Abstract
Purpose
Progression or recurrence due to resistance to aromatase inhibitors (AIs) is a significant clinical problem for a considerable number of patients with breast cancer. Program cell death 4 (PDCD4), a tumor suppressor protein, is targeted for degradation during tumor progression. We aimed to examine PDCD4 expression and regulation in AI-resistant breast cancer cells and association with survival in estrogen receptor (ER)-positive breast cancer patients.
Methods
We determined PDCD4 expression levels in AI-resistant breast cancer cell lines and ER-positive breast cancer tumors, investigated the regulation of PDCD4 in AI-resistant breast cancer cell lines, and carried out a Kaplan-Meier survival analysis in two independent cohorts that included a total of 420 patients with ER-positive breast cancer.
Results
PDCD4 expression was down-regulated in AI-resistant breast cancer cells, and this down-regulation was inversely correlated with activation of HER2 signaling. Moreover, lower expression of PDCD4 was significantly associated with HER2 positive status in ER-positive breast tumors. Down-regulation of PDCD4 was mediated through up-regulation of HER2 via the mitogen-activated protein kinase (MAPK), protein kinase B (PKB/AKT), and miR-21 in AI-resistant breast cancer cells. miR-21 inhibitor and fulvestrant induced PDCD4 expression and decreased cell proliferation in AI-resistant breast cancer cells. Furthermore, forced overexpression of PDCD4 resensitized AI-resistant cells to AI or hormone deprivation. Finally, we identified that down-regulation of PDCD4 was associated with a lower rate of disease-free survival in ER-positive breast cancer and higher histologic grade of breast tumors.
Conclusions
Expression of PDCD4 is down-regulated by HER2 signaling in AI-resistant breast cancer cells. Down-regulation of PDCD4 is associated with AI resistance and a poor prognosis in patients with ER-positive breast cancer.
Keywords: PDCD4, aromatase inhibitor, HER2, miR-21, breast cancer, prognosis
1. Introduction
Endocrine therapy plays a central role in the treatment of patients with estrogen receptor (ER)-positive advanced breast cancer. In postmenopausal patients, selective aromatase inhibitors (AIs) (i.e., letrozole, anastrozole, and exemestane), which block the peripheral conversion of androgens into estrogens and reduce the estrogen levels, are used as first-line treatment [1]. Unfortunately, resistance to AI treatment is present in a considerable number of patients, who may have no response to AIs (primary or de novo resistance) or will eventually relapse despite an initial response (acquired resistance) [1].
There is increasing evidence suggesting that cross-talk between the ER and the human epidermal growth factor receptor 2 (HER2) signaling is involved in the development of resistance to AIs.
ER can interact with and activate HER2 and its downstream signaling intermediates, such as the mitogen-activated protein kinase (MAPK) and protein kinase B (PKB/AKT) [2]. On the other hand, activation of HER2 signaling pathway, including the MAPK and phosphatidylinositol 3'-kinase (PI3K)/AKT, can phosphorylate and activate ER in a ligand-independent manner [3,4], which has been implicated in endocrine therapy resistance [5–7]. In patients treated with AIs, HER2 signaling is often up-regulated in breast tumors [8]. These findings suggest that targeting the ER pathway may lead to the up-regulation of HER2 pathway due to the extensive cross-talk, and ultimately results in endocrine resistance.
Program cell death 4 (PDCD4) is a tumor suppressor protein that was originally found to be induced by apoptosis [9,10]. PDCD4 binds to the translation initiation factor eIF4A and inhibits its RNA-helicase activity, thus inhibiting protein translation [11,12]. PDCD4 has been linked to tumorigenesis and tumor progression [13,14], and its expression is decreased in several type of cancers, including lung, colon, liver and breast cancers [15–18]. Like the well-known tumor suppressor phosphatase and tensin homolog (PTEN) [19], PDCD4 has been established as an important functional target of the oncogenic microRNA, miR-21, which is commonly up-regulated in solid tumors and contributes to the down-regulation of PDCD4 [20,21].
Translational abberations are known to be associated with a poor prognosis in hormone receptor-positive breast cancer [22]. The expression of PDCD4, a translation inhibitor, is decreased during the progression of many cancers. We thus hypothesized that PDCD4 could be down-regulated during the development of AI resistance in breast cancer. In the current study, we found that PDCD4 expression was down-regulated by HER2 signaling in AI-resistant breast cancer and associated with survival outcomes in patients with ER-positive breast cancer.
2. Materials and Methods
2.1. Cell lines
Human breast cancer cell line MCF7 derived cell lines MCF7aro, LTEDaro, LET-R, HER2aro, AKTaro were generated in this laboratory and were characterized and described previously [23–25]. Details of culture conditions are provided in the Supplementary Appendix.
2.2. Antibodies and reagents
Antihuman PDCD4 (#9535), PTEN (#9188), p-HER2 (Tyr1248) (#2247), p-MAPK (#9101), MAPK (#9102), p-AKT (Ser473) (#9271), AKT (#9272), GAPDH (#2118) antibodies were obtained from Cell Signaling Technology. Antihuman HER2 antibody (#06–562) was from Abcam Inc. Antihuman ERα (HC-20) antibody (sc-543) was from Santa Cruz Biotechnology. The p44/42 MAPK siRNA (#6560) was obtained from Cell Signaling Technology. The nontargeting control siRNA (sc-37007) was from Santa Cruz Biotechnology. The AKT inhibitor MK-2206 was obtained from Selleck Chemicals. The ER antagonist fulvestrant was from Sigma-Aldrich. The anti-miR-21 inhibitor and inhibitor negative control were purchased from Ambion.
2.3. Plasmid construct
The DNA fragment coding human PDCD4 was generated by RT-PCR using RNA from MCF7 cells as the template and the following oligonucleotides as the PCR primers (forward primer: 5’-CTGGGATCCCACCATGGATGTAGAAAATGAGCAG-3’; reverse primer: 5’- GGGGCTAGCTCAGTAGCTCTCTGGTTTAAGACG -3’). The interest fragment was then cloned into the mammalian expression vector pMG-H2 using Bgl II-Nhe I restriction sites. The insert was verified by DNA sequencing.
2.4. Western blotting
Western blotting was performed as previously described [26]. Relative expression of proteins was normalized against the internal control GAPDH. Details are provided in the Supplementary Appendix.
