Abstract
Influenza A viruses counteract host antiviral activities, especially the production of interferons (IFNs) and the activities of IFN-induced proteins that inhibit virus replication. The viral NS1 protein is largely responsible for countering these IFN antiviral responses, but there are functional differences between the NS1 proteins of different virus strains. The NS1 protein inhibits IFN production by two mechanisms: inhibition of the activation of IRF3 and IFN transcription; and inhibition of the processing of IFN pre-mRNAs. The NS1 proteins of several virus strains do not inhibit IRF3 activation, and the NS1 protein of one virus strain does not inhibit the processing of IFN pre-mRNAs. Many issues remain concerning the mechanisms of action of the various NS1 proteins in countering the IFN response.
General features of the NS1 protein
The NS1 proteins of influenza A viruses range in size from 215 to 237 amino acids long, and are comprised of two functional domains connected by a short linker: N-terminal RNA-binding domain (RBD) (amino acids 1–73); and C-terminal effector domain (ED) (amino acids 85-end) [1]. The RBD forms a unique six-helical homodimer which binds double-stranded RNA (dsRNA) [2–4]. Only one amino acid, the R at position 38, is absolutely required for dsRNA binding [4], but other adjacent basic amino acids also participate in dsRNA binding [5]. A nuclear location sequence (NLS) (amino acids 35–41) overlaps with the sequence required for dsRNA binding [6,7]. The ED, which also dimerizes [8–11], binds several cellular proteins, two of which are discussed below. The EDs of most NS1 proteins also contain a nuclear export signal [12]. Consequently, the NS1 protein can carry out functions in both the cytoplasm and nucleus. The sequence of NS1 proteins from different virus strains is largely conserved. The largest sequence variations occur in the C-terminal region of the ED [1].
Mechanisms by which the NS1 protein inhibits the production of IFN
The differences in the C-terminus of the NS1 ED account for many of the functional differences between the NS1 proteins of different virus strains. One such functional difference is evident in the mechanisms by which NS1 proteins of different virus strains inhibit the production of IFN. In one mechanism, the NS1 protein inhibits the activation of the IRF3 and NFκB transcription factors that are required for the activation of IFN transcription [1,13–15]. Only the NS1 proteins of some virus strains inhibit IRF3 activation [16]. Specifically, IRF3 activation is inhibited in cells infected with viruses expressing NS1 proteins of some, but not all seasonal H1N1 viruses, or expressing the NS1 protein of the 2009 pandemic H1N1 virus or H5N1 virus. In contrast, IRF3 and IFN-β transcription are strongly activated in cells expressing NS1 proteins of seasonal H3N2 and H2N2 viruses, as well as some seasonal H1N1 viruses, demonstrating that these NS1 proteins do not inhibit these activations [16]. The C-terminal region of the ED is largely responsible for conferring the IRF3 and IFN-β transcription phenotype of the NS1 protein. The identity of an amino acid in this region, at position 196, closely correlates with the IRF3 and IFN-β transcription phenotype of the NS1 protein [16]. The NS1 proteins that do not block the activation of IRF3 and IFN-β transcription contain K at this position, whereas the NS1 proteins that block these activations contain E at this position. One possibility is that the C-terminal region of the ED contains a binding site for a protein, with the amino acid at position 196 playing an important role in this binding, and that binding of this putative protein results in either activation of IRF3 or inhibition of IRF3 activation. However, such a protein has not yet been identified.
