated from the largest evaluable area as the percentage of pixels among >150,000 evaluated with a luminosity above the cutoff level set to include 95% of pixels for untreated mice. Different animals were used to quantify depigmentation at each time point with the exception of Vitesse mice beyond 30 weeks of age. These later time points were included to evaluate when depigmentation tapers off in both groups. As relevant, mice were photographed using a DSC-S950 SteadyShot digital camera (Sony, Tokyo, Japan).
Phenotypic characterization of circulating T cells by flow cytometry
Cell surface markers were evaluated among splenocytes from 7 wk old transgenic mice using antibodies CD3-AlexaFluor647 (145-2C11; BioLegend, San Diego, CA) or CD3-FITC (145-2C11, BD Biosciences, San Jose, CA), CD4-V450 (RM4-5; BD Biosciences), CD8-BrilliantViolet711 (53-6.7; BD Biosciences), HLA-A2-FITC (BB7.2; BD Biosciences), CD107a-PeCy7 (ID4B, Biolegend) and Vβ12-FITC (511; Thermo Scientific, Hanover Park, IL). For detection of intracellular markers, paraformaldehyde-fixed splenocytes were stained for FoxP3-PE (150D, BioLegend), IL17a-allophycocyanin-Cy7 (TC11-18H10, BD Biosciences), IFN-γ-PerCP/Cy5.5 (XMG1.2, Biolegend) and RORγT-allophycocyanin (AFKJS-9, eBioscience, San Diego, CA) in presence of 0.3% saponin. To analyze effects of SCF on expansion of Treg and Th17 compartments, splenocytes from C57BL/6, HLA-A2, K14-SCF, h3TA2 and Vitesse mice were pre-stimulated using Cell Stimulation Cocktail plus protein transport inhibitor (eBioscience, San Diego, CA) for 5 hrs. prior to immunostaining. Surface molecules were detected before intracellular staining. Cell staining data were acquired using a FACSCanto or LSR-II flow cytometer (BD Biosciences).
Cytokine Profiles
Peptide reactive T cells were quantified using ELISPOT kits (Mabtech, Inc., Mariemont, OH). Briefly, plates were coated with capture anti-IFN-γ or anti-IL-17 antibodies. Splenocytes from C57BL/6, HLA-A2, K14-SCF, h3TA2 or Vitesse mice were cultured in RPMI1640 plus 10%FBS in presence of 10-30μg/ml mouse (FMDGTMSQV) and human (YMDGTMSQV) tyrosinase peptides (Pi Proteomic, LLC, Huntsville, AL) or PBS and plated at 5×10(5) cells/well. After 24h for IFN-γ or 48h for IL-17 detection, cytokine secreting cells were detected using biotinylated anti-cytokine antibodies, followed by horseradish peroxidase-conjugated streptavidin (Mabtech, Inc.) and 3-amino-9-ethylcarbazole substrate (Sigma-Aldrich). Cytokine secreting cells were quantified using a CTL-ImmunoSpot S6 Micro Analyzer (Cellular Technology Ltd, Shaker Heights, OH). For intracellular cytokine staining, see above section
Immunostaining of skin
Abdominal skin biopsies were snap-frozen in OCT (Sakura Finetek, Torrance, CA). Cryosections were fixed in cold acetone and stored at -20°C until use. For immunohistochemical stains, indirect double staining was performed as described (Le Poole et al., 1993b; Le Poole et al, 2008). Briefly, 8μm sections were pretreated with SuperBlock and Biotin Block (ScyTek, Logan, UT). Primary antibodies to CD3-biotin (145-2C11; BD Biosciences), TRP-1 (Ta99; Covance, Princeton, NJ) and HLA-A2-FITC (BB7.2; BD Biosciences) were added, followed by incubation with anti-mouse IgG2a-AP (SouthernBiotech, Birmingham, AL) and streptavidin-HRP (SouthernBiotech) or anti-FITC (MyBioSource, SanDiego, CA). Enzymatic detection was initiated using Fast Blue BB substrate (Sigma-Aldrich) and finalized using 3-amino-9-ethylcarbazole (Sigma-Aldrich). Where single immunoperoxidase stainings were performed, sections were counterstained using haematoxylin (Sigma-Aldrich). For immunofluorescent detection, acetone or paraformaldehyde fixed sections were blocked as above and exposed to antibodies reactive with CD3-FITC (145-2C11, BD Biosciences), FoxP3-PE (MF-14, BioLegend) or IL-17a (TC11-18H10, BioLegend). For IL-17a detection, secondary anti-rat-biotin (Polyclonal, product #13-4813-85, eBioscience) was added followed by streptavidin-PE (product # 7100-09M, SouthernBiotech). For nuclear counterstaining, 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA) was used. Staining was imaged on an Olympus AX80T Microscope (Melville, NY) and quantified as the number of cells per mm2 from the epidermis to 5-fold depth below by Adobe Photoshop software.
Measuring responses of cKIT+ APC to SCF
To understand phenotypic changes induced by SCF exposure, splenocytes from h3TA2 mice (3/group) were stimulated with 15 ng/ml of recombinant mouse SCF (BioLegend) in vitro for 24 hrs prior fluorocytometric analysis using antibodies CD117-allophycocyanin (2B8; eBioscience), CD11b-PercpCy5.5 (M1/70; BD-Pharmingen), CD11c-FITC (HL3; BD-Pharmingen), CD41-PECy7 (MWReg30; eBioscience), CD151-PE (455807, R&D systems, Minneapolis, MN). The percentage of CD117+CD41+CD151+ among CD11b-CD11c- antigen presenting cells (MM cells) was compared before and after SCF exposure using the FACS Canto-II. Indirect consequences for T cell activation were measured in supernatants 20 hrs after adding mouse tyrosinase peptide FMDGTMSQV (conc. 0-30μg/μl) to SCF-treated splenocytes, using IFN-γ and IL-17 ELISA kits (Mabtech, Inc., Mariemont, OH). In separate experiments, SCF-exposed splenocytes were T-cell enriched using mouse CD3+ enrichment cocktail (Stem Cell Technologies, Vancouver, BC, Canada). Human T2 cells which cannot process antigen, are MHCII- and will present HLA-A2 restricted antigens upon addition of peptides (ATCC# CRL-1992, Manassas, VA) were pulsed with mTYR for 2 hrs prior to adding to enriched T-cells at a 1:1 ratio for 5 hrs. T cell stimulation was measured by intracellular cytokine staining for cells pretreated with 10μg/mL brefeldinA prior to staining with primary antibodies to IL-17A-AlexaFluor700 (TC11-18H10.1, BioLegend) and IFN-γ-PerCP/Cy5.5 (XMG1.2, BioLegend), as well as CD107a-PE-Cy7 (1D4B, BioLegend), to probe the cytotoxic function of cytokine secreting cells after SCF treatment in response to pulsed T2 cells. Stained cells were analyzed using the FACS Fortessa LSR II.
