Abstract
Tumor resistance to cytotoxic drugs is one of the main obstacles to successful cancer therapy. Emerging evidence suggests that chemoresistance is promoted by substances released from dead and damaged cells that activate the host repair program orchestrated by Toll-like receptor-4 (TLR4). TLR4 is often overexpressed in malignant and tumor infiltrating immune cells. In addition to endogenous ligands released by therapy-induced tumor destruction, TLR4 is directly activated by paclitaxel, one of the most commonly used chemotherapeutic drugs against various human cancers. TLR4 activation promotes local and systemic inflammation leading to induction of multiple circuits that create a regenerative environment favoring local recurrence and metastasis. Of particular importance is TLR4-mediated recruitment of endothelial progenitors derived from immature myeloid cells. These cells play a major role in rebuilding tumor-associated lymphatic and blood vessels thereby promoting lymphatic and hematogenous metastasis. The latter is further enhanced by the premetastatic niche generated by mobilization of myeloid provascular cells to distant organs. This review summarizes the recent evidence demonstrating that paclitaxel and other clinically used anticancer drugs actively induce metastasis even while shrinking the primary tumor. Better understanding of the mechanisms underlying TLR4 dependent chemotherapy-driven metastasis might be the key to overcoming challenges of cancer eradication.
Keywords: TLR4, paclitaxel, chemoresistance, recurrence, vessel formation, metastasis
INTRODUCTION
Cytotoxic drugs remain the main, and sometimes the only, therapeutic modality for advanced, refractory, and metastatic tumors as well as for those cancers lacking a specific molecular target. The main drugs used clinically for anticancer therapy are anthracyclines, platin-based, and various taxanes. Although these drugs are highly efficacious in killing cultured tumor cells, application of chemotherapy to cancer patients results in up to 40% of recurrence at a primary, locoregional or distant site (1). The underlying mechanisms of recurrence are multifaceted. Recent studies suggest that, in addition to genetic alterations in malignant cells, the local tumor environment and systemic host responses to injury strongly contribute to reducing drug efficacy (2–4). Moreover, some studies show that chemotherapy by itself can instigate metastatic spread while simultaneously restraining growth of the primary tumor (5–7). Such counterintuitive outcomes of cytotoxic therapies might be explained by two synergistic events: the natural tendency of the host to protect and repair sites of injury coupled with limitations of the immune system to recognize all malignant cells as non-self. The host response to chemotherapy-inflicted tumor destruction is remarkably similar to that occurring during sepsis, chronic inflammation, and other deviations from homeostasis. In all of these instances, the main host receptor that senses and responds to tissue damage is Toll-like receptor-4 (TLR4) (8). Activation of TLR4 and similar receptors in immune cells fulfills several important defense functions such as increased survival, motility, and invasion of pathogen-fighting cells that allow them to quickly access the affected site and destroy the invaders. One of the important functions of TLR4 signaling is to rebuild damaged vasculature at the site of injury in order to maintain continuous communication with the host. This is achieved by expanding the pools of provascular progenitors in the bone marrow (BM) and spleen followed by their recruitment to inflamed or remodeled tissue. In the context of cancer, these normal host responses to injury promote metastasis because they endow the tumor cells surviving chemotherapy with increased invasive capacity while simultaneously providing the means for their transportation. To make things worse, one of the most commonly used drugs, paclitaxel (PXL), directly stimulates TLR4 (9). Coupled with tumor-protective host mechanisms, PXL therapy might be a driving force for metastasis, particularly in patients with advanced tumor burden or genetic predisposition to evasion of apoptosis. This review will summarize the current understanding of the mechanisms mediated by host immune cells and cytotoxic drugs that cumulatively promote rather than inhibit metastatic behavior. Validation of this concept could fundamentally change the clinical paradigms for cancer therapy to account for unwanted consequences of an activated TLR4 pathway and the host responses to tissue loss.
