Abstract
Background
As physicians have increased opioid prescribing, overdose deaths from pharmaceutical opioids have substantially increased in the United States. Naloxone hydrochloride (naloxone), an opioid antagonist, is the standard of care for treatment of opioid induced respiratory depression. Since 1996, community-based programs have offered overdose prevention education and distributed naloxone for bystander administration to people who use opioids, particularly heroin. There is growing interest in translating overdose education and naloxone distribution (OEND) into conventional medical settings for patients who are prescribed pharmaceutical opioids. For this review, we summarized and classified existing publications on overdose education and naloxone distribution to identify evidence of effectiveness and opportunities for translation into conventional medical settings.
Methods
For this review, we searched English language PubMed for articles on naloxone based on primary data collection from humans, including feasibility studies, program evaluations, surveys, qualitative studies and studies comparing the effectiveness of different routes of naloxone administration. We also included cost-effectiveness studies.
Results
We identified 41 articles that represented 5 categories: evaluations of OEND programs, effects of OEND programs on experiences and attitudes of participants, willingness of medical providers to prescribe naloxone, comparisons of different routes of naloxone administration, and the cost-effectiveness of naloxone.
Conclusions
Existing research suggests that people who are at risk for overdose and other bystanders are willing and able to be trained to prevent overdoses and administer naloxone. Counseling patients about the risks of opioid overdose and prescribing naloxone is an emerging clinical practice which may reduce fatalities from overdose while enhancing the safe prescribing of opioids.
Keywords: overdose, opioids, prevention, primary care
INTRODUCTION
Unintentional poisoning represents a significant, growing problem in the United States.1–5 Drug poisoning fatalities now exceed deaths from motor vehicle crashes.6 In 2010, opioid poisonings accounted for over 16,000 deaths.7 Unintentional poisoning from pharmaceutical opioids has become an epidemic in the last decade, in part due to increasing opioid analgesic availability.8 Overdose education and provision of naloxone is one approach to address this epidemic.
Naloxone is a short-acting opioid antagonist used by medical practitioners to reverse opioid overdose since 1971. In the United States, it is approved by the Food and Drug Administration (FDA) for prescription use.9 Naloxone antagonizes opioid effects by displacing opioid agonists from opioid receptors in the central nervous system, reversing respiratory depression. Naloxone can be administered intranasally (IN), intramuscularly (IM), intravenously (IV), or subcutaneously and is effective against all opioid agonists, including morphine, heroin, oxycodone, and methadone. To reverse long-acting opioids, the dose may need to be repeated. The major adverse effect of naloxone in opioid-dependent patients is precipitated opioid withdrawal. This effect results from the rapid displacement of opioid agonist from the opioid receptor, the same mechanism by which naloxone also reverses respiratory depression. Naloxone has no psychoactive properties, is not a scheduled drug, and has no abuse potential.10
Community-based and public health organizations have developed overdose education and naloxone distribution (OEND) programs to prevent opioid overdose fatalities among people who use heroin, and, more recently, among people who use pharmaceutical opioids. In a survey of OEND programs completed in 2010,188 programs located in 15 states and the District of Colombia provided take-home naloxone to people who used opioids.11 From 1996 to 2010, these programs had trained and distributed naloxone to over 50,000 persons and received reports of over 10,000 overdose reversals.11 Prevention strategies employed by these OEND programs may be applicable to the prevention of pharmaceutical opioid overdose deaths in primary care and specialty medical practices.
Provision of naloxone as a part of a strategy to address opioid overdose has been endorsed by several US Federal agencies.12 In 2013, the Substance Abuse and Mental Health Services Administration released the Opioid Overdose Prevention Toolkit to provide communities and local governments information to develop policies to prevent opioid related deaths.13 Scotland and Wales recently developed national naloxone distribution programs.14 In early 2014, Norway began offering naloxone for the first time in intranasal form.15 Other countries to allow for the distribution of naloxone include Sweden,16 England,17 Germany,18 Italy,19 Canada,20 and Australia.21
Conventional medical settings, such as primary care, pain clinics, emergency departments, and addiction treatment centers are potential venues for overdose education and naloxone prescription. These sites provide opioid prescriptions or medications and patients may present to these sites with complications from opioid use. Our aim was to review and classify existing publications on OEND and naloxone in community-based settings. We sought to identify evidence of effectiveness and opportunities for translation of these practices into conventional medical settings.
METHODS
Search Strategy and Article Selection
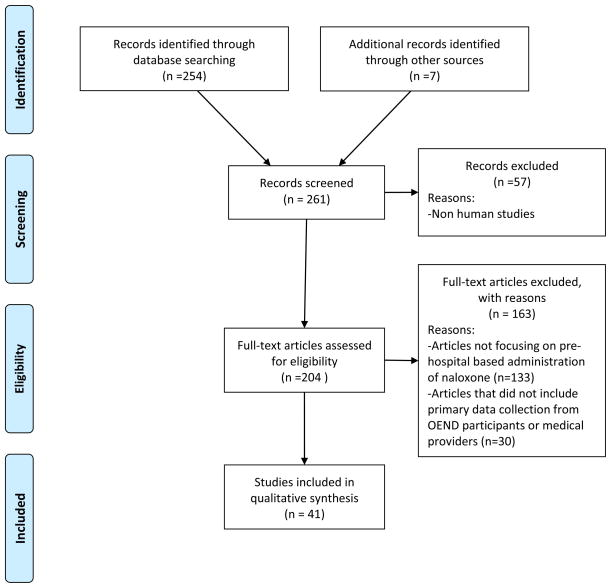
One author searched English language PubMed for peer-reviewed, original research articles through May 2014 using the following Medical Subject Heading (MeSH) terms: naloxone, drug overdose. This search yielded 254 articles. Two authors reviewed the abstracts of the 254 articles and excluded 221 articles because they were non-human studies, studies that did not focus on pre hospital-based administration of naloxone, efficacy studies in controlled settings, commentaries and perspectives, medical news articles, and policy or legal reviews. Based on the aim of our review to inform OEND programming in conventional medical settings, we included original peer-reviewed articles that involved primary data collection from patients or medical providers about OEND programs, including feasibility studies and program evaluations (if they included data collected from participants), surveys and qualitative studies of attitudes towards take-home naloxone, and studies comparing the effectiveness of different routes of naloxone administration in pre- and non-hospital settings. We also included cost-effectiveness studies. We also consulted national content experts and 3 of the authors searched the reference lists of the included articles, producing 7 additional articles which met inclusion criteria. A final consensus was reached by these 3 authors on the 41 articles included in this review. For reporting purposes, we then classified the articles into 5 major topic areas. A PRISMA diagram (Figure 1) summarizes articles that were included in our initial search and were excluded based on our article selection criteria.22
Figure 1.
PRISMA Flowchart of Included and Excluded Studies
Article Abstraction
Two of the authors reviewed each article and recorded the location, the number of participants, the population, the study design, the questions addressed by the article, and a summary of key findings. Given the early stage of research in this area and the heterogeneous methods and outcomes employed, we chose not to apply systematic methods, such as meta-analysis, to summarize outcomes.
RESULTS
We identified 41 articles that met our inclusion criteria (Table 1). After reviewing articles that met inclusion criteria, we categorized the articles into 5 topical categories. Nineteen articles evaluated overdose prevention programs. These studies were largely observational in nature and included evaluations of programming. They also included 4 prospective cohort studies which followed participants over time.18, 23–25 The next set of articles (n=11) evaluated the effects of OEND programs on the experiences and attitudes of participants. These included qualitative (n=4) and survey (n=7) studies. Four articles described willingness of medical providers to prescribe naloxone. Five studies compared routes of naloxone administration in pre-hospital settings. In this category were 4 prospective studies, of which 2 were observed cohorts and 2 were randomized trials. Finally, two studies evaluated the cost-effectiveness of naloxone. The following results summarize our findings.
