Abstract
Purpose
Adults residing in rural areas have been linked with higher bone mineral density (BMD). We aimed to determine if this difference is due in part to air pollution by examining the relationships between traffic metrics and ambient air pollution with total body and pelvic BMD.
Methods
Mexican-American adults (n=1,175; mean 34 years; 72% female) who had participated in the BetaGene study of air pollution, obesity and insulin resistance were included in this analysis. Total body and pelvic BMD were estimated using dual-energy X-ray absorptiometry. Traffic and ambient air pollutant exposures were estimated at residences using location and ambient monitoring data. Variance component models were used to analyze the associations between residential distance to the nearest freeway and ambient air pollutants with BMD.
Results
Residential proximity to a freeway was associated with lower total body BMD (p-trend=0.01) and pelvic BMD (p-trend=0.03) after adjustment for age, sex, weight and height. The adjusted mean total body and pelvic BMD in participants living within 500m of a freeway were 0.02 g/cm2 and 0.03 g/cm2 lower than participants living greater than 1,500m from a freeway. These associations did not differ significantly by age, sex or obesity status. Results were similar after further adjustment for body fat and weekly physical activity minutes. Ambient air pollutants (NO2, O3 and PM2.5) were not significantly associated with BMD.
Conclusions
Traffic-related exposures in overweight and obese Mexican-Americans may adversely affect BMD. Our findings indicate that long-term exposures to traffic may contribute to the occurrence of osteoporosis and its consequences.
Keywords: osteopenia, osteoporosis, bone mineral density, BMD, traffic-related pollution, air pollution
INTRODUCTION
Osteoporosis is characterized by reduced bone mineral density (BMD) which leads to an increased risk of fracture [1]. Over 10 million people in the U.S. are affected by osteoporosis and by the year 2020, it is projected to impact approximately 14 million adults over the age of 50 [2]. The underlying cellular mechanisms in bone mineralization and remodeling are influenced by multiple factors including smoking [3, 4], nutrition [5], physical activity [6], alcohol consumption [7], sex hormone deficiency [8], and genetic factors [9].
A growing body of evidence has documented that people living in rural areas have higher body BMD [10, 11] and lower fracture rates [12, 13] than people living in urban areas. However, differences in BMD and fracture rates are not explained by risk factors including lifestyle factors and body size. Studies have shown personal smoking [3] and exposure to second-hand smoke [4] were associated with lower BMD and increased risk of fracture. Since combustion products from smoking were associated with decreased BMD, we hypothesized that chronic exposure to traffic combustion products also would be associated with decreased BMD, which may also explain the previously published work documenting lower BMD in urban compared to rural populations [10, 11]. Ambient air pollutants such as fine particulate matter (PM2.5), nitrogen oxides (NOx), and near-roadway tailpipe emissions are produced directly by combustion of fossil fuels or via subsequent atmospheric chemistry, such as ozone (O3). A limited number of studies of children and the elderly have reported that concentrations of air pollutants, particularly PM (PM2.5 and PM10), were inversely related with BMD [14-16]. Because chronic exposure to elevated levels of these pollutants is a growing problem in many regions of the world [17], a better understanding of the contribution of air pollutant exposures to bone health is needed.
In this study, we assessed the effects of traffic exposures and ambient air pollutants (NO2, O3, and PM2.5) on total body and pelvic BMD among subjects who participated in the BetaGene study, a family-based observational study of obesity, insulin resistance and beta-cell function in Mexican Americans. We hypothesize that higher traffic and ambient air pollution exposures are associated with lower total body and pelvic BMD.
METHODS
Study Participants
BetaGene participants were recruited during the period 2002-2008. The participants are Mexican American adults (both parents and at least 3 grandparents are Mexican or of Mexican descent) who are either (a) women who had gestational diabetes mellitus (GDM) within the previous 5 years, (b) siblings or cousins of women with a history of GDM, or (c) women with normal glucose levels during pregnancy in the past 5 years. Women with and without previously documented GDM were identified from the Los Angeles County/University of Southern California Medical Center, Kaiser Permanente Southern California's delivery population, and obstetrical/gynecological clinics at local Southern California hospitals. Women without previous GDM were frequency-matched to GDM cases by age, body mass index (BMI), and parity. Details regarding recruitment have been previously described [18]. All protocols for BetaGene were approved by the Institutional Review Boards of participating institutions, and all participants provided written informed consent.
Clinical Assessment
Health outcome data were collected in two separate visits to the John T. Nicoloff General Clinical Research Center at the University of Southern California. The first visit consisted of a physical examination, dietary and physical activity questionnaires, a 2-hour, 75-gram oral glucose tolerance test (oGTT), and fasting blood draw for lipid measurements. Participants with fasting glucose < 7.0 mmol/l (<126 mg/dL) were invited for a second visit, which consisted of a dual-energy X-ray absorptiometry (DXA) scan for body composition including BMD measurements and an insulin-modified intravenous glucose tolerance test (ivGTT) for measurement of insulin sensitivity and beta-cell function.
Physical Activity and Dietary Assessment
Trained bilingual (English/Spanish) interviewers administered both physical activity and dietary questionnaires. The amount and intensity of physical activity was assessed by questionnaires developed in the Hawaii-Los Angeles Multi-ethnic Cohort Study [19]. This questionnaire is comprised of a list of structured questions describing various types of activity (sitting, strenuous sports, vigorous work, and moderate activities including sports and work) during the past year. Responses were then used to estimate the total minutes of moderate and vigorous activity per week. Dietary intake was assessed using the 126-item semi-quantitative Harvard food-frequency questionnaire (FFQ) [20]. The FFQ consisted of a list of foods with a standardized serving size and a selection of 9 frequency categories ranging from never or <1 serving per month to > 6 servings per day, during the past year. An open-ended free text section was utilized to capture food items that did not appear on the standard list, and included information on usual serving size and number of servings consumed per week for incorporation in the dietary intake calculation for each subject. Total caloric and nutrient intakes were calculated by the Harvard Channing Laboratory.