Biological modifiers of the tumor response to chemotherapy in vivo
Initially, overexpression of drug-excluding pumps in tumor cells was thought to be the main reason for resistance to cytotoxic therapy (10). However, after decades-long exploration, pharmacological inhibition of these pumps failed to eliminate the problem. This suggested that alternative powerful mechanisms in vivo may contribute to limitations of cytotoxic therapies for cancer treatment. This conclusion is supported by extensive evidence showing the protective effect of the tumor environment (11) including upregulation of prosurvival proteins in tumor and tumor-associated cells. Superficially, the idea that cytotoxic drugs enhance survival of malignant cells appears counterintuitive. It is consistent, however, with well-known physiological responses to the organ damage, necrosis, and hypoxia that invariably occur due to collapse of blood vasculature during tissue loss. Such outcomes of successful chemotherapy are typically compensated by massive influx of progenitors programmed to restore homeostasis by rebuilding epithelial, stromal, and vascular structures in damaged tissue. Therefore, tumor eradication in vivo may depend not only on genetically-dictated sensitivity of malignant cells to cytotoxic drugs but also on an inflammation-amplifying host response caused by drug-induced damage (11).
Paclitaxel as a prototype of anticancer drugs with functionally opposing effects on tumor growth
PXL is an excellent example of an anticancer drug that both efficiently kills tumor cells and promotes their survival. PXL cytotoxicity is mediated by binding to beta-tubulin, an event that over-stabilizes microtubules leading to interruption of the cell cycle, blockade of mitosis, and eventually, cell death (12). While early studies demonstrated undeniable efficacy of PXL against many types of metastatic and refractory cancers (13), they also showed drug-specific activation of the NF-κB pathway leading to transcriptional induction of numerous inflammatory genes (14). The shift to pro-inflammatory phenotype induced by PXL was detected in mouse macrophages (9) and human tumor cells (14) as well as in the blood of breast cancer patients receiving PXL monotherapy (15). Studies in vitro showed that the kinetics and expression profile of PXL-altered genes strongly resemble those induced by lipopolysaccharide (LPS) (16,17), a pathogen-derived molecule with strong inflammatory properties. Initially, PXL-mediated inflammatory response was thought to enhance the tumoricidal effects of immune cells, and therefore, was regarded as beneficial for cancer patients (14). Subsequent studies showed that PXL-induced inflammatory mediators promote tumor cell survival (18) suggesting that drug-associated inflammation blunts tumor sensitivity to anticancer drugs (19) rather than helping to mount the anti-tumor defense. Although many questions in this area of investigation are still open, multiple studies using human clinical cancers favor the hypothesis that PXL-induced inflammation leads to progression of tumor growth. Studies in breast, ovarian, prostate, and other tumors showed that TLR4, a natural LPS receptor, is highly upregulated in malignant epithelial cells (20–22), and PXL signals through TLR4 (21,23). Because PXL is a functional mimetic of LPS (9), both compounds elicit nearly identical responses in TLR4-positive myeloid cells and in cancer cells with upregulated TLR4 expression. PXL similarly to LPS causes dimerization of TLR4 which, in turn, activates the NF-kB pathway (23) leading to upregulation of numerous inflammatory, migratory, and prosurvival proteins (e.g., VEGF-A, COX2, IL-6, IL-8, MMP9, XIAP, and Bcl-2) (24–26). These proteins cumulatively confer resistance to cell death (26), evasion from immunosurveillance (27), and increased metastasis (6). Consistently, silencing TLR4 expression restores tumor cell sensitivity to PXL in vitro (21,23) and correlates with significant increase in tumor-free animals after chemotherapy in breast tumor models in vivo (6,23). These studies suggest that while PXL is highly toxic to TLR4-negative or low-expressing tumor cells, it is cytoprotective or even growth-promoting for cancer cells expressing high TLR4 levels. Therefore, TLR4 could be an important discriminating marker to distinguish between PXL-responsive and -resistant cancers.
Mechanisms by which paclitaxel therapy promotes metastasis
If cytotoxic drugs could be administrated at the unlimited dose and time required for tumor eradication, the ability of these drugs to kill tumor cells would probably prevail over tumor-promoting actions. However, since all cytotoxic therapies are restricted by toxicities, the drug pro-oncogenic effects on surviving tumor cells can translate into local and distant recurrence. PXL, specifically, has been shown to promote metastasis by multiple mechanisms. The primary effects on TLR4-positive tumor cells are: 1) enhancement of tumor inflammation (23), the hallmark of the activated TLR4 pathway and a well-known promoter of tumor growth; 2) induction of a migratory/invasive phenotype in tumor cells (28), an essential prerequisite for escaping the primary tumor site; and 3) upregulation of prosurvival Bcl-2, XIAP, and Bcl-XL proteins (29) that promote resistance to anoikis and successful establishment of metastatic lesions. Although all these activities promote metastatic behavior, PXL’s ability to increase tumor cell invasion might be of particular significance. In the screen using 1,300 compounds and a variety of human cancer lines, PXL was shown as one of the most potent inducers of invadopodia, i.e., cell protrusion required for invasion (28). This prometastatic effect of PXL is exponentially magnified by a regenerative environment of the primary tumor created by inflamed tumor cells and bone marrow recruited monocytes (23).
