Abstract
The beginning of the millennium saw the discovery of a new bone-matrix protein, Matrix Extracellular PhosphogloprotEin (MEPE) and an associated C-terminal motif called ASARM. This motif and other distinguishing features occur in a group of proteins called SIBLINGs. These proteins include dentin matrix protein 1 (DMP1), osteopontin, dentin-sialophosphoprotein (DSPP), statherin, bone sialoprotein (BSP) and MEPE. MEPE, DMP1 and ASARM-motifs regulate expression of a phosphate regulating cytokine FGF23. Further, a trimeric interaction between phosphate regulating endopeptidase homolog X-linked (PHEX), DMP1, and α5β3-integrin that occurs on the plasma-membrane of the osteocyte mediates FGF23 regulation (FAP pathway). ASARM-peptides competitively inhibit the trimeric complex and increase FGF23. A second pathway involves specialized structures, matrix vesicles (MVP pathway). This review will discuss the FAP and MVP pathways and present a unified model for mineral and energy metabolism.
Introduction
The endoskeleton is not just a lifeless frame designed to defeat gravity it is also a complex endocrine gland. Throughout life the hidden artisanal mining of the osteoblast and osteoclast constantly remodel this exquisite mineral structure. Hidden deep within mineral caves and communicating through a network of dendritic tunnels, a third cell-type the osteocyte regulates a continuing process of bone formation (osteoblasts) and resorption (osteoclasts). Unlike any other cell, the osteocyte, related to the osteoblast survives entombed within the hydroxyapatite matrix for decades [1]. Recent discoveries have revealed an emerging cornucopia of growth factors, hormones, matrix molecules and neuronal outputs that interact with these cells [2]. These factors are also responsible for centrally and peripherally regulating energy metabolism and mineral balance in the skeleton, kidney, muscle and gut. The roles of these molecules has emerged by studying the effects caused by mutations in humans and in rodent models. At times, comparing rodents and humans has proved to be difficult. This is perhaps not surprising given the difference in size, life span and basal metabolism of humans and mice. The size difference for example heightens the structural design needed to oppose gravity and this indirectly impacts energy and mineral metabolism. Thus, when using rodent models to assess the usefulness of human drug targets, species differences may sometimes confound interpretation. Despite these difficulties, murine models have undoubtedly provided powerful tools for the study of bone-mineral metabolism [3]. Also, unraveling the evolutionary history of the skeleton and kidney from marine to freshwater to terrestrial environments is helping to close the gaps [4-6]. It is clear the ancient evolutionary paths of bone and kidney have remarkable associations. This review discusses and provides evidence for: (1). FAP and MVP pathways and the key players (PHEX, FGF23, DMP1, MEPE, ASARM, α5β3-inegrin, family with sequence similarity 20 member C kinase (FAM20C), tissue non-specific alkaline phosphatase (TNAP), and ectonucleotide phosphodiesterase pyrophosphatase (ENPP1)); (2). The mechanism (s) linking both MVP and FAP pathways; and (3). A unified model for mineral and energy metabolism incorporating both pathways. An understanding of these pathways will increase our knowledge and potential treatments for inherited forms of hypophosphatemic bone mineral loss disorders, chronic and end stage kidney diseases, cardiovascular soft tissue calcification diseases, obesity and diabetes.
The FGF23, PHEX, DMP1, ASARM and α5β3 integrin (FAP) Pathway
A detailed review describing this pathway has been published [4]. The following discussion therefore summarizes the pathway and presents new findings. Matrix Extracellular PhosphoglycoprotEin (MEPE) was cloned in 2000 from the resected intracranial tumor of a patient suffering with tumor induced osteomalcia (OHO) [7]. Patients with OHO present with pathophysiologies that overlap with X-linked (HYP) and autosomal forms of hypophosphatemic rickets (ARH) [4]. Later research showed MEPE is a substrate and ligand for PHEX, a Zn metalloendopeptidase that when mutated results in X-linked hypophosphatemic rickets [4]. The earlier MEPE paper also characterized a conserved MEPE C-terminal ASARM motif (Acidic Serine Aspartate Rich MEPE Associated Motif). This motif with other distinguishing features occurs in a group of proteins now classed as a single family called SIBLINGs (Short Integrin Binding Ligand Interacting Glycoproteins) [4,7]. These proteins include DMP1, Osteopontin, DSPP, Statherin, BSP and MEPE. MEPE, DMP1 and associated ASARM-motifs regulate expression of a preeminent phosphate regulating cytokine FGF23 [4,8-10]. Further, a trimeric interaction between PHEX, DMP1, and α5β3 integrin that occurs on the plasma membrane of the osteocyte mediates FGF23 regulation [4]. ASARM-peptides competitively inhibit the trimeric complex and increase FGF23 expression. ASARM-peptides and motifs (MEPE, DMP1 and osteopontin derived) are the only known biological substrates and or ligands for PHEX [4,8-14]. Compelling evidence suggests the ratio of ASARM-peptide to SIBLING-protein plays a role in regulating the mineral matrix and FGF23 production that then moderates systemic phosphate and vitamin-D metabolism [9,15-18]. ASARM-peptides are also responsible for the mineralization defect and component to the hypophosphatemia in HYP and ARHR [4,8-10,13,14,19-21]. Recent in vivo and in vitro experiments using a bio-engineered synthetic PHEX related peptide (SPR4; 4.2 kDa) that sequesters DMP1 and MEPE ASARM-motifs delivered additional support [10,13,14,22]. Administration of SPR4-peptide to wild type and HYP mice validated the ASARM-model and provided a new promising treatment strategy. Strikingly, this peptide suppresses bone, renal and serum sclerostin (SOST), increases active β-catenin and corrects energy metabolism defects in the HYP mouse. Figure 1 illustrates the FAP pathway and both in vitro and in vivo pharmacologic effects of SPR4-peptide have been reported [10,13,14].