Statistical analyses
Each experiment represented in the body of the manuscript was performed at least twice, with the exception of data shown in Fig. 1 and in supplemental figs S1 and S4. Data were presented as mean ± SEM and analyzed for statistical significance of differences among two groups using two tailed student t-tests accounting for unequal variance, except for the depigmentation curve. The Pearson chi-square statistic was used to compare depigmentation rates. Statistical modeling was performed in R (2.15.2) using the generalized estimating equations (GEE) library for Pearson's chi-square test.
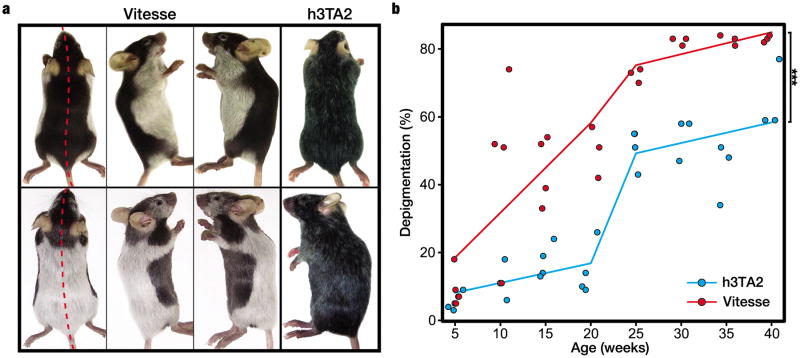
Figure 1.
Sharply demarkated and accelerated depigmentation develops in Vitesse mice. (a) Symmetrical depigmentation patterns of the pelage in Vitesse mice. Littermates, both heterozygous for all 3 transgenes (h3T, HLA-A2 and SCF), showed human-like vitiligo symmetry and complete depigmentation (rather than hair graying observed in h3TA2) at 14 weeks of age. (b) Vitesse mice (n=4) showed significantly accelerated and more complete depigmentation as compared to h3TA2 mice (n=6); Vitesse mice showed half-maximum depigmentation at 14 weeks of age whereas h3TA2 mice showed it at 23 weeks. Depigmentation was calculated for 48 mice total by including individual animals except for time points 30, 35 and 40 weeks calculated for the same Vitesse mice.
Results
Vitesse mice show depigmentation patterns resembling human vitiligo
Lesional symmetry and complete depigmentation are hallmarks of human vitiligo lesions. We monitored depigmentation in triple transgenic mice expressing melanocyte reactive T cells, HLA-A2 and epidermal melanocytes, and observed varied patterns of sharply demarcated, symmetrical depigmentation similar to human disease. Aberrant growth, development or behavior was not observed in triple transgenic mice, named ‘Vitesse’ for reasons explained below. Skin depigmentation precedes that of the pelage in Vitesse mice and by 5-7 weeks, pigment loss from the ears and extremities is essentially complete. Fig.1a shows littermates, heterozygous for all transgenes, with strikingly opposing yet human-like symmetry in hair depigmentation at 14 weeks of age, suggesting environmental factors may impact depigmentation patterns. To quantify differences in depigmentation kinetics compared to h3TA2 mice, hair pigmentation was evaluated in a total of 48 animals as shown in Fig. 1b. Depigmentation was calculated by scanning the animals. Following ventral depigmentation over time, Vitesse mice reached maximum depigmentation (82.3±0.7%) at 30 weeks of age, but h3TA2 mice remained less depigmented (65±6%) by 40 weeks. Half maximum depigmentation was observed at 14 and 23 wks for Vitesse and h3TA2 mice, respectively. Depigmentation was significantly accelerated in triple transgenic mice at P=0.00006, which prompted us to name the model ‘Vitesse’. Taking into account that the average depigmentation rate (which can vary over time within each patient) appears close to 1% per year for human patients (Cedercreutz et al, 2011), we can carefully infer that depigmentation in Vitesse mice occurs on a roughly 50-fold reduced time scale.
Transgenic T cells are predominantly CD4–CD8– in Vitesse mice
We analyzed whether overexpressed SCF affects T cell development and function. Coreceptor expression was compared among TCR transgenic T cells in Vitesse, h3TA2, and h3T mice (Fig. 2). In Vitesse mice, 3.4±1.1% were CD8+, 1.2±1.2% were CD4+, and 94.7±3.2% were CD4-CD8- double negative (DN) T cells among the Vβ12+ cell population. The percentage of DN cells was similar in h3TA2 mice but different from h3T mice without HLA-A2, where 55.8±9.2% of TCR transgenic T cells were CD8+, 16.4±8.7% were CD4+, and 25.7±0.7% were DN (P=<0.0001). Thus, coreceptor expression was not significantly different between h3TA2 and Vitesse mice, and whereas expression of the HLA-A2 transgene affects coreceptor expression, the SCF transgene does not. The expression of the h3T T cell receptor primarily on CD4-CD8- T cells in h3TA2 and Vitesse mice reinforces the co-receptor independence of this TCR, which functions in the absence of either CD4 or CD8 (Mehrotra et al, 2012).
Figure 2.
