Abstract
The systematic increase in O2 uptake and O2 extraction with increasing work rates conceals a substantial heterogeneity of O2 delivery (Q̇O2)-to-V̇O2 matching across and within muscles and other organs. We hypothesize that whether increased/decreased Q̇O2/V̇O2 heterogeneity can be judged as “good” or “bad”, for example after exercise training or in aged individuals or with disease (heart failure, diabetes), depends on the resultant effects on O2 transport and contractile performance.
Keywords: blood flow, oxygen utilization, heterogeneity, muscle, exercise, aging, diseases
Introduction
Within the realms of physiology and medicine it is axiomatic that cardiac output is distributed among and within tissues according to their specific needs (14; 32; 39). Those needs vary widely from substrate delivery and utilization (O2, glucose, free fatty acids, La−), metabolite (CO2, H+, La−) and heat removal, fluid homeostasis, hormonal function, and absorption to the sensory functions of, for example, the carotid bodies. Consequently, it is not surprizing that the venous effluent from different organs, even at rest, contains a range of O2 contents reflecting disparate fractional O2 extractions. Specifically, the left ventricle of the heart extracts >60% of its inflowing arterial O2 whereas that for the carotid body is a vanishingly small 1–2%. This broad range is tacitly ignored when the whole body average of ~25% at rest is reported. Even within skeletal muscle in the resting individual there exists a substantial range of blood flows (Q̇m) (29). This range becomes far wider during exercise depending, in part, upon the exercise intensity (moderate, heavy, severe) and mode (small muscle mass such as knee extensor exercise or large muscle mass such as cycling or running) and how these dictate recruitment patterns across muscles and muscle groups. Thus, during severe intensity exercise when cardiac output may increase 5+-fold above resting (Figure 1), 80–90% of that blood flow is directed to skeletal muscle and whole body fractional O2 extraction approaches 90% (29).
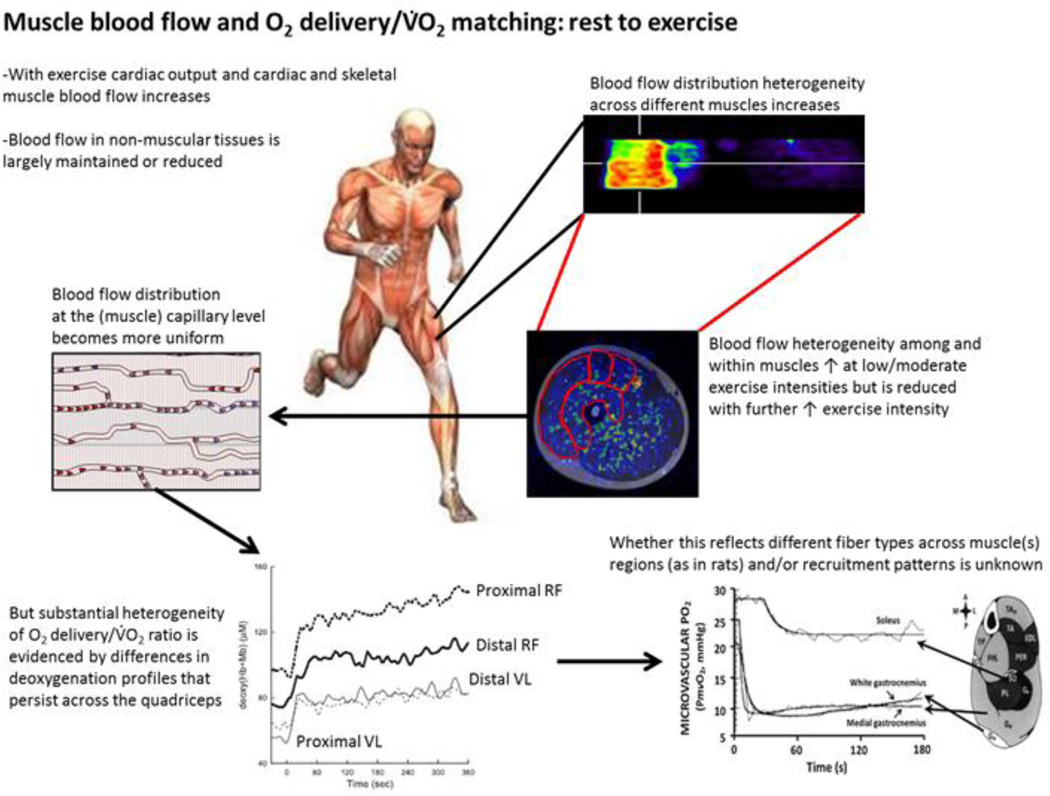
Figure 1.
Blood flow and thus oxygen supply distribution cascade to tissues illustrated at systemic whole body and locally at the tissue level. As cardiac output and coronary perfusion increases with increasing exercise intensity, distribution heterogeneity of blood flow between tissues and muscles also increases, whereas blood flow in non-muscular organs is mostly maintained at the resting levels or even reduced. Within exercising muscles there is generally a biphasic response in blood flow heterogeneity, thus it initially increases, but is followed by a reduction with increasing exercise intensities that results in more uniform muscle capillary perfusion at the tissue level. Nevertheless, substantial blood flow heterogeneity still persists in different exercising muscles, which may reflect differences in fibre types in different regions and/or muscle recruitment patterns and thus varying O2 demands.
Bottom left figure is from (28). [Adapted from (28). Copyright © 2011 the American Physiological Society. Used with permission.]
Bottom right figure is from (34). [Adapted from (34). Copyright © 2005 John Wiley and Sons. Used with permission.]
At these severe intensities of exercise there may be a 10-fold range in Q̇m between and within individual muscles and muscle portions (31; 41). Because muscle fractional O2 extraction can only increase ~3.5-fold above rest (25–90%) and yet muscle V̇O2 can rise 100-fold it is evident that directing Q̇m according to local V̇O2 requirements is essential to support sustained contractile performance. This brief review focusses on the nature of the Q̇m/V̇O2 or, more correctly, Q̇O2/V̇O2, matching during rest and especially exercise in health and the causes and consequences of its derangement in aging and disease (heart failure, diabetes) among/within muscles.