Table 1.
Articles Included in Review
| Article | Location | N | Population | Study Design | Question Addressed by Study | Summary of Key Findings |
|---|---|---|---|---|---|---|
|
Evaluation of Overdose Education and Naloxone Distribution Programs
| ||||||
| Albert et al., 201138 | North Carolina, USA | N/A | Overdose (OD) deaths in Wilkes County | Program description and evaluation | Determine if the OD death rate decreased over time after 5-strategy community-level overdose prevention program implementation | County OD death rate dropped from 46.6/100,000 to 29/100,000 with program implementation, but this occurred prior to the large rollout in the naloxone rescue kit portion of the intervention |
| Bennett et al., 201126 | Pittsburgh, Pennsylvania, USA | 426 | Needle exchange program participants | Program description and evaluation | Describe the experiences of program participants | 89 individuals reported administering naloxone in response to 249 OD episodes. In cases where naloxone was administered 96% of ODs were reversed. |
| Bennett et al., 201227 | Wales, UK | 525 | 521 opioid users, 4 non-opioid users | Repeated measure design | Determine the effectiveness of naloxone training on knowledge of opiate overdose and the willingness to use naloxone before and after training | Knowledge to recognize an OD, perceived confidence on treating an overdose, and willingness to carry out recommended procedures increased with training; Naloxone was used 28 different times (27 reversals and 1 fatality). |
| Doe-Simkins et al., 200928 | Boston, Massachusetts, USA | 385 | Potential overdose bystanders | Program evaluation | Determine the feasibility of overdose prevention education and distribution intranasal naloxone as a public health intervention | After 15 months, follow up was made with 72% of program participants, with 50 reporting the use of naloxone to reverse an overdose; 74 opioid overdoses were successfully reversed. |
| Dettmer et al., 200118 | Berlin, Germany and Jersey, Channel Islands | 124 | Opiate users attending a mobile health care project | Prospective cohort study | Determine the effectiveness of take-home naloxone in reversing opiate OD | 22 out of 40 who reported back gave naloxone to 29 people, all of whom recovered (Berlin); it was appropriate in 90% of cases and caused abrupt opioid withdrawal in 34%; more risky consumption with availability of naloxone was not reported. In Jersey, 5 reported resuscitations with naloxone were reported, all recovered and no adverse consequences were reported. |
| Enteen et al., 201029 | San Francisco, California, USA | 1942 | People who inject drugs recruited from syringe exchange programs and other community sites | Program monitoring and evaluation | Determines outcomes of naloxone program | 24% of naloxone recipients requested refills; of 399 ODs where naloxone was used, 89% were reversed; 83% attributed the reversal to the naloxone; 13% reported vomiting after administration, 9% reported anger or discomfort, 3 (<1%) reported seizures, and 4 did not survive. |
| Galea et al., 200623 | New York City, NY, USA | 25 | People who inject drugs recruited from a syringe exchange program | Prospective cohort study (3 month follow-up) | Assess a pilot OEND program | 11 of 22 participants who followed up at 3 months reported witnessing 26 ODs: naloxone was administered in 17 of these and all of these 17 live; at 7 witnessed ODs where naloxone was not given, 1 person died and one outcome was unknown. 15 of 20 participants felt comfortable using naloxone. |
| Strang et al., 200824 | South East, South West, Midlands, and Northern Regions of England (UK) | 239 | Opiate users in treatment | Prospective cohort study (3 month follow-up) | Examine the impact of training in OD management and naloxone provision on participant knowledge and confidence and to assess subsequent management of OD | 78% follow up with high retention of physical/behavioral characteristics of OD and actions to be taken at about 3 months; 17 OD occurred in 3 mo.; naloxone used 12 times with successful reversals; 4 of these were dissatisfied with being given naloxone (e.g. angry); When naloxone not used (n=6), 1 death occurred |
| Gaston et al., 200925 | Birmingham and London, UK | 70 | Opioid dependent patients attending detoxification or drug treatment | Prospective cohort study (6 month follow-up) | Assess whether drug users are able to manage opiate overdose through peer interventions, including the administration of naloxone | Of 46 participants interviewed at baseline, 3 months and 6 months, 37 retained the naloxone that was prescribed them at 6 months, and knowledge of overdose recognition and intervention was strong. However, 30 participants did not consistently carry the naloxone with them and would not have it available in the event of an overdose. |
| Heller et al., 200730 | New York City, New York, USA | 1800 | Syringe exchange program participants | Program evaluation | Describe the development of a naloxone distribution program | After 18 months, there were 162 OD reversals reported by 1800 program participants (9% reversal rate) |
| Lankenau et al., 201331 | Los Angeles, California, USA | 30 | OEND participants who use injection drugs | Program evaluation | Evaluate the effectiveness of two OEND programs using closed and open ended interview questions | 30 ODs were witnessed after training, 29 victims recovered, 1 outcome was unknown; naloxone was administered in 17 out of 30 episodes. |
| Leece et al., 201332 | Toronto, Canada | 209 | People who use opioids by any route | Program evaluation | Describe the first OEND program offered by a Canadian public health unit | After 8 months, 209 clients were trained and naloxone was administered in 17 ODs all of whom survived. |
| McAuley et al., 201039 | Scotland | 23 | Drug users, friends and family of drug users | Program evaluation | Assess whether drug users and their friends and family could be trained to manage an OD and administer naloxone safely and effectively | 3 ODs were witnessed, with 2 of the ODs reversed with naloxone. One OD led to death, as the participant did not have naloxone at the time. A majority of participants still had their naloxone after 6 months. |
| Maxwell et al., 200633 | Chicago, Illinois, USA | 319 | Peer overdose reversals | Program evaluation | Describe and assess Chicago Recovery Alliance’s OEND program | Out of >3500 multidose vials of naloxone distributed, 319 reports of peer OD reversals obtained; 1 unsuccessful revival; 5 cases where the victim required 2 injections; 1 case of severe withdrawal symptoms, 1 case of seizures; 1/3rd of the ODs occurred in people who were reinstituting heroin after a period of abstinence. |
| Piper et al., 200734 | New York City, New York, USA | 1004 | Syringe exchange program participants and current or former drug users | Program description | Describe challenges to developing and running a naloxone distribution program and lessons learned | Challenges included political climate, prescription drug laws, recruitment into program, development of training methodologies, program evaluation, and evolution of program response to naloxone. Overcoming barriers to naloxone distribution programs include flexibility during program planning and implementation, developing feasible evaluation tools, and incorporating participant feedback. |
| Seal et al., 200535 | San Francisco, California, USA | 24 (in pairs) | People who injected heroin and had had prior heroin ODs | Prospective cohort study (6 month follow-up) | Determine the safety and feasibility of training people who inject heroin to perform CPR and administer naloxone in heroin ODs | 20 OD events; naloxone was administered in 15; CPR and naloxone given in 6; CPR, 911 call and naloxone in 3 rescue breathing and naloxone in 3; naloxone in 3. The number of heroin ODs experienced by participants similar before and after the intervention. Frequency of heroin injection decreased and 14 participants entered drug treatment in the follow-up period. |
| Walley et al., 201337 | Massachusetts, USA | 1553 | OEND participants taking methadone | Program evaluation | Describe an OEND program enrolling people taking methadone | 92 OD reversals were reported from September 2008 to December 2010 by 62 enrollees who were taking methadone in the past 30 days; OEND is feasible among people who take methadone. |
| Walley et al., 201340 | Massachusetts, USA | 2912 | Opioid users, social service agency staff, family, and friends of opioid users | Interrupted time series analysis | Measure the effect of a state supported OD education and nasal naloxone distribution program on opioid OD deaths | 327 OD reversals were reported; communities with more intensive OEND saturation experienced lower opioid OD death rates. |
| Yokell et al., 201136 | Rhode Island, USA | 160 | Participants of the Preventing Overdose and Naloxone Intervention program | Program description | To describe the OEND program | 10 participants interviewed at follow-up: 5 used OD response training and did not need naloxone. 5 reported successfully administering intramuscular naloxone to reverse an OD. |
|
| ||||||
|
Effects of Overdose Education and Naloxone Distribution on Experiences and Attitudes of Participants
| ||||||
