Abstract
Use of marijuana during pregnancy is fairly commonplace and can be expected increase in frequency as more states legalize its recreational use. The cannabinoids present in marijuana have been shown to be immunosuppressive, yet the effect of prenatal exposure to cannabinoids on the immune system of the developing fetus, its long term consequences during adult stage of life, and transgenerational effects have not been well characterized. Confounding factors such as coexisting drug use make the impact of cannabis use on progeny inherently difficult to study in a human population. Data from various animal models suggests that in utero exposure to cannabinoids results in profound T cell dysfunction and a greatly reduced immune response to viral antigens. Furthermore, evidence from animal studies indicates that the immunosuppressive effects of cannabinoids can be mediated through epigenetic mechanisms such as altered microRNA, DNA methylation and histone modification profiles. Such studies support the hypothesis that that parental or prenatal exposure to cannabis can trigger epigenetic changes that could have significant immunological consequences for offspring as well as long term transgenerational effects.
Keywords: Marijuana, Pregnancy, Cannabinoids, Endocannabinoids, CB1, CB2, THC, Immune system, Epigenetic, Transgenerational, DNA methylation, Histone modification, MicroRNA
Introduction
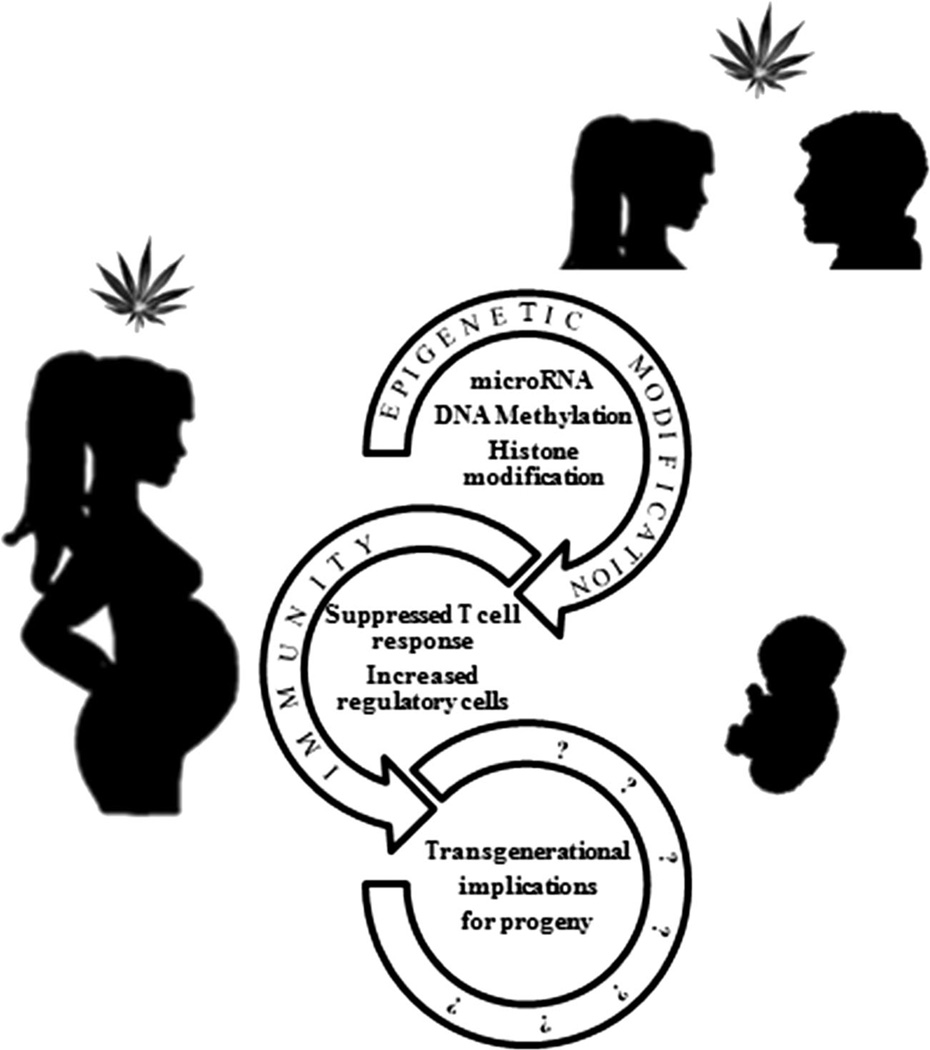
As the decriminalization of marijuana spreads across the United States, the scientific community is working to fully comprehend dangers associated with recreational usage. According to the National Survey on Drug Use and Health, greater than 43 % of Americans 12 years of age and older have used marijuana in their lifetime (http://www.drugabuse.gov). At the time of writing, 22 states and the District of Columbia have legalized marijuana for medicinal purposes. Four of these states and Washington DC, have also legalized recreational use of marijuana. This change in legislation is limited to the state level since federal law still maintains that cannabis, a Schedule one substance, has no medical uses and a high potential for abuse (The Controlled Substances Act of 1970). With the legalization of marijuana for recreation, its use is likely become more socially acceptable, which in turn will lead to increased use among adolescents and young adults as well as women who are pregnant. Chronic abuse of marijuana has been shown to have significant deleterious effects on certain physiological functions such as cognitive processes, in humans. Also, experimental studies have shown that marijuana cannabinoids are highly immunosuppressive (Klein et al. 1998; Klein et al. 2000; McKallip et al. 2002; Hegde et al. 2008; Nagarkatti et al. 2009; Hegde et al. 2010). The developing fetus is highly susceptible to various environmental insults. Thus, abuse of marijuana during pregnancy has been shown to be associated with many deleterious effects on the developing fetus (Hingson et al. 1982; Tanasescu and Constantinescu 2010; Morris et al. 2011; Hayatbakhsh et al. 2012; Behnke et al. 2013; Jaques et al. 2014). Whether exposure to marijuana cannabinoids during pregnancy can cause immunological alterations in the human fetus remains to be studied. The “fetal basis of adult disease” concept proposed by Barker originated from his observation that low birth weight in humans was strongly associated with coronary heart disease later in life (Barker 2007). This has led to the hypothesis that exposure of the fetus to environmental insults may have profound impact on health even during the adult stage of life. Additionally, recent studies have suggested that epigenetic alterations brought about by such environmental insults in the fetus may have transgenerational effects. This has been clearly established in epidemiological studies of pregnant mothers that were treated with diethylstilbestrol, which led to increased susceptibility of their daughters and granddaughters to cervical cancers and increased susceptibility to autoimmune disorders in sons (Reed and Fenton 2013). It is therefore necessary to better understand the possible long term immunological ramifications of marijuana use during pregnancy on offspring. Here, we offer a review of research that supports our hypothesis that parental exposure to cannabinoids triggers epigenetic changes that could have potential for long term immunological consequences for offspring as well as well as such effects being carried transgenerationally (Fig. 1).
