Abstract
Background
The aim of this study was to estimate the cost-effectiveness of injectable extended release naltrexone (XR-NTX) compared to methadone maintenance and buprenorphine maintenance treatment (MMT and BMT respectively) for adult males enrolled in treatment for opioid dependence in the United States from the perspective of state-level addiction treatment payers.
Methods
We used a Markov model with daily time cycles to estimate the incremental cost per opioid-free day in a simulated cohort of adult males ages 18–65 over a six-month period from the state health program perspective.
Results
XR-NTX is predicted to be more effective and more costly than methadone or buprenorphine in our target population, with an incremental cost per opioid-free day gained relative to the next-most effective treatment (MMT) of $72. The cost-effectiveness of XR-NTX relative to MMT was driven by its effectiveness in deterring opioid use while receiving treatment.
Conclusions
XR-NTX is a cost-effective medication for treating opioid dependence if state addiction treatment payers are willing to pay at least $72 per opioid-free day.
Keywords: XR-NTX, opioid dependence, cost-effectiveness
INTRODUCTION
Opioid dependence is a chronic, relapsing brain disease characterized by physical dependence and subjective need and craving for an opioid. In 2013, there were approximately 2.4 million people in the United States suffering from dependence or abuse of heroin or opioid analgesics.1 This number has increased significantly over the past 10 years.1 Beginning in 2009, drug overdose became the leading cause of accidental death in the United States.2 Opioid dependence has a high cost to society,3,4 as well as to the health care system,3–5 and identifying cost-effective treatments for opioid dependence is a priority for federal, state and local policy makers as well as private and public insurers.
In October 2010, the Food and Drug Administration (FDA) approved extended-release naltrexone (XR-NTX) for the treatment of opioid dependence, primarily based on findings from a randomized trial conducted in Russia.6 XR-NTX was the first medication approved for treating opioid dependence in eight years. XR-NTX is a novel therapy; unlike other approved pharmacotherapies for opioid dependence that require daily or every other day administration, one intramuscular injection of XR-NTX blocks an individual’s response to opioids for up to 28 days.7
While pharmacotherapy is considered cost-effective for treatment of opioid dependence relative to treatment with only therapy or only medication,8,9 utilization is relatively low. In 2012, less than 40% of Americans who abused or were dependent on opioids received any form of medication-assisted treatment (MAT), including methadone maintenance treatment (MMT) and buprenorphine maintenance treatment (BMT), for opioid dependence.10
MMT and BMT pharmacotherapy faces considerable barriers to utilization. Methadone is only available at specialty MMT clinics; buprenorphine can only be provided by physicians that have received mandated special training and licensing.10–12 Unlike MMT and BMT, XR-NTX can be administered by general practitioners, and patients only need to receive an injection every 28 days.12,13 Unlike MMT and BMT, there is no known diversion potential for XR-NTX, increasing its relative public health appeal. However, barriers to XR-NTX utilization also exist. XR-NTX is relatively expensive, with a state-average per-diem price of $48.36, compared to $13.31 and $21.16 for MMT and BMT, respectively. Patients must also abstain from opioids seven to ten days before starting XR-NTX, a significant barrier for many opioid dependent people.12,13
METHODS