| Baca et al., 200745 | Albuquerque, New Mexico, USA | 101 | Current heroin users | Survey | Describe OD experiences of heroin users, both the ODs they themselves experienced and those witnessed | 65 reported personal OD events, average 4.5 OD per person; 36 never OD’ed; 95 witnessed an average of 7.7 OD events; generally 3 or more people present during 80–95 of the OD events; 100/101 willing to use rescue breathing and naloxone if trained |
| Green et al., 200849 | Baltimore, MD, San Francisco, CA, Chicago, IL, New York City, NY (2 programs), New Mexico, USA | 62 | Trained and untrained current or former opioid users and syringe exchange staff | Survey | Compare OD and naloxone administration knowledge among trained and untrained participants | 45.8% experienced a prior OD, 72% witnessed a prior OD; trained participants recognized more OD scenarios and situations where naloxone was indicated more accurately than untrained participants |
| Kerr et al., 200841 | Melbourne, Australia | 99 | Needle exchange participants | Survey | Assess attitudes to administration of naloxone to others after heroin OD and preferences for administration method | 89% of participants reported positive attitudes about naloxone distribution; 86% would accept naloxone treatment by a peer, and 74% preferred intranasal naloxone |
| Lagu et al., 200643 | Providence, Rhode Island, USA | 329 | Individuals who used heroin or cocaine by injection and non injection routes | Survey | Determine willingness of people who use drugs to administer naloxone and assess fear of calling police at OD | 64% had witnessed an OD; 34.6% had overdosed; 88.5% willing to administer naloxone; 14% afraid to call for help |
| Piper et al., 200846 | New York City, NY, USA | 122 | OEND program participants who requested a refill of naloxone | Survey | Describe the experience of people who used naloxone | Naloxone was administered 82 times; 83% lived; 82% were comfortable using naloxone; 86% would want naloxone administered on them if they were overdosing |
| Sherman et al., 200951 | Baltimore, Maryland, USA | 25 | Program participants who completed the training and had used naloxone to revive an OD victim | Qualitative interviews | Examine the diffusion of information and innovation among participants in the Staying Alive program. | Through peer diffusion of information, participants of the program were able to demonstrate correct responses to an OD and shared their knowledge with others who did not participate in the training. |
| Sherman et al., 200852 | Chicago, Illinois, USA | 31 | Syringe exchange participants who had witnessed an OD in the past 6 months | Qualitative interviews | Determine what informed participants’ choice in OD response. | Naloxone was administered in 58% of the last witnessed ODs. Participants’ fear of legal consequences and a desire to save a life weighed into their decision on whether to administer naloxone and call emergency responders. |
| Tobin et al., 200953 | Baltimore, Maryland, USA | 85 | Participants in the Staying Alive OEND program | Survey | Describe the results of pre- and post-test (6 month) evaluation surveys | 51% witnessed an OD at baseline and follow-up. Pre training 911 call: 22 reversals with naloxone by 19 individuals. After training, inappropriate OD responses decreased and appropriate responses increased. |
| Wagner et al., 201048 | Los Angeles, California, USA | 69 | Program participants who agreed to be enrolled in the study | Survey | Evaluate an OD prevention and response training program at 3 months | 15% overdosed in past 3 mo; 49% witnessed OD in past 3 mo.; 22 participants responded to 35 ODs, 26 recovered, 4 died; response techniques included: staying w/victim (85%), naloxone (80%), rescue breathing (66%), and EMS call (60%) |
| Worthington et al., 200682 | New York City, NY, USA | 13 | Opiate users and individuals who completed the Overdose Prevention and Reversal Program | Focus groups | Describe experiences with naloxone and the program | Participants were supportive of using naloxone to revive an overdosing friend or family member. Barriers to take-home naloxone included difficulty of administering naloxone, fear of withdrawal, and fear of police. |
| Wright et al., 200674 | United Kingdom | 27 | People with a past or current history of drug use, past or current history of homelessness, and experience with heroin OD | Qualitative interviews | To explore the acceptability and risk of peer naloxone use among homeless drug users | Participants preferred naloxone distribution in the context of training. Participants unlikely to use naloxone inappropriately or use more heroin as a result of naloxone for a range of reasons including cost considerations and desire to avoid withdrawal symptoms. |
|
| ||||||
| Willingness of Medical Providers to Prescribe Naloxone | ||||||
|
| ||||||
| Beletsky et al., 200754 | USA | 588 | Nationally representative sample of physicians in the American Medical Association master file | Survey | Physicians knowledge and willingness to prescribe naloxone to people who inject drugs | <1/4 had heard of naloxone prescription as an intervention; the majority of respondents reported they would not consider prescribing it; factors predicting a favorable attitude towards prescribing naloxone included fewer negative perceptions of people who inject drugs |
| Coffin et al., 200355 | New York City, New York, USA | 363 | Random sample of prescription-authorized health care providers in New York City | Survey | Determine willingness to prescribe naloxone to patients at risk of an opioid OD | Willing to prescribe naloxone to patients at risk for OD=Total: 33% yes, 29% unsure; 37% no |
| Green et al., 201356 | Connecticut and Rhode Island, USA | 24 | Emergency department, substance treatment, pain, and generalist providers | Qualitative interviews | Assess providers’ support and concerns regarding take-home naloxone | Providers expressed concerns that naloxone may condone riskier drug use, may not be provided a proper education on how to use naloxone, and may be used as a medical providers’ focal prevention effort |
| Tobin et al., 200547 | Baltimore, Maryland, USA | 176 | Emergency Medical Services providers | Survey | Describe Emergency Medical Service providers attitudes towards take-home naloxone | 56% responded that take-home naloxone training would not be effective in reducing deaths. Concerns included users’ ability to administer naloxone appropriately, promotion of illicit drug use, and disposal of used needles. |
|
| ||||||
| Comparing Routes of Naloxone Administration in Pre-Hospital Settings | ||||||
|
| ||||||
| Barton et al., 200562 | Denver, Colorado, USA | 95 | Adult patients in a prehospital setting with a suspected opiate OD, found down, or with and altered mental status who received intranasal (IN) naloxone | Prospective cohort study | Determine if intranasal naloxone is effective for suspected OD in prehospital settings | 83% of patients who responded to naloxone (n=52) responded to IN and did not require intravenous naloxone |
| Buajordet et al., 200463 | Oslo, Norway | 1192 | Patients who received naloxone by paramedics for heroin OD | Prospective observational study | Determine the frequencies and characteristics of adverse events related to out of hospital administration of naloxone by paramedics over a one year period | Adverse events of naloxone administration included 32% with confusion, 22% with headache, 9% with nausea/vomiting, 8% with aggressiveness and 6% with tachycardia. Serious adverse events from naloxone requiring hospitalization occurred in only three cases (0.3%). |
| Kelly et al., 200559 | Victoria, Australia | 155 | Patients who received naloxone by paramedics | Prospective, randomized, unblinded trial of 2 mg intramuscular naloxone or 2 mg intranasal naloxone (in 5 mL) | Compare the effectiveness of intramuscular and intranasal naloxone | Response to intramuscular naloxone was more rapid than intranasal naloxone but required rescue naloxone was equivalent in both groups; For 74% of patients receiving intranasal naloxone, that intervention alone was sufficient to reverse OD |
| Kerr et al., 200964 | Victoria, Australia | 172 | Patients requiring treatment for suspected opiate OD by ambulance services | Prospective, randomized, unblinded trial of 2 mg intramuscular naloxone or 2 mg intranasal naloxone (in 1 mL) | To assess whether IN naloxone is as effective as IM naloxone in treating suspected opiate ODs. | Mean response time, hospitalizations and minor adverse events were equivalent in patients administered IN or IM naloxone; More patients receiving IN naloxone required a rescue dose. |
| Merlin et al., 201065 | Urban setting, USA | 277 | Patients who received naloxone by paramedics | Retrospective cohort study with chart review | Determine if intranasal naloxone is noninferior to intravenous naloxone | Intranasal naloxone was noninferior to intravenous naloxone at reversing the effects of opioid OD in terms of changes in Glasgow Coma Score and respiratory rate, but 42% of the intranasal naloxone recipients required redosing compared with 20% of the intravenous naloxone recipients. |
|
| ||||||
| Cost-effectiveness | ||||||
|
| ||||||
| Coffin et al., 201366 | USA | N/A | Hypothetical 21 year old US heroin users | Cost effectiveness analysis | Estimate the cost effectiveness of distributing naloxone to comparing distribution of naloxone to 20% of heroin users with no distribution | In probabilistic analysis, 6% of OD deaths were prevented with naloxone distribution, equivalent to one death prevented for every 227 kits distributed. Naloxone distribution increased costs by $53 and quality-adjusted life years by 0.119 |
