Abstract
In the central nervous system, NG2-glia represent a neural cell population that is distinct from neurons, astrocytes and oligodendrocytes. While in the past the main role ascribed to these cells was that of progenitors for oligodendrocytes, in the last years it has become more obvious that they have further functions in the brain. Here, we will discuss some of the most current and highly debated issues regarding NG2-glia: Do these cells represent a heterogeneous population? Can they give rise to different progenies, and does this change under pathological conditions? How do they respond to injury or pathology? What is the role of neurotransmitter signaling between neurons and NG2-glia? We will first give an overview on the developmental origin of NG2-glia, and then discuss whether their distinct properties in different brain regions are the result of environmental influences, or are due to intrinsic differences. We will then review and discuss their in vitro differentiation potential and in vivo lineage under physiological and pathological conditions, together with their electrophysiological properties in distinct brain regions and at different developmental stages. Finally, we will focus on their potential to be used as therapeutic targets in demyelinating and neurodegenerative diseases. Therefore, this review article will highlight the importance of NG2-glia not only in the healthy, but also in the diseased brain.
Keywords: heterogeneity, lineage potential, physiology, disease, oligodendrocyte progenitors
Introduction
Nerve/glial antigen 2 (NG2) is a chondroitin sulfate proteoglycan that in the brain occurs mostly in a class of glial cells called NG2-glia(Nishiyama et al. 2009) or in pericytes associated with blood vessels (Ozerdem et al. 2001) – two cell types differentiated by their expression of other protein markers. Why have NG2-glia attracted so much attention in recent years? Perhaps the best approach to this question is to clarify the type of cells identified as NG2-glia. More than any other cell type in the brain, NG2-glia have been given many names: NG2 progenitor cells; polydendrocytes; synantocytes; NG2 cells; or more often oligodendrocyte progenitor cells (OPCs). We prefer the term NG2-glia, as NG2-expressing cells during development can generate not only oligodendrocytes but also astrocytes, leading to the conclusion that NG2 expression is the common feature to all cells. To further distinguish these cells from other non-glial cells in the brain that also express NG2, i.e. pericytes or macrophages that can transiently express it after some specific injury conditions(Bu et al. 2001), we refer to them as NG2-glia. Cellular heterogeneity is so incredibly high in the brain that specific cell types must be identified and quantified using a combination of morphological, antigenic, and functional criteria. These criteria have been successfully applied to NG2-glia, producing a complete anatomical map of their development and distribution in different brain regions. There is the general agreement that NG2-glia represent an immature neural cell population that, under differing environmental conditions, can terminally differentiate into mature neural cell types (see (Richardson et al. 2011) for a review). However, in most instances, NG2-glia are characterized only by their expression of a specific antigen: the proteoglycan NG2.
One issue debated for many years is whether NG2-glia – besides uniformly expressing the proteoglycan NG2 – collectively represent a homogeneous and different class of glia, separate from oligodendrocytes and astrocytes. These questions are intimately linked, as NG2-glia were thought to be exclusively progenitors of oligodendrocytes, representing an immature stage of this class of macroglia. However, more specific genetic tools have been generated to study these cells in thebrain, and it appears that: i) NG2-glia may not be a homogeneous population and ii) they might give rise to distinct progenies in different brain regions, different developmental stages and/or in pathological vs. physiological conditions. More advanced experimental approaches must be used to determine whether – in intactand in pathological brain – different subtypes of NG2-glia exist, and whether these are distinct from other central nervous system (CNS) macroglial cells, e.g. astrocytes.
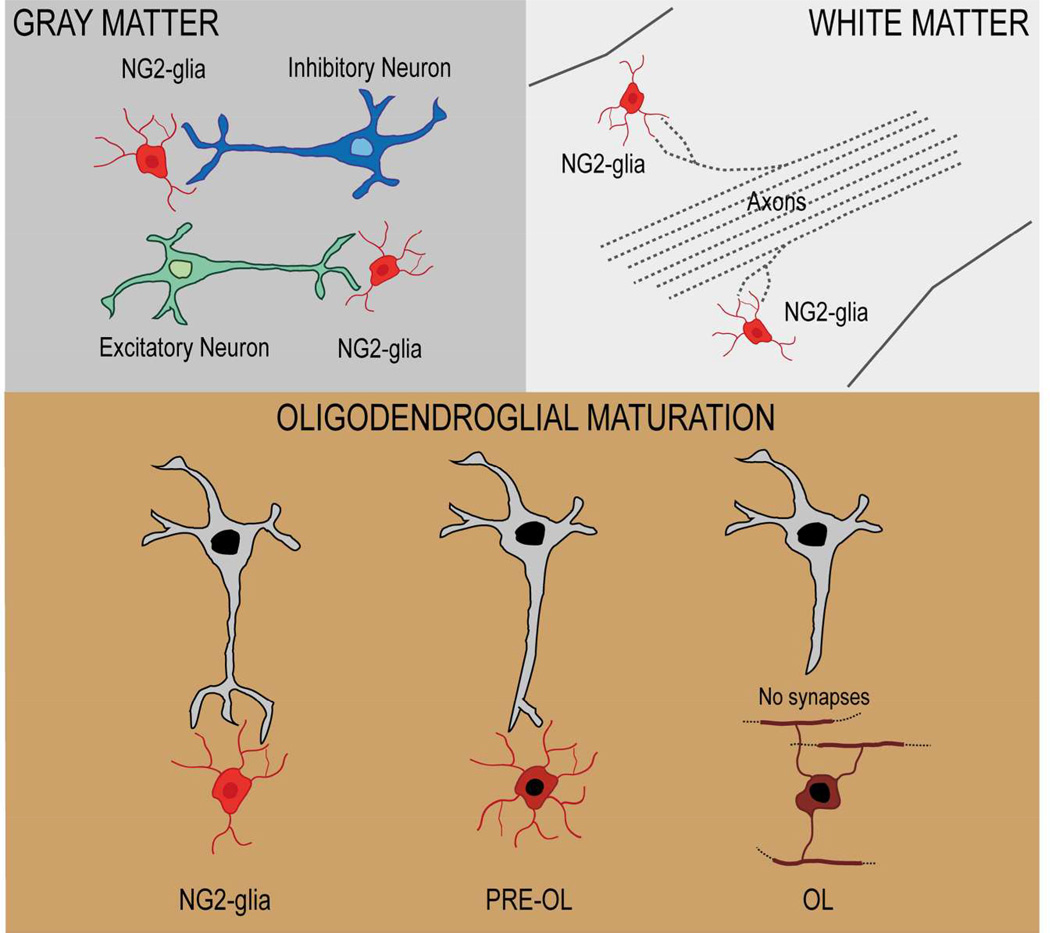
An important and functionally relevant feature of NG2-glia is their presence in all brain regions – both gray and white matter – throughout postnatal development and into adulthood (Nishiyama et al. 2009). NG2-glia are also present in neurogenic niches, including the subventricular zone (SVZ) and the hippocampal dentate gyrus (Aguirre and Gallo 2004; Aguirre et al. 2004; Passlick et al. 2013). It is well established that, in the postnatal and adult brain, NG2-glia represent the largest population of endogenous/resident progenitor cells (4–8% of total cells, depending on the brain region), capable of rapidly “reacting” to any type of injury and with a strong potential to repopulate areas of lesion(Aguirre et al. 2007; Filous et al. 2014; Scafidi et al. 2014; Simon et al. 2011; Whittaker et al. 2012). NG2-glia morphology and their functional properties (e.g., membrane channels and receptors) have also been found to be distinct in different brain regions(Bakiri et al. 2009; Chittajallu et al. 2004). Finally, interactions between NG2-glia and other neural cell types may vary between brain areas (Hammond et al. 2014; Miron et al. 2013; Wigley et al. 2007; Wu et al. 2010).
To add another critical piece to the puzzle, it is now well established that NG2-glia receive direct excitatory and inhibitory synapses from neurons in all brain regions (reviewed in (Bergles et al. 2010; Frohlich et al. 2011; Gallo et al. 2008; Sakry et al. 2011). Although first discovered in the hippocampus, neuron-glial synapses are now considered a “standard feature” of NG2-glia. Their precise physiological role(s) is still rather undefined, but these synapses clearly indicate that neurotransmitter-mediated signaling between neurons and NG2-glia may play a major modulatory role in the developmental biology of these glial cells.
In summary, as we understand more about the role of NG2-glia in the brain, questions continue to arise. Because of the great interest in NG2-glia, several excellent review articles on these cells have been published in the past few years (Dimou and Gotz 2014; Nishiyama et al. 2009; Richardson et al. 2011). Therefore, our contribution will focus on some of the most current and undefined issues concerning NG2-glia development, biology, and physiology. Rather than providing a comprehensive review of the topic, we will attempt to discuss various perspectives that could form the basis for future research on NG2-glia. Among these are: their developmental properties, their role in neuroprotection and cell regeneration after injury, their role in surveillance of the extracellular environment, and their participation to neural circuit physiology and plasticity.
Are NG2-glia a homogeneous or heterogeneous cell population?
The best established role of NG2-glia in development as well as in the adult CNS is to generate oligodendrocytes, a reason why they are often referred to as OPCs. However, it is also known that, depending on the developmental stage and the brain area, they can also produce astrocytes (Zhu et al. 2008a; Zhu et al. 2011) or remain as self-renewing NG2-glia (Simon et al. 2011). The finding that NG2-expressing cells are able to generate more than one neural cell type besides oligodendrocytes indicates that expression of the proteoglycan NG2 is one of the features common to all these cells. Therefore, to further distinguish these cells from other non-glial cells in the brain that also express NG2, i.e. pericytes, we refer to them as NG2-glia.
Besides their multiple differentiation potential, it is also established that subsets of these cells express a variety of voltage- and ligand-dependent channels, thereby exhibiting unique electrophysiological properties. These distinct properties of NG2-glia raise the still unanswered question whether the NG2-glia population includes a homogeneous cell population that displays different developmental and physiological properties solely depending on environmental influences, or is an intrinsically heterogeneous cell population. In this section, we will discuss different properties of brain NG2-glia, as well as evidence in favor and against the notion of NG2-glia heterogeneity.
Developmental origin
In the developing brain, NG2-glia emerge in different temporal and regional waves. In vivo fate-mapping analysis using a Nkx2.1-Cre transgenic mouse line that labels neural progenitors in the basal forebrain demonstrated that the first oligodendrocyte progenitors appear in the cerebral cortex at approximately E16 (embryonic day 16), and migrate from ventral areas of the medial ganglionic eminence. These cells populate the entire cortex by E18, and are followed by a second wave of NG2-glia – as shown in a Gsh2-Cre mouse line -arising in the lateral and/or caudal ganglionic eminence. Finally, a third wave arises from Emx1-positive cells within the postnatal cortex (Kessaris et al. 2006). Therefore, at E18, all oligodendrocytes originate from the ventral telencephalon, whereas after E18 the contribution of ventral cells decreases and gradually disappears, and NG2-glia almost exclusively originate within the cortex itself. Similarly, experiments of retrovirus injection in the subventricular zone (SVZ) of postnatal brains demonstrated that oligodendrocytes are generated from progenitor cells that reside in this neurogenic region (Levison et al. 1993; Levison and Goldman 1997). Separate studies also showed that the SVZ, a region derived from the embryonic lateral eminence and lateral cortex, is the major source of NG2-glia and oligodendrocytes in the postnatal brain (Aguirre and Gallo 2007; Menn et al. 2006). In contrast to these findings, recent studies using live imaging and single cell tracking demonstrated that NG2-glia and neurons are generated by distinct stem cells and that NG2-glia are mainly generated from the dorsal and not the lateral wall of the ventricle (Ortega et al. 2013). Independently from their origin, NG2-glia migrate out of the SVZ into white matter regions, where they undergo extensive proliferation before they terminally differentiate into myelinating oligodendrocytes.
The different origins of NG2-glia (ventral and dorsal, depending on the developmental stage) raise the important question of whether these cells are also intrinsically different. In fact, it has been shown that signals such as sonic hedgehog (SHH) are important for oligodendrocyte specification in ventral but not dorsal areas (Nery et al. 2001; Spassky et al. 2001; Tekki-Kessaris et al. 2001), pointing to possible heterogeneity between NG2-glia of different origins. On the other hand, if these cells were intrinsically different, would they still be able to generate the very same progeny – oligodendrocytes - and even be able to functionally replace each other? Analysis of the properties of ventrally- and dorsally-derived NG2-glia did not identify differences in proliferation rates, cell cycle length or membrane properties (Psachoulia et al. 2009; Tripathi et al. 2011). Additionally, when ventrally- or dorsally-derived populations were separately ablated by targeted expression of diphtheria toxin, the surviving cells could migrate in, fill the space and functionally replace each other (Kessaris et al. 2006). This finding hints towards common functional properties among different NG2-glia populations, or at least strong intrinsic plasticity between cell subpopulations of different origins. Interestingly, while all ventrally-derived NG2-glia disappear in the adult brain, ventrally-derived oligodendrocytes contribute about 20% of total mature oligodendrocytes in the corpus callosum, while the gray matter of the cerebral cortex almost entirely consists of dorsally-derived cells (Tripathi et al. 2011).