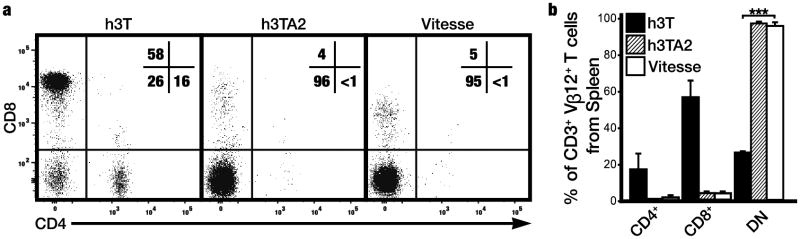
T cell receptor transgene expression is observed primarily among circulating CD4-CD8- T cells. (a) The h3T transgenic TCR was identified by staining for the Vβ12 subunit and CD4 and CD8 expression was assessed among gated h3T, h3TA2, and Vitesse splenocytes. (b) Similar to h3TA2 mice, expression of the TCR transgene is observed for 3.4±1.1% on CD8+ T cells, 1.2±1.2% on CD4+ T cells, and 94.7±3.2% on DN T cells in Vitesse mice. A significant difference in transgene expression among T cell subsets was observed between h3T and Vitesse mice. (n=3) ***P <0.001.
Presence of the SCF transgene is associated with increased abundance of IL-17A+ T cells
We quantified IL-17 producing T cells, important in autoimmunity, in h3TA2 and Vitesse mice (Fig. 3). IL-17A+ T cells were visualized among splenocytes in Fig. 3a and quantified in Fig. 3b. In control K14-SCF transgenic mice, RORγt+ cells (0.3%) and IL17A+ cells (0.5%) were equally abundant compared to control C57BL/6 mice. Interestingly, the number of spontaneous IL-17A producing cells was significantly (2.5 fold) increased in Vitesse compared to h3TA2 mice (P=0.0281), not shown. These differences were further accentuated upon activation (Fig. 3b); Vitesse mice showed increased percentages of both RORγt+ (10.1 fold) as well as IL-17A+ cells (6.3 fold) upon PMA and ionomycin stimulation (P=0.0246 and 0.0091, respectively) when compared to C57BL/6 mice. IL-17a secretion was likewise significantly increased among Vitesse versus h3TA2 splenocytes (P=0.0281). In Figs. 3c (example histogram) and 3d (quantification), we further show that Vitesse mice contain a negligible IFN-γ+IL-17+ double positive, plastic population of 0.5% that is not significantly increased compared to other mouse strains, indicating that functional aspects ascribed to IL-17+ cells in Vitesse mice can be assigned to a single positive, committed T cell population rather than a subpopulation of cells with IFN-γ expression potential. Taken together with the data presented in Figs. 3a and b, this indicates that SCF increased the compartment of committed cells in TCR transgenic mice, producing IL-17A upon activation.
Figure 3.
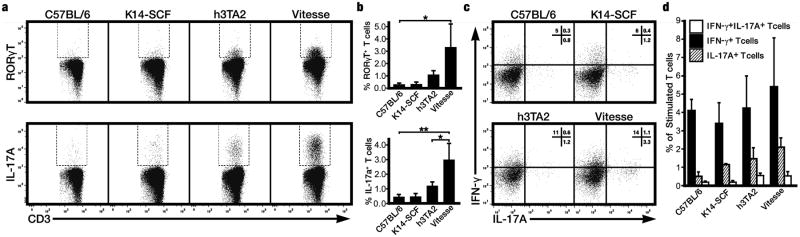
Presence of the SCF transgene is accompanied by an enlarged IL-17 producing T cell compartment. (a) In flow cytometric analysis, Vitesse mice showed a markedly increased percentage of RORγt+ and IL-17+ cells among splenocytes as compared to C57BL/6 mice upon stimulation with PMA and ionomycin (b) Percentages of RORγt+ and IL-17+ cells were 10.1 and 6.3 fold increased, respectively, when comparing Vitesse to C57BL/6 mice at n=3. Moreover, in Vitesse mice, IL-17A producing cells were significantly (2.5 fold) more abundant compared to h3TA2 mice. *P <0.05; **P <0.01. (c) Though a trend is visible, no significant increase in the plastic, IL-17 and IFN-γ double positive T cell population was observed in Vitesse mice.
Vitesse mice display enhanced antigen-specific IL-17A+ T cell responses
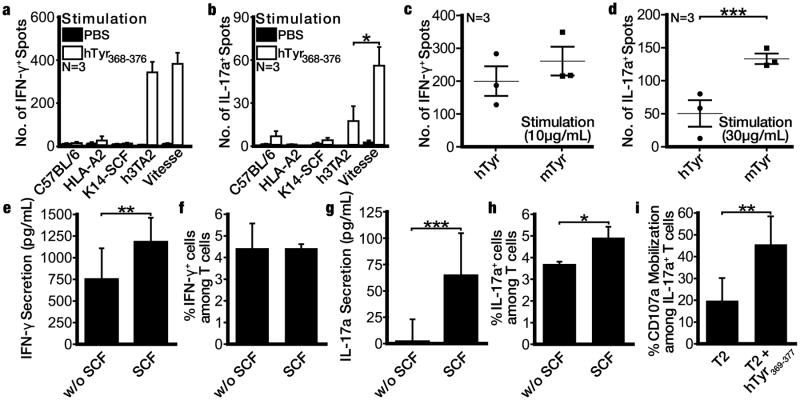
To assign a cytokine profile to accelerated depigmentation, we next compared antigen specific T cell responses to 3-30 μg/ml peptide among Vitesse and h3TA2 mice in IFN-γ or IL-17A ELISPOT plates. The number of cytokine secreting cells increased with higher peptide concentrations for either cytokine and overall, IFN-γ secreting T cells were more abundant than IL-17 secreting cells. IFN-γ responses, best measured at 3 or 10μg/ml peptide were similar between both strains. Fig. 4a-b shows results for optimal peptide concentrations to measure responses for either cytokine. Best measured at 30 μg/ml peptide, T cells from Vitesse mice showed 3.2 fold increased IL-17 responses over h3TA2 mice (P=0.0184). Additional data are found in Fig. S1; at the highest peptide concentration, the number of IFN-γ secreting T cells was too numerous to count. We also tested whether the h3T TCR can recognize the HLA-A2 restricted mouse tyrosinase-derivative peptide FMDGTMSQV (mTyr) most relevant to mouse depigmentation. T cells from Vitesse mice showed similar IFN-γ responses to hTyr and mTyr, but 2.7 fold elevated IL-17 responses in response to mTyr (Fig. 4c-d) (P=0.0090). To probe the role of SCF in antigen-specific IL-17 responses enhanced in Vitesse mice, we prestimulated h3TA2 splenocytes with 15 ng/mL SCF for 24 hrs and added 10 μg/ml hTyr to measure resulting cytokine expression; (e) IFN-γ secretion was enhanced 1.6 fold (P=0.0054), but (f) not the number of IFN-γ secreting cells whereas (g) the amount (P=0.0004) and (h) the number of IL-17 secreting cells (P=0.039) was increased 27.2-fold and 1.3-fold, respectively in an SCF-dependent fashion. Importantly, (i) IL-17 secreting cells showed increased expression of CD107a, indicating their involvement in cytotoxic activity against tyrosinase peptide-presenting cells (P=0.0016). We also observed a significant, 46.9% reduction in Treg among h3TA2, 30 μg/μl peptide-stimulated T cells following SCF addition (P=0.0279) as shown in Fig. S2. Thus accelerated depigmentation correlated with more IL-17A producing T cells in mice, supported by overexpression of SCF.
