Abstract
Hexavalent chromium (Cr(VI)) is present in the marine environment and is a known carcinogen and reproductive toxicant. Cr(VI) is the form of chromium that is well absorbed through the cell membrane. It is also the most prevalent form in seawater. We measured the total Cr levels in skin biopsies obtained from healthy free-ranging fin whales from the Gulf of Maine and found elevated levels relative to marine mammals in other parts of the world. The levels in fin whale biopsies ranged from 1.71 ug/g to 19.6 ug/g with an average level of 10.07 ug/g. We also measured the cytotoxicity and genotoxicity of Cr(VI) in fin whale skin cells. We found that particulate and soluble Cr(VI) are both cytotoxic and genotoxic to fin whale skin cells in a concentration-dependent manner. The concentration range used in our cell culture studies used environmentally relevant concentrations based on the biopsy measurements. These data suggest that Cr(VI) may be a concern for whales in the Gulf of Maine.
Keywords: Fin whale, chromium, genotoxicity, cytotoxicity, whale, marine mammal, hexavalent chromium
Introduction
Metals have emerged as a major concern for ocean health. Chromium is a naturally occurring metal commonly used as an anticorrosive and a dye in many paints. It gets into the environment through leaching from marine paints and through storm water and industrial runoff as well as deposition from air. The mean Cr level in the ocean is 0.3 ug/L [1] and the primary species in salt water is Cr(VI) [2, 3]. The primary source of environmental chromium is through industrial pollution to the atmosphere. One-third of all atmospheric Cr released by anthropogenic sources is Cr(VI) [1]. More recently, we reported that Cr was a global marine pollutant with the high levels implicating Cr(VI) as the probable cause [4].
Hexavalent chromium [Cr(VI)] is a known human carcinogen with the ability to damage DNA and impair reproduction and development [5-12]. The vast majority of Cr(VI) studies consider its impacts on human and laboratory animals or cell lines derived from them. By contrast, levels and impacts of Cr in marine species are poorly understood. In the ocean, marine mammals such as whales are the closest relatives to humans and effects in them reflect both threats to the animals as well as likely threats to humans.
We have been using marine mammals to study metal pollution, including Cr, in the ocean and its impacts on endangered species and populations as well as harbingers of threats to human health. Considering baleen whales, we found Cr levels of concern in the highly endangered Northern right whale (Eubalaena glacialis) off the coast of Maine [13]. In this study, Cr skin levels were found to be similar to skin levels of occupationally exposed workers with Cr-induced cancer. This concern appears to be regionally specific as a study of the Northern right whale's close cousin, the Southern right whale (Eubalaena australis), in Argentina, showed very low skin levels of Cr [14]. These two populations of whales are geographically distinct and do not intermix. Thus, they reflect different regions. These observations suggest a concern in the Gulf of Maine, though beyond these two reports, there are no apparent data of Cr levels in baleen whales from these regions.
The impact of Cr on whales is poorly understood. In baleen whales, the Northern right whale is the only species to be considered. The data show both particulate and soluble Cr(VI) compounds are cytotoxic and genotoxic to right whale skin, lung and testes cells, indicating Cr is likely to be a carcinogen and reproductive hazard in whales [13, 15]. Data also indicate that Northern right whale cells are more resistant to the toxic effects of Cr(VI) than human cells [16]. However, because right whales are the only baleen whale species considered, it is unknown if they represent other whales or are particularly resistant.
Fin whales (Balaenoptera physalus) are considered endangered under the Endangered Species Act with the Western North Atlantic population only being 3,522 individuals [17]. Currently, there are no studies of Cr in fin whales. Accordingly, in this study, we measured Cr levels in fin whales from the Gulf of Maine and measured the cytotoxic and genotoxic effects of Cr(VI) in fin whale cells to further consider the hypothesis that Cr is a marine health concern.
Methods
Cell culture
Fin whale skin fibroblasts were isolated from live animal skin biopsies. The cells were cultured in a 50:50 mixture of Dulbecco's’ minimal essential medium and Ham's F12 medium plus 15% cosmic calf serum, 1% L-glutamine and 1% penicillin/streptomycin. All cells were maintained in a 33°C, humidified incubator with 5% CO2.
Chemical Preparations
Sodium chromate (Na2CrO4) was used as a representative soluble Cr(VI) compound and desired dilutions were made with sterile distilled water as previously described [18]. Lead chromate (PbCrO4) was used as a particulate model for Cr (VI) and particles were suspended in sterile distilled water as previously described [18].
Cytotoxicity
Cytotoxicity was measured with a clonogenic assay using our published methods [13]. Briefly, cells were seeded as a monolayer in a 6-well cell culture plate and allowed to grow for 48 h. Cells were then treated with either sodium or lead chromate at concentrations ranging from 0-10 uM of and 1-100 ug/cm2, respectively for 24 h. Then the cells were reseeded at colony forming density and allowed to grow for about 2 weeks. Then the colonies were stained using crystal violet. The colonies were counted and cell survival was calculated relative to the control. Experiments were repeated at least three times.
Genotoxicity
Genotoxicity was measured with a chromosome damage assay using our published methods [13]. Briefly, cells were seeded as a monolayer in 100 mm Petri dishes and allowed to grow for 48 h. Cells were then treated with either sodium or lead chromate at concentrations ranging from 0-10 uM of and 1-10 ug/cm2, respectively for 24 h. Cells were arrested in metaphase using 0.1 ug/ml demecolcine. Then the cells were resuspended in 0.075M potassium chloride hypotonic solution for 17 minutes and then fixed in methanol:acetic acid (3:1). Cells were washed twice with fix then dropped onto a microscope slide and stained with 5% Giemsa stain in Gurr's buffer. 100 metaphases were scored per concentration for each experiment.
