Abstract
Objective
To compare the characteristics and hospital outcomes of patients with an acute exacerbation of chronic obstructive lung disease (COPD) treated in the ICU with initial noninvasive (NIV) or invasive mechanical ventilation (IMV).
Design
Retrospective, multicenter cohort studyof prospectively collected data. We used propensity matching to compare the outcomes of patients treated with NIV to those treated with IMV. We also assessed predictors for NIV failure.
Setting
Thirty-eight hospitals participating in the Acute Physiology and Chronic Health Evaluation (APACHE) database from 2008 through 2012.
Subjects
A total of 3,520 with a diagnosis of COPD exacerbation including 27.7% who received NIV and 45.5% who received IMV.
Measurements and Main Results
NIV failure was recorded in 13.7% from patients ventilated noninvasively. Hospital mortality was 7.4% for patients treated with NIV; 16.1% for those treated with IMV; and 22.5% for those who failed NIV. In the propensity matched analysis, patients initially treated with NIV had a 41% lower risk of death compared with those treated with IMV (RR: 0.59, 95% CI 0.36, 0.97). Factors that were independently associated with NIV failure were SAPS-II score (relative risk = 1.04 per point increase, 95% CI: 1.03, 1.04) and the presence of cancer (2.29, 95% CI: 0.96, 5.45).
Conclusions
Among critically ill adults with COPD exacerbation, the receipt of NIV was associated with a lower risk of in-hospital mortality compared to IMV; NIV failure was associated with the worst outcomes. These results support the use NIV as a first line therapy in appropriately selected critically ill patients with COPD while also highlighting the risks associated with NIV failure and the need to be cautious in the face of severe disease.
Keywords: COPD, noninvasive ventilation, invasive mechanical ventilation, intensive care unit, acute respiratory failure, SAPS-II score
Background
Chronic obstructive pulmonary disease (COPD) is a highly prevalent condition which is responsible for approximately 1 million hospitalizations each year and it is the third leading cause of death in the United States.1–3 There is a wide range of disease severity among hospitalized patients with an acute exacerbation of chronic obstructive lung disease (AE-COPD), ranging from brief hospital admission to prolonged hospitalization and death.4, 5 Approximately 12–18% of patients hospitalized with an AE-COPD are treated in the intensive care unit (ICU)6 and mortality in this population approaches 15%.5
The efficacy of NIV in patients with AE-COPD has been extensively studied. Several randomized control trials7–9 and meta-analyses10, 11 found a reduction in intubation rate, hospital-acquired pneumonia and mortality when NIV was added to supportive care. A number of guidelines strongly recommend NIV versus standard care alone in moderate to severe COPD exacerbation. However, only two small randomized controlled trials (RCT) directly compared the efficacy of NIV and IMV and found that NIV use resulted in fewer complications and lower readmission rate without changes in mortality.12, 13 One survey study of 99 patients with AE-COPD admitted to 42 French ICUs14 and one recent large US study using an administrative dataset15 showed that NIV use was associated with significant reduction in mortality compared to IMV. Because of insufficient evidence the Canadian Practice Guidelines and the US Agency for Healthcare Research and Quality comparative effectiveness review make no recommendations about the use of NIV versus IMV in patients with severe acute respiratory failure (ARF) secondary to COPD.16, 17
There is limited recent data about the use of NIV and its associated outcomes in patients with severe AE-COPD admitted to ICU, and what has been learned recently about the comparative effectiveness of NIV to IMV comes mainly from studies based only on claims data.
We sought to take advantage of a large, multicenter ICU database which contains physiological data to compare the characteristics and short term outcomes of patients hospitalized with severe COPD exacerbation and treated with NIV and IMV. We hypothesized that after adjusting for severity of illness and other patient and hospital characteristics, patients treated with NIV would have better outcomes than patients treated with IMV.
Methods
Design, setting, and subjects
We conducted a cohort study of patients hospitalized from January 2008 to December 2012 at 38 structurally diverse US hospitals that participate in the Acute Physiology and Chronic Health Evaluation (APACHE) outcomes database. The APACHE project is a US prospective, multicenter, clinical registry that was created to provide feedback to hospital ICUs on risk-adjusted outcomes for quality improvement purposes. All patient data are entered on-site by trained data collectors using specific software and standardized definitions. The database has been used extensively for research.18, 19
We included patients 40 years of age or older with a primary diagnosis of bronchitis/emphysema or respiratory arrest paired with a secondary diagnosis of COPD. In the APACHE database, the admission diagnosis reflects the primary reason for ICU admission, and is selected by a physician within the first 24 hours. We did not include patients with any other primary diagnosis (e.g., pneumonia or sepsis with a secondary diagnosis of COPD) because our primary goal was to analyze patients with acute respiratory failure secondary to COPD, and not patients with comorbid COPD.