PXL induced inflammation in tumor cells is phenotypically identical to that induced by LPS in normal immune cells. However, the magnitude of the response might be much higher in the context of cancer because particularly large tumors can generate inflammatory conditions greatly surpassing the outcome of tissue-recruited mononuclear infiltrates. Qualitatively, activation of TLR4 in either situation aims at restoration of homeostasis by remodeling the damaged tissue while protecting the residual cells during repair. Consistent with this concept, we recently demonstrated in breast cancer models that PXL supports TLR4+ tumors by expanding provascular BM progenitors and recruiting them to the damaged site (6) as well as by the local upregulation of inflammatory cytokines and their receptors in tumor cells thus creating autocrine tumor-protecting loops (23).
One of the most deleterious consequences of tumor activation of TLR4 results is mobilization of provascular progenitors to the damaged site. BM-derived cells (BMDC) that include hematopoietic, myeloid, and endothelial progenitors have been repeatedly implicated in promoting the growth of primary tumors and priming the distant sites to favor extravasation and metastatic outgrowth (30). Human BMDC are significantly increased in PXL-treated tumors as shown, for instance, by a 5-fold increase of CD14+ myeloid progenitors in breast cancers of treated women relative to untreated patients (31). PXL can also directly mobilize BMDC to the lungs as shown in non-tumor bearing mice with a significantly higher number of lung homing tumor cells in treated animals relative to controls (7). Consequently, drug-treated hosts had a significantly higher number of pulmonary metastases relative to untreated mice (7). PXL can also activate TLR4 in blood vascular endothelial cells, which may explain the direct effect of PXL on pulmonary vessel remodeling (4). The exposure of endothelial cells to PXL in vivo impaired the vascular barrier leading to increased tumor cell lodging and consequent metastasis (4). We recently showed that these effects of PXL on preparing the distant organs for potential tumor invasion are amplified by systemic inflammation recorded in the blood, lungs, spleen, lymph nodes, and BM. Induction of these systemic inflammatory circuits substantially increases pools of myeloid-derived provascular progenitors that subsequently are recruited to the tumor and distant organs and promote vascular outgrowth (6).
One of the earliest events in either physiological tissue repair or tumor recovery after chemotherapy is regeneration of blood vasculature. Supporting this concept, multiple studies show that chemotherapy-induced provascular BMDC play a paramount role in induction of tumor angiogenesis and consequent hematogenous metastasis (32). We recently expanded this concept by demonstrating that PXL therapy of TLR4-positive (but not negative) breast tumors also increases the number of lymphatic vessels that were highly permissive for invasion by malignant cells (6). The preferential invasion of the lymphatics might be due to their defective formation deep inside the recurring tumor - an unusual site for tumor-induced lymphatics even in metastatic cancers. Albeit deformed, the new intratumoral lymphatics (in mice with TLR4-positive tumors treated with PXL) increased the burden of lymph node metastases by more than 250-fold relative to untreated animals or tumors lacking TLR4 (6). These studies attest to the alarming potential of PXL to augment metastasis of TLR4-positive tumors by direct upregulation of prometastatic genes in neoplastic cells, and indirectly, through recruitment of immature BMDC that contribute to formation of new tumor vasculature.
Other cytotoxic drugs with prometastatic activities
The pro-oncogenic effects of chemotherapy are not limited to PXL by virtue of the fact that TLR4 can be activated by 20 or more endogenous ligands released from damaged or dead cells (33). The list of TLR4-activating factors includes many danger-sensing proteins known as “alarmins” such as HMGB1, S100A8/A9, and various heat-shock proteins (33). It should be mentioned that host response to alarmins is very complex and the endogenous ligands of TLR4 can generate pro-oncogenic effects via various TLRs (34) as well as additional homeostasis-maintaining receptors (35). In fact, it has not been firmly established that functional effects of PXL are mediated through physical binding to TLR4. Alternatively, PXL might activate TLR4 indirectly by alarmins released from dead cells. If alarmins are ultimately responsible for activation of TLR4, any cytotoxic drug can potentially generate a pro-inflammatory response which might be sufficient to rescue residual cells from apoptosis thereby initiating tumor re-growth.