Air Pollution and Traffic Exposure Assessments
Air pollution and traffic exposures were assigned based at the participant's residential address provided at the time of testing. Addresses were standardized and their locations were geocoded using Google Earth Pro (www.googleearth.com) and TomTom's EZ-Locate service (www.geocode.com). In most cases the EZ-Locate results were only used to verify the Google Earth Pro geocodes. Addresses that were not recognized by Google Earth Pro were geocoded using the EZ-Locate service. For residences that were geocoded with sufficient accuracy to permit traffic exposure assignment (97% of the subjects), we calculated a proximity metric defined as the distance from the residence to the nearest roadway for each of the four Feature Class Codes (FCC): Freeway or highway (FCC1), major collector (FCC2), minor collector (FCC3), and arterial road (FCC4)] using the Esri Streetmap Premium database in ArcGIS (version 10.1, Esri, Redlands, CA).
Ambient air quality data in California were obtained from the U.S. Environmental Protection Agency's Air Quality System (AQS, http://www.epa.gov/ttn/airs/airsaqs) for the years 2002-2008 [21]. The O3 and NO2 data were collected using Federal Reference Method (FRM) monitors. The PM2.5 data were primarily collected using FRM or Federal Equivalent Method (FEM) monitors. When FRM or FEM data were not available from AQS for a monitoring location, continuous PM data were used instead. The station-specific air quality data were spatially interpolated to the residence locations using inverse distance-squared weighting (IDW2). Data from up to 4 air quality measurement stations were included in each interpolation. Due to the regional nature of NO2, O3 and PM2.5 concentrations in southern California, a maximum interpolation radius of 50 km was used for all pollutants. However, when a residence was located within 5 km of one or more stations with valid observations, the interpolation was based solely on the nearest station values. We calculated average concentrations for the 12 months prior to each subject's test date to estimate longer-term air pollution exposure.
Contextual Variables
Contextual variables were collected to quantify the social-economic status of participants. Demographic data including median household income, poverty rate, unemployment rate, and proportion of respondents over age 25 with highest attained education (no education, lower than or equal to high school, some college or technical school, more than 4 years of college) were obtained from the U.S. Bureau of Census website (http://www.census.gov/) for all the counties within the U.S. that contained at least one geocoded address. The spatial analysis tools in ArcGIS (version 10.2) were used to calculate the proportion of the 2000 Census block groups that fell within 300 m buffers of each of the geocoded addresses. The proportions were applied to the demographic data and the numbers, rates, and proportions were estimated for each buffer.
Additional data for fast foods, grocery stores, and parks and recreation areas were extracted from Esri's Business Analyst database (version 2013). The ArcGIS buffer tool was used to create 500 m buffers for each residential address, and the numbers of fast food and grocery stores, and proportions of parks or recreation areas located inside these buffers were calculated and assigned to each geocoded address. Crime data was extracted from Esri's Community Analyst database (version 2013) at the ZIP code level. The same spatial tools in ArcGIS were used to calculate the proportion of each ZIP code that fell within each of the 500 m buffers and a crime index (based on information about murder, rape, robbery, assault, burglary, and motor vehicle theft) was then calculated by delineating and summing the portions inside the buffer area and assigning these values to the appropriate address.
Data Analysis
Characteristics of the study participants were summarized using means, medians and inter-quartile ranges. Distances to freeways and major roadways were log transformed to approximate the normal distribution and fit the exponential decay characteristic of freeway-related pollution. Geometric means are presented for these variables. The major and minor collectors coded as FCC2 and FCC3 were classified as major roadways. Based on previous results that air pollutant concentrations were significantly higher within 500m of freeways and within 75m of a major road [22], residential distances from the nearest freeway were categorized as ≤500m, 500m, 500-1,000m, 1,000m, 1,0 00-1,500m, and >1,500m; whereas, residential distances from the nearest major road were categorized as ≤75m, 75m, 75-150m, 150-300m, and >300m. The relationships of total body and pelvic BMD with distances from the nearest freeway and major road, as well as the air pollutants NO2, O3 and PM2.5 were assessed under a variance components framework using SOLAR (version 4.3.1) to account for correlations among related individuals within families. Residential distances from the nearest freeway and major road were analyzed as both continuous and categorical exposures. A trend test was performed to test significant linear trend association between BMD and four incremental categories of traffic exposures. Age, sex, weight, height, percent body fat, total physical activity minutes per week, parity, contextual variables including the median household income, the percentage of households with incomes less than the federally designated poverty rate, adult unemployment rate, the proportion of respondents over age 25 with highest attained education, crime index, the number of fast food and grocery stores, and the acreages of parks or recreation areas within the buffers of each residential address, plus daily calcium and vitamin D intakes during the past year were included as covariates to adjust for potential confounders. The interactions of age, sex and obesity status with residential distances from the nearest freeway or major road were also tested by including multiplicative interaction terms in the model. Individual ambient pollutants were evaluated for their associations with BMD. Multiple-pollutant models were used to test joint impact of air pollutant combinations.
