Abstract
Borrelia miyamotoi is a relapsing fever Borrelia group spirochete that is transmitted by the same hard-bodied (ixodid) tick species that transmit the agents of Lyme disease. It was discovered in 1994 in Ixodes persulcatus ticks in Japan. B. miyamotoi species phylogenetically cluster with the relapsing fever group spirochetes, which usually are transmitted by soft-bodied (argasid) ticks or lice. B. miyamotoi infects at least six Ixodes tick species in North America and Eurasia that transmit Lyme disease group spirochetes and may use small rodents and birds as reservoirs. Human cases of B. miyamotoi infection were first reported in 2011 in Russia and subsequently in the United States, Europe, and Japan. These reports document the public health importance of B. miyamotoi, as human B. miyamotoi infection appears to be comparable in frequency to babesiosis or human granulocytic anaplasmosis in some areas and may cause severe disease, including meningoencephalitis. The most common clinical manifestations of B. miyamotoi infection are fever, fatigue, headache, chills, myalgia, arthralgia, and nausea. Symptoms of B. miyamotoi infection generally resolve within a week of the start of antibiotic therapy. B. miyamotoi infection should be considered in patients with acute febrile illness who have been exposed to Ixodes ticks in a region where Lyme disease occurs. Because clinical manifestations are non-specific, etiologic diagnosis requires confirmation by blood smear examination, PCR, antibody assay, in vitro cultivation, and/or isolation by animal inoculation. Antibiotics that have been used effectively include doxycycline for uncomplicated B. miyamotoi infection in adults and ceftriaxone or penicillin G for meningoencephalitis.
Keywords: Borrelia miyamotoi, relapsing fever, Lyme disease, Ixodes, tick-borne disease, spirochete
Introduction
Borrelia miyamotoi is a relapsing fever spirochete transmitted by the same hard-bodied (ixodid) ticks that are vectors of Borrelia burgdorferi and other Lyme disease agents [1–10]. As early as 1985, spirochetes that were likely B. miyamotoi were observed in ticks in the United States. They were mistakenly thought to be B. burgdorferi as a consequence of cross-reactive antibodies that were used in direct immunoassays. For example, two reports identified putative B. burgdorferi in Ixodes scapularis and Ixodes pacificus adult ovarial tissue, eggs and/or larvae [11–12]. This lead to the false conclusion that B. burgdorferi was transovarially transmitted by ticks. Recent experimental evidence has confirmed transovarial (vertical) transmission of B. miyamotoi but not B. burgdorferi in I. scapularis [13]. Misidentification not only led to false conclusions about the transovarial transmission of B. burgdorferi in Ixodes ticks but may have delayed recognition of B. miyamotoi as an etiologic agent.
It was not until 1994 that spirochetes identified as B. miyamotoi were isolated from field-collected Ixodes persulcatus ticks and the small Japanese field mouse Apodemus argenteus in Japan [1]. In 2000 a novel spirochete was serendipitously identified in laboratory-reared I. scapularis ticks that were expected to be free from B. burgdorferi infection. Sequencing of the 16S ribosomal gene and other loci revealed that this newly-discovered organism from the northeastern United States was closely related to B. miyamotoi isolates of Japan [2,4]. B. miyamotoi has subsequently been identified in all other tick species that are vectors of Lyme disease and probably occurs throughout much of the Holarctic Region [2–10, 13–30]. The discovery of B. miyamotoi expands the potential geographic range of relapsing fever group Borrelia species. Most other relapsing fever spirochetes are transmitted by soft-bodied ticks (Argasidae) and lice that have different ecologies and only occasionally are found in the same geographic locations as Lyme disease vectors [31].
Although the novelty and wide geographic distribution of B. miyamotoi have been recognized for several years now, this spirochete received comparatively little attention until human cases of a relapsing fever-like disease from B. miyamotoi infection were reported in 2011 in Russia, and subsequently in the United States, Europe, and Japan [10,32–38]. These reports have documented the public health importance of B. miyamotoi. Human B. miyamotoi infection appears to be comparable in frequency to babesiosis or human granulocytic anaplasmosis (HGA) in the northeastern U.S. and may cause severe disease, including meningoencephalitis in immunocompromised individuals, as well as coinfection with other Ixodes-borne pathogens [10,32–38]. Additionally, antigenic cross-reactivities in immunoassays between Borrelia species in North America may complicate diagnosis of both Lyme disease and relapsing fever [39].
The organism
B. miyamotoi was not the first relapsing fever-group species shown to use a hard tick species as its primary vector. The association of the cattle pathogen B. theileri with Boophilus (now named Rhipicephalus) microplus hard ticks was noted by Arnold Theiler a century ago [40]. More recently, B. lonestari was discovered in Amblyomma species [41] and the reptile pathogen B. turcica was shown to be transmitted by Hyalomma species hard ticks [42]. Nucleotide sequences of these organisms, including the complete chromosomes of isolates of B. miyamotoi from North America [43] and Japan (GenBank accession number CP004217) confirmed that B. miyamotoi and the other hard-tick associated species phylogenetically cluster with the relapsing fever Borrelia species [44]. These include both New World species B. hermsii and B. turicatae and the Old World species, such as B. crocidurae, that are transmitted by soft ticks (Figure 1). A real-time quantitative PCR based on the same primers but different probes for the 16S ribosomal RNA gene distinguishes between the relapsing fever group species (including B. miyamotoi) and the Lyme disease group species [45].