This study used a Markov model to estimate the incremental cost-effectiveness of XR-NTX, MMT and BMT for adult males in the United States initiating pharmacotherapy for opioid dependence from the perspective of state addiction treatment payers. Non-pharmaceutical treatments and placebo, which have been found not cost-effective relative to pharmacotherapy,8,9,14–16 were not included as comparators in this study. We also excluded the once-daily oral formulation of naltrexone prescribed, which has been found to be less effective than either MMT or BMT.17 For all three treatments, our model assumed flexible dosing of each medication in accordance with best clinical practice.18–20
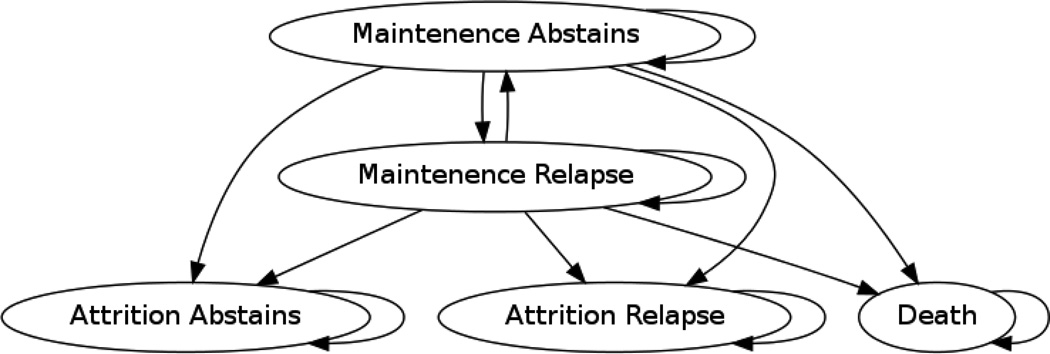
The Markov model used daily time cycles to simulate health outcomes and expenditures under alternative treatment regimens over a 168 day (approximately six-month) horizon. Five states were used to describe the process of opioid dependence treatment: (1) maintenance in a treatment program and abstaining from using opioids; (2) maintenance in a treatment program but relapsing to opioid use; (3) attrition from treatment and abstaining from using opioids; (4) attrition from treatment and relapsing to opioid use; or (5) death (see Figure 1).
Figure 1.
Markov Model of Transition States
Transition probabilities for MMT and BMT were estimated from a Cochrane library meta-analysis of 24 clinical trials published in 2008;16 transition probabilities for XR-NTX were based on a clinical trial with 24-week follow-up conducted in a population of Russian adult males (see Table 1).6 We calculated the daily probability of remaining in treatment from the observed percentage of patients in treatment 24 weeks after initiation,6,16 assuming retention declined geometrically at a constant daily rate based on retention patterns observed in MMT and BMT trials.16 We estimated the probability of transitioning between abstinence and opioid use from the percentage of opioid-positive urine drug screens.6,9,16 Because our data sources did not report off-treatment abstinence rates, we assumed equal daily probabilities of off-treatment abstinence based on the average one in four day rate of use observed in trial placebo groups.16 We assumed a constant, state-independent death rate over the 24-week period based on a meta-analysis of mortality among individuals dependent on opioids.21
Table 1.
Transition Probabilities by Treatment
| Treatment | P(Transition Out of Treatment) |
P(Opioid Use In Treatment) |
P(Opioid Use Out of Treatment) |
P(Mortality) |
|---|---|---|---|---|
| Methadone | .0062 | .5940 | .7990 | .0001 |
| Buprenorphine | .0090 | .6250 | .7990 | .0001 |
| XR-NTX | .0087 | .1000 | .7990 | .0001 |
Truncated list of transition probabilities. Opioid free status in and out of treatment simply calculated as 1-P (use). Categories assumed to be mutually exclusive and exhaustive.
As all estimates were made using secondary public use statistics and no individual level data is used, this study has not been reviewed by an institutional review board.
To estimate the cost of pharmacotherapy from the state addiction treatment payer perspective, we obtained data from a sample of 11 state Medicaid programs and Single State Agencies (SSAs) chosen for geographic and population variation. MMT is predominantly billed to state payers as a weekly bundle (using HCPCS code H0020), including the costs of the drug, counseling usually delivered in a group setting, medication management and oversight. Services for BMT and XR-NTX are currently not billed at a bundled rate in most states. We estimated BMT and XR-NTX treatment costs from the state payer perspective assuming best clinical practices were followed.22 Average reimbursement rates for pharmaceuticals and their administration were added to the average costs of weekly group counseling (HCPCS code H0005 or CPT code 90853) and monthly physician medication management (CPT codes 90862 and 99213). Although some states do not provide reimbursement for all of the services related to XR-NTX, BMT, and MMT, we believe our costs are representative of the majority of U.S. state payers. Given the short time horizon of the simulation, we did not apply discounting to costs or outcomes.