RESULTS
A total of 1,173 participants with complete measurements of body composition were included in this study. Characteristics of the participants are shown in Table 1. The mean age was 34.4 (range: 17.9 – 65.5) years, 849 (72.3%) participants were females, and 207 women were diagnosed with GDM. Mean BMI was 29.5 (range: 17.1 – 55.4) kg/m2, with 442 (37.7%) categorized as overweight defined as BMI from 25-30 kg/m2 and 474 (40.4%) categorized as obese defined as BMI≥30 kg/m2 [23]. Mean percent body fat was 34.5%. Compared to the Centers for Disease Control and Prevention (CDC) referenced mean BMD among Mexican Americans [24], no subjects met clinical criteria for osteoporosis and only three subjects had T-scores of pelvic BMD lower than -1, which matches the 16th percentile of the entire population. The geographic areas where our participants lived had a mean of 0.2% households below the federally designated poverty rate, a mean of 18.8% people over age 25 had highest attained education at or below high school, and a mean of 0.3% people over age 25 had highest attained at least some college education (Table 2).
Table 1.
Selected characteristics and exposures for BetaGene study participants at the time of intravenous glucose tolerance tests (n=1173)
| Variable | n | Mean | Median | Inter-quartile Range |
|---|---|---|---|---|
| Age (yrs) | 1173 | 34.4 | 34.2 | (29 – 39.1) |
| Height (cm) | 1173 | 160.66 | 160.00 | (155 – 166) |
| Weight (kg) | 1173 | 76.27 | 73.70 | (64.5 – 85.7) |
| Body mass index (BMI kg/m ) | 1173 | 29.51 | 28.70 | (25.4 – 32.6) |
| Percent body fat (%) | 1173 | 34.51 | 35.80 | (28.5 – 40.6) |
| Waist-to-hip ratio×100 | 1152 | 88.50 | 88.00 | (84 – 93) |
| Average weekly total physical activity in past 1 year (mins/week) | 1126 | 361.3 | 139.2 | (32.1 – 460.5) |
| Moderate activity (mins/week) | 1138 | 201.8 | 32.1 | (0 – 214.2) |
| Vigorous activity (mins/week) | 1126 | 157.6 | 32.1 | (0 – 214.2) |
| Average daily Calcium intake in past 1 year (mg/day)a | 1133 | 922.5 | 917.3 | (679.4 – 1269.6) |
| Average daily Vitamin D intake in past 1 year (IU/day)a | 1133 | 219.6 | 215.7 | (134.4 – 386.6) |
| Total body BMD (g/cm2 × 100) | 1173 | 110.12 | 109.44 | (103.81 – 115.78) |
| Pelvic BMD (g/cm2 × 100) | 1173 | 124.15 | 123.43 | (114.46 – 132.37) |
| Residential distance from the nearest freeway (m) a | 1137 | 1149 | 1296 | (593 – 2342) |
| Residential distance from the nearest major road (m) a | 1137 | 174.39 | 191.76 | (105.78 – 358.51) |
| Annual average nitrogen dioxide (NO2, ppb) | 1163 | 25.64 | 26.08 | (20.72 – 30.76) |
| Annual average eight-hour ozone (O3, ppb) | 1167 | 40.65 | 39.61 | (36.29 – 45.84) |
| Annual average fine particulate pollution (PM25, μg/m ) | 1164 | 17.42 | 17.36 | (15.29 – 20.36) |
Variables are log transformed for the data analysis, and are presented as geometric means.
Table 2.
Distribution of contextual variables of BetaGene study participants collected from U.S. Bureau of Census 2000 block group and U.S. Postal Service ZIP code data
| Variable | n | Mean | Median | Inter-quartile Range |
|---|---|---|---|---|
| Median household income (US $) a | 1173 | 38705 | 36660 | (28480 – 46786) |
| Proportions of households below federally designated poverty rate a | 1173 | 0.22 | 0.21 | (0.12 – 0.29) |
| Proportions of adult who are unemployed a | 1173 | 0.06 | 0.05 | (0.04 – 0.07) |
| Proportion of respondents over age 25 with highest attained education a | ||||
| No education | 1173 | 0.07 | 0.07 | (0.03 – 0.11) |
| Lower than or equal to high school | 1167 | 18.72 | 5.86 | (2.43 – 12.63) |
| Some college or technical school | 1173 | 0.23 | 0.22 | (0.14 – 0.31) |
| More than 4 years of college | 1173 | 0.11 | 0.08 | (0.04 – 0.15) |
| Crime Index b | 1173 | 123 | 120 | (89 – 152) |
| Park or recreation areas (km2) b | 1173 | 0.015 | 0 | (0 – 0.017) |
| Number of fast food restaurants b | 1173 | 0.49 | 0 | (0 – 1) |
| Number of grocery stores b | 1173 | 1.72 | 1 | (0 – 2) |
Different GIS tools (intersect, dissolve and calculate field) were applied to calculate the proportion of the census block groups that fell within each of the 300 m buffers for each of the geocoded addresses.
GIS buffer tool was used to create 500 m buffer for each geocoded addresses.