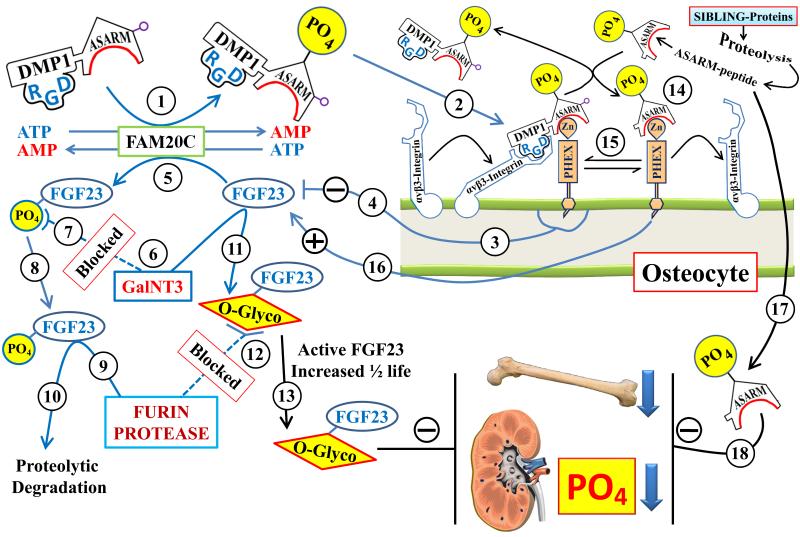
Figure 1.
Scheme illustrating the ASARM-model and the FAM20C kinase link to the FAP pathway: The numbers highlighted in the circles refer to the explanations in the following text. The interactions depicted on the osteocyte cell-surface between DMP1, PHEX, integrin and ASARM-peptides are dynamic and competitive occurring on the extracellular cell-surface. Arrows linking other factors represented in the cartoon illustrate positive and negative effector relationships (paracrine, autocrine, allosteric, signal transduction or gene expression): (1) FAM20C kinase phosphorylates the DMP1 C-terminal ASARM-motif; (2) Phosphorylation of the DMP1-ASARM motif is required for binding to PHEX and the RGD motif of DMP1 binds to α5β3 integrin to form a [PHEX-DMP1-α5β3-integrin] trimeric complex. This interaction occurs on the cell surface of the osteocyte where PHEX and α5β3-integrin have an intramembranous, domain a short intracellular domain and a large extracellular domain; (3 & 4) Formation of the [PHEX-DMP1-α5β3 integrin] trimeric complex initiates a signaling pathway (MAPK/Erk) that suppresses FGF23 expression; (5) FAM20C kinase also phosphorylates FGF23 (Ser180); (6 & 7) FAM20C phosphorylation of Ser180 inhibits O-glycosylation of FGF23 by polypeptide N-acetylgalactosaminyltransferase 3 (GalNT3); (8, 9 & 10) The under-glycosylated and phosphorylated FGF23 is targeted for Furin degradation and proteolysis; (6 & 11) In contrast, non-phosphorylated FGF23 is O-glycosylated by GalNT3; (12 & 13) O-Glycosylation of FGF23 increases resistance to Furin degradation and increases ½ life of full length active FGF23. Of relevance, mutations in GalNT3 are responsible for reduced circulating full-length FGF23 resulting in hyperphosphatemia and tumoral calcinosis [48,78,79]. This is the opposite phenotype to high FGF23 or hypophosphatemia. In summary FAM20C is responsible for suppressing FGF23 expression via the [PHEX-DMP1-α5β3-integrin] trimeric complex and decreasing full-length active FGF23 by targeting the hormone for furin degradation. This is consistent with the high circulating active FGF23, increased FGF23 mRNA expression, rickets and hypophosphatemia (ARHR 2) reported in mice that are null for bone expressed FAM20C [45,46]; (14 & 15) The binding of PHEX to the DMP1-ASARM-motif is also competitively regulated by free ASARM-peptide. Specifically, free ASARM-peptide can directly bind to PHEX preventing the binding to DMP1 and thereby disrupting the [PHEX-DMP1-α5β3-integrin] trimeric complex; (16) This in turn results in increased expression of FGF23; (17 & 18) Also, free ASARM-peptide inhibits mineralization and renal phosphate uptake. In X-linked and autosomal recessive rickets there are high levels of circulating ASARM-peptides. Both in vitro and in vivo (bolus and infusion) administration of ASARM-peptides and transgenic mice over expressing ASARM-peptides causes mineralization defects and hypophosphatemia [4,8-10,16-18,22,80]. The bimodal effects of SPR4-peptide administration are not shown in the scheme but are discussed in detail in recent publications [10,13,14]. The dual pharmacological effects depend on phosphate diet and mode of administration (bolus or continuous fusion). Briefly, SPR4-peptide binds to either the DMP1-ASARM motif or free ASARM-peptide. Binding to the DMP1-ASARM motif results in increased expression of FGF23 whereas binding to ASARM-peptides decreased FGF23 expression.