2.5. Real-time PCR
The human PDCD4 gene was amplified using the forward primer 5′-ACAGGTGTATGATGTGGAGGA-3′ and the reverse primer 5′-TTCTCAAATGCCCTTTCATCCAA-3′ (PrimerBank ID 313760536c3) [27]. The ACTB (β-actin) gene was amplified using the forward primer 5′-CACCAACTGGGACGACAT-3′ and the reverse primer 5′- GCACAGCCTGGATAGCAAC-3′. The miR-21 and U6 RNA were amplified using miR-21 or U6 RNA specific primer in conjunction with the universal PCR primer, all purchased from Quanta Biosciences. Experimental details are provided in the Supplementary Appendix.
2.6. Transfection
Details of transfection of cells with small interfering RNA (siRNA), estrogen responsive element (ERE) reporter plasmid, and human PDCD4 construct are provided in the Supplementary Appendix.
2.7. Luciferase reporter assay
Details of luciferase reporter assay are provided in the Supplementary Appendix.
2.8. Cell proliferation assay
Cell proliferation was measured by an MTT assay. Experimental details are provided in the Supplementary Appendix.
2.9. Gene expression analysis
PDCD4 differential expression analysis was performed in The Cancer Genome Atlas (TCGA) cohort in breast cancer patients with ER and HER status [28]. The differential expression p-values were determined via t-test using R [29] (http://www.R-project.org/). Disease-free survival analyses according to PDCD4 expression were determined in two independent prospective cohorts in breast cancer patients with ER status and disease-free survival outcomes [30,31]. The high PDCD4 expression and low PDCD4 expression groups were segregated based on median expression values. Kaplan-Meier survival analysis was used to determine the disease-free survival differences between the high PDCD4 expression and low PDCD4 expression groups among patients with ER-positive breast cancer in two cohorts, and visualized by Kaplan-Meier plots and compared using Cox regression analysis, with p-values calculated by log-rank test using the survival package in R [29]. Details are provided in the Supplementary Appendix.
3. Results
3.1. Down-regulation of PDCD4 expression is inversely correlated with activation of HER2 signaling in AI-resistant breast cancer cells and ER-positive breast cancer tumors
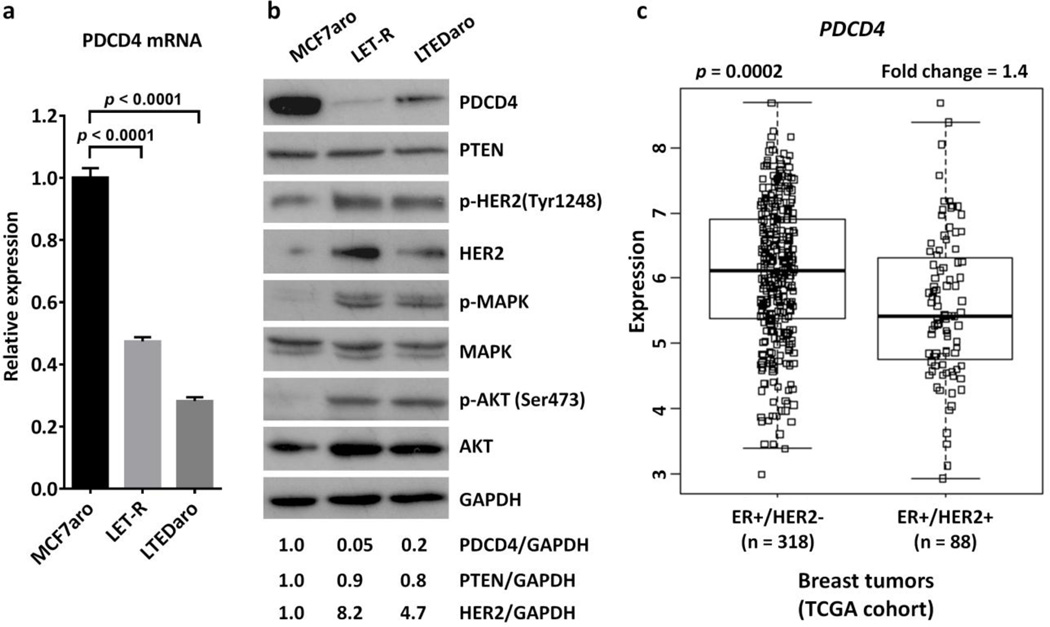
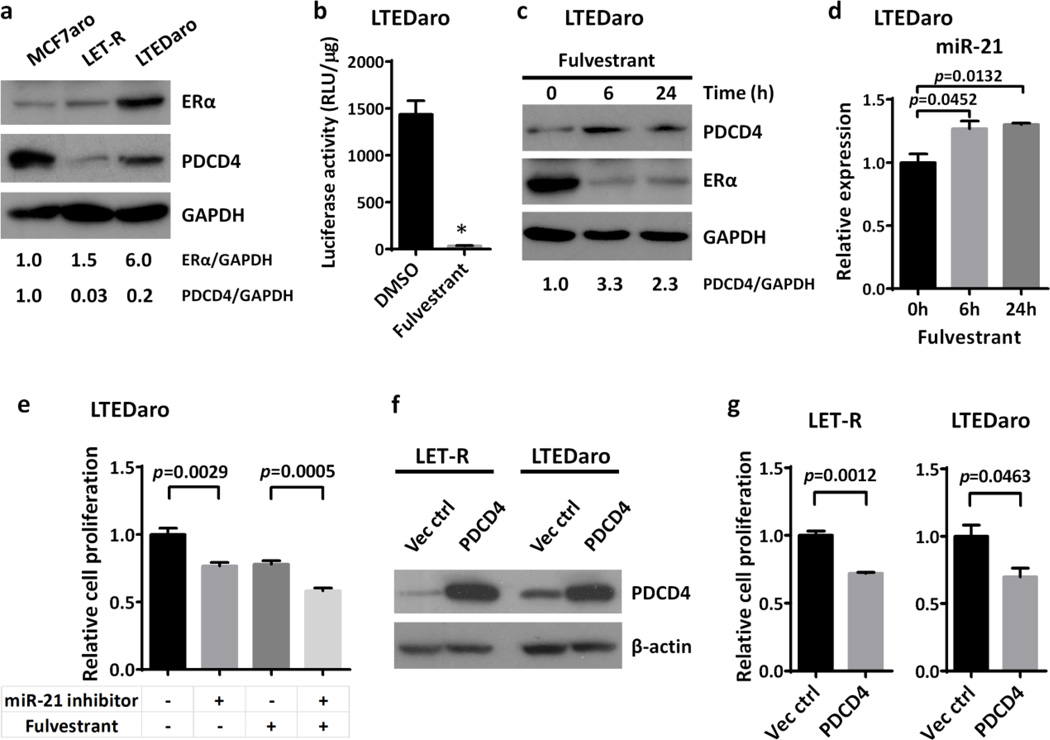
PDCD4, a tumor suppressor protein, has been linked to tumor progression [14]. To test our hypothesis that PDCD4 is down-regulated when breast cancer becomes resistant to AIs, we evaluated the mRNA and protein levels of PDCD4 in LET-R, a MCF7aro-derived breast cancer cell line resistant to the AI letrozole, and LTEDaro, a MCF7aro-derived breast cancer cell line after long-term estrogen deprivation. We found that both the mRNA and protein levels of PDCD4 were significantly decreased in LET-R and LTED cells, as compared with the MCF7aro parental cells (Fig. 1a–b). In contrast, the levels of PTEN, another tumor suppressor protein, were not significantly changed in LET-R or LTEDaro cells (Fig. 1b).