Fig. 1.
Schematic showing possible impact of cannabinoid usage on progeny. This figure highlights that cannabinoid use causes both epigenetic and immune dysregulation, both of which have been associated with deleterious impact on progeny as well as future generations
Cannabinoids and Cannabinoid Receptors
Marijuana contains over 80 cannabinoids, the most studied of which is the aromatic terpenoid Δ-9-tetrahydrocannabinol (THC) which is the major psychotropic component of cannabis (Gaoni and Mechoulam 1971). Nearly 30 years after the discovery of THC, two G-coupled protein receptors, identified as cannabinoid receptor 1 (CB1) and 2 (CB2), were discovered with other less studied cannabinoid receptors discovered in the ensuing years (Matsuda et al. 1990; Munro et al. 1993). CB1 is predominantly expressed in the CNS but is also present on activated immune cells, albeit to a lesser degree (Sido et al. 2014). In contrast, CB2 is most prominently expressed on the cells of the immune system and activation of CB2 can lead to significant alterations in the immune response including apoptosis, cytokine suppression, altered T cell differentiation towards T regulatory (Treg) cells, induction of Myeloid-Derived Suppressor Cells (MDSCs) and a shift of the immunological status from a proinflammatory (Th1) to an antiinflammatory (Th2) profile (McKallip et al. 2002; Klegeris et al. 2003; Do et al. 2004; Lombard et al. 2007; Hegde et al. 2008; Nagarkatti et al. 2009; Nagarkatti et al. 2010; Singh et al. 2012b; Sido et al. 2014).
While THC works as a ligand for the CB1 and CB2 receptors, the discovery of a specialized cannabinoid receptor suggested that endogenous ligands must also exist. These endogenous ligands for the cannabinoid receptors, called endocannabinoids, are arachidonic acid precursors which are derived from cellular membranes (Rossi et al. 2010). The first endocannabinoids discovered, and still the mostly widely studied, are N-arachidonyl ethanolamide (AEA or anandamide) and 2-arachidonoyl glycerol (2-AG) (Mechoulam et al. 1995; Sugiura et al. 1995; Tanasescu and Constantinescu 2010).
The endogenous cannabinoids, along with the CB1 and CB2 receptors make up the endocannabinoid system (Tanasescu and Constantinescu 2010). In the CNS the endocannabinoid system is associated with the regulation of motor control, memory, cognitive processes, and neurotransmitter release (Mackie 2008; Pertwee 2008; Kano et al. 2009). While it was originally believed that the endocannabinoid system was limited to the central nervous system, it is now known that the immune and reproductive systems also express cannabinoid receptors and are thus impacted by cannabinoids (Smita et al. 2007; Nagarkatti et al. 2009; Pandey et al. 2009a; Rieder et al. 2010; Sido et al. 2014). Indeed, endocannabinoids play an integral role in autoimmune diseases, with CB1 specifically involved in the regulation and amelioration of autoimmune induced inflammation (Sido et al. 2014).
Prenatal Exposure to Cannabis
Environmental influences during early development, particularly in utero, are hypothesized to be associated with long term consequences on health and disease predisposition. This “developmental origins of adult disease hypothesis”, also known as “the Barker hypothesis”, came from David J.P. Barker’s observations that low birth weight was strongly associated with coronary heart disease later in life (Barker 2007). Likewise, there is an abundance of evidence that prenatal exposure to cannabis adversely affects neurodevelopment with negative consequences for neuropsychiatric, behavior and executive functions (Jaques et al. 2014). Approximately 2.5 % of pregnant women admit to consistent use of cannabis, thus actual use is probably higher (Hayatbakhsh et al. 2011). Importantly, cannabinoids can cross the placenta as well as the blood brain barrier and can also be concentrated in breast milk (Schou et al. 1977; Bar-Oz et al. 2003; Jaques et al. 2014).
While it is difficult to separate the impact of cannabis use from other frequent co-exposures such as nicotine and alcohol, research suggests that these effects include a variety of adverse birth outcomes such as low birth weight, preterm labor and increased admission to the neonatal intensive care unit (Hatch and Bracken 1986; Hayatbakhsh et al. 2012). Numerous studies have looked at the impact on the neurological function of the developing child. For example, researchers have shown that prenatal marijuana exposure has a significant negative impact on intelligence as early as age 6, depressive symptoms at age 10, and school achievement at age 14 (Gray et al. 2005; Goldschmidt et al. 2008; Goldschmidt et al. 2012). There is also an increased risk of psychosis, increased aggression, attention problems, impaired memory, and cognitive dysfunction associated with exposure to cannabis during pregnancy (Jaques et al. 2014). Thus, there exists a fairly extensive body of research indicating a marked impact of cannabinoid exposure during pregnancy on neurological development. Yet, while cannabinoids are well known to have suppressive effects on the immune system, no human studies have been conducted to investigate if adverse immunological outcomes result from in utero exposure.