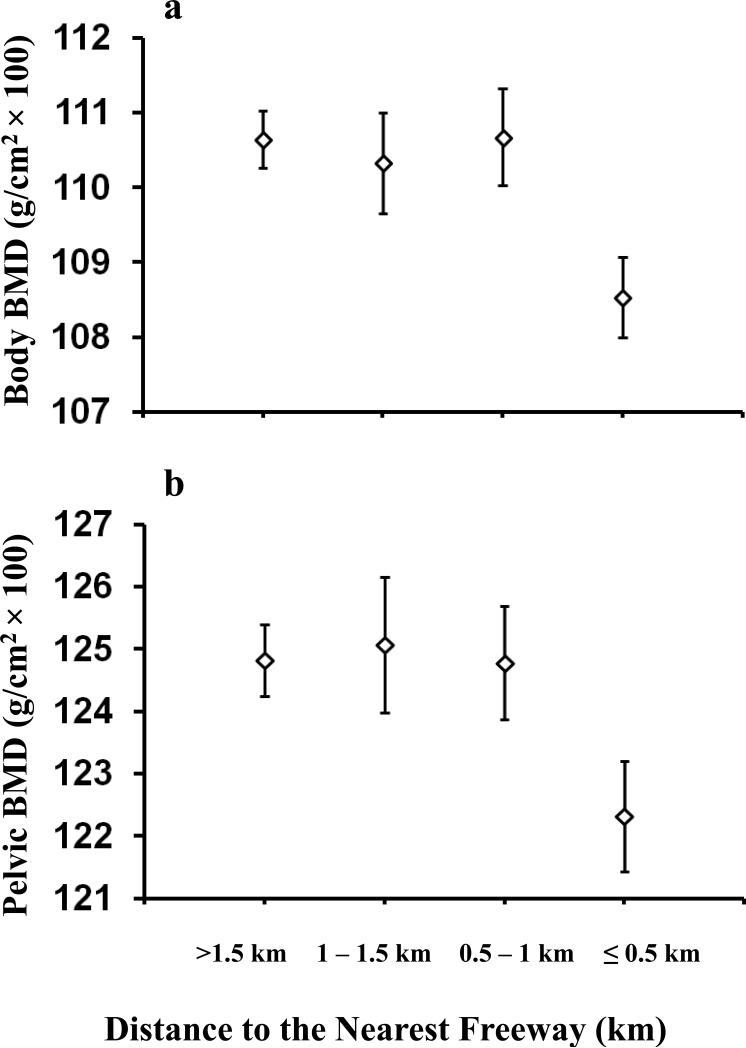
Table 3 presents the associations between total body and pelvic BMD with traffic exposures after adjusting for age, sex, weight and height. Using traffic exposure categories, residential proximity to the nearest freeway was associated with lower total body BMD (p-trend = 0.012) and lower pelvic BMD (p-trend = 0.033) (Table 3, Figure 1). Compared to the reference distance (>1,500m) from the nearest freeway, participants living within 500m of a freeway had on average 0.02 g/cm2 lower body BMD (p<0.001) and 0.03 g/cm2 lower pelvic BMD (p=0.012) (Table 3, Figure 1). Similar associations were found for both body (p<0.001) and pelvic BMD (p=0.007) when comparing participants within 500m of a freeway to the remainder of the cohort who lived farther than 500m from a freeway (Table 3). Additional analysis using log-transformed residential distances from the nearest freeway as a continuous variable showed similar results, with living closer to freeways being significantly associated with lower total body BMD (p=0.032) and marginally significant for pelvic BMD (p=0.12).
Table 3.
Associations between traffic exposures and BMD measurements among BetaGene participantsa
| Traffic Exposures | n (%) | Total Body BMD (g/cm2 × 100) | Pelvic BMD (g/cm2 × 100) | ||||
|---|---|---|---|---|---|---|---|
| Adjusted Mean (SE)c | β | p-value | Adjusted Mean (SE)c | β | p-value | ||
| Residential distance from the nearest freeway (m) | |||||||
| > 1500 m | 510 (44.9) | 110.6 (0.4) | Reference | 124.8 (0.6) | Reference | ||
| 1000 – 1500 m | 161 (14.2) | 110.3 (0.7) | −0.313 | 0.65 | 125.1 (1.1) | 0.250 | 0.82 |
| 500 – 1000 m | 220 (19.4) | 110.7 (0.6) | 0.029 | 0.97 | 124.8 (0.9) | −0.036 | 0.97 |
| ≤ 500 m | 246 (21.6) | 108.5 (0.5) | −2.112 | <0.001 | 122.3 (0.9) | −2.500 | 0.012 |
| Trend test | −0.497 | 0.012 | −0.466 | 0.033 | |||
| > 500 m | 891 (78.4) | 110.6 (0.3) | Reference | 124.9 (0.5) | Reference | ||
| ≤ 500 m | 246 (21.6) | 108.5 (0.5) | −2.062 | <0.001 | 122.3 (0.9) | −2.536 | 0.007 |
| Residential distance from the nearest major road (m)b | |||||||
| > 300 m | 365 (32.1) | 110.8 (0.5) | Reference | 124.8 (0.8) | Reference | ||
| 150 – 300 m | 335 (29.5) | 110.4 (0.5) | −0.334 | 0.60 | 124.2 (0.8) | −0.597 | 0.54 |
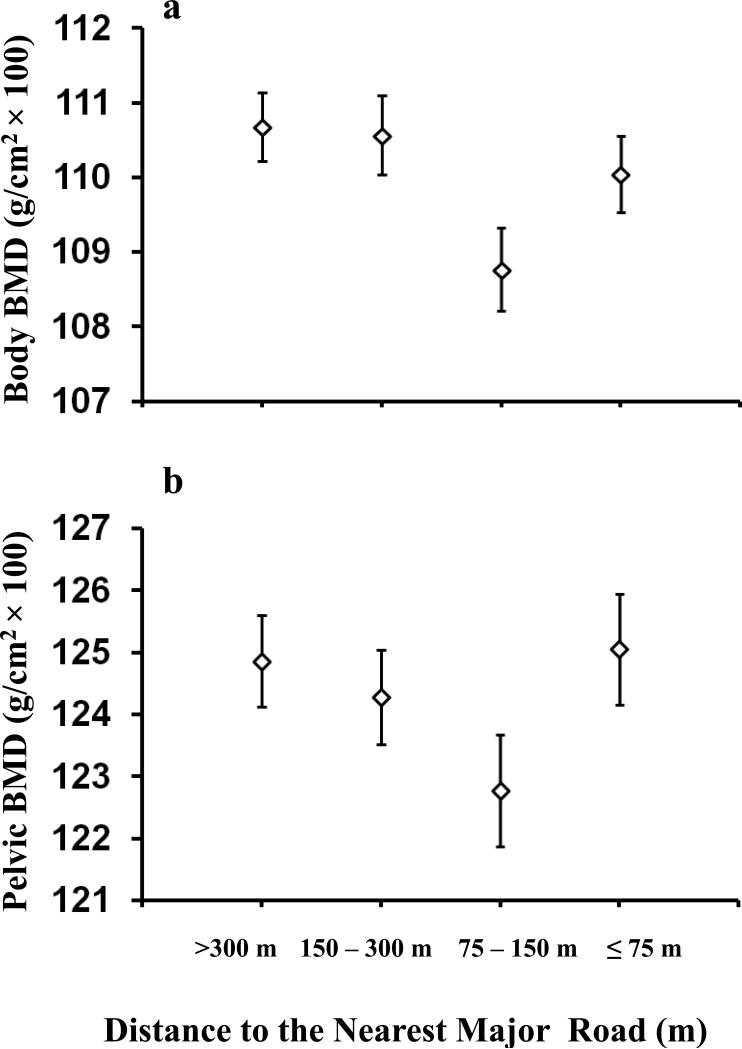
| 75 – 150 m | 225 (19.8) | 108.8 (0.6) | −1.950 | 0.003 | 122.8 (0.9) | −2.033 | 0.08 |
| ≤ 75 m | 212 (18.7) | 110.2 (0.5) | −0.512 | 0.40 | 125.2 (0.9) | 0.386 | 0.70 |
| Trend test | −0.634 | 0.036 | −0.253 | 0.45 | |||
| > 15 0 m | 700 (61.6) | 110.6 (0.4) | Reference | 124.0 (0.7) | Reference | ||
| ≤ 150 m | 437 (38.4) | 109.5 (0.4) | −1.085 | 0.013 | 124.5 (0.6) | −0.561 | 0.46 |
Associations were adjusted for age, sex, weight and height. Regression coefficients (β) and test statistics p-values are presented in the table.
Large roadways are defined as both major and minor collectors coded as FCC2 and FCC3.
Age-, sex-, weight-, and height-adjusted means and standard errors of BMD over different categories of traffic exposures are presented.
Figure 1.
Adjusted means and standard errors of total body BMD and pelvic BMD over categories of the distance from the nearest freeway. The model is adjusted for age, sex, weight and height
A significant trend was also found for the association of residential distance from the nearest major road and total body BMD (p-trend = 0.036) although the pattern did not follow a monotonic increase (Table 3, Figure 2). Those living within 150m of a major road had a 0.01 g/cm3 lower BMD compared to those living greater than 150m of a major road (p=0.013). No significant trend was found between the distance to the nearest major road and pelvic BMD (p=0.60, Table 3, Figure 2).