Our key measure of treatment cost effectiveness is the incremental cost effectiveness ratio (ICER). The ICER represents the additional cost required to achieve an additional unit of outcome (opioid free day) under a specified treatment relative to a comparator:
If a treatment is both more costly and less effective than the comparator, it is said to be “dominated”by the comparator.
We conducted scenario-based deterministic sensitivity analyses to check the robustness of our findings to parameters for which there is substantial uncertainty. Two treatment cost scenarios were conducted: one with most favorable relative cost of XR-NTX (where the cost of MMT and BMT are held at the maximum value observed in the state data and the cost of XR-NTX is held at the minimum value), and the other with the least favorable relative cost of XR-NTX (where the costs of MMT and BMT are held at the lowest value observed in state data and the cost of XR-NTX is held at the maximum observed in state data). We also explored how varying the probability of opioid use while under treatment with XR-NTX would affect the incremental cost-effectiveness of XR-NTX. To do this, we again explored two extreme scenarios: assigning the probability of opioid use while under XR-NTX equal to 50% (comparable to the opioid use for patients treated with methadone) and 0% (the lowest possible value). Finally, we systematically varied patient retention rates in all treatments from 45% (retention observed in a U.S. based study which looked at the effectiveness of an XR-NTX variant)23 to 70% (one of the highest retention rates observed in U.S. based trials of methadone and the retention rate observed in a recent 12 week trial of XR-NTX).24 Two-way sensitivity analysis was conducted by varying both patient retention and opioid use within treatment.