The discovery of the central portal of the FAP pathway, the PHEX gene in 1995 and its role in HYP was instrumental in advancing the field of bone-mineral hypophosphatemic disorders [23]. Since 1995, several new hypophosphatemic bone-mineral loss disorders and their associated primary gene defects have surfaced [24]. A common denominator of these diseases is increased levels or increased half-life of FGF23, a cytokine and phosphatonin. The FAP pathway provides a model for the regulation of this important cytokine. FGF23 regulates Vitamin D metabolism, renal phosphate homeostasis and plays an indirect role in the mineralization defects [25]. Despite intense research and the discovery of several genes responsible for X-linked and autosomal forms of hypophosphatemic rickets, effective treatments for these diseases are elusive. Classic treatments involve combined high phosphate and calcitriol supplements that partially correct the growth plate abnormalities but are ineffective at resolving the endochondral mineralization defects. For example, high calcitriol supplements lead to increased FGF23 levels and an exacerbation of the bone disease (vicious cycle) [26]. Also, high phosphate diets and supplements do not correct the hyperosteoidosis and the systemic hypophosphatemia per se is not the sole reason for the mineralization defect [27,28]. Recent research using HYP mice has raised optimism for possible treatment by targeting PC2 proprotein-convertase processing of FGF23. Specifically, Hexa-D-Arginine treatment is reported to enhance PC2 activity, normalize FGF23 levels and rescue the HYP-mice phenotype [29]. Remarkably, although Hexa-L-Arginine (levorotary enantiomer) enhances PC2 activity 1.4 fold, Hexa-D-Arginine (dextrorotary enantiomer) is reported to have no stimulatory or inhibitory effect on PC2 activity in vitro [30]. Thus, by implication the Hexa-D-Arginine form used in the HYP mice study [29] must either behave differently in vivo or affect the HYP phenotype via a PC2 independent-mechanism. Relevant to this, although a partial reduction in serum full-length active FGF23 levels occurred with HYP mice treated with Hexa-D-Arginine, the levels were still very high compared to WT mice (HYP non treated=2300 pg/mL, HYP treated=1800 pg/mL and wild type (WT) mice=70 pg/mL). Also, since both enantiomers (L and D) are potent “inhibitors” of related proprotein-convertases (Furin, PACE4 and PC1/3 for example) [30] careful toxicity evaluations and further studies are required before Hexa-D-Arginine is used clinically for long-term treatment. Two recent reviews provide a more detailed discussion and provide a model involving BMP1, PC2, 7B2 in the context of inherited hypophosphatemic rickets [4,31]. Finally, several studies have shown encouraging results using a different approach. Notably, FGF23 neutralizing antibodies were used with some success to treat X-linked hypophosphatemic rickets (HYP) patients and mice [32-34]. There are however concerns that FGF23 neutralizing antibody treatment may also have adverse outcomes [35,36].
The Matrix Vesicle (MVP) pathway
A second pathway involving specialized Matrix Vesicles (MVs) is also clearly involved in bone-mineralization, arterial calcification, phosphate regulation and energy metabolism [37]. The MVP pathway involves the microcrystalline formation of nascent mineral that occurs inside the MV structure. Key to this process is the generation of inorganic phosphate (Pi) by hydrolysis of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) by PHOSPHO1 [37-39]. The cataclysmic eruption of the MV marks the geniture of the nascent hydroxyapatite crystals into the extracellular matrix and the resulting binding of these crystals with collagen fibers. The growth of the incipient mineralized bone matrix is dependent on hydrolysis of ATP and nucleotide phosphates by Tissue Non-Specific Alkaline Phosphatase (TNAP) and Ectonucleotide Phosphodiesterase Pyrophosphatase (ENNP1) [37]. The link between the DMP1-PHEX-ASARM-FGF23 (FAP) and MVP pathways remains uncharacterized. Of relevance, a common denominator of the matrix vesicle and FAP pathways is ATP; the energy currency of the cell. ATP is also the chief source of PO4 substrate used by the matrix vesicle pathway (MVP). A recent new discovery promises to provide the nexus to the MVP and FAP pathways. Specifically, FAM20C, an ATP-dependent osteocyte kinase that specifically phosphorylates ASARM-motifs [40-42] and FGF23 [43,44] may provide a link. Targeted bone deletion of FAM20C in mice results in autosomal hypophosphatemic rickets [45,46]. This adds to the growing number of X-linked and autosomal forms of rickets consequent to mutations in genes involved in both MVP and FAP pathways. The FAM20C link is discussed in more detail in the next section and Figure 2 sketches the MVP pathway and the proposed connections through the FAP pathway, ATP and FAM20C.
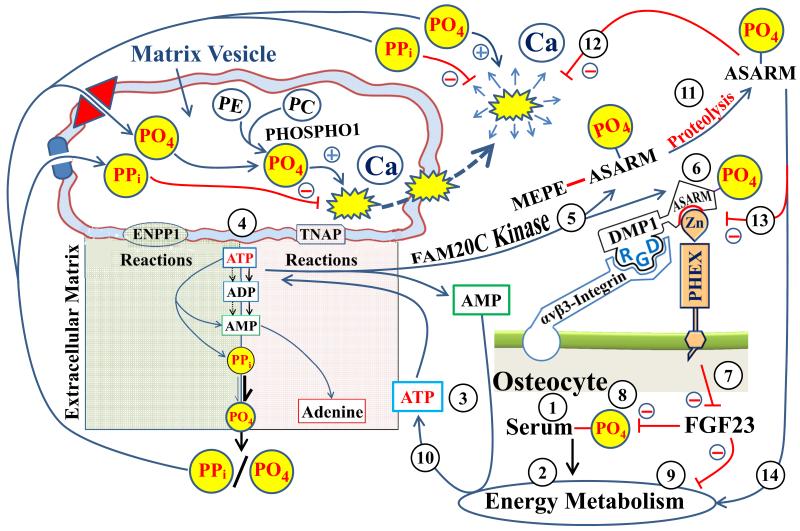
Figure 2.