A separate set of experiments further supporting the concept of NG2-glia plasticity was performed by the Duncan lab, who isolated NG2-glia from the optic nerve - which solely consists of small calibre axons - and transplanted these cells into the spinal cord. Interestingly, after grafting, optic nerve NG2-glia were able to differentiate to oligodendrocytes that myelinated both small and large calibre axons (Fanarraga et al. 1998). Although, it cannot be completely excluded that the optic nerve is comprised by different NG2-glia subpopulations, it appears that these cells are able to differentiate to oligodendrocyte types, myelinating both small- or large-diameter axons. Further studies are necessary to fully understand whether different developmental origins lead to distinct subpopulations of NG2-glia that display differences in their properties and functions.
Regional heterogeneity
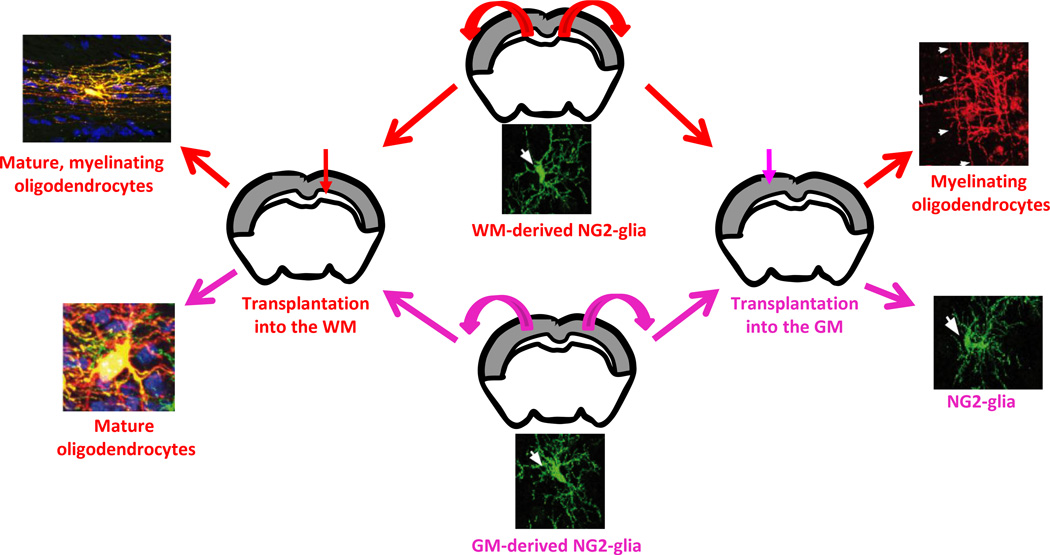
Fate-mapping studies using distinct mouse lines expressing inducible Cre-recombinase under oligodendrocyte lineage specific promoters or BrdU label-retaining experiments showed region-dependent differences in NG2-glia differentiation. While the majority of adult NG2-glia located in the white matter (WM) of the cerebral cortex differentiate mostly into mature, myelinating oligodendrocytes, gray matter (GM) NG2-glia generate fewer mature oligodendrocytes (Dimou et al. 2008; Kang et al. 2010; Simon et al. 2011). Interestingly, recent transplantation experiments also demonstrated intrinsic differences between NG2-glia from these two regions (Vigano et al. 2013). While cells from the WM differentiated with the same efficiency into mature myelinating oligodendrocytes in a more (WM) and a less supportive (GM) environment, GM-derived NG2-glia did not display the same properties (Figure 1). It is still unclear to what extent these intrinsic differences are due to distinct developmental origins, or the result from long-term exposure of these cells to different extracellular environments that have modified their properties. However, independently from the cellular and molecular mechanisms underlying these differences, defining region-specific properties of NG2-glia is fundamentally important to understand the distinct remyelination potential of these cells in different brain regions (Albert et al. 2007; Gudi et al. 2009) and to identify new molecular candidates influencing NG2-glia differentiation.
Figure 1. Regional heterogeneity of NG2-glia in the adult cerebral cortex.
Transplantations of NG2-glia isolated either from the corpus callosum (white matter; WM) or the cerebral cortex (gray matter; GM) of the adult brain revealed that WM-derived cells generate mature, myelinating oligodendrocytes independently of the area of transplantation. In contrast, GM-derived NG2-glia remained as progenitors when transplanted homotopically, but differentiated into mature non-myelinating oligodendrocytes when grafted into the more permissive environment of the WM.
Regional differences between NG2-glia have also been evidenced based on expression of voltage- and ligand-dependent ionic channels (for more details, see chapter below), as well as proliferation properties. Several studies demonstrated that the cell cycle length of NG2-glia is different not only between WM and GM, but also between WM tracts of different brain regions. Although the proportion of proliferating NG2-glia is very similar in both WM and GM (80% – 98%, depending on the study; (Clarke et al. 2012; Kang et al. 2010; Simon et al. 2011), cumulative BrdU or EdU-labeling in mice revealed that NG2-glia in the WM display a much shorter cell cycle length compared to GM cells (2.7 days (d) vs. 18.6 d at postnatal day (P)21 and 9.5 d vs. 36.3d at P60 in corpus callosum and cerebral cortex respectively; (Young et al. 2013). Cell cycle times do not only vary between WM and GM, but also between WM-and GM-tracts in different areas. For example, while the cell cycle length of NG2-glia in WM-tracts of the spinal cord is longer compared to the corpus callosum (14.9 d vs. 9.5 d at P60), it is the opposite in GM-tracts (27.4 d vs. 36.3 d at P60 in spinal cord vs. cerebral cortex; (Young et al. 2013). It is important to note, however, that these studies report average cell cycle length times, and do not take into account any variability in the cell cycle length between individual NG2-glia within the same brain region. A recent clonal analysis of NG2-glia showed a strong variability in cluster size of NG2-clones, varying from 40 to 340 cells / clone in the cerebral cortex (Garcia-Marques et al. 2014). This could at least in part be explained by heterogeneity in NG2-glia proliferation properties. Another clonal analysis study by Kang et al. (2010) suggested that NG2-glia in all brain regions retain mitotic activity until they differentiate into oligodendrocytes. In the future, it will be very interesting to determine whether different populations of NG2-glia exist within the same regions, which display slow or fast cycling properties, and how their proliferation can be influenced by environmental changes, including injury or extrinsic cellular factors. Notably, PDGF has been identified as a factor influencing the proliferative response of WM- but not GM-derived NG2-glia (Hill et al. 2013), further pointing to intrinsic differences between these two populations.
NG2-glia heterogeneity could be observed not only between NG2-glia in different brain regions, but also between cells within the very same area. For example, the transcription factor Ascl1 is only expressed by a subset of NG2-glia in the adult cerebral cortex (Battiste et al. 2007; Parras et al. 2007), and it is still unresolved whether these two NG2-glia subtypes also display differences in their proliferation or differentiation properties. Recent studies demonstrated that the G-protein coupled receptor GPR17 labels a subset of NG2-glia in the adult brain (Boda et al. 2011; Zhang et al. 2014). This receptor has been shown to modulate NG2-glia differentiation in vitro and in vivo (Chen et al. 2009; Fumagalli et al. 2011). Analysis of a new GPR17-iCreERT2 line demonstrated that GPR17+ NG2-glia differentiated at a slower rate, as compared to GPR17− cells in both the WM and GM, a difference that was however re-normalized after a cerebral damage induced by acute injury or ischemia (Vigano and Dimou, unpublished observations). Whether GPR17 is actively involved in maintaining a non-differentiative state, or is just a marker that identifies a cell subpopulation will also have to further been investigated in the future. However, this is the first demonstration that different subsets of NG2-glia with distinct modes of differentiation and maturation speed might exist, opening new avenues to further identification of different subpopulations of NG2-glia.
Lineage potential of NG2-glia
NG2-glia are cells with a highly proliferative capacity (Clarke et al. 2012; Simon et al. 2011; Young et al. 2013) and it is nowadays broadly accepted that they can generate oligodendrocytes. Genetic fate mapping analysis from different laboratories demonstrated the oligodendrocyte potential of NG2-, PDGFRa- and Olig2-positive cells in the brain and spinal cord parenchyma at all ages of the mouse (Dimou et al. 2008; Huang et al. 2014; Kang et al. 2010; Nishiyama et al. 2009; Rivers et al. 2008; Zhu et al. 2011). However, the rate of oligodendrocyte generation differs between brain regions and age, namely NG2-glia generate oligodendrocytes faster in the white than in the gray matter, and in younger than in older animals (Dimou et al. 2008; Huang et al. 2014; Kang et al. 2010; Zhu et al. 2011). These results were also confirmed by an independent genetic fate mapping study, where BrdU labeling-retaining experiments were used to analyse the potential of NG2-glia in the adult brain (Simon et al. 2011).
Under physiological conditions, NG2-glia can also self-renew (Simon et al. 2011), but it remains still unresolved whether all NG2-glia are able to differentiate into oligodendrocytes or if a specific subpopulation of NG2-glia always remains undifferentiated and is responsible for the self-renewal capacity of these cells. In any case, this ability points to further functions for NG2-glia beyond being only progenitors for oligodendrocytes.
During development, NG2-glia not only generate oligodendrocytes, but also astrocytes in a temporal- and regional-restricted manner. Two independent studies using either a BAC-transgenic NG2-CreERT2 or a mouse line generated by homologous recombination (knock-in mouse line) showed that - when induced embryonically - a significant number of astrocytes could be generated, specifically in the ventral forebrain gray matter, including cortex, striatum, thalamus and hypothalamus (Huang et al. 2014; Nishiyama et al. 2009; Zhu et al. 2011). Interestingly, these astrocytes persisted in the adult brain and did not die, although their density decreased, probably due to brain growth. In contrast, a very low number of astrocytes generated from NG2-glia could be observed in the dorsal brain and in white matter tracts, such as corpus callosum, while no astrocytes were found in other areas than the forebrain, e.g. brainstem, cerebellum or olfactory bulb (Huang et al. 2014). When the very same mice (NG2-CreERT2) - as well as other NG2-glia specific inducible mouse lines (e.g. Olig2-CreERT, PDGFRa-CreERT2; (Dimou et al. 2008; Kang et al. 2010; Rivers et al. 2008)-were induced with tamoxifen in adult ages, no astrocyte generation from NG2-glia could be observed, although in some studies a low number of astrocytes was recombining directly after the tamoxifen application. These results hint to the existence of embryonic NG2-glia with astrogenic potential that reside or migrate in/into the ventral forebrain and differentiate into astrocytes in the healthy brain. Another possibility could be that a small subset of astrocyte progenitors expresses NG2 in a short temporal and regional manner.This as well as the question whether a single NG2-glia can generate both NG2-glia and astrocytes has still to be answered. It has also to be resolved whether these NG2-glia resemble a specific subset of astrogenic cells that then disappear in the neonatal brain, or whether these cells exist also in the adult brain, but lose their astrogenic ability after birth. An alternative scenario could be that these NG2-glia still exist in the adult ventral forebrain, but the intrinsic or environmental signals important for astrogliogenesis are missing, and these cells can be reactivated only after injury to generate astrocytes (see also below).
A recent study by Zhu et al. (2012) demonstrated that when the transcription factor Olig2 - an essential regulator of oligodendrocyte progenitor, oligodendrocyte and motoneuron development (Ligon et al. 2006a; Ligon et al. 2006b; Takebayashi et al. 2002) - was specifically deleted in NG2-glia either constitutively or perinataly, a conversion of NG2-glia into astrocytes could be observed, also in the dorsal forebrain, leading to hypomyelination (Zhu et al. 2012). Interestingly, although NG2-glia in the healthy embryonic ventral forebrain downregulate Olig2 expression before they differentiate into astrocytes, a deletion of Olig2 in this area does not lead to a further increase in astrogenesis. This suggests different roles of Olig2 in NG2-glia arising from different sources, e.g. either Nkx2.1+- and/or Gsx2+- derived in the ventral forebrain, versus Emx1+-progenitors in the dorsal forebrain (Kessaris et al. 2006) and above).