Patient and hospital information
Patient demographics, location prior to ICU admission, detailed clinical and physiological variables, duration and type of ventilator therapy, advance care directives, readmission to ICU, discharge status and hospital characteristics were collected. The severity of illness was assessed using the Simplified Acute Physiology Score II (SAPS-II).20 Only comorbidities known to impact ICU mortality are collected in the APACHE dataset, including immunosuppression, cancer, cirrhosis, COPD, diabetes and renal disease requiring renal replacement therapy. Severity of COPD is assessed, based on the functional limitation caused by the chronic pulmonary disease and is classified as: “severe”, “moderate”, or “no limitation with activities of daily living.” Time to discharge from the ICU and from the hospital and to death was recorded for all patients. The majority of the variables were complete and only a few variables were not recorded in all patients (e.g., advance directives, albumin, paO2).
Receipt of mechanical ventilation
The mode of initial mechanical ventilation at admission to ICU was the primary exposure variable. For the purpose of this study we classified patients according to ventilatory strategy into two groups: “NIV Initial” (patients started on NIV) and “IMV Initial” (patients who were initially intubated). We noted any changes in the type of mechanical ventilation during the hospitalization to ICU. Patients were considered to have failed NIV if they were intubated after an NIV trial. We excluded from the NIV or IMV groups but not from the study in general, a small number of cases in which we were unable to determine the order of ventilation. For the comparison of the outcomes of NIV to IMV we restricted analysis to patients admitted to ICU from the emergency room; we excluded patients transferred from the hospital ward because their outcomes were more likely to be influenced by management prior to ICU admission. In a sensitivity analysis we included all patients admitted to the ICU irrespective of the source of admission.
Outcomes
The primary outcomes were ICU and in-hospital mortality. Secondary outcomes were ICU and hospital length of stay (LOS).
Analysis
We calculated descriptive statistics including counts and percentages for categorical factors and means, standard deviations, and percentiles for continuous factors to characterize the study population. Chi-square inference tests, t-tests and nonparametric analogs were used to compare patients and hospital characteristics including demographics, physiological data and outcomes for patients who received NIV or IMV as initial ventilation strategies.
To adjust for confounding when comparing outcomes by initial ventilation strategy, we first created a multilevel mixed-effects (hierarchical) logistic regression model with hospitals as a random effect to estimate the propensity of initial NIV treatment as the outcome. In the propensity score model, the predictors were age, and the patient demographics, comorbidities, and SAPS-II score as shown in Table 1. We then matched patients who received NIV initial to patients with similar propensity who received IMV to compare mortality using a 5:1 Greedy matching algorithm.
Table 1.
Main characteristics of patients with acute COPD exacerbation admitted to intensive care unit: overall and by ventilation strategy
| Variable | All Patients N = 3,520 |
Initial NIV N = 974 (27.7%) |
Initial IMV N = 1603 (45.5%) |
P-value* |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr [median, IQR] | 67 [59, 74] | 67 [59, 75] | 66 [58, 74] | 0.014 |
| Male (n, %) | 1,610 (45.7) | 398 (40.9) | 752 (46.9) | 0.003 |
| Race (n, %) | ||||
| White | 2980 (84.7) | 803 (82.4) | 1,362 (85.0) | |
| Black | 446 (12.7) | 153 (15.7) | 188 (11.7) | 0.002 |
| Hispanic | 31 (0.9) | 10 (1.0) | 17 (1.1) | |
| Other/Unknown Race | 63 (1.8) | 8 (0.8) | 36 (2.2) | |
| Location Prior to ICU Admission n, % | ||||
| Emergency Department | 1778 (50.5) | 529 (54.3) | 765 (47.7) | |
| Other Hospital | 645 (18.3) | 84 (8.6) | 385 (24.0) | |
| Hospital ward | 547 (15.5) | 185 (19.0) | 222 (13.9) | |