Fig. 1. Down-regulation of PDCD4 is inversely correlated with activation of HER2 signaling in aromatase inhibitor-resistant breast cancer cells and ER-positive breast cancer tumors.
a. Real time PCR analysis of PDCD4 expression in MCF7aro, LET-R and LTEDaro cells cultured in defined culture media described in the Materials and Methods. Values were normalized to ACTB and plotted relative to the expression of the MCF7aro cells. Data are means ± SE (n=3).
b. Western blotting analysis of PDCD4, PTEN and the activation of the HER2, MAPK and AKT signaling pathways in MCF7aro, LET-R and LTEDaro cells. The quantification of PDCD4 was normalized to the loading control GAPDH, then normalized to the MCF7aro cells.
c. Gene expression analysis in 406 patients with ER-positive breast cancer in the TCGA cohort. Differential expression analysis for PDCD4 shows a lower level of PDCD4 in patients with ER+ and HER2+ breast tumors than in patients with ER+ and HER2- breast tumors.
Because the up-regulation of HER2 signaling pathway, including the MAPK and PI3K/AKT, has been implicated in endocrine therapy resistance [5–7], we sought to determine whether PDCD4 expression is correlated with HER2 signaling in AI-resistant breast cancer cells. To investigate the activation state of the HER2 pathways in our LET-R and LTEDaro cell lines, we evaluated the levels of phosphorylated and total HER2, MAPK and AKT by Western blotting. As shown in Fig. 1b, total HER2 expression and phospho-HER2, phospho-MAPK and phospho-AKT were increased in both LTEDaro and LET-R cells as compared with MCF7aro cells, which were inversely correlated with the PDCD4 expression.
To further determine the correlation between PDCD4 and HER2 signaling pathways, we performed a differential expression analysis for PDCD4 in 406 patients with ER-positive breast cancer in the TCGA cohort [28], including 318 patients with ER-positive and HER2-negative (ER+ and HER2−) breast tumors, and 88 patients with ER-positive and HER2-positive (ER+ and HER2+) breast tumors. We found that lower expression of PDCD4 was significantly associated with HER2 positive status (p = 0.0002, fold change = 1.4), supporting that down-regulation of PDCD4 is inversely correlated with the levels of HER2 in ER-positive breast cancer (Fig. 1c).
3.2. Up-regulation of HER2 and MAPK signaling inhibits PDCD4 expression in AI-resistant breast cancer cells
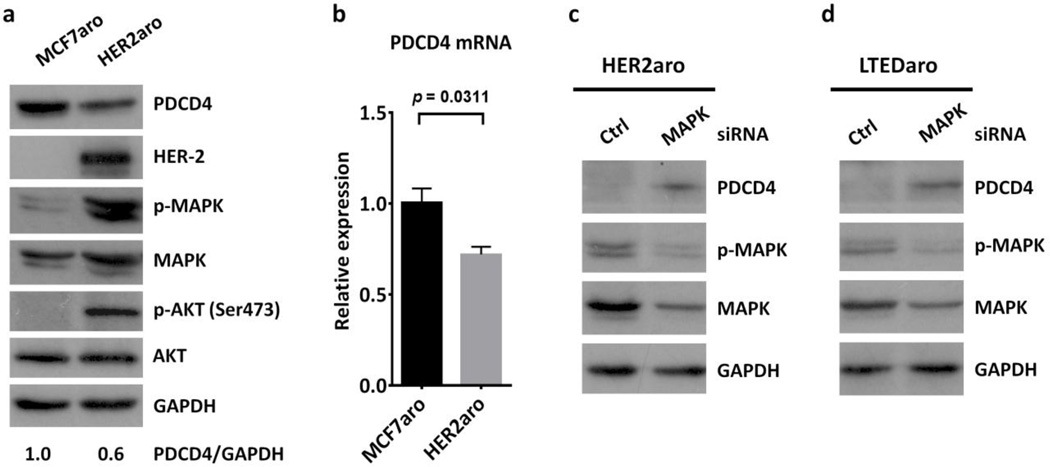
Given the inverse correlation between low PDCD4 expression and HER2 levels in both ER-positive breast cancer patients and AI-resistant breast cancer cells, we sought to determine whether up-regulation of the HER2 pathway decreases the expression of PDCD4. We thus did comparisons between MCF7aro and HER2aro, a MCF7 derived breast cancer cell line overexpressing HER2 and a model for primary resistance to AIs [25]. We found that HER2 expression and the phosphorylation of MAPK and AKT were significantly increased, whereas both the PDCD4 protein and mRNA levels were significantly decreased in HER2aro cells as compared with MCF7aro cells (Fig. 2a–b), indicating that up-regulation of the HER2 signaling down-regulates PDCD4 expression.