These studies correlate also to analysis performed in vitro, demonstrating that NG2-glia can differentiate into type 2 astrocytes (Behar et al. 1988; Power et al. 2002; Raff et al. 1983b) that are limited in number and might contribute to the structure of nodes of Ranvier (Ffrench-Constant and Raff 1986). Type 2 astrocytes differ from the more common type 1 astrocyte, based on their expression of the cell surface ganglioside A2B5 (Kondo and Raff 2000), and the absence of GFAP and Ran2 (Johnstone et al. 1986; Raff et al. 1983a). Interestingly, type 2 astrocytes develop later than type 1 astrocytes and oligodendrocytes and, in general, O2A cells (oligodendrocyte-type-2 astrocyte progenitors that can give rise to both, oligodendrocytes and type 2 astrocytes but not neurons under normal in vitro conditions; (Noble et al. 2003a; Rao et al. 1998) seem to need factors secreted from type 1 astrocytes, e.g. PDGF or CNTF, Shh and BMPs, to proliferate or differentiate in vitro (Lillien et al. 1988; Lu et al. 2000; Mabie et al. 1997; Noble et al. 2003b; Raff and Lillien 1988).. Upon postnatal transplantation, these cells give rise to myelinating oligodendrocytes (Espinosa de los Monteros et al. 1993; Sawamura et al. 1995). However, when O2A cells are transplanted into the adult brain, they differentiate into GFAP+ astrocytes, suggesting that the differentiation of O2A cells is controlled by stage-specific factors in the brain (Sawamura et al. 1995).
In the last two decades, it has become clear that specific cell culture conditions can induce a subpopulation of cortical O2A progenitors to self-renew and to give rise not only to oligodendrocytes and type 2 astrocytes, but also type 1 astrocytes and even neurons (Kondo and Raff 2000; Kondo and Raff 2004; Noble et al. 2003a). This finding suggests that extracellular signals can reverse glial cell specification and convert specified precursors into multipotent stem cells. Similarly, Bartlett and colleagues demonstrated that the adult CNS contains a pool of neural precursors that can divide and differentiate to mature neurons – as determined by morphology and antigenic markers - when treated with growth factors such bFGF and EGF (Richards et al. 1992). The identity of these precursors was not determined, but in a separate study NG2-expressing multipotent precursor cells were identified in the cerebral cortex and in the white matter in rodents (Gensert and Goldman 1996) and humans (Roy et al. 1999), as these were the only proliferating cells outside the neurogenic niches. When isolated and cultured in vitro, or transplanted into the brain, NG2-glia from the white matter can either give rise to oligodendrocytes only, or to neurons, astrocytes and oligodendrocytes, depending on cell density and the presence or absence of serum, respectively (Aguirre and Gallo 2004; Belachew et al. 2003; Nunes et al. 2003; Windrem et al. 2002). This lead to the conclusion that an abundant pool of neurogenic progenitors exists in the adult white matter, which is restricted to generate glia due to its environment, rather than cell autonomous lineage commitment. At variance with these results, more recent studies using NG2-dsRed transgenic mice revealed that purified NG2-glia do not give rise to neurons in culture (Zhu et al. 2008a).
The studies described above were performed in vitro, raising the important question of the potential of NG2-glia to generate neurons in vivo. This question has been highly debated in the last few years, as fate-mapping experiments using genetic tracing with different mouse lines lead to controversial results. Rivers et al. (2008) described for the first time that NG2-glia can generate neurons in the piriform cortex of the adult brain by using an inducible PDGFRα-CreERT2 mouse line. This approach was challenged by a subsequent study from the same laboratory (Clarke et al. 2012) and from a different group using a distinct PDGFRα-CreERT2 line (Kang et al. 2010), who found no neurons generated by NG2-glia in the very same brain region. Another study using a PLP-CreERT mouse line could also detect some neurons in the piriform cortex (Guo et al. 2009; Guo et al. 2010), but as the transgene is also expressed in cells other than oligodendrocytes or their progenitors, the real precursors of these neurons are still unidentified.
In other studies using a PDGFRα-, a knock-in NG2- or an Olig2-CreERT mouse line, some neurons could also be observed in ventral areas of the brain, or in the CA3 of the hippocampus (Dimou et al. 2008; Huang et al. 2014; Kang et al. 2010), but also in these studies generation of neurons by NG2-glia was not unequivocally demonstrated. Firstly, mature neurons were detected very early after tamoxifen administration (3–4 days later). If these neurons had been generated by NG2-glia, they would have differentiated at a very fast rate, at variance with other studies demonstrating that neurons generated from neural stem cells require at least 10 to 14 days to display NeuN-immunoreactivity (a marker for mature neurons; (Zhao et al. 2008). Secondly, in all these studies, the number of newly generated neurons remained stable. If NG2-glia served as neuronal progenitors, the number of newly generated neurons should increase with time, as observed by Rivers et al. (2008). Finally, none of these neurons appeared to be generated by proliferating cells. As almost all NG2-glia divide (Clarke et al. 2012; Simon et al. 2011), then one would expect that at least some neurons should be generated by NG2-glia that have incorporated the thymidine analogue BrdU, which labels proliferating cells. This was not the case, neither in the Huang et al. nor in the Rivers et al. study discussed above. These results, together with further negative observations from fate mapping analysis using transgenic lines (Zhu et al. 2008a; Zhu et al. 2011) or BrdU label-retaining experiments (Simon et al. 2011), suggest that the potential of NG2-glia in the healthy vertebral cortex is purely gliogenic, and that the reporter expression in neurons probably solely results from direct expression of CreER by neurons, rather than from direct differentiation of NG2-glia. Another possibility could be that NG2-glia secrete exosomes carrying the Cre-recombinase DNA that is taken up by neurons, thereby causing recombination, as already described for oligodendrocytes (Fruhbeis et al. 2013). However, whether NG2-glia can release exosomes has not yet been studied.
Importantly, a recent study demonstrated that a small subset of proliferating NG2-glia in the hypothalamus expressed the stem cell marker Sox2, and that a low number of BrdU+ neurons can be specifically generated from these cells in this area of the brain (Robins et al. 2013), pointing once again towards regional heterogeneity of NG2-glia.
Reactive NG2-glia after acute injury or other pathological conditions
Morphology and behavior
In general, NG2-glia react to many types of injury or pathological conditions by changes in cell morphology and proliferation rate. However, the nature and the time course of their response very strongly depend on the nature of the insult and the developmental stage at which this occurs. Here, we will discuss the different responses of NG2-glia observed in distinct injury and disease paradigms.
Reaction of NG2-glia to acute injury
NG2-glia respond to traumatic injury - including stab wound lesion (Buffo et al. 2005; Dimou et al. 2008; Levine 1994), ischemia (Zhang et al. 2013) and spinal cord injury (McTigue et al. 2001) - very fast (within one day; (Horky et al. 2006; Simon et al. 2011) and not only with morphological changes, but also with increased proliferation rate. NG2-glia around the lesion become hypertrophic, and display an enlarged cell body, many shorter and thicker processes, and a more intensive immunostaining for the proteoglycan NG2 (Levine 1994). In vivo imaging has revealed that NG2-glia processes after injury (demyelination or focal laser lesion) are highly dynamic (Hill et al. 2014; Hughes et al. 2013) and that cells migrate towards the injury site (Hughes et al. 2013); von Streitberg and Dimou, unpublished observations) This migratory process, together with enhanced proliferation and increased number of cellular processes leads to accumulation of NG2-glia in the lesioned area shortly after injury (Levine 1994; Matsumoto et al. 2008), thereby overcoming the homeostatic control of their density described by Hughes et al. (2013). The numbers of NG2-glia progressively decrease with increasing post-lesion time, leading to restoration of their physiological density by 28 days after injury (von Streitberg and Dimou, unpublished data).
The role of NG2-glial reaction at the injury site is still unresolved. A possible role in scar formation has been speculated, as NG2-glia - together with microglia - are the first cells to react and strongly accumulate around the injury, implicating these glial cells in functions previously thought to be taken over by astrocytes. A recent in vivo imaging study showed that only a small number of astrocytes react to traumatic injury within 5–7 days after lesion with hypertrophy and low proliferation rate, while no migration towards the injury site could be observed (Bardehle et al. 2013). This late and low level response of astrocytes makes these cells less important for scar formation and wound healing, supporting the hypothesis that NG2-glia are responsible for wound closure and scar formation by building a high cell density area.
An additional, negative role of NG2-glia due to excessive accumulation of the proteoglycan NG2 has also been suggested for axonal growth inhibition (Chen et al. 2002; Tan et al. 2005). Consistent with this role, blockage of cell proliferation with the antimitotic drug AraC with subsequent inhibition of the increase in number of NG2-glia after injury, lead to enhanced axonal regeneration (Rhodes et al. 2003). However, other studies demonstrated opposite effects of the NG2 proteoglycan and support the notion that NG2-glia provide an adhesive substrate for axonal growth cones, even when elevated NG2-protein levels are present (Yang et al. 2006). Further studies will have to be performed to resolve the issue of the role of NG2-glial response after injury. An accepted role of NG2-glia after traumatic injury is their contribution to repair by generating new oligodendrocytes, as shown after cortical stab wound and spinal cord injury, as well as after ischemia (Ishii et al. 2001; Komitova et al. 2011; Tanaka et al. 2003; Watanabe et al. 2002); see also below). However, similar to other pathological paradigms including adult demyelination, this repair process is never complete. Possible reasons for this will be discussed below.
Reaction of NG2-glia to neurodegeneration
NG2-glia not only respond to traumatic injury, but also to progressive neurodegenerative insults, including Alzheimer´s disease (AD) and Amyotrophic lateral sclerosis (ALS). Interestingly, several studies have recently shown that in both disease models defects in oligodendrocyte metabolism and myelin maintenance precede neurodegeneration (Behrendt et al. 2013; Desai et al. 2009; Mackenzie et al. 2011). In an ALS-mouse model carrying a mutation of the superoxide dismutase (SOD), the number of oligodendrocytes does not change - despite their degeneration and death - due to increased proliferation and differentiation of NG2-glia (Kang et al. 2013; Magnus et al. 2008; Philips et al. 2013). However, newly generated oligodendrocytes appear to be dysfunctional, due to maturation failure, as they are impaired both in terms of myelination and metabolic/trophic support of axons (Kang et al. 2013; Philips et al. 2013). Interestingly, when the mutated SOD protein was specifically removed in the oligodendroglial lineage, the disease onset was delayed and mice survived longer, supporting a direct role of the mutated protein in oligodendrocytes (Kang et al. 2013).
In mouse models of AD, myelin aberrations were also observed prior to the disease onset. Notably, in the APP/PS1 mouse model of AD, in which mutations in both proteins are restricted to neurons, myelin defects could be observed, hinting to a direct or indirect effect of the extracellular plaque deposition or of the mutated neurons. In this mouse model, similar to the ALS-models, a significant increase in the proliferation and differentiation of NG2-glia was observed. This led to recovery in oligodendrocyte numbers and repair of myelin abnormalities in aged brains (Behrendt et al. 2013). It should be noted that, although an increase in proliferation in the chronic amyloidosis model was observed, this was much lower than that observed after an invasive lesion, such as stab wound injury, further supporting the idea of distinct reactions of NG2-glia depending on the insult.
In contrast to the findings in the disease models described above, when NG2-glia were analyzed in a mouse model in which massive neuronal cell death in the cerebral cortex was caused by overexpression of p25 in neurons (CK/p25 mice; (Cruz et al. 2006; Cruz et al. 2003), no cellular response of NG2-glia could be observed, either morphologically nor in proliferation (Sirko et al. 2013). Similarly, in a rat model of Parkinson´s disease (PD), there was only a slight decrease in numbers of NG2-glia in the substantia nigra (Steiner et al. 2006). Thus, neuronal cell death alone does not trigger a cellular response in NG2-glia, despite their close cellular interactions with neurons, suggesting that the response of NG2-glia observed in AD and ALS is due to demyelination, rather than neurodegeneration.
Reaction of NG2-glia to demyelination
Consistent with findings described above on the cellular response of NG2-glia to injury, these cells also react to all types of demyelinating diseases by becoming hypertrophic (Keirstead et al. 1998; Levine and Reynolds 1999), and undergoing rapid proliferation (Di Bello et al. 1999; Reynolds et al. 2002) and differentiation into myelinating oligodendrocytes (Franklin et al. 2002; Gensert and Goldman 1997; Guo et al. 2011; Komitova et al. 2011; Tripathi et al. 2010). Interestingly, when inflammation is not accompanied by demyelination (Di Bello et al. 1999) or after viral injection (Levine et al. 1998), a rapid proliferative response of NG2-glia could not be observed, suggesting that increased proliferation is the result of signaling from exposed axons and/or damaged oligodendrocytes (Franklin 2002).