There is some evidence that prenatal marijuana exposure results in an increase in certain cancers, such as neuroblastoma and leukemia in offspring (Robison et al. 1989; Bluhm et al. 2006). With some in the medical community suggesting that cannabinoids could be used to relieve severe nausea in pregnancy and the potential abuse of cannabis for recreational use during pregnancy, a greater understanding of the long term immunological effects on the exposed child is needed. The difficulty of studying such effects in humans makes the use of animal models of prenatal cannabinoid exposure essential (Table 1).
Table 1.
Summary of research: cannabinoid exposure impact on fetus
| Cannabinoid exposure | System | Model | Study |
|---|---|---|---|
| Perinatal | Immune | Mouse | (Lombard et al. 2011) |
| Perinatal | CNS | Rat | (Suarez et al. 2004a) |
| Perinatal | CNS | Rat | (Bonnin et al. 1996) |
| Perinatal | CNS | Rat | (Garcia-Gil et al. 1999) |
| Perinatal | CNS | Rat | (Garcia-Gil et al. 1999) |
| Gestation/ Lactation | Endocrine/ Immune | Rat | (del Arco et al. 2000) |
| Prenatal | CNS | Rat | (Suarez et al. 2004b) |
| Perinatal | Behavior | Mouse | (Newsom and Kelly 2008) |
| Prenatal | CNS | Human OPPS cohort MHPCD cohort | As reviewed by (Wu et al. 2011) |
Notably, THC exposure in pregnant mice resulted in markedly defective T cell differentiation and impaired T cell function in offspring (Lombard et al. 2011). These defects were reversed by CB1 and CB2 agonists. Thymic cellularity was also reduced in mouse pups resulting in thymic atrophy mediated by apoptosis of thymocytes. Importantly, these changes had a profound impact on the post-natal immune response in 5 week old mice that had been exposed prenatally (Lombard et al. 2011). These young mice had a decrease in T cell proliferation and decreased Tcell and antibody responses to HIV-1 antigens. Thus, cannabinoid exposure during pregnancy resulted in a profound T cell dysfunction, which could lead to increased susceptibility to certain infections and cancers in offspring.
Cannabinoids and Immune Suppression
The anti-inflammatory properties of THC, including suppression of the antitumor response, have been well studied (Klein et al. 1998; Hegde et al. 2008; Nagarkatti et al. 2009; Pandey et al. 2011). Cannabinoids have been shown to suppress inflammatory responses using various mechanisms such as apoptosis, altering the cytokine milieu, induction of MDSCs, and upregulation of Tregs as detailed below. As such, cannabinoids have been explored as possible treatments for numerous diseases such as malignant lymphoblastic disease, leukemia, colitis, autoimmune hepatitis, rheumatoid arthritis, and transplant rejection (McKallip et al. 2002; Lombard et al. 2005; Pandey et al. 2009b; Nagarkatti et al. 2010; Singh et al. 2012b) THC exposure has been linked to cannabinoid receptor dependent apoptosis in splenic and thymic T cells, dendritic cells (DCs), and macrophages (Zhu et al. 1998; McKallip et al. 2002; Do et al. 2004; Hegde et al. 2011). In addition to apoptosis, THC can induce immune suppression by shifting the cytokine profile from proinflammatory (Th1 or Th17) to anti-inflammatory (Th2) (Hegde et al. 2008; Pandey et al. 2011; Jackson et al. 2014a). Cannabinoids have also been observed to decrease lymphocyte driven immune responses through the activation of regulatory cells such as T regulatory cells (Tregs) and myeloid derived suppressor cells (MDSCs) (Zhu et al. 2000; McKallip et al. 2005; Hegde et al. 2010; Jackson et al. 2014b).
Cannabinoids and Effect on Fertility and Fetus
As early as 1978, research looking into the role that the cannabinoids were playing in both reproduction and development was initiated (Bloch et al. 1978). The uterus expresses CB1, but not CB2, receptors and has the ability to synthesize AEA suggesting that the endocannabinoid system could play a role in uterine function (Das et al. 1995). Additionally, AEA levels in utero change during implantation and pregnancy (Westerlind et al. 1997; Wang et al. 1999; Wang et al. 2004). When these data were taken together with reports of blighted ovum, early stage pregnancy failure, and miscarriage due to marijuana exposure, the impact of endocannabinoid system dysregulation during pregnancy and the effect on the fetus became even more pertinent (Bloch et al. 1978; Smith and Asch 1987). It has been shown, in a 2009 National survey on drug use and health, that marijuana is the most common illegal drug found in pregnant women, at 11 % (HHS publication no. SMA10-4609). Furthermore, it has been shown that THC can bio-accumulate in fetal tissue such as the brain (Harbison and Mantilla-Plata 1972; Kennedy and Waddell 1972; Hutchings et al. 1989). Timing and expression of the CB1 receptor, and its agonists, is vital to embryo survival. However additional studies are necessary to address the mechanistic role of endogenous cannabinoids and their receptors during pregnancy and the impact of exogenous cannabinoids. Exposures to environmental insults during pregnancy can not only alter the immune status in the fetus (Camacho et al. 2004; Singh et al. 2011; Singh et al. 2012a) but also can have transgenerational effects (Manikkam et al. 2012b; Manikkam et al. 2012a). One of the most well documented epidemiological observations in humans is the exposure to DES, a synthetic estrogen, during pregnancy. Millions of pregnant women were given DES only to find out later on that the DES-mothers became more susceptible to breast cancers and DES-daughters and even granddaughters became more susceptible to cervical cancers (Hatch et al. 1998). Exposure to DES has also been linked to a wide range of abnormalities in DES sons and daughters including immune system disorders, psychosexual effects, and reproductive disorders (Giusti et al. 1995). Such transgenerational effects of DES can be explained primarily through epigenetic changes brought about by external agents through their effect not only on somatic cells but also on germinal cells (Walker and Haven 1997; Newbold et al. 2006; Sato et al. 2009).