In fact, the mechanism by which some NS1 proteins inhibit IRF3 and NFκB activation has not been established. The RIG-I pathway is responsible for the activation of these two transcription factors [17]. Cytoplasmic RIG-I recognizes and binds a specific structure/sequence in viral RNA (e.g., the 5’ triphosphate end of a double-stranded region), and then undergoes a conformational change that includes exposure of its N-terminal CARD domains [18–20]. The CARD domain is then activated either by ubiquitination catalyzed by an E3 ligase, either TRIM25 [21] or Riplet [22], or by binding polyubiquitin chains previously synthesized by TRIM25 [23]. The activated RIG-I CARD domain is required for downstream interactions leading to the activation of IFN transcription [17,21]. Because the NS1 protein of all virus strains was found to bind TRIM25 [24], it was proposed that sequesteration of TRIM25 by the NS1 protein causes the inhibition of the activation of the RIG-I CARD domain and hence inhibition of IRF3 activation. However, the NS1 proteins of H3N2 viruses also efficiently bind TRIM25 but do not inhibit the activation of IRF3 and IFN transcription [16], indicating that the binding of TRIM25 by the NS1 protein does not necessarily lead to inhibition of IRF3 activation. It is therefore possible that the NS1 protein binds TRIM25 to prevent a different antiviral activity of TRIM25. Riplet binding to the NS1 protein was only tested with NS1 proteins that inhibit IRF3 activation [25], so that it is not known whether Riplet binds to the NS1 proteins of H3N2 viruses that do not inhibit IRF3 activation. In addition, the NS1 protein of the laboratory-generated influenza A PR/8/34 virus has been reported to interact with RIG-I itself [15,26,27]. However, it has not been established that such a NS1-RIG-I interaction plays a role in the inhibition of IRF3 activation in cells infected with the PR/8/34 virus or infected with seasonal influenza A viruses. The NS1 protein binds dsRNA, albeit with low affinity [28], and it has been established that this dsRNA-binding is not involved in inhibiting the activation of IRF3 and IFN transcription (see below). Consequently, experiments that have examined the binding of the NS1 protein to TRIM25, RIG-I, and dsRNA have not established that these NS1 interactions are responsible for inhibiting the activation of IRF3 and IFN transcription.
The two groups of viruses that differ in the effects of their NS1 proteins on IRF3 activation also differ in the effects of LGP2 on IRF3 activation and IFN production [29]. LGP2 contains domains similar to those of RIG-I that recognize and bind a sequence/structure in viral RNA, but LGP2 lacks the N-terminal CARD domains required for the downstream interactions that lead to IRF3 activation [30]. With seasonal influenza A viruses that block IRF3 activation, LGP2 has no effect [29]. In contrast, with seasonal influenza A viruses that activate IRF3 and IFN transcription, LGP2, which is induced during infection, downregulates the synthesis of IFN [29]. Such a downregulation of IFN production by LGP2 has not been observed during infection with any other virus [29,31]. It seemed counterintuitive that LGP2, a host protein induced during infection, would downregulate the host antiviral response. However, it was subsequently shown that LGP2 downregulation of cytokine expression is beneficial to the host in that it reduces the detrimental inflammatory response resulting from influenza A virus infection, thereby reducing pathogenesis [32]. It is not known how LGP2 causes downregulation of the IFN and cytokine response.
The NS1 protein inhibits IFN production by another mechanism. It binds the 30kDa subunit of the cellular cleavage and polyadenylation specificity factor (CPSF30) that is required for the 3’ end processing of cellular pre-mRNAs [33]. As a consequence of the sequestering of CPSF30 by the NS1 protein, unprocessed cellular pre-mRNAs accumulate in the nucleus, and cellular mRNA production in the cytoplasm is inhibited [34–37]. These cellular mRNAs include interferon mRNAs and other antiviral mRNAs. The structure of a complex of the NS1 ED with a fragment of CPSF30 comprising two of its zinc fingers elucidated the NS1-CPSF30 interface [34]. The CPSF30 binding pocket is comprised of hydrophobic amino acids, including W187, an amino acid required for ED dimerization. Consequently, CPSF30 binding disrupts ED dimerization, and each NS1 ED chain binds a CPSF30 molecule [10,11]. CPSF30 binding is stabilized by two NS1 amino acids, F103 and M106, which are outside the CPSF30 binding pocket [34,38,39]. As is the case for the inhibition of IRF3 activation, inhibition of pre-mRNA processing via CPSF30 binding varies between virus strains. The NS1 proteins of H3N2 and H2N2 viruses, which do not inhibit IRF3 activation, efficiently bind CPSF30 and effectively inhibit the processing of IFN pre-mRNA [16]. In contrast, the binding of CPSF30 to the NS1 protein of the pandemic 2009 H1N1 is hindered by three amino acids [40]. It was reported that restoration of efficient CPSF30 binding by substituting non-blocking amino acids decreased replication and virulence in mice [40]. However, in contrast, my laboratory found that restoration of efficient CPSF30 binding increased virus replication approximately 10-fold (Smith, B. and Krug, R.M., manuscript in preparation). Other results confirm that suboptimal CPSF30 binding to the NS1 protein attenuates virus replication. Thus, the NS1 protein of the H7N9 virus, an avian virus that has recently caused substantial human deaths, contains I rather than M at position 106. Replacing M for I at this position, enhanced virus replication in vivo [41]. The NS1 protein of pathogenic H5N1 viruses isolated in Hong Kong in 1997, for example the A/Hong Kong/483/97 (HK97) virus, contains L instead of F at 103 and I instead of M at 106. Replacing these two amino acids with the consensus amino acids at these positions led to a 20-fold increase in the rate of virus replication in tissue culture cells coupled with a 9-fold increase in IFN-β mRNA production [38]. Remarkably, this replacement of amino acids resulted in a 300-fold increase in the lethality of the virus in mice by increasing the systemic spread of the virus from the lungs, particularly to the brain [42]. The NS1 proteins of all H5N1 isolates from humans in subsequent years contain the consensus amino acids at positions 103 and 106.