It is currently well accepted that remyelination observed after demyelination is achieved by local NG2-glia residing at or close to the demyelination lesions. However, it is also known that stem cells of the neurogenic SEZ can give rise to NG2-glia during development and in the adult brain (Jackson et al. 2006; Levison and Goldman 1993). Retroviral labeling experiments revealed that after a demyelinating lesion in the corpus callosum, the number of oligodendrocytes derived from stem cells in the SEZ strongly increased, indicating the participation of these stem cells in myelin repair (Menn et al. 2006). A recent study could demonstrate by fate mapping that SEZ neural precursors contribute substantially to remyelination after cuprizone induced demyelination, at least in regions of the rostral corpus callosum adjacent to the SEZ. In contrast, SEZ-derived cells that migrate into the caudal corpus callosum do not manage to differentiate into mature oligodendrocytes although the local NG2-glia do so with a high efficiency (Xing et al. 2014). Notably, myelin derived by SEZ-progenitors was significantly thicker than that generated by local NG2-glia and was equivalent to the myelin originating during development.
As mentioned above, although endogenous NG2-glia respond to pathological insults with proliferation and enhanced remyelination, this remyelination process does not reach completion. Interestingly, while acute demyelination is repaired efficiently, remyelination fails in chronic lesions (Wilson et al. 2006). Therefore, a very important question emerges, in particular why are endogenous NG2-glia unable to fully remyelinate nude axons after pathology? At present, we could only speculate, as different hypotheses can be suggested: i) NG2-glia can be recruited to the site of injury, but their differentiation program is halted by either lack of molecular cues and growth factors needed for remyelination, or by the inhibitory lesion environment (McDonald and Belegu 2006); ii) as NG2-glia manage to remyelinate acute but not chronic lesions, a depletion of progenitors could also prevent full remyelination (Keirstead et al. 1998; Mason et al. 2004). However, a study using the cuprizone demyelination model showed opposite results, i.e. an increase in NG2-glia after chronic demyelination (Armstrong et al. 2006); iii) the regional heterogeneity of NG2-glia in regard to their ability to differentiate (see above; (Vigano et al. 2013) could also lead to limited remyelination ability, e.g. in the cerebral gray matter; iv) as the ability of NG2-glia to proliferate and differentiate decreases with aging, this could also lead to an age-related decrease in the remyelination properties of these cells (Sim et al. 2002); v) axonal damage and degeneration of demyelinated neurons due to the loss of support by myelin (Raine and Cross 1989); vi) as autoantibodies to the NG2-proteoglycan have been discovered in the cerebrospinal fluid of multiple sclerosis patients, it is possible that NG2-glia are damaged and/or killed by these antibodies (Trotter 2005). All these hypotheses highlight the importance and need for further studies to fully elucidate these questions, which could also lead to new therapeutic strategies.
Lineage potential after injury
As described above, it is well accepted that, depending on the injury model, NG2-glia can generate mature, myelinating oligodendrocytes. Several genetic fate mapping studies of NG2-expressing cells have been performed after brain stab wound injury (Dimou et al. 2008; Komitova et al. 2011), spinal cord injury (Barnabe-Heider et al. 2010), demyelination (Tripathi et al. 2010; Zawadzka et al. 2010) and in models of ALS (Kang et al. 2010). All these investigations support the notion that NG2-glia mostly differentiate into oligodendrocytes (for summary see Figure 2). However, do NG2-glia also generate other neural cell types after injury?
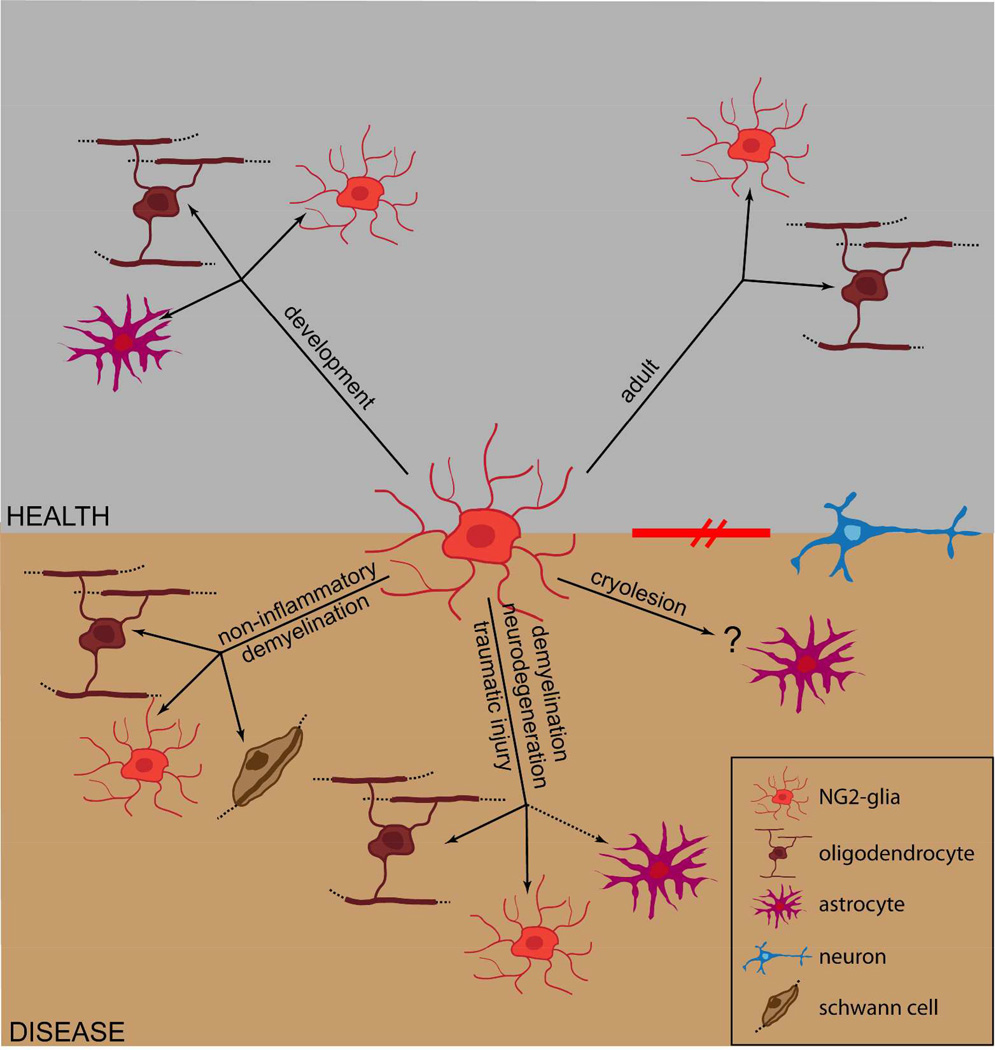
Figure 2. Progeny of NG2-glia at different developmental stages and in disease.
While during development NG2-glia generate more NG2-glia, oligodendrocytes and astrocytes, in the adult CNS their progeny remains purely oligodendrogenic. However, under some pathological conditions of the adult brain, NG2-glia can also generate astrocytes and myelinating Schwann cells. Evidence in favor of generation of neurons from NG2-glia still remains highly contentious.
Indeed, some studies demonstrated a low rate of astrocyte production by NG2-glia in both brain and spinal cord (Barnabe-Heider et al. 2010; Komitova et al. 2011; Zawadzka et al. 2010), while other studies performed either in transgenic Cre-mouse lines or with BrdU label-retaining experiments found virtually no astrocyte generation (Dimou et al. 2008; Kang et al. 2010; Simon et al. 2011). A study, where the reaction of NG2-glia was analyzed in an ALS-model, described that a subset of NG2-glia isolated from ALS-mice transform into astrocytes in vitro, most likely due to proinflammatory cytokine signaling (Magnus et al. 2008). In contrast, in vivo fate-mapping analysis of a PDGFRα-CreERT2 mouse line revealed that the majority - if not all NG2-glia - remain committed to the oligodendrocyte lineage, suggesting that NG2-glia do not play a major role in astrogliosis and astrogenesis (Kang et al. 2010). Also when Komitova et al. (2010) deleted the transcription factor Olig2 by using the NG2-CreERT mouse line after stab wound injury, they could not observe an increase in the production of astrocytes, further supporting the idea that NG2-glia are not the major source of reactive astrocytes in the cerebral cortex.
In contrast to all other studies, one study using the Olig2-CreERT mouse line reported the generation of a high number of scar-forming astrocytes - but not oligodendrocytes - from NG2-glia after cold-induced injury (Tatsumi et al. 2008). However, when the very same line was used in a brain stab wound (Dimou et al., 2008) or spinal cord injury paradigm (Warnabe-Heider et al., 2010), very little or no astrogenesis could be observed. This discrepancy could be due to the lesion model used, pointing to either different injury-dependent signals leading to distinct fates of NG2-glia, or to different subpopulations of NG2-glia with specific differentiation potential. Therefore, it would be important to use the same lesion paradigm with different Cre-mouse lines in order to fully elucidate the differentiation potential of NG2-glia under pathological conditions.
Surprisingly, in models of lysolecithin- or ethidium bromide-induced demyelination in the spinal cord, NG2-glia fate-mapped by a PDGFRα- or Olig2-CreERT mouse lines differentiated into Schwann cells, which contributed to the remyelination process. The fraction of newly generated Schwann cells was significant, as 35 to 56% of the recombined cells were positive for Schwann cell markers such as Periaxin and SCIP. When the very same lab analyzed the progeny of NG2-glia after experimental autoimmune encephalomyelitis (EAE; a strong neuroinflammatory model) they could only observe a very low number of newly generated Schwann cells, pointing to the essential role played by the injury environment on the differentiation properties of NG2-glia.
Currently, no direct evidence has been provided in favor of the idea that NG2-glia can generate neurons after injury, at least without any molecular or genetic manipulation. Different studies demonstrated low rate neurogenesis from NG2-glia that could be attained after in vivo injury when a repressor of the gliogenic transcription factor Olig2 was overexpressed in proliferating glial cells (Buffo et al., 2005). Similar results could also be obtained after overexpression of neurogenic determinants, such as Pax6 or Neurogenin-2 (Buffo et al. 2005; Ohori et al. 2006). In a recent study the combination of the transcription factors Sox2 and Ascl1 - or Sox2 alone - after stab wound injury induced conversion of NG2-glia (genetically fate-mapped in the Sox10-iCreERT2 mouse line; (Simon et al. 2012) into fully functional neurons; (Heinrich et al. 2014). These studies highlight the potential of NG2-glia to generate new neurons in the cerebral cortex after genetic manipulation, identifying them as a potential target for cortical repair.
Neuron-NG2-glia synapses and their role in development and plasticity
NG2-glia exhibit unique electrophysiological properties, including a variety of voltage- and ligand-activated channels, which confer a specific physiological phenotype on these cells. The type of receptors and channels expressed by NG2-glia have been extensively studied and reviewed (Bakiri et al. 2009; Bergles et al. 2010), but their function in vivo is not yet completely defined. In particular, it is still unknown how different membrane signaling pathways are integrated in NG2-glia during development and in adulthood, under both physiological and pathological conditions. The discovery of neuron-NG2 synapses provides an opportunity to investigate this problem and to define the functional relationship between neurons and NG2-glia in the CNS.
NG2-glia in gray matter
Following the first demonstration that NG2-glia in the hippocampus receive synapses from excitatory neurons in young and in adult mice (Bergles et al. 2000), the function of these synapses was and continues to be the object of many studies. The availability of transgenic lines that allow direct visualization of NG2-glia or selective gene deletion in this cell population has greatly facilitated physiological studies and continues to support investigation of neuron-NG2 synapses (Dimou et al. 2008; Kang et al. 2010; Rivers et al. 2008; Zhu et al. 2008b). Both physiological and morphological/ ultrastructural studies have established that NG2-glia receive direct synaptic contacts from neurons (Bergles et al. 2000; Kukley et al. 2007; Lin et al. 2005; Ziskin et al. 2007). However, with the role of NG2-glia in the developing and adult CNS not yet completely defined, the physiological role of neuron-NG2 synapses is still largely undetermined. We will summarize “where and when” these synapses are found in NG2-glia, which may suggest their role(s) in development and neuron-glia interactions at large.