This idea is strongly supported by mounting evidence indicating that several structurally unrelated drugs induce tumor and host responses that strikingly resemble the effects of PXL. Activation of NF-κB and resultant inflammatory response have been noted after tumor or host cell exposure to at least five drugs other than PXL including doxorubicin, 5-fluoracil (5-FU), oxaliplatin, cyclophosphamide (CTX), and cisplatin (36). Similarly to PXL, stimulation of the NF-κB pathway by these drugs led to activation of the MAPK pathway, upregulation of the pro-inflammatory AP-2 transcription factor (37), and overexpression of inflammatory (36), prosurvival (29), and migratory genes (38). The TLR4 dependent inflammatory response induced by various chemodrugs was also implicated in promoting epithelial-mesenchymal transition (36) and selecting tumor cells with the stem-like phenotype (39). Acquisition of these traits is strongly associated with metastatic behavior reflecting the diversity of mechanisms by which TLR4, in conjunction with chemotherapy, can promote metastasis.
PXL and other cytotoxic agents share an additional functional aspect: all chemodrugs prompt BM to dispatch massive amounts of myeloid progenitors to the tumor. Studies with various chemodrugs also showed that the circulating BM-derived progenitors can be absorbed by the lungs (4,7,37). This event may initiate the preparation of the premetastatic niche because lung-recruited BMDC secrete inflammatory and vascular remodeling factors that increase extravasation of circulating tumor cells (4,40), and create a tissue-regenerative environment (4). Thus, inflammatory factors produced by tumor TLR4 initiate the development of the niche while cytokines produced by lung-recruited BMDC advance its formation.
Prometastatic effects of several cytotoxic drugs have recently been reported in multiple animal models and clinical studies. Doxorubicin injected into non-tumor bearing mice has been shown to induce the formation of the inflammasome (a multi-protein complex that generates mature IL-1β) in BM cells (41). This is significant because fully-processed IL-1β induces a secondary inflammatory wave by upregulating IL-6, G-CSF, and other mediators (41). An unrelated drug, CTX, has been shown to promote the growth of primary tumors (42), BMDC homing to the lungs (43), enhancement of bone (5) and lung (43) metastases in mice, and increased circulating myeloid-derived suppressor cells in cancer patients (44). The impact of the CTX-induced and host-propagated prometastatic effect can be as high as a 1,000-fold increase in pulmonary lesions formed by circulating tumor cells in drug-pretreated mice relative to controls (45). Similarly, pretreatment with cisplatin increased influx of provascular BMDC to primary tumors and the burden of pulmonary metastases by 6–10 fold (4). Cisplatin also significantly shortened tumor growth doubling time in lung cancer patients (46). A similar compound, oxaliplatin, increased spontaneous metastasis in an orthotopic mouse cancer model (38). Collectively, these studies suggest that damage-sensing receptors might be ultimately responsible for induction of the cytoprotective and provascular inflammatory responses that negate the clinical benefits of cytotoxic therapies.
Summary and Perspectives – how to eliminate tumor-promoting effects of chemotherapy
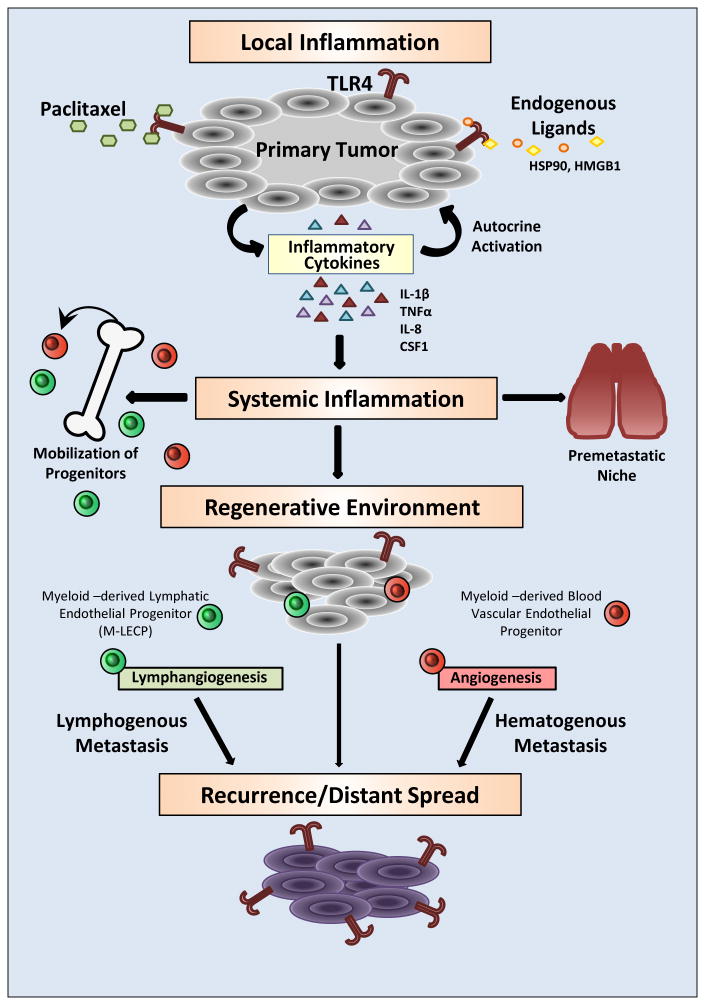