Fig. 2. Up-regulation of HER2 and MAPK signaling inhibits PDCD4 expression in aromatase inhibitor-resistant breast cancer cells.
a. Western blotting analysis of PDCD4 expression and the activation of the HER2 signaling pathway in MCF7aro and HER2aro cells cultured in defined culture media. The quantification of PDCD4 and HER2 was normalized to GAPDH, and then normalized to the MCF7aro cells.
b. Real time PCR analysis of PDCD4 expression in MCF7aro and HER2aro cells. Values were normalized to ACTB and plotted relative to the expression of the MCF7aro cells. Data are means ± SE (n=3).
c. PDCD4 protein levels in whole-cell lysates from HER2aro cells transfected with a nontargeting control (Ctrl) siRNA or a siRNA targeting MAPK. The quantification of PDCD4 was normalized to GAPDH, then normalized to the MCF7aro cells.
d. PDCD4 protein levels in whole-cell lysates from LTEDaro cells transfected with a nontargeting control (Ctrl) siRNA or a siRNA targeting MAPK. The quantification of PDCD4 was normalized to GAPDH, then normalized to the control siRNA
To further investigate whether the MAPK pathway is involved in the regulation of PDCD4, we determined whether reduction of MAPK expression by short interfering RNA (siRNA) would alter the expression of PDCD4. We found that knockdown of MAPK significantly increased PDCD4 expression in both HER2aro and LTEDaro cells (Fig. 2c–d), suggesting that PDCD4 is down-regulated by the MAPK pathway.
3.3. Up-regulation of AKT inhibits PDCD4 expression in AI-resistant breast cancer cells
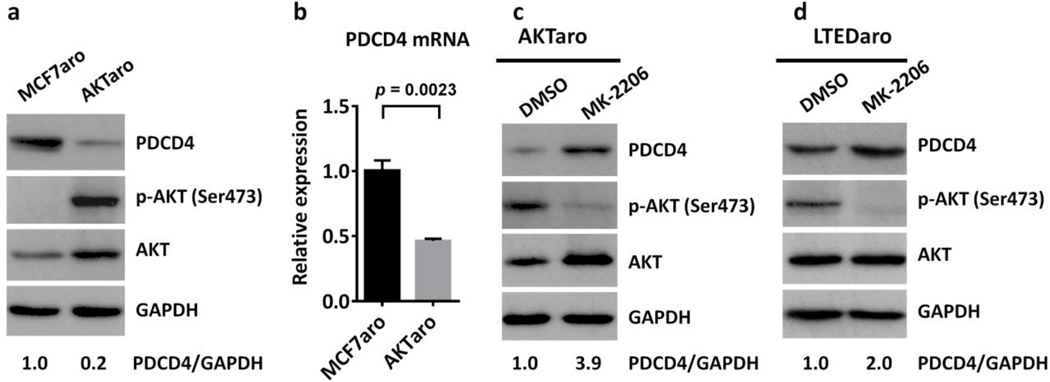
Protein kinase S6K1, a downstream mediator of the PI3K/AKT pathway, promotes the phosphorylation-dependent degradation of PDCD4 [32]. Because the AKT signaling pathway was up-regulated in AI-resistant breast cancer cells, we hypothesized that up-regulation of AKT signaling could reduce the expression of PDCD4. To test this hypothesis, we did comparisons between MCF7aro and AKTaro, a MCF7 derived breast cancer cell line overexpressing AKT and a model for primary resistance to AIs [25]. We found that the phosphorylation of AKT was significantly increased, whereas both the protein and mRNA levels of PDCD4 were significantly decreased in AKTaro cells as compared with MCF7aro cells (Fig. 3a–b), suggesting that activation of AKT down-regulates PDCD4 expression.
Fig. 3. Up-regulation of AKT inhibits PDCD4 expression in aromatase inhibitor-resistant breast cancer cells.
a. Western blotting analysis of PDCD4 expression and the activation state of AKT signaling in MCF7aro and AKTaro cells cultured in defined culture media. The quantification of PDCD4 was normalized to GAPDH, then normalized to the MCF7aro cells.
b. Real time PCR analysis of PDCD4 expression in MCF7aro and AKTaro cells. Values were normalized to ACTB and plotted relative to the expression of the MCF7aro cells. Data are means ± SE (n=3).
c. PDCD4 protein levels in whole-cell lysates from AKTaro cells treated with DMSO or MK-2206 1µM for 24 hr. The quantification of PDCD4 was normalized to GAPDH, then normalized to the control siRNA.
d. PDCD4 protein levels in whole-cell lysates from LTEDaro cells treated with DMSO or MK-2206 1µM for 24 hr. The quantification of PDCD4 was normalized to GAPDH, and then normalized to the control siRNA
To further confirm that AKT down-regulates PDCD4, we determined whether inhibition of AKT increases the expression of PDCD4. We found that treatment with MK-2206, an AKT inhibitor, significantly increased PDCD4 expression in both AKTaro and LTEDaro cells (Fig. 3c–d), confirming that PDCD4 is down-regulated by AKT.
3.4. Down-regulation of PDCD4 is associated with up-regulation of miR-21 in AI-resistant breast cancer cells
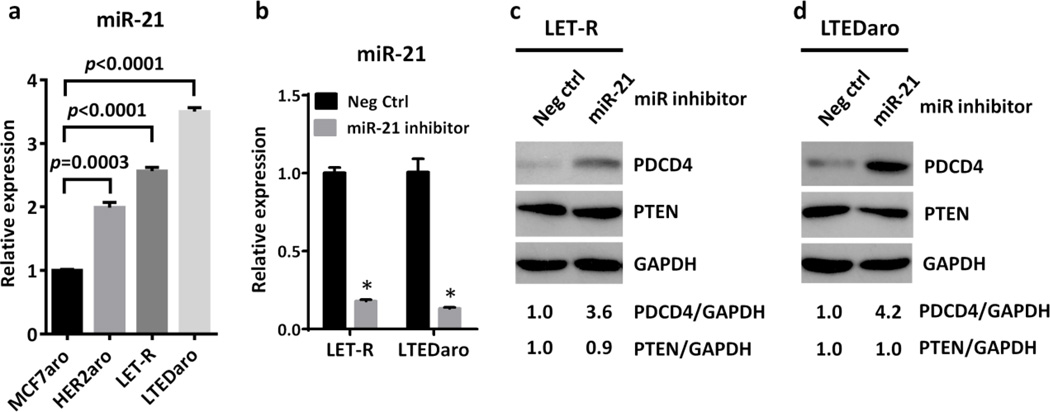
PDCD4 has been established as a target of the oncogenic microRNA, miR-21, whose expression is up-regulated by HER2 [20,21,33]. High miR-21 expression is found to be associated with HER2 positive status in primary breast tumors [34]. As HER2 is up-regulated in AI-resistant breast cancer cells, we wondered whether miR-21 is increased in these cells. We thus evaluated the levels of miR-21 in MCF7aro, HER2aro, LET-R, and LTEDaro cells by quantitative real-time PCR. We found that the levels of miR-21 were significantly increased in HER2aro, LET-R and LTEDaro cells as compared with MCF7aro cells (Fig. 4a).