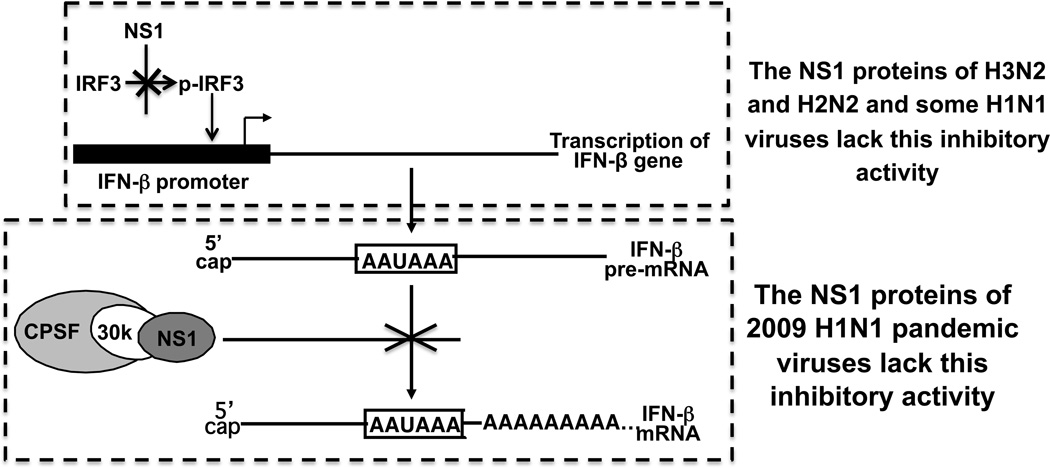
Consequently, the NS1 proteins encoded by influenza A viruses isolated from humans fall into three categories with respect to the inhibition of IFN production. The NS1 proteins of some viruses (a subset of H1N1 viruses prior to 2009 and H5N1 viruses after 1997) inhibit IFN production by two mechanisms: inhibition of the activation of IRF3 and IFN transcription, and inhibition of pre-mRNA processing via strong binding of CPSF30. In contrast, other virus strains employ only one of these NS1-mediated mechanisms, either inhibiting only the activation of IRF3 and IFN transcription (pandemic 2009 H1N1 viruses) [41], or only inhibiting pre-mRNA processing efficiently via strong binding of CPSF30 (H3N2 and H2N2 viruses and a subset of H1N1 viruses prior to 2009) [16] (Figure 1). It is not clear why only one of these NS1-mediated mechanisms is sufficient for some virus strains, whereas some virus strains employ both mechanisms.
Figure 1.
The NS1 proteins of influenza A virus strains that either do not inhibit the activation of IRF3 and IFN transcription, or do not bind CPSF30 and hence do not inhibit the 3' end processing of IFN pre-mRNAs.