Epigenetic Immunosuppressive Mechanisms of Cannabinoids
MicroRNA
The evidence of the immunosuppressive effects of cannabinoids such as THC is becoming ever more robust. However, the mechanisms for such immunosuppressive activity are only beginning to emerge. Epigenetic mechanisms underlying establishment and maintenance of differential gene expression in T cells have been uncovered (Araki et al. 2009). Epigenetic modifications can thus regulate T cell differentiation by altering the expression of cytokines and transcription factors such as Ifn-γ and FoxP3 (Morinobu et al. 2004). Thus, it can be predicted that cannabinoids act to suppress inflammation via similar means. CB1 and CB2 are known to be epigenetically regulated by both histone modification and DNA methylation and this has been demonstrated in a variety of experimental systems (D’Addario et al. 2013). Because studies on epigenetic effects of cannabinoid use in humans, including prenatal exposure, are completely lacking, animal models must be used to offer a glimpse into their potential impact. As such, there are several recent studies using animal models that suggest that exposure to THC may impart downstream immunological effects via epigenetic pathways (Table 2).
Table 2.
Summary of research: cannabinoid impact on epigenetic regulation
| Cannabinoid | Model | Study | |
|---|---|---|---|
| microRNA | |||
| AEA | Mouse | (Jackson et al. 2014a) | |
| THC | Mouse | (Hegde et al. 2013) | |
| HU210 synthetic cannabinoid | Rat | (Hollins et al. 2014) | |
| THC | Nonhuman primate | (Chandra et al. 2014) | |
| THC | Nonhuman primate | (Molina et al. 2011) | |
| DNA methylation | |||
| AEA | Human keratinocytes in vitro | (Paradisi et al. 2008) | |
| THC | Nonhuman primate | (Molina et al. 2011) | |
| Histone modification | |||
| THC | Mouse | (Yang et al. 2014) | |
| THC | Mouse | (Khare et al. 2006) | |
| THC | Rat | *(DiNieri et al. 2011) |
indicates results from progeny of prenatal exposure to cannabinoids
Epigenetic mechanisms consist of the regulation of gene expression via microRNA, DNA methylation and histone modification. MicroRNAs are temporal, tissue specific, posttranscriptional regulators (Bartel 2004). MicroRNAs are endogenous small non-coding RNA molecules of approximately 22 nucleotides that can bind to target mRNAs thereby marking them for destruction or interference with translation. Each microRNA can have hundreds of potential targets and each mRNA can be targeted by numerous microRNAs, making meaningful evaluation of dysregulated microRNAs initially dependent on a robust bioinformatics analysis. Nonetheless, several studies have documented the dysregulation of microRNAs in animals treated with cannabinoids and tied this to the anti-inflammatory effects that are observed.
Both endogenous and exogenous cannabinoids have been found to exert immunosuppressive effects via microRNA regulation of inflammatory targets (D’Addario et al. 2013). AEA is an endocannabinoid that acts on the same receptors (CB1 and CB2) as exogenous cannabinoids such as THC (Sido et al. 2014). AEA is most concentrated in the CNS but is also present in the periphery. As such, AEA treatment was found to reduce the production of inflammatory cytokines such as IL-17 and IFN-γ and increase the anti-inflammatory cytokine IL-10 in the draining lymph nodes in a murine model of delayed type hypersensitivity (Jackson et al. 2014a). The lymph nodes from AEA treated mice had a markedly altered profile of microRNA expression with dysregulation of 100 of 609 microRNAs. A subset of the upregulated microRNAs targeted members of proinflammatory pathways, offering a mechanism for the observed reduction in Th17 cells, which are proinflammatory, following AEA treatment. Exogenous cannabinoids, such as THC, which utilize the same receptors as endocannabinoids, can thus be predicted to induce an antiinflammatory state by similar mechanisms.
Activation of cannabinoid receptors CB1 and CB2 can trigger immunosuppression by a very strong induction of MDSCs, as demonstrated by administration of THC to mice (Hegde et al. 2010). This induction is mediated by the chemokines, G-CSF and CXCL1. Importantly, microRNAs were also found to play a critical role in the THC mediated induction of MDSCs (Hegde et al. 2013). The distinct immunosuppressive microRNA profile of 13 differentially expressed microRNAs in MDSCs from THC treated mice was shown to target transcription factors with known roles in hematopoiesis and myeloid cell differentiation, such as RUNX1, PU.1, C/EBPα and c-JUN. One of the dysregulated microRNAs in particular, miR-690, was highly expressed in THC-induced MDSCs and was shown to regulate C/EBPα. This is significant because C/EBPα is an important transcription factor in development and terminal differentiation of granulocyte-monocyte progenitors (Collins et al. 2001) (Fukuchi et al. 2006) (Wang et al. 2006).
Another study examining the effects of in utero immune activation followed by adolescent cannabinoid exposure on neuropathology associated with schizophrenia yielded findings with potential neuro-immunological significance (Hollins et al. 2014). There is some evidence that adolescent cannabis use is one of numerous possible environmental insults that could bring on neuropathologies such as schizophrenia in humans (Dudley et al. 2011). This study explored microRNA biogenesis disturbances induced by cannabinoids using a rat model and found an alteration in expression of microRNAs, many of which came from a single imprinted locus. This genomic region in the rat, containing the Dlk1-Dio3 microRNA cluster, encodes a large number of microRNAs which are also encoded by the syntenic human locus (14q32). Interestingly, these microRNAs are differentially expressed in the peripheral blood lymphocytes of schizophrenia patients, pointing to an interrelatedness of the neurological and immunological consequences of cannabinoid exposure (Gardiner et al. 2012).