Over the past three decades our view of Q̇m (and Q̇O2) heterogeneity has undergone radical revision from purely pejorative to the formulation of the novel hypothesis that exquisite temporal and spatial Q̇O2/V̇O2 matching is fundamental for effective muscle and exercise performance, in health and disease. However, key questions remain:
What is the optimal Q̇O2/V̇O2 ratio (or distribution/heterogeneity thereof) where blood-myocyte O2 flux and intramyocyte PO2 are high enough to sustain optimal oxidative function, which also minimizes changes in high energy phosphates required for a given V̇O2 (i.e., ensure no O2 supply-dependency of mitochondrial V̇O2 kinetics, limit glycolytic activation)?
How does the Q̇O2/V̇O2 ratio differ among muscles comprised of different fiber types and could this help explain or influence their metabolic behaviour?
How does prior exercise and exercise training impact the Q̇O2/V̇O2 ratio and when are such changes intrinsic to faster V̇O2 kinetics and improved exercise performance?
Aging and disease states, such as diabetes and chronic heart failure, each compromise the Q̇O2/V̇O2 ratio such that, across the rest-exercise transition microvascular PO2 falls below that seen in healthy young individuals and V̇O2 kinetics are slowed. What are the mechanistic bases for this effect and can they be reversed?
The heart is a highly oxidative muscle and sustains very high (and variable) V̇O2 sat rest and especially during exercise. Can this muscle help us better understand skeletal muscle Q̇O2/V̇O2 relationships during exercise and after exercise training?
This review will present what is known at present, in these regards, and identify fruitful directions for further investigation.
What is the optimal Q̇O2/V̇O2 ratio or blood flow distribution/heterogeneity?
The regional Q̇O2/V̇O2 ratio is extremely important because it determines the upstream driving pressure (i.e., microvascular PO2) crucial for achieving a given blood-myocyte O2 flux and is also an important determinant of intramyocyte PO2 and therefore metabolic regulation (4; 6; 34). On the other hand, heterogeneity in the present context can be defined as uneven distribution of blood or substrate delivery within or among/between muscles. It can be temporal or spatial in nature, the latter being the principal focus herein. Blood flow heterogeneity determines with the efficacy of oxygen (and nutrient) delivery and, ultimately, tissue oxygenation levels. Although less heterogeneous blood flow has been regarded as the superior condition for good tissue oxygenation this judgement cannot be made without considering the local muscle V̇O2 demands and the efficacy of microvascular hemodynamics, muscle O2 diffusing capacity and mitochondrial energetics across all muscles and muscle regions recruited. Whereas it is true that a wide range of Q̇O2/V̇O2 ratios can, in theory, lead to regions where blood-muscle O2 flux is compromised, heterogeneity is not ipso facto counterproductive. Rather, this situation may also be beneficial, reflecting system 'flexibility' and the capacity to sustain the V̇O2 kinetics response across a range of Q̇O2/V̇O2 ratios (see Table 1). Thus, the presence of microvessel blood flow heterogeneity is not necessarily a sign of poor vascular function, and findings need to be contextualized using multiple techniques/models where possible to develop a greater understanding of the relationship(s) between Q̇O2/V̇O2 heterogeneities and function/dysfunction. In this regard, it is pertinent that both near infrared spectroscopy (NIRS) and phosphorescence quenching have high temporal fidelity and can follow the dynamics of muscle microvascular oxy/deoxygenation across rest-exercise transitions whereas positron emission tomography (PET) and proton magnetic resonance spectroscopy (H+MRS) are principally used for steady-state responses.
Table.
Conditions and perspectives highlighted in this review. Notice that detriment/benefit of Q̇O2/V̇O2 heterogeneity, and changes thereof, depends upon context: When muscle mass increases (from knee extension exercise (KE) to cycling) the available cardiac output (CO) must be distributed more heterogeneously to help optimize muscle O2 delivery and O2 extraction. Exercise training helps direct more O2 delivery (Q̇O2) towards highly oxidative fibers whereas aging does the opposite. Whether ↑or ↓heterogeneity is beneficial may depend more upon corollary adaptations in capillarity, microcirculation and mitochondria. The ↑heterogeneity with chronic/congestive heart failure (CHF) is clearly pernicious as capillaries adjacent to some muscle fibers may be devoid of RBC flux entirely. In this situation fractional O2 extraction may be high but this reflects the very low Q̇O2/V̇O2 ratio (and hence higher diffusing capacity (DO2)/Q̇O2 ratio caused by a low DO2 accompanied by a disappearingly small Q̇O2). ↑NO bioavailability helps correct this deficit and reduce this source of heterogeneity. See text for more complete explanation. V̇O2, O2 consumption; V̇O2 max, condition specific maximal V̇O2; PmvO2, mean microvascular O2 partial pressure; RBCs, red blood cells.