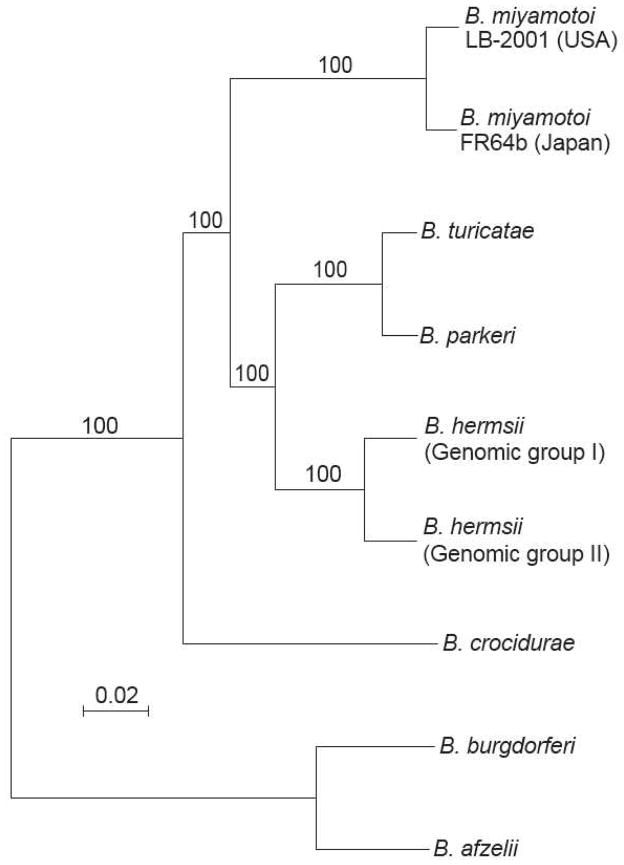
Figure 1.
Phylograms of aligned syntenic chromosome sequences of nine selected relapsing fever group and Lyme disease group Borrelia species by BioNJ neighbor-joining protocol for observed differences at 850,377 ungapped sites by a procedure described in reference 44. Nodes with bootstrap values of ≥70% support after 100 replicates are shown. The bar represents nucleotide substitutions per site. The organisms (with GenBank accession numbers) were B. miyamotoi strain LB-2001 from Connecticut, USA (CP006647); B. miyamotoi strain FR64b from Japan (CP004217); North American tickborne relapsing fever species B. turicatae strain 91E135 (CP000049), B. parkeri strain HR1 (CP007022), B. hermsii strain DAH of genomic group I (CP000048), B. hermsii strain YOR of genomic group II (CP004146); the Old World tickborne relapsing fever species B. crocidurae strain DOU (CP004267); and two Lyme disease species, B. burgdorferi strain B31 (AE000783) and B. afzelii strain PKo (CP002933).
Differences exist between B. miyamotoi isolates according to tick vector and geographic region, but so far little genetic difference has been found between isolates within a given geographic area or with the same tick vector association [4,18,29]. The overall genetic difference between a North American B. miyamotoi isolate (LB-2001) and a Japanese B. miyamotoi isolate (FR64b) is about the same as between B. turicatae and B. parkeri, two North American relapsing fever species with different host and vector associations [31], but less than between the two major genomic groups of B. hermsii strains [46] (Figure 1). In our opinion, the designation “sensu lato” is provisionally applicable for the North American strains, with “sensu stricto” reserved for the original Japanese isolates until further genetic and phenotypic characterization is carried out [44].
Besides its overall genetic distance from B. burgdorferi and other Lyme disease species, B. miyamotoi shares with other relapsing fever group species the distinctive feature of carriage and expression of a GlpQ (glycerophosphodiester phosphodiesterase) biosynthetic gene [47]. An antibody response to Borrelia GlpQ antigen is a characteristic of relapsing fever that distinguishes it from the immune response to Lyme disease (see below) [47]. On the other hand, there are many antigenic similarities between B. miyamotoi and other relapsing fever group species and the Lyme disease species, which may account for cross-reactive antibody binding in diagnostic assays based on whole cell antigens [39]. These common antigens include 4 of the 10 antigens specified in standard Western blot criteria for Lyme disease testing [48].
Genetic differences correspond to biological differences between B. miyamotoi and the Lyme disease group of disease agents. As is the case for other relapsing fever group species, much higher densities of the spirochetes are observed in the blood of infected wild rodents and laboratory mice than are observed with Lyme disease species in either natural or experimental infections [49]. While in vitro cultivation of B. miyamotoi isolates from Japan was achieved shortly after discovery, the North American isolates appear to be more difficult to cultivate than B. burgdorferi (A.G.B., unpublished findings). There are reports of in vitro passage of a North American isolate in modified BSK medium that has been used for other Borrelia species but the yields of in vitro cultures have been low to date [50–52]. Transovarial transmission is another biological feature that distinguishes B. miyamotoi and several other relapsing fever species from B. burgdorferi [13].