Scheme illustrating the matrix vesicle (MVP) mineralization pathway and the PHEX/FGF23/DMP1 & ASARM extracellular matrix mineralization pathway (FAP). The encircled numbers refer to the following explanation of the model: (1) changes in serum phosphate (PO4) will impact energy metabolism (2) and ATP production (3 & 10). ATP levels are pivotal for the generation of pyrophosphate (PPi) and PO4 by two key matrix vesicle (MV) membrane bound phosphatases (ENPP1 and TNAP; see text and 4). PPi inhibits mineralization and PO4 is a required substrate both are transported into the MV by specific transporters (Pit and ANK; depicted by the blue cartouche and red diamond). The ratio of PPi/PO4 is key for mineralization to proceed normally. Within the MV, PHOSPHO1 also generates PO4 from phosphatidylethanolamine (PE) and phosphatidylcholine (PC) providing additional substrate for the growth of nascent MV hydroxyapaptite (depicted by yellow explosion cartoon). FAM20C kinase (5) also utilizes ATP to phosphorylate (6) ASARM-motif (DMP1) and ASARM-peptide (MEPE). Phosphorylation of DMP1 ASARM motif is requisite for the formation of a PHEX/DMP1/α5β3-integrin complex on the surface of osteocytes (6). This results in down regulation of FGF23 (7). This in turn affects renal PO4 handling and energy metabolism (8 & 9). A feedback is provided by proteolysis and release of ASARM peptides (11) that inhibits the PHEX/DMP1/α5β3-integrin complex (13). FGF23 increases and ASARM-peptides inhibit mineralization and energy metabolism (12 & 14). The ATP/FAM20C component (3, 5 & 10) provides a nexus between the MV, FAP and energy metabolism pathways.
FAM20C Kinase: A Nexus to the FAP/MVP pathways and Energy Metabolism through ATP
Mineralization is a time and spatially coordinated process involving dispositional-crystallization of hydroxyapatite (HA) mineral. This process not only requires cytokines and hormones but also downstream structurally important matrix-proteins. These matrix-proteins are integral biophysical components needed to bioengineer the growing HA lattice by systematic sequestration and deposition of phosphate and calcium [4]. The specific phosphorylation of these proteins plays an important role. For example, murine bone FAM20C-kinase inactivation causes autosomal hypophosphatemic rickets 2 (ARHR 2) [40,45-47]. Also, FAM20C-kinase specifically phosphorylates SIBLING-protein ASARM-motifs and ASARM-peptides (MEPE, DMP1, osteopontin) [40,41]. Also, a reduced DMP1 expression occurs with FAM20C-kinase null mice [45,47]. Of relevance, phosphorylation of the DMP1 ASARM-motif is important for binding and substrate hydrolysis by PHEX (PHEX mutations cause X-linked hypophosphatemic rickets). Formation of the [PHEX-DMP1-α5β3-integrin] trimeric complex on the surface of the osteocyte signals suppression of FGF23 expression [4], see also Figure 1 (FAP pathway). So, defective phosphorylation of the DMP1 C-terminal ASARM-motif results in compromised [(PHEX)-(DMP1-ASARM)-(α5β3-integrin)] binding, increased FGF23 mRNA expression and increased circulating FGF23 protein (as seen in ARHR2 and HYP mice). Recent research suggests FAM20C also controls FGF23 levels by directly affecting FGF23 protein stability. Specifically, FAM20C phosphorylates FGF23 and this inhibits FGF23 O-glycosylation by N-acetylgalactosaminyltransferase (GalNac-T3) [43,44]. Normal O-glycosylation by GalNac-T3 is needed to protect FGF23 from proteolytic-cleavage by Furin(s) thereby increasing circulating half-life [48]. Mutations in GalNac-T3 impair O-glycosylation of FGF23 and cause tumoral calcinosis, hyperphosphatemia with reduced FGF23 half-life [48]. In summary, loss of bone FAM20C in ARHR2 mice has two effects on FGF23: (1). Reduced expression because of impaired [PHEX-DMP1-α5β3-integrin] formation; and (2). Reduced FGF23 stability and half-life because of impaired O-glycosylation of FGF23 (Figure 1). This is consistent with the mineralization defects (rickets) and hypophosphatemia noted with these mice. Also, WT and HYP mice infused with SPR4-peptide show marked suppression of bone FAM20C kinase with improved energy metabolism [13,14]. This suggests a link with phosphate, FAM20C, ATP, energy and mineral metabolism. Figure 2 explains the FAM20C nexus between the FAP and MVP pathways and the proposed connections through ATP, ENPP1 and TNAP.
The powerful new approach of integrative physiology and the use of murine models has shown a clear link with energy and bone-kidney mineral metabolism [49]. Peripherally, insulin and osteocalcin a bone protein regulate bone turnover and glucose metabolism. The adipokine leptin mediates central control and does this by crossing the blood brain barrier to regulate the biosynthesis of serotonin, a neurotransmitter. Leptin suppresses synthesis of serotonin in the brain stem and this reduces serotonergic signaling and sympathetic-tone in the hypothalamic arcuate nucleus (ARC) and ventrolateral medial nucleus (VLM). Serotonergic signaling in the VLM nucleus increases bone-formation and decreases bone-resorption by activating β-adrenergic osteoblast receptors. Serotonergic signaling in ARC ganglion affects appetite [50]. More recent research has shown peripheral circulating serotonin has an opposite effect on bone turnover. This effect is mediated by Low-density lipoprotein receptor-related protein 5 (Lrp5) regulation of serotonin synthesis in the gut [51,52]. LRP5 also plays a major role in directly regulating bone homeostasis through the Wnt/β-catenin pathway [53]. Since serotonin does not cross the blood-brain barrier the peripheral and central serotonin pools have distinct catabolic and anabolic effects respectively on bone turnover. The new serotonin gut findings have major medical implications for treating osteoporosis, obesity and diabetes. However, the pathways and models proposed are currently controversial since recent studies contradict key findings likely because of strain and murine model differences [54-58].