Intracellular Chromium Measurement
Intracellular chromium ion levels were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES) using our published methods [16]. A monolayer of cells was treated with various concentrations of sodium chromate and lead chromate for 24 h. The cells were harvested and treated with a hypotonic solution followed by 2% SDS. This solution was sheered through a needle seven times and filtered into a vial. Chromium ion concentrations of the samples were then measured using standard ICP methods.
Biopsies
Biopsies were collected from healthy free ranging fin whales in the Gulf of Maine between 2010 and 2012 based on published methods [13]. Sampling efforts were conducted jointly with photo-identifications of individual whales to minimize duplication. Samples were collected with stainless steel cylindrical biopsy tips 6 mm in diameter, with a flared rim and stop collar to limit penetration to the skin and blubber interface. Biopsy tips were attached to an arrow and delivered to the animal via a crossbow with a maximum draw weight of 68 kg (150 lbs). Arrows were retrieved through the use of floatation collars. Tissue samples were removed from the biopsy tip with clean, sterile forceps. The skin was removed and a portion for metal analysis immediately placed in a sterile, labeled tube, and stored frozen at -20°C. We previously demonstrated there is no contamination by the biopsy dart or the forceps [4].
Tissue Chromium Measurement
Total Cr present in skin biopsies was measured using inductively coupled plasma mass spectroscopy (ICPMS). All samples were analyzed at the Center for Environmental Sciences and Engineering at the University of Connecticut, Storrs [13]. Each sample was frozen and stored at -20°C. Samples were analyzed for chromium using inductively coupled plasma mass spectrometry (ICPMS). Each sample, between 0.3 and 0.7g, was rinsed with deionized water, air dried in a laminar flow hood, weighed, placed in a digestion tube with nitric acid and refluxed for 4 h at 95°C. Each sample was then cooled, and 2 ml deionized water and 3 ml of trace metal grade hydrogen peroxide were added. Each sample was then heated in a hot block until the effervescence subsided. The samples were then cooled and brought to a final volume of 10 ml with deionized water.
Samples were analyzed on a Perkin-Elmer/Sciex ELAN ICPMS using standard protocols. Samples were run undiluted, and when over range, diluted five-fold. Interference check solutions were analyzed (ICS A and ICS A+B; High Purity Standards, Charleston, SC) with all sample runs to compensate for any matrix effects which may interfere with sample analysis. Standard quality assurance procedures were employed. Instrument response was evaluated, every 10 samples, and at the end of an analytical run using a calibration verification standard and blank. For the Cr measurements, the analysis of duplicate samples had a relative percent difference of 12.4 ± 3.2 %; the method blanks measured below detection limit (0.08 ug/g); the spiked samples had a 87.8 ± 3.4 % recovery; laboratory control samples (LCS) had an 100.3 ± 0.6 % recovery; and the standard reference materials (SRM) (DORM-3;NRC Canada) had an 114.6 ± 6.3% recovery. All quality control parameters, including all standard reference material data, were within method specifications. Data are presented in units of ug/g wet weight (w.w.).
Statistics
Responses for individual doses were compared using t-tests. LC50's and EC20's were estimated using linear regression analysis, and variances were calculated using the variance-covariance matrix. No adjustment was made for multiple comparison.
Results
Chromium Skin Levels
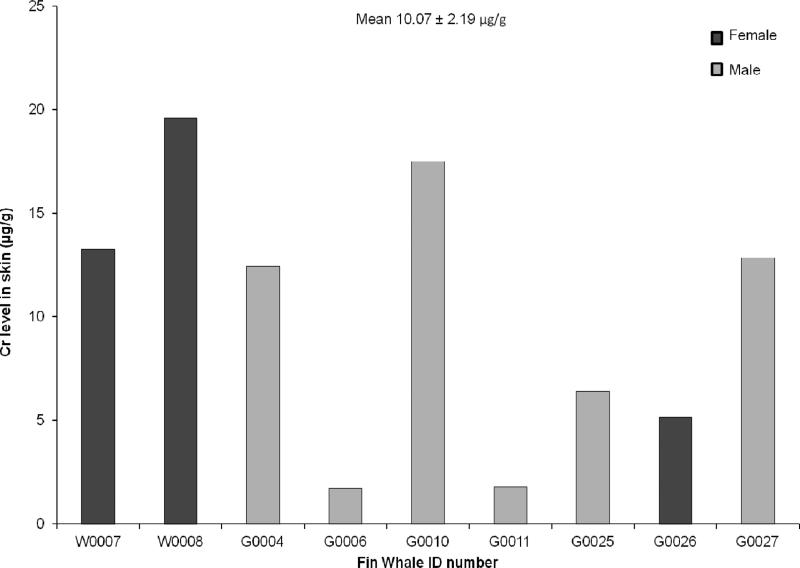
In organisms and cells, Cr(VI) is rapidly and immediately reduced to Cr(III), thus it is not technically possible to directly identify which valence of Cr the animal was originally exposed to and measurements are simply made of total Cr in the tissue. We measured the level of total Cr in skin biopsies from nine free ranging fin whales including 6 males and 3 females. All animals appeared to be adult-sized. All nine animals had measureable levels of Cr. Individual Cr levels ranged from 1.71 ug/g to 19.6 ug/g with an average level of 10.07 ug/g (Figure 1) indicating the presence of Cr in the fin whales’ environment.