Figure 2.
Adjusted means and standard errors of total body BMD and pelvic BMD over categories of the distance from the nearest major road. A major road is defined as both major and minor collectors. The model is adjusted for age, sex, weight and height
Since post-menopausal women experience expedited bone loss due to estrogen deficiency, we performed a sensitivity analysis excluding women (n=13) above 51 years, which is the national average age for menopause in the U.S. [25] and was also the median menopause age among Hispanics [26]. Associations of distances to a freeway with body (p-trend=0.020) and pelvic BMD (p-trend=0.047) remained significant in premenopausal women. In addition, women with histories of GDM have higher risk for bone loss among Hispanics [27, 28] and other populations [29]. In our cohort, women with GDM had on average 0.9% lower total body BMD and 0.7% lower pelvic BMD than non-GDM women, though the difference was not statistically significant. Therefore, we conducted a restricted subgroup analysis among 207 women with GDM, and found the direction of associations between distances from a freeway with body and pelvic BMD were the same as previous observations in the entire cohort, but the magnitude of the associations were smaller and tests of associations were not significant (all p>0.45), probably due to small sample size. Additionally, there were no statistically significant effect modifications by age, sex, and obesity status in the above associations (all interaction p values > 0.39, Figure A1-A3 in online supplement).
Previous studies documented that percent body fat was negatively associated with BMD [30] and positively associated with air pollution [31], so body fat was tested as a potential confounder. Significant associations between distance to the nearest freeway and total body and pelvic BMD remained after adjusting for percent body fat (p-trend=0.006 and 0.032, respectively). Adjustment for percent body fat reduced the trend association between distances from the nearest major road and total body BMD by 16% (p=0.06). Further adjustment for parity, total physical activity minutes per week, contextual variables, daily calcium intake and vitamin D intake did not significantly influence the association between residential proximity to a major freeway and total body BMD (changes in regression coefficients were all less than 10%, Table A1 in online supplement).
And finally, we did not find significant associations between the ambient air pollutants NO2, O3 and PM2.5 and total body and pelvic BMD after adjusting for age, sex, weight and height (all p>0.47, Table A2 in online supplement). However, the directions of associations were consistent with observed results from traffic exposures. Higher annual NO2 and PM2.5 exposures were associated with lower total body and pelvic BMD. Because NO2 was negatively correlated with BMD and O3 was negatively correlated with NO2 (Pearson correlation coefficient = -0.7), the positive relationship between O3 and BMD most likely reflects the relationship between NO2 and BMD. Multiple-pollutant models did not reveal significant joint associations of multiple pollutants with BMD (all p>0.40). However, significant associations between distances from the nearest freeway and BMD remained after adjusting for ambient air pollutants.