| Coffin et al., 201367 | Russia | N/A | Hypothetical 18 year old Russian heroin users | Cost effectiveness analysis | Estimate the cost effectiveness of distributing naloxone to comparing distribution of naloxone to 20% of heroin users with no distribution | In probabilistic analysis, 7.6% of OD deaths were prevented with naloxone distribution, equivalent to one death prevented for every 89 kits distributed. Naloxone distribution increased costs by $13 and quality-adjusted life years by 0.137 |
Evaluation of Overdose Education and Naloxone Distribution Programs
Community based organizations and a number of state public health departments began conducting and sponsoring OEND programs in 1996.11 OEND programs typically make naloxone directly available to people who use opioids, outside of a medical setting, and include training on opioid overdose prevention, recognition, and response. The overdose response training includes seeking help from the emergency medical system, rescue breathing, administering naloxone, and staying with the victim until recovery or help arrives.
The articles representing program evaluations of OEND programs in Table 1 suggests that mortality from overdose can be prevented by providing overdose education and naloxone to a variety of participants, including people who used needle exchange programs and injected heroin,18, 23, 26–36 people using pharmaceutical opioids,37, 38 people who use opioids in treatment,24, 25 and the family and friends of people who use drugs.39, 40 These studies demonstrated that OEND trainings improved participants’ knowledge of opioid overdoses and equipped them to administer naloxone safely and effectively when witnessing an overdose. One study suggested that participants reduced their frequency of injecting drugs and were more likely to enter treatment six months after naloxone training compared to baseline.35 In Chicago, overdose deaths were reduced after the introduction of the OEND program.33 An analysis that compared communities in Massachusetts with no OEND implementation to those with low implementation (1–100 people trained per 100,000 population) and high implementation (greater than 100 people trained per 100,000 population), demonstrated 27% and 46% reductions in opioid overdose mortality rates, respectively, after adjusting for community level demographic and substance use factors.40
Effects of OEND Programs on Experiences and Attitudes of Participants
A number of articles support the feasibility of OEND programs. One concern that may inhibit naloxone prescribing is that potential bystanders or witnesses may not wish to intervene in response to an overdose. Several studies confirm that witnesses are willing to take action to revive victims.19, 41–44 One study of people who use heroin showed that nearly every participant was willing to administer naloxone and perform rescue breathing if they had been trained.45 The majority of participants from a needle exchange program who used heroin (92 percent) in an Australian study also reported a willingness to participate in an OEND program. Other studies assessed the willingness of participants to have naloxone used on them in an overdose event, with most participants responding that they would want naloxone to be administered to them in an overdose.46
Because naloxone must be administered by a bystander, concerns that lay bystanders cannot accurately identify an opioid overdose and properly administer naloxone have been raised.47 Several studies suggest that bystanders, including people who use opioids, are capable of recognizing an opioid overdose and administering naloxone.48, 49 In addition to targeting people who use opioids, some OEND programs focus on educating family members and/or bystanders who may witness an opioid overdose.50 An evaluation of six OEND programs concluded that trained participants were more likely to recognize overdose scenarios and identify when naloxone administration was indicated compared to those who had not received training.49 Trained respondents scored similarly to medical experts in accurately recognizing overdose scenarios and identifying instances when naloxone was indicated.49 In a prospective study of overdose training and naloxone provision in 239 people who use opioids, participants had significant improvements in their knowledge of the risk factors for overdose, characteristics of an overdose, and the appropriate actions to reverse a potentially fatal overdose.24 In Massachusetts, where a state sponsored OEND program has been in existence since 2007, methadone maintenance and medically supervised withdrawal (inpatient detoxification) patients have been successfully trained in overdose prevention, equipped with naloxone rescue kits, and rescued people in the community.37 One study investigated the ability of participants to accurately share information about overdose prevention and naloxone administration with their peers and family, finding that they were able to successfully diffuse information from the program to others.51
Naloxone may be particularly beneficial in populations that may avoid or delay calling for emergency services (e.g. 911) when they witness an overdose due to fear of arrest for heroin or opioid analgesic possession, a pre-existing warrant, or because they are afraid of jeopardizing their housing.45, 52 While overdose education typically includes instruction on calling emergency services, trained bystanders may feel more capable to handle an overdose without help from paramedics or medical personnel. A survey of prospective OEND trainees in Baltimore reported that fewer subjects would call for help after naloxone training.53 These concerns may be reduced through legislation and collaboration with law enforcement to shield bystanders from legal consequences when calling 911 or administering naloxone.35
Medical Providers Willingness to Prescribe Naloxone
Prescribers in general medical practice have limited experience regarding naloxone for take-home use and potential misconceptions about naloxone. In one study of 571 physicians conducted from 2002 to 2003, 23% of those surveyed were aware of the option of prescribing take-home naloxone as an intervention to prevent the development of overdose symptoms in people who use injection drugs.54 Most physicians (54%) indicated that they would never consider prescribing naloxone to a patient who injected drugs, suggesting that providers may either be uncomfortable or lack knowledge about providing care for these patients.54 This data was collected before pharmaceutical opioid overdose rates rapidly increased and community programs were well known, and did not assess physicians’ willingness to prescribe naloxone to patients receiving prescription opioids. In another study conducted from 2001 to 2003, one-third of 363 nurse practitioners, physicians, and physician assistants surveyed said they would consider prescribing naloxone.55 In a recent investigation of medical provider attitudes towards prescribing naloxone, providers expressed concerns that naloxone may condone riskier drug use.56
Studies Comparing Routes of Naloxone Administration in Pre-Hospital Settings
The intranasal route of administration is not currently FDA approved, but its safety, convenience, and effectiveness (compared with IM naloxone) has been reported in controlled trials in pre-hospital settings.57–62 IN naloxone is available for off-label use and is the local standard of care in many emergency departments.62 In a study of people who used heroin, researchers reported a preference for IN naloxone administration over naloxone administered by needle injection due to its ease of use, reduced risk of blood-borne viruses, and less pain and risk from needle injection.41
In a study of adverse events after IM and IV naloxone treatment, by paramedics, the most common adverse events in 1,192 overdose episodes were withdrawal-related, including gastrointestinal discomfort, physical aggressiveness, tachycardia, shivering, sweating, tremors, confusion, and restlessness.63 Overall, only 0.3% of patients were hospitalized for adverse events related to the administration of naloxone. Another study of 155 participants administered IM (n=71) or IN (n=84) naloxone involved no major adverse events.59 Other studies have shown that while there is a longer mean response time and an additional dose of naloxone required when using IN naloxone, there were no additional adverse outcomes associated with its use.59, 64, 65
Cost Effectiveness
Two studies, one in the US and one in Russia, estimated the cost-effectiveness of distributing naloxone to people who use heroin and concluded that naloxone distribution is cost-effective.66, 67
DISCUSSION
Existing research suggests that training people who are at risk for overdose and their peers is a feasible and effective way to prevent mortality from overdose. The articles included in this review indicate that people are willing to be trained about the risk factors for an overdose and are capable of responding appropriately when witnessing an overdose. Both IM and IN naloxone have been shown to be effective at reversing an overdose in pre-hospital settings without considerable risks of adverse outcomes.