Fig. 1.
This figure shows the presence of chromium in fin whale skin samples. Chromium was found at levels ranging between 1.71 and 19.6 ug/g tissue wet weight in nine individuals
Cytotoxicity of Particulate and Soluble Cr(VI) in Fin Whale Skin Cells
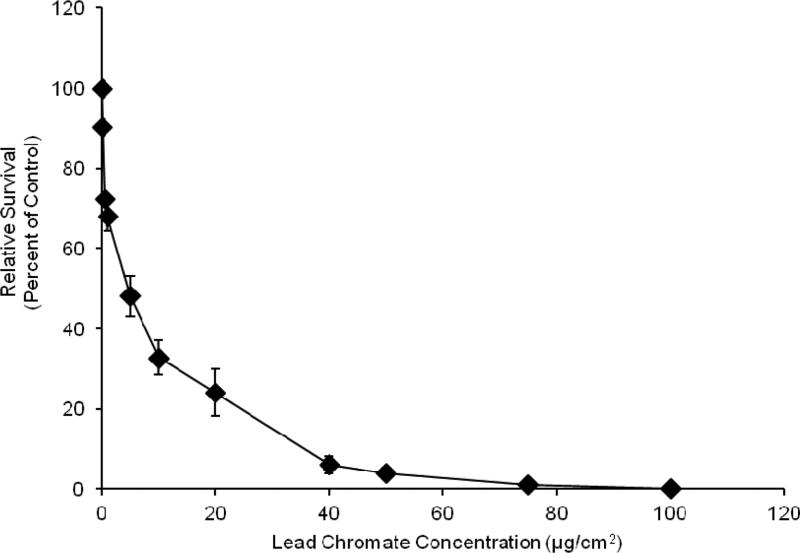
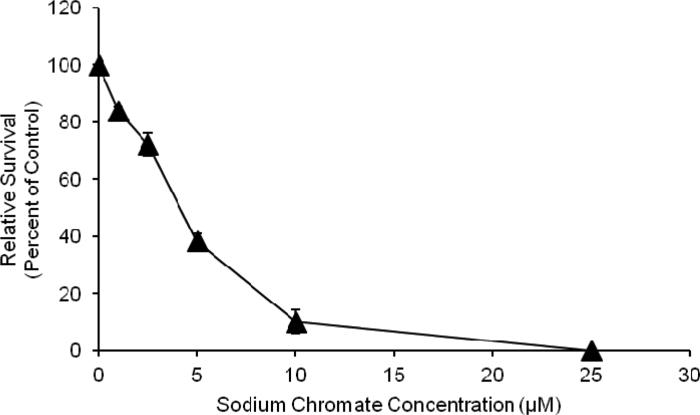
Both particulate and soluble Cr(VI) induced a concentration-dependent increase in cytotoxicity. Concentrations of 0.1, 1.0, 5, 10 and 20 ug/cm2 lead chromate induced 91, 72, 55, 40 and 38 percent relative survival, respectively (Figure 2). Concentrations of 1, 2.5, 5 and 10 uM sodium chromate induced 84, 72, 38 and 10 percent relative survival, respectively (Figure 3). Because the particulate compound (lead chromate) does not fully dissolve in water, but the soluble compound (sodium chromate) does, it is not possible to accurately compare the data based on administered dose.
Fig. 2.
This figure shows lead chromate is cytotoxic to fin whale cells in a concentration dependent manner. The data represent an average of 3-7 experiments ± standard error of mean. Concentrations of 0.1 and 1-100 ug/cm2 were significantly different from control (p<0.0001). For comparisons between concentrations: 0.1 ug/cm2 was significantly different from and 0.5-100 ug/cm2 (p<0.001); 0.5 ug/cm2 was significantly different from and 0.1, 10-100 ug/cm2 (p<0.001); 1 ug/cm2 was significantly different from and 0.1, 5-100 ug/cm2 (p<0.001); 5 ug/cm2 was significantly different from and 0.1, 1-100 ug/cm2 (p<0.01); 10 ug/cm2 was significantly different from 0.1-5 ug/cm2 (p<0.001) and 40-100 ug/cm2 (p<0.001); 20 ug/cm2 was significantly different from 0.1-5 ug/cm2 (p<0.001) and 40-100 ug/cm2 (p<0.01); 40 ug/cm2 was significantly different from 0.1-20 ug/cm2 (p<0.001) and 75-100 ug/cm2 (p<0.01); 50 ug/cm2 was significantly different from 0.1-20 ug/cm2 (p<0.001) and 75-100 ug/cm2 (p<0.037); 75 ug/cm2 was significantly different from 0.1-50 ug/cm2 (p<0.001) and 100 ug/cm2 (p<0.012)
Fig. 3.