RESULTS
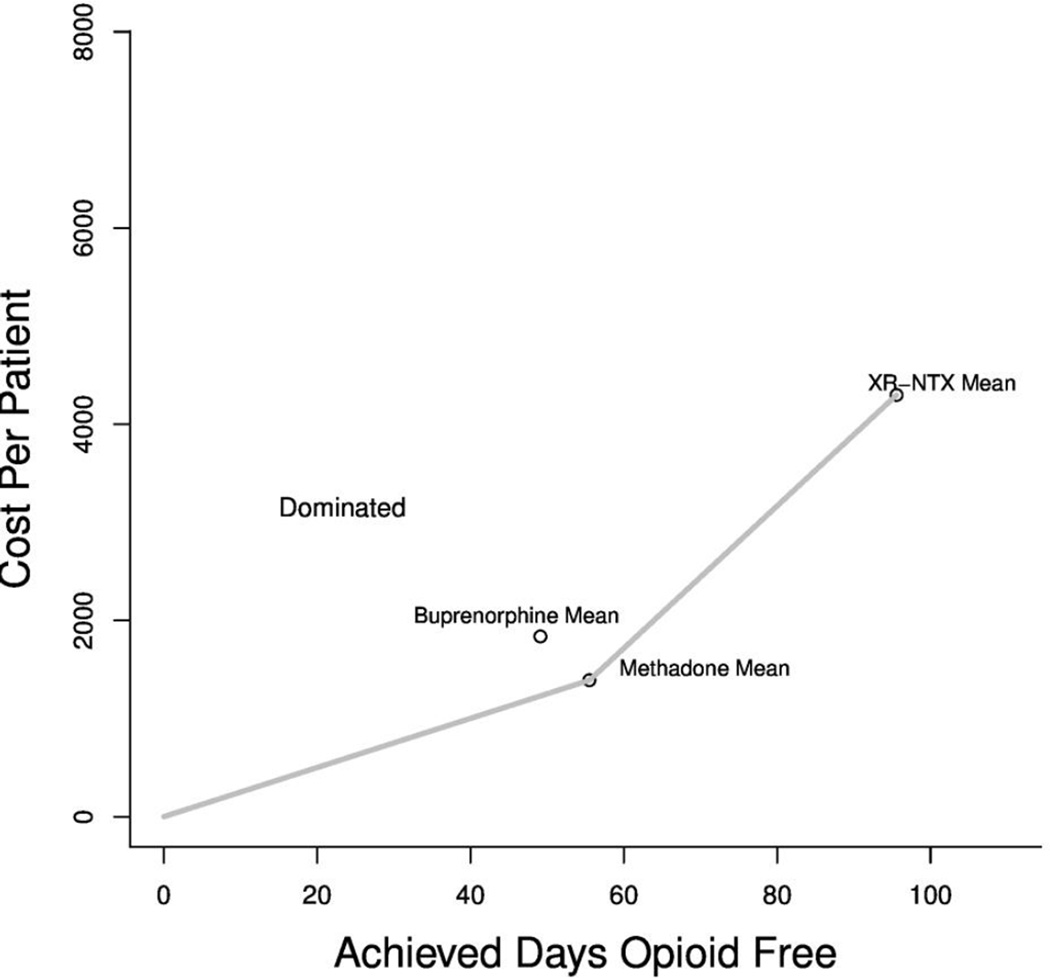
Based on our 24 week (168 day) model, patients are expected to remain opioid free for approximately 56, 49, and 96 days when treated with MMT, BMT and XR-NTX respectively (see Table 2). While days spent on treatment among patients treated with MMT was slightly higher compared to those treated with BMT, patients treated with BMT had slightly lower predicted rates of opioid use while on treatment (45% of days spent using opioids versus 47% of days spent using opioids in MMT). In addition to having the highest predicted number of days on treatment, XR-NTX appears to be significantly more effective than MMT or BMT at discouraging opioid use while on treatment, with patients treated with XR-NTX spending an average of 6% of their time in maintenance treatment using opioids.
Table 2.
Incremental Cost Effectiveness of Treatments for Opioid Dependence
| Medication | Number of drug free days |
Cost per day of Treatment |
Average cost per patient over study period* |
Incremental cost effectiveness ratio |
|---|---|---|---|---|
| Methadone | 56 | $13.31 | $1,390.98 | $24.83 (base case) |
| Buprenorphine | 49 | $21.16 | $1,837.40 | dominated** |
| XR-NTX | 96 | $48.36 | $4,287.73 | $72.42 |
Average cost over all patients including those that left treatment
The base case is both more effective and less expensive