Another NS1-mediated inhibition of antiviral gene expression
The NS1 proteins of H3N2 viruses isolated since 1989 inhibit transcription of cellular antiviral genes by another mechanism. These NS1 proteins contain the sequence ARSK (positions 226 to 229) at their C-terminus that is analogous to the N-terminal ARTK sequence of histone H3 (denoted as the H3K4 site of histone H3). Similar to the histone H3K4 sequence, host enzymes methylate the terminal K in the NS1 ARTK sequence in vitro and in vivo [43]. The methylated NS1 sequence acts as a “histone mimic” that binds to PAF1C, a cellular transcription-elongation complex to which methylated H3K4 also binds. The binding of this NS1 sequence to PAF1C inhibits inducible expression of specific genes, specifically including antiviral genes, presumably by competing with methylated H3K4 for binding to PAF1C [43]. The evidence for this conclusion was based on the absence of this activity in cells infected by a recombinant virus that expresses a NS1 protein containing a deletion of ten C-terminal amino acids that includes the ARTK sequence. It might be argued that this NS1 countermeasure against antiviral gene expression via its “histone mimic” sequence compensates for the inability of these H3N2 NS1 proteins to inhibit the activation of IRF3 and IFN transcription. One might then expect that the H3N2 viruses isolated prior to 1989 would be less pathogenic because their NS1 proteins do not inhibit activation of IRF3 and IFN transcription but also lack a C-terminal “histone mimic”. The NS1 proteins of these H3N2 viruses have a 7-amino acid extension at their C-terminal ends that masks the ARTK “histone mimic” sequence.
The NS1 protein inhibits PKR and OAS
The NS1 protein also inhibits two IFN-induced proteins, PKR (protein kinase R) and OAS (2’-5’-oligo A synthetase). PKR is constitutively expressed in mammalian cells, and its level is increased by IFN treatment. PKR is activated by dsRNA or by the cellular PACT protein, resulting in autophosphorylation and phosphorylation of target proteins, one of which is the α subunit of the eIF2 translation initiation factor (eIF2α) [44]. Phosphorylation of eIF2α results in the inhibition of all protein synthesis in infected cells, thereby inhibiting virus replication. PKR is not activated in influenza A virus-infected cells. This block in PKR activation is not due to the sequestration of dsRNA by the NS1 RBD which has an affinity for dsRNA that is much lower than that of PKR [28]. In fact, PKR is not activated in cells infected with a virus that encodes a NS1 protein lacking dsRNA-binding activity (see below). Rather the inhibition of PKR activation by dsRNA as well as by PACT results from direct binding of PKR to NS1 ED amino acids 123–127 [45,46]. It is presumed that binding of PKR to the NS1 ED prevents PKR from undergoing its activating conformational change that is induced by dsRNA or PACT binding. Because activated PKR was not detected in tissue culture cells infected with influenza viruses expressing wild-type NS1 protein, it was postulated that the inhibition of PKR activation is due solely to the action of the NS1 protein [46]. However, others have provided evidence that a host factor activated during viral infection, p58IPK, has an important role in inhibiting PKR activation in influenza A virus-infected cells [47,48]. These two sets of results have not been reconciled.
OAS is activated by dsRNA to produce poly A chains with 2’-5’ phosphodiester bonds. These poly A chains bind to and activate constitutively expressed RNase L that then cleaves viral and cellular single-stranded RNAs, thereby inhibiting virus replication [49]. In addition, some of these degradation products have been reported to bind to and activate RIG-I, thereby enhancing activation of IFN transcription. The NS1 protein via the dsRNA-binding activity of its RBD inhibits OAS activation. This role was established using a H3N2 influenza A/Udorn/72 (Ud) virus encoding a NS1 protein with a R38A mutation [50]. This mutation eliminates at least two functions of the NS1 protein: dsRNA binding and the NLS in the RBD. However, nuclear localization of the Ud NS1 protein was not affected by the R38A mutation, due to the presence of a second NLS in the ED, which is present in the NS1 proteins of only some influenza A virus strains [7]. The R38A mutation resulted in a large (1000-fold) attenuation of virus replication during multiple cycle growth. Activation of the OAS/RNase L pathway accounted for most of this attenuation [51]. No increase in IFN production or activation of PKR was detected in Ud R38A-infected cells [46,51]. These results showed that NS1 dsRNA-binding activity does not function in counteracting IFN production or PKR activation, but rather the primary role of NS1 dsRNA binding activity is the inhibition of the activation of the OAS/RNase L pathway. The dsRNA-binding activity of the NS1 RBD would be expected to effectively compete with the even lower affinity dsRNA-binding activity of OAS, and thus sequester dsRNA away from OAS. Some of the attenuation of the R38A mutant virus was not attributable to activation of the OAS/RNase L pathway [51]. The defect responsible for this residual attenuation is not known.