| Step-down unit or Telemetry | 493 (14.0) | 157 (16.1) | 203(12.7) | <0.001 |
| ICU Transfer | 45 (1.3) | 15 (1.5) | 23 (1.4) | |
| Home/Other | 12 (0.3) | 4 (0.4) | 3 (0.2) | |
| Comorbidities | ||||
| Severe COPD | 1884 (53.5) | 530 (54.4) | 851 (53.1) | 0.51 |
| Immunosuppression | 220 (6.2) | 64 (6.6) | 84 (5.2) | 0.16 |
| Cirrhosis | 47 (1.3) | 13 (1.3) | 22 (1.4) | 0.93 |
| Cancer (any) | 136 (3.9) | 31 (3.2) | 70 (4.4) | 0.13 |
| Diabetes | 1338 (38.0) | 381 (39.1) | 640 (39.9) | 0.68 |
| Dialysis | 106 (3.0) | 36 (3.7) | 48 (3.0) | 0.33 |
| Advance directives | ||||
| No advance directive restriction | 1891, (N=2111) (89.6%) | 597 (N=684) (87.3) | 908 (N=991) (91.6) | 0.004 |
| Vitals | ||||
| RR (N = 3515) | 27 [14, 33] | N = 972 29 [23, 35] |
N = 1601 22 [12, 31] |
<0.001 |
| GCS (N = 2891) | 15 [13, 15] | N =957 15 [14, 15] |
N= 1006 13 [10, 15] |
<0.001 |
| ABG at admission | ||||
| FiO2 (N = 2591) | 45 [36, 60] | N = 736 40 [35, 50] |
N = 1419 50 [40, 80] |
<0.001 |
| PaO2 (N = 2591) | 82 [66, 120] | N = 736 76 [63, 94] |
N = 1419 94 [70, 155] |
<0.001 |
| PaCO2 mmHg (n = 2936) | 54 [41, 69] | N = 857 62 [46, 77] |
N = 1491 52 [42, 64] |
<0.001 |
| pH (N = 2591) | 7.35[7.29, 7.41] | N = 736 7.34 [7.29, 7.39] |
N = 1419 7.36 [7.29, 7.43] |
<0.001 |
| SAPS II score (n = 3518) | 37 [26, 51] | 34 [24, 44] | 44 [32, 58] | <0.001 |
| Hospital Characteristics | ||||
| Patients treated at a hospital with ≤400 beds | 1334 (37.9) | 380 (39.0) | 639 (39.9) | 0.66 |
| Number of ICU Beds | 20 [16, 26] | 18 [14, 30] | 20 [16, 26] | 0.001 |
| Patients treated at a Non-Teaching Hospital | 793 (22.5) | 270 (27.7) | 300 (18.7) | <0.001 |
Data are given as N (sample Size) and Percent or Median [25th percentile, 75th Percentile]
“All Patients” refers to all patients admitted to an ICU, meeting the inclusion criteria for COPD
p value compares initial NIV to initial IMV
Definition of abbreviations: ABG = arterial blood gases; FI O2 = fraction of inspired oxygen; NIV = noninvasive ventilation; PaCO2 = arterial carbon dioxide tension; SAPS = Simplified Acute Physiology Score, RR= respiratory rate, GCS = Glasgow coma score
Advance directives and vitals were not recorded consistently; the N equals the total number of patients with the variable recorded
We performed a multivariable logistic regression analysis to identify factors associated with NIV failure also adjusting for age, patient demographics, comorbidities, location prior to admission to the ICU and SAPS-II score. For this analysis we included patients without any advanced directive restriction and all patients with NIV failure (although for a small number of patients the code status was not specified). In a sensitivity analysis we restricted the cohort to only patients with a known code status.
Stata/MP 13.1 for Windows (StataCorp, College Station, TX) was used for statistical analyses.
This project was approved by the Baystate Medical Center Institutional Review Board for the Protection of Human Subjects.
Results
Study Population Characteristics
A total of 3,520 patients from 38 hospitals were included in this analysis. The median age was 67 years; 54.0% were women; 84.0% were white and the median SAPS-II score was 37. The majority of admissions were from the emergency department. Sixty-two percent of patients were admitted to hospitals with more than 400 beds, and 23% were admitted to non-teaching hospitals. (Table 1)
At the time of ICU admission, 918 patients (26.1%) were not ventilated, 974 (27.7%) were treated with NIV, and 1603 (45.5%) with IMV. 25 patients (0.7%) received both NIV and IMV on the same first day and so were indeterminate as to what treatment was first started. NIV failure was recorded in 13.7% from patients ventilated noninvasively.
The median ICU and hospital lengths of stay were 2.7 (IQR: [1.5, 5.1]) and 7.3 (IQR: [4.6, 15.9]) days respectively, and 6.5% of patients were readmitted to the ICU during their same hospitalization. Patients spent a median of 2.2 days on IMV, and 15% of patients were on IMV for more than 7 days. Overall ICU and hospital mortality rates were 6.5% and 11.1% respectively.