Several studies unambiguously demonstrated that NG2-glia receive glutamatergic synapses in a variety of brain regions including hippocampus (Bergles et al. 2000), dentate gyrus (Mangin et al. 2008), cerebral cortex (Chittajallu et al. 2004; Mangin et al. 2012), brainstem (Muller et al. 2009), corpus callosum (Etxeberria et al. 2010; Kukley et al. 2007; Ziskin et al. 2007), cerebellar white matter (De Biase et al. 2010; Karadottir et al. 2008) and cerebellar cortex (Lin et al. 2005). In hippocampus, NG2-glia of the CA1 region receive synapses from CA3 pyramidal neurons (Bergles et al. 2000); and in cerebral cortex, thalamocortical projections form synapses with NG2-glia located in the barrel cortex (Mangin et al. 2012). In the brainstem, NG2-glia establish synaptic contacts with the large calyx of Held (CoH) excitatory terminal of the medial nucleus of the trapezoid body (MNTB; (Muller et al. 2009). Finally, in the molecular layer of the cerebellar cortex, glutamate released from climbing fibers triggers AMPA receptor channel-mediated currents (Lin et al. 2005). In all these gray matter regions, excitatory synapses on NG2-glia – i.e., glutamatergic currents – display several common features, including: i) small amplitudes; ii) rapid kinetics; and iii) high sensitivity to AMPA receptor antagonists. However, possible functional differences between synapses on NG2-glia of different brain regions still require further investigation.
In addition to glutamatergic synapses, NG2-glia also receive GABAergic synapses in hippocampus, dentate gyrus, and cortex (Ge et al. 2009; Lin and Bergles 2004; Mangin et al. 2008; Tanaka et al. 2009). In the CA1 region of the hippocampus, NG2-glia receive direct GABAergic inputs from interneuron collaterals (Lin and Bergles, 2004). These inputs elicit inhibitory postsynaptic currents (IPSCs) in NG2-glia by activating GABAA receptor channels (Lin and Bergles 2004). Spontaneous inward currents with a decay slower than those due to activation of AMPA receptors could be reliably observed when NG2-glia were loaded with Cl− in order to increase the amplitude of the currents flowing through the GABAA channel. Based on their rise time and insensitivity to benzodiazepines and GABA reuptake blockers, these GABA-mediated responses were ascribed to synapses from local circuit interneurons (Lin and Bergles 2004). Further immunohistochemical and ultrastructural analysis confirmed that interneuronal terminals make direct synaptic contacts with hippocampal NG2-glia. Interestingly, firing of these interneurons was associated with NG2-glia depolarization due to GABAA receptor activation; application of GABA induced a transient inhibition of AMPA receptor-mediated currents in NG2-glia, indicating that GABA may regulate the efficacy of glutamatergic signaling in NG2-glia (Lin and Bergles 2004). Finally, interneuron-NG2 synapses were also characterized in the developing cerebral cortex (Valez-Fort et al., 2010). Interestingly, the number of GABAergic synaptic inputs decreased in cortical NG2 cells during postnatal development, and in the adult mouse GABA signaling mainly involved spillover and activation of extrasynaptic receptors (Valez-Fort et al., 2010).
Together, these studies indicate that integration of glutamate- and GABA-mediated signaling likely occurs in NG2-glia. Further analysis in other brain regions where NG2-glia may receive inputs from GABAergic neurons, including interneurons, will define whether GABA is a major neuronal signal released on NG2-glia and the physiological response associated with activation of GABAergic synapses. It remains to be determined whether GABA also depolarizes NG2-glia in other brain regions, or whether it elicits IPSCs similar to those observed in neurons.
NG2-glia in white matter
NG2-glia are present in large numbers in white matter regions, where they give rise to oligodendrocytes through a complex series of developmental events and cellular changes culminating in myelin synthesis and the wrapping of many axons. Thus, the question of whether NG2-glia also receive synapses in the white matter, and the type of neurons/synaptic terminals that would generate these contacts, was compelling and potentially of great physiological relevance. It was already known that neurons display functional structures dedicated to neurotransmitter release at specialized sites along their axons in white matter regions (Kriegler and Chiu 1993; Li et al. 1999), a finding that raised the crucial question: Was this machinery dedicated to engage in functional interactions with white matter glia?
Two separate studies in the mouse brain reported this functional interaction through direct, excitatory synaptic contacts between collaterals of corpus callosum axons and NG2-glia (Kukley et al., 2007; Ziskin et al., 2007). These synapses are also found in optic nerve and occur between unmyelinated axons and NG2-glia (Kukley et al., 2007), suggesting that they might mediate developmental signaling between neurons and glial progenitors in white matter. Since unmyelinated axons represent only about 30% of the total axons in corpus callosum, it is likely that preferential association of NG2-glia with this subset of axons might have functional relevance to the myelination process. Both studies demonstrated that neurotransmitter release along white matter axons was rather similar to vesicle fusion occurring at neuron-neuron synapses, as: i) it depended on action potential propagation and intensity; ii) it was reliable; and iii) it depended on Ca2+ microdomain signaling. Furthermore, NG2-glia in white matter expressed Ca2+-permeable AMPA receptor channels (Kukley et al., 2007; Ziskin et al., 2007) – well-equipped to respond to a glutamatergic input that would cause a substantial postsynaptic response and associated Ca2+-dependent intracellular events. Finding glutamatergic synaptic currents with fast kinetics on NG2-glia of the human white matter (Gallo et al. 2008) indicated that this type of neuron-glia transmission likely occurred also in larger brains where the relative volume occupied by the white matter is much larger than in rodents (approximately 50% in humans vs. 15% in rodents).
The presence of GABAergic synapses in NG2-glia of the white matter region is still being defined. In corpus callosum, spontaneous or evoked GABA receptor-mediated responses have not been demonstrated (Kukley et al., 2007; Ziskin et al., 2007). Other white matter regions, including other subcortical areas and cerebellum, must be explored more thoroughly.
Note that different types of neuron-NG2-glia communication – both synaptic and nonsynaptic – might simultaneously occur in white matter regions. A recent elegant study of the premotor cortex of awake, behaving mice demonstrated that selective optogenetic stimulation of neurons in this cortical region triggered OPC proliferation and enhanced oligodendrogenesis and myelination in both the deep layers of the premotor cortex and in subcortical white matter (Gibson et al. 2014). Importantly, this activity-dependent myelination also enhanced motor function of the corresponding limb, indicating that activity-dependent oligodendrogenesis and myelination are causally linked with behavioral improvement (Gibson et al., 2014). The relative contribution of synaptic and nonsynaptic mechanisms, and their influence on NG2-glia development and function, might change during white matter maturation in the postnatal brain. Further studies will help determine how these mechanisms are integrated in NG2-glia and how they influence oligodendrogenesis and myelination.
NG2-glia in the subventricular zone
Functional synaptic contacts appear to occur between all NG2-glia and neurons. This raises important questions: Are there any brain regions where NG2-glia do not receive synapses from neurons? and What could this reveal about the possible function of these synapses? NG2-glia are present in high numbers in the SVZ, where they express OPC markers (Olig2), a type C cell phenotype, and actively divide (Aguirre et al. 2004; Menn et al. 2006). Although NG2-glia of the SVZ respond to a variety of extracellular signals (Young et al. 2011), they do not receive neuronal synapses. Therefore, we could speculate that premigratory NG2-glia are not predisposed to make synaptic contacts, as migration would be incompatible with the presence of synapses. We tested this hypothesis in a model of demyelination, in which NG2-glia migration out of the SVZ is greatly enhanced by focal demyelination of the adjacent corpus callosum (Aguirre et al. 2007; Etxeberria et al. 2010). We found that neither NG2-glia in the SVZ nor migratory NG2-glia establish synaptic contacts with neurons before terminating their migration in the corpus callosum (Etxeberria et al. 2010). However, once NG2-glia reach the white matter, they engage in a functional relationship with axons through glutamatergic synapses before differentiating into myelinating OLs (Etxeberria et al. 2010). These synapses are functionally similar to those established between local NG2-glia and axons of the corpus callosum (Kukley et al., 2007; Ziskin et al., 2007).
These findings raise important questions about the cellular and molecular mechanisms that control formation of neuron-NG2-glia synapses and how these synapses are regulated during development. In particular, are synapses maintained by NG2-glia during their entire “developmental history”, i.e., during proliferation, migration, and differentiation?
Developmental regulation of synapses on NG2-glia
In both mouse and rat brains, synaptic inputs on NG2-glia can initially be detected during the first week postnatal (Bergles et al. 2000; Kukley et al. 2008; Lin and Bergles 2004; Mangin et al. 2008). In some brain regions, the intensity of synaptic activity on NG2-glia increases during postnatal development into adulthood, indicating that NG2 synapses undergo a maturation process. However, the molecular mechanisms directing the formation of either glutamatergic or GABAergic synapses on NG2-glia are still unknown.
NG2-glia are the most proliferative cells of the postnatal and adult brain, and nearly all of them connect synaptically with neurons. Thus, NG2-glia synapses probably modulate cell proliferation during development and regeneration (see section below). Some estimate that approximately 50% of the NG2-glia population actively divides every 3 days (Kukley et al., 2008); therefore, formation and maintenance of synapses in NG2-glia should be very efficient processes. At least one reason that NG2-glia appear to form synapses so efficiently is that – in the parenchyma – they maintain synaptic connections while dividing and repositioning during cell proliferation (Kukley et al., 2008). In both gray and white matter regions, functional GABAergic and glutamatergic synapses are still active while NG2-glia undergo mitosis (Ge et al. 2009; Kukley et al. 2008).
Interestingly, although almost all NG2-glia show synapses with neurons, these disappear – or are undetectable – at more mature stages of NG2-glia development, i.e., in differentiated oligodendrocytes (De Biase et al. 2010; Kukley et al. 2010). A very detailed analysis of NG2-glia in situ demonstrated that loss of synaptic inputs occurs at the premyelinating stage (NG2-negative, DM20/PLP+, O1+), i.e., after cell cycle exit (De Biase et al., 2010). This event also corresponds to a significant downregulation of AMPA and NMDA receptors, and Nav channels (De Biase et al., 2010). Transition of the cells to an NG2-negative stage and downregulation of AMPA receptors might even be synchronized events, as a biochemical and morphological study demonstrated that interaction between Glutamate Receptor Interacting Protein (GRIP) and the proteoglycan NG2 may orient the glial AMPA receptors towards sites of neuronal glutamate release (Stegmuller et al. 2003). These important findings restrict our hypotheses about the function of neuron-NG2 synapses, which are likely to be dispensable after NG2-glia stop dividing and differentiate.
Functional role of synapses on NG2-glia: Development
How can we directly study the functional role of neuron-NG2 synapses in intact brain? Different approaches are possible, relying on a combination of genetic tools, electrophysiology, and developmental analysis. Although NG2-glia-specific ablation of glutamate or GABA receptor channel subunits could, in principle, be a viable approach as different gene promoters allow for cell-selective expression of genes in NG2-glia, this strategy would be extremely time consuming and labor intensive, with many potential side effects. Because AMPA and GABA receptor channels comprise many distinct subunits encoded by separate genes, genetic ablation of an individual subunit gene is likely to reduce – but not completely silence – activity at its respective synapses, requiring ablation of more than one gene. Furthermore, compensatory mechanisms might be induced by ablating distinct subunits, thereby changing the composition and functional properties of recombinant AMPA and GABA receptor channels.
Channel rhodopsin expression in specific neuronal populations, combined with optogenetic stimulation, has recently been used to investigate the effect of neuronal electrical activity on NG2-glia and myelination (Gibson et al., 2014). A similar approach could be used to directly and continuously activate synapses on subsets of NG2-glia by stimulating selected groups of neurons, then determining the consequences of this process for NG2-glia proliferation and differentiation.
Another experimental strategy relies on eliminating specific synapses on NG2-glia in selected areas of the brain, where such inputs can be anatomically manipulated. When used in transgenic mice with NG2-glia being permanently labeled and can be directly visualized, this approach has the advantages of maintaining the molecular integrity of native glutamate and GABA receptor channels and avoiding genetic modification of NG2-glia. In some brain regions, degeneration of specific inputs can be induced by anatomical or pharmacological manipulation (Fox 1992). Expression of toxins in specific neuronal populations can also be used as an approach to selectively ablate synaptic inputs to specific cell populations (Revy et al. 2014; Saito et al. 2001).
The rodent barrel cortex has been used as a model system to investigate plasticity in primary somatosensory cortex (Fox 1992; Fox 2002). This brain area displays an orderly anatomical map that matches the array of facial whiskers: Each whisker is represented in the cortex, one-to-one with barrel structures, and ablating, plucking, or trimming the facial whiskers cause changes in the barrel structures (Stern et al. 2001). Therefore, experience-dependent plasticity has been extensively studied by depriving the barrel cortex of selected sensory inputs.
In a recent study, we demonstrated that NG2-glia receive glutamatergic synapses from thalamocortical fibers during development of the mouse barrel cortex (Mangin et al., 2012). While the barrel cortex is being formed, NG2-glia preferentially accumulate along the septa separating the barrels. We also found that deprivation of whisker sensory input significantly reduced thalamocortical inputs on NG2-glia and simultaneously increased their proliferation (Mangin et al., 2012), causing a more uniform distribution in the deprived barrels. These findings indicate that, under normal physiological conditions, tonic, glutamate-mediated depolarization of the cell membrane constrains NG2-glia proliferation. Furthermore, early sensory experience regulates thalamocortical innervation on NG2-glia, as well as their distribution during development.