Some of the issues of implementing OEND programming into wider settings include medical providers’ reluctance to prescribe naloxone. Medical providers may be concerned about bystanders ability to accurately recognize an overdose and administer naloxone,47 the cost of naloxone to patients,11 and condoning riskier drug use.56 Legal concerns may also be part of the reason for low engagement of prescribers in overdose education and naloxone prescription.68 In a legal review of naloxone prescribing, Burris et al. concluded that if medical providers prescribe naloxone to people who use opioids they are doing so in a way that is consistent with state and federal laws regulating drug prescribing and the risks of malpractice are very low.69
Between 2001 and 2013, 24 states and the District of Columbia (DC) enacted laws promoting the accessibility of naloxone in the community through limiting liability for prescribing, possessing, and/or administering naloxone.70 Twenty-one of these states enacted laws promoting the prescription of naloxone to third parties, meaning those who are not themselves at risk for overdose, but may be in such a person’s social network. In the absence of special legislation or standing orders permitting third party prescribing, providing naloxone to people who are not themselves at risk of overdose, but who may be friends or family of people who use opioids might be outside of the prescriber-patient relationship.69
Concerns about police involvement may prevent individuals with criminal justice involvement or using non-prescription opioids from carrying prescribed naloxone with them and/or calling emergency services during an overdose.25, 31 Further regulatory or legislative action and community education/outreach to inform the public about their protections related to calling emergency services or administering naloxone may be necessary.71 States increasingly recognize the importance of bystanders’ responding to overdose and are providing some immunity from arrest and/or prosecution for drug possession crimes and/or liability protection for administering naloxone.69 Twenty-one states and the District of Columbia have enacted “Good Samaritan” provisions providing some protection from prosecution for people who provide help at the scene of an overdose.70
The potential absence of medical personnel at naloxone reversals has led some to express concern that individuals who have been revived from overdose outside of a medical setting have less opportunity to enter substance use treatment.72 Advocates for naloxone distribution respond that it is an intervention that prevents death and allows for future possibility of recovery.73 One study suggested education may promote treatment entry35 Further work is needed about whether OEND or administration of naloxone increases treatment admissions for the individual trained or the person who overdosed.35 Another common concern is that people may use larger doses of opioids, believing they can be rescued from an overdose but this is unlikely because of the unpleasant effects of naloxone on opioid dependent individuals, who rapidly experience symptoms of withdrawal with naloxone administration.74
Implications for Medical Practice
In 2012, the American Medical Association and Massachusetts Medical Society issued endorsements of OEND programs.75 Recently, OEND programs have expanded access to naloxone in many states, but a number of states with high drug overdose death rates remain without OEND programs.11 Furthermore, OEND programs were originally established to address overdose people who inject heroin, but many others are at risk, including people who take pharmaceutical opioids for pain. Additional risk groups have since been proposed as potential targets of overdose education and naloxone distribution (see Table 2).
Table 2.
Characteristics of Patients at Risk for Overdose
|
While not addressed in the studies identified by this review, rising rates of pharmaceutical opioid use and overdose require novel prevention approaches to reduce risk. These approaches could include co-prescription of naloxone with opioids, insurance reimbursement for take-home naloxone, pharmacy dispensing of naloxone without a prescription, and over-the-counter naloxone distribution.13 More broadly, these interventions could be considered within the context of other opioid safety efforts, such as safe disposal of excess opioids,38 prescription drug monitoring programs,76 risk evaluation and mitigation strategies (REMS),77 and abuse-deterrent medications.78 New administration devices, such as Evzio, an auto-injector device, which was fast-tracked for approval by the FDA because of the severity of the opioid overdose epidemic, should be evaluated further for its effectiveness in pre-hospital settings and its limitations, such as cost and availability. 79
Opioid prescribers have a responsibility to assess the overdose risk in their patients and educate them about potential adverse events, including overdose.80 Physicians have an opportunity to apply their clinical assessment skills to identify patients as candidates for overdose education and naloxone prescription based on known risk factors for overdose. A thorough clinical history would include asking patients about a history of prior overdose, chronic medical illness (pulmonary, renal or hepatic disease), drug use, incarceration history, and use of other sedating medications. Key elements of counseling patients may include not taking more milligrams or more frequently than prescribed, self-monitoring of functional status while on opioids, and letting others in one’s family or social network know about the risks of overdose and what to do in the event of an overdose (e.g. calling 911). Prescribers should consider advising patients to secure opioids and other sedating medications, such as benzodiazepines, by keeping them locked up in the home to avoid diversion and to avoid sharing medications.80
For patients with overdose risk, medical providers should prepare patients with instructions to follow in the event of an overdose. Prescribing take-home naloxone could be part of this preparation. The prescribing of naloxone should not be seen as a discrete event, but as part of an ongoing process that includes patient education, monitoring, and opioid dose adjustment.81 Because patients who have been prescribed naloxone are unable to use the drug on themselves, their peers and family members must be involved in overdose education and management training.73
Barriers to prescribing naloxone may need to be overcome through efforts by physicians, pharmacists, policy-makers, patient advocates and health care systems. Pharmacies should consider stocking naloxone, intramuscular needles or nasal atomizers, and educational materials on administration. Patients may have to pay out-of-pocket for naloxone until insurance companies and public payers (e.g. Medicaid) cover naloxone, administration devices, and associated counseling/education costs. The Appendix includes several web resources produced by a variety of community-based OEND programs, government agencies, researchers, and activists which currently aim to educate medical providers about their patients’ risk of opioid overdose and provide information about prescribing naloxone. This list is not intended to be all inclusive or exhaustive, but provides a sample of resources available for medical providers interested in prescribing naloxone.