Comparison of NIV and IMV therapy
Main demographic and physiological characteristics of all patients admitted to an ICU meeting the inclusion criteria for COPD treated either with NIV or IMV at admission to the ICU are presented in Table 1. Compared with patients initially treated with IMV, patients treated with NIV had lower SAPS-II scores, were more likely to be admitted from the emergency department and have a DNR order.
The receipt of NIV was associated with lower ICU and hospital mortality compared to the receipt of IMV (3.1% vs 10.5% and 7.4% vs 16.1% respectively); patients treated with NIV had shorter ICU and hospital LOS and were less likely to be discharged to nursing home. When we analyzed LOS for survivors by age we found that patients who received NIV had a shorter LOS than those receiving IMV across all ages. Among NIV patients, length of stay tended to be longer at both extremes of age (Figure 1).
Figure 1.
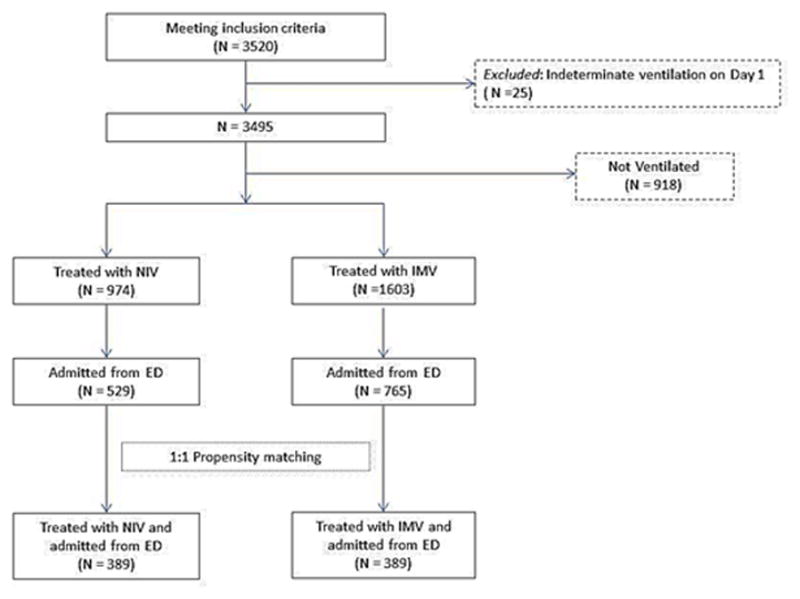
Flow chart of the study cohort
In the propensity matched analysis which included patients admitted directly from the emergency department, we found that those treated with NIV had a 61% lower risk of dying in the ICU (relative risk 0.39, 95% CI (0.18, 0.85) and 41% lower risk of dying in the hospital (relative risk of 0.59; 95% CI 0.36 to 0.97) compared with patients treated with IMV. The propensity analysis was based on 389 matched pairs which means that 71.1% of the eligible NIV patients were successfully matched to an IMV patient within a propensity difference no larger than 0.1. All predictors in the model were statistically non-significant between IMV and NIV after matching. As a sensitivity analysis, we re-calculated the propensity score analysis using the same predictors on all patients and not just those from the ED. NIV patients has a statistically similar survival benefit (RR = 0.63, 95% CI (0.46, 0.86), p=0.004) when compared to the subset directly admitted from the ED. This result is based on 679 matched pairs or 69.7% of the eligible NIV patients.
Patients who were transitioned from NIV to IMV had the highest mortality (13.5% in ICU and 22.5% in the hospital) and the longest hospital stay. (Table 2) Compared with patients who avoided intubation, those who failed NIV had higher respiratory rate, higher SAPS-II score, and were more likely to have a GCS <15. Table 3 presents these unadjusted results. Factors that were independently associated with NIV failure were SAPS-II score (relative risk = 1.04, per point 95% CI (1.03, 1.04) and the presence of cancer (2.29, 95% CI 0.96, 2.44). (Table 4) Age was not significantly associated with NIV failure. These results were similar in a sensitivity analysis that was restricted to patients with known advanced directives. (Table 4) When we grouped SAPS-II scores by quintiles we observed that the rate of NIV failure increased as the SAPS II scores increased. (Figure 3)
Table 2.