This figure shows soluble Cr(VI) is cytotoxic to fin whale skin fibroblasts in a concentration-dependent manner. The data represent an average of 3 experiments ± standard error of mean. Concentrations of 1-100 uM were significantly different from control (p<0.02). For comparisons between concentrations: 1 uM was significantly different from and 2.5-100 uM (p<0.001); 2.5 uM was significantly different from 1 uM and 5-100 uM (p<0.001); 5 uM was significantly different from 1-2.5 uM and 10-100 uM (p<0.001); 10 uM was significantly different from 1-5 uM and 25-100 uM (p<0.001); 25 uM was significantly different from 1-10 uM and 50-100 uM (p<0.001); 50 uM was significantly different from 1-25 uM and 100 uM (p<0.001);
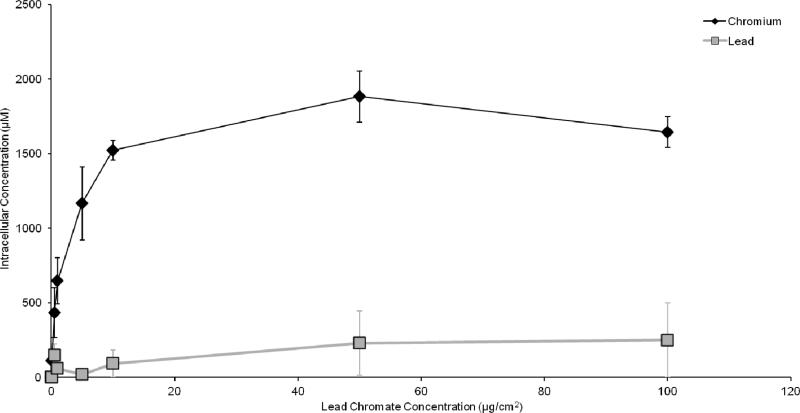
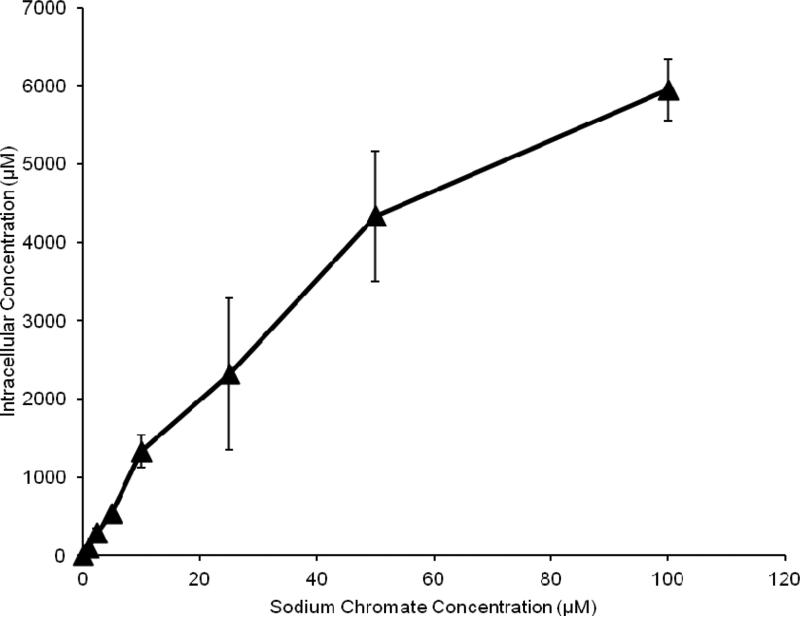
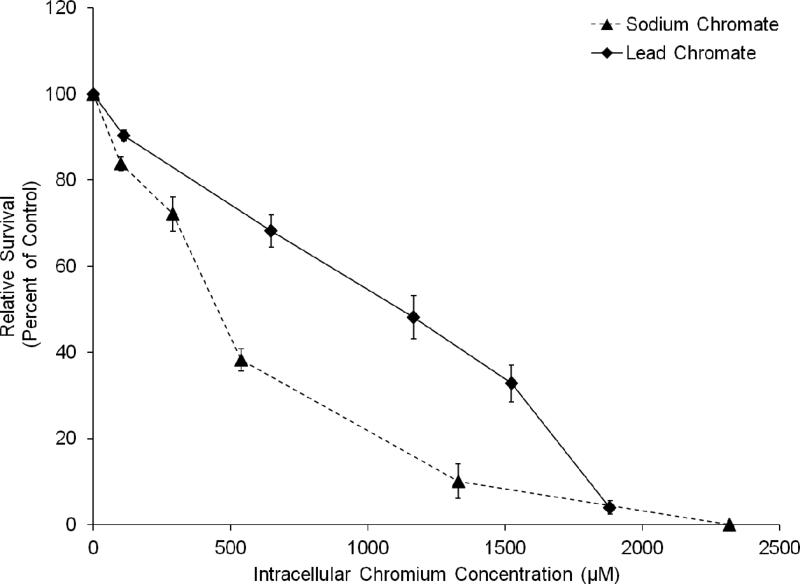
In order to compare the two compounds, we measured intracellular Cr ion levels. Both compounds increased the intracellular Cr levels in a concentration-dependent manner (Figures 4 and 5). Considering the cytotoxicity data based on intracellular Cr level, Figure 6 shows soluble chromate is more cytotoxic to fin whale skin cells than particulate chromate. For example, the LC50 for sodium chromate, based on intracellular concentration was 390 uM, which is 3-fold lower than the 1,125 uM LC50 for lead chromate (p<0.001).
Fig. 4.
This figure shows intracellular chromium concentrations in fin whale skin cells increase after lead chromate treatment. Intracellular lead ions area also produced, but do not increase with concentration. The data represent an average of 3 experiments ± standard error of mean
Fig. 5.
This figure shows intracellular concentrations in fin whale skin cells increase after sodium chromate treatment. The data represent an average of 3 experiments ± standard error of mean
Fig. 6.