Figure 4.
SCF drives more prominent tyrosinase-specific IL-17 responses in Vitesse mice. Total splenocytes were stimulated with hTyr peptide in (a) IFN-γ and (b) IL-17A ELISPOT plates, showing enhanced antigen-specific IL-17 responses by Vitesse T cells compared to h3TA2 mice (and other strains) whereas IFNγ responses are similar for both vitiligo-prone strains. Comparing reactivity to hTyr and mTyr by Vitesse splenocytes, (c) IFN-γ responses were similar but (d) numerically greater IL-17 responses were observed to mTyr than hTyr. To measure the contribution of SCF to cytokine responses, h3TA2 splenocytes were incubated with hTyr in presence and absence of SCF and (e) the amount of IFN-γ secreted but (f) not the number of IFN-γ secreting cells increased, whereas both the (g) amount and (h) number of IL-17 secreting cells increased after 24 hrs SCF exposure in vitro. (i) IL-17 secreting cells are cytotoxic (express surface CD107a) in response to tyrosinase peptide. Peptide concentrations used were 10μg/ml except for Fig. 4b and 4d, where results are shown for 30μg/ml. For each graph (n=3), *P <0.05, **P<0.01, ***P <0.001.
SCF supports the development of MM cells driving IL-17 responses
A subset of cKIT+ antigen presenting cells was held responsible for enhanced IL-17 responses (Harrington et al., 2005, Stockinger et al., 2011, Kang et al., 2012). Others have assigned enhancement of IL-17 responses to a CD11b+ subset of antigen presenting cells (Yi et al., 2012). Here, we examined the SCF-responsive population among h3TA2 splenocytes and found that a CD117+CD11b-CD11c-CD41+CD151+ subset of antigen presenting cells (megakaryocyte and/or bipotent megakaryocyte/erythroid progenitors or MM cells) was increased 1.6-fold in response to 15 ng/mL SCF for 24 hrs (P=0.016), similar to observations made in models of systemic lupus erythematosus (Kang et al, 2012). Taken together with the above results measuring IL17A secretion among peptide-stimulated splenocytes, the proposed role of SCF in driving IL-17 responses in our model is further substantiated.
SCF overexpression allows for spotted repigmentation of the epidermis
Rapid depigmentation in Vitesse mice is accompanied by the development of pigmented lesions, increasing in number and pigmentation over time (Fig. 5a). Vitesse mice revealed such lesions even underlying the depigmenting pelage. This phenomenon may reflect resilient melanocytes in a process mimicking repigmentation, as SCF supports melanocyte retention in the epidermis (Vanover et al., 2009). For spotted repigmentation to occur, melanocytes must survive against a gradient of melanocyte-reactive T cells, and migrate, differentiate and proliferate within that environment. We thus studied the distribution of melanocytes and reactive T cells. There was no overall difference in density of Vβ12+ T cells between the skin of h3TA2 and Vitesse mice (data not shown). Melanocytes were observed in skin of C57BL/6, HLA-A2, and K14-SCF mice, but not in h3TA2 and Vitesse mice except for pigmented areas in the latter where epidermal melanocytes were found in proximity to T cells (Fig. 5b). Thus such lesions contain resilient melanocytes coexisting with infiltrating T cells, likely supported by SCF overexpressing keratinocytes. Such findings suggest that SCF may be supportive of repigmentation in vitiligo. In supplemental figure S4a, a similar phenomenon is observed in FH mice that are crossbred with K14-SCF mice; skin depigmentation is greatly accelerated and mice display spotted repigmentation. Thus repigmentation can be assigned to SCF overexpression in both mouse models.
Figure 5.
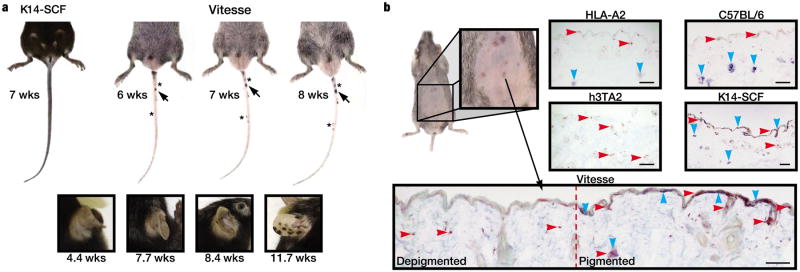
SCF overexpression is associated with formation of pigmented lesions. (a) Rapid depigmentation is accompanied by the gradual development of repigmenting lesions. Pigmented lesions increased in pigmentation (arrow) and number (asterisks) over time (b) Epidermal repigmentation underneath the pelage of Vitesse mice. Expression of melanocyte marker, Trp-1 (blue) and pan-T cell marker, CD3 (red) were observed in the skin of C57BL/6, HLA-A2, and K14-SCF mice. Melanocytes were absent from skin of h3TA2 and Vitesse mice except within pigmented lesions, where a near original melanocyte distribution was accompanied by infiltrating T cells. n=3. Scale bar = 0.2mm.