Ecology
B. miyamotoi is transmitted to humans by ticks that had acquired the organism either horizontally from a vertebrate reservoir host or possibly by ticks infected transovarially from the female tick. B. miyamotoi has been found in several of the tick species known to be vectors of Lyme disease group Borrelia species. These include I. scapularis in the northeastern and north-central United States and adjoining areas of Canada; Ixodes pacificus in the far-western United States and British Columbia; Ixodes ricinus in Europe, and Ixodes persulcatus in Europe and Asia (Figure 2) [1–10,14–30]. Ixodes ovatus and Ixodes pavlovskyi in northern Asia are two other species that have been shown to carry B. miyamotoi [29]. B. miyamotoi infection rates in field collected nymphal I. scapularis populations range between 0% and 10% [2,5,7–8,17]. I. pacificus nymphal infection rates vary from 0% to 15% [18,20–21,28]. The ratios of B. burgdorferi to B. miyamotoi infection in I. scapularis nymphs in the northeastern United States range from 4:1 to 16:1. In California the prevalence of B. burgdorferi in I. pacificus is approximately 10-fold lower than that observed in the Northeast. Under these circumstances, B. miyamotoi infection prevalences in nymphal and adult I. pacificus approach and may exceed those of B. burgdorferi [20,28]. In Europe, the range of B. miyamotoi infection rates reported in I. ricinus nymphal ticks is 0–3.2% [3,9,15–16,18,22–26,30]. Infection rates in I. scapularis in Canada and I. persulcatus in Russia fall within the range of B. miyamotoi infection detected in I. scapularis ticks in the United States [10,19].
Figure 2.
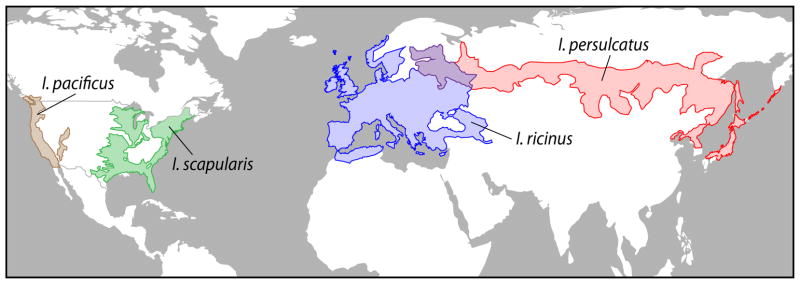
Approximate current geographic distributions of the main tick vectors of Borrelia miyamotoi: I. scapularis (green) and I. pacificus (brown) in North America and I. ricinus (blue) and I. persulcatus (red) in Eurasia. I. ovatus and I. pavlovskyi are two other species that have been shown to carry B. miyamotoi.
The reservoir hosts of B. miyamotoi throughout much of its distribution are poorly known or unknown. The white-footed mouse (Peromyscus leucopus) is a competent reservoir host of B. miyamotoi in the northeastern United States [2,7] but other species including birds [17], also may serve as reservoirs. In a study of 556 P. leucopus captured in eastern Connecticut, B. miyamotoi was identified in the blood of 7% of the mice and in skin tissue of 2% of mice; the corresponding prevalences for B. burgdorferi were 12% and 76% in blood and skin of this large sample [7]. While prevalence of B. miyamotoi was less than for B. burgdorferi, the densities of B. miyamotoi spirochetes in the blood were generally much higher than the density of B. burgdorferi spirochetes. Potential B. miyamotoi reservoir host species include species of field mice, voles, and birds in Europe, and field mice in Japan [25–26, 49]. Unlike Lyme disease Borrelia species, B. miyamotoi are carried by some unfed larvae, the consequence of transovarial acquistion. If larvae can transmit the infection to vertebrates, as a report from Japan indicates, there is the potential extension of the peak transmission season of certain species, such as I. scapularis into the later summer, when larval activity is at its highest [13,29,53].
Epidemiology
There is limited knowledge of the full geographic distribution of human B. miyamotoi infection but it is likely to be similar to that of Lyme disease. Human cases of B. miyamotoi have been reported in Russia, the United States, the Netherlands, and Japan. Two reports of B. miyamotoi seroprevalence have been published in healthy residents of southern New England. In the first study about 1% of 584 archived sera collected from 1990–2010 in healthy residents of Block Island, RI; Prudence Island, RI; and Brimfield, MA were seropositive for B. miyamotoi compared with 3% of 277 suspected Lyme disease patients [33]. In the second study, of 639 sera obtained from healthy residents of southern New England between 1991 and 2012, 3.9% were B. miyamotoi seropositive and 9.4% were B. burgdorferi seropositive [34]. A similar B. miyamotoi seroprevalence rate of 2% was noted in healthy (blood donor) residents of the Netherlands, while higher rates were noted in forestry workers (10%), and patients experiencing HGA (14.6%) and Lyme disease (7.4%) [38].
The geographic range of B. miyamotoi infection in tick vectors is much broader than the countries where human infection has been reported. B. miyamotoi has been found in ticks from Asia (Japan and central Russia), North America (the United States and Canada), and Europe (Czech Republic, Denmark, England, Estonia, France, Germany, Netherlands, Poland, Sweden, and Switzerland). It likely coexists with B. burgdorferi or other Lyme disease Borrelia species throughout its distribution [1–10, 14–30].
B. miyamotoi infections might occur in residents living outside its known enzootic geographic range through blood transfusion. Recurrent high density spirochetemia is characteristic of relapsing fever and increases the risk of transfusion transmission. Transfusion-transmitted relapsing fever caused by Borrelia recurrentis and Borrelia duttoni have been reported [54–56]. A recent study showed that B. miyamotoi can be transmitted through blood transfusion in a mouse experimental model [57]. Motile spirochetes were observed microscopically in the blood of both immunocompromised and immunocompetent mouse recipients after transfusion of murine B. miyamotoi–infected blood that was either fresh or stored for 7 days under conditions used in human blood banks. Spirochetemia was observed in immunocompetent mice up to five days and in immunocompromised mice 28 days after transfusion with fresh or stored B. miyamotoi-infected blood. These data suggest that transfusion transmission of B. miyamotoi may occur in humans.