Outcomes according to the initial ventilation strategy (unadjusted outcomes)
| Initial NIV N = 974 (27.7%) |
Initial IMV N = 1603 (45.5%) |
NIV failure N = 89 of 974 |
P-value comparing Initial NIV vs Initial IMV | |
|---|---|---|---|---|
| ICU mortality | 30 (3.1%) | 168 (10.5%) | 12 (13.5%) | <0.001 |
| Hospital mortality | 72 (7.4%) | 258 (16.1%) | 20 (22.5%) | <0.001 |
| ICU LOS | 2.1 [1.3, 3.5] | 4.2 [2.5, 7.9] | 7.0 [4.7, 11.8] | <0.001 |
| Hospital LOS | 6.7 [4.3, 10.4] | 9.0 [5.6, 15.1] | 11.9 [7.9, 17.8] | <0.001 |
| Duration of IMV | ||||
| Median [25th, 75th] | N/A | 2.2 [1.1, 4.5] | 3.7 [1.7, 7.4] | N/A |
| < 3 Days on IMV | N/A | 1002 (62.5%) | 40 (44.9%) | N/A |
| 7 or More Days on IMV | N/A | 225 (14.0%) | 26 (29.2%) | N/A |
| Readmission to ICU | 53 (5.4%) | 126 (7.9%) | 6 (6.7%) | 0.019 |
| Discharge from Hospital for Survivors | ||||
| Other Hospital | 27 (3.0%) | 64 (4.8%) | 5 (7.3%) | <0.001 |
| Home | 537 (59.5%) | 714 (53.1%) | 29 (42.0%) | |
| Nursing Home/ Long-term acute care | 241 (26.7%) | 419 (31.2%) | 28 (40.6%) | |
| Other | 30 (3.3%) | 86 (6.4%) | 5 (7.3%) | |
| Hospice | 67 (7.4%) | 62 (4.6%) | 2 (2.9%) |
Data are given as N (sample Size) and Percent or Median [25th percentile, 75th Percentile]
Table 3.
Comparison of clinical characteristics of NIV success and NIV failure
| Variable | Initial NIV and no DNR, N= 561 (86.3%) | NIV Failure N = 89 (13.7%) |
p-value |
|---|---|---|---|
| Age, yr (median, IQR) | 67 [59, 74] | 68 [61, 74] | 0.53 |
| Male (no, %) | 221(39.4) | 43 (48.31) | 0.111 |
| SAPS II score | 32 [22, 42] | 46 [38, 55] | <0.001 |
| Comorbidities | |||
| Severe COPD | 275 (49.0) | 49 (55.1) | 0.29 |
| Immunosuppression | 36 (6.4) | 4 (4.5) | 0.48 |
| Cirrhosis | 5 (0.9) | 1 (1.1) | 0.58 |
| Cancer (any) | 16 (2.9) | 6 (6.7) | 0.06 |
| Diabetes | 235 (41.9) | 27 (30.3) | 0.04 |
| Dialysis | 19 (3.4) | 3 (3.4) | 1.00 |
| ABG at admission | |||
| PaCO2 mmHg | N = 472 64 [50, 78] |
N = 88 62 [44, 76] |
0.17 |
| PF-Ratio mmHg | N = 426 188 [150, 243] |
N = 77 173 [120, 247] |
0.15 |
| pH | N = 426 7.34 [7.29, 7.39] |
N = 77 7.33 [7.24, 7.38] |
0.08 |
| Respiratory rate | N = 560 28 [16, 33] |
N = 89 33 [27, 38] |
<0.001 |
| GCS | N =559 15 [14, 15] |
N= 86 15 [13, 15] |
0.005 |
| GCS 15 | 64.9% | 51.2% | 0.01 |
Data are given as N (sample Size) and Percent or Median [25th percentile, 75th Percentile]
Definition of abbreviations: ABG = arterial blood gases; NIV = noninvasive ventilation; PaCO2 = arterial carbon dioxide tension; SAPS = Simplified Acute Physiology Score, GCS = Glasgow coma score, PF ration = FIO2/PaO2
Table 4.
Predictors of NIV Failure in Initial NIV Patients
| Variable | RR; 95% CI N = 649 |
P-value | Sensitivity Analysis RR; 95% CI N = 596 |
Sensitivity Analysis P-value |
|---|---|---|---|---|
| Age 65 or older | 0.77; (0.47, 1.26) | 0.291 | 0.96; (0.39,2.32) | 0.922 |
| SAPS II Score per unit | 1.04; (1.03, 1.04) | <0.001 | 1.03; (1.01, 1.05) | 0.001 |
| Cancer | 2.29; (0.96, 5.45) | 0.061 | 4.18; (1.67, 10.44) | 0.002 |
| Diabetes | 0.64; (0.42, 0.97) | 0.037 | 0.57; (0.37, 0.90) | 0.015 |
Outcome variable: NIV Failure vs NIV. In the primary analysis, we assume that all patients with IMV after NIV are failures, even if their DNR code status is unknown. In the Sensitivity Analysis, we only look at patients with known “full code” (no code restriction) status.