The discovery of several new genes responsible for inherited hypophosphatemic rickets diseases are beginning to reveal additional players [59]. For example mice and humans with inherited hypophosphatemic rickets diseases show accompanying defects in insulin sensitivity, glucose and fat metabolism [13,14,60]. Also, obesity, insulin resistance and cardiovascular disease (CVD) show correlations with FGF23 a major hormone regulating phosphate and mineralization [61,62]. In obese patients with non-insulin-dependent diabetes mellitus (NIDDM) a causal link with low serum phosphate has been suggested [63]. Specifically, the hypophosphatemia is proposed to affect ATP production and thus glucose metabolism. This results in hyperglycemia with increased risk of NIDDM, hypertension and stroke [63]. Also, hyperphosphatemia plays an important physiological role in obesity by impairing thermogenesis and the basal metabolic rate [63,64]. Consistent with this, major increases in red blood cell oxygen affinity with changes in oxygen transport and glycolytic intermediates occur in hypophosphatemic subjects [65]. These changes are accompanied by reduced renal cortical ribonucleoside triphosphate pools and defective ATP synthesis in hypophosphatemic rickets mice models and humans [66,67]. Defective thermoregulatory control, defective ATP synthesis with increased metabolic rate and oxygen consumption are key features of X-linked hypophosphatemic rickets mice (HYP) [67,68]. This is accompanied by increased osteoblastic pH, increased gluconeogenesis, increased glucose-6-phosphatase activity and decreased renal and bone GAPDH expression [13,14,60]. Oxygen tension and thus normal ATP synthesis is also required for osteoblast to osteocyte transformation [69]. The osteocyte, embedded within the lacuno-canalicular complex is subject to an environment with low partial pressures of oxygen (pO2) [70]. Cell culture studies have shown hypoxia has major influence on the expression of osteocyte expressed proteins DMP1, MEPE, FGF23 Cx43 [69]. Also, osteoblast to osteocyte transformation was positively affected under hypoxic conditions and this was associated with changes in osteocalcin and alkaline phosphatase [69].
Phosphorylation also directly impacts glucose metabolism by influencing the activity of a key bone matrix protein osteocalcin and thus insulin secretion and sensitivity. Specifically, osteotesticular protein tyrosine phosphatase (OST-PTP) dephosphorylates and inactivates the osteoblast insulin receptor and this increases γ-carboxylation of osteocalcin. The γ-carboxylation of osteocalcin reduces bioactivity and suppresses osteocalcin mediated insulin secretion. This results in hyperglycemia, glucose intolerance and reduced insulin sensitivity [71,72]. In counterbalance, osteoclastic bone resorption and the accompanying increased acidity of the bone milieu is responsible for the γ-decarboxylation of osteocalcin. The active decarboxylated osteocalcin then increases insulin secretion and improves glucose tolerance and insulin sensitivity [71,72]. An important component of the MVP-pathway discussed earlier, Ectonucleotide Phosphodiesterase Pyrophosphatase (ENNP1) also plays a major role in hyperglycemia, insulin resistance and diabetes [73,74]. ENNP regulates matrix vesicle mineralization and mutations in this gene cause autosomal recessive hypophosphatemic rickets (ARHR2; MIM 173335) in mice and man [37,75,76]. ENPP1 like OST-PTP interacts with the insulin receptor (IR) and decreases IR β-subunit auto-phosphorylation [74,77]. Over expression of ENPP1 induces hyperglycemia, insulin resistance and diabetes [74]. Thus, energy metabolism, mineralization and bone mass are tightly linked to mineral phosphate regulation with FAM20C and ATP central players linking the different facets (see Figures 1 and 2).
Conclusion
In conclusion, DMP1 interacts with PHEX via an ASARM-motif and α5β3-integrin on the surface of the osteocyte. This trimeric complex then suppresses FGF23 expression through the FAP pathway. Bone-derived ASARM-peptides control these interactions and thus regulate mineralization, bone turnover and phosphate. Indeed, ASARM-peptides are the only known physiological substrate(s) and ligand(s) for PHEX [10-12,15,20]. Formation of the trimeric complex and consequent suppression of FGF23 gene expression needs FAM20C kinase phosphorylation of ASARM-motif and free ASARM-peptide. FAM20C also phosphorylates FGF23 protein and decreases protein stability by preventing FGF23 O-glycosylation. A second pathway involves matrix vesicles, ATP and specific phosphatases (MVP pathway). FAM20C kinase and ATP provide the nexus between the FAP, MVP and energy metabolism pathways. There is a compelling medical need to study the functional implications of these associations in inherited bone-kidney mineral diseases, chronic kidney disease and mineral bone disorder (CKD-MBD), obesity, diabetes and osteoporosis.
Highlights.
FGF23 is an important regulator of Vitamin D, phosphate and mineral metabolism.
An osteocyte membrane complex of PHEX, DMP1 α5β3-integrin suppresses FGF23.
SIBLING ASARM-peptides disrupts this complex and increases FGF23 (FAP pathway).
Phosphorylation of ASARM and FGF23 by FAM20C-Kinase also regulates this pathway.
FAM20C-Kinase and ATP provide a nexus to matrix vesicle mineralization the FAP pathway and energy metabolism.
Acknowledgements
The authors would like to thank the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases) for grant support to P. S. N. Rowe (Grant 5R01AR051598-10).
Footnotes
Conflict of Interest:
Nothing declared for author Peter S Rowe
References
* of special interest
** of outstanding interest
- ••1.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. Outstanding review on the role of the osteocyte in bone mineral homeostasis.
- 2.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe PS. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem Funct. 2012;30:355–375. doi: 10.1002/cbf.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado JP, Johnson WE, O’Brien SJ, Vasconcelos V, Antunes A. Adaptive evolution of the Matrix Extracellular Phosphoglycoprotein in mammals. BMC evolutionary biology. 2011;11:342. doi: 10.1186/1471-2148-11-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •6.Grantham JJ, Wallace DP. Return of the secretory kidney. Am J Physiol Renal Physiol. 2002;282:F1–9. doi: 10.1152/ajprenal.2002.282.1.F1. Outstanding review on the evolution of the kidney from marine, freshwater and terrestrial environments.