This figure shows when adjusted for the amount of intracellular chromium sodium chromate is more cytotoxic to fin whale skin cells than lead chromate. The data represent an average of 3 experiments ± standard error of mean
Clastogenicity of Particulate and Soluble Cr(VI) in Fin Whale Skin Cells
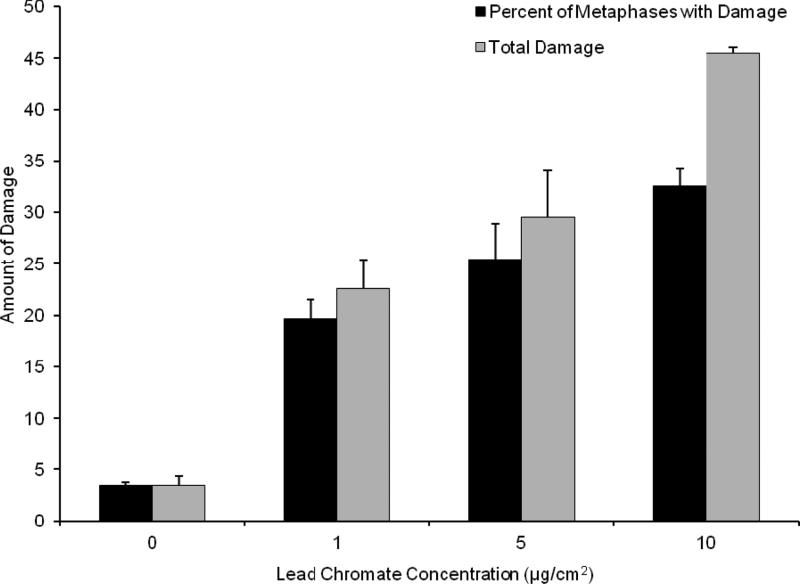
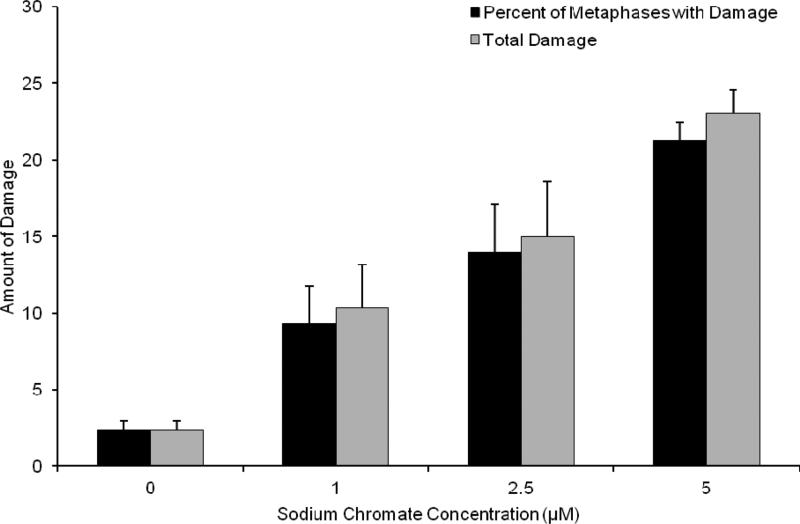
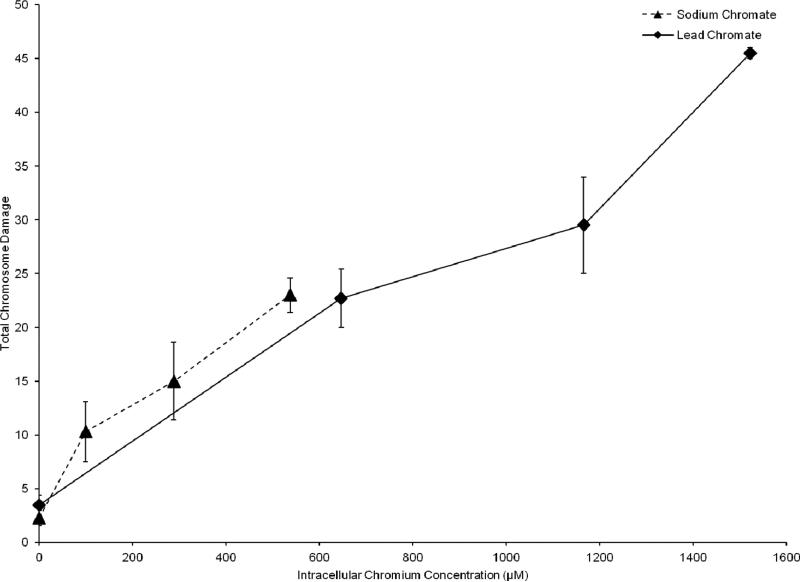
The genotoxicity of particulate and soluble Cr(VI) was determined through the production of chromosome aberrations quantified through two measures: 1) The percent of metaphases with damage, which uses the cell as the unit of comparison; and 2) Total chromosome damage, which uses the chromosome as the unit of comparison. Both particulate and soluble Cr(VI) induced a concentration-dependent increase in genotoxicity. Lead chromate concentrations of 0, 1, 5 and 10 ug/cm2 lead chromate induced aberrations in 4, 20, 25 and 33 percent of metaphases and a total of 4, 23, 30 and 45 aberrations in 100 metaphases (Figure 7). Sodium chromate concentrations of 0, 1, 2.5, and 5 uM induced aberrations in 2, 9, 12 and 21 percent of metaphases and a total of 2, 10, 15 and 23 aberrations in 100 metaphases (Figure 8). Considering the data based on intracellular Cr levels, (Figure 9), shows sodium chromate and lead chromate were similarly potent. For example, the EC20s for sodium chromate and lead chromate, based on intracellular concentration were 611 uM and 820 uM, respectively for the percent of metaphases with damage, and 507 and 709 uM, respectively for the total aberrations in 100 metaphases.