The expected per patient cost of each treatment 168 days is $1,390.98 for MMT, $1,837.40 for BMT, and $4,287.73 for XR-NTX. When considering both effectiveness and costs, BMT is predicted to be dominated by MMT (see Figure 2). The predicted incremental cost-effectiveness ratio (ICER) of XR-NTX compared to MMT is approximately $72 per opioid-free day gained.
Figure 2.
Frontier Plot of Incremental Cost Effectiveness Across Treatments
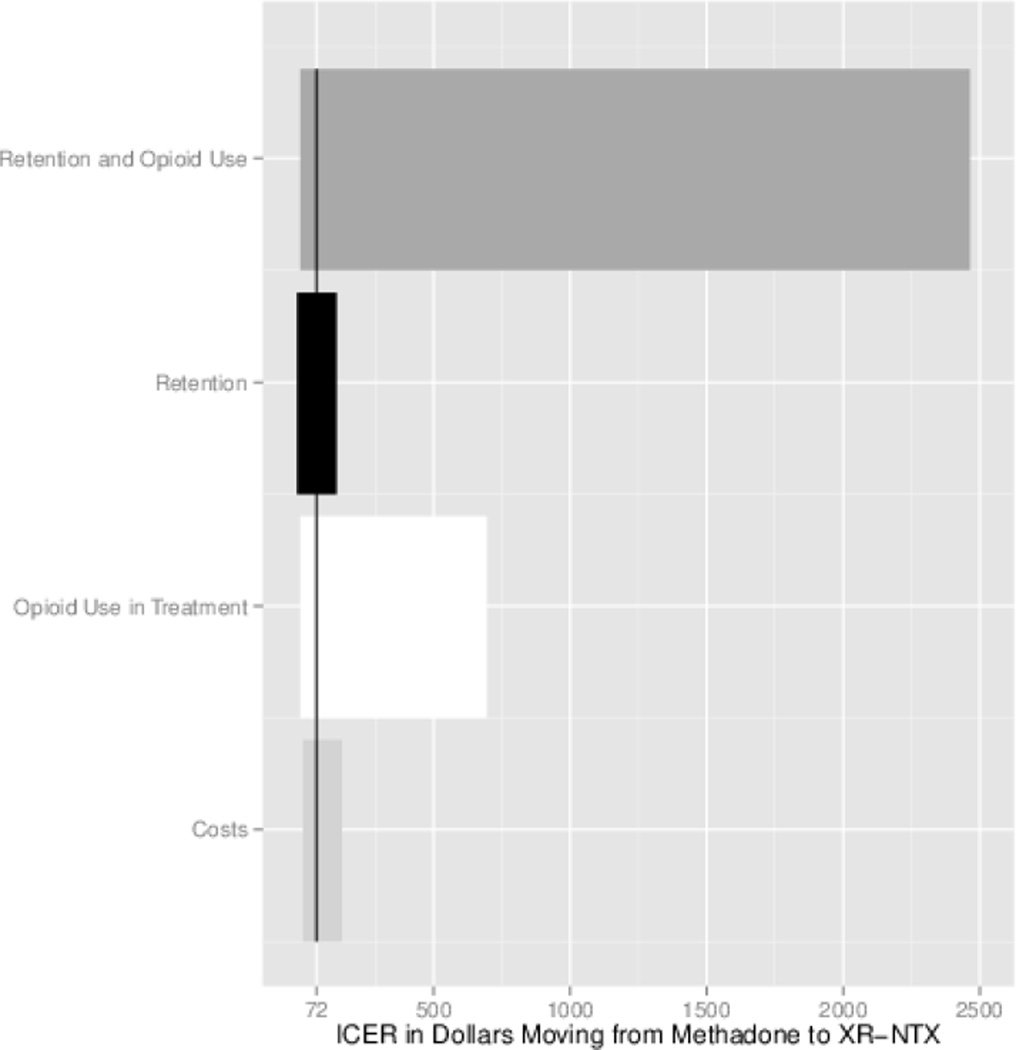
Our best-worst case scenario analysis with respect to treatment cost suggested a lower and upper bound of cost-effectiveness of XR-NTX compared to MMT of $50 and $93 per opioid-free day gained, respectively. Scenario analysis varying the probability of opioid use while under XR-NTX treatment suggested a lower bound of cost-effectiveness of XR-NTX compared to MMT of $60 when there is no opioid use while in XR-NTX treatment to $633.38 when the probability of opioid use under XR-NTX treatment is 50%. Notably, the ICER point estimate of $72 per opioid-free day gained is much closer to the lower bound than the upper bound. Varying only patient retention in treatment in line with the studies conducted in the U.S. produces only slight changes in estimated cost-effectiveness, with a lower and upper bound of $71 and $73 per opioid-free day gained, respectively, when daily probability of remaining in treatment is 70% (as observed in Bisaga et al.)24 and 45% (as observed in Comer et al.).23 When both patient retention and abstinence are varied, the ICER ranges from $59.80 when 70% of patients are retained in treatment and opioid use in treatment is held at 0 to $2,392.75 when 45% of patients are retained in treatment and opioid use in treatment is held at 50%.