Fig. 4. Up-regulation of miR-21 inhibits PDCD4 in aromatase inhibitor-resistant breast cancer cells.
a. Real time PCR analysis of miR-21 expression in MCF7aro, HER2aro, LET-R and LTEDaro cells cultured in defined culture media. Values were normalized to U6 RNA and plotted relative to the expression of the MCF7aro cells. Data are means ± SE (n=3).
b. Real time PCR analysis of miR-21 expression in LET-R and LTEDaro cells transfected with an inhibitor negative control (Neg Ctrl) or a miR-21 inhibitor for 48 hr. Values were normalized to U6 RNA and plotted relative to the expression of the inhibitor negative control. Data are means ± SE (n=3). Asterisk (*) denotes P <0.0005 (t test).
c. Western blotting analysis of PDCD4 and PTEN expression in LET-R and LTEDaro cells transfected with an inhibitor negative control (Neg ctrl) or a miR-21 inhibitor for 72 hr. The quantification of PDCD4 was normalized to GAPDH, then normalized to the control siRNA
Next, to investigate whether PDCD4 is a target of miR-21 in AI-resistant breast cancer cells, we determined whether inhibition of miR-21 increases the expression of PDCD4 in LET-R and LTEDaro cells. MiR-21 inhibitor significantly decreased the miR-21 levels in both LET-R and LTEDaro cells (Fig. 4b). After the treatment with miR-21 inhibitor, PDCD4 levels were remarkably elevated in both LET-R and LTEDaro cells (Fig. 4c–d). Interestingly, the levels of PTEN, another target of miR-21 [19], were not changed (Fig. 4c–d), suggesting that PDCD4 rather than PTEN is a target of miR-21 in AI-resistant breast cancer cells.
3.5. Forced overexpression of PDCD4 resensitizes AI-resistant breast cancer cells to AI or hormone deprivation
Activation of HER2 signaling pathway can lead to the activation of ER in a ligand-independent manner [3,4]. Many groups, including us [24,35,36], have shown that ERα is up-regulated and constitutively activated in AI-resistant breast cancer cells, especially in LTEDaro that has a low level of PDCD4 (Fig. 5a). To test whether ERα plays a role in the down-regulation of PDCD4 in AI-resistant breast cancer cells, we examined the effect of fulvestrant treatment on PDCD4 expression in LTEDaro cells. Fulvestrant treatment resulted in abolished ERα transcriptional activity, confirming that ERα in LTEDaro cells responds to fulvestrant (Fig. 5b). After treatment with fulvestrant, the expression of PDCD4 was significantly increased (Fig. 5c), which is consistent with a previous report [37], indicating a role for PDCD4 in the treatment of AI-resistant breast cancer.
Fig. 5. Forced overexpression of PDCD4 resensitizes aromatase inhibitor-resistant breast cancer cells to aromatase inhibitor or hormone deprivation.
a. Western blotting analysis of ERα and PDCD4 expression in MCF7aro, LET-R and LTEDaro cells cultured in defined culture media. The quantification of ERα and PDCD4 was normalized to GAPDH, then normalized to the MCF7aro cells.
b. Luciferase reporter assay in cell lysates from LTEDaro cells transfected with ERE reporter plasmid, and then treated with DMSO or fulvestrant 100nM for 24h. The relative luciferase activity was calculated by dividing the luciferase activity by the protein concentration. Data are means ± SE (n=4). Asterisk (*) denotes P <0.0001 (t test).
c. PDCD4 protein levels in LTEDaro cells treated with fulvestrant. Cells were harvested at the indicated time points. The quantification of PDCD4 was normalized to GAPDH, then normalized to the t=0 time point.
d. Real time PCR analysis of miR-21 expression in LTEDaro cells treated with fulvestrant at the indicated time points. Values were normalized to U6 RNA and plotted relative to the t=0 time point. Data are means ± SE (n=3).
e. Cell proliferation of LTEDaro cells treated with miR-21 inhibitor and/or fulvestrant. Cells were transfected with inhibitor negative control (−) or 50nM miR-21 inhibitor (+) for 24 hr, and then treated with DMSO (−) or 100nM fulvestrant (+). MTT was measured after 72 hr. Data are means ± SE (n=5).
f. PDCD4 protein levels in whole-cell lysates form LET-R and LTEDaro cells transfected with pMG-H2-PDCD4 expression construct or empty vector for 72 hr.
g. Cell proliferation of LET-R and LTEDaro cells transfected with pMG-H2-PDCD4 expression vector or empty vector. MTT was measured after 72 hr. Data are means ± SE (n=3).
Because PDCD4 is a target of miR-21, we determined the effect of fulvestrant on miR-21 expression. We found that miR-21 levels were increased in LTEDaro cells following fulvestrant treatment (Fig. 5d), suggesting that the induced expression of PDCD4 by fulvestrant is not through miR-21, and that there may be a compensational increase of miR-21 after fulvestrant treatment.
As miR-21 was up-regulated in AI-resistant breast cancer cells and its expression was further increased following fulvestrant treatment, we sought to examine the effect of miR-21 inhibitor or a combination of miR-21 inhibitor and fulvestrant in AI-resistant breast cancer cells. We found that miR-21 inhibitor treatment alone decreased cell proliferation in LTEDaro cells (Fig. 5e). Combination treatment of miR-21 inhibitor and fulvestrant further decreased cell proliferation in LTEDaro cells, in an additive manner (Fig. 5e).
Next, to test whether increasing the expression of PDCD4, which is induced by miR-21 inhibitor and fulvestrant treatment, can resensitize AI-resistant breast cancer cells to AI, we generated a pMG-H2-PDCD4 expression construct. LET-R and LTEDaro cells were transfected with pMG-H2-PDCD4 expression construct or empty vector (Fig. 5f). We found that forced overexpression of PDCD4 resensitized LET-R and LTEDaro to letrozole and hormone deprivation, respectively (Fig. 5g). These data strongly suggest that PDCD4 plays a role in AI resistance of breast cancer.