Fig. 7.
This figure shows lead chromate is genotoxic to fin whale cells in a concentration dependent manner. The data represent an average of 3-4 experiments ± standard error of mean. For 5 ug/cm2 lead chromate only 284 metaphases were scored because in one experiment on 84 metaphases could be found. For 10 ug/cm2 lead chromate only 176 metaphases were scored because in one experiment no metaphases were found and in another on 76 could be found. The reduction in metaphase availability typically reflects cell cycle arrest, presumably due to high levels of damage. Concentrations of 1-10 ug/cm2 were significantly different from control for both measurements (p<0.001). For comparisons between concentrations: 1 and 10 ug/cm2 were significantly different from each other for percent of metaphases with damage (p<0.001); 1 and 10 ug/cm2 (p<0.001) and 5 and 10 ug/cm2 (p<0.002) were significantly different from each other for total damage in 100 metaphases
Fig. 8.
This figure shows soluble Cr(VI) is genotoxic to fin whale skin fibroblasts in a concentration-dependent manner. The data represent an average of 3 experiments ± standard error of mean. For 5 uM sodium chromate only 292 metaphases were scored as on one experiment only 92 metaphases could be found. The reduction in metaphase availability typically reflects cell cycle arrest, presumably due to high levels of damage. Concentrations of 1-10 uM were significantly different from control for both measurements (p<0.001). For comparisons between concentrations: 1 and 5 uM and 1 and 10 uM were significantly different from each other for percent of metaphases with damage (p<0.001) and 2.5 and 5 uM and 2.5 and 10 uM were also significantly different from each other for percent of metaphases with damage (p<0.006); 1 and 5 uM, 1 and 10 uM and 2.5 and 10 uM were significantly different from each other for total damage in 100 metaphases (p<0.001) and 5 and 10 uM were also significantly different from each other for total damage in 100 metaphases (p<0.004)
Fig. 9.
This figure shows sodium chromate and lead chromate exhibit a similar genotoxic response based upon intracellular uptake. The data represent an average of 3-4 experiments ± standard error of mean
Discussion
Cr(VI) is emerging as a global health concern for the marine environment [4]. This study is the first to report metal toxicity in fin whales. The data extend our current knowledge of metal genotoxicity in whales from cells from two whale species, Northern right whales (a baleen whale) and sperm whales (a toothed whale) to add fin whales (a second baleen whale). The data indicate both that fin whales are exposed to Cr and that Cr has the potential for cytotoxic and genotoxic outcomes in this species.
The data also suggest there is a marine Cr exposure concern in the Gulf of Maine or along the Eastern seaboard of the United States. Previous data from Northern right whales sampled prior to 2008, off the coast of Maine, revealed a mean total Cr level of 7.1 ug/g w.w. [13]. A similar study of Southern right whales sampled off the coast of Argentina in 2011 reported a mean skin Cr level of 0.64 ug/g wet weight [14]. In this study of fin whales sampled in the Gulf of Maine from 2010-2012, we found a mean skin Cr level 1.4-times higher than that of the Northern right whales. Given the geographic overlap of Northern right and fin whale compared to their geographically distinct Southern right whale cousins, the data suggest a significant Cr input in the habitat of the northern two whale species. These high levels are not normal for marine mammals as they exceed levels previously reported [19-22], nor are they normal for mammals in general as they are 32-times higher than levels reported for humans without occupational exposure [23].
Of course, it cannot be directly determined which valence of Cr the whales encountered. This uncertainty arises simply because Cr(VI) is so quickly reduced to Cr(III) in cells and tissues, once reduced it quickly complexes with intracellular compounds including amino acids and proteins [24]. However, it can indirectly be inferred that a substantial amount of the exposure most likely included Cr(VI) because Cr(III) is very poorly absorbed in mammalian systems [1]. Substantial amounts of Cr(VI) are released into the environment and Cr(VI) is the major valence of Cr in marine waters [2], therefore, given its availability and high level, the data suggest a significant portion of the exposure most likely involved Cr(VI).
The observations that particulate Cr(VI) is cytotoxic in a concentration-dependent manner is consistent with previous data showing lead chromate is cytotoxic to North Atlantic right whale cells [13, 15-16]. Comparing the two species suggests fin whales are less susceptible to Cr(VI)-induced cell death. For example, 5 ug/cm2 lead chromate induced 5-fold less cell death in fin whale cells than in North Atlantic right whale skin cells [15]. The explanation for this difference is probably due to differences in lead chromate-induced genotoxicity between the two species.
The observations that particulate Cr(VI) is genotoxic in a concentration-dependent manner is consistent with previous data showing lead chromate is genotoxic to North Atlantic right whale cells [13,15-16]. Comparing the two species suggests fin whales are also less susceptible to Cr(VI)-induced chromosome damage. For example, 5 ug/cm2 lead chromate induced 25% fewer aberrations in fin whale skin cells than a 4 ug/cm2 lead chromate treatment did in North Atlantic right whale skin cells [15]. The 4 ug/cm2 lead chromate dose was the highest concentration with reported damage in the right whale study. The explanation for this difference is uncertain. It is tempting to speculate this difference is due to either Cr(VI) or lead uptake, but we have found fin whale cells take up 2.5-fold more Cr(VI) and 3.3-fold less lead than right whale skin cells (unpublished observations), thus this does not appear to be the explanation. Instead, it is most likely some undiscovered, species-related difference in the response to either or both of these two metals. Soluble Cr(VI) toxicity has not been reported for a baleen whale skin cell before so such comparisons are not possible.