- 7.Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 8.David V, Martin AC, Hedge AM, Drezner MK, Rowe PS. ASARM peptides: PHEX-dependent & independent regulation of serum phosphate. Am J Physiol Renal Physiol. 2011;300:F783–791. doi: 10.1152/ajprenal.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David V, Martin A, Hedge AM, Rowe PS. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology. 2009;150:4012–4023. doi: 10.1210/en.2009-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addison W, Masica D, Gray J, McKee MD. Phosphorylation-Dependent Inhibition of Mineralization by Osteopontin ASARM Peptides is Regulated by PHEX Cleavage. J Bone Miner Res. 2009;25:695–705. doi: 10.1359/jbmr.090832. [DOI] [PubMed] [Google Scholar]
- 12.Addison W, Nakano Y, Loisel T, Crine P, McKee M. MEPE-ASARM Peptides Control Extracellular Matrix Mineralization by Binding to Hydroxyapatite - An Inhibition Regulated by PHEX Cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 13.Zelenchuk L, Hedge A, Rowe PS. SPR4-peptide Alters Bone Metabolism of Normal and HYP Mice. Bone. 2015;72:23–33. doi: 10.1016/j.bone.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelenchuk LV, Hedge AM, Rowe PS. PHEX Mimetic (SPR4-Peptide) Corrects and Improves HYP and Wild Type Mice Energy-Metabolism. PLoS One. 2014;9:e97326. doi: 10.1371/journal.pone.0097326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe PS, Matsumoto N, Jo OD, Shih RN, Oconnor J, Roudier MP, Bain S, Liu S, Harrison J, Yanagawa N. Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone. 2006;39:773–786. doi: 10.1016/j.bone.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirley DG, Faria NJ, Unwin RJ, Dobbie H. Direct micropuncture evidence that matrix extracellular phosphoglycoprotein inhibits proximal tubular phosphate reabsorption. Nephrol Dial Transplant. 2010;25:3191–3195. doi: 10.1093/ndt/gfq263. [DOI] [PubMed] [Google Scholar]
- 17.Marks J, Churchill LJ, Edward SD, Unwin R. The Phosphatonin Matrix Extracellular Phosphoglycoprotein (MEPE) Inhibits Renal Intestinal Phosphate Transport in vivo. J Am Soc Nephrol. 2008;19:2313–2320. doi: 10.1681/ASN.2008030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobbie H, Unwin RJ, Faria NJ, Shirley DG. Matrix extracellular phosphoglycoprotein causes phosphaturia in rats by inhibiting tubular phosphate reabsorption. Nephrol Dial Transplant. 2008;23:730–733. doi: 10.1093/ndt/gfm535. [DOI] [PubMed] [Google Scholar]
- 19.Staines KA, Mackenzie NC, Clarkin CE, Zelenchuk L, Rowe PS, MacRae VE, Farquharson C. MEPE is a novel regulator of growth plate cartilage mineralization. Bone. 2012;51:418–430. doi: 10.1016/j.bone.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barros NM, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res. 2013;28:688–699. doi: 10.1002/jbmr.1766. [DOI] [PubMed] [Google Scholar]
- 21.Salmon B, Bardet C, Khaddam M, Naji J, Coyac BR, Baroukh B, Letourneur F, Lesieur J, Decup F, Le Denmat D, et al. MEPE-Derived ASARM Peptide Inhibits Odontogenic Differentiation of Dental Pulp Stem Cells and Impairs Mineralization in Tooth Models of X-Linked Hypophosphatemia. PLoS One. 2013;8:e56749. doi: 10.1371/journal.pone.0056749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM. Sclerostin is a locally acting regulator of late-osteoblast/pre-osteocyte differentiation and regulates mineralization through a MEPE-ASARM dependent mechanism. J Bone Miner Res. 2011;26:1425–1436. doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HYP-consortium. Francis F, Hennig S, Korn b, Reinhardt R, de Jong D, Poustka A, Lehrach H, Rowe PSN, Goulding JN, et al. The HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter TO. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab. 2012;30:1–9. doi: 10.1007/s00774-011-0340-2. [DOI] [PubMed] [Google Scholar]
- 25.Rowe PS. Regulation of Bone-Renal Mineral and Energy Metabolism: The PHEX, FGF23, DMP1, MEPE ASARM Pathway. Critical reviews in eukaryotic gene expression. 2012;22:61–86. doi: 10.1615/critreveukargeneexpr.v22.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. The Journal of clinical endocrinology and metabolism. 2010;95:1846–1850. doi: 10.1210/jc.2009-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marie PJ, Travers R, Glorieux FH. Healing of bone lesions with 1,25-dihydroxyvitamin D3 in the young X-linked hypophosphataemic male mouse. Endocrinology. 1982;111:904–911. doi: 10.1210/endo-111-3-904. [DOI] [PubMed] [Google Scholar]
- 28.Marie PJ, Travers R, Glorieux FH. Bone response to phosphate and vitamin D metabolites in the hypophosphatemic male mouse. Calcif Tissue Int. 1982;34:158–164. doi: 10.1007/BF02411227. [DOI] [PubMed] [Google Scholar]
- ••29.Yuan B, Feng JQ, Bowman S, Liu Y, Blank RD, Lindberg I, Drezner MK. Hexa-D-Arginine treatment increases 7B2*PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. Journal of bone and mineral research. 2013;28:56–72. doi: 10.1002/jbmr.1738. This paper shows murine X-linked rickets can be cured by treatment with Hexa-D-Arginine. This is proposed to occur by: 1) increasing 7B2*PC2 proprotein-protease activity and thus increasing FGF23 degradation; and 2). increasing conversion of pro-BMP1 to BMP1 and thus increasing processed DMP1. Also, the authors present a new model for the pathology of X-linked rickets.
- ••30.Cameron A, Appel J, Houghten RA, Lindberg I. Polyarginines are potent furin inhibitors. J Biol Chem. 2000;275:36741–36749. doi: 10.1074/jbc.M003848200. This paper shows HEXA-D-arginine does not increase PC2 activity in vitro and HEXA-L-arginine has a mild stimulatory effect. This suggests a more complex model than that proposed by Yuan et al 2013 (29).