3.6. Down-regulation of PDCD4 is associated with a poor prognosis in ER-positive breast cancer
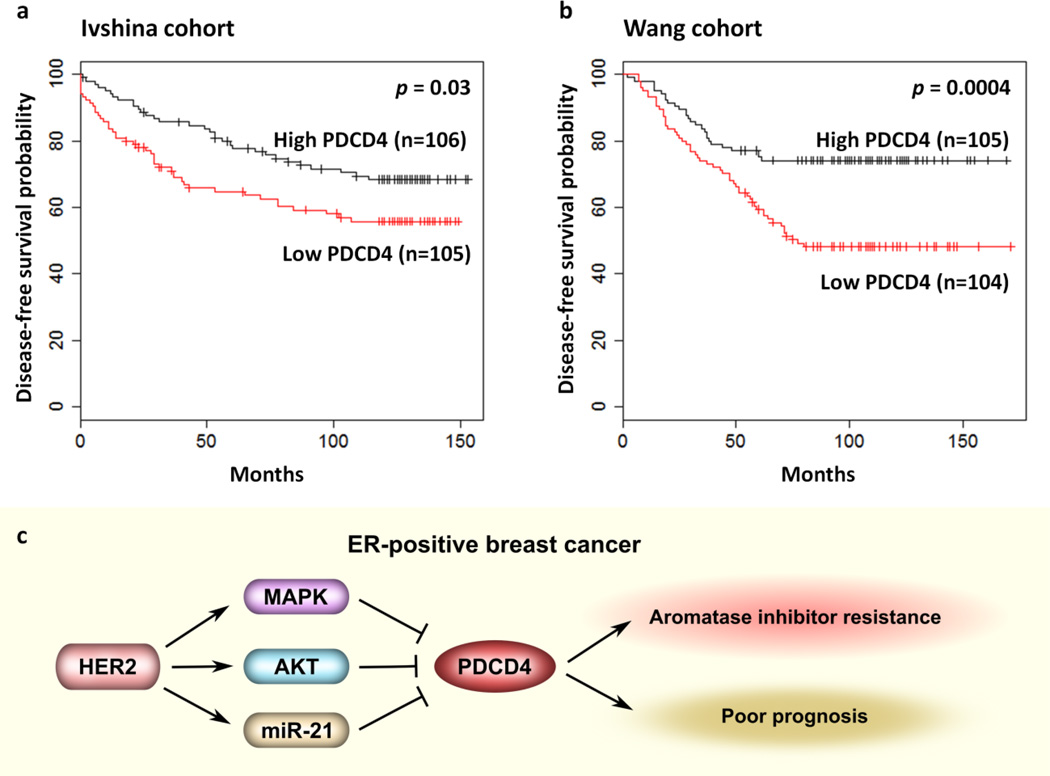
To analyze the clinical relevance of PDCD4 down-regulation in ER-positive breast cancer, we carried out a Kaplan-Meier survival analysis. Because data on disease-free survival in the TCGA cohort are lacking, we did the analysis in other two independent cohorts that included a total of 420 patients with ER-positive breast cancer [30,31]. This analysis showed that patients with a low level of PDCD4 expression had a worse prognosis than patients with a high level of PDCD4 expression. A Kaplan-Meier analysis of disease-free survival among 211 ER-positive breast cancer patients in the Ivshina cohort [30] showed that higher PDCD4 expression conferred a significant survival advantage (p = 0.03) (Fig. 6a). Similarly, lower PDCD4 expression was associated with a poor disease-free survival among 209 patients in the Wang cohort [31] with ER-positive breast tumors (p = 0.0004) (Fig. 6b). These data suggest that PDCD4 expression has a prognostic value for patients with ER-positive breast cancer.
Fig. 6. Poor prognosis predicted by low expression of PDCD4 in ER-positive breast cancer.
a. Kaplan-Meier estimates of disease-free survival are shown for 211 patients with ER-positive breast cancer in the Ivshina cohort. The curves show a lower rate of disease-free survival with low-PDCD4 breast tumors.
b. Kaplan-Meier estimates of disease-free survival are shown for 209 patients with ER-positive breast cancer in the Wang cohort. The curves show a lower probability of disease-free survival with low-PDCD4 breast tumors.
c. Proposed model of PDCD4 down-regulation by HER2 in ER-positive breast cancer.
Next, we sought to determine the correlation between PDCD4 and histologic grade of tumors. Data on tumor grade are available in the Ivshina cohort [30] but not in the Wang cohort [31]. We thus performed a differential expression analysis for PDCD4 in 289 patients in the Ivshina cohort, including 68 patients with Elston grade 1 tumors, 166 with Elston grade 2, and 55 with Elston grade 3. The analysis showed a lower level of PDCD4 in patients with high grade (Elston grade 3) tumors than in patients with low grade (Elston grade1) tumors (p = 4.62e-08, fold change 1.5) (Supplementary Fig. S1).
In summary, our study shows that PDCD4 expression is in part down-regulated by HER2 via the MAPK, AKT and miR-21 in AI-resistant breast cancer cells. Down-regulation of program cell death 4 (PDCD4) is associated with AI resistance and a poor prognosis in ER-positive breast cancer (Fig. 6c).
4. Discussion
Progression or recurrence due to resistance to AIs is a significant clinical problem for a considerable number of ER-positive breast cancer patients. In this study, we found that the tumor suppressor PDCD4 was down-regulated in AI-resistant breast cancer cells. We also identified an association between the down-regulation of PDCD4 and the up-regulation of HER2 signaling in AI-resistant breast cancer cells and ER-positive breast tumors. More importantly, a poor prognosis was found in ER-positive breast cancer patients with a low level of PDCD4, further sustaining a potential contribution of PDCD4 to the development and/or outcome of AI resistance in breast cancer.