| Boundary Conditions | Change | Positive /Negative Consequences with respect to O2 flux and metabolic control |
|---|---|---|
| Healthy | ||
| No CO Limitation (e.g., KE exercise) | ↑ Q̇O2 → ↑Q̇O2/V̇O2 → ↑ PmvO2 | Overall positive for blood-myocyte O2 flux – highest V̇O2 max to date. Little/no RBC-capillary transit time limitation. |
| In 1 region ↗ ↑ Q̇O2 → ↑Q̇O2/V̇O2 → ↑ PmvO2 | ||
| CO Limitation (cycling) | necessitates redistribution/↑heterogeneity | Does improvement/refinement of Q̇O2/V̇O2 “matching” benefit function more than any detriment incurred by re-distributing Q̇O2 among compartment(s)? |
| In another region↘ ↓ Q̇O2 → ↓Q̇O2/V̇O2 → ↓ PmvO2 | ||
| Highly oxidative fibers ↗ ↑ Q̇O2 → ↑Q̇O2/V̇O2 → ↑ PmvO2 | ||
| Exercise training | redistribution/may ↓↔↑heterogeneity | Change benefits function more than any detriment. Works in concert with ↑DO2 (↑ capillarity & #RBCs adjacent to myocytes) & ↑mitochondrial volume. |
| Low oxidative fibers↘ | ↓ Q̇O2 → ↓Q̇O2/V̇O2 → ↓ PmvO2 ↑exercise capacity. | |
| Low oxidative fibers ↗ ↑ Q̇O2 → ↑Q̇O2/V̇O2 → ↑ PmvO2 | Change detrimental to function. Also ↓ DO2 (altered capillary RBC hemodynamics) & ↓mitochondrial function. ↓exercise capacity. | |
| Aging | redistribution/↓heterogeneity | |
| Highly oxidative fibers↘ ↓ Q̇O2 → ↓Q̇O2/V̇O2 → ↓ PmvO2 | ||
| Disease - CHF | ||
| CO Limitation | ↓ Q̇O2 → ↓Q̇O2/V̇O2 → ↓ PmvO2 | ↑↑heterogeneity with regions devoid of capillary RBC flow. Inability to↑Q̇O2 & distribute O2 effectively → impaired V̇O2 /kinetics,↓exercise capacity. Also ↓DO2 (↓ capillarity & #RBCs adjacent to myocytes) & ↓mitochondrial volume. ↓exercise capacity. |
| CHF+↑NO bioavailability | ↑ Q̇O2 → ↑Q̇O2/V̇O2 → ↑ PmvO2 | ↓heterogeneity. Blood-myocyte O2 flux, V̇O2 kinetics speeded, ↑ V̇O2 max, ∆PmvO2 dependent upon resultant ΔDO2m/Δ Q̇O2. ↑exercise capacity. |
How does the Q̇O2/V̇O2 ratio differ among muscles comprised of different fiber types?
In animals and humans Q̇m at rest and during exercise, is usually the highest in muscle parts that consist mostly of slow-twitch highly oxidative muscle (29). Across the spectrum of exercise intensities there is a biphasic profile of Q̇m heterogeneity: Greater recruitment of these highly oxidative fibers increases their Q̇m at low-to-moderate exercise intensities, but this response is absent in their lower oxidative counterparts, increasing Q̇m heterogeneity (17; 31). This Q̇m heterogeneity is subsequently reduced during severe intensity exercise as the entire spectrum of fibers is recruited (22). It is important to note, however, that even at supra-V̇O2 max running speed there may be an order of magnitude higher Q̇m in high versus low oxidative muscles or muscle parts (41). There is also the imperative here to note here that recruitment of muscle fibers and increased Q̇m does not necessarily imply that more microvascular units or capillaries are recruited but rather, that blood flow (i.e., red blood cell and plasma flux) increases within already flowing capillaries (38). In muscles comprised of highly oxidative fibers vasoactive mechanisms including endothelium-mediated vasodilation (e.g., (33)) are up-regulated and α-adrenergic mediated vasoconstriction is reduced (3) compared with their low oxidative counterparts. Thus, even when the muscles are recruited and increase their oxygen demand microvascular PO2 falls faster and to a far lower absolute level in fast-twitch than slow twitch muscles or muscle parts (7; 34).
With respect to the increase of muscle glucose uptake during exercise both inter- and intra-muscular heterogeneity decrease from rest to exercise (9). Interestingly, at least during moderate intensity exercise, muscle regional FFA uptake is correlated with Q̇m (30), but this is not the case for glucose uptake in humans (30). Whether this is the case for higher exercise intensities where there is a more uniform muscle fiber recruitment and glucose is the primary energy substrate remains to be determined.
When addressing the participation of different vasoactive mechanisms on the Q̇m response to exercise and Q̇O2-to-V̇O2 matching it must be recognized that animal and human studies have not always been in agreement. In the case of nitric oxide (NO) it may not be possible ethically to get full NOS blockade in humans (19) whereas acetylcholine challenge affirms that this is achievable in animals (i.e., rats) (9–11; 23). Accordingly, both eNOS and nNOS-derived NO contribute significantly to the Q̇m responses to moderate/heavy and severe intensity exercise in the rat (9–11; 23). Reducing Q̇m via L-NAME reduces the contracting muscle Q̇O2/V̇O2 ratio and decreases microvascular PO2 (12; 13). Interestingly, nNOS contributes proportionally more of the Q̇m response to fast twitch muscles when these are recruited at the faster (severe) running intensities (11). Downregulation of NO bioavailability may therefore constitute an important mechanism for impairment of Q̇O2-to-V̇O2 matching evident in aged individuals (especially nNOS, (5; 22)) and in diseases such as chronic heart failure and diabetes (12; 23; 36).
Investigations in humans suggest that neither inhibition of NO formation (19), α-adrenergic stimulation or blockade (20), nor combined exercise and systemic hypoxia (21) affect significantly resting or exercising Q̇m heterogeneity. This is true despite the fact that resting mean Q̇m is usually drastically altered. Interestingly however, NOS inhibition with L-NMMA does influence utilization of circulating energy substrates (18). Moreover, α-adrenergic inhibition alters limb Q̇m distribution markedly, and adenosine may play a role in distributing Q̇ to adipose tissue (15) as well as influencing Q̇m heterogeneity (17). Thus, our knowledge of the mechanistic bases for Q̇m (and Q̇O2/V̇O2) heterogeneity particularly in animals is well advanced and is helping to facilitate design and testing of provocative hypotheses in humans. It is anticipated that so doing will elucidate key elements responsible for exercise intolerance especially in in aged and patient populations.
How do prior exercise (acute response) and exercise training (chronic adaptations) impact the Q̇O2/V̇O2 ratio?