This discovery may add importantly to our understanding of how synaptic activity in specific circuits and brain regions controls a large population of cells and contributes to regulating their developmental program (see section below). From a developmental and mechanistic perspective, we need to define how synaptic activity in NG2-glia synchronizes or modulates the mode of NG2-glia division. In particular, how are spontaneous synaptic currents arising in the cell body and/or in cellular processes integrated to regulate specific pathways that functionally regulate NG2-glia cycle progression? Some of these pathways have been identified (e.g., p27Kip1 and p21CIP1) and regulate G1-S transition, as well as cell cycle exit and differentiation (Casaccia-Bonnefil et al. 1997; Ghiani et al. 1999; Jablonska et al. 2012)). In addition, since application of either glutamate or GABA depolarizes the cell membrane and induces Ca2+ transients in NG2-glia (Ge et al. 2006), it is crucially important to investigate the relationship between the spatiotemporal properties of these Ca2+ events and the activation of specific intracellular signaling pathways that regulate NG2-glia development. We must also determine whether neuronal inputs are sufficient to induce Ca2+ transients in these cells under physiological conditions in vivo – especially as the extent of membrane depolarization caused by individual neuron-NG2-glia synapses is rather small, and NG2-glia display a strongly hyperpolarized membrane resting potential (Lin and Bergles 2004).
A recent study of postnatal development demonstrated that the majority of NG2-glia that stop dividing will differentiate during a critical time window of 3–8 days (Hill et al. 2014). The length of this gap changes during maturation of the brain (Hill et al. 2014). An attractive hypothesis for future testing is whether synaptic activity contributes to regulating this critical time window and its length. It would be particularly interesting to analyze whether synaptic activity contributes to the timing of cell cycle exit and initiation of differentiation, and to identify the mechanism(s) involved.
Previous analysis in cell cultures identified a mechanism by which glutamate enhances p27Kip1 and inhibits NG2-glia proliferation. Dividing NG2-glia – but not differentiated oligodendrocytes – display large outward K+ currents that are regulated during cell cycle progression and blocked by glutamate-induced membrane depolarization (Gallo et al. 1996). Similar effects of glutamate on NG2-glia were also demonstrated in a more intact system – cultured cerebellar slices from early postnatal tissue – where endogenously released glutamate inhibits NG2-glia proliferation by activating AMPA receptors (Yuan et al. 1998). This is consistent with the finding that cerebellar NG2-glia exhibit AMPA receptor-mediated currents at the same developmental stage (Karadottir et al. 2008).
Together, the studies performed in cultured cells and tissue explants, along with more recent evidence in vivo, consistently point to a role of glutamate in regulating NG2-glia proliferation. An important question arising from these findings is whether GABAergic synapses on NG2-glia might also play a role in regulating their development, since GABA also causes cell membrane depolarization at early postnatal developmental stages (Ganguly et al. 2001). GABA has been clearly shown to regulate the development of different types of neural progenitors (Alfonso et al. 2012; Liu and Wong-Riley 2005; LoTurco et al. 1995), which indicates GABA’s potential to function as a developmental signal for NG2-glia as well. Future investigations in brain regions where NG2-glia receive well-identified and functional GABAergic synapses will determine not only whether these synapses are involved in regulating NG2-glia development, but also how glutamatergic and GABAergic signals cooperate in these progenitor cells.
Further information about the role of NG2-glia synapses in various brain regions could derive from analyzing NG2-glia after pathological insults or during regenerative processes. In fact, a common feature of the response of NG2-glia to different types of injury – in developing and in adult brain – is their enhanced proliferation, which produces significant expansion of the endogenous pool of NG2-glia in gray and white matter regions (Gallo and Deneen 2014). Electroconvulsive seizures also cause NG2-glia proliferation in the hippocampus (Wennstrom et al. 2003). Therefore, it will be crucial to determine whether NG2-glia are partially or completely “unleashed” from their functional relationship with neurons after insult, and how their activation and proliferation relate to the loss or reduction of synaptic inputs. Finally, some models of brain injury will allow us to follow up NG2-glia while they undergo “rewiring” by neurons during regeneration and recovery, to determine how newly formed synapses modify NG2-glia behavior.
Functional role of synapses on NG2-glia: Physiology and plasticity
It is very likely that neurons regulate NG2-glia development and function through synaptic contacts, so a crucial question is: Do NG2-glia modulate neuronal function? Some interesting hypotheses currently being generated are based on a more detailed functional analysis of neuron-NG2 synapses in the context of specific neural circuits.
Several studies have investigated in detail the type and origin of presynaptic terminals on NG2-glia. Are synaptic inputs on NG2-glia dedicated to this cell population, or do presynaptic neurons innervate both NG2-glia and other neurons? The question is important, as one could imagine that sharing the same presynaptic input increases the likelihood of synchronized postsynaptic activity in NG2-glia and specific neuronal populations. We investigated the origin of presynaptic terminals on NG2-glia of the hilus of the mouse dentate gyrus between postnatal days 3–21 and found that, in this gray matter region, NG2-glia are closely associated with interneurons (Mangin et al., 2008). This anatomical association also reflected a physiological interaction between these cell types, as both hilar interneurons and associated NG2-glia received excitatory inputs from granule cells and CA3 pyramidal neurons (Mangin et al., 2008). In hilar NG2-glia, the frequency and amplitude of sEPSCs increase during the first 3 postnatal weeks; conversely, the rise and decay times of sEPSCs significantly decrease, suggesting that glutamatergic synapses on NG2-glia undergo a maturation process similar to that of their neuronal counterparts during the same time period.
Interestingly, pair recordings demonstrated that bursts of activity induced by GABAergic antagonists caused synchronized bursts of activity between both cell types, and the amplitude of these bursts was positively correlated. Furthermore, carbachol increased EPSC activity, and closely apposed cells were more likely to exhibit synchronized EPSCs than were cells separated by gaps of more than 200 microns.
Investigations in other brain regions demonstrated that NG2-glia frequently share presynaptic terminals with neurons. For example, in the medial nucleus of the trapezoid body (MNTB), each glutamatergic terminal from the calyx of Held (CoH) forms an axosomatic synapse with a single principal neuron of the MNTB (Muller et al. 2009). In this region, also, NG2-glia receive an excitatory input from the CoH; however, unlike the principal neurons – which receive a glutamatergic and a GABAergic input – NG2-glia only receive AMPA receptor-mediated synaptic inputs (Muller et al., 2009). Similar to the dentate gyrus, simultaneous recordings from neurons and NG2 glia indicated that they receive synchronized spontaneous inputs.
A further example of shared presynaptic input is provided by cerebellar NG2 and Purkinje cells (PCs), found to be simultaneously innervated by climbing fiber (CF) terminals (Lin and Bergles, 2005). However, each PC is contacted by only one climbing fiber, while NG2-glia receive inputs from several climbing fibers, providing the opportunity to monitor and integrate the activity arising from several neighboring PCs.
Not only are NG2-glia engaged in synchronized activity with neurons, they are also directly involved in long-term potentiation (LTP). Much like their neuronal counterparts, neuron-NG2 synapses also undergo activity-dependent modifications equivalent to LTP at neuronal excitatory synapses (Ge et al., 2006). This specifically occurs in neuron-NG2 synapses between Schaffer collaterals and NG2-glia in CA1. Yet, distinct from neuron-neuron synapses, induction and expression of LTP in neuron-NG2-glia synapses depends on activating Ca2+-permeable AMPA receptors in the glial progenitors. Note that the NG2 proteoglycan has been demonstrated to associate with postsynaptic AMPA receptors via the PSD protein GRIP (Stegmuller et al., 2003). This indicated that expression of NG2 and AMPA receptors in the NG2-glia membrane might be co-regulated and, therefore, LTP could be modulated by different levels of the proteoglycan NG2.
A more recent study elegantly demonstrates that NG2-glia engage in bidirectional crosstalk with surrounding neurons mediated by the NG2 proteoglycan itself, and contributes to elucidating the function of NG2-glia in neuronal networks (Sakry et al. 2014). In particular, NG2 is cleaved by the α-secretase ADAM10 in an activity-dependent fashion to release an ectodomain present in the extracellular matrix. Pharmacological inhibition of this process or genetic ablation of NG2 in expressing cells causes a large reduction in NMDA and AMPA receptor currents in pyramidal neurons of the somatosensory cortex, as well as in NMDA-dependent LTP in the same neurons (Sakry et al. 2014). Consistent with the physiological results, NG2-knockout mice also displayed abnormal sensorimotor function. In conclusion, although the mechanism by which the NG2 ectodomain regulates both NMDA and AMPA receptor currents is still undefined, this study demonstrates a novel physiological role for OPC in regulating information processing and plasticity at neuronal synapses. Furthermore, based on this study, one could also speculate that NG2-glia may influence synaptic activity in neurons by releasing factors able to modulate synaptic networks in developing and mature brain.
NG2-glia in the human brain
NG2-glia in rodents have been studied extensively and in detail, however much less is known about their counterpart in the human brain, as they can only be studied in postmortem or neurosurgical material. This has prevented analysis of human NG2 cell development, including cell proliferation and differentiation.
In the early `90s, human NG2-glia were first isolated from white matter and taken into culture (Armstrong et al. 1992; Gogate et al. 1994). While they were found to express similar markers as rodent NG2-glia - including O4, PDGFRa and MyT1 - their response to different growth factors was remarkably different. In fact, although PDGF and bFGF strongly promote cell proliferation of rodent NG2-glia in culture, human NG2-glia do not respond to either of these factors by mitosis, but rather by morphological changes, i.e. process formation (Armstrong et al. 1992; Gogate et al. 1994; Yong et al. 1988). Immunohistochemical analysis from human post mortem material revealed a rather low number of PDGFRa-expressing cells in the adult human brain (1–3% of all brain cells), which interestingly did not change in the vicinity of multiple sclerosis lesions (Scolding et al. 1998).
More recently, the identification of further markers for NG2-glia, including NG2 and Olig2, lead to a better characterization of these cells in human brain tissue (Chang et al. 2000). However, only in 2009 two independent studies demonstrated for the first time that NG2-glia can proliferate also in the adult human cerebral cortex in vivo (Geha et al. 2010; Rhee et al. 2009). Rhee et al. (2009) estimated that 1/2500 to 1/5000 cells in the subcortical white matter from chronic epileptic patients are dividing NG2-/Olig2-positive cells. Conversely, Geha et al. (2009) analyzed both healthy and chronic epileptic specimens, and showed that diving cells in the adult human CNS are in their vast majority NG2-glia. They also suggested that human NG2-cells are either slowly dividing with an extended G1-phase, or are blocked at different G1 check points, similar to mouse NG2-glia (Dimou et al. 2008; Simon et al. 2011).
These findings unambiguously demonstrated that human NG2-glia could proliferate in the adult brain, but what about their differentiation potential? Several observations in the rodent brain support the idea that oligodendrocyte turnover contributes to myelin remodeling and experience-dependent myelin plasticity. A recent study by Yeung et al. (2014) demonstrated that the number of oligodendrocytes in the human corpus callosum remains rather stable during adulthood, and that around 1/300 oligodendrocytes is exchanged every year. Although these results show that adult NG2-glia also have the ability to differentiate in the human brain, the low numbers of these newly-generated oligodendrocytes cannot account by itself for the experience-dependent changes observed in white matter volume (Yeung et al. 2014). Therefore, these findings still leave us with the important open question of how our knowledge of NG2-glia in rodents and humans can be translated into development of new therapeutic approaches aimed at curing demyelinating diseases.
Several strategies have been applied to determine whether different types of neural progenitor populations - including NG2-glia – could promote myelination in animal models of hypomyelination or demyelination. A major focus of these strategies has been on transplantation of human-derived glial progenitor cells or neural stem cells into hypomyelinated mouse brains (Uchida et al. 2012; Windrem et al. 2008), which was demonstrated to lead to an almost fully remyelinated and humanized white matter. Recently, a phase 1 study was published, where human-derived neural stem cells were transplanted in four patients with early severe Pelizaeus-Merzbacher disease, a disorder where oligodendrocytes fail to myelinate axons during development, leading to global neurological dysfunction. Notably, although magnetic resonance analysis suggested donor-derived myelin production in the grafted areas, only modest gains in neurological functions were observed (Gupta et al. 2012).