DISCUSSION
This study used a Markov model to conduct a cost-effectiveness analysis of XR-NTX compared to other commonly-used pharmacological treatments (MMT and BMT) for U.S. adult males with opioid dependence initiating pharmacological treatment from the state addiction treatment payer’s perspective. Our base case results suggest that XR-NTX is cost-effective if state health payers are willing to pay at least $72 per opioid-free day gained, about the cost of treating three patients with methadone for one day. Public payers are well aware of the trade-off between the number of people treated and the cost of treatment. Because payers set different cost-effectiveness thresholds, the implications of this study will vary by payer. The cost effectiveness analysis is meant to be an input to the decision process and not the sole factor for consideration. Other factors such as patient preferences, medication side effects, and efficacy in treating other conditions may be important payer concerns and factor into decision-making. Payers should also consider limitations to these cost-effectiveness estimates, which are discussed in detail below.
Because payers, treatment seekers, and the general public value different outcomes, examining a variety of costs and consequences when considering whether to implement a new technology is important.25 We believe our chosen effectiveness measure of opioid-free days is significant for public payers. Abstinence within treatment has long been theoretically associated with improved post treatment outcomes22 and is often the most important outcome for payers as well as the patient and public at large.
Ideally, our study would have also included potential variation in mortality and quality of life under conditions of abstinence and opioid use, allowing us to express cost-effectiveness in terms of incremental cost per QALY gained. Such a measure would allow for broader health resource allocation decisions across a wide range of conditions. We would have ideally also included the cost of adverse events. However, because the probability of an adverse event was extremely low in the study time frame, and high attrition makes it difficult to measure adverse events associated with treatment, we lacked adequate data to include the costs or consequences of adverse treatment events in our model. Some payers may be interested in the effectiveness of each medication in deterring other forms of substance abuse as XR-NTX has been approved for treating alcohol dependence as well.26 Yet, following cost-effectiveness analyses of buproprion,27 we did not look at any additional positive benefits because of limited information on the efficacy of these medications for simultaneously deterring different types of substance abuse.
We are aware of only one other study addressing the economic consequences of XR-NTX utilization since its approval for use among opioid dependent patients in 2011. In that study, Baser and colleagues used retrospective data from a U.S. commercial health plan to assess the cost and utilization outcomes of XR-NTX compared to oral naltrexone, BMT and MMT, finding that although the drug cost of XR-NTX is much higher than the other comparators, total healthcare costs among patients receiving XR-NTX were lower compared to patients receiving MMT.15 Our study extends upon the work by Baser and colleagues by also considering health outcomes related to opioid use and examining a different population. Cases in Baser’s study were covered by a commercial health care plans, while our study estimates costs assuming retention and opioid use rates observed in clinical trials. Similarly, as patients enrolling in clinical trials may differ from populations served by payers, some patient factors not examined here, like patient preference, race, gender, age and other co-occurring conditions, may affect the cost estimates and payer recommendations. Future work in this policy area should include a full budget impact analysis.
Unlike Baser et al.,15 we did not consider the effect of each treatment on other, non-addiction treatment, healthcare costs. Public sources currently finance a majority of specialty substance abuse treatment.28 Much of this funding is administered separately from other healthcare spending, either through carve-out programs or categorical spending,29 so other healthcare costs may not be a priority for decision makers currently involved with the consideration of cost of addiction treatment. This study examined costs and consequences only from the state addiction treatment payer’s perspective and did not consider other societal or patient costs or consequences. Studies that consider costs and consequences for patients or communities may come to different conclusions about the cost-effectiveness of these treatments. However, the state substance abuse agencies’ perspective is likely to be the most policy relevant perspective as it is the one most likely to affect access to treatment. Decision makers in state substance abuse agencies may not be swayed by cost offsets to other systems because those savings do not have an impact on the payer’s budget.30
Based on sensitivity analysis, we believe our most significant limitation involves a relative lack of evidence on the effectiveness of XR-NTX in a U.S. population. Our base case source of clinical evidence for XR-NTX comes from a study conducted in a Russian population6 which may involve cultural factors associated with greater compliance with XR-NTX treatment than would be expected in the United States.31 It is noteworthy, however, that the Russian study was cited as pivotal evidence in the FDA’s approval of XR-NTX. One study, conducted in the U.S. but using a non-licensed formulation of XR-NTX, found considerably lower patient retention and opioid abstinence in a population of patients in the U.S.23 A second U.S. study, using a licensed formulation of XR-NTX but lasting only 12 weeks, seemed to support the previous findings that patient retention and opioid abstinence may be substantially lower for a U.S. population. In this study, only 70% of patients were retained for the 12 week period and some 64% of patients reported using opioids at least once in the month following their first injection of XR-NTX and 43% reported using opioids at least once in the month following the second injection.24 While it cannot be determined to what extent these two U.S.-based studies’ results differed from the Russian study due to differences in patient population, medication formula or study design, we felt it useful to allow the relatively poor performance of XR-NTX in the U.S. to inform our wide range of effectiveness for sensitivity analysis.