Clinical manifestations
The most commonly reported clinical presentation of B. miyamotoi infection is a febrile illness consisting of fever that may exceed 40°C, fatigue, headache, chills, myalgia, arthralgia, and nausea (Table 1). In the first reported case series of B. miyamotoi infection, patients were enrolled who had a history of recent tick bite [10]. Five of the 46 (11%) B. miyamotoi cases experienced two to three episodes of fever, each lasting 2 to 5 days with a mean interval of 9 days between episodes (range 2 days to 2 weeks). The frequency of cases with a relapse of illness might have been higher if most patients had not been given empiric antibiotic therapy during their first episode of fever. The fever associated with B. miyamotoi infection generally lasts less than a week without antibiotic therapy while other symptoms such as fatigue may persist for as long as several weeks following antibiotic therapy [32–37]. The course of relapsing fever transmitted by soft-bodied ticks is similar. It generally consists of two or more episodes of fever lasting 1 to 5 days each with intervals of well being lasting at least 2 days and no more than 7 days between febrile episodes in untreated patients [58]. Patients infected with relapsing fever spirochetes have experienced as many as six episodes of recurrent fever [58]; the maximum noted with B. miyamotoi thus far is three episodes [10].
Table 1.
Clinical manifestations in patients with Borrelia spp. infection, Yekaterinburg City, Russia, 2009, and northeastern United States, 1991–2008. Adapted from Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 2011;17:1816–23 [10].
| Manifestation | % Patients
|
p value
|
||||
|---|---|---|---|---|---|---|
| B. miyamotoi, n=46 | B. garinii, n = 21 | B. burgdorferi, n = 92 | B. miyamotoi vs. B. garinii | B. miyamotoi vs. B. burgdorferi | B. garinii vs. B. burgdorferi | |
| Individual | ||||||
| EM | 9 | 91 | 89 | <0.001 | <0.001 | >0.999 |
| Multiple EM | 0 | 14 | 7 | 0.03 | 0.18 | 0. 36 |
| Fever† | 98 | 67 | 32 | 0.001 | <0.001 | 0.005 |
| Fatigue | 98 | 86 | 74 | 0.09 | <0.001 | 0.4 |
| Headache | 89 | 57 | 63 | 0.007 | 0.001 | 0.63 |
| Chills | 35 | 10 | 43 | 0.04 | 0.36 | 0.005 |
| Myalgia | 59 | 52 | 63 | 0.8 | 0.71 | 0.46 |
| Arthralgia | 28 | 29 | 62 | >0.999 | <0.001 | 0.007 |
| Nausea | 30 | 10 | 24 | 0.07 | 0.420 | 0.24 |
| Vomiting | 7 | 5 | 7 | >0.999 | >0.999 | >0.999 |
| Neck stiffness | 2 | 0 | 38 | >0.999 | <0.001 | <0.001 |
|
| ||||||
| Overall | ||||||
| No. symptoms, mean ± SD | 4.5 ± 1.4 | 4.2 ± 2.0 | 5.0 ±2.3 | 0.43 | 0.13 | 0.13 |
| No. symptoms (excluding EM and multiple EM), mean ± SD | 4.5 ± 1.4 | 3.1 ± 1.9 | 4.1 ± 2.3 | 0.007 | 0.46 | 0.09 |
EM, erythema migrans.
Maximum axillary temperature >37.2°C for patients in Russia and maximum oral temperature >37.7°C for patients in the United States.
In contrast to the clinical course of the majority of reported B. miyamotoi cases, two patients with well-documented B. miyamotoi infections of the central nervous system experienced symptoms and signs of meningoencephalitis. They each had a progressive decline in cognition and unsteady gait over several months but no fever [32,35]. One was an 80 year old woman from the United States and the other a 70 year old man from the Netherlands. Both had a lymphoproliferative disorder (non-Hodgkin’s and diffuse large B-cell lymphoma, respectively) and had received chemotherapy that was discontinued 6 months or more prior to the onset of symptoms. The diagnosis of B. miyamotoi infection was confirmed in both cases by cerebrospinal fluid analysis, which showed spirochetes on Giemsa staining of a cytospin of CSF sediment or direct darkfield examination of CSF, increased white blood cell count and protein, and amplification and sequencing of B. miyamotoi DNA. The patient in the United States was given one dose of ceftriaxone and four weeks of intravenous penicillin. The patient from the Netherlands was given two weeks of ceftriaxone. Both patients had a full recovery, including neurologic function, a month after the start of antibiotic therapy.
B. miyamotoi and B. burgdorferi coinfection has been documented in reports from the United States and Japan [34,37]. In the Russian B. miyamotoi case series, 9 of the 46 cases had an erythema migrans rash. Although there was no confirmation, B. burgdorferi coinfection was the most likely cause because erythema migrans rash has not been associated with soft tick transmitted relapsing fever [10, 58]. Previous studies have shown that coinfections of B. burgdorferi with either Babesia microti or with Anaplasma phagocytophilum are associated with more severe disease compared with that B. burgdorferi infection alone [59–61]. Further studies with a large sample size will be needed to determine whether B. miyamotoi and B. burgdorferi coinfection influences the outcome of disease in humans.