Repigmentation is accompanied by increased Treg infiltration
We evaluated HLA-A2 expression to examine melanocyte protection from T cell cytotoxicity by loss of HLA-A2 expression in repigmented areas, as reported in melanoma (Natali et al., 1989). Expression was however similar in pigmented and depigmented areas (Fig. 6a). Alternatively, transgenic T cell activity may be suppressed by Treg. Compared to C57BL/6 mice at 1.68%, Foxp3+ regulatory T cells were systemically reduced among both splenocytes from both vitiligo models h3TA2 and Vitesse, to 1.02% (P=0.0072) and 1.01% (P=0.0345) respectively, consistent with reduced Treg abundance in skin of human patients compared to controls (Klarquist et al., 2010). Percentages were unaffected at 1.93% and 2.02% in HLA-A2 and K14-SCF mice (Fig. 6b). IL-17+CD3+ cells were similarly abundant in depigmented and repigmented Vitesse mouse skin, but importantly, Foxp3+CD3+cells were 2.6 fold more frequent in repigmented than depigmented areas (P=0.0164) (Fig. 6c-f). In a preliminary experiment, a similar increase in skin infiltrating Treg was found in repigmenting skin from FH mice expressing the SCF transgene (P=0.0008), whereas IL-17 producing T cell populations remained unchanged (Fig. S4b). These data suggest that repigmentation is supported by increased/ enhanced Treg infiltrates.
Figure 6.
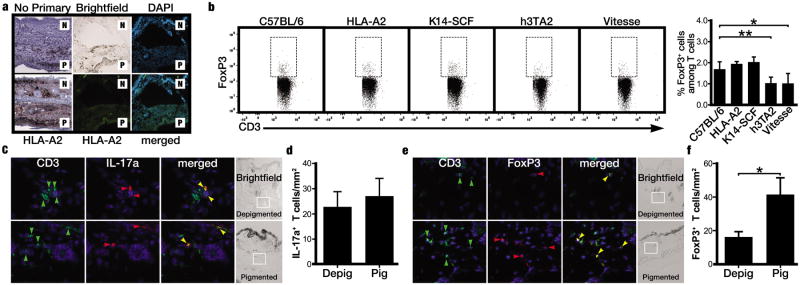
Repigmentation is accompanied by increased Treg infiltration to the skin. (a) HLA-A2 immunostaining of Vitesse mouse skin (above: negative control images) revealing no differences between depigmented (‘N’ for non-pigmented) and repigmented (‘P’ for pigmented) areas. (b) Fluorocytometry reveals less Treg among splenocytes of h3TA2 and Vitesse than of C57BL/6 mice (n=3). (c) Immunofluorescent staining of Vitesse mouse skin revealed IL-17+CD3+ cells (orange arrow) in both depigmented and repigmented areas. (d) IL-17+ cells quantified in Vitesse mouse skin (n=4). (e) Foxp3+CD3+ cells (orange arrows) were more abundant in repigmented relative to depigmented areas. (f) Quantification of Treg in Vitesse mouse skin samples (n=4). *P <0.05; **P <0.01.
Discussion
In the Vitesse mouse, spontaneous depigmentation of the skin and hair models patterns commonly observed in generalized vitiligo, now commonly named ‘vitiligo’ to include all previously recognized subforms except segmental vitiligo (Ezzedine et al., 2012). The depigmentation patterns are strikingly symmetrical and initiate in areas also commonly affected in human vitiligo, including periocular areas and extremities. Symmetry is possibly explained by T cells extravasating from blood vessels with comparable adhesion molecule expression on opposite sides of the body (Goudie et al., 1990).
A mouse model faithfully representing human disease is important to investigate the pathomechanism and develop therapies. The C57BL/J6-vit/vit mouse was developed as a spontaneous vitiligo model (Boissy et al., 1987; Lerner et al., 1986) but underlying mutations in the gene encoding microphthalmia-associated transcription factor (Mitf) are not found in patients (Tripathi et al., 1999). Vaccine-induced depigmentation can serve as a mouse model of vitiligo but does not express spontaneous disease (Denman et al., 2008; Guan et al., 2006).
TCR transgenic mouse models reflect T cell mediated autoimmunity. Existing strains such as FH (Gregg et al., 2010; Nichols et al., 2007), Pmel-1 (Overwijk et al., 2003), and h3TA2 (Mehrotra et al., 2012) display spontaneous hair depigmentation with different kinetics, depending on TCR affinity and the match between cognate peptide and its murine counterpart. T cells may not seek out neighboring follicular melanocytes as readily as neighboring epidermal melanocytes, and depigmentation is more reflective of hair graying. By contrast, depigmentation of human skin is a more complete, centripetal process where few melanocytes escape the cytotoxic events leading to vitiligo. Importantly, a mouse model with epidermal depigmentation was described using adoptive T cell transfer (Harris et al., 2012), yet this model again lacks spontaneous disease. Epidermal depigmentation is likewise observed in the Vitesse model.
The process of repigmentation in Vitesse mice is reminiscent of perifollicular repigmentation observed in UV treated human patients (Yang et al., 2010). Human perifollicular repigmentation is initiated from immune privileged sites, where T cells supposedly cannot act (Meyer et al., 2008; Yagi et al., 1997). Particularly as repigmentation is unlikely in areas of hair depigmentation in vitiligo, this observation suggests that SCF may offer a powerful means to advance differentiation of melanocyte stem cells and support repigmentation. Note that in Vitesse mice, repigmenting lesions do centrifugally increase in size over time as in perifollicular repigmentation in patients, but whether repigmentation can be considered perifollicular in the mice is difficult to determine due to the abundance of hair follicles within the skin.
In melanoma, tumor cells and reactive T cells can sometimes co-exist due to lack of expression of the targeted antigen (Lee et al., 1999). In pigmented lesions in Vitesse mice, however, as well as in similar lesions from offspring of FH mice that were crossed to the K14-SCF transgenic model, the presence of tyrosinase is obvious. Alternatively, skin-repopulating melanocytes may lack expression of relevant MHC molecules, when arising from stem cells unable to present antigen (Menendez et al., 2005). Immunostainings indicate HLA-A2 expression is not lacking from repigmenting skin. Instead, we observed increased percentages of Treg potentially protecting newly differentiating melanocytes from autoimmune attack, measured as an increase in CD3+FoxP3+ cells in situ (Gjerdrum et al, 2008, Gorczynski et al, 2011).