DISCUSSION
In this study, we observed that Mexican American adults living near freeways had lower total body and pelvic BMD, independent of weight, height, percent body fat, physical activity, contextual variables, and daily calcium and vitamin D intakes. Additionally, trends were consistent across different age groups, between males and females, and between normal weight and overweight or obese subjects. Similar trends were also found for distances to a major road and BMD. However, no associations were found between BMD and ambient air pollutants including NO2, O3 and PM2.5. These findings suggest that exposures to traffic may lead to decreased bone density and therefore increase risk for osteopenia/osteoporosis in Mexican American adults.
The inverse association between smoking and BMD has been observed in many studies [3, 32]. However, few studies have evaluated the impact of air pollution on BMD. A sub-study of the Oslo Health Study (2000-2001) with 590 men aged around 75 years documented that higher concentrations of annual average PM2.5 and PM10 during years 1992-2001 were related to lower total body BMD, but not total hip BMD [16]. The adjusted odds ratio [95% CI] for low total body BMD (Z-score ≤ -1) was 1.33 [1.05-1.70] and 1.28 [1.00-1.63] for a standard deviation increase of PM2.5 and PM10, respectively. Another study of 105 Romanian subjects with mean age of 62 found the air pollution index (the sum of proportions of mean concentrations of annual average NO2 and PM10 over their maximum permissible concentrations) was inversely associated with total body BMD, which suggested that NO2 and PM10 might have a synergistically detrimental effect on BMD [15]. We did not find significant associations of individual or multiple ambient air pollutants including NO2, O3, and PM2.5 with total body or pelvic BMD. The lack of significant findings might be due to small variations in BMD among our relatively younger participants, which limited our power to detect significant associations. However, we found residential proximity to the nearest freeway was associated with lower total body and pelvic BMD. These results suggest that automotive exhaust might have adverse effects on bone mass. One group of chemicals in automotive exhaust potentially related to BMD is the polycyclic aromatic hydrocarbons (PAHs), which are also found in the tar fraction of cigarette smoke. A rat study revealed that PAHs caused a loss of bone mass and bone strength, possibly through enhanced bone turnover [33]. Taken together, our results suggest the negative impact of combustion-related pollutants on bone density.
The biological mechanism underlying the impact of traffic-related pollution on BMD is unclear; but several mechanisms can be hypothesized. First, enhanced inflammatory responses to air pollution exposures can be considered as a contributory factor. Accumulating evidence indicates that air pollution is associated with chronic inflammatory conditions [34]. Chronic inflammation characterized by elevated levels of pro-inflammatory markers including interleukin (IL-6), tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP) have been implicated as the mediators of air pollution associated risk of these conditions. Osteoporosis is closely linked with chronic inflammation. Experimental and in vitro studies indicate that pro-inflammatory cytokines have stimulatory effects on osteoclastogenesis [35, 36]. Age-associated increases in TNF-α and IL-6 partially explain bone loss in older age, particularly in women as lack of estrogen is associated with increases in TNF-α and IL-6 [37]. Consistent with the experimental and in vitro study findings, higher levels of IL-6, TNF-α, and CRP were consistently inversely associated with BMD in postmenopausal women [38] and men [39].
In addition to creating a heightened inflammatory response, air pollution can induce immune activation [40]. While there is no direct evidence linking immune activation and BMD, increased incidence and prevalence of osteoporosis were noted in subjects with inflammatory and immunologic disorders [41]. In addition, IFN-γ released from Th1 response of activated T-cells has an inhibitory effect on bone loss [42]. Therefore, it is possible that our finding of an association between traffic exposures and lower BMD can be mediated by activated immune system, a hypothesis that is yet to be tested.
Finally, vitamin D deficiency should be considered in understanding the mechanism underlying the association between traffic-related pollution and low BMD. A study among 35 six-year-old children found exposure to urban air pollutants was significantly associated with higher IL-6 and vitamin D deficiency [14]. Traffic emissions are a major source of urban air pollution. Vitamin D is critical for intestinal absorption of dietary calcium. Insufficient vitamin D can cause low serum calcium level, which induces osteoclast activity and mobilization of calcium from the skeleton into the extracellular space [43]. Living near traffic is also related with fewer outdoor activities [44], which can lead to reduced solar ultraviolet B (UVB) exposures. Insufficient cutaneous absorption of UVB is one of the major causes of vitamin D deficiency. Clearly, more studies are warranted to understand the mechanistic pathways explaining the association between traffic exposure and lower BMD.
We acknowledge our study has some limitations. First, the majority of our cohort was overweight and obese people, which restricted the interpretation of results to a general population. However, adjustment for body fat did not significantly influence our results. Stratified analysis by obesity status showed the association effect sizes were not significantly different between normal weight and overweight or obese people. Second, the calculation of residential distances from the nearest freeway and major road at study entry is only an approximate measure of exposure to traffic-related pollutants. This method of exposure assessment is likely to result in non-differential exposure misclassification, which would bias risk estimates towards the null. Third, we did not collect information on some covariates such as noise levels, medication, and smoking history. However, it has been reported that the smoking prevalence among Hispanic men was almost twice the smoking prevalence among Hispanic women (20.7% for men and 10.7% for women) [45], and we did not find the associations between the proximity to freeways and BMD was significantly different between men and women. This suggests that adjustment for smoking may have little influence on our observed associations. Also, future studies are warranted to examine the impact of residential and occupational exposures on BMD. Lastly, the cross-sectional design of our study precludes us from examining the dynamic impact of air pollution on the change of BMD over time. Another limitation is that we do not have information of how long participants had lived in the address as provided for this study. Future research with improved exposure assessment and a longitudinal design is needed to confirm and extend these findings and to better understand the magnitude of the impacts of traffic-related air pollutants on BMD and osteoporosis-related morbidity.