In contrast to XR-NTX, MMT has been used for over 50 years and BMT for more than 10 years in the U.S., so the cost-effectiveness of both treatments compared to each other and to placebo has been extensively studied.32,33 Meta-analyses have clearly shown that both treatments are significantly more effective than placebo.16 However, comparisons between MMT and BMT have had mixed results, depending on the outcome evaluated and the perspective taken. Generally, as reflected in our simulation results, retention in treatment is higher for MMT, while BMT has slightly lower opioid use within treatment.9,16 Sensitivity analyses show that while uncertainty in relative costs of pharmacotherapies across states may alter the estimated cost-effectiveness of XR-NTX compared to MMT, the uncertainty in effectiveness is more consequential.
Given the current budget climate among most public payers in the United States, as well as the drastic increase in opioid dependence over the past ten years, identifying cost-effective strategies to treat opioid dependence is a public health priority. Our model suggests that XR-NTX is cost-effective for treatment for males initiating pharmacotherapy for opioid dependence if state addiction payers are willing to pay at least $72 per opioid free day gained. Conclusions about cost-effectiveness may vary slightly based on relative treatment costs across states and could be limited to the extent that available data on the effectiveness of XR-NTX is limited to a single clinical trial conducted in a non-US population with possibly different patterns of treatment adherence.
Figure 3.
Scenario Based Sensitivity Analyses
Acknowledgments
sHeide Jackson is supported by the Center for Demography and Ecology Center Grant (P2C HD047873) and Population, Life Course and Aging (T32 AG00129). Kara Mandell is supported by the Agency for Healthcare Research and Quality (T32 HS00083; Principal Investigator [PI]: M. Smith). Debanjana Chatterjee is supported by the Bureau of Health Professions T32HP22239 (PI: Borowsky). Kimberely Johnson has previously received funding from Alkermes.
Footnotes
The authors declare that they currently have no conflicts of interest.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 2.Web-based Injury Statistics Query and Reporting System (WISQARS) [online] [Accessed 5 November 2014];2014 http://www.cdc.gov/injury/wisqars/fatal.html.
- 3.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 4.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61(2):195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 5.Lynch FL, McCarty D, Mertens J, et al. Costs of care for persons with opioid dependence in commercial integrated health systems. Addict Sci Clin Pract. 2014;9(1):16. doi: 10.1186/1940-0640-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. The Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 7.Rappaport R. Overview of the September 16, 2010 PDAC Meeting to Discuss Efficacy Supplement to NDA 21-897 for Vivitrol (naltrexone for extended-release injectable suspension) for prevention of relapse in recently-detoxified opioid dependent patients [Memorandum] [Accessed 05 November, 2014];2010 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/ucm225655.pdf.