Gaps Identified and Further Research Needs
Based on current available evidence, prescribers should consider providing overdose education and naloxone in medical practice. Further study of barriers and facilitators to OEND in conventional clinical settings with more diverse populations of people at risk for overdose is needed. Future research should investigate how to select patients for naloxone prescription, how to engage patients and potential bystanders in overdose education and management training, the optimal breadth and depth of overdose education, the proper roles for different healthcare team members in disseminating OEND, the safety of take-home naloxone across a broad range of patient characteristics, and the reach and effectiveness of overdose education and naloxone prescription in traditional health care settings. These issues are particularly important since OEND programs may not meet the needs of all people who use pharmaceutical opioids due to the limited geographic availability of OEND programs, stigma against accessing community-based OEND programs, which have traditionally served people who use heroin and people who inject drugs, and costs of naloxone and related counseling or educational services. Access through traditional medical and pharmacy settings may offer some advantages including scale and insurance coverage. At the same time, clinical settings may not offer the degree of training or sensitivity to the needs of populations at risk demonstrated in dedicated community based programs. Additionally, more research should be conducted to understand what may be limiting medical providers’ willingness to prescribe naloxone. Finally, more research using empirical data is needed to examine the cost-effectiveness of providing naloxone to patients treated with pharmaceutical opioids. While overdose education and naloxone distribution may be a key component of a public health effort to reduce opioid overdose deaths, our findings suggests further research is needed on the role of naloxone in conventional medical practice. Medical providers are in an ideal position to prescribe take-home naloxone to reduce mortality for opioid overdose amongst their patients.14 Data from observational, health services, and randomized controlled trials could further inform physician practice and establish a new standard of care, with regards to naloxone prescription to patients receiving opioids in medical practice settings.
Acknowledgments
We wish to acknowledge the Harm Reduction Action Center, Lisa Raville, Jane Kennedy, DO, Edward M. Gardner, MD, and Steve Koester, PhD for their assistance and thoughtful contributions.
FUNDING
Work on this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R34DA035952 and R21DA31041. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
SR Mueller, IA Binswanger, and AY Walley conceived of the review. SR Mueller, IA Binswanger, AY Walley and JM Glanz formulated the methods for the review. SR Mueller conducted the search of the literature. SR Mueller, IA Binswanger, AY Walley and SL Calcaterra reviewed the articles. All authors interpreted the review findings. SR Mueller drafted the manuscript. All authors reviewed and provided critical revisions to the manuscript. All authors give final approval for publication.
The authors declare that they have no conflicts of interest.
References
- 1.Paulozzi LJ. Drug-induced deaths - united states, 2003–2007. MMWR Surveill Summ. 2011 Jan 14;60( Suppl):60–61. [PubMed] [Google Scholar]
- 2.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010 Nov 18;363(21):1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the united states. Pharmacoepidemiol Drug Saf. 2006 Sep;15(9):618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 4.Vital signs: Risk for overdose from methadone used for pain relief - united states, 1999–2010. MMWR Morb Mortal Wkly Rep. 2012 Jul 6;61:493–497. [PubMed] [Google Scholar]
- 5.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend. 2013 Jan 4;131(3):263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. Drug poisoning deaths in the united states, 1980–2008. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- 7.Jones C, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, united states, 2010. JAMA. 2013;309(7):657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Vital signs: Overdoses of prescription opioid pain relievers -- united states, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011 Nov 4;60:1487–1492. [PubMed] [Google Scholar]
- 9.Campana M. Overdose mortality and naloxone distribution in italy. Preventing Heroin Overdose: Pragmatic Approaches. 2000 [Google Scholar]
- 10.Darke S, Hall W. The distribution of naloxone to heroin users. Addiction. 1997 Sep;92(9):1195–1199. [PubMed] [Google Scholar]
- 11.Community-based opioid overdose prevention programs providing naloxone - united states, 2010. MMWR Morb Mortal Wkly Rep. 2012 Feb 17;61(6):101–105. [PMC free article] [PubMed] [Google Scholar]
- 12.Throckmorton DC, Compton WM, Lurie P. Management of opioid analgesic overdose. N Engl J Med. 2012 Oct 4;367(14):1371. doi: 10.1056/NEJMc1209707. author reply 1372–1373. [DOI] [PubMed] [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration; Substance Abuse and Mental Health Services Administration, editor. Samhsa opioid overdose prevention toolkit. Hhs publication no. (sma) 13–4742. Rockville MD: 2013. [Google Scholar]
- 14.Matheson C, Pflanz-Sinclair C, Aucott L, et al. Reducing drug related deaths: A pre-implementation assessment of knowledge, barriers and enablers for naloxone distribution through general practice. BMC Fam Pract. 2014;15(1):12. doi: 10.1186/1471-2296-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen A. Norway tries naloxone in spray form to prevent deaths from drug overdose. BMJ. 2014;348:g1686. doi: 10.1136/bmj.g1686. [DOI] [PubMed] [Google Scholar]
- 16.Håkansson A, Vedin A, Wallin C, AHK distribution of naloxone to prevent death from heroin overdose. Study of opioid dependent patients’ attitudes to be part of the antidote program. Lakartidningen. 2013 Jul-Aug;110(29–31):1340–2. [PubMed] [Google Scholar]
- 17.Strang J, Kelleher M, Best D, Mayet S, Manning V. Emergency naloxone for heroin overdose. BMJ. 2006 Sep 23;333(7569):614–615. doi: 10.1136/bmj.333.7569.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettmer K, Saunders B, Strang J. Take home naloxone and the prevention of deaths from opiate overdose: Two pilot schemes. BMJ. 2001 Apr 14;322(7291):895–896. doi: 10.1136/bmj.322.7291.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seal KH, Downing M, Kral AH, et al. Attitudes about prescribing take-home naloxone to injection drug users for the management of heroin overdose: A survey of street-recruited injectors in the san francisco bay area. J Urban Health. 2003 Jun;80(2):291–301. doi: 10.1093/jurban/jtg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzemis D, Al-Qutub D, Amlani A, Kesselring S, Buxton JA. A quantitative and qualitative evaluation of the british columbia take home naloxone program. CMAJ Open. 2014 Jul;2(3):E153–161. doi: 10.9778/cmajo.20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenton S, Dietze P, Olsen A, Wiggins N, McDonald D, Fowlie C. Working together: Expanding the availability of naloxone for peer administration to prevent opioid overdose deaths in the australian capital territory and beyond. Drug Alcohol Rev. 2014 Oct 1; doi: 10.1111/dar.12198. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009 Aug 18;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 23.Galea S, Worthington N, Piper TM, Nandi VV, Curtis M, Rosenthal DM. Provision of naloxone to injection drug users as an overdose prevention strategy: Early evidence from a pilot study in new york city. Addict Behav. 2006 May;31(5):907–912. doi: 10.1016/j.addbeh.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Strang J, Manning V, Mayet S, et al. Overdose training and take-home naloxone for opiate users: Prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction. 2008 Oct;103(10):1648–1657. doi: 10.1111/j.1360-0443.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- 25.Gaston RL, Best D, Manning V, Day E. Can we prevent drug related deaths by training opioid users to recognise and manage overdoses? Harm Reduct J. 2009;6:26. doi: 10.1186/1477-7517-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett AS, Bell A, Tomedi L, Hulsey EG, Kral AH. Characteristics of an overdose prevention, response, and naloxone distribution program in pittsburgh and allegheny county, pennsylvania. J Urban Health. 2011 Dec;88(6):1020–1030. doi: 10.1007/s11524-011-9600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett T, Holloway K. The impact of take-home naloxone distribution and training on opiate overdose knowledge and response: An evaluation of the thn project in wales. Drugs-Education Prevention and Policy. 2012;19(4):320–328. [Google Scholar]