PDCD4 has been known as a tumor suppressor [38]. PDCD4 inhibits protein translation by binding to the translation initiation factor eIF4A and suppressing its activity [11,12]. PDCD4 is targeted for degradation during tumor progression [14]. Breast cancer may progress or relapse because of resistance to AIs, and we demonstrated that PDCD4, a tumor suppressor, was down-regulated in AI-resistant breast cancer cells, and that forced overexpression of PDCD4 resensitized AI-resistant cells to AI or hormone deprivation. Furthermore, our survival analysis suggests that the down-regulation of PDCD4 is clinically significant in breast cancer, since ER-positive breast cancer patients with a low level of PDCD4 tend to have a lower rate of disease-free survival based on microarray datasets in two independent patient cohorts [30,31] that included a total of 420 patients. Consistent with our finding that low PDCD4 predicts a poor prognosis, Meric-Bernstam et al. have reported that low protein expression of PDCD4 is associated with worse overall survival but not recurrence-free survival in hormone receptor-positive breast cancer with the use of reverse phase protein arrays in 190 patients [22]. In addition, our study showed that lower PDCD4 was significantly correlated with high histologic grade of breast tumors. Identification of reliable biomarkers that can be used to predict patient outcomes ensure more effective clinical management, and we propose that PDCD4 is such a biomarker in ER-positive breast cancer.
Up-regulation of HER2 signaling pathway has been found in AI-resistant breast cancer cells [5,6] and breast tumors of patients treated with AIs [8]. HER2 signaling promotes cell proliferation and survival through the MAPK and PI3K/AKT pathways [39]. In our study, we found an inverse correlation between the PDCD4 expression and the activation of HER2 signaling pathways in AI-resistant breast cancer cells. Consistently, our differential gene expression analysis in the TCGA patient cohort [28] showed that lower expression of PDCD4 was significantly associated with HER2 positive status in patients with ER-positive breast cancer. We further demonstrated that HER2 down-regulated PDCD4 via the MAPK, AKT and miR-21 in AI-resistant breast cancer cells. These findings strongly suggest that the down-regulation of PDCD4 is, at least in part, mediated through HER2 in AI-resistant breast cancer. In addition to HER2 signaling, other growth factor receptors, such as the insulin-like growth factor 1 receptor (IGF1R) [40,41] and epidermal growth factor receptor (EGFR) [42], have been associated with AI resistance. As these growth factor receptors can also activate the MAPK and PI3K/AKT [43], further studies are needed to investigate whether they are involved in the down-regulation of PDCD4.
Up-regulated and constitutively activated ERα has been shown to play a role in AI resistance [24,35,36]. PDCD4 can be induced by anti-estrogen treatment [37]. Consistently, our results showed that PDCD4 expression was significantly increased following fulvestrant treatment in AI-resistant breast cancer cells, suggesting that ERα may play a role in the down-regulation of PDCD4. The mechanisms of ERα regulation of PDCD4 might be complicated. We evaluated the effect of fulvestrant on miR-21 expression. Interestingly, we found that fulvestrant increased the expression of miR-21 rather than decreased it, which is consistent with a report showing that estradiol represses the expression of miR-21 by activating ER in MCF7 cells [44]. These findings suggest that the regulation of PDCD4 by ERα is not through miR-21 and that there are other mechanisms responsible for it. One possibility is that ERα activation can activate PI3K/AKT/p-70S6K signaling [45] and even can directly transcriptionally up-regulate expression of S6K1 [46], which promotes the phosphorylation-dependent degradation of PDCD4 [32]. Our results showed that activation of AKT down-regulated PDCD4 expression in support of this possible mechanism. In addition, activating mutations of ESR1, which encodes ERα, have been identified in hormone-resistant metastatic breast cancer [47,48]. It is possible that ERα mutant signaling may also play a role in the regulation of PDCD4, but we do not know whether it is involved in our AI-resistant models. Therefore, the mechanisms of ERα regulation of PDCD4 need to be further investigated.
Down-regulation of PDCD4 is often associated with up-regulation of the oncogenic microRNA miR-21 [20,21]. High miR-21 expression is associated with HER2 positive status in primary breast tumors[34], and overexpression of HER2 significantly induces miR-21 in breast cancer cells [33]. Consistent with the up-regulation of HER2 signaling, we found an increase of miR-21 levels in AI-resistant breast cancer cells. miR-21 has also been linked to MAPK or PI3K/AKT pathways [49,50], and the two major pathways can be activated by HER2. There is increasing evidence suggesting that microRNAs are involved in AI resistance [51–53], including miR-21, which has been shown to have elevated expression following combine treatment of tamoxifen and exemestane [52]. In this study, we found that treatment with miR-21 inhibitor remarkably increased the expression of PDCD4 and decreased cell proliferation in AI-resistant breast cancer cells. Furthermore, we showed that forced overexpression of PDCD4 resensitized AI-resistant cells to AI or hormone deprivation. These data suggest potential roles of miR-21 and PDCD4 in AI resistance of breast cancer.
In conclusion, our study shows that the tumor suppressor PDCD4 expression is down-regulated by HER2 signaling in AI-resistant breast cancer cells. The down-regulation of PDCD4 is associated with AI resistance and may predict a poor prognosis in patients with ER-positive breast cancer.
Supplementary Material
Acknowledgements
The study was supported by the National Institutes of Health (R01 ES08258 to SC). Research reported in this publication included work performed at the Bioinformatics Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572.
Abbreviations
- AI
aromatase inhibitor
- AKT/PKB
protein kinase B
- ER
estrogen receptor
- FBS
fetal bovine serum
- HER2
human epidermal growth factor receptor 2
- HR
hormone-receptor
- MAPK
mitogen-activated protein kinase
- MEM
minimal Eagle's medium
- PDCD4
Program cell death 4
- PI3K
phosphatidylinositol 3'-kinase
- PTEN
phosphatase and tensin homolog
- siRNA
short interfering RNA
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest.