It is interesting to note that when compared on an intracellular level, both Cr(VI) compounds induced a similar amount of genotoxicity, but soluble Cr(VI) was more cytotoxic. This outcome suggests the particulate compound, perhaps through the action of the lead cation, can induce DNA damage, but some damaged cells survive. Such an outcome is consistent with observations in humans and rodents that the particulate Cr(VI) compounds are a greater health concern and the more potent carcinogens [25, 26]. It suggests a similar potency difference exists for whales exposed to airborne Cr(VI) particles.
It is difficult to directly compare cell culture concentrations with tissue levels. The most basic approach is to express all levels in units of ppm, although this simplified approach ignores important confounding factors. Under this approach, our administered doses of 1, 2.5, 5 and 10 uM sodium chromate convert to 0.052, 0.130, 0.260, and 0.520 ppm total Cr, respectively. For lead chromate, the administered doses of 0.1, 1.0, 5, 10 and 20 ug/cm2 convert to 0.068, 0.68, 3.4, 6.8, and 13.6 ppm total Cr, respectively. Thus, our treatments ranged from 0.052 to 13.6 ppm. The fin whale skin Cr tissue levels are ug/g, which directly converts to ppm. Thus, the total Cr levels in fin whale skin range from 1.71 ppm to 19.6 ppm with a mean level of 10.07 ppm. Using this simplified approach, all of our cell culture treatments were lower than the mean Cr tissue levels except for our highest lead chromate treatment. Furthermore, all of them were below the high end of the range, found in the tissue and several of the lower treatments were 2.5-33 fold lower than the low end of the whale tissue range.
A second approach is to consider the doses based on a hypothetical exposure. In this instance, one can be posited for whale inhalation. Such an exposure is an important route given the large whale lung volumes and their lifestyle requirements to hold their breaths for lengthy periods of time, which may increase deposition and absorption of inhaled particles [27]. Fin whales lung volume and respiration rate are estimated to be 2 m3 and 24 breaths/hour, respectively [28]. The fin whales sampled in this study have a range along the Eastern U.S. seaboard. Marine atmospheric chromium levels are not commonly measured, but based on an average marine air level of 0.226 ug/m3 reported for Baltimore harbor [1], the fin whales near Baltimore could inhale about 260 ug in 24 h (0.226 ug/m3 X 2m3/breath X 24 breaths/h X 24 h = 260 ug). If 35% of this total airborne level was hexavalent as predicted [1], then a whale would inhale 91 ug Cr(VI) in 24 h. In our studies, we treated for 24 h with 0.88–176 ug Cr(VI). Thus, by this approach our concentrations also model a potential environmental exposure.
In conclusion, our data show that Cr is present in fin whale skin tissue and is cytotoxic and genotoxic to fin whale skin cells. The data further suggest that particulate Cr(VI) is a particular concern with some damaged cells surviving. These data, along with the data from the Northern right whale testes and lung cell cultures suggest that Cr could be a reproductive or an inhalation hazard for fin whales as well. Altogether, the data support a hypothesis that chromium in a current marine health concern for whales and particularly for the Gulf of Maine. Further studies are needed to elucidate the potential sources and pathways of exposure to chromium to fin whales, and how these may be affecting their health and reproductive success.
Acknowledgements
We would like to thank Christy Gianios, Jr. for technical support. This work was supported by the National Institute of Environmental Health Sciences (NIEHS) grant ES016893 (J.P.W.); the Pacific Life Foundation Marine Mammal Research Fund at the Ocean Foundation; and National Oceanic and Atmospheric Administration (NOAA) grant NA03NMF4720478 (J.P.W.). Marine mammal cell line development and use by the Wise Laboratory for Environmental and Genetic Toxicology is performed under National Marine Fisheries Service (NMFS) Permit #16305. This paper was developed under Greater Research Opportunities (GRO) Fellowship Assistance Agreement No. MA-91739301-0 awarded by the United States Environmental Protection Agency (EPA). It has not been formally reviewed by EPA. The views expressed in this manuscript are solely those of Catherine F. Wise, and EPA does not endorse any products or commercial services mentioned.