To our knowledge, this is the first study demonstrating an association between traffic exposures and lower BMD among Mexican American adults. While previously published work had shown lower BMD in men and women living in urban areas compared to those living in rural areas [10, 11], our work indicates that higher traffic-related pollution in urban compared to rural areas could be one explanation for the urban vs. rural difference in BMD. Our novel findings require confirmation, but have important public health implications for implementing regulatory strategies to reduce traffic-related exposures thereby delaying bone loss and preventing osteoporosis.
In conclusion, our study indicates that traffic exposures in overweight and obese Mexican Americans may adversely affect BMD. Our findings raise the possibility that air pollutants, especially traffic-related air pollutions, may contribute to osteoporosis and its consequences.
Supplementary Material
ACKNOWLEDGEMENTS
Authors’ roles: Z.C. and F.D.G. conducted the analyses and wrote the article. E.T., R.M.W, T.A.B, M.T., J.W., and F.L. contributed to study design and data collection. M.T.S., R.K., C.M.T., R.M.W, A.H.X., R.H., T.M.B., M.T., J.P.W., and T.A.B revised the article and contributed to data analyses and discussion. All authors reviewed the article. Z.C. and F.D.G. are the guarantors of this work, and as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by National Institutes of Health grants DK-061628, M01-RR-0043 and UL1-TR000130; Clinical Research Grant 7-09-CT-09 from the American Diabetes Association Research Award ; the Southern California Environmental Health Sciences Center (grant 5P30ES007048) funded by the National Institute of Environmental Health Sciences; National Institute of Environmental Health Sciences (grant 5P01ES011627); and the Hastings Foundation.
Footnotes
Zhanghua Chen, Muhammad T. Salam, Roksana Karim, Claudia M. Toledo-Corral, Richard M. Watanabe, Anny H. Xiang, Thomas A. Buchanan, Rima Habre, Theresa Bastain, Fred Lurmann, Maryam Taher, John Wilson, Enrique Trigo, and Frank D. Gilliland declare that they have no conflict of interest.
REFERENCES
- 1.WHO . WHO Technical Report Series. World Health Organization; Geneva: 2003. Prevention and management of osteoporosis. [PubMed] [Google Scholar]
- 2.National Osteoporosis Foundation [18 March 2014];America's bone health: the State of osteoporosis and low bone mass in our nation. 2002 Available: http://nof.org/files/nof/public/content/file/63/upload/49.pdf.
- 3.Lorentzon M, Mellström D, Haug E, Ohlsson C. Smoking Is Associated with Lower Bone Mineral Density and Reduced Cortical Thickness in Young Men. Journal of Clinical Endocrinology & Metabolism. 2007;92:497–503. doi: 10.1210/jc.2006-1294. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg T, Bech M, Curtis T, Juel K, Gronbaek M, Brixen K. Association between passive smoking in adulthood and phalangeal bone mineral density: results from the KRAM study--the Danish Health Examination Survey 2007-2008. Osteoporosis International. 2011;22:2989–2999. doi: 10.1007/s00198-010-1506-9. [DOI] [PubMed] [Google Scholar]
- 5.Heaney RP. Nutritional factors in osteoporosis. Annual review of nutrition. 1993;13:287–316. doi: 10.1146/annurev.nu.13.070193.001443. [DOI] [PubMed] [Google Scholar]
- 6.Pigozzi F, Rizzo M, Giombini A, Parisi A, Fagnani F, Borrione P. Bone mineral density and sport: effect of physical activity. The Journal of sports medicine and physical fitness. 2009;49:177–183. [PubMed] [Google Scholar]
- 7.Sommer I, Erkkila AT, Jarvinen R, Mursu J, Sirola J, Jurvelin JS, Kroger H, Tuppurainen M. Alcohol consumption and bone mineral density in elderly women. Public health nutrition. 2013;16:704–712. doi: 10.1017/S136898001200331X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Laughlin GA, Li H, et al. The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2012;27:2306–2313. doi: 10.1002/jbmr.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoback D. Update in Osteoporosis and Metabolic Bone Disorders. Journal of Clinical Endocrinology & Metabolism. 2007;92:747–753. doi: 10.1210/jc.2007-0042. [DOI] [PubMed] [Google Scholar]
- 10.Gu W, Rennie KL, Lin X, Wang Y, Yu Z. Differences in bone mineral status between urban and rural Chinese men and women. Bone. 2007;41:393–399. doi: 10.1016/j.bone.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Specker B, Binkley T, Fahrenwald N. Rural versus nonrural differences in BMC, volumetric BMD, and bone size: a population-based cross-sectional study. Bone. 2004;35:1389–1398. doi: 10.1016/j.bone.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Omsland TK, Ahmed LA, Gronskag A, et al. More forearm fractures among urban than rural women: the NOREPOS study based on the Tromso study and the HUNT study. J Bone Miner Res. 2011;26:850–856. doi: 10.1002/jbmr.280. [DOI] [PubMed] [Google Scholar]
- 13.Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. J Epidemiol Community Health. 2002;56:466–470. doi: 10.1136/jech.56.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderon-Garciduenas L, Mora-Tiscareno A, Francolira M, et al. Exposure to urban air pollution and bone health in clinically healthy six-year-old children. Arh Hig Rada Toksikol. 2013;64:23–34. doi: 10.2478/10004-1254-64-2013-2219. [DOI] [PubMed] [Google Scholar]
- 15.Cevei M, Stoicanescu D. Air pollution and genetic influences on bone mineral density and osteoporosis. Analele Universitatii din Oradea. Fascicula Biologie TOM. 2010;XVII(1):84–89. [Google Scholar]
- 16.Alvær K, Meyer HE, Falch JA, Nafstad P, Søgaard AJ. Outdoor air pollution and bone mineral density in elderly men - the Oslo Health Study. Osteoporosis International. 2007;18:1669–1674. doi: 10.1007/s00198-007-0424-y. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) [Dec. 12th 2014];Air quality deteriorating in many of the world's cities. 2014 Available at http://www.who.int/mediacentre/news/releases/2014/air-quality/en/.