Because of their epidermal melanocytes and spontaneous disease development, Vitesse mice are uniquely suited to test topical treatment strategies for vitiligo. For example, treatment efficacy of topical steroids and calcineurin inhibitors can be compared (Falabella and Barona, 2009; Gawkrodger et al., 2010). Enhanced bleaching phenol treatment can be tested (Gawkrodger et al., 2010). UV treatment, advantageous by inhibiting depigmentation and stimulating repigmentation, is testable in Vitesse mice (Fisher and Kripke, 2002). The repigmentation phase is otherwise difficult to study in models without epidermal melanocytes. Even skin grafting can be studied (Fongers et al., 2009). With the Vitesse model, some obstacles to a cure can hopefully be overcome.
Melanocyte resilience is further supported by observations of one in six Vitesse mice where skin depigmentation was not accompanied by hair depigmentation. Depigmentation was not quantifiable and these animals were not included in Fig. 1; a pigmented pelage persisted beyond 40 weeks (not shown). Stem cell factor supports melanocyte survival (Botchkareva et al., 2001) and keratinocyte expression of SCF may protect melanocyte stem cells and support differentiation to follicular melanocytes (Mak et al, 2006). Here, depigmentation may further be focally suppressed by Treg. In fact, a causative role for epidermal melanocytes in the appearance of Treg cannot presently be excluded.
We investigated T cell profiles found within depigmenting animals. IL-17 producing cells are comparatively numerous in patients with active vitiligo (Kotobuki et al., 2012). Interestingly, mouse SCF transgene expression is associated with a greater Th17 cell compartment. Here we observed increased numbers of mouse RORγt+ T cells committed to IL-17 production. Increased numbers of IL-17 producing cells were found among T cells in Vitesse mice, where transgenic T cells continuously encounter antigen. Thus, SCF is causing the increase in IL-17+ cells whereas the TCR and A2 transgenes influence the significance of this increase. The cytotoxic ability of IL-17 producing T cells is often debated, yet antigen-specific CD107a mobilization shown here strongly supports cytotoxicity of this T cell subset and as such, a role in depigmentation (Chatterjee et al, 2013).
SCF has been implicated in Th17 polarization as supported by a unique population of antigen-presenting cells, the MM cells, that accelerate lupus in mice and men (Kang et al., 2012). SCF is similarly required to maintain melanocytes within human epidermis; reduced expression of SCF in lesional vitiligo skin may or may not contribute to lesional stability (Berti et al., 2011). The same SCF overexpression likely contributes to repigmentation observed in Vitesse mice (Vanover et al, 2009, Yamaguchi and Hearing, 2009). The K14 promoter is active in epidermal keratinocytes as well as in thymic epithelium where transgenic SCF overexpression can support IL-17 producing T cell-polarization similarly observed in human vitiligo (Kunisada et al., 1998; Sukseree et al., 2012; Wang et al., 2011).
Tyrosinase is a relevant antigen in vitiligo (Jin et al., 2010). The murine tyrosinase peptide is naturally processed and presented in HLA-A2 transgenic mice (Colella et al., 2000). However, the human peptide (YMDGTMSQV) differs in one amino acid from its murine homologue (FMDGTMSQV) the natural target peptide in FH mice. As h3TA2 and Vitesse mice spontaneously depigment, h3T transgenic T cells are non-tolerant for murine tyrosinase. Coreceptor independence of the TCR is indicative of high affinity T cells, effectively eliminating melanocytes expressing the homologous self-antigen (Nishimura et al., 1999). This is congruent with recent reports of CD4-CD8- double negative T cell prevalence in autoimmune disease (Tarbox et al, 2014; Quandt et al, 2014), possibly due in part to chronic stimulation (Grishkan et al, 2013). Vitesse T cells showed similar IFN-γ responses to hTyr and mTyr epitopes, but surprisingly, increased IL-17 responses to mTyr, again suggesting that IL-17 producing T cells contribute to depigmentation. This is further supported by enhanced IL-17 responses in FH mice overexpressing SCF under the K14 promotor (not shown), and may in part be caused by SCF transgene expression resulting in expansion of an antigen presenting cell type that favors Th17 development, similar to observations in a mouse model of lupus (Kang et al., 2012).
The observed changes in Th17 and Treg in Vitesse skin may seem subtle. These changes would appear to be physiologically relevant if we take into account that vitiligo was initially not recognized as a T cell mediated autoimmune response because T cell infiltrates to the skin are likewise subtle as well as transient, whereas a pathologic role for T cells in vitiligo is now commonly accepted. To further this example, Treg abundance is approximately five-fold higher in healthy controls than in patient skin (Klarquist et al., 2010). Dermal T cell infiltrates in patients with inflammatory vitiligo were two to three-fold greater than in those in skin from unaffected individuals (Le Poole et al, 1996). IL-17 producing T cell abundance, as reported in patient skin is even more subtle (Kotobuki et al., 2012). Thus, subtle numerical changes can be associated with a remarkably apparent phenotype. In this evaluation, the lineage commitment of Treg versus Th17 should be considered (Betelli et al, 2006).
Mouse models of human disease are limited by species differences, and differences inherent to the model in question. In Vitesse mice, such differences include the abundance of melanocyte-reactive T cells, which may be a minor limitation as we found that among T cells isolated from vitiligo skin, greater than 15% were reactive with even a single gp100 derivative peptide, indicating that the majority of T cells on site are likely melanocyte reactive (Oyarbide et al, 2006). Whereas the presence of the TCR transgene theoretically impacts on the animals' ability to respond to other antigens, they appear healthy, and it is possible that the T cells express dual TCRs to maintain a broad arsenal of immune reactivity. T cells in this model respond primarily to tyrosinase whereas in human disease, several antigens may be targeted within a single patient. The epidermis of a mouse is thinner than that in humans and topically applied agents may traverse the epidermis with relative ease. Another difference is the abundance of hair follicles found in mice, offering an abundance of stem cells and thus relatively improved odds of repigmentation. Overall however, the Vitesse mouse offers unique opportunities to address cutaneous vitiligo in a spontaneous disease model and displays limited repigmentation, offering an opportunity to study means of modulating vitiligo development.
Supplementary Material
Significance.