Figure 3.
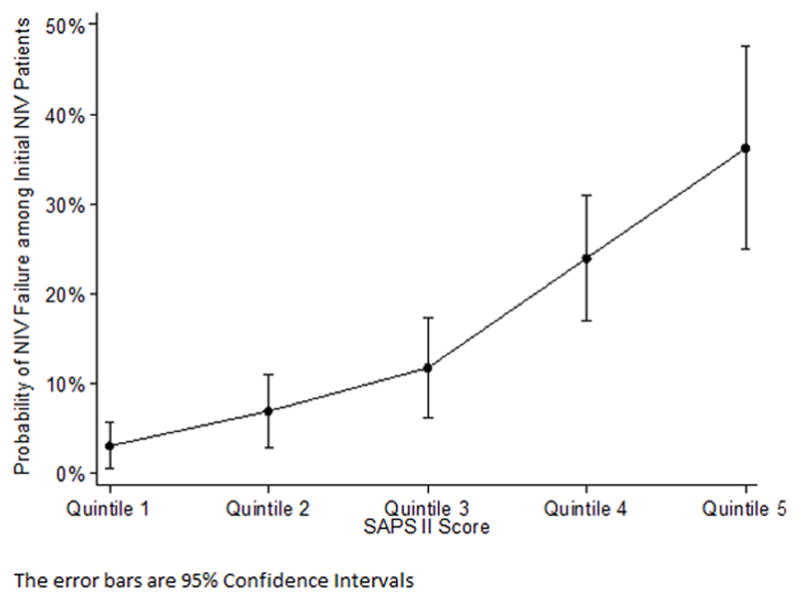
Probability of NIV failure among quintiles of the SAPS II score
Discussion
In this contemporary observational study of more than 3,000 patients admitted with an AE-COPD to 38 ICUs in the US, we found that after adjusting for severity of illness using propensity score matching, receipt of NIV was associated with lower ICU and in-hospital mortality and shorter length of stay compared with IMV. Patients who failed NIV had the worst outcomes and SAPS-II score was independently associated with the need for intubation.
Several current guidelines recommend early use of NIV in patients hospitalized with moderate to severe AE-COPD based on the results of randomized controlled trials (RCTs) which found that NIV improves outcomes compared with supportive care alone.16, 21–23 For example, the Canadian Practice guidelines published in 2011 give a level 1A recommendation for NIV use in severe exacerbation of COPD versus standard therapy (no ventilation).17 However, the guidelines state: “we make no recommendation about the use of NIV vs. conventional mechanical ventilation in patients who have a severe COPD exacerbation that requires ventilator support, because of insufficient evidence.” There are only two RCTs which compared NIV with IMV; the small number of studies may reflect the general current belief that avoiding invasive ventilation is strongly desired. These trials, which included patients with severe acute respiratory failure who failed standard treatment, found that NIV lowered hospital-acquired pneumonia but did not reduce mortality or length of stay and had a high rate of NIV failure.12, 24 Our results are consistent with those from a survey study in 42 ICUs in France in 1997 which found that NIV use was associated with lower risk of death than IMV (10% vs 28%). In our study in the propensity matched analysis, NIV therapy was associated with a 41% lower risk of death than IMV. Our results are also similar with the findings from the study by Tsai et al, a recent retrospective study of 67,000 emergency department visits for AE-COPD with acute respiratory failure in US; in this study patients treated with NIV alone had a mortality rate of 8% compared with 16% in patients treated with IMV alone.15 Although we did reach some of the same conclusions as this study, our target population was different, and our methodology was much stronger and overcomes a number of the biases in the Nationwide Inpatient Sample, on which Tsai’s work was based.
In our study, the percentage of patients treated with NIV who avoided endotracheal intubation was 86%, and the SAPS-II score was predictive of NIV failure which concurs with prior research.25 In a prospective randomized study, Antonelli et al reported that a SAPS-II score >34 was independently associated with the need for endotracheal intubation.26 Consistent with the findings of Antonelli et al, we also found the likelihood of NIV failure rose sharply at higher SAPS-II scores. Patients who transition from NIV to IMV had a high ICU and hospital mortality in keeping with the results from other studies.15, 27
Study Strengths and limitations
This study has several strengths. To the best of our knowledge, this is the largest contemporary US multicenter study of patients admitted to intensive care with respiratory failure secondary to an acute COPD exacerbation, and provides an estimate of the ventilation practices and outcomes in the ICU for these patients. All the data were collected prospectively by trained, on-site investigators, the advance directive status was known, and the primary diagnosis was assigned at the time of admission, not retrospectively by coders. The dataset comprises clinical and laboratory data and the SAPS-II score is calculated allowing better adjustment for the severity of illness than other observational studies with administrative datasets.