Diagnosis
Diagnosis of B. miyamotoi infection should be considered in any patient who resides in or has recently traveled to a region where Lyme disease is endemc in the North American or Eurasian continents during tick disease transmitting season and develops fever. Unlike Lyme disease, babesiosis, and HGA, it is conceivable that B. miyamotoi infection can be acquired by humans from the bite of a larval tick, because of transovarial transmission [29]. Consistent clinical findings such as fever, fatigue, and headache provide support for the diagnosis but similar symptoms may occur with other Ixodes transmitted diseases (such as Lyme disease, babesiosis, human granulocytic anaplasmosis, tick-borne encephalitis in Eurasia and deer tick virus encephalitis in North America) and acute viral infections. Diagnosis therefore requires confirmation using specific laboratory tests that include blood smear, polymerase chain reaction (PCR), and/or antibody determination [10,32–38]. At least one commercial laboratory is offering an antibody and a PCR test for B. miyamotoi and these tests are likely to be available in the near future from other commercial companies. B. miyamotoi can be isolated following in vitro culture or inoculation into immunodeficient laboratory mice but these methods are confined to a limited number of research laboratories.
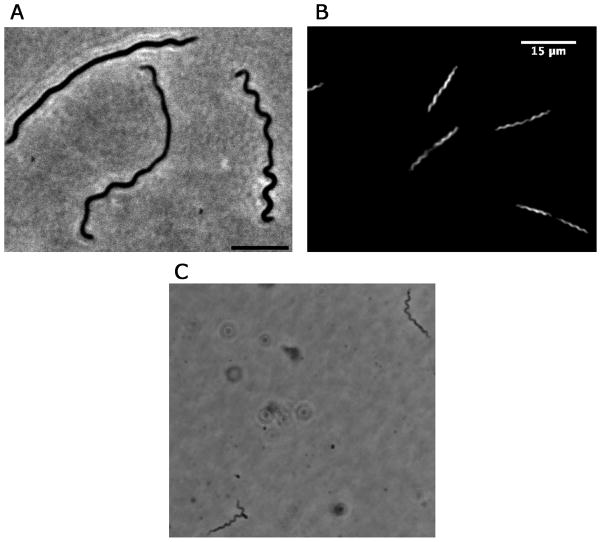
If the density of spirochetes in the blood is ≥104 per milliliter, B. miyamotoi might be identified by examining several high-power fields of a thin blood smear or spun sample of cerebrospinal fluid (for those suspected of meningitis or menigoencephalitis) stained with Giemsa or Wright stain. The blood smear used for a manual white blood cell differential will serve. A thick smear, such as prepared for a malaria screen, that has been de-hemoglobinized and then stained with acridine orange may reveal spirochetes present at lower densities in the blood. Motile spirochetes may be detected by dark-field or phase contrast of a wet mount of anticoagulated blood mixed with an equal volume of physiologic saline (Figure 3).
Figure 3.
A. B. miyamotoi grown In vitro (phase contrast, 100x [bar 5 um]), B. B. miyamotoi grown In vitro (dark-field, 40x [bar 15 um]), C. B. miyamotoi in infected mouse blood (phase contrast of wet mount of plasma [400x]).
Several PCR assays have been described for the detection of B. miyamotoi in whole blood, plasma, CSF, and tissues using primers specific for 16S ribosomal RNA and for the flaB and glpQ genes [3,7,32,35]. One of these discriminates between the Lyme disease and relapsing fever groups of Borrelia species, is quantitative, and has been shown to amplify B. miyamotoi DNA in ticks and in animal blood and tissues [7]. In vitro cultivation of B. miyamotoi isolates from Japan and North America have been achieved in specialized media but the reported yields of cultures for a North American isolate were lower than that expected for B. burgdorferi and several other Borrelia species [50–52].
Serologic testing can help confirm B. miyamotoi diagnosis. A 2-tiered antibody assay based on recombinant B. miyamotoi GlpQ protein has been developed that detects GlpQ-specific antibodies during the acute and convalescent stages of infection [33–34,47]. B. miyamotoi GlpQ protein is no more than 50% identical to the GlpQ proteins of some other bacterial pathogens, such as Klebsiella pneumoniae and Salmonella enterica. On the basis of this distance, B. miyamotoi GlpQ is not expected to cross-react with anti-GlpQ antibodies elicited by other disease agents, but this has not been established as yet. More clinically relevant is the presumption that sera from Lyme disease patients will not cross-react in a GlpQ assay because Lyme disease Borrelia species (as well as Anaplasma, Babesia and Erhlichia species) lack the gene for GlpQ [47]. In contrast, the sera of 10% of B. miyamotoi infected patients met the criteria for seropositivity in a standard 2-tier B. burgdorferi antibody assay [34]. Possible causes included a prior B. burgdorferi infection, acute co-infection with B. burgdorferi, a false-positive test reaction, and/or cross-reactivity of antibodies elicited by the B. miyamotoi infection against one or more B. burgdorferi antigens. The results of whole cell B. burgdorferi EIA tests were positive in some patients who apparently had B. miyamotoi alone, possibly due to cross-reactivity of B. miyamotoi-elicited antibodies with some B. burgdorferi proteins [10,34]. B. miyamotoi genes encode the homologues of many B. burgdorferi proteins, including the flagellin FlaB protein, GroEL heat shock proteins, P66 outer membrane protein, and the BmpA (P39)-type basic membrane protein. B. miyamotoi antibody probably cross reacts with the GlpQ proteins of other relapsing fever Borrelia species, as well as B. lonestari. These organisms are not transmitted by Ixodes ticks and generally have different areas of distribution than B. miyamotoi in North America and Eurasia, although there are some areas in California where both B. miaymotoi and B. hermsii are enzootic [34,47,58, new ref].
