Abstract
In the peripheral nervous system, Schwann cells are glial cells that are in intimate contact with axons throughout development. Schwann cells generate the insulating myelin sheath and provide vital trophic support to the neurons that they ensheathe. Schwann cell precursors arise from neural crest progenitor cells, and a highly ordered developmental sequence controls the progression of these cells to become mature myelinating or non-myelinating Schwann cells. Here, we discuss both seminal discoveries and recent advances in our understanding of the molecular mechanisms that drive Schwann cell development and myelination with a focus on cell-cell and cell-matrix signaling events.
Keywords: Schwann cell, Peripheral nervous system, neural crest, Schwann cell precursor, immature Schwann cell, radial sorting, myelinating Schwann cell, Remak Schwann cell
Neural Crest Cells – A Brief Introduction
During early vertebrate development, neural crest cells delaminate from the dorsal-most region of the neural tube; these progenitor cells are migratory and proliferative, and they disperse throughout the embryo to give rise to a remarkable variety of cell types including cardiac cells, melanocytes, skeletal and connective tissue components of the head, and neurons and glia of the peripheral nervous system (PNS). Induction of the neural crest from ectoderm requires bone morphogenetic protein (BMP), fibroblast growth factor (FGF), and Wnt signaling pathways. This induction and the gene regulatory network that controls subsequent steps of neural crest development are reviewed elsewhere (e.g., (Sauka-Spengler and Bronner-Fraser 2008; Stuhlmiller and Garcia-Castro 2012)).
Upon delamination from the dorsal neural tube, neural crest cell migration is directed by both instructive (e.g., receptor tyrosine kinase signaling) and permissive (e.g. extracellular matrix (ECM) components) cues (reviewed in (Theveneau and Mayor 2012)). Neural crest cells can be classified according to the region of the neural tube along the anterior-posterior axis from which they delaminate: cranial, cardiac, vagal, trunk, and sacral, and this regional origin greatly impacts subsequent development. For example, both cranial and trunk neural crest cells can form pigment cells, glial cells, and peripheral neurons, but only cranial neural crest cells can form bone and cartilage. Moreover, when trunk neural crest cells are transplanted into the head region, they follow cranial crest migratory routes but do not generate cranial crest derivatives. In contrast, transplanted cranial neural crest cells migrate and differentiate similarly to trunk neural crest. It is thought that the ability to form bone is an ancient property of neural crest cells, which has been lost during the course of evolution in trunk and other non-cranial neural crest cells (Smith and Hall 1993).
Importantly for the purposes of this review, the majority of neural crest-derived cells in the PNS, including Schwann cells (SCs), develop from trunk neural crest. Trunk neural crest cells migrate along two developmentally distinct pathways: (1) a ventral pathway, which occurs first, in which neural crest cells travel ventrally through the anterior sclerotome; and (2) a dorsolateral pathway between the dermis and the epidermis. SCs derive from ventrally migrating neural crest cells, as do sympathetic neurons, sensory dorsal root ganglia (DRG) neurons, and other glia associated with these neurons (Le Douarin and Teillet 1974; Weston 1963).
The multipotency vs. fate restriction of migrating neural crest cells is an area of active research. Some studies support the notion that neural crest cells are highly plastic during migration. Marker analyses indicate that there is little heterogeneity before delamination and during the earliest migratory stages (Prendergast and Raible 2014) and some lineage tracing studies in chick embryos show that a single neural crest cell can give rise to many cell types (Bronner-Fraser and Fraser 1988; Frank and Sanes 1991). A very recent fate mapping study demonstrated that most neural crest cells are multipotent in vivo in mouse (Baggiolini et al. 2015). Conversely, other lineage tracing studies in zebrafish and chick suggest that fate restriction occurs early, even before migration has commenced (reviewed in (Prendergast and Raible 2014)). Current models incorporating all data posit that an original multipotent neural crest cell divides and progressively defines its developmental potential. However, individual neural crest cells can differ greatly in their developmental potential and commitments, and these fates may be specified prior to delamination and migration, or these fates may be influenced by the migratory pathway and final location that a given neural crest cell experienced. For further reading, we suggest several excellent reviews and primary research articles (e.g., (Baggiolini et al. 2015; Hall 2000; Le Douarin 1980; Newbern 2015; Prendergast and Raible 2014; Rogers et al. 2013)).