- 31.Feng JQ, Clinkenbeard EL, Yuan B, White KE, Drezner MK. Osteocyte Regulation of Phosphate Homeostasis and Bone Mineralization Underlies the Pathophysiology of the Heritable Disorders of Rickets and Osteomalacia. Bone. 2013;54:213–221. doi: 10.1016/j.bone.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••32.Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124:1587–1597. doi: 10.1172/JCI72829. This paper shows encouraging results following treatment of X-linked hypophosphatemic rickets patients with anti FGF23 antibodies.
- 33.Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S, et al. Therapeutic Effects of Anti-FGF23 Antibodies in Hypophosphatemic Rickets/Osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 34.Shimada T, Fukumoto S. FGF23 as a novel therapeutic target. Adv Exp Med Biol. 2012;728:158–170. doi: 10.1007/978-1-4614-0887-1_10. [DOI] [PubMed] [Google Scholar]
- 35.Shalhoub V. Chronic kidney disease: FGF23 neutralization associated with increased risk of death. Nat Rev Nephrol. 2012;8:492. [Google Scholar]
- ••36.Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543–2553. doi: 10.1172/JCI61405. This paper shows anti FGF23 antibody treatment may have adverse effects in certain groups. This has implications regarding anti FGF23 treatment of X-linked rickets see also Carpenter et al 2014 and Shalhoub, V. 2012 (32, 35).
- ••37.Millan JL. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. Outstanding review on matrix vesicles and the role of key phosphatases on mineralization.
- 38.McKee MD, Yadav MC, Foster BL, Somerman MJ, Farquharson C, Millan JL. Compounded PHOSPHO1/ALPL deficiencies reduce dentin mineralization. J Dent Res. 2013;92:721–727. doi: 10.1177/0022034513490958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiffer-Moreira T, Yadav MC, Zhu D, Narisawa S, Sheen C, Stec B, Cosford ND, Dahl R, Farquharson C, Hoylaerts MF, et al. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J Bone Miner Res. 2013;28:81–91. doi: 10.1002/jbmr.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••40.Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336:1150–1153. doi: 10.1126/science.1217817. Demonstration that FAM20C–Kinase (protein responsible for an autosomal form of rickets) specifically phosphorylates FGF23, prevents O-glycosylation by GalNAc-T3 and decreases FGF23 stability (consistent with the ASARM model and FAP pathway).
- 41.Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One. 2012;7:e42988. doi: 10.1371/journal.pone.0042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinoshita Y, Hori M, Taguchi M, Fukumoto S. Functional analysis of mutant FAM20C in Raine syndrome with FGF23-related hypophosphatemia. Bone. 2014;67:145–151. doi: 10.1016/j.bone.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Tagliabracci A, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Appaiah HN, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. PNAS. 2014;111:5520–5525. doi: 10.1073/pnas.1402218111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••44.Lindberg I, Pang HW, Stains JP, Clark D, Yang AJ, Bonewald L, Li KZ. FGF23 is endogenously phosphorylated in bone cells. J Bone Miner Res. 2015;30:449–454. doi: 10.1002/jbmr.2354. Demonstration that FAM20C–Kinase (protein responsible for an autosomal form of rickets) specifically phosphorylates FGF23 (consistent with the ASARM model and FAP pathway).
- ••45.Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A, Sun Y, Hao J, George A, Lu Y, et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 2012;8:e1002708. doi: 10.1371/journal.pgen.1002708. Seminal paper showing bone inactivation of FAM20C–Kinase results in hypophosphatemic rickets with increased FGF23.
- 46.Vogel P, Hansen GM, Read RW, Vance RB, Thiel M, Liu J, Wronski TJ, Smith DD, Jeter-Jones S, Brommage R. Amelogenesis Imperfecta and Other Biomineralization Defects in Fam20a and Fam20c Null Mice. Veterinary pathology. 2012;49:998–1017. doi: 10.1177/0300985812453177. [DOI] [PubMed] [Google Scholar]
- 47.Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S, Bjerknes R. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res. 2013;28:1378–1385. doi: 10.1002/jbmr.1850. [DOI] [PubMed] [Google Scholar]
- 48.Yancovitch A, Hershkovitz D, Indelman M, Galloway P, Whiteford M, Sprecher E, Kilic E. Novel mutations in GALNT3 causing hyperphosphatemic familial tumoral calcinosis. Journal of bone and mineral metabolism. 2011;29:621–625. doi: 10.1007/s00774-011-0260-1. [DOI] [PubMed] [Google Scholar]
- 49.Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310:21–29. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav VK, Oury F, Tanaka K, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208:41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karsenty G, Gershon MD. The importance of the gastrointestinal tract in the control of bone mass accrual. Gastroenterology. 2011;141:439–442. doi: 10.1053/j.gastro.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 54.Kode A, Obri A, Paone R, Kousteni S, Ducy P, Karsenty G. Lrp5 regulation of bone mass and serotonin synthesis in the gut. Nat Med. 2014;20:1228–1229. doi: 10.1038/nm.3698. [DOI] [PubMed] [Google Scholar]