Vitiligo is an autoimmune disorder of the skin mediated by melanocyte-reactive T cells. Here, we present a spontaneous mouse model of epidermal depigmentation, and define the association between T cell subset abundance and disease parameters. The rapid depigmentation process observed in Vitesse mice is best understood in the context of great melanocyte target abundance and enhanced IL-17 responses. The resulting ‘Vitesse’ mouse model incorporates T cell mediated autoimmune responses towards epidermal melanocytes and a partial repigmentation phenotype. The Vitesse mouse faithfully reproduces human vitiligo, offering opportunities to study modulators of disease development and reversal.
Acknowledgments
This work was supported by NIH RO1 research grant AR057643-03 to CLP, NIH R21 AR056524 to SM, AI068836 to VHE and PO1 CA154778 to MIN. The authors would like to acknowledge Pat Simms for skillful support of the fluorocytometric analysis presented in this manuscript.
Footnotes
Conflict Of Interest: The authors have no conflicts of interest to declare.
References
- Abu Tahir M, Pramod K, Ansari SH, Ali J. Current remedies for vitiligo. Autoimmun Rev. 2010;9:516–20. doi: 10.1016/j.autrev.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Berti S, Amato L, Arunachalam M, Prignano F, Nassini R, Massi D, Colucci R, Moretti S. Mast cells do not play a role in vitiligo. Eur J Dermatol. 2011;21:800–1. doi: 10.1684/ejd.2011.1459. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;41:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Moellmann GE, Lerner AB. Morphology of melanocytes in hair bulbs and eyes of vitiligo mice. Am J Pathol. 1987;127:380–8. [PMC free article] [PubMed] [Google Scholar]
- Botchkareva NV, Khlgatian M, Longley BJ, Botchkarev VA, Gilchrest BA. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 2001;15:645–58. doi: 10.1096/fj.00-0368com. [DOI] [PubMed] [Google Scholar]
- Buggiani G, Tsampau D, Hercogova J, Rossi R, Brazzini B, Lotti T. Clinical efficacy of a novel topical formulation for vitiligo: compared evaluation of different treatment modalities in 149 patients. Dermatol Ther. 2012;25:472–6. doi: 10.1111/j.1529-8019.2012.01484.x. [DOI] [PubMed] [Google Scholar]
- Carter EL, O'Herrin S, Woolery C, Longley BJ. Epidermal stem cell factor augments the inflammatory response in irritant and allergic contact dermatitis. J Invest Dermatol. 2008;128:1861–3. doi: 10.1038/sj.jid.5701247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedercreutz K, Denman CJ, Klarquist J, Vaitla Rama, Boissy Raymond E, Westerhof Wiete, Hernandez Claudia, Le Poole I Caroline. Vitiligo Etiology and Treatment: Parameters Derived From a Patient Survey. J Dermatol Nur Assoc. 2010;2:265–72. [Google Scholar]
- Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang HK, Kaur N, Naga OS, Murali A, Nishimura MI, Le Poole IC, Mehrotra S. A quantitative increase in regulatory cells controls development of vitiligo. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.540. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, Pierce RA, Restifo NP, Engelhard VH. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J Exp Med. 2000;191:1221–32. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PK, van den Wijngaard RM, Wankowicz-Kalinska A, Le Poole IC. A symbiotic concept of autoimmunity and tumour immunity: lessons from vitiligo. Trends Immunol. 2001;22:130–6. doi: 10.1016/s1471-4906(00)01844-5. [DOI] [PubMed] [Google Scholar]
- Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patiño JA, Le Poole IC. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–8. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R. Decreased regulatory T-cells and CD4(+)/CD8(+) ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res. 2013;26:586–91. doi: 10.1111/pcmr.12105. [DOI] [PubMed] [Google Scholar]
- Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, Goh BK, Anbar T, Silva de Castro C, Lee AY, et al. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25:E1–13. doi: 10.1111/j.1755-148X.2012.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falabella R, Barona MI. Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res. 2009;22:42–65. doi: 10.1111/j.1755-148X.2008.00528.x. [DOI] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. 1977. Bull World Health Organ. 2002;80:908–12. [PMC free article] [PubMed] [Google Scholar]
- Fongers A, Wolkerstorfer A, Nieuweboer-Krobotova L, Krawczyk P, Tóth GG, van der Veen JP. Long-term results of 2-mm punch grafting in patients with vitiligo vulgaris and segmental vitiligo: effect of disease activity. Br J Dermatol. 2009;161:1105–11. doi: 10.1111/j.1365-2133.2009.09367.x. [DOI] [PubMed] [Google Scholar]
- Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J, Young K. Vitiligo: concise evidence based guidelines on diagnosis and management. Postgrad Med J. 2010;86:466–71. doi: 10.1136/pgmj.2009.093278. [DOI] [PubMed] [Google Scholar]
- Gjerdrum LM, Woetmann A, Odum N, Hother C, Hendrik-Nielsen R, Giandecki R, Ralfkiaer E. FOXP3 positive regulatory T cells in cutaneous and systemic CD30 positive T-cell lympoproliferations. Eur J Haematology. 2008;80:483–9. doi: 10.1111/j.1600-0609.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Chen Z, Khatri I, Yu K. Graft-infiltrating cells expressing a CD200 transgene prolong allogeneic skin graft survival in association with local increases in FoxP3(+) Treg and mast cells. Transpl Immunol. 2011;25:187–93. doi: 10.1016/j.trim.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Goudie RB, Soukop M, Dagg JH, Lee FD. Hypothesis: symmetrical cutaneous lymphoma. Lancet. 1990;335:316–8. doi: 10.1016/0140-6736(90)90607-7. [DOI] [PubMed] [Google Scholar]
- Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–17. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishkan IV, Ntranos A, Calabresi PA, Gocke AR. Helper T cells downregulate CD4 expression upon chronic stimulation giving rise to double negative T cells. Cell Immunol. 2013;284:68–74. doi: 10.1016/j.cellimm.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Shindler KS, Tabuena P, Rostami AM. Retinal ganglion cell damage induced by spontaneous autoimmune optic neuritis in MOG-specific TCR transgenic mice. J Neuroimmunol. 2006;178:40–8. doi: 10.1016/j.jneuroim.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–76. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, Mailloux CM, Sufit AJ, Hutton SM, Amadi-Myers A, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–97. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HK, Chiang MY, Ecklund D, Zhang L, Ramsey-Goldman R, Datta SK. Megakaryocyte progenitors are the main APCs inducing Th17 response to lupus autoantigens and foreign antigens. J Immunol. 2012;188:5970–80. doi: 10.4049/jimmunol.1200452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, Mehrotra S, Nishimura MI, Le Poole IC. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–86. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA, Quesniaux V, Ryffel B, Liu Z, Brand D, et al. Antigen-specific transforming growth factor β-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg balance from Th17 predominance to Treg cell predominance. Arthritis Rheum. 2012;64:2548–58. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, Fujimoto M, Serada S, Naka T, Katayama I. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012;25:219–30. doi: 10.1111/j.1755-148X.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams DA, Wang X, Longley BJ. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–73. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol. 1993a;2:145–53. doi: 10.1111/j.1600-0625.1993.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993b;100:816–22. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Denman CJ, Arbiser JL. Immunosuppression may be present within conduyloma accuminata. J Am Acad Dermatol. 2008;59:967–74. doi: 10.1016/j.jaad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Shiohara T, Boissy RE, Jacobson KA, Lamoreux ML, Moellmann GE. A mouse model for vitiligo. J Invest Dermatol. 1986;87:299–304. doi: 10.1111/1523-1747.ep12524353. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Tyrrell L, Ma Y, Williams DA, Halaban R, Langley K, Lu HS, Schechter NM. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci U S A. 1997;94:9017–21. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak SS, Moriyama M, Nishioka E, Osawa M, Nishikawa S. Indispensable role of Bcl2 in the development of the melanocyte stem cell. Dev Biol. 2006;291:144–53. doi: 10.1016/j.ydbio.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Al-Khami AA, Klarquist J, Husain S, Naga O, Eby JM, Murali AK, Lyons GE, Li M, Spivey ND, et al. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J Immunol. 2012;189:1627–38. doi: 10.4049/jimmunol.1103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez P, Bueno C, Wang L, Bhatia M. Human embryonic stem cells: potential tool for achieving immunotolerance? Stem Cell Rev. 2005;1:151–8. doi: 10.1385/SCR:1:2:151. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, Sinclair R, Paus R. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159:1077–85. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- Misfeldt ML, Grimm DR. Vet Immunol Immunopathol. 1994;43:167–75. doi: 10.1016/0165-2427(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Bigotti A, Venturo I, Marcenaro L, Giacomini P, Russo C. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc Natl Acad Sci U S A. 1989;86:6719–23. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- Nishimura MI, Avichezer D, Custer MC, Lee CS, Chen C, Parkhurst MR, Diamond RA, Robbins PF, Schwartzentruber DJ, Rosenberg SA. MHC class I-restricted recognition of a melanoma antigen by a human CD4+ tumor infiltrating lymphocyte. Cancer Res. 1999;59:6230–8. [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarbide-Valencia K, van den Borrn JG, Denman CJ, Li M, Carlson JM, Hernandez C, Nishimura MI, Das PK, Luiten RM, Le Poole IC. Therapeutic implications of autoimmune vitiligo T cells. Autoimmun Rev. 2006;5:486–92. doi: 10.1016/j.autrev.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes. 2003;1:58. doi: 10.1186/1477-7525-1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS One. 2014;9:e93293. doi: 10.1371/journal.pone.0093293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Krishnamoorthy N, Ray A. Emerging functions of c-kit and its ligand stem cell factor in dendritic cells: regulators of T cell differentiation. Cell Cycle. 2008;7:2826–32. doi: 10.4161/cc.7.18.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23:99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Sukseree S, Mildner M, Rossiter H, Pammer J, Zhang CF, Watanapokasin R, Tschachler E, Eckhart L. Autophagy in the thymic epithelium is dispensable for the development of self-tolerance in a novel mouse model. PLoS One. 2012;7:e38933. doi: 10.1371/journal.pone.0038933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360:160–9. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- Tarbox JA, Keppel MP, Topcagic N, Mackin C, Ben Abdallah M, Baszis KW, White AJ, French AR, Cooper MA. Elevated double negative T cells in pediatric autoimmunity. J Clin Immunol. 2014 doi: 10.1007/s10875-014-0038-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RK, Flanders DJ, Young TL, Oetting WS, Ramaiah A, King RA, Boissy RE, Nordlund JJ. Microphthalmia-associated transcription factor (MITF) locus lacks linkage to human vitiligo or osteopetrosis: an evaluation. Pigment Cell Res. 1999;12:187–92. doi: 10.1111/j.1600-0749.1999.tb00512.x. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard R, Wankowicz-Kalinska A, Pals S, Weening J, Das P. Autoimmune melanocyte destruction in vitiligo. Lab Invest. 2001;81:1061–7. doi: 10.1038/labinvest.3780318. [DOI] [PubMed] [Google Scholar]
- Vanover JC, Spry M, Hamilton L, Wakamatsu K, Ito S, D'Orazio JA. Stem cell factor rescues tyrosinase expression and pigmentation in discreet anatomic locations in albino mice. Pigment Cell Melanoma Res. 2009;22:827–38. doi: 10.1111/j.1755-148X.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, Gilleaudeau P, Cohen JA, Krueger JG. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6:e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Tokura Y, Furukawa F, Takigawa M. Vitiligo with raised inflammatory borders : involvement of T cell immunity and keratinocytes expressing MHC class II and ICAM-1 molecules. Eur J Dermatol. 1997;7:19–22. [Google Scholar]
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–9. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang X, Zou H, Chu Y, Li M. Recovery of the immune balance between Th17 and regulatory T cells as a treatment for systemic lupus erythematosus. Rheumatology. 2011;50:1366–72. doi: 10.1093/rheumatology/ker116. [DOI] [PubMed] [Google Scholar]
- Yang YS, Cho HR, Ryou JH, Lee MH. Clinical study of repigmentation patterns with either narrow-band ultraviolet B (NBUVB) or 308 nm excimer laser treatment in Korean vitiligo patients. Int J Dermatol. 2010;49:317–23. doi: 10.1111/j.1365-4632.2009.04332.x. [DOI] [PubMed] [Google Scholar]
- Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune arthritis. J Immunol. 2014;189:4295–304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.