- 8.McCarty D, Perrin NA, Green CA, Polen MR, Leo MC, Lynch F. Methadone maintenance and the cost and utilization of health care among individuals dependent on opioids in a commercial health plan. Drug Alcohol Depend. 2010;111(3):235–240. doi: 10.1016/j.drugalcdep.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009:3. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-Assisted Therapies — Tackling the Opioid-Overdose Epidemic. N Engl J Med. 2014;370(22):2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 11.Drug Addiction Treatment Act of 2000 (DATA 2000), Title XXXV, Section 3502 [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration. An Introduction to Extended-Release Injectable Naltrexone for the Treatment of People With Opioid Dependence. Advisory. 2012;11(1) [Google Scholar]
- 13.Ling W, Mooney L, Wu LT. Advances in opioid antagonist treatment for opioid addiction. Psychiatr Clin North Am. 2012;35(2):297–308. doi: 10.1016/j.psc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobmaier P, Kornor H, Kunoe N, Bjorndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev. 2008:2. doi: 10.1002/14651858.CD006140.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(8):S235. [PubMed] [Google Scholar]
- 16.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:2. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2006:1. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Fareed A, Vayalapalli S, Casarella J, Drexler K. Treatment outcome for flexible dosing buprenorphine maintenance treatment. Am J Drug Alcohol Abuse. 2012;38(2):155–160. doi: 10.3109/00952990.2011.643988. [DOI] [PubMed] [Google Scholar]
- 19.Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35(1):28–33. doi: 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health and Clinical Excellence. London: National Institute for Health and Clinical Excellence; 2007. [Accessed 05 November 2014]. NICE Technology Guidance: Methadone and buprenorphine for the management of opioid dependence [TA114] http://www.nice.org.uk/guidance/TA114/chapter/1-Guidance. [Google Scholar]
- 21.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Geneva: World Health Organization; 2009. [Accessed 05 November 2014]. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. http://www.who.int/substance_abuse/publications/opioid_dependence_guidelines.pdf. [PubMed] [Google Scholar]
- 23.Comer SD, Sullivan MA, Yu E, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(2):210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisaga A, Sullivan MA, Glass A, et al. A placebo-controlled trial of memantine as an adjunct to injectable extended-release naltrexone for opioid dependence. J Subst Abuse Treat. 2014;46(5):546–552. doi: 10.1016/j.jsat.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.French MT, Zavala SK, McCollister KE, Waldron HB, Turner CW, Ozechowski TJ. Cost-effectiveness analysis of four interventions for adolescents with a substance use disorder. J Subst Abuse Treat. 2008;34(3):272–281. doi: 10.1016/j.jsat.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Substance Abuse and Mental Health Services Administration. Naltrexone for Extended-Release Injectable Suspension for Treatment of Alcohol Dependence. Advisory. 2007;6(1) [Google Scholar]
- 27.Hall S, Lightwood J, Humfleet G, Bostrom A, Reus V, Muñoz R. Cost-effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. J Behav Health Serv Res. 2005;32(4):381–392. doi: 10.1007/BF02384199. [DOI] [PubMed] [Google Scholar]
- 28.Mark T, Levit K, Buck J, Coffey R, Vandivort-Warren R. Mental health treatment expenditure trends, 1986–2003. Psychiatr Serv. 2007;58(8):1041–1048. doi: 10.1176/ps.2007.58.8.1041. [DOI] [PubMed] [Google Scholar]
- 29.Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Affair. 2011;30(8):1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- 30.Humphreys K, Wagner TH, Gage M. If substance use disorder treatment more than offsets its costs, why don't more medical centers want to provide it?: A budget impact analysis in the Veterans Health Administration. J Subst Abuse Treat. 2011;41(3):243–251. doi: 10.1016/j.jsat.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Krupitsky E, Zvartau E, Woody G. Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr Psychiatry Rep. 2010;12(5):448–453. doi: 10.1007/s11920-010-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doran CM, Shanahan M, Mattick RP, Ali R, White J, Bell J. Buprenorphine versus methadone maintenance: a cost-effectiveness analysis. Drug Alcohol Depend. 2003;71(3):295–302. doi: 10.1016/s0376-8716(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 33.Harris AH, Gospodarevskaya E, Ritter AJ. A randomised trial of the cost effectiveness of buprenorphine as an alternative to methadone maintenance treatment for heroin dependence in a primary care setting. Pharmacoeconomics. 2005;23(1):77–91. doi: 10.2165/00019053-200523010-00007. [DOI] [PubMed] [Google Scholar]