- 18.Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes. 2007;56:1481–1485. doi: 10.2337/db06-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A Multiethnic Cohort in Hawaii and Los Angeles: Baseline Characteristics. American Journal of Epidemiology. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Benson PE, Pinkerman KO, Laboratory COoT, Administration USFH . CALINE4 - A dispersion model for predicting air pollution concentrations near roadways. State of California, Dept. of Transportation, Division of Engineering Services, Office of Transportation Laboratory; 1984. [Google Scholar]
- 22.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 23.WHO . Physical status: the use and interpretation of anthropometry. World Health Organization; 1995. [April 7th 2014]. Available at: http://whqlibdoc.who.int/trs/WHO_TRS_854.pdf. [Google Scholar]
- 24.Looker A, Borrud L, Hughes J, B F, Shepherd J, Melton LI. Lumbar Spine and Proximal Femur Bone Mineral Density, Bone Mineral Content, and Bone Area: United States, 2005-2008. Dept. of Health and Human Services, Public Health Service, National Center for Health Statistics; Hyattsville, Md.: 2012. p. 10. [PubMed] [Google Scholar]
- 25.National Institute on Aging . Menopause. National Institute on Aging, National Institutes of Health, U.S. Department of Health and Human Services; 2013. [Dec. 1st 2014]. Available: http://www.nia.nih.gov/health/publication/menopause. [Google Scholar]
- 26.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors Associated with Age at Natural Menopause in a Multiethnic Sample of Midlife Women. American Journal of Epidemiology. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 27.Chau DL, Edelman SV. Osteoporosis and Diabetes. Clinical Diabetes. 2002;20:153–157. [Google Scholar]
- 28.Chau DL, Mulvihill M, Moore T, Bouno M, Ramsdell J, Hovell M. Oral presentation: “Post-partum bone loss in Latino women with prior gestational diabetes.”.. Western Regional Meeting of the American Federation of Medical Research.2002. [Google Scholar]
- 29.To WWK, Wong MWN. Bone mineral density changes in gestational diabetic pregnancies–a longitudinal study using quantitative ultrasound measurements of the os calcis. Gynecological Endocrinology. 2008;24:519–525. doi: 10.1080/09513590802288184. [DOI] [PubMed] [Google Scholar]
- 30.Lu H, Fu X, Ma X, Wu Z, He W, Wang Z, Allison D, Heymsfield S, Zhu S. Relationships of percent body fat and percent trunk fat with bone mineral density among Chinese, black, and white subjects. Osteoporosis International. 2011;22(12):3029–3035. doi: 10.1007/s00198-010-1522-9. [DOI] [PubMed] [Google Scholar]
- 31.Rundle A, Hoepner L, Hassoun A, et al. Association of Childhood Obesity With Maternal Exposure to Ambient Air Polycyclic Aromatic Hydrocarbons During Pregnancy. American Journal of Epidemiology. 2012;175(11):1163–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oncken C, Prestwood K, Kleppinger A, Wang Y, Cooney J, Raisz L. Impact of smoking cessation on bone mineral density in postmenopausal women. J Womens Health (Larchmt) 2006;15:1141–1150. doi: 10.1089/jwh.2006.15.1141. [DOI] [PubMed] [Google Scholar]
- 33.Lee LL, Lee JS, Waldman SD, Casper RF, Grynpas MD. Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone. 2002;30:917–923. doi: 10.1016/s8756-3282(02)00726-3. [DOI] [PubMed] [Google Scholar]
- 34.Brook RD, Rajagopalan S, Pope CA, et al. Particulate Matter Air Pollution and Cardiovascular Disease: An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocrine reviews. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocrine reviews. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 37.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocrine reviews. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 38.Giuliani N, Sansoni P, Girasole G, Vescovini R, Passeri G, Passeri M, Pedrazzoni M. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Experimental gerontology. 2001;36:547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 39.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. The Journal of clinical endocrinology and metabolism. 2008;93:1952–1958. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 40.Deiuliis JA, Kampfrath T, Zhong J, Oghumu S, Maiseyeu A, Chen LC, Sun Q, Satoskar AR, Rajagopalan S. Pulmonary T cell activation in response to chronic particulate air pollution. American journal of physiology Lung cellular and molecular physiology. 2012;302:L399–409. doi: 10.1152/ajplung.00261.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikuls TR, Saag KG, Curtis J, et al. Prevalence of osteoporosis and osteopenia among African Americans with early rheumatoid arthritis: the impact of ethnic-specific normative data. Journal of the National Medical Association. 2005;97:1155–1160. [PMC free article] [PubMed] [Google Scholar]
- 42.Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 43.Moller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect. 2010;118:1126–1136. doi: 10.1289/ehp.0901725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank L, Engelke P, Schmid T. Health and Community Design: The Impact Of The Built Environment On Physical Activity. Island Press; 2003. [Google Scholar]
- 45.American Lung Association [Dec. 17 2014];Tobacco Use. 2010 Available at: http://www.lung.org/assets/documents/publications/solddc-chapters/tobacco.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.