- 28.Doe-Simkins M, Walley AY, Epstein A, Moyer P. Saved by the nose: Bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health. 2009 May;99(5):788–791. doi: 10.2105/AJPH.2008.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enteen L, Bauer J, McLean R, et al. Overdose prevention and naloxone prescription for opioid users in san francisco. J Urban Health. 2010 Dec;87(6):931–941. doi: 10.1007/s11524-010-9495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller DI, Stancliff S. Providing naloxone to substance users for secondary administration to reduce overdose mortality in new york city. Public Health Rep. 2007 May-Jun;122(3):393–397. doi: 10.1177/003335490712200313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lankenau SE, Wagner KD, Silva K, et al. Injection drug users trained by overdose prevention programs: Responses to witnessed overdoses. J Community Health. 2013 Feb;38(1):133–141. doi: 10.1007/s10900-012-9591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leece PN, Hopkins S, Marshall C, Orkin A, Gassanov MA, Shahin RM. Development and implementation of an opioid overdose prevention and response program in toronto, ontario. Can J Public Health. 2013 May-Jun;104(3):e200–204. doi: 10.17269/cjph.104.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: A program to reduce heroin overdose deaths. J Addict Dis. 2006;25(3):89–96. doi: 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- 34.Piper TM, Rudenstine S, Stancliff S, et al. Overdose prevention for injection drug users: Lessons learned from naloxone training and distribution programs in new york city. Harm Reduct J. 2007;4:3. doi: 10.1186/1477-7517-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seal KH, Thawley R, Gee L, et al. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: A pilot intervention study. J Urban Health. 2005 Jun;82(2):303–311. doi: 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokell MA, Green TC, Bowman S, McKenzie M, Rich JD. Opioid overdose prevention and naloxone distribution in rhode island. Med Health R I. 2011 Aug;94(8):240–242. [PMC free article] [PubMed] [Google Scholar]
- 37.Walley AY, Doe-Simkins M, Quinn E, Pierce C, Xuan Z, Ozonoff A. Opioid overdose prevention with intranasal naloxone among people who take methadone. J Subst Abuse Treat. 2013 Feb;44(2):241–247. doi: 10.1016/j.jsat.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Albert S, Brason FW, 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B. Project lazarus: Community-based overdose prevention in rural north carolina. Pain Med. 2011 Jun;12( Suppl 2):S77–85. doi: 10.1111/j.1526-4637.2011.01128.x. [DOI] [PubMed] [Google Scholar]
- 39.McAuley A, Lindsay G, Woods M, Louttit D. Responsible management and use of a personal take-home naloxone supply: A pilot project. Drugs (Abingdon Engl) 2010;17(4):388–399. [Google Scholar]
- 40.Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in massachusetts: Interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr D, Dietze P, Kelly AM, Jolley D. Attitudes of australian heroin users to peer distribution of naloxone for heroin overdose: Perspectives on intranasal administration. J Urban Health. 2008 May;85(3):352–360. doi: 10.1007/s11524-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakeman SE, Bowman SE, McKenzie M, Jeronimo A, Rich JD. Preventing death among the recently incarcerated: An argument for naloxone prescription before release. J Addict Dis. 2009;28(2):124–129. doi: 10.1080/10550880902772423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagu T, Anderson BJ, Stein M. Overdoses among friends: Drug users are willing to administer naloxone to others. J Subst Abuse Treat. 2006 Mar;30(2):129–133. doi: 10.1016/j.jsat.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Strang J, Powis B, Best D, et al. Preventing opiate overdose fatalities with take-home naloxone: Pre-launch study of possible impact and acceptability. Addiction. 1999 Feb;94(2):199–204. doi: 10.1046/j.1360-0443.1999.9421993.x. [DOI] [PubMed] [Google Scholar]
- 45.Baca CT, Grant KJ. What heroin users tell us about overdose. J Addict Dis. 2007;26(4):63–68. doi: 10.1300/J069v26n04_08. [DOI] [PubMed] [Google Scholar]
- 46.Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in new york city. Subst Use Misuse. 2008;43(7):858–870. doi: 10.1080/10826080701801261. [DOI] [PubMed] [Google Scholar]
- 47.Tobin KE, Gaasch WR, Clarke C, MacKenzie E, Latkin CA. Attitudes of emergency medical service providers towards naloxone distribution programs. J Urban Health. 2005 Jun;82(2):296–302. doi: 10.1093/jurban/jti052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner KD, Valente TW, Casanova M, et al. Evaluation of an overdose prevention and response training programme for injection drug users in the skid row area of los angeles, ca. Int J Drug Policy. 2010 May;21(3):186–193. doi: 10.1016/j.drugpo.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: An evaluation of six overdose training and naloxone distribution programs in the united states. Addiction. 2008 Jun;103(6):979–989. doi: 10.1111/j.1360-0443.2008.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, Irwin KS, Khoshnood K. Expanded access to naloxone: Options for critical response to the epidemic of opioid overdose mortality. Am J Public Health. 2009 Mar;99(3):402–407. doi: 10.2105/AJPH.2008.136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman SG, Gann DS, Tobin KE, Latkin CA, Welsh C, Bielenson P. The life they save may be mine”: Diffusion of overdose prevention information from a city sponsored programme. Int J Drug Policy. 2009 Mar;20(2):137–142. doi: 10.1016/j.drugpo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Sherman SG, Gann DS, Scott G, Carlberg S, Bigg D, Heimer R. A qualitative study of overdose responses among chicago idus. Harm Reduct J. 2008;5:2. doi: 10.1186/1477-7517-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobin KE, Sherman SG, Beilenson P, Welsh C, Latkin CA. Evaluation of the staying alive programme: Training injection drug users to properly administer naloxone and save lives. Int J Drug Policy. 2009 Mar;20(2):131–136. doi: 10.1016/j.drugpo.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Beletsky L, Ruthazer R, Macalino GE, Rich JD, Tan L, Burris S. Physicians’ knowledge of and willingness to prescribe naloxone to reverse accidental opiate overdose: Challenges and opportunities. J Urban Health. 2007 Jan;84(1):126–136. doi: 10.1007/s11524-006-9120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coffin PO, Fuller C, Vadnai L, Blaney S, Galea S, Vlahov D. Preliminary evidence of health care provider support for naloxone prescription as overdose fatality prevention strategy in new york city. J Urban Health. 2003 Jun;80(2):288–290. doi: 10.1093/jurban/jtg031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green TC, Bowman SE, Zaller ND, Ray M, Case P, Heimer R. Barriers to medical provider support for prescription naloxone as overdose antidote for lay responders. Subst Use Misuse. 2013 May;48(7):558–567. doi: 10.3109/10826084.2013.787099. [DOI] [PubMed] [Google Scholar]
- 57.Loimer N, Hofmann P, Chaudhry HR. Nasal administration of naloxone is as effective as the intravenous route in opiate addicts. Int J Addict. 1994 Apr;29(6):819–827. doi: 10.3109/10826089409047912. [DOI] [PubMed] [Google Scholar]
- 58.Leavitt SB. Intranasal naloxone for at-home opioid rescue. Pract Pain Manag. 2010 Oct;42:46. [Google Scholar]