Concluding Remarks
The influenza A virus NS1 protein was shown to counteract the IFN response more than 10 years ago, but many of the underlying mechanisms still have not been resolved, as discussed above. In fact, it is not yet known how the NS1 proteins of some influenza A virus strains inhibit IRF3 activation, whereas the NS1 proteins of other virus strains do not inhibit IRF3 activation. One of the problems is that many investigators initially focused largely on the NS1 protein of the laboratory-generated PR8 virus strain, which differs in several respects from the NS1 proteins of circulating influenza A viruses. Only in the last few years have many investigators turned their focus to elucidating the functional differences between the NS1 proteins of different circulating influenza A virus strains.
Influenza A NS1 protein blocks interferon (IFN) production by one or two mechanisms
NS1 proteins of several virus strains do not inhibit activation of IFN transcription
NS1 proteins of one virus strain do not inhibit 3’ end processing of IFN pre-mRNAs
NS1 proteins of other virus strains inhibit both steps in IFN production
Acknowledgements
Research in the Author’s laboratory is funded by NIH grant AI11772.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krug RM, Garcia-Sastre A. The NS1 protein: A master regulator of host and viral functions. In: Webster RGMA, Braciale TJ, Lamb RA, editors. Influenza Textbook. 2nd edition. Wiley-Blackwell; 2013. pp. 114–132. [Google Scholar]
- 2.Chien CY, Tejero R, Huang Y, Zimmerman DE, Rios CB, Krug RM, Montelione GT. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nat Struct Biol. 1997;4:891–895. doi: 10.1038/nsb1197-891. [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Lynch PA, Chien CY, Montelione GT, Krug RM, Berman HM. Crystal structure of the unique RNA-binding domain of the influenza virus NS1 protein. Nat Struct Biol. 1997;4:896–899. doi: 10.1038/nsb1197-896. References 2 and 3 provide the first description of the unique structure of the RNA-binding domain of the NS1 protein
- 4. Wang W, Riedel K, Lynch K, Chien C, Montelione GT, Krug RM. RNA-binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA. 1999;5:195–205. doi: 10.1017/s1355838299981621. This study identifies the NS1 amino acids that participate in dsRNA binding, enabling the generation of recombinant influenza A viruses with specific mutations ihat inactiivate dsRNA binding by the NS1 protein.
- 5.Cheng A, Wong SM, Yuan YA. Structural basis for dsRNA recognition by NS1 protein of influenza A virus. Cell Res. 2009;19:187–195. doi: 10.1038/cr.2008.288. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan D, Palese P, Krystal M. Two nuclear location signals in the influenza virus NS1 nonstructural protein. J. Virol. 1988;62:3020–3026. doi: 10.1128/jvi.62.8.3020-3026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melén K, Kinnunen L, Fagerlund R, Ikonen N, Twu KY, Krug RM, Julkunen I. Nuclear and nucleolar targeting of influenza A virus NS1 protein: striking differences between different virus subtypes. J. Virol. 2007;81:5995–6006. doi: 10.1128/JVI.01714-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia S, Monzingo AF, Robertus JD. Structure of NS1A effector domain from the influenza A/Udorn/72 virus. Acta Crystallogr. D. Biol. Crystallogr. 2009;65:11–17. doi: 10.1107/S0907444908032186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hale BG, Barclay WS, Randall RE, Russell RJ. Structure of an avian influenza A virus NS1 protein effector domain. Virology. 2008;378:1–5. doi: 10.1016/j.virol.2008.05.026. The first description of the structure of the effector domain of the NS1 protein
- 10.Aramini JM, Ma LC, Zhou L, Schauder CM, Hamilton K, Amer BR, Mack TR, Lee HW, Ciccosanti CT, Zhao L, et al. Dimer interface of the effector domain of non-structural protein 1 from influenza A virus: an interface with multiple functions. J Biol Chem. 2011;286:26050–26060. doi: 10.1074/jbc.M111.248765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerry PS, Ayllon J, Taylor MA, Hass C, Lewis A, Garcia-Sastre A, Randall RE, Hale BG, Russell RJ. A transient homotypic interaction model for the influenza A virus NS1 protein effector domain. PLoS One. 2011;6:e17946. doi: 10.1371/journal.pone.0017946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Yamakita Y, Krug RM. Regulation of a nuclear export signal by an adjacent inhibitory sequence: The effector domain of the influenza virus NS1 protein. Proc. Natl. Acad. Sci. USA. 1998;95:4864–4869. doi: 10.1073/pnas.95.9.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, Garcia-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 2000;74:7989–7996. doi: 10.1128/jvi.74.17.7989-7996.2000. The first demonstration that the NS1 protein can inhibit the activation of IRF3