Regulation of microRNA appears to be a mechanism by which cannabinoids reduce inflammation, not only in murine models, but in primate models as well. In a recent study, THC was regularly administered to SIV-infected rhesus macaques to study the impact on intestinal inflammation, a common problem in both SIV infected macaques and HIV infected humans (Chandra et al. 2014). THC treatment resulted in a slower SIV disease progression with reduced viral replication and inflammation. A number of microRNAs known to target pro-inflammatory targets were upregulated, including: miRs-10a, −24, −99b, −145, −149 and −187. miR-99b in particular targeted NOX4, resulting in downregulation of this protein known for inducing damage to intestinal epithelial cells by an oxidative stress mechanism and which is also known to be regulated by cannabidiol (McKallip et al. 2006). In another study looking at THC treatment of SIV infected animals, microRNA was found to be alternatively expressed in CD4+ T cells, as well as other tissues (Molina et al. 2011). Specifically, this study found that miR-21, a micro-RNA associated with inflammation, was downregulated upon THC treatment. That THC has been found to regulate inflammation via microRNA in a primate model strongly indicates that such mechanisms are also likely to take effect following human exposure to exogenous cannabinoids such as those present in marijuana.
DNA Methylation
Nearly a decade ago, it was hypothesized that cannabinoid exposure might impact DNA methlylation (Onaivi et al. 2006). However, the data on the effects of human in utero exposure to drugs of abuse on DNA methylation is extremely limited, and completely absent with regards to marijuana (Morris et al. 2011). DNA methylation, which results in suppression of transcription, involves the covalent modification of DNA with methyl groups at CpG islands in the promoter region of genes, carried out by DNA methyltransferases. Interestingly, human in utero exposure to tobacco is known to alter DNA methylation of specific genetic loci within the placenta, including CYP1A1, which has known roles in inflammation (Suter et al. 2010). However, with regard to cannabinoids, the scant studies of their impact on DNA methylation are limited to in vitro assays or animal models (Shirazi et al. 2013). AEA treatment of human keratinocytes was found to result in an inhibition in differentiation by increasing in DNA methylation in a p38 and p42/44 mitogen-activated protein kinase-dependent manner triggered by CB1 activation (Paradisi et al. 2008). These alterations surely impact the expression of numerous genes, including immunologically relevant pathways, although further characterization is needed. Furthermore, in THC treated SIV infected rhesus macaques, at least half of the differentially expressed genes had altered DNA methylation, as compared to vehicle-treated SIV infected animals (Molina et al. 2011). Importantly, a subset of the hypermethylated genes in this study was involved in inflammation, such as C/EBPD, which in turn regulates the expression of IL-1, IL-6 and TNF-α. Therefore, DNA methylation appears to be an important mechanism by which cannabinoids impact the immune system and further investigation into methylation changes of immunologically relevant genes during in utero cannabinoid exposure is needed.
Histone Modification
While extremely limited information exists on the effect of cannabinoids on histone modification, a couple of recent studies point to histone modification as a mechanism impacting immunological and neurological functions (DiNieri et al. 2011; Yang et al. 2014). Post-translational modification of the core histone proteins (H3, H4, H2A and H2B) alters chromatin structure, which results in activation or repression of the associated gene. Modifications can include methylation, acetylation, phosphorylation and ubiquitination, among others, and occur most frequently on specific amino acid residues. For example, histone modifications such as H3K4me3 and H3K36me3 are associated with gene activation, and H3K9me3 and H3K27me3 involved in gene repression, may also play a role in cell differentiation. Studies have shown that histone modifications can affect CD4+ Tcell differentiation at the Inf-γ locus in Th1, the Il-4 locus in Th2 and the Il-17 locus in Th17 cells (Ansel et al. 2006; Hatton et al. 2006; Wei et al. 2009).
Inasmuch as THC exposure in mice is known to alter the CD4+ T cell differentiation profile, the role of histone modification in this model has been explored. Mice exposed to staphylococcal enterotoxin B (SEB), a superantigen, results in a massive upregulation of Th1 CD4 subset and a cytokine storm. THC treatment of these mice led to suppressive histone markings in the Th1 associated genes and activating histone marks of Th2 associated genes (Yang et al. 2014). This suggests that modulation of histone markings is a mechanism by which THC induces the shift from a pro-inflammatory Th1 profile to an anti-inflammatory Th2 profile. H3K4me3, H3K27me3 and H3K36me3 were all found to be altered in key Th1/Th2 cytokine genes that are affected by THC treatment. While the amount of histone methylation and acetylation markings was also altered at the global level, the overall distribution was not significantly changed, suggesting that THC has a pleiotropic effect on gene expression, potentially impacting many cellular functions. Interestingly, THC treatment also altered histone methylation signals in the transcriptional start sites of immunologically relevant microRNAs.
Other evidence exists demonstrating that histone modification plays an important role in the mechanism by which cannabinoids exert immunological effects. For instance, cannabinoid receptor agonists can increase the number of H3K9me3 positive glioma stem cells, an effect which is blocked by CB agonists (Aguado et al. 2007). Additionally, in Huntingdon’s disease, there is an overall increase in H3 acetylation and a decreased level of CB1 (Sadri-Vakili et al. 2007). THC has also been found to alter histone deacetylase three in a dose dependent manner as wells as PI3K/AKT signaling, a pathway known to impart global effects on H3K27me3 (Khare et al. 2006; Zuo et al. 2011). Finally, studies with CB1 knock-out mice revealed a role for CB1 in the regulation of chromatin remodeling during spermatogenesis. Thus, while the research on the epigenetic regulation of immunological effects of cannabinoids is clearly at its infancy, there are numerous lines of evidence tying in histone modification as an underlying regulatory mechanism.
As far as research on epigenetic changes induced on progeny, following a prenatal exposure to cannabinoids, there is a single published study, albeit from a neurological perspective (DiNieri et al. 2011). DiNieri et al. demonstrated that prenatal THC exposure of rats resulted reduced dopamine D2 gene expression via alteration of 2meH3K9 and 3meH3K4 markings in progeny. The prenatally exposed rats also had reduced dopamine D2 receptor binding sites and were more sensitive to opiate rewards as adults. Therefore, prenatal exposure to cannabinoids can induce long term epigenetic changes that persist into adulthood, likely setting the stage for enhanced addictive behavior and possibly as yet unknown immunological consequences such as increased susceptibility to infections or cancers.