Whereas V̇O2 max is inarguably O2 delivery limited the presiding view is that, for most young healthy individuals performing moderate intensity exercise, this is not the case for V̇O2 kinetics. Specifically, these individuals lie to the right of the O2 delivery limitation “tipping point” hypothesized by Poole and Jones (40). Specifically, unlike aged or patient (heart failure, diabetes) populations (40), the kinetics of Q̇O2 appear sufficiently fast to support unimpeded mitochondrial V̇O2 kinetics (though see Murias et al. 2014 for a provocative counter-position). One intriguing consequence of the fiber-type differences in microvascular PO2 response described above is that, when a greater fast twitch fiber population is recruited these fibers will necessarily be closer to the O2 delivery tipping point than their slow twitch counterparts. It is also pertinent that, even though there may be no O2 delivery dependence of either V̇O2 or V̇O2 kinetics, this does not mean that raising (↓Δ[PCr], [ADPfree], [Pi], [NADH]) or lowering (↑Δ[PCr], [ADPfree], [Pi], [NADH]) the intracellular PO2 will not have metabolic consequences (rev. (40)).
NIRS–derived muscle deoxygenation (i.e., the increase in deoxygenated hemoglobin and myoglobin ([HHb+Mb]) reflects O2 extraction (i.e., Q̇O2/V̇O2). The primary component kinetics of muscle [HHb+Mb] following the onset of exercise is spatially heterogeneous (29). However, in keeping with the concept that the speed of the V̇O2 kinetics is not O2 delivery limited, the degree of dynamic heterogeneity in [HHb+Mb] is not associated with any systematic variation of the pulmonary phase II V̇O2 (thus muscle V̇O2) profile following the onset of cycling exercise.
Prior exercise (acute response)
The compelling weight of evidence supports that heavy intensity priming exercise does not speed V̇O2 kinetics during moderate intensity exercise (36). In marked contrast, Murias et al. (2014) did observe priming-induced faster kinetics in a cohort of subjects whose initial (non-primed) kinetics were considered to be relatively slow (i.e., time constant >20 s) (35). Furthermore, because their [HHb+Mb]-to-V̇O2 ratio during the rest-exercise transition decreased in proportion to the speeded V̇O2 kinetics this was considered as evidence of O2 supply limitation in the non-primed condition. A plausible alternative is simply that priming induced relatively greater speeding of vascular (Q̇O2) versus mitochondrial (V̇O2) dynamics.
The mechanistic bases for the changes in pulmonary V̇O2 kinetics induced by prior heavy exercise on subsequent heavy exercise (i.e., increased primary component amplitude, decreased slow component) are more robust across subject populations than those for moderate intensity exercise above. These presumably involve enhanced mitochondrial function and/or increased O2 delivery mediated by an accentuated vasodilatation and/or a right shift in the oxyhemoglobin dissociation curve. Importantly, prior heavy exercise reduces the spatial heterogeneity of muscle oxygenation kinetics (29) the slowing of deoxygenation kinetics supports the presence of a greater Q̇O2/V̇O2 ratio at multiple intramuscular sites. However, this reduced spatial heterogeneity of muscle [HHb+Mb] kinetics did not translate into faster primary component pulmonary V̇O2 kinetics, suggesting that the heterogeneity of microvascular O2 delivery (in relation to pulmonary/muscle O2) was not associated with any discernible O2 delivery limitation of the primary phase of muscle O2 kinetics in healthy young subjects (29). However, inter-subject variations in heterogeneity and V̇O2 kinetics may have been too small to test rigorously whether these variables were indeed correlated.
Exercise training (chronic adaptations)
Exercise training increases the speed of pulmonary (41) and muscle (27) V̇O2 kinetics. It is well documented that speeding occurs concomitant with enhanced vasodilatory capacity within skeletal muscle and that there is also a redistribution of Q̇m away from low oxidative muscles or muscle parts towards their more oxidative counterparts (32). Recently, it has been determined that training slows the fall of muscle microvascular PO2 during the rest-contractions transition indicating that, despite a training-induced increase in muscle oxidative capacity and thus faster V̇O2 kinetics, the speeding of the vascular response (and Q̇O2) must have occurred to a greater extent (22). Intriguingly, when the progressive adaptations to training were documented the dynamics of bulk muscle(s) Q̇m was speeded (44), as was V̇O2 (37) and this occurred prior to a detectable elevation of muscle oxidative enzyme activity. Whether this finding represents evidence for faster Q̇O2 dynamics facilitating those of V̇O2 or rather a limitation of the muscle biopsy technique remains uncertain. Irrespective of this possibility and, whether or not Q̇O2 were limiting in the untrained condition (6), it was even less likely to be so after training. These findings are consistent with the training-induced reduction of the [HHb+Mb]-to-V̇O2 ratio following the onset of exercise found by Murias et al. (2014) in humans. Thus, even if Q̇O2 is not limiting for V̇O2 kinetics the elevation of microvascular and consequently intramyocyte PO2 after training may enhance metabolic control and muscle contractile function.
Regarding NIRS, this technique has to date only investigated relatively superficial muscles/muscle parts in humans. However, current technical advances using high power time resolved spectroscopy (TRS) will allow studies of deeper muscles such as m. vastus intermedius. Furthermore, in combination with indocyanine green dye to measure Q̇m (and Q̇O2) the standard oxygenation/deoxygenation measurements could feasibly monitor regional matching of Q̇m and V̇O2 with high temporal/ spatial fidelity throughout the entire recruited muscle mass. This technology thus has substantial potential to answer pressing questions regarding heterogeneity and its relationship with metabolic control in health and disease.