- 14.Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J Virol. 2000;74:11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr, García-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 2007;81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuo RL, Zhao C, Malur M, Krug RM. Influenza A virus strains that circulate in humans differ in the ability of their NS1 proteins to block the activation of IRF3 and interferon-beta transcription. Virology. 2010;408:146–158. doi: 10.1016/j.virol.2010.09.012. The demonstration that the NS1 proteins of only some circulating influenza A virus strains inhibit the activation of IRF3 and IFN transcription and that the binding of TRIM25 by the NS1 protein does not necessarily lead to the inhibition of IRF3 activation.
- 17.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myong S, Cui S, Cornish PV, Kirchhofer A, Gack MU, Jung JU, Hopfner KP, Ha T. Cytosolic viral sensor RIG-I is a 5'-triphosphate-dependent translocase on double-stranded RNA. Science. 2009;323:1070–1074. doi: 10.1126/science.1168352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Gack MU, Kirchhofer A, Shin YC, Inn KS, Liang C, Cui S, Myong S, Ha T, Hopfner KP, Jung JU. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci U S A. 2008;105:16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshiumi H, Miyashita M, Inoue N, Okabe M, Matsumoto M, Seya T. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe. 2009;5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog. 2012;8:e1003059. doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Chen LM, Zeng H, Gomez JA, Plowden J, Fujita T, Katz JM, Donis RO, Sambhara S. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell. Mol. Biol. 2007;36:263–269. doi: 10.1165/rcmb.2006-0283RC. [DOI] [PubMed] [Google Scholar]
- 27.Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 2007;9:930–938. doi: 10.1111/j.1462-5822.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 28.Chien CY, Xu Y, Xiao R, Aramini JM, Sahasrabudhe PV, Krug RM, Montelione GT. Biophysical characterization of the complex between double-stranded RNA and the N-terminal domain of the NS1 protein from influenza A virus: evidence for a novel RNA-binding mode. Biochemistry. 2004;43:1950–1962. doi: 10.1021/bi030176o. [DOI] [PubMed] [Google Scholar]
- 29.Malur M, Gale M, Jr, Krug RM. LGP2 downregulates interferon production during infection with seasonal human influenza A viruses that activate interferon regulatory factor 3. J Virol. 2012;86:10733–10738. doi: 10.1128/JVI.00510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 31.Satoh T, Kato H, Kumagai Y, Yoneyama M, Sato S, Matsushita K, Tsujimura T, Fujita T, Akira S, Takeuchi O. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc Natl Acad Sci U S A. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si-Tahar M, Blanc F, Furio L, Chopy D, Balloy V, Lafon M, Chignard M, Fiette L, Langa F, Charneau P, et al. Protective role of LGP2 in influenza virus pathogenesis. J Infect Dis. 2014;210:214–223. doi: 10.1093/infdis/jiu076. [DOI] [PubMed] [Google Scholar]
- 33. Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. This study demonstrated that the NS1 protein binds the cellular CPSF30 factor, thereby causing inhibition of the 3' end processing of cellular pre-mRNAs and hence inhibition of the production of mature cellular mRNAs, including interferon mRNAs.
- 34. Das K, Ma L-C, Xiao R, Aramini J, Marklund J, Kuo R-L, Arnold E, Krug RM, Montelione GT. Structural basis for suppression by influenza A virus of a host antiviral response. Proc. Natl. Acad. Sci. USA. 2008;105:13093–13098. doi: 10.1073/pnas.0805213105. The description of the structure of the NS1 effector domain complexed with a fragment of CPSF30 identifies the NS1 amino acids that are critical for CPSF30 binding, enabling the generation of recombinant viruses with mutations that inactivate CPSF30 binding by the NS1 protein..