Transgenerational Effects of Cannabinoid Exposure
There are currently very few studies looking at the transgenerational effects of cannabinoid exposure and none that explore the potential immunological consequences. It is known, in humans, that in utero cannabinoid exposure is associated with a myriad of neurological and behavioral consequences for the individual as both a child and adolescent, including an increased tendency for drug abuse (Jaques et al. 2014). Interestingly, adolescent female rats given a brief exposure to cannabinoid agonist WIN-55,212, after a drug free period had progeny that had a significantly enhanced response to morphine (Vassoler et al. 2013). The progeny had complete absence of in utero exposure to the cannabinoid agonist, yet had behavioral, gene transcriptional and endocrine alterations. This study offers clues underlying the altered response to drugs of abuse, such as opiates, seen in humans. These results also suggest that exposure to cannabis, even well before pregnancy, may have a detrimental effect on offspring. It is known that female reproductive tissues including the ovaries express CB1 and endocannabinoids (Bari et al. 2011) and that in males, THC can disrupt gonadal functions (Banerjee et al. 2011). Therefore, it is not unreasonable to suspect that cannabinoids could impact the progeny following either maternal or paternal exposure. The epigenetic mechanisms described in this review offer a potential process by which transgenerational effects may occur.
Conclusion
Marijuana is being legalized for recreational purposes, and is used by adolescents, adults of child-bearing age and women who are breastfeeding. Cannabis clearly has important neurological consequences. However, research into the long term impact of cannabinoids on the immune system and neuroimmunology is emerging. Studies on the potential maternal and paternal transgenerational and/or epigenetic effects of cannabinoid abuse are also critical and urgently needed. As cannabis is legalized in more states, surely a proliferation of products containing the drug will follow suit. The results of such research will have far reaching consequences on human health and therefore aid in guiding future medical and public policy.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- Aguado T, Carracedo A, Julien B, Velasco G, Milman G, Mechoulam R, Alvarez L, Guzman M, Galve-Roperh I. Cannabinoids induce glioma stem-like cell differentiation and inhibit gliomagenesis. J Biol Chem. 2007;282:6854–6862. doi: 10.1074/jbc.M608900200. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Araki Y, Wang Z, Zang C, Wood WH, 3rd, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, Becker KG, Zhao K, Weng NP. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Res B Dev Reprod Toxicol. 2011;92:195–205. doi: 10.1002/bdrb.20295. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Pirazzi V, Maccarrone M. Themanifold actions of endocannabinoids on female and male reproductive events. Front Biosci. 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Bar-Oz B, Klein J, Karaskov T, Koren G. Comparison of meconium and neonatal hair analysis for detection of gestational exposure to drugs of abuse. Arch Dis Child Fetal Neonatal Ed. 2003;88:F98–F100. doi: 10.1136/fn.88.2.F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Behnke M, Smith VC Committee on Substance A, Committee on F, Newborn. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009–e1024. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E, Thysen B, Morrill GA, Gardner E, Fujimoto G. Effects of cannabinoids on reproduction and development. Vitam Horm. 1978;36:203–258. doi: 10.1016/s0083-6729(08)60985-1. [DOI] [PubMed] [Google Scholar]
- Bluhm EC, Daniels J, Pollock BH, Olshan AF Children’s Oncology G. Maternal use of recreational drugs and neuroblastoma in offspring: a report from the children’s oncology group (United States) Cancer Causes Control. 2006;17:663–669. doi: 10.1007/s10552-005-0580-3. [DOI] [PubMed] [Google Scholar]
- Bonnin A, de Miguel R, Castro JG, Ramos JA, Fernandez-Ruiz JJ. Effects of perinatal exposure to delta 9-tetrahydrocannabinol on the fetal and early postnatal development of tyrosine hydroxylase-containing neurons in rat brain. J Mol Neurosci. 1996;7:291–308. doi: 10.1007/BF02737066. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on maternal immune response during pregnancy. Arch Toxicol. 2004;78:290–300. doi: 10.1007/s00204-003-0538-8. [DOI] [PubMed] [Google Scholar]
- Chandra LC, Kumar V, Torben W, Stouwe CV, Winsauer P, Amedee A, Molina PE, Mohan M. Chronic administration of Delta9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute SIV infection of rhesus macaques. J Virol. 2014 doi: 10.1128/JVI.01754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SJ, Ulmer J, Purton LE, Darlington G. Multipotent hematopoietic cell lines derived from C/EBPalpha(−/−) knockout mice display granulocyte macrophage-colony-stimulating factor, granulocyte- colony-stimulating factor, and retinoic acid-induced granulocytic differentiation. Blood. 2001;98:2382–2388. doi: 10.1182/blood.v98.8.2382. [DOI] [PubMed] [Google Scholar]
- D’Addario C, Di Francesco A, Pucci M, Finazzi Agro A, Maccarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280:1905–1917. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci US A. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Arco I, Munoz R, Rodriguez De Fonseca F, Escudero L, Martin-Calderon JL, Navarro M, Villanua MA. Maternal exposure to the synthetic cannabinoid HU-210: effects on the endocrine and immune systems of the adult male offspring. Neuroimmunomodulation. 2000;7:16–26. doi: 10.1159/000026416. [DOI] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–2382. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]
- Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci Biobehav Rev. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Fukuchi Y, Shibata F, Ito M, Goto-Koshino Y, Sotomaru Y, Ito M, Kitamura T, Nakajima H. Comprehensive analysis of myeloid lineage conversion using mice expressing an inducible form of C/EBP alpha. EMBO J. 2006;25:3398–3410. doi: 10.1038/sj.emboj.7601199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil L, de Miguel R, Romero J, Perez A, Ramos JA, Fernandez-Ruiz JJ. Perinatal delta9-tetrahydrocannabinol exposure augmented the magnitude of motor inhibition caused by GABA(B), but not GABA(A), receptor agonists in adult rats. Neurotoxicol Teratol. 1999;21:277–283. doi: 10.1016/s0892-0362(98)00058-0. [DOI] [PubMed] [Google Scholar]