Footnotes
Conflict of Interest
Author John Pierce Wise Sr. received grants from NIH, the Ocean Foundation and NOAA (described above) which helped support this work. Author Catherine Wise received a fellowship from EPA (described above) which helped support this work.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Chromium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2012. [PubMed] [Google Scholar]
- 2.Pettine M, Millero FJ. Chromium speciation in seawater: The probable role of hydrogen peroxide. Limnol Oceanogr. 1990;35:730–736. [Google Scholar]
- 3.Geisler CD, Schmidt D. An overview of chromium in the marine environment. Dtsch hydrogr Z. 1991;44:185–196. [Google Scholar]
- 4.Wise JP, Sr, Payne R, Wise SS, LaCerte C, Wise J, Gianios C, Jr, Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Kerr I. A global assessment of chromium pollution using sperm whales (Physeter macrocephalus) as an indicator species. Chemosphere. 2009;75(11):1461–1467. doi: 10.1016/j.chemosphere.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Holmes AL, Wise SS, Wise JP., Sr Carcinogenicity of hexavalent chromium. Ind J Med Res. 2008;128:353–372. [PubMed] [Google Scholar]
- 6.Wise SS, Holmes AL, Wise JP., Sr Hexavalent chromium-induced DNA damage and repair mechanisms. Rev Environ Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- 7.IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel and Welding. Vol. 49. International Agency for Research on Cancer; Lyons, France: 1990. [PMC free article] [PubMed] [Google Scholar]
- 8.Witmer CM, Park H-S, Shupack SI. Mutagenicity and disposition of chromium. Sci Total Environ. 1989;86:131–148. doi: 10.1016/0048-9697(89)90200-3. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury AR, Mitra C. Spermatogenic and steroidogenic impairment after chromium treatment in rats. Indian J Exp Biol. 1995;33:480–484. [PubMed] [Google Scholar]
- 10.Bataineh H, Al-Hamood MH, Elbetieha A, Bani Hani I. Effect of long-term ingestion of chromium compounds on aggression, sex behavior and fertility in adult male rat. Drug Chem Toxicol. 1997;20:133–149. doi: 10.3109/01480549709003875. [DOI] [PubMed] [Google Scholar]
- 11.Mancuso TF. Chromium as an industrial carcinogen. II. Chromium in human tissues. Am J Ind Med. 1997;2:140–147. doi: 10.1002/(sici)1097-0274(19970204)31:2<140::aid-ajim2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hamood MH, Elbetieha A, Bataineh H. Sexual maturation and fertility of male and female mice exposed prenatally and postnatally to trivalent and hexavalent chromium compounds. Reprod Fertil Dev. 1998;10:179–183. doi: 10.1071/r97001. [DOI] [PubMed] [Google Scholar]
- 13.Wise JP, Wise SS, Kraus S, Shaffiey F, Grau M, Li Chen T, Perkins C, Thompson WD, Zheng T, Zhang Y, Romano T, O'Hara T. Hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and testes fibroblasts. Mutat Res Genet Toxicol Environ Mutagen. 2008;650(1):30–38. doi: 10.1016/j.mrgentox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Martino J, Wise SS, Perkins C, Sironi M Wise JP., Sr Metal Levels in Southern Right Whales (Eubalaena australis) from Península Valdés, Argentina. J Environ Anal Toxicol. 2013;3:190. doi: 10.4172/2161-0525.1000190. doi: 10.4172/2161-0525.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Chen T, Wise SS, Kraus S, Shaffiey F, Levine KM, Thompson WD, Romano T, O'Hara T, Wise JP., Sr Particulate hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and skin fibroblasts. Environ Mol Mutagen. 2009;50(5):387–393. doi: 10.1002/em.20471. [DOI] [PubMed] [Google Scholar]
- 16.Li Chen T, Wise SS, Holmes A, Shaffiey F, Wise JP, Jr, Thompson WD, Kraus S, Wise JP., Sr Cytotoxicity and genotoxicity of hexavalent chromium in human and North Atlantic right whale (Eubalaena glacialis) lung cells. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:487–494. doi: 10.1016/j.cbpc.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waring GT, Josephson E, Maze-Foley K, Rosel PE, editors. U.S. Atlantic and Gulf of Mexico Marine Mammal Stock Assessments -- 2013. NOAA Tech Memo NMFS NE. 2014;228:464. [Google Scholar]
- 18.Wise JP, Sr, Wise SS, Little JE. The Cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 19.Kunito T, Watanabe I, Yasunaga G, Fujise Y, Tanabe S. Using trace elements in skin to discriminate the populations of minke whales in southern hemisphere. Mar Environ Res. 2002;53:175–197. doi: 10.1016/s0141-1136(01)00119-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Miyazaki N, Kunito T, Tanabe S. Trace elements and butyltins in a Dall's porpoise (Phocoenoides dalli) from the Sanriku coast of Japan. Chemosphere. 2002;63:449–457. doi: 10.1016/j.chemosphere.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 21.Bryan CE, Christopher SJ, Balmer BC, Wells RS. Establishing baseline levels of trace elements in blood and skin of bottlenose dolphins in Sarasota Bay, Florida: implications for noninvasive monitoring. Sci Tot Environ. 2007;388:325–342. doi: 10.1016/j.scitotenv.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Stavros HC, Bossart GD, Hulsey TC, Fair PA. National Oceanic and Trace element concentrations in skin of free-ranging bottlenose dolphins (Tursiops truncatus) from the southeast Atlantic coast. Sci Tot Environ. 2007;388:300–315. doi: 10.1016/j.scitotenv.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder HA, Nason AP, Tipton IH. Chromium deficiency as a factor in atherosclerosis. J Chronic Dis. 1970;23:123–142. doi: 10.1016/0021-9681(70)90071-8. [DOI] [PubMed] [Google Scholar]
- 24.De Flora S, Wetterhahn KE. Mechanisms of chromium metabolism and genotoxicity. Life Chem Rep. 1989;7:169–244. [Google Scholar]
- 25.Wise SS, Holmes AL, Ketterer ME, Hartsock WJ, Fomchenko E, Katsifis SP, Thompson WD, Wise JP., Sr Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutat Res. 2004;560:79–89. doi: 10.1016/j.mrgentox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Xie H, Holmes AL, Wise SS, Gordon N, Wise JP., Sr Lead chromate-induced chromosome damage requires extracellular dissolution to liberate chromium ions but does not require particle internalization or intracellular dissolution. Chem Res Toxicol. 2004;17:1362–1367. doi: 10.1021/tx0498509. [DOI] [PubMed] [Google Scholar]
- 27.Rawson J, Anderson HF, Patton GW, Beecher T. Anthracosis in the Atlantic Bottlenose Dolphin (Tursiops truncatus). Marine Mammal Sci. 1991;7:413–416. [Google Scholar]
- 28.Brodie PF. Cetacean energetics, an overview of intraspecific size variation. Ecology. 1975;56:152–161. [Google Scholar]