- 55.Cui Y, Niziolek PJ, MacDonald BT, Alenina N, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Powell DR, He X, et al. Reply to Lrp5 regulation of bone mass and gut serotonin synthesis. Nat Med. 2014;20:1229–1230. doi: 10.1038/nm.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee GS, Simpson C, Sun BH, Yao C, Foer D, Sullivan B, Matthes S, Alenina N, Belsky J, Bader M, et al. Measurement of plasma, serum, and platelet serotonin in individuals with high bone mass and mutations in LRP5. J Bone Miner Res. 2013;29:976–981. doi: 10.1002/jbmr.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goltzman D. LRP5, serotonin and bone: Complexity, contradictions and conundrums. J Bone Miner and Res. 2011;26:2002–2011. doi: 10.1002/jbmr.462. [DOI] [PubMed] [Google Scholar]
- 58.Cui Y, Niziolek PJ, Macdonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, et al. Lrp5 functions in bone to regulate bone mass. Nature medicine. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nanes MS. Phosphate wasting and fibroblast growth factor-23. Curr Opin Endocrinol Diabetes Obes. 2013;20:523–531. doi: 10.1097/01.med.0000436189.80104.80. [DOI] [PubMed] [Google Scholar]
- 60.Xie W, Mechin MC, Dubois SG, Lajeunesse D, van de Werve G. Up-regulation of liver glucose-6-phosphatase in x-linked hypophosphatemic mice. Horm Metab Res. 2002;34:288–292. doi: 10.1055/s-2002-33256. [DOI] [PubMed] [Google Scholar]
- 61.Wojcik M, Dolezal-Oltarzewska K, Janus D, Drozdz D, Sztefko K, Starzyk JB. FGF23 contributes to insulin sensitivity in obese adolescents - preliminary results. Clin Endocrinol (Oxf) 2012;77:537–540. doi: 10.1111/j.1365-2265.2011.04299.x. [DOI] [PubMed] [Google Scholar]
- 62.Wojcik M, Janus D, Dolezal-Oltarzewska K, Drozdz D, Sztefko K, Starzyk JB. The association of FGF23 levels in obese adolescents with insulin sensitivity. J Pediatr Endocrinol Metab. 2012;25:687–690. doi: 10.1515/jpem-2012-0064. [DOI] [PubMed] [Google Scholar]
- 63.Haglin L. Hypophosphataemia: cause of the disturbed metabolism in the metabolic syndrome. Med Hypotheses. 2001;56:657–663. doi: 10.1054/mehy.2000.1272. [DOI] [PubMed] [Google Scholar]
- 64.Levi E, Fadda GZ, Ozbasli C, Massry SG. Evolution of metabolic and functional derangements of pancreatic islets in phosphate depletion. Endocrinology. 1992;131:2182–2188. doi: 10.1210/endo.131.5.1330495. [DOI] [PubMed] [Google Scholar]
- 65.Travis SF, Sugerman HJ, Ruberg RL, Dudrick SJ, Delivoria-Papadopoulos M, Miller LD, Oski FA. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N Engl J Med. 1971;285:763–768. doi: 10.1056/NEJM197109302851402. [DOI] [PubMed] [Google Scholar]
- 66.Hettleman BD, Sabina RL, Drezner MK, Holmes EW, Swain JL. Defective adenosine triphosphate synthesis. An explanation for skeletal muscle dysfunction in phosphate-deficient mice. J Clin Invest. 1983;72:582–589. doi: 10.1172/JCI111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabina RL, Drezner MK, Holmes EW. Reduced renal cortical ribonucleoside triphosphate pools in three different hypophosphatemic animal models. Biochem Biophys Res Commun. 1982;109:649–655. doi: 10.1016/0006-291x(82)91989-1. [DOI] [PubMed] [Google Scholar]
- 68.Vaughn LK, Meyer RA, Jr., Meyer MH. Increased metabolic rate in X-linked hypophosphatemic mice. Endocrinology. 1986;118:441–445. doi: 10.1210/endo-118-1-441. [DOI] [PubMed] [Google Scholar]
- 69.Hirao M, Hashimoto J, Yamasaki N, Ando W, Tsuboi H, Myoui A, Yoshikawa H. Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. J Bone Miner Metab. 2007;25:266–276. doi: 10.1007/s00774-007-0765-9. [DOI] [PubMed] [Google Scholar]
- 70.Bonewald LF. Osteocytes: a proposed multifunctional bone cell. J Musculoskelet Neuronal Interact. 2002;2:239–241. [PubMed] [Google Scholar]
- 71.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Lorenzo C, Greco A, Fiorentino TV, Mannino GC, Hribal ML. Variants of insulin-signaling inhibitor genes in type 2 diabetes and related metabolic abnormalities. Int J Genomics. 2013;2013:376454. doi: 10.1155/2013/376454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maddux BA, Chang YN, Accili D, McGuinness OP, Youngren JF, Goldfine ID. Overexpression of the insulin receptor inhibitor PC-1/ENPP1 induces insulin resistance and hyperglycemia. Am J Physiol Endocrinol Metab. 2006;290:E746–749. doi: 10.1152/ajpendo.00298.2005. [DOI] [PubMed] [Google Scholar]
- 75.Li Q, Guo H, Chou DW, Berndt A, Sundberg JP, Uitto J. Mutant Enpp1asj mice as a model for generalized arterial calcification of infancy. Dis Model Mech. 2013;6:1227–1235. doi: 10.1242/dmm.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••76.Mackenzie NC, Zhu D, Milne EM, van ’t Hof R, Martin A, Darryl Quarles L, Millan JL, Farquharson C, MacRae VE. Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS One. 2012;7:e32177. doi: 10.1371/journal.pone.0032177. Seminal paper showing bone inactivation of ENPP1 results in mineralization defects and increased FGF23.
- 77.Goldfine ID, Maddux BA, Youngren JF, Reaven G, Accili D, Trischitta V, Vigneri R, Frittitta L. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities. Endocr Rev. 2008;29:62–75. doi: 10.1210/er.2007-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gattineni J, Baum M. Genetic disorders of phosphate regulation. Pediatric nephrology. 2012 doi: 10.1007/s00467-012-2103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farrow EG, Imel EA, White KE. Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and alphaKlotho) Best practice & research. Clinical rheumatology. 2011;25:735–747. doi: 10.1016/j.berh.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Opsahl Vital S, Gaucher C, Bardet C, Rowe PS, George A, Linglart A, Chaussain C. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone. 2012;50:989–997. doi: 10.1016/j.bone.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]