Emerging evidence from multiple tumor models and clinical studies suggests a distinct possibility that commonly used anticancer drugs promote tumor cell survival and facilitate metastasis in addition to their cytotoxic effects. The main culprit underlying the prometastatic effects of chemotherapy appears to be drug-induced inflammation (Fig. 1). This response is induced by TLR4 and similar damage-sensing receptors that bear natural responsibility for restoring homeostasis to the injured tissue. In the context of TLR4-positive tumors, this local response is rapidly translated to systemic inflammation through excessive production of mediators released to the blood that expand the pools of BM and spleen progenitors. While drug-induced apoptosis shrinks the tumor, upregulated products of the activated TLR4 pathway protect the residual tumor cells and enhance their dissemination. This prometastatic trend is strongly amplified by the host “misreading” the damage signals from the drug-destroyed tumor. The host translates these signals into a massive regenerative effort led by provascular and immunosuppressive BMDC. The widely documented immunosuppressive phenotype of tumor BMDC (47) is congruent with the late immune cell function of re-building the injured tissue rather than their early role in attacking undesirable cells. Once mobilized to the tumor, BMDC promote tumor vasculature that connects new blood and lymphatic vessels to systemic circulation. Access to new vasculature might be a deciding event in post-therapy tumor spread, also favored by the cytoprotective atmosphere of the remodeled tumor mass. BMDC also support distant metastasis because circulating immature progenitors lodge into the lungs and other organs where their vascular and tissue remodeling properties enhance extravasation of tumor cells and sustained expansion of metastatic colonies, respectively (Fig. 1).
Figure 1. Prometastatic consequences of TLR4 activation in cancer cells driven by chemotherapy.
TLR4 expressed in tumor cells can be activated by a chemotherapeutic drug, paclitaxel, and numerous endogenous ligands including HSP90 and HMGB1. TLR4-activated NF-κB signaling leads to upregulation of metalloproteinases and inflammatory cytokines that enhance tumor motility and local inflammation. This proinflammatory shift protects tumor cells from the toxicity of anticancer drugs and endows them with better capacity to migrate out of the primary site. Excessive cytokines released to the blood generate systemic inflammation that causes expansion of bone marrow progenitors and their mobilization to the tumor. Blood-circulating progenitors also lodge in the lungs where they generate a highly receptive environment for passing tumor cells. Myeloid-derived blood vascular and lymphatic endothelial progenitors (M-LECP) create a regenerative environment typified by accelerated formation of new blood and lymphatic vessels. Increased migratory and survival potentials of tumor cells, coupled with increased blood and lymphatic vessel densities, results in local recurrence as well as lymphatic and distant metastasis. Systemic dissemination is also supported by generation of the premetastatic niche in lungs and other organs. Cumulatively, these events lead to multifocal disease largely resistant to the tumoricidal effects of cytotoxic therapies.
While TLR4 activation in malignant cells clearly benefits the tumor, stimulation of this pathway in immune cells might have both pro- and anticancer consequences. For instance, immune cells depend on TLR4 activation for effective antigen presentation and recruitment of cytotoxic T-cells. This may result in decreased growth of primary tumors due to chemotherapy-induced TLR4 signaling in BMDC and other immune cells (48). This “double-edged sword” role of immune cells in chemotherapy highlights the pressing need to define local tumor and systemic circumstances that lead to TLR4 tumor-suppressing effects as opposed to pro-metastatic actions.