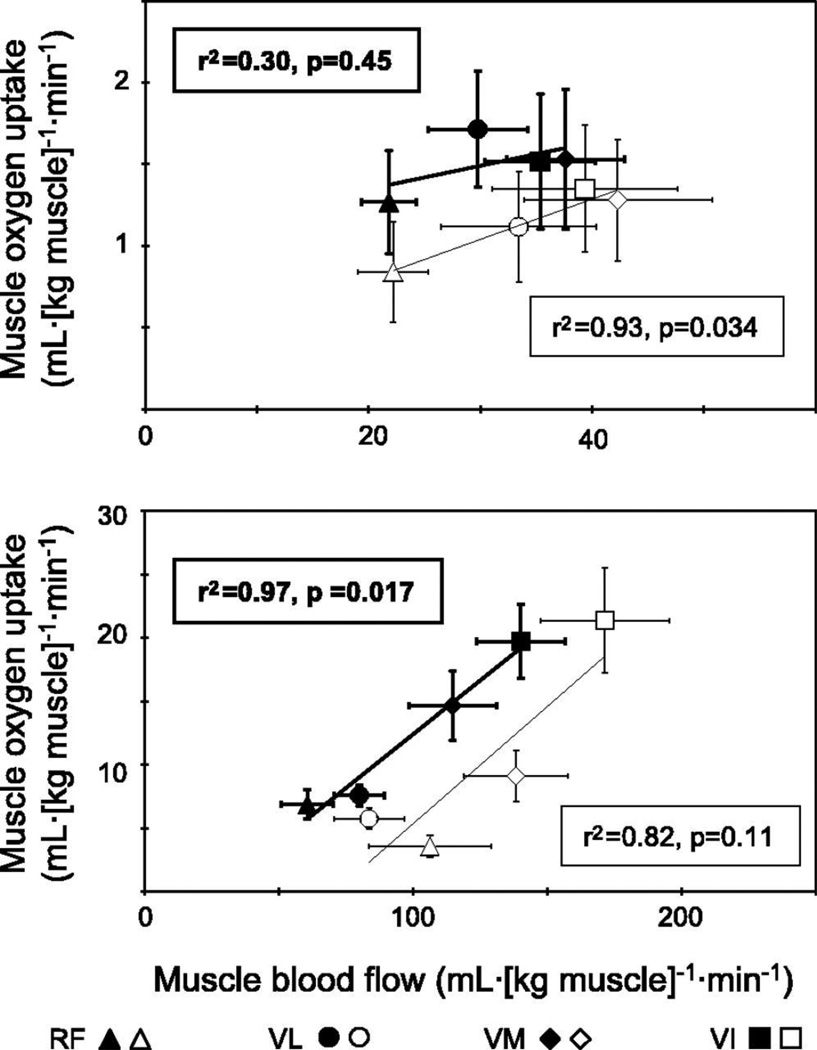
Regarding investigations with other techniques, Richardson and colleagues (42) used MRS at the individual voxel level (1 cm3) to determine that, in healthy young subjects, the spatial matching of Q̇O2 and V̇O2 (assessed via phosphocreatine depletion) was far from perfect (Figure 2). This perspective, however, was changed when the sampled volume was increased to encompass the four individual parts of m. quadriceps femoris (25). In endurance-trained subjects, Q̇O2 and V̇O2 are better matched than in their untrained counterparts (25) (Figure 3): possibly as a consequence of less hyperperfused and heterogeneous Q̇m-to-V̇O2 distributions. Although capillary red blood cell transit times are not generally considered to limit fractional O2 extraction the improvements in muscle O2 diffusing capacity (DO2) resulting from a greater capillary and red blood cell volume adjacent to the contracting myocytes may serve to reduce Q̇O2 spatial heterogeneity and enhance Q̇O2 and/or metabolic control (26).
Figure 2.
Absence of any proportional relationship between phosphocreatine (PCr) depletion (a proxy for V̇O2) and exercise-induced muscle blood flow when examined at the 1-cm3 voxel level by Richardson et al. (42).
(Reprinted from (42). Copyright © 2001 the American Physiological Society. Used with permission.)
Figure 3.
Relationship between blood flow and oxygen uptake in the resting (upper) and exercising (bottom) muscle in the endurance-trained (solid symbols) and untrained (open symbols) men (25). RF, rectus femoris; VL, vastus lateralis; VM, vastus medialis; VI, vastus intermedius.
(Reprinted from (25). Copyright © 2005 the American Physiological Society. Used with permission.)
What are the mechanistic bases for compromised Q̇O2/V̇O2 ratio and slowed V̇O2 kinetics in aging, diabetes and chronic heart failure (CHF) and can they be reversed?
Compromised muscle O2 delivery (either as decreased Q̇O2 or arterial/ microvascular PO2) slows V̇O2 kinetics, necessitating greater intracellular perturbations (i.e., Δ[PCr], [ADPfree], [Pi],[H+]) accelerated glycogen utilization and ultimately impaired exercise tolerance (8; 40). Aging, diabetes and CHF all reduce exercise capacity through a complex array of physiological/pathophysiological changes. Common features among conditions include a reduced maximal cardiac output, V̇O2 max and slowed V̇O2 kinetics. With respect to the question at hand, each condition is also characterized by various degrees of vascular and microvascular dysfunction that alters bulk muscle Q̇O2 lowering the overall Q̇O2/V̇O2 ratio (Table 1). More distally, there is profound arteriolar dysfunction and red blood cell flux may cease in as much as 50% of the capillary bed (diabetes, CHF) creating a condition where the capacity to spatially distribute Q̇O2 relative to V̇O2 within and between muscles is degraded (39). Not surprisingly the microvascular Q̇O2/V̇O2 ratio, measured via phosphorescence quenching (i.e., microvascular PO2, animals) or NIRS ([HHb+Mb], humans/animals), is reduced either during the dynamic transition (aging, moderate CHF) and/or in the subsequent steady-state (severe CHF, diabetes) (5; 39; 40).
When vascular/microvascular PO2 (the product of a low Q̇O2/V̇O2 ratio) is reduced, exercising skeletal muscle(s) experience a decreased capacity to spatially distribute microvascular O2 delivery to meet local energetic requirements (i.e., V̇O2, (8)). Although aging and CHF and, to a degree, diabetes are all associated with a reduced structural capacity for muscle Q̇O2 (e.g., decreased vascularity) there is an often profound down-regulation of NO bioavailability. Accordingly, acute NO increases achieved by means of NO/nitrite/nitrate supplementation or sildenafil treatment improve the overall Q̇O2/V̇O2 ratio (12; 45) and enhance exercise tolerance, at least in CHF (45). Moreover, increased NO and endothelium-mediated function may constitute an important (though certainly not sole) contributor to the improved Q̇O2/V̇O2 ratios found after exercise training in young rats (22) and its counterpart ([HHb+Mb]-to-V̇O2) in young and older humans (35). In this latter instance the training-induced ΔV̇O2 kinetics correlated with the reduced [HHb+Mb]-to-V̇O2 ratio which may, or may not, be indicative of an O2 delivery limitation to V̇O2 kinetics in the untrained state. Further, for the support of physical activity and exercise training in healthy aging, there is evidence that aerobic exercise training can correct the cardiac output distribution impairments (2) and facilitate a more effective exercise hyperaemic response to muscle contractions despite smaller muscles in the elderly people (43).