- 59.Kelly AM, Kerr D, Dietze P, Patrick I, Walker T, Koutsogiannis Z. Randomised trial of intranasal versus intramuscular naloxone in prehospital treatment for suspected opioid overdose. Med J Aust. 2005 Jan 3;182(1):24–27. doi: 10.5694/j.1326-5377.2005.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 60.Kerr D, Dietze P, Kelly AM. Intranasal naloxone for the treatment of suspected heroin overdose. Addiction. 2008 Mar;103(3):379–386. doi: 10.1111/j.1360-0443.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- 61.Wermeling DP. Opioid harm reduction strategies: Focus on expanded access to intranasal naloxone. Pharmacotherapy. 2010 Jul;30(7):627–631. doi: 10.1592/phco.30.7.627. [DOI] [PubMed] [Google Scholar]
- 62.Barton ED, Colwell CB, Wolfe T, et al. Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the prehospital setting. J Emerg Med. 2005 Oct;29(3):265–271. doi: 10.1016/j.jemermed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Buajordet I, Naess AC, Jacobsen D, Brors O. Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur J Emerg Med. 2004 Feb;11(1):19–23. doi: 10.1097/00063110-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Kerr D, Kelly AM, Dietze P, Jolley D, Barger B. Randomized controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose. Addiction. 2009 Dec;104(12):2067–2074. doi: 10.1111/j.1360-0443.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- 65.Merlin MA, Saybolt M, Kapitanyan R, et al. Intranasal naloxone delivery is an alternative to intravenous naloxone for opioid overdoses. Am J Emerg Med. 2010 Mar;28(3):296–303. doi: 10.1016/j.ajem.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013 Jan 1;158(1):1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- 67.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal in russian cities. J Med Econ. 2013 Aug;16(8):1051–1060. doi: 10.3111/13696998.2013.811080. [DOI] [PubMed] [Google Scholar]
- 68.Advisory Council on the Misuse of Drugs. Consideration of naloxone. London: England: 2012. [Google Scholar]
- 69.Burris S, Beletsky L, Castagna C, Coyle C, Crowe C, McLaughlin J. Stopping an invisible epidemic: Legal issues in the provision of naloxone to prevent opioid overdose. Drexel L Rev. 2009;1:273. [Google Scholar]
- 70.Davis C, Chang S. Legal interventions to reduce overdose mortality: Naloxone access and overdose good samaritan laws. The Network for Public Health Law, Robert Wood Johnson Foundation; 2014. [Google Scholar]
- 71.Davis C, Webb D, Burris S. Changing law from barrier to facilitator of opioid overdose prevention. J Law Med Ethics. 2013 Mar;41( Suppl 1):33–36. doi: 10.1111/jlme.12035. [DOI] [PubMed] [Google Scholar]
- 72.Bazazi AR, Zaller ND, Fu JJ, Rich JD. Preventing opiate overdose deaths: Examining objections to take-home naloxone. J Health Care Poor Underserved. 2010 Nov;21(4):1108–1113. doi: 10.1353/hpu.2010.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dasgupta N, Sanford C, Albert S, Brason FW., II Opioid drug overdoses: A prescription for harm and potential for prevention. Am J Lifestyle Med. 2010;4(1):32–37. [Google Scholar]
- 74.Wright N, Oldham N, Francis K, Jones L. Homeless drug users’ awareness and risk perception of peer “take home naloxone” use--a qualitative study. Subst Abuse Treat Prev Policy. 2006;1:28. doi: 10.1186/1747-597X-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medical Student Section. Promoting prevention of fatal opioid overdose. Delegates. American Medical Association House of Delegates. 2012;503(A-12):79. [Google Scholar]
- 76.Green TC, Zaller N, Rich J, Bowman S, Friedmann P. Revisiting paulozzi et al. ’S “prescription drug monitoring programs and death rates from drug overdose”. Pain Med. 2011 Jun;12(6):982–985. doi: 10.1111/j.1526-4637.2011.01136.x. [DOI] [PubMed] [Google Scholar]
- 77.US Food and Drug Administration. Guidance for industry-format and content of proposed risk evaluation and mitigation strategies (rems), rems assessments, and proposed rems modifications. 2009. [Google Scholar]
- 78.Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of oxycontin. N Engl J Med. 2012 Jul 12;367(2):187–189. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- 79.Knopf A. Naloxone auto-injector gains fda approval. Behav Healthc. 2014 May-Jun;34(3):48–49. [PubMed] [Google Scholar]
- 80.Beletsky L, Rich JD, Walley AY. Prevention of fatal opioid overdose. JAMA. 2012 Nov 14;308(18):1863–1864. doi: 10.1001/jama.2012.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.George S, Moreira K. A guide for clinicians on” take home” naloxone prescribing. Addict Disord Their Treat. 2008;7(3):163. [Google Scholar]
- 82.Worthington N, Markham Piper T, Galea S, Rosenthal D. Opiate users’ knowledge about overdose prevention and naloxone in new york city: A focus group study. Harm Reduct J. 2006;3:19. doi: 10.1186/1477-7517-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Substance Abuse and Mental Health Services Administration OAS. Drug abuse warning network, 2003: Area profiles of drug-related mortality. United States Department of Health and Human Services; Rockville, MD: 2005. [Google Scholar]
- 84.Coffin PO, Tracy M, Bucciarelli A, Ompad D, Vlahov D, Galea S. Identifying injection drug users at risk of nonfatal overdose. Acad Emerg Med. 2007 Jul;14(7):616–623. doi: 10.1197/j.aem.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Darke S, Hall W. Heroin overdose: Research and evidence-based intervention. J Urban Health. 2003 Jun;80(2):189–200. doi: 10.1093/jurban/jtg022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sporer KA. Acute heroin overdose. Ann Intern Med. 1999 Apr 6;130(7):584–590. doi: 10.7326/0003-4819-130-7-199904060-00019. [DOI] [PubMed] [Google Scholar]
- 87.Darke S, Mills KL, Ross J, Teesson M. Rates and correlates of mortality amongst heroin users: Findings from the australian treatment outcome study (atos), 2001–2009. Drug Alcohol Depend. 2011 Jun 1;115(3):190–195. doi: 10.1016/j.drugalcdep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 88.Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: Implications for overdose prevention. Drug Alcohol Depend. 2012 Jan 1;120(1–3):168–173. doi: 10.1016/j.drugalcdep.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: Follow up study. BMJ. 2003 May 3;326(7396):959–960. doi: 10.1136/bmj.326.7396.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007 Jan 11;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Binswanger IA, Nowels C, Corsi KF, et al. Return to drug use and overdose after release from prison: A qualitative study of risk and protective factors. Addict Sci Clin Pract. 2012;7(1):3. doi: 10.1186/1940-0640-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011 Apr 6;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 93.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010 Jan 19;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Evans JL, Tsui JI, Hahn JA, Davidson PJ, Lum PJ, Page K. Mortality among young injection drug users in san francisco: A 10-year follow-up of the ufo study. Am J Epidemiol. 2012 Feb 15;175(4):302–308. doi: 10.1093/aje/kwr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008 Dec 10;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 96.Green TC, Grau LE, Carver HW, Kinzly M, Heimer R. Epidemiologic trends and geographic patterns of fatal opioid intoxications in connecticut, USA: 1997–2007. Drug Alcohol Depend. 2011 Jun 1;115(3):221–228. doi: 10.1016/j.drugalcdep.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warner-Smith M, Darke S, Lynskey M, Hall W. Heroin overdose: Causes and consequences. Addiction. 2001 Aug;96(8):1113–1125. doi: 10.1046/j.1360-0443.2001.96811135.x. [DOI] [PubMed] [Google Scholar]
- 98.Toblin RL, Paulozzi LJ, Logan JE, Hall AJ, Kaplan JA. Mental illness and psychotropic drug use among prescription drug overdose deaths: A medical examiner chart review. J Clin Psychiatry. 2010 Apr;71(4):491–496. doi: 10.4088/JCP.09m05567blu. [DOI] [PubMed] [Google Scholar]
- 99.Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000 May;95(5):687–695. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- 100.Havens JR, Oser CB, Knudsen HK, et al. Individual and network factors associated with non-fatal overdose among rural appalachian drug users. Drug Alcohol Depend. 2011 May 1;115(1–2):107–112. doi: 10.1016/j.drugalcdep.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan GM, Stajic M, Marker EK, Hoffman RS, Nelson LS. Testing positive for methadone and either a tricyclic antidepressant or a benzodiazepine is associated with an accidental overdose death: Analysis of medical examiner data. Acad Emerg Med. 2006 May;13(5):543–547. doi: 10.1197/j.aem.2005.12.011. [DOI] [PubMed] [Google Scholar]