This study has also several limitations. First, although a mixture of teaching and non-teaching and small and large hospitals were included in this database, hospitals participating in the APACHE project may not be representative of all hospitals in the US. Second, we did not have spirometric data. However all diagnoses of COPD were assigned by an intensivist and our comparative analysis included only ventilated patients with a diagnosis of COPD and so their COPD was most likely severe. Third, although we adjusted for the SAPS-II score and other patient characteristics using propensity score matching, the survival benefit we observed with NIV use assumes that all patients treated with NIV or IMV had similar degrees of severity of respiratory failure and this may not have been true. This was an observational study and although we adjusted for severity via SAPS-II and other physiological variables we cannot exclude that unmeasured confounders at the patient and hospital level distorted our findings. In addition, the initial decision for NIV or IMV was based physician judgment rather than on unified criteria. Fourth, unlike most randomised studies in this area, the population included in this study is of patients receiving ventilatory support for an exacerbation of COPD and not necessarily with hypercapnic acidotic exacerbation. Lastly, we examined in-hospital mortality since we did not have any information regarding the survival of patients with COPD after discharge though we note that 90 day mortality has been reported to be high.
Conclusion
Our observational study suggested that NIV use is associated with lower mortality than IMV use in patients with severe acute respiratory failure secondary to an exacerbation of COPD and that NIV failure is associated with the worst outcomes. These results support the use NIV as a first line therapy in appropriately selected critically ill patients with COPD while also highlighting the risks associated with NIV failure and the need to be cautious in the face of severe disease.
Figure 2.
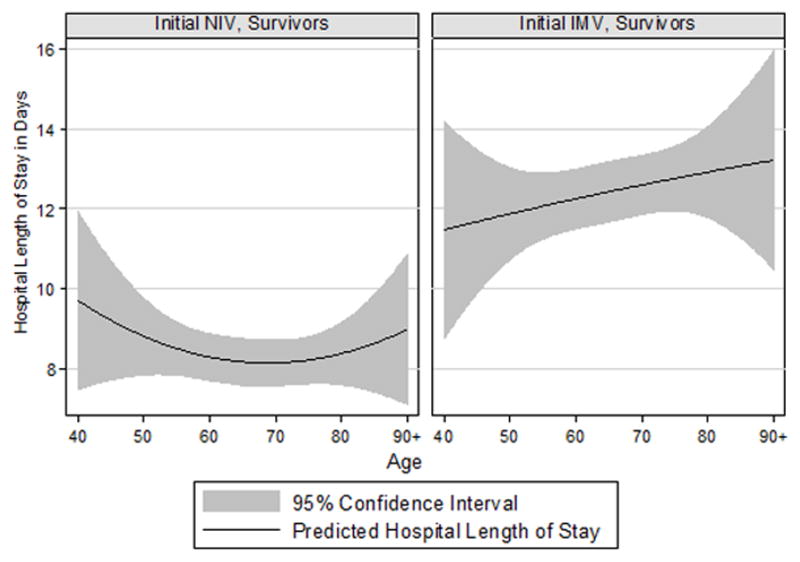
Hospital length of stay for patients treated with initial NIV and initial IMV
Acknowledgments
Source of Funding: Dr. Stefan is supported by grant 1K01HL114631-01A1 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The project was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number UL1 TR000073. Dr. Lindenauer and Rothberg are supported by grant 1R18HL108810-01 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Lagu is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K01HL114745. Dr. Steingrub is supported by contract HHSN268200536165C (NHLBI).
We thank Andrew A. Kramer, PhD from Cerner Corporation for providing the data for the study. Although Cerner Corporation administers the data, the authors are exclusively responsible for the analysis and conclusion of the study.
Footnotes
Conflicts of Interest:
Authors report no conflicts of interest.