Future studies could clarify this point by providing answers to several outstanding questions. First, it should be clearly determined in clinical cancers whether the expression of TLR4 or other TLRs correlates with their metastatic and therapy-resistant potentials. Some work on this aspect has already been done (20) but the direct link between recurrence and cancer expression of TLRs has not been established. Second, future studies should determine whether TLR expression in malignant or tumor-associated cells can serve as a biomarker for sensitivity to cytotoxic therapy. Potential utility of TLR tumor expression might significantly improve early clinical recognition of difficult-to-treat tumors and tailor the therapy accordingly. Third, it is essential to define the prometastatic and therapy-evasion mechanisms mediated by TLR-dependent damage-responsive pathways. It has been established that activation of these pathways protects tumor cells, generates new vessels, and enhances tumor spread. However, many details of these processes are unknown or poorly understood. We do not know the identity of all receptors sensing chemotherapy-induced tumor injury nor do we clearly understand the molecular basis of interactions of TLRs with anticancer drugs and endogenous ligands. It is possible, for instance, that analogous to LPS, drug activation of TLR4 requires specific co-receptors; however, this important aspect of TLR4 activation by cytotoxic drugs is currently unknown. Lastly, the use of experimental models can help to assess the ability of TLR-targeting drugs to sensitize tumors to cytotoxic therapies as well as to intercept lymphatic and distant metastasis. Here, translational science should benefit from the plethora of existing TLR4 antagonists generated to combat sepsis that have already demonstrated an acceptable safety profile in human subjects. Cumulatively, exploration of these questions should provide the mechanistic details of TLR-mediated resistance to cytotoxic therapies and be a significant step forward in addressing current challenges to eradicating cancer.
Acknowledgments
This work was supported by NIH grant 5R01CA140732, and by grants from the Simmons Cancer Institute at the Southern Illinois University School of Medicine and the William E. McElroy Charitable Foundation awarded to Sophia Ran. The author is grateful to Kelly Hall for help with constructing the Figure 1 and to Susan Ryherd with the Center for Clinical Research at SIU for very helpful suggestions and thorough editing of the manuscript.
Abbreviations
- 5-FU
5-fluoracil
- BC
breast cancer
- BM
bone marrow
- BMDC
bone marrow derived cells
- CTX
cyclophosphamide
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- LPS
lipopolysaccharide
- MMPs
metalloproteinases
- PXL
paclitaxel
- TLRs
Toll-like receptors
- TLR4
Toll-like receptor-4
Footnotes
Disclosure of Conflict of Interests: The author discloses no potential conflicts of interest
References
- 1.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 2.Roodhart JM, He H, Daenen LG, Monvoisin A, Barber CL, et al. Notch1 regulates angio-supportive bone marrow-derived cells in mice: relevance to chemoresistance. Blood. 2013;122:143–153. doi: 10.1182/blood-2012-11-459347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daenen LG, Houthuijzen JM, Cirkel GA, Roodhart JM, Shaked Y, et al. Treatment-induced host-mediated mechanisms reducing the efficacy of antitumor therapies. Oncogene. 2014;33:1341–1347. doi: 10.1038/onc.2013.94. [DOI] [PubMed] [Google Scholar]
- 4.Daenen LG, Roodhart JM, van AM, Dehnad M, Roessingh W, et al. Chemotherapy enhances metastasis formation via VEGFR-1-expressing endothelial cells. Cancer Res. 2011;71:6976–6985. doi: 10.1158/0008-5472.CAN-11-0627. [DOI] [PubMed] [Google Scholar]
- 5.Park SI, Liao J, Berry JE, Li X, Koh AJ, et al. Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Res. 2012;72:2522–2532. doi: 10.1158/0008-5472.CAN-11-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk-Draper L, Hall K, Griggs C, Rajput S, Kohio P, et al. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014;74:5421–5434. doi: 10.1158/0008-5472.CAN-14-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gingis-Velitski S, Loven D, Benayoun L, Munster M, Bril R, et al. Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice. Cancer Res. 2011;71:6986–6996. doi: 10.1158/0008-5472.CAN-11-0629. [DOI] [PubMed] [Google Scholar]
- 8.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, et al. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 9.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 11.Atsumi T, Singh R, Sabharwal L, Bando H, Meng J, et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014;74:8–14. doi: 10.1158/0008-5472.CAN-13-2322. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5 (Suppl 6):S3–S6. [PubMed] [Google Scholar]