Can the heart help us to better understand muscular Q̇O2/V̇O2 matching during exercise and especially after exercise training?
Exercise training increases fractional O2 extraction by elevating muscle(s) O2 diffusing capacity (DO2) to a greater extent than maximal Q̇m (i.e., higher DO2/ Q̇m ratio) (29). In contrast, CHF increases fractional O2 extraction, at a given V̇O2, by reducing Q̇m more than DO2m (39). Interpretation of fractional O2 extraction is therefore context-dependent (Table 1) and must be viewed relative to the extant muscle V̇O2. Whereas we have seen that the normal response following the onset of exercise is for microvascular PO2 to fall reflecting an increased fractional O2 extraction (a consequence of the decreasing Q̇O2/V̇O2 ratio), a lower microvascular PO2 than normal, as is manifested in aged and diseased muscle(s), is a consequence of pathology and may lead to slowed V̇O2 kinetics and exercise intolerance.
The heart, like the brain, is critically dependent on adequate Q̇O2 and, given its extremely high V̇O2 and rapid response (i.e., increased heart rate < 1 s) following the onset of exercise must effectively match Q̇O2-to-V̇O2 on a second-by-second basis. The relevant question here is whether the heart can support these fast kinetics in the presence of a low Q̇O2/V̇O2 ratio (and therefore low microvascular PO2)? Both dogs (46) and humans (24) at the onset of moderate-severe exercise evince a biphasic coronary venous O2 saturation profile. Specifically, whilst myocardial O2 demands are increasing most rapidly, coronary venous O2 saturation falls precipitously (indicative of increased fractional O2 extraction and lowered microvascular PO2) prior to a brief recovery and subsequent reduction to its lower exercising value (46). Compared with skeletal muscle in these species, the heart has an impressively high capillarity and mitochondrial volume density (i.e., ~23% vs <10%). These attributes may be crucial for supporting very fast myocardial V̇O2 kinetics even in the face of low coronary venous O2 saturation and thus microvascular PO2s. This suggests that peripheral skeletal muscle adaptations, for example, in response to exercise training might facilitate faster V̇O2 kinetics and associated improvements in exercise tolerance even in disease conditions where compromised cardiac output places a low limiting ceiling on muscle Q̇O2 and therefore microvascular PO2.
In contrast to skeletal muscles the work rate of the heart in vivo cannot be set with precision and consequently certain facets of the training response may be determined by extraneous adaptations. These adaptations include a reduced peripheral resistance and increased blood volume which will both modify the cardiac workload at a given pulmonary V̇O2. Notwithstanding this situation, there is recent evidence that endurance exercise training reduces myocardial Q̇ (and Q̇O2) and enhances fractional O2 extraction both at rest and during submaximal exercise in humans (16). These adaptations are attributed, in part, to enhanced myocardial vascular resistance and longer mean red blood cell transit times in the myocardial capillaries, respectively. These findings contrast with the unchanged bulk hindlimb blood flows seen in trained rats (1), faster Q̇m kinetics in humans (44) and higher transient microvascular PO2s in the contracting rat spinotrapezius (unchanged steady-state) (22). Laughlin and colleagues also reported that, although bulk hindlimb Q̇ was unchanged after training in rats there was an enhanced redistribution away from the low oxidative towards the highly oxidative fibers (1; 31). It is unknown whether the trained human myocardium and/or skeletal muscles are able to better distribute Q̇m to improve Q̇O2- to-V̇O2 matching. Alternatively, it is possible that post-training either prolonged capillary red blood cell transit times (due to lower Q̇m and possibly capillary neogenesis) and/or enhanced DO2m enable adequate blood-myocyte O2 flux at a reduced microvascular PO2. Interestingly, this latter response occurs in mouse gastrocnemius muscle after PGC-1α induced upregulation of capillarity and oxidative capacity (27).
One crucial consideration here is where the muscle, be it skeletal or myocardial, sits on the Q̇O2 continuum (40). If there is an O2 dependency of mitochondrial function (left of tipping point) blood-myocyte O2 flux will be compromised by any reduction in microvascular PO2: This condition is more likely to be present in muscles of aged individuals as well as diabetic and CHF patients and especially when the exercise necessitates recruitment of fast twitch fibers (see: How does the Q̇O2/V̇O2 ratio differ among muscles comprised of different fiber types? above). Alternatively, by occupying any position to the right of that tipping point there is a tolerance for lowering microvascular PO2 that may be enhanced by adaptations such as exercise training or upregulation of PGC-1α (27).
CONCLUSIONS AND FUTURE PERSPECTIVES
The temporal and spatial distribution of Q̇m and therefore Q̇O2 between and within muscles in proportion to their energetics requirements is crucial to sustain mitochondrial and thus contractile function. To accomplish this Q̇O2-to-V̇O2 requirement balance increases in cardiac output and sympathetic tone must work in concert with local vasoactive control within the active muscle(s). That local control is mediated by a complex interaction of fiber recruitment patterns and multiple vasoactive pathways (including NO, cyclooxygenase, purinergic, adrenergic, hydrogen peroxide, vasoactive metabolites) that operate in a fiber type-specific manner to control the local Q̇O2/V̇O2 ratio and thus microvascular PO2.