The development of a method to grow cultures of B. miyamotoi strains in vitro provides an opportunity to identify additional antigenic B. miyamotoi proteins that would not be expected to cross-react with Lyme disease Borrelia antigens or other clinically relevant etiologic agents [43, 50–52]. The database of the deduced proteins from the genome sequences provides a basis for identifying informative antigens that are expressed in vivo but not in vitro, as was carried with a genome-wide protein array for B. burgdorferi [62]. If the immunodominant antigens during B. miyamotoi infection include the variable membrane proteins, which are the basis for antigenic variation during relapsing fever from other species [62], then there may need to be several variant antigens in a microarray-based or bead-based assay to detect the spectrum of antibody responses in infected patients.
Treatment and prevention
Treatment recommendations for B. miyamotoi infection are based on the few case reports and series that have been published thus far and supplemented here by the recommendations for treatment of other relapsing fever Borrelia infections [32–37,58]. There have been no therapeutic trials nor any experimental data published on the antibiotic susceptibility either in vitro or in vivo of B. miyamotoi. Therefore, optimal antibiotics of choice, their dosages, and treatment duration have yet to be determined.
Doxycycline (100 mg orally every 12 hours) given for 7 to 14 days is the most commonly prescribed antibiotic for patients experiencing uncomplicated B. miyamotoi infection to date [10,32–37]. This tetracycline appears to have been effective based on fever defervescence within 5 days of initiation and absence of further fever episodes. Intravenous ceftriaxone (2 grams once a day) given for two weeks or penicillin G (24 million U per day) given for four weeks were used successfully in the two patients with meningoencephalitis.
Amoxicillin or cefuroxime given orally are suitable alternatives to doxycycline or other tetracycline, which are generally contraindicated for children less than 9 years of age and for pregnant and nursing women. These antibiotics are commonly used for treatment of Lyme disease and would be expected to be effective against B. miyamotoi infection based on susceptibilities of other relapsing fever Borrelia species. Penicillin G or cefotaxime are alternatives to ceftriaxone for parenteral therapy of documented or suspected CNS involvement or other severe infection. Azithromycin would also probably clear infection but macrolides are generally less effective than tetracyclines and most beta-lactam antibiotics for Borrelia infections. Some first generation cephalosporins like cephalexin are likely not as effective against B. miyamotoi as other beta-lactam antibiotics. On the basis of other Borrelia species, B. miyamotoi is likely to be relatively resistant to fluroquinolones and aminoglycosides. If human granulocytic anaplasmosis is suspected, then doxycycline, but not beta-lactam antibiotics or macrolides, would probably be effective for both infections.
The Jarisch-Herxheimer reaction is a commonly encountered, mild to serious adverse effect of the first dose or two of antibiotic therapy for relapsing fever [58,63]. The fever returns or increases to ≥40°C. There are accompanying chills and rigors followed by diaphoresis. Hypotension and a shock-like state may occur suddenly and be life-threatening. It has been reported in up to half of people given antibiotics for tick-borne relapsing fever and 15% of people treated for B. miyamotoi infection in Russia [10,58]. A patient from the United States experiencing meningoencephalitis developed manifestations of a Jarisch-Herxheimer reaction, including hypotension, within nine hours after her initial dose of ceftriaxone [32]. Effective management of this problem includes anticipation of the reaction with the initiation of antibiotics and the provision of monitoring and supportive measures, such as volume expansion and antipyretics.
Effective preventive measures for B. miyamotoi infection are expected to be the same as for other Ixodes tick-borne diseases, such as Lyme disease. They include personal protective measures to avoid tick bite, landscape modification, and environmental measures to reduce tick abundance. No vaccine has been developed and approved for B. miyamotoi or any other relapsing fever Borrelia species.
Summary
B. miyamotoi is a recently discovered human pathogen belonging to the relapsing fever group of species in the spirochete genus of Borrelia. It is transmitted by the same hard-bodied tick species that are the vectors of Lyme disease and is likely to be found wherever Lyme disease occurs. Human infection has been shown to be prevalent in the northeastern United States. Disease severity is variable but patients most commonly present with fever and non-localizing symptoms. Meningoencephalitis requiring hospital admission has been reported. There is limited availability of laboratory testing for infection at this time. Laboratory procedures that can be widely implemented but are of uncertain sensitivity include examination of a stained blood smear for spirochetes and amplification of B. miyamotoi DNA using PCR. Assays of acute and convalescent sera for antibodies to the GlpQ protein of B. miyamotoi may provide confirmation of a diagnosis; other immunoassays are in development. Procedures with very limited availability for direct detection are in vitro cultivation and animal inoculation. The most common antimicrobial agents that have been used to achieve cure are doxycycline and ceftriaxone, but other antibiotics that are used to treat tick-borne relapsing fever and Lyme disease are likely to be as effective as well.