- 35.Kim MJ, Latham AG, Krug RM. Human influenza viruses activate an interferon-independent transcription of cellular antiviral genes: Outcome with influenza A virus is unique. Proc Natl Acad Sci U S A. 2002;99:10096–10101. doi: 10.1073/pnas.152327499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noah DL, Twu KY, Krug RM. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3' end processing of cellular pre-mRNAs. Virol. 2003;307:386–395. doi: 10.1016/s0042-6822(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 37.Twu KY, Noah DL, Rao P, Kuo R-L, Krug RM. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 2006;80:3957–3965. doi: 10.1128/JVI.80.8.3957-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twu KY, Kuo RL, Marklund J, Krug RM. The H5N1 influenza virus NS genes selected after 1998 enhance virus replication in mammalian cells. J Virol. 2007;81:8112–8121. doi: 10.1128/JVI.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol. 2007;81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hale BG, Steel J, Medina RA, Manicassamy B, Ye J, Hickman D, Hai R, Schmolke M, Lowen AC, Perez DR, et al. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. J. Virol. 2010;84:6909–6922. doi: 10.1128/JVI.00081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayllon J, Domingues P, Rajsbaum R, Miorin L, Schmolke M, Hale BG, Garcia-Sastre A. A single amino acid substitution in the novel H7N9 influenza A virus NS1 protein increases CPSF30 binding and virulence. J Virol. 2014;88:12146–12151. doi: 10.1128/JVI.01567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spesock A, Malur M, Hossain MJ, Chen LM, Njaa BL, Davis CT, Lipatov AS, York IA, Krug RM, Donis RO. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 2011;85:7048–7058. doi: 10.1128/JVI.00417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marazzi I, Ho JS, Kim J, Manicassamy B, Dewell S, Albrecht RA, Seibert CW, Schaefer U, Jeffrey KL, Prinjha RK, et al. Suppression of the antiviral response by an influenza histone mimic. Nature. 2012;483:428–433. doi: 10.1038/nature10892. This study demonstrates that the ARSK sequence at the C-terminus of the H3N2 influenza A viruses isolated since 1989 acts as a "histone mimic" that inhibits the expression of a subset of cellular genes, including antiviral genes.
- 44.Gale MJ, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Min JY, Krug RM, Sen GC. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology. 2006;349:13–21. doi: 10.1016/j.virol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46. Min J-Y, Li S, Sen GC, Krug RM. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology. 2007;363:236–243. doi: 10.1016/j.virol.2007.01.038. This study shows that the NS1 protein inhibits PKR activation by binding PKR to a specific region of the NS1 effector domain.
- 47.Melville MW, Tan SL, Wambach M, Song J, Morimoto RI, Katze MG. The cellular inhibitor of the PKR protein kinase, P58(IPK), is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J. Biol. Chem. 1999;274:3797–3803. doi: 10.1074/jbc.274.6.3797. [DOI] [PubMed] [Google Scholar]
- 48.Goodman AG, Smith JA, Balachandran S, Perwitasari O, Proll SC, Thomas MJ, Korth MJ, Barber GN, Schiff LA, Katze MG. The cellular protein P58IPK regulates influenza virus mRNA translation and replication through a PKR-mediated mechanism. J. Virol. 2007;81:2221–2230. doi: 10.1128/JVI.02151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman RH. Viral encounters with 2',5'-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81:12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Min JY, Krug RM. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: Inhibiting the 2'–5' oligo (A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. (USA) 2006;103:7100–7105. doi: 10.1073/pnas.0602184103. This study shows that inhibition of the IFN-induced OAS/RNase L pathway is a primary function of dsRNA binding by the RNA-binding domain of the NS1 protein and that activation of IFN transcription is not a function of the NS1 RNA-binding domain in infected cells.
- 51.Min J-Y, Krug RM. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2'–5' OAS/RNase L pathway. Proc. Natl. Acad. Sci. USA. 2006;103:7100–7105. doi: 10.1073/pnas.0602184103. [DOI] [PMC free article] [PubMed] [Google Scholar]