In conclusion, the finding that NG2-glia found in both healthy and pathological human brain can be maintained in culture and are capable of actively dividing has important implications not only for our understanding of oligodendrocyte turnover and myelination under normal physiological conditions, but also for development of future strategies to promote myelin repair. Further analysis and characterization of adult human NG2-glia, together with in vitro studies, will lead to the identification of factors that control proliferation and differentiation of these cells, and may help elucidate mechanisms underlying the failure of the adult human CNS to remyelinate after pathological insults that cause demyelination of white matter and gray matter regions of the brain.
NG2-glia and therapeutic approaches
Endogenous cells
Demyelinating diseases
As described and discussed above, NG2-glia exist in high number in the adult brain and can differentiate into myelinating oligodendrocytes. This together with the fact that they exhibit robust homeostasis (Hughes et al. 2013), leading to their rapid replacement after differentiation, makes them the ideal candidates for myelin repair after demyelination. The most common demyelinating disease in humans is multiple sclerosis, an inflammatory disease leading to plaques in the CNS due to oligodendrocyte death and myelin breakdown. Endogenous NG2-glia respond to this pathological insult, differentiate into oligodendrocytes and partially remyelinate naked axons. However, as already discussed above in detail, remyelination is never complete and the newly formed myelin is abnormal, i.e. thinner, and not compacted and with shorter internodes. Although this defective myelin is sufficient to partially protect axons from degeneration and enhance electrical conduction velocity, it is likely that improving the remyelination ability of endogenous NG2-glia would be beneficial to remyelination and functional recovery. Therefore it is of extreme importance to identify candidate molecules and to understand the mechanisms that regulate the proliferation and differentiation of NG2-glia not only after demyelination, but also in the healthy brain, as these cells may be used in therapeutic approaches promoting regeneration and preventing demyelination.
Importantly, several growth factors have already been identified that regulate NG2-glia proliferation, differentiation and migration. It would be of great interest to understand whether these factors are altered in the damaged brain and whether signaling pathways are shared between development - when physiological myelination takes place - and pathological remyelination. Indeed, in the last decade several growth factors were identified in demyelination lesions that are known to be important during development for proliferation, migration and differentiation of NG2-glia, including PDGF (Armstrong et al. 1990), FGF (Messersmith et al. 2000), EGF (Aguirre et al. 2007; Scafidi et al. 2014), CNTF (Albrecht et al. 2003) and BDNF (VonDran et al. 2011). The fact that these factors are also found in the brain under pathological conditions raises the crucial question of why is the remyelination capacity of NG2-glia is limited. A first explanation could be that some factors that positively regulate NG2-glia proliferation and differentiation under physiological conditions switch their physiological effects after injury. It has indeed been shown for FGF that its ablation in demyelinated lesions leads to enhanced remyelination due to an increase in NG2-glia differentiation (Armstrong et al. 2002). Another reason for the repair failure could be that inhibitory cellular signals are present at high levels after demyelination. Several studies demonstrated that some cellular factors that negatively regulate oligodendrocyte differentiation during development are upregulated after injury, such as Notch (Hammond et al. 2014; Scafidi et al. 2014; Wang et al. 1998), Wnt (Fancy et al. 2009) and BMP (Colak et al. 2008; Hampton et al. 2007).
Another important aspect to be considered is the regional heterogeneity within NG2-glia (see above). Therefore, it is necessary to also identify factors and molecular signals that either overcome the limited proliferation and differentiation properties of gray matter cells, or promote migration of white matter cells into demyelinated areas. To this end, we need not only to identify gene expression patterns and dominant signaling pathways that are differentially expressed in distinct subtypes of NG2-glia, but also to elucidate the homeostatic self-renewal mechanisms that ultimately govern their behavior.
Neuronal replacement
Different types of injury and pathologies cause both oligodendrocyte and neuronal death in the CNS. In contrast to oligodendrocytes, neurons cannot be replaced by progenitors or other cells, e.g. by reactive NG2-glia. However, recent studies have demonstrated that forced expression of key neurogenic fate determinants in glial cells can instruct them towards neurogenesis (Buffo et al. 2005; Kronenberg et al. 2010; Torper et al. 2013). In particular, astrocytes were thought to be the main cells available to be reprogrammed into neurons; however it is now clear that also NG2-glia could provide a pool of viable progenitors in close proximity to the injury that can be instructed towards neurogenesis.
In most studies, crucial neurogenic transcription factors were introduced into the brain by the use of retroviruses, which specifically integrate in proliferating cells. By using this approach, it was shown that infected cells were reprogrammed into neurons in the intact and injured brain, and in spinal cord (Grande et al. 2013; Ohori et al. 2006). However, the identity of the infected cells was often not determined. As NG2-glia are the only cells proliferating in the intact brain outside neurogenic niches, it is very likely that they would be the main target cells to be reprogrammed after retroviral injection. Similarly, since NG2-glia - rather than astrocytes –are the main dividing cells after injury, this cell population is the most susceptible to retroviral manipulation. The idea that NG2-glia can be reprogrammed into neurons also correlates with experiments by Kondo and Raff showing that NG2-glia can reverse to a neural stem cell-like phenotype in vitro after sequential exposure to a specific set of growth factors. However, this does not seem to be the case in vivo - at least after stab wound injury -as NG2-glia failed to display stem cell behavior and did not form neurospheres (Sirko et al., 2013).
In favor of the notion that NG2 cells can be efficiently reprogrammed to generate neurons, a recent study demonstrated by genetic fate mapping that neurons generated after ectopic expression of Sox2, or Sox2 and Ascl1, are indeed progeny of NG2-glia (Heinrich et al. 2014). However, this fate switch was only observed in the injured cerebral cortex and not under normal physiological conditions. In a separate study in stab wounded and AD mice, a very high rate of reprogramming efficiency of NG2-glia was obtained after ectopic expression of the neurogenic transcription factor NeuroD1 driven by the NG2-promoter. These newly formed neurons were fully functional and displayed synaptic inputs. Interestingly, NG2-glia generated both glutamatergic and GABAergic neurons, while astrocytes only converted into glutamatergic cells, underlining a higher degree of plasticity of NG2-glia (Guo et al. 2014). However, not only overexpression of neurogenic factors can lead to reprogramming of NG2-glia. Ju et al. showed that antagonizing the EGFR after spinal cord injury caused a significant number of NG2-glia to acquire a neuronal phenotype, which ultimately resulted in improved behavioral performance in these animals (Ju et al. 2012).
Although very encouraging, all these results raise important questions that should be addressed before NG2-glia are considered as a feasible therapeutic target in humans: Are the environmental changes taking place after injury and/or the intrinsic changes occurring in reactive NG2-glia functionally relevant to the reprogramming potential of these cells? As the newly generated neurons appear to be fully functional, are the active synapses on these neurons derived from pre-existing neuron - NG2-glia synapses, or are they newly generated? Do the newly generated neurons fully integrate into synaptic networks and neural circuitry? What is their impact in brain repair, and do they fully rescue behavioral deficits and lead to cognitive improvement? The answers to these questions are still largely undefined; however it is becoming progressively clearer that endogenous NG2-glia represent a viable and effective target to regenerate not only oligodendrocytes, but also neurons.
Main points.
NG2-glia are heterogeneous and display distinct properties
NG2-glia receive synapses from neurons which play a modulatory role in their development and regeneration
NG2-glia respond to pathological insults by changing their properties
Acknowledgements
V.G. was supported by the NIH (R01NS045702, R01NS056427, and P01NS062686), the National Multiple Sclerosis Society (RG4706) and by Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Centers P30HD40677. LD was supported by the ERA-NET ‘Network of European Funding for Neuroscience Research’ (NEURON) project “RENEW IT” and the BMBF - Federal Ministry of Education and Research- Germany. Additional support for L.D. was provided by the SFB 870 of the Deutsche Forschungsgemeinschaft and the Friedrich Bauer Stiftung. We thank Sarah Schneider for Figure 2 and Jonathan Ritter for Figure 3.
Figure 3. Neuron-NG2 synapses in the brain.
NG2-glia form synaptic contacts with neurons in gray and white matter regions. In gray matter, NG2-glia receive synaptic inputs from both excitatory and inhibitory neurons. In white matter, NG2-glia receive excitatory synaptic inputs from axon collaterals. During development, functional synapses are detected at early developmental stages in NG2-glia (oligodendrocyte progenitors), but are downregulated in pre-oligodendrocytes, and are undetectable in mature oligodendrocytes. PRE-OL = pre-oligodendrocyte; OL = oligodendrocyte.
References
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10(8):990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24(46):10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3(3):209–220. doi: 10.1017/S1740925X08000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165(4):575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17(2):129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht PJ, Murtie JC, Ness JK, Redwine JM, Enterline JR, Armstrong RC, Levison SW. Astrocytes produce CNTF during the remyelination phase of viral-induced spinal cord demyelination to stimulate FGF-2 production. Neurobiol Dis. 2003;13(2):89–101. doi: 10.1016/s0969-9961(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell. 2012;10(1):76–87. doi: 10.1016/j.stem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Armstrong R, Friedrich VL, Jr, Holmes KV, Dubois-Dalcq M. In vitro analysis of the oligodendrocyte lineage in mice during demyelination and remyelination. J Cell Biol. 1990;111(3):1183–1195. doi: 10.1083/jcb.111.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992;12(4):1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Flint NC, Vana AC, Zhou YX. Endogenous cell repair of chronic demyelination. J Neuropathol Exp Neurol. 2006;65(3):245–256. doi: 10.1097/01.jnen.0000205142.08716.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J Neurosci. 2002;22(19):8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Attwell D, Karadottir R. Electrical signalling properties of oligodendrocyte precursor cells. Neuron Glia Biol. 2009;5(1–2):3–11. doi: 10.1017/S1740925X09990202. [DOI] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134(2):285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Behar T, McMorris FA, Novotny EA, Barker JL, Dubois-Dalcq M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 1988;21(2–4):168–180. doi: 10.1002/jnr.490210209. [DOI] [PubMed] [Google Scholar]
- Behrendt G, Baer K, Buffo A, Curtis MA, Faull RL, Rees MI, Gotz M, Dimou L. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 2013;61(2):273–286. doi: 10.1002/glia.22432. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161(1):169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhauser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010;63(1–2):130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405(6783):187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Boda E, Vigano F, Rosa P, Fumagalli M, Labat-Gest V, Tempia F, Abbracchio MP, Dimou L, Buffo A. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia. 2011;59(12):1958–1973. doi: 10.1002/glia.21237. [DOI] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34(4):296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102(50):18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V, Jr, Chao MV, Koff A. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 1997;11(18):2335–2346. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20(17):6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci. 2009;12(11):1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31(6–7):481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561(Pt 1):109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Young KM, Hamilton NB, Li H, Richardson WD, Attwell D. Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J Neurosci. 2012;32(24):8173–8185. doi: 10.1523/JNEUROSCI.0928-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Gotz M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28(2):434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci. 2006;26(41):10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40(3):471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30(10):3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57(1):54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28(4–5):365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Dimou L, Gotz M. Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiol Rev. 2014;94(3):709–737. doi: 10.1152/physrev.00036.2013. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28(41):10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Zhang M, De Vellis J. O2A progenitor cells transplanted into the neonatal rat brain develop into oligodendrocytes but not astrocytes. Proc Natl Acad Sci U S A. 1993;90(1):50–54. doi: 10.1073/pnas.90.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13(3):287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanarraga ML, Griffiths IR, Zhao M, Duncan ID. Oligodendrocytes are not inherently programmed to myelinate a specific size of axon. J Comp Neurol. 1998;399(1):94–100. [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23(13):1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319(6053):499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, Kang SH, Bergles DE, Lee SI, Levine JM, et al. Entrapment via Synaptic-Like Connections between NG2 Proteoglycan+ Cells and Dystrophic Axons in the Lesion Plays a Role in Regeneration Failure after Spinal Cord Injury. J Neurosci. 2014;34(49):16369–1684. doi: 10.1523/JNEUROSCI.1309-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12(5):1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111(4):799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3(9):705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Zhao C, Sim FJ. Ageing and CNS remyelination. Neuroreport. 2002;13(7):923–928. doi: 10.1097/00001756-200205240-00001. [DOI] [PubMed] [Google Scholar]