References
- 1.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98(18):1285–1291. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 2.Nemere I, Pietras RJ, Blackmore PF. Membrane receptors for steroid hormones: signal transduction and physiological significance. J Cell Biochem. 2003;(3):438–445. doi: 10.1002/jcb.10409. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276(13):9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 4.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 5.Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M. Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem. 2003;278(33):30458–30468. doi: 10.1074/jbc.M305226200. [DOI] [PubMed] [Google Scholar]
- 6.Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005;65(12):5380–5389. doi: 10.1158/0008-5472.CAN-04-4502. [DOI] [PubMed] [Google Scholar]
- 7.Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, Morita M, Kakeji Y, Baba H, Maehara Y. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13(2):137–144. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- 8.Flageng MH, Moi LL, Dixon JM, Geisler J, Lien EA, Miller WR, Lonning PE, Mellgren G. Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br J Cancer. 2009;101(8):1253–1260. doi: 10.1038/sj.bjc.6605324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene. 1995;166(2):297–301. doi: 10.1016/0378-1119(95)00607-9. [DOI] [PubMed] [Google Scholar]
- 10.Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, Matsuhashi S, Colburn NH. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci U S A. 1999;96(24):14037–14042. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Molecular and cellular biology. 2003;23(1):26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Molecular and cellular biology. 2004;24(9):3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65(14):6034–6041. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- 14.Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68(5):1254–1260. doi: 10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, Ozaki I, Petersen I. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol. 2003;200(5):640–646. doi: 10.1002/path.1378. [DOI] [PubMed] [Google Scholar]
- 16.Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, Post S, Jansen A, Colburn NH, Allgayer H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110(8):1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25(45):6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- 18.Wen YH, Shi X, Chiriboga L, Matsahashi S, Yee H, Afonja O. Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol Rep. 2007;18(6):1387–1393. [PubMed] [Google Scholar]
- 19.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283(2):1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27(31):4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- 22.Meric-Bernstam F, Chen H, Akcakanat A, Do KA, Lluch A, Hennessy BT, Hortobagyi GN, Mills GB, Gonzalez-Angulo A. Aberrations in translational regulation are associated with poor prognosis in hormone receptor-positive breast cancer. Breast Cancer Res. 2012;14(5):R138. doi: 10.1186/bcr3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou DJ, Pompon D, Chen SA. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50(21):6949–6954. [PubMed] [Google Scholar]
- 24.Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008;68(12):4910–4918. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 25.Wong C, Wang X, Smith D, Reddy K, Chen S. AKT-aro and HER2-aro, models for de novo resistance to aromatase inhibitors; molecular characterization and inhibitor response studies. Breast Cancer Res Treat. 2012;134(2):671–681. doi: 10.1007/s10549-012-2105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Wang O, Nie M, Elison K, Zhou D, Li M, Jiang Y, Xia W, Meng X, Chen S, Xing X. Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: Effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. (Database issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Team RC. R: A Language and Environment for Statistical Computing. 2013 [Google Scholar]
- 30.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, Wong JE, Liu ET, Bergh J, Kuznetsov VA, Miller LD. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66(21):10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 32.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314(5798):467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 33.Huang TH, Wu F, Loeb GB, Hsu R, Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo YY, Goga A, McManus MT. Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J Biol Chem. 2009;284(27):18515–18524. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JA, Lee HY, Lee ES, Kim I, Bae JW. Prognostic Implications of MicroRNA-21 Overexpression in Invasive Ductal Carcinomas of the Breast. Journal of breast cancer. 2011;14(4):269–275. doi: 10.4048/jbc.2011.14.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin LA, Farmer I, Johnston SR, Ali S, Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocr Relat Cancer. 2005;12(Suppl 1):S75–S84. doi: 10.1677/erc.1.01023. [DOI] [PubMed] [Google Scholar]
- 36.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J, Jr, Berstein L, Yue W. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer. 2005;12(Suppl 1):S61–S73. doi: 10.1677/erc.1.01018. [DOI] [PubMed] [Google Scholar]
- 37.Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene. 2004;23(49):8135–8145. doi: 10.1038/sj.onc.1207983. [DOI] [PubMed] [Google Scholar]
- 38.Lankat-Buttgereit B, Goke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biology of the cell / under the auspices of the European Cell Biology Organization. 2009;101(6):309–317. doi: 10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- 39.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews Molecular cell biology. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 40.Stephen RL, Shaw LE, Larsen C, Corcoran D, Darbre PD. Insulin-like growth factor receptor levels are regulated by cell density and by long term estrogen deprivation in MCF7 human breast cancer cells. J Biol Chem. 2001;276(43):40080–40086. doi: 10.1074/jbc.M105892200. [DOI] [PubMed] [Google Scholar]
- 41.Fox EM, Miller TW, Balko JM, Kuba MG, Sanchez V, Smith RA, Liu S, Gonzalez-Angulo AM, Mills GB, Ye F, Shyr Y, Manning HC, Buck E, Arteaga CL. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71(21):6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilani RA, Kazi AA, Shah P, Schech AJ, Chumsri S, Sabnis G, Jaiswal AK, Brodie AH. The importance of HER2 signaling in the tumor-initiating cell population in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat. 2012;135(3):681–692. doi: 10.1007/s10549-012-2148-8. [DOI] [PubMed] [Google Scholar]
- 43.Roop RP, Ma CX. Endocrine resistance in breast cancer: molecular pathways and rational development of targeted therapies. Future Oncol. 2012;8(3):273–292. doi: 10.2217/fon.12.8. [DOI] [PubMed] [Google Scholar]
- 44.Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells (Nucleic acids research) Nucleic Acids Res. 2009;37(8):2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61(16):5985–5991. [PubMed] [Google Scholar]
- 46.Maruani DM, Spiegel TN, Harris EN, Shachter AS, Unger HA, Herrero-Gonzalez S, Holz MK. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene. 2012;31(49):5073–5080. doi: 10.1038/onc.2011.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L, Gursky A, Siddiqui J, Tomlins SA, Roychowdhury S, Pienta KJ, Kim SY, Roberts JS, Rae JM, Van Poznak CH, Hayes DF, Chugh R, Kunju LP, Talpaz M, Schott AF, Chinnaiyan AM. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45(12):1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, Dvir A, Soussan-Gutman L, Jeselsohn R, Yelensky R, Brown M, Miller VA, Sarid D, Rizel S, Klein B, Rubinek T, Wolf I. D538G mutation in estrogen receptor-alpha: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 2013;73(23):6856–6864. doi: 10.1158/0008-5472.CAN-13-1197. [DOI] [PubMed] [Google Scholar]
- 49.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285(26):20281–20290. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6(4):e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masri S, Liu Z, Phung S, Wang E, Yuan YC, Chen S. The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells. Breast Cancer Res Treat. 2010;124(1):89–99. doi: 10.1007/s10549-009-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, Vagner S. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69(21):8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- 53.Hayes EL, Lewis-Wambi JS. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17:40. doi: 10.1186/s13058-015-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.