- Gardiner E, Beveridge NJ, Wu JQ, Carr V, Scott RJ, Tooney PA, Cairns MJ. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2012;17:827–840. doi: 10.1038/mp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254–263. doi: 10.1097/CHI.0b013e318160b3f0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford JA, Severtson SG, Day NL. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol. 2012;34:161–167. doi: 10.1016/j.ntt.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KA, Day NL, Leech S, Richardson GA. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol. 2005;27:439–448. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Harbison RD, Mantilla-Plata B. Prenatal toxicity, maternal distribution and placental transfer of tetrahydrocannabinol. J Pharmacol Exp Ther. 1972;180:446–453. [PubMed] [Google Scholar]
- Hatch EE, Bracken MB. Effect of marijuana use in pregnancy on fetal growth. Am J Epidemiol. 1986;124:986–993. doi: 10.1093/oxfordjournals.aje.a114488. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Palmer JR, Titus-Ernstoff L, Noller KL, Kaufman RH, Mittendorf R, Robboy SJ, Hyer M, Cowan CM, Adam E, Colton T, Hartge P, Hoover RN. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA. 1998;280:630–634. doi: 10.1001/jama.280.7.630. [DOI] [PubMed] [Google Scholar]
- Hatton RD, Harrington LE, Luther RJ, Wakefield T, Janowski KM, Oliver JR, Lallone RL, Murphy KM, Weaver CT. A distal conserved sequence element controls Ifng gene expression by Tcells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Kingsbury AM, Flenady V, Gilshenan KS, Hutchinson DM, Najman JM. Illicit drug use before and during pregnancy at a tertiary maternity hospital 2000–2006. Drug Alcohol Rev. 2011;30:181–187. doi: 10.1111/j.1465-3362.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, Najman JM. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215–219. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–3371. doi: 10.1002/eji.201040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. doi: 10.1371/journal.pone.0018281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Tomar S, Jackson A, Rao R, Yang X, Singh UP, Singh NP, Nagarkatti PS, Nagarkatti M. Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Delta9-tetrahydrocannabinol in vivo: regulation of CCAAT/enhancer-binding protein alpha by microRNA-690. J Biol Chem. 2013;288:36810–36826. doi: 10.1074/jbc.M113.503037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Alpert JJ, Day N, Dooling E, Kayne H, Morelock S, Oppenheimer E, Zuckerman B. Effects of maternal drinking and marijuana use on fetal growth and development. Pediatrics. 1982;70:539–546. [PubMed] [Google Scholar]
- Hollins SL, Zavitsanou K, Walker FR, Cairns MJ. Alteration of imprinted Dlk1-Dio3 miRNA cluster expression in the entorhinal cortex induced by maternal immune activation and adolescent cannabinoid exposure. Transl Psychiatry. 2014;4:e452. doi: 10.1038/tp.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS One. 2014a;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AR, Hegde VL, Nagarkatti PS, Nagarkatti M. Characterization of endocannabinoid-mediated induction of myeloid-derived suppressor cells involving mast cells and MCP-1. J Leukoc Biol. 2014b;95:609–619. doi: 10.1189/jlb.0613350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques SC, Kingsbury A, Henshcke P, Chomchai C, Clews S, Falconer J, Abdel-Latif ME, Feller JM, Oei JL. Cannabis, the pregnant woman and her child: weeding out the myths. J Perinatol Off J Calif Perinatal Assoc. 2014;34:417–424. doi: 10.1038/jp.2013.180. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kennedy JS, Waddell WJ. Whole-body autoradiography of the pregnant mouse after administration of 14 C-9-THC. Toxicol Appl Pharmacol. 1972;22:252–258. doi: 10.1016/0041-008x(72)90175-5. [DOI] [PubMed] [Google Scholar]
- Khare M, Taylor AH, Konje JC, Bell SC. Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Mol Hum Reprod. 2006;12:321–333. doi: 10.1093/molehr/gal036. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–115. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti PS. Targeting cannabinoid receptors to treat leukemia: role of cross-talk between extrinsic and intrinsic pathways in Delta9-tetrahydrocannabinol (THC)-induced apoptosis of Jurkat cells. Leuk Res. 2005;29:915–922. doi: 10.1016/j.leukres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard C, Hegde VL, Nagarkatti M, Nagarkatti PS. Perinatal exposure to Delta9-tetrahydrocannabinol triggers profound defects in T cell differentiation and function in fetal and postnatal stages of life, including decreased responsiveness to HIV antigens. J Pharmacol Exp Ther. 2011;339:607–617. doi: 10.1124/jpet.111.181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286:S60–S65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012a;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012b;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, Nagarkatti PS, Nagarkatti M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174:3281–3289. doi: 10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Molina PE, Amedee A, LeCapitaine NJ, Zabaleta J, Mohan M, Winsauer P, Vande Stouwe C. Cannabinoid neuroimmune modulation of SIV disease. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol. 2011;6:516–527. doi: 10.1007/s11481-011-9301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinobu A, Kanno Y, O’Shea JJ. Discrete roles for histone acetylation in human T helper 1 cell-specific gene expression. J Biol Chem. 2004;279:40640–40646. doi: 10.1074/jbc.M407576200. [DOI] [PubMed] [Google Scholar]
- Morris CV, DiNieri JA, Szutorisz H, Hurd YL. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur J Neurosci. 2011;34:1574–1583. doi: 10.1111/j.1460-9568.2011.07884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti M, Rieder SA, Hegde VL, Kanada S, Nagarkatti P. Do cannabinoids have a therapeutic role in transplantation? Trends Pharmacol Sci. 2010;31:345–350. doi: 10.1016/j.tips.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–S17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- Newsom RJ, Kelly SJ. Perinatal delta-9-tetrahydrocannabinol exposure disrupts social and open field behavior in adult male rats. Neurotoxicol Teratol. 2008;30:213–219. doi: 10.1016/j.ntt.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Sugiura T, Di Marzo V. Endocannabinoids the brain and body’s marijuana and beyond. Boca Raton: Taylor & Francis; 2006. In, p 1 online resource (563 p.) ill. [Google Scholar]
- Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Endocannabinoids and immune regulation. Pharmacol Res Off J Ital Pharmacol Soc. 2009a;60:85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Hegde VL, Singh NP, Hofseth L, Singh U, Ray S, Nagarkatti M, Nagarkatti PS. Use of cannabinoids as a novel therapeuticmodality against autoimmune hepatitis. Vitam Horm. 2009b;81:487–504. doi: 10.1016/S0083-6729(09)81019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Hegde VL, Nagarkatti M, Nagarkatti PS. Targeting cannabinoid receptors as a novel approach in the treatment of graft-versus-host disease: evidence from an experimental murine model. J Pharmacol Exp Ther. 2011;338:819–828. doi: 10.1124/jpet.111.182717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi A, Pasquariello N, Barcaroli D, Maccarrone M. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J Biol Chem. 2008;283:6005–6012. doi: 10.1074/jbc.M707964200. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today. 2013;99:134–146. doi: 10.1002/bdrc.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010;215:598–605. doi: 10.1016/j.imbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LL, Buckley JD, Daigle AE, Wells R, Benjamin D, Arthur DC, Hammond GD. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group) Cancer. 1989;63:1904–1911. [PubMed] [Google Scholar]
- Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp Neurol. 2010;224:92–102. doi: 10.1016/j.expneurol.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Bouzou B, Benn CL, Kim MO, Chawla P, Overland RP, Glajch KE, Xia E, Qiu Z, Hersch SM, Clark TW, Yohrling GJ, Cha JH. Histones associated with downregulated genes are hypoacetylated in Huntington’s disease models. Hum Mol Genet. 2007;16:1293–1306. doi: 10.1093/hmg/ddm078. [DOI] [PubMed] [Google Scholar]
- Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr J. 2009;56:131–139. doi: 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- Schou J, Prockop LD, Dahlstrom G, Rohde C. Penetration of delta-9-tetrahydrocannabinol and 11-OH-delta-9-tetrahydrocannabinol through the blood–brain barrier. Acta Pharmacol Toxicol. 1977;41:33–38. doi: 10.1111/j.1600-0773.1977.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Shirazi J, Shah S, Sagar D, Nonnemacher MR, Wigdahl B, Khan ZK, Jain P. Epigenetics, drugs of abuse, and the retroviral promoter. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol. 2013;8:1181–1196. doi: 10.1007/s11481-013-9508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sido JM, Nagarkatti PS, Nagarkatti M. Role of endocannabinoid activation of peripheral CB1 receptors in the regulation of autoimmune disease. International reviews of immunology. 2014 doi: 10.3109/08830185.2014.921165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh US, Nagarkatti M, Nagarkatti PS. Resveratrol (3,5,4′-trihydroxystilbene) protects pregnant mother and fetus from the immunotoxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Nutr Food Res. 2011;55:209–219. doi: 10.1002/mnfr.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Guan H, Nagarkatti P, Nagarkatti M. Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PLoS One. 2012a;7:e45054. doi: 10.1371/journal.pone.0045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(−/−) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol. 2012b;258:256–267. doi: 10.1016/j.taap.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smita K, Sushil Kumar V, Premendran JS. Anandamide: an update. Fundam Clin Pharmacol. 2007;21:1–8. doi: 10.1111/j.1472-8206.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- Smith CG, Asch RH. Drug abuse and reproduction. Fertil Steril. 1987;48:355–373. doi: 10.1016/s0015-0282(16)59400-x. [DOI] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Fernandez-Ruiz J, Ramos JA, Rubio M, Fernandez B. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre- and perinatal delta9-tetrahydrocannabinol exposure. Cerebellum. 2004a;3:66–74. doi: 10.1080/14734220310017230. [DOI] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Rubio M, Fernandez-Ruiz JJ, Ramos JA, Fernandez B. Prenatal cannabinoid exposure down-regulates glutamate transporter expressions (GLAST and EAAC1) in the rat cerebellum. Dev Neurosci. 2004b;26:45–53. doi: 10.1159/000080711. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabc Clin Exp. 2010;59:1481–1490. doi: 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215:588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Johnson NL, Byrnes EM. Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. J Psychopharmacol. 2013;27:1015–1022. doi: 10.1177/0269881113503504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BE, Haven MI. Intensity of multigenerational carcinogenesis from diethylstilbestrol in mice. Carcinogenesis. 1997;18:791–793. doi: 10.1093/carcin/18.4.791. [DOI] [PubMed] [Google Scholar]
- Wang J, Paria BC, Dey SK, Armant DR. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol Reprod. 1999;60:839–844. doi: 10.1095/biolreprod60.4.839. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- Wang D, D’Costa J, Civin CI, Friedman AD. C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108:1223–1229. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O’Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, Turner RT. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci U S A. 1997;94:4199–4204. doi: 10.1073/pnas.94.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Jew CP, Lu HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Futur Neurol. 2011;6:459–480. doi: 10.2217/fnl.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M. Histone modifications are associated with Delta9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem. 2014;289:18707–18718. doi: 10.1074/jbc.M113.545210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Friedman H, Klein TW. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. J Pharmacol Exp Ther. 1998;286:1103–1109. [PubMed] [Google Scholar]
- Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, Dubinett SM. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. 2000;165:373–380. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- Zuo T, Liu TM, Lan X, Weng YI, Shen R, Gu F, Huang YW, Liyanarachchi S, Deatherage DE, Hsu PY, Taslim C, Ramaswamy B, Shapiro CL, Lin HJ, Cheng AS, Jin VX, Huang TH. Epigenetic silencing mediated through activated PI3K/AKT signaling in breast cancer. Cancer Res. 2011;71:1752–1762. doi: 10.1158/0008-5472.CAN-10-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]