It is intuitive that “good” matching of Q̇O2 and V̇O2 is better than “bad” matching but what constitutes each is not readily apparent and may differ under certain circumstances in health and disease (Table 1). Specifically, does a lower Q̇O2/V̇O2 ratio (and therefore lower microvascular PO2 and higher fractional extraction) mean better matching or, alternatively, lower microvascular PO2 such that blood-myocyte O2 flux and metabolic control are sub-optimal? Within a given region a high Q̇O2/V̇O2 ratio raises microvascular (and intracellular) PO2 better facilitating blood-myocyte O2 flux and metabolic control. However, unless there is no limit imposed upon bulk blood flow by a cardiac output ceiling, for example, small muscle mass exercise (2–3 kg active muscle) versus conventional cycling (~15 kg active muscle), this high Q̇O2/V̇O2 ratio in one region will come at the expense of a lower ratio (and microvascular PO2) in another region and may ultimately compromise function (Table 1). Rather than relegating these decisions (akin to “robbing Peter to pay Paul”) to the periphery an overall greater increase in cardiac output may be desirable: If such can be achieved without negative cardiac consequences. CHF and diabetes lower bulk muscle Q̇O2, resulting in an overall lower Q̇O2/V̇O2 ratio and, due in part to widespread capillary hemodynamic dysfunction/stasis, an inability to distribute O2 according to V̇O2 requirements at the microvascular level. These conditions reduce muscle O2 diffusing capacity and result in very low microvascular PO2s crippling blood-myocyte O2 flux and impairing metabolic and contractile function. Whereas this situation inarguably presents a problem, what constitutes an ideal Q̇O2-to-V̇O2 distribution is far from certain and is likely context-dependent. For example, for exercise at submaximal levels endurance-type training redistributes Q̇ (and Q̇O2) away from low oxidative fibers towards their more oxidative counterparts: An example of better Q̇O2-to-V̇O2 matching. Aging does the opposite in the presence of lowered or unchanged overall muscle Q̇. How much of the increased (training) or decreased (aging) function can be attributed to this phenomenon remains unknown. It is recognized that the heart achieves effective blood-cardiomyocyte O2 flux and rapid V̇O2 kinetics despite low microvascular PO2’s. Understanding the structural and functional bases for this capability will help inform and direct therapeutic approaches to improving oxidative function in compromised muscle(s).
In CHF evidence is emerging that therapeutic treatments which raise the Q̇O2-to-V̇O2 ratio, via strategies that augment NO bioavailability by either nitrate/nitrite supplementation or phosphodiesterase inhibition (sildenafil), speed V̇O2 kinetics and improve exercise performance. Further investigations using established and novel technological approaches (e.g., deep muscle and time-resolved NIRS) may help define the key control features of truly effective Q̇O2-to-V̇O2 matching and the responsible mechanisms. Undoubtedly the role of chronic exercise and other, NO or perhaps non-NO based, therapeutic strategies to correct dysfunction will feature strongly in this endeavor. These further studies might also usefully define the extent to which increased or decreased Q̇O2-to-V̇O2 heterogeneity is beneficial/detrimental under physiological and/or pathophysiological conditions.
Summary statement.
Muscle Q̇O2/V̇O2 heterogeneity may be key to subserving different regional exercise O2 demands in health but derangements in disease may be detrimental to blood-myocyte O2 flux and contractile performance in disease states.
Acknowledgments
The authors recognize and acknowledge the wealth of outstanding work of other researchers and apologize that all that work could not be cited due to reference limitations.
The research of the authors I.H and K.K has been supported by The Academy of Finland, The Finnish Foundation for Cardiovascular Research, Finnish Diabetes Research Foundation, and Ministry of Education and Culture, State of Finland. S.K. was supported by grants from Japan Society for the Promotion of Science (KAKENHI-18207019, 20650103, 21370111, 22370091, 22650151, 24650401, 24247046, 26560362). DCP and TIM were funded by grants from the National Institutes of Health (HL-50306 and HL-108328) and the American Heart Association, Heartland Affiliate.
Footnotes
Disclosures: None.
References
- 1.Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol. 1984;246:H59–H68. doi: 10.1152/ajpheart.1984.246.1.H59. [DOI] [PubMed] [Google Scholar]
- 2.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 3.Behnke BJ, Armstrong RB, Delp MD. Adrenergic control of vascular resistance varies in muscles composed of different fiber types: influence of the vascular endothelium. Am J Physiol Regul Integr Comp Physiol. 2011;301:R783–R790. doi: 10.1152/ajpregu.00205.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol. 2002;133:229–239. doi: 10.1016/s1569-9048(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 5.Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol. 2005;146:259–268. doi: 10.1016/j.resp.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol. 2001;126:53–63. doi: 10.1016/s0034-5687(01)00195-5. [DOI] [PubMed] [Google Scholar]
- 7.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen TS, Rossiter HB, Benson AP, Amano T, Kondo N, Kowalchuk JM, Koga S. Slowed oxygen uptake kinetics in hypoxia correlate with the transient peak and reduced spatial distribution of absolute skeletal muscle deoxygenation. Exp Physiol. 2013;98:1585–1596. doi: 10.1113/expphysiol.2013.073270. [DOI] [PubMed] [Google Scholar]
- 9.Copp SW, Hirai DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirculation. 2011;18:501–511. doi: 10.1111/j.1549-8719.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- 10.Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol. 2010;588:1321–1331. doi: 10.1113/jphysiol.2009.183723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol. 2013;591:2885–2896. doi: 10.1113/jphysiol.2013.251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 2006;188:3–13. doi: 10.1111/j.1748-1716.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol (Oxf) 2006;186:223–232. doi: 10.1111/j.1748-1716.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen I, Kalliokoski KK, Hannukainen J, Duncker DJ, Nuutila P, Knuuti J. Organ-Specific physiological responses to acute exercise and long-term training in humans. Physiology. 2014;29(6):421–436. doi: 10.1152/physiol.00067.2013. [DOI] [PubMed] [Google Scholar]