Authors contribution
Drs. Stefan, Lindenauer, Rothberg, Higgins, Lagu and Steingrub conceived and designed the study. Dr. Higgins and Dr. Stefan acquired the data used in the analysis. Drs. Stefan, Lindenauer, Nathanson, Higgins, Lagu and Steingrub were involved in the analysis and interpretation of the data. Dr. Stefan drafted the manuscript and Drs. Lindenauer, Nathanson, Steingrub, Higgins, Lagu and Rothberg reviewed and contributed to revisions prior to submission.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Copyright form disclosures:
Dr. Stefan received grant support from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (grant 1K01HL114631-01A1). Dr. Nathanson’s institution consulted for OptiStatim, LLC (Dr. Nathanson’s company, OptiStatim, LLC, has a consulting agreement with Baystate Medical Center to do statistical analyses and help with manuscript preparation for various research projects.) and received other support from Cerner Corporation (OptiStatim, LLC, has a consulting agreement with Cerner Corporation to do statistical analyses and help with manuscript preparation for various research projects). Dr. Lagu consulted for the Institute for Healthcare Improvement (IHI) (Dr. Lagu has received consulting fees from IHI, under contract to CMS, for her work on a project to help health systems achieve disability competence) and The Island Peer Review Organization (IPRO) (Dr. Lagu has received consulting fees from The IPRO, under contract to CMS, for her work on development of episodes of care for CMS payment purposes.) and she received support for article research from the NIH (Dr. Lagu is supported by the NHLBI of the NIH under Award Number K01HL114745). Her institution received grant support from the NHLBI (Dr. Lagu is supported by the NHLBI of the NIH under Award Number K01HL114745. 75,000 in salary per year paid to her institution). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Wier LM, Elixhauser A, Pfuntner A, Au DH. Overview of Hospitalizations among Patients with COPD. 2008: Statistical Brief #106. 2006 Feb; [PubMed] [Google Scholar]
- 2.Hill NS. Complications of noninvasive ventilation. Respir Care. 2000 May;45(5):480–481. [PubMed] [Google Scholar]
- 3.Gershon AS, Guan J, Victor JC, Goldstein R, To T. Quantifying health services use for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013 Mar 15;187(6):596–601. doi: 10.1164/rccm.201211-2044OC. [DOI] [PubMed] [Google Scholar]
- 4.Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003 May 26;163(10):1180–1186. doi: 10.1001/archinte.163.10.1180. [DOI] [PubMed] [Google Scholar]
- 5.Afessa B, Morales IJ, Scanlon PD, Peters SG. Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med. 2002 Jul;30(7):1610–1615. doi: 10.1097/00003246-200207000-00035. [DOI] [PubMed] [Google Scholar]
- 6.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. May 26;303(20):2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 7.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995 Sep 28;333(13):817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 8.Bott J, Carroll MP, Conway JH, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993 Jun 19;341(8860):1555–1557. doi: 10.1016/0140-6736(93)90696-e. [DOI] [PubMed] [Google Scholar]
- 9.Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995 Jun;151(6):1799–1806. doi: 10.1164/ajrccm.151.6.7767523. [DOI] [PubMed] [Google Scholar]
- 10.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003 Jan 25;326(7382):185. doi: 10.1136/bmj.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med. 2003 Jun 3;138(11):861–870. doi: 10.7326/0003-4819-138-11-200306030-00007. [DOI] [PubMed] [Google Scholar]
- 12.Conti G, Antonelli M, Navalesi P, et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002 Dec;28(12):1701–1707. doi: 10.1007/s00134-002-1478-0. [DOI] [PubMed] [Google Scholar]
- 13.Scala R, Nava S, Conti G, et al. Noninvasive versus conventional ventilation to treat hypercapnic encephalopathy in chronic obstructive pulmonary disease. Intensive Care Med. 2007 Dec;33(12):2101–2108. doi: 10.1007/s00134-007-0837-2. [DOI] [PubMed] [Google Scholar]
- 14.Carlucci A, Richard JC, Wysocki M, Lepage E, Brochard L. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001 Mar;163(4):874–880. doi: 10.1164/ajrccm.163.4.2006027. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA., Jr Comparative effectiveness of noninvasive ventilation vs invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013 Apr;8(4):165–172. doi: 10.1002/jhm.2014. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed March, 2014];Noninvasive positive pressure ventilation for acute respiratory failure. http://www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/
- 17.Keenan SP, Sinuff T, Burns KE, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011 Feb 22;183(3):E195–214. doi: 10.1503/cmaj.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006 Jul 6;355(1):41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 19.Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. May 31;366(22):2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993 Dec 22–29;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 21.Non-invasive ventilation in acute respiratory failure. Thorax. 2002 Mar;57(3):192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004 Jun;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 23.Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Accessed March, 21, 2014];NHLBI/WHI Global Strategy for the Diagnosis, Management and Prevention of COPD. http://www.goldcopd.org.
- 24.Jurjevic M, Matic I, Sakic-Zdravcevic K, Sakic S, Danic D, Bukovic D. Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study) Coll Antropol. 2009 Sep;33(3):791–797. [PubMed] [Google Scholar]
- 25.Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998 Aug 13;339(7):429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 26.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001 Nov;27(11):1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 27.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2011 Jan 15;185(2):152–159. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