- 13.Guchelaar HJ, ten Napel CH, de Vries EG, Mulder NH. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol. 1994;6:40–48. doi: 10.1016/s0936-6555(05)80367-x. [DOI] [PubMed] [Google Scholar]
- 14.Bewick M, Conlon M, Lee H, Parissenti AM, Zhang L, et al. Evaluation of sICAM-1, sVCAM-1, and sE-Selectin levels in patients with metastatic breast cancer receiving high-dose chemotherapy. Stem Cells Dev. 2004;13:281–294. doi: 10.1089/154732804323099217. [DOI] [PubMed] [Google Scholar]
- 15.Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Henricson BE, Carboni JM, Burkhardt AL, Vogel SN. LPS and Taxol activate Lyn kinase autophosphorylation in Lps(n), but not in Lpsd), macrophages. Mol Med. 1995;1:428–435. [PMC free article] [PubMed] [Google Scholar]
- 17.Manthey CL, Brandes ME, Perera PY, Vogel SN. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992;149:2459–2465. [PubMed] [Google Scholar]
- 18.Duan Z, Feller AJ, Penson RT, Chabner BA, Seiden MV. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology: analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res. 1999;5:3445–3453. [PubMed] [Google Scholar]
- 19.Volk LD, Flister MJ, Bivens CM, Stutzman A, Desai N, et al. Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia. 2008;10:613–623. doi: 10.1593/neo.08302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez LO, Del Casar JM, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10:665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Reyes S, Fernandez JM, Gonzalez LO, Aguirre A, Suarez A, et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2010;60:217–226. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajput S, Volk-Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther. 2013;12:1676–1687. doi: 10.1158/1535-7163.MCT-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 25.Volk LD, Flister MJ, Chihade D, Desai N, Trieu V, et al. Synergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia. 2011;13:327–338. doi: 10.1593/neo.101490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Zhao J, Li H, He KL, Chen Y, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 28.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal. 2011;4:ra49. doi: 10.1126/scisignal.2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang AC, Su QB, Wu FX, Zhang XL, Liu PS. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur J Clin Invest. 2009;39:157–164. doi: 10.1111/j.1365-2362.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 30.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 31.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellerman JE, Brown CK, de VM, Zeh HJ, Billiar T, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 36.Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015–1023. doi: 10.2147/OTT.S60114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi K, Yang M, Hayashi K, Jiang P, Yamamoto N, et al. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res. 2008;68:516–520. doi: 10.1158/0008-5472.CAN-07-3063. [DOI] [PubMed] [Google Scholar]
- 38.Xiong W, Ren ZG, Qiu SJ, Sun HC, Wang L, et al. Residual hepatocellular carcinoma after oxaliplatin treatment has increased metastatic potential in a nude mouse model and is attenuated by Songyou Yin. BMC Cancer. 2010;10:219. doi: 10.1186/1471-2407-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latifi A, Abubaker K, Castrechini N, Ward AC, Liongue C, et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J Cell Biochem. 2011;112:2850–2864. doi: 10.1002/jcb.23199. [DOI] [PubMed] [Google Scholar]
- 40.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer Biol Ther. 2011;11:1008–1016. doi: 10.4161/cbt.11.12.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu YJ, Muldoon LL, Dickey DT, Lewin SJ, Varallyay CG, et al. Cyclophosphamide enhances human tumor growth in nude rat xenografted tumor models. Neoplasia. 2009;11:187–195. doi: 10.1593/neo.81352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Man S, Zhang Y, Gao W, Yan L, Ma C. Cyclophosphamide promotes pulmonary metastasis on mouse lung adenocarcinoma. Clin Exp Metastasis. 2008;25:855–864. doi: 10.1007/s10585-008-9201-3. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Putten LM, Kram LK, van Dierendonck HH, Smink T, Fuzy M. Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int J Cancer. 1975;15:588–595. doi: 10.1002/ijc.2910150408. [DOI] [PubMed] [Google Scholar]
- 46.El Sharouni SY, Kal HB, Battermann JJ. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br J Cancer. 2003;89:2184–2189. doi: 10.1038/sj.bjc.6601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2014;74:436–445. doi: 10.1158/0008-5472.CAN-13-1265. [DOI] [PubMed] [Google Scholar]