Acknowledgments
This work was supported by grants (AI-088079 to D.F. and P.J.K. and AI-100236 to A.G.B.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Additional support was provided by the Gordon and Llura Gund Foundation and the G. Harold and Leila Y. Mathers Charitable Foundation. We wish to thank Dr. Brandon Jutras and Ms. Elizabeth Scott for providing the B. miyamotoi dark-phase images. We are grateful to Wendolyn Hill for creation of Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov. isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Internat J System Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 2.Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 3.Richter D, Schlee DB, Matuschka FR. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg Infect Dis. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10:1661–1664. doi: 10.3201/eid1009.040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- 7.Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. American J Trop Med Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tokarz R, Jain K, Bennett A, Briese T, Lipkin WI. Assessment of polymicrobial infections in New York State. Vector Borne Zoonotic Dis. 2010;10:217–221. doi: 10.1089/vbz.2009.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelmsson P, Fryland L, Borjesson S, Nordgren J, Bergstrom S, Ernerudh J, Forsberg P, Lindgren PE. Prevalence and diversity of Borrelia species in ticks that have bitten humans in Sweden. J Clin Microbiol. 2010;48:4169–4176. doi: 10.1128/JCM.01061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgdorfer W, Lane RS, Barbour AG, Gresbrink RA, Anderson JR. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am J Trop Med Hyg. 1985;34:925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- 12.Piesman J, Donahue JG, Mather TN, Spielman A. Transovarially acquired Lyme disease spirochetes (Borrelia burgdorferi) in field-collected larval Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1986;23:219. doi: 10.1093/jmedent/23.2.219. [DOI] [PubMed] [Google Scholar]
- 13.Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Richter D, Debski A, Hubalek Z, Matuschka FR. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 2012;12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- 15.Geller J, Nazarova L, Katargina O, Jarvekulg L, Fomenko N, Golovljova I. Detection and genetic characterization of relapsing fever spirochete Borrelia miyamotoi in Estonian ticks. PLoS One. 2012;7:e51914. doi: 10.1371/journal.pone.0051914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian G, Sekeyova Z, Raoult D, Mediannikov O. Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks Tick Borne Dis. 2012;3:406–410. doi: 10.1016/j.ttbdis.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Hamer SA, Hickling GJ, Keith R, Sidge JL, Edward D, Walker ED, Tsao J. Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasites & Vectors. 2012;5:231. doi: 10.1186/1756-3305-5-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowder CD, Carolan HE, Rounds MA, Honig V, Mothes B, Haag H, Nolte O, Luft BJ, Grubhoffer L, Ecker DJ, Schutzer S, Eshoo M. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis. 2014;20:1678–1682. doi: 10.3201/eid2010.131583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dibernardo A, Cote T, Ogden NH, Lindsay LR. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasit Vectors. 2014;15:183. doi: 10.1186/1756-3305-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padgett K, Bonilla D, Kjemtrup A, Vilcins IM, Yoshimizu MH, Hui L, Sola M, Quintana M, Kramer V. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS One. 2014;21:9, e110853. doi: 10.1371/journal.pone.0110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorova N, Kleinjan JE, James D, Hui LT, Peeters H, Lane RS. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick-borne Dis. 2014;5:951–961. doi: 10.1016/j.ttbdis.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Kiewra D, Stańczak J, Richter M. Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in Lower Silesia, Poland-preliminary study. Ticks Tick Borne Dis. 2014;5:892–897. doi: 10.1016/j.ttbdis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, Chirico J, van der Wal FJ, Sprong H, Boye Pihl TP, Klitgaard K, Bodker R, Fach P, Moutailler S. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol. 2014;29:1–13. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansford KM, Fonville M, Jahfari S, Sprong H, Medlock JM. Borrelia miyamotoi in host-seeking Ixodes ricinus ticks in England. Epidemiol Infect. 2014;14:1–9. doi: 10.1017/S0950268814001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lommano E, Dvořak C, Vallotton L, Jenni L, Gern L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis. 2014;5:871–882. doi: 10.1016/j.ttbdis.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Cosson JF, Michelet L, Chotte J, Le Naour E, Cote M, Devillers E, Poulle ML, Huet D, Galan M, Geller J, Moutailler S, Vayssier-Taussat M. Genetic characterization of the human relapsing fever spirochete Borrelia miyamotoi in vectors and animal reservoirs of Lyme disease spirochetes in France. Parasit Vectors. 2014;7:233. doi: 10.1186/1756-3305-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamer SA, Hickling GJ, Walker ED, Tsao JI. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the Midwestern United States. Infect Genet Evol. 2014;27:531–542. doi: 10.1016/j.meegid.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Salkeld D, Cinkovich S, Nieto NC. Tick-borne pathogens in northwestern California, USA. Emerg Infect Dis. 2014;20:493–494. doi: 10.3201/eid2003.130668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takano A, Toyomane K, Konnai S, Ohashi K, Nakao M, Ito T, Andoh M, Maeda K, Watarai M, Sato K, Kawabata H. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One. 2014;11:9, e104532. doi: 10.1371/journal.pone.0104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eshoo MW, Crowder CD, Carolan HE, Rounds MA, Ecker DJ, Haag H, Mothes B, Nolte O. Broad-range survey of tick-borne pathogens in Southern Germany reveals a high prevalence of Babesia microti and a diversity of other tick-borne pathogens. Vector Borne Zoonotic Dis. 2014;14:584–591. doi: 10.1089/vbz.2013.