- Frohlich N, Nagy B, Hovhannisyan A, Kukley M. Fate of neuron-glia synapses during proliferation and differentiation of NG2 cells. J Anat. 2011;219(1):18–32. doi: 10.1111/j.1469-7580.2011.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Daniele S, Lecca D, Lee PR, Parravicini C, Fields RD, Rosa P, Antonucci F, Verderio C, Trincavelli ML, et al. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J Biol Chem. 2011;286(12):10593–10604. doi: 10.1074/jbc.M110.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83(2):283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586(16):3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16(8):2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Marques J, Nunez-Llaves R, Lopez-Mascaraque L. NG2-glia from pallial progenitors produce the largest clonal clusters of the brain: time frame of clonal generation in cortex and olfactory bulb. J Neurosci. 2014;34(6):2305–2313. doi: 10.1523/JNEUROSCI.3060-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312(5779):1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci U S A. 2009;106(1):328–333. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, Chneiweiss H, Daumas-Duport C, Varlet P. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20(2):399–411. doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia. 1996;17(1):39–51. doi: 10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Yuan X, Eisen AM, Knutson PL, DePinho RA, McBain CJ, Gallo V. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27(Kip1) and p21(CIP1) in glial progenitor cells. J Neurosci. 1999;19(13):5380–5392. doi: 10.1523/JNEUROSCI.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogate N, Verma L, Zhou JM, Milward E, Rusten R, O’Connor M, Kufta C, Kim J, Hudson L, Dubois-Dalcq M. Plasticity in the adult human oligodendrocyte lineage. J Neurosci. 1994;14(8):4571–4587. doi: 10.1523/JNEUROSCI.14-08-04571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande A, Sumiyoshi K, Lopez-Juarez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi V, Moharregh-Khiabani D, Skripuletz T, Koutsoudaki PN, Kotsiari A, Skuljec J, Trebst C, Stangel M. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127–138. doi: 10.1016/j.brainres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29(22):7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Delgado M, Sohn J, Miers L, Ko EM, Bannerman P, Xu J, Wang Y, et al. Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J Neurosci. 2011;31(33):11914–11928. doi: 10.1523/JNEUROSCI.1759-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30(36):12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4(155) doi: 10.1126/scitranslmed.3004373. others 155ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A, Gallo V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron. 2014;81(3):588–602. doi: 10.1016/j.neuron.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26(11):3024–3035. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Bergami M, Gascon S, Lepier A, Vigano F, Dimou L, Sutor B, Berninger B, Gotz M. Sox2-Mediated Conversion of NG2 Glia into Induced Neurons in the Injured Adult Cerebral Cortex. Stem Cell Reports. 2014;3(6):1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci. 2014;17(11):1518–1527. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci. 2013;33(36):14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498(4):525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia. 2014;62(6):896–913. doi: 10.1002/glia.22648. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Toda M, Nakai Y, Asou H, Watanabe M, Nakamura M, Yato Y, Fujimura Y, Kawakami Y, Toyama Y, et al. Increase of oligodendrocyte progenitor cells after spinal cord injury. J Neurosci Res. 2001;65(6):500–507. doi: 10.1002/jnr.1180. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Scafidi J, Aguirre A, Vaccarino F, Nguyen V, Borok E, Horvath TL, Rowitch DH, Gallo V. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32(42):14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Johnstone SR, Levi G, Wilkin GP, Schneider A, Ciotti MT. Subpopulations of rat cerebellar astrocytes in primary culture: morphology, cell surface antigens and [3H]GABA transport. Brain Res. 1986;389(1–2):63–75. doi: 10.1016/0165-3806(86)90173-2. [DOI] [PubMed] [Google Scholar]
- Ju P, Zhang S, Yeap Y, Feng Z. Induction of neuronal phenotypes from NG2+ glial progenitors by inhibiting epidermal growth factor receptor in mouse spinal cord injury. Glia. 2012;60(11):1801–1814. doi: 10.1002/glia.22398. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16(5):571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11(4):450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22(2):161–170. [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia. 2011;59(5):800–809. doi: 10.1002/glia.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289(5485):1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004;18(23):2963–2972. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13(10):4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Gertz K, Cheung G, Buffo A, Kettenmann H, Gotz M, Endres M. Modulation of fate determinants Olig2 and Pax6 in resident glia evokes spiking neuroblasts in a model of mild brain ischemia. Stroke. 2010;41(12):2944–2949. doi: 10.1161/STROKEAHA.110.583039. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10(3):311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are born with synapses. FASEB J. 2008;22(8):2957–2969. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30(24):8320–8331. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14(8):4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Enquist LW, Card JP. Reactions of oligodendrocyte precursor cells to alpha herpesvirus infection of the central nervous system. Glia. 1998;23(4):316–328. [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160(2):333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119(3):611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10(2):201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J Neurosci Res. 1997;48(2):83–94. [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19(14) doi: 10.1523/JNEUROSCI.19-14-j0002.1999. RC16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006a;54(1):1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006b;103(20):7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien LE, Sendtner M, Rohrer H, Hughes SM, Raff MC. Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron. 1988;1(6):485–494. doi: 10.1016/0896-6273(88)90179-1. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47(3):290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46(5):773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol (1985) 2005;98(4):1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15(6):1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25(2):317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17(11):4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Ansorge O, Strong M, Bilbao J, Zinman L, Ang LC, Baker M, Stewart H, Eisen A, Rademakers R, et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011;122(1):87–98. doi: 10.1007/s00401-011-0838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia. 2008;56(2):200–208. doi: 10.1002/glia.20604. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008;28(30):7610–7623. doi: 10.1523/JNEUROSCI.1355-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JM, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci. 2012;15(9):1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Toews A, Hostettler JD, Morell P, Suzuki K, Goldman JE, Matsushima GK. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am J Pathol. 2004;164(5):1673–1682. doi: 10.1016/S0002-9440(10)63726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Chuai M, Imai Y, Takahashi H, Tanaka J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2008;28(1):149–163. doi: 10.1038/sj.jcbfm.9600519. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23(3–4):345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith DJ, Murtie JC, Le TQ, Frost EE, Armstrong RC. Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res. 2000;62(2):241–256. doi: 10.1002/1097-4547(20001015)62:2<241::AID-JNR9>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Reyes-Haro D, Pivneva T, Nolte C, Schaette R, Lubke J, Kettenmann H. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol. 2009;134(2):115–127. doi: 10.1085/jgp.200910194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128(4):527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Noble M, Arhin A, Gass D, Mayer-Proschel M. The cortical ancestry of oligodendrocytes: common principles and novel features. Dev Neurosci. 2003a;25(2–4):217–233. doi: 10.1159/000072270. [DOI] [PubMed] [Google Scholar]
- Noble M, Smith J, Power J, Mayer-Proschel M. Redox state as a central modulator of precursor cell function. Ann N Y Acad Sci. 2003b;991:251–271. doi: 10.1111/j.1749-6632.2003.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9(4):439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26(46):11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F, Gascon S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15(6):602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222(2):218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27(16):4233–4242. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passlick S, Grauer M, Schafer C, Jabs R, Seifert G, Steinhauser C. Expression of the gamma2-subunit distinguishes synaptic and extrasynaptic GABA(A) receptors in NG2 cells of the hippocampus. J Neurosci. 2013;33(29):12030–12040. doi: 10.1523/JNEUROSCI.5562-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, Hersmus N, Kusters B, Van Den Bosch L, Van Damme P, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136(Pt 2):471–482. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J, Mayer-Proschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev Biol. 2002;245(2):362–375. doi: 10.1006/dbio.2002.0610. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5(3–4):57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983a;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Lillien LE. Differentiation of a bipotential glial progenitor cell: what controls the timing and the choice of developmental pathway? J Cell Sci. 1988;(Suppl 10):77–83. doi: 10.1242/jcs.1988.supplement_10.6. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983b;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raine CS, Cross AH. Axonal dystrophy as a consequence of long-term demyelination. Lab Invest. 1989;60(5):714–725. [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95(7):3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revy D, Jaouen F, Salin P, Melon C, Chabbert D, Tafi E, Concetta L, Langa F, Amalric M, Kerkerian-Le Goff L, et al. Cellular and behavioral outcomes of dorsal striatonigral neuron ablation: new insights into striatal functions. Neuropsychopharmacology. 2014;39(11):2662–2672. doi: 10.1038/npp.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31(6–7):523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Rhee W, Ray S, Yokoo H, Hoane ME, Lee CC, Mikheev AM, Horner PJ, Rostomily RC. Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia. 2009;57(5):510–523. doi: 10.1002/glia.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120(1):41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992;89(18):8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70(4):661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11(12):1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Trudel E, Rotondi O, Liu X, Djogo T, Kryzskaya D, Bourque CW, Kokoeva MV. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One. 2013;8(10):e78236. doi: 10.1371/journal.pone.0078236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19(22):9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19(8):746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Sakry D, Karram K, Trotter J. Synapses between NG2 glia and neurons. J Anat. 2011;219(1):2–7. doi: 10.1111/j.1469-7580.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Biname F, Perera SS, Endres K, Lutz B, Radyushkin K, et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014;12(11):e1001993. doi: 10.1371/journal.pbio.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura S, Sawada M, Ito M, Nagatsu T, Nagatsu I, Suzumura A, Shibuya M, Sugita K, Marunouchi T. The bipotential glial progenitor cell line can develop into both oligodendrocytes and astrocytes in the mouse forebrain. Neurosci Lett. 1995;188(1):1–4. doi: 10.1016/0304-3940(95)11378-a. [DOI] [PubMed] [Google Scholar]
- Scafidi J, Hammond TR, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter RJ, Jr, et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506(7487):230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121(Pt 12):2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59(6):869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- Simon C, Lickert H, Gotz M, Dimou L. Sox10-iCreERT2 : a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis. 2012;50(6):506–515. doi: 10.1002/dvg.22003. [DOI] [PubMed] [Google Scholar]
- Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected] Cell Stem Cell. 2013;12(4):426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Spassky N, Heydon K, Mangatal A, Jankovski A, Olivier C, Queraud-Lesaux F, Goujet-Zalc C, Thomas JL, Zalc B. Sonic hedgehog-dependent emergence of oligodendrocytes in the telencephalon: evidence for a source of oligodendrocytes in the olfactory bulb that is independent of PDGFRalpha signaling. Development. 2001;128(24):4993–5004. doi: 10.1242/dev.128.24.4993. [DOI] [PubMed] [Google Scholar]
- Stegmuller J, Werner H, Nave KA, Trotter J. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells. Implications for glial-neuronal signaling. J Biol Chem. 2003;278(6):3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Steiner B, Winter C, Hosman K, Siebert E, Kempermann G, Petrus DS, Kupsch A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson’s disease. Exp Neurol. 2006;199(2):291–300. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31(2):305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12(13):1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. J Anat. 2005;207(6):717–725. doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989(2):172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, Hisatsune T. Excitatory GABAergic activation of cortical dividing glial cells. Cereb Cortex. 2009;19(9):2181–2195. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, et al. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res. 2008;86(16):3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128(13):2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110(17):7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Clarke LE, Burzomato V, Kessaris N, Anderson PN, Attwell D, Richardson WD. Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. J Neurosci. 2011;31(18):6809–6819. doi: 10.1523/JNEUROSCI.6474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30(48):16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J. NG2-positive cells in CNS function and the pathological role of antibodies against NG2 in demyelinating diseases. J Neurol Sci. 2005;233(1–2):37–42. doi: 10.1016/j.jns.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4(155) doi: 10.1126/scitranslmed.3004371. others 155ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano F, Mobius W, Gotz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16(10):1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31(40):14182–14190. doi: 10.1523/JNEUROSCI.6595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21(1):63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69(6):826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wennstrom M, Hellsten J, Ekdahl CT, Tingstrom A. Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat hippocampus. Biol Psychiatry. 2003;54(10):1015–1024. doi: 10.1016/s0006-3223(03)00693-0. [DOI] [PubMed] [Google Scholar]
- Whittaker MT, Zai LJ, Lee HJ, Pajoohesh-Ganji A, Wu J, Sharp A, Wyse R, Wrathall JR. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia. 2012;60(2):281–294. doi: 10.1002/glia.21262. [DOI] [PubMed] [Google Scholar]
- Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210(6):661–670. doi: 10.1111/j.1469-7580.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176(1–2):162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, 2nd, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69(6):966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2(6):553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yoo S, Wilcock D, Lytle JM, Leung PY, Colton CA, Wrathall JR. Interaction of NG2(+) glial progenitors and microglia/macrophages from the injured spinal cord. Glia. 2010;58(4):410–422. doi: 10.1002/glia.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing YL, Roth PT, Stratton JA, Chuang BH, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci. 2014;34(42):14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26(14):3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159(4):766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Yong VW, Kim SU, Kim MW, Shin DH. Growth factors for human glial cells in culture. Glia. 1988;1(2):113–123. doi: 10.1002/glia.440010203. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur J Neurosci. 2011;33(6):1123–1132. doi: 10.1111/j.1460-9568.2011.07611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125(15):2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135(1):145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138(4):745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4(1):19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A. Olig2-dependent developmental fate switch of NG2 cells. Development. 2012;139(13):2299–2307. doi: 10.1242/dev.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10(3):321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]