- 15.Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R, Kalliokoski KK. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol (1985) 2012;112:1059–1063. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 16.Heinonen I, Kudomi N, Kemppainen J, Kiviniemi A, Noponen T, Luotolahti M, Luoto P, Oikonen V, Sipila HT, Kopra J, Mononen I, Duncker DJ, Knuuti J, Kalliokoski KK. Myocardial blood flow and its transit time, oxygen utilization, and efficiency of highly endurance-trained human heart. Basic Res Cardiol. 2014;109:413. doi: 10.1007/s00395-014-0413-1. [DOI] [PubMed] [Google Scholar]
- 17.Heinonen I, Nesterov SV, Kemppainen J, Nuutila P, Knuuti J, Laitio R, Kjaer M, Boushel R, Kalliokoski KK. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J Appl Physiol (1985) 2007;103:2042–2048. doi: 10.1152/japplphysiol.00567.2007. [DOI] [PubMed] [Google Scholar]
- 18.Heinonen I, Saltin B, Kemppainen J, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Effect of nitric oxide synthase inhibition on the exchange of glucose and fatty acids in human skeletal muscle. Nutr Metab (Lond) 2013;10:43. doi: 10.1186/1743-7075-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a PET study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol. 2011;300:H1510–H1517. doi: 10.1152/ajpheart.00996.2010. [DOI] [PubMed] [Google Scholar]
- 20.Heinonen I, Wendelin-Saarenhovi M, Kaskinoro K, Knuuti J, Scheinin M, Kalliokoski KK. Inhibition of alpha-adrenergic tone disturbs the distribution of blood flow in the exercising human limb. Am J Physiol Heart Circ Physiol. 2013;305:H163–H172. doi: 10.1152/ajpheart.00925.2012. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol. 2010;299:R72–R79. doi: 10.1152/ajpregu.00056.2010. [DOI] [PubMed] [Google Scholar]
- 22.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Exercise training and muscle microvascular oxygenation: functional role of nitric oxide. J Appl Physiol (1985) 2012;113:557–565. doi: 10.1152/japplphysiol.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol (1985) 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- 24.Kaijser L, Kanstrup I-L. Coronary blood flow and cardiac hemodynamics. In: Saltin B, Boushel R, Secher N, Mitchell, editors. Exercise and circulation in health and disease. Human Kinetics; 2000. [Google Scholar]
- 25.Kalliokoski KK, Knuuti J, Nuutila P. Relationship between muscle blood flow and oxygen uptake during exercise in endurance-trained and untrained men. J Appl Physiol (1985) 2005;98:380–383. doi: 10.1152/japplphysiol.01306.2003. [DOI] [PubMed] [Google Scholar]
- 26.Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001;280:E1015–E1021. doi: 10.1152/ajpendo.2001.280.6.E1015. [DOI] [PubMed] [Google Scholar]
- 27.Kano Y, Miura S, Eshima H, Ezaki O, Poole DC. The effects of PGC-1alpha on control of microvascular P(O2) kinetics following onset of muscle contractions. J Appl Physiol (1985) 2014;117:163–170. doi: 10.1152/japplphysiol.00080.2014. [DOI] [PubMed] [Google Scholar]
- 28.Koga S, Poole DC, Fukuoka Y, Ferreira LF, Kondo N, Ohmae E, Barstow TJ. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2011;301:R534–R541. doi: 10.1152/ajpregu.00101.2011. [DOI] [PubMed] [Google Scholar]
- 29.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc. 2014;46:860–876. doi: 10.1249/MSS.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 30.Laaksonen MS, Kemppainen J, Kyrolainen H, Knuuti J, Nuutila P, Kalliokoski KK. Regional differences in blood flow, glucose uptake and fatty acid uptake within quadriceps femoris muscle during dynamic knee-extension exercise. Eur J Appl Physiol. 2013;113:1775–1782. doi: 10.1007/s00421-013-2609-8. [DOI] [PubMed] [Google Scholar]
- 31.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- 32.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 33.McAllister RM. Endothelium-dependent vasodilation in different rat hindlimb skeletal muscles. J Appl Physiol (1985) 2003;94:1777–1784. doi: 10.1152/japplphysiol.00901.2002. [DOI] [PubMed] [Google Scholar]
- 34.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murias JM, Spencer MD, Paterson DH. The critical role of O2 provision in the dynamic adjustment of oxidative phosphorylation. Exerc Sport Sci Rev. 2014;42:4–11. doi: 10.1249/JES.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 36.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;291:H2439–H2444. doi: 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- 37.Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal exercise. J Appl Physiol (1985) 1995;79:1914–1920. doi: 10.1152/jappl.1995.79.6.1914. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol. 2013;98:1645–1658. doi: 10.1113/expphysiol.2013.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012;2:933–996. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- 41.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol (1985) 2000;88:186–194. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- 42.Richardson RS, Haseler LJ, Nygren AT, Bluml S, Frank LR. Local perfusion and metabolic demand during exercise: a noninvasive MRI method of assessment. J Appl Physiol (1985) 2001;91:1845–1853. doi: 10.1152/jappl.2001.91.4.1845. [DOI] [PubMed] [Google Scholar]
- 43.Rudroff T, Weissman JA, Bucci M, Seppanen M, Kaskinoro K, Heinonen I, Kalliokoski KK. Positron emission tomography detects greater blood flow and less blood flow heterogeneity in the exercising skeletal muscles of old compared with young men during fatiguing contractions. J Physiol. 2014;592:337–349. doi: 10.1113/jphysiol.2013.264614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoemaker JK, Phillips SM, Green HJ, Hughson RL. Faster femoral artery blood velocity kinetics at the onset of exercise following short-term training. Cardiovasc Res. 1996;31:278–286. [PubMed] [Google Scholar]
- 45.Sperandio PA, Oliveira MF, Rodrigues MK, Berton DC, Treptow E, Nery LE, Almeida DR, Neder JA. Sildenafil improves microvascular O2 delivery-to-utilization matching and accelerates exercise O2 uptake kinetics in chronic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H1474–H1480. doi: 10.1152/ajpheart.00435.2012. [DOI] [PubMed] [Google Scholar]
- 46.von RW, Holtz J, Bassenge E. Exercise induced augmentation of myocardial oxygen extraction in spite of normal coronary dilatory capacity in dogs. Pflugers Arch. 1977;372:181–185. doi: 10.1007/BF00585334. [DOI] [PubMed] [Google Scholar]