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwan TG, Piesman J. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg Infect Dis. 2002;8:115–121. doi: 10.3201/eid0802.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gugliotta JL, Goethert HK, Berardi VP, Telford SR., 3rd Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. New Engl J Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. Human Borrelia miyamotoi Infection in the United States. New Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, Jahfari S, Pals ST, Horlings HM, Fikrig E, Sprong H, Oers MHJ. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowdri HR, MD, Gugliotta JL, Berardi VP, Goethert HK, ScD, Molloy PJ, Sterling SL, Telford SL., III Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med. 2013;159:217. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Takano A, Konnai S, Nakao M, Ito T, Koyama K, Kaneko M, Ohnishi M, Kawabata H. Human infections with Borrelia miyamotoi, Japan. Emerg Infect Dis. 2014;20:1391–1393. doi: 10.3201/eid2008.131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahfari S, Herremans T, Platonov AE, Kuiper H, Karan LS, Vasilieva O, Koopmans MPG, Hovius JWR, Sprong H. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect. 2014;2:144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnarelli LA, Anderson JF, Johnson RC. Cross-reactivity in serologic tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 40.Theiler A. Spirillosis of cattle. J Comp Pathol Therapeutics. 1904;17:47–55. [Google Scholar]
- 41.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 42.Takano A, Goka K, Une Y, Shimada Y, Fujita H, Shiino T, Watanabe H, Kawabata H. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ Microbiol. 2010;12:134–146. doi: 10.1111/j.1462-2920.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 43.Hue F, Ghalyanchi Langeroudi A, Barbour AG. Chromosome sequence of Borrelia miyamotoi, an uncultivable tick-borne agent of human infection. Genome Announc. 2013;1 doi: 10.1128/genomeA.00713-13. Epub 2013/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbour AG. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect Genet Evol. 2014;27:551–558. doi: 10.1016/j.meegid.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukhacheva TK, Kovalev SY. Borrelia spirochetes in Russia: Genospecies differentiation by real-time PCR. Ticks Tick Borne Dis. 2014;5:722–726. doi: 10.1016/j.ttbdis.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Porcella SF, et al. Variable tick protein in two genomic groups of the relapsing fever spirochete Borrelia hermsii in western North America. Infect Immun. 2005;73:6647–6658. doi: 10.1128/IAI.73.10.6647-6658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Konkel ME. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dressler F, et al. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 49.Taylor KR, Takano A, Konnai S, Shimozuru M, Kawabata H, Tsubota T. Borrelia miyamotoi infections among wild rodents show age and month independence and correlation with Ixodes persulcatus larval attachment in Hokkaido, Japan. Vector Borne Zoonotic Dis. 2013;13:92–97. doi: 10.1089/vbz.2012.1027. [DOI] [PubMed] [Google Scholar]
- 50.Teegler A, Herzberger P, Margos G, Fingerle V, Kraiczy P. The relapsing fever spirochete Borrelia miyamotoi resists complement-mediated killing by human serum. Ticks Tick Borne Dis. 2014;5:898–901. doi: 10.1016/j.ttbdis.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasites & Vectors. 2014;7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margos G, Stockmeier S, Hizo-Teufel C, Fish D, Dautel H, Sing A, Dzaferovic E, Rieger MS, Jungnick S, Binder K, Straubinger RK, Fingerle V. Long-term in vitro cultivation of Borrelia miyamotoi. Ticks and Tick-Borne Dis. doi: 10.1016/j.ttbdis.2014.12.001. in press. [DOI] [PubMed] [Google Scholar]
- 53.Daniels TJ, Falco RC, Curran KL, Fish D. Timing of Ixodes scapularis (Acari: Ixodidae) oviposition and larval activity in southern New York. J Med Entomol. 1996;33:140–147. doi: 10.1093/jmedent/33.1.140. [DOI] [PubMed] [Google Scholar]
- 54.Nadelman RB, Wormser GP, Sherer C. Blood transfusion associated relapsing fever. Transfusion. 1990;30:380–381. doi: 10.1046/j.1537-2995.1990.30490273451.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang CW, Lee CU. Malaria and relapsing fever following blood transfusion including the report of a case of congenital transmission of relapsing fever. Chin Med J. 1936;50:241–248. [Google Scholar]
- 56.Hira PR, Husein SF. Some transfusion-induced parasitic infections in Zambia. J Hyg Epidemiol Microbiol Immunol. 1979;23:436–444. [PubMed] [Google Scholar]
- 57.Krause PJ, Hendrickson JE, Steeves TK, Fish D. Blood transfusion transmission of the tick-borne relapsing fever spirochete Borrelia miyamotoi in mice. Transfusion. 2014 Sep 23; doi: 10.1111/trf.12879. [DOI] [PubMed] [Google Scholar]
- 58.Dworkin MS, Anderson DE, Jr, Schwan RG, et al. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- 59.Krause PJ, Telford S, Spielman A, Sikand VJ, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis: Evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 60.Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, Closter L, Christianson D, Telford SR, Persing D, Radolf JD, Spielman A the deer-associated infection study group. Disease-specific diagnosis of coinfecting tick-borne zoonoses: Babesiosis, human granulocytic ehrlichiosis and Lyme disease. Clin Inf Dis. 2002;34:1184–1191. doi: 10.1086/339813. [DOI] [PubMed] [Google Scholar]
- 61.Belongia EA, Reed KD, Mitchell PD, Chyou PH, Mueller-Rizner N, Finkel MF, Schriefer ME. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472–1477. doi: 10.1086/313532. [DOI] [PubMed] [Google Scholar]
- 62.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, Felgner PL. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerrier G, Doherty T. Comparison of antibiotic regimens for treating louse-borne relapsing fever: a meta-analysis. Trans Royal Society Trop Med Hygiene. 2011;105:483–490. doi: 10.1016/j.trstmh.2011.04.004. [DOI] [PubMed] [Google Scholar]