While most SCs arise from neural crest-derived SC precursors (SCPs; discussed in more detail below), it is important to note that some SCs arise from neural crest-derived boundary cap cells. Boundary cap cells are a transient population of progenitor cells located at embryonic motor exit points and dorsal root entry zones that give rise to SCs of the dorsal and ventral roots (Maro et al. 2004). These progenitor cells form an essential barrier between the PNS and the central nervous system (CNS) such that loss of boundary cap cells results in mislocalization of oligodendrocytes and motor neurons in the PNS (reviewed in (Newbern 2015)). Boundary cap cells can be distinguished molecularly from neural crest cells and SCPs by several markers including the Wnt inhibitory factor-1 gene (Wif1), L20, which encodes a protein of unknown function, and early expression of the transcription factor Krox-20 (Egr2) (Coulpier et al. 2009). Although boundary cap cells are relatively understudied, in the future, it will be interesting to compare differences in the development and biology between boundary cap cells present in the dorsal and ventral roots as well as to determine how mature SCs derived from boundary cap cells vary in development and/or in injury response compared to non-boundary cap-derived SCs.
Establishment of the Schwann Cell Lineage from Neural Crest Cells
Although the molecular mechanisms that govern the development of SCPs from neural crest cells are incompletely understood, some key players are known. Sox10, a basic helix-loop-helix (bHLH) transcription factor, is expressed in neural crest cells; Sox10 expression persists in PNS glia and melanocytes, but is downregulated in neurons and other crest derivatives (Kuhlbrodt et al. 1998; Woodhoo and Sommer 2008). Sox10 mutant mice and zebrafish lack peripheral glia (Britsch et al. 2001; Kelsh and Eisen 2000); however, while Sox10 is necessary for SC specification, it is not sufficient. Seminal clonal analysis studies of rat neural crest showed that Neuregulin-1 (NRG1) suppresses neuronal differentiation and promotes glial specification (Shah et al. 1994). More recently, Jacob and colleagues demonstrated that the histone deacetylases 1 and 2 (HDAC1/2) induce expression of Pax3, a paired box family transcription factor known to be important for SC differentiation and proliferation (Blanchard et al. 1996; Doddrell et al. 2012; Kioussi et al. 1995). Pax3 in turn is required to maintain high levels of Sox10 in SC lineage cells and to induce expression of the key SC lineage genes, Fatty acid binding protein 7 (Fabp7) and Myelin protein zero (P0) (Jacob et al. 2014).
Schwann Cell Precursors and the Transition to Immature Schwann cells
SCPs, like neural crest cells, are migratory and proliferative. SCPs comigrate with axons in the developing PNS and they are dependent upon axonal signals for survival (Dong et al. 1995). Recent data demonstrates that cranial SCPs can also give rise to parasympathetic ganglia (Dyachuk et al. 2014; Espinosa-Medina et al. 2014), although we focus on the transition from SCP to immature SC for the purposes of this review. To this end, NRG1 is an essential regulator of SCP development. NRG1 binds ErbB2/3, an obligate heteromeric receptor tyrosine kinase pair, on SCPs to activate key downstream signal transduction cascades. NRG1 activation of ErbB2/3 is essential for both SCP proliferation and directed migration. The NRG1-ErbB2/3 signaling axis in SCPs has been recently and extensively reviewed elsewhere (e.g., (Newbern and Birchmeier 2010; Raphael and Talbot 2011)).
In time, SCPs cease migration and develop into immature SCs. Although the molecular mechanisms that govern this transition are incompletely understood, Notch signaling is one mediator of the SCP to immature SC transition. Loss of Notch1 activity or of the downstream transcriptional activator RBP-J (Recombining binding protein suppressor of hairless) in SCPs decreases SCP proliferation and inhibited immature SC formation, while overactivation of the pathway increases SCP proliferation and immature SC number (Woodhoo et al. 2009).
Immature SCs populate axons that are still projecting to their innervating target and acquire a set of properties that clearly distinguish them from SCPs. They cease migrating, they become dependent on autocrine, not axonal, factors to survive, and they deposit an organized a basal lamina. We refer the reader to the seminal review by Jessen and Mirsky for more details on these properties (Jessen and Mirsky 2005).
Biology of Immature Schwann cells
Functionally, immature SCs contribute to nerve morphogenesis by accomplishing two important steps: (1) in a process called radial sorting, they separate axons destined to become myelinated from those that will remain in non-myelinated Remak bundles; and (2) they signal to perineurial cells and other nerve components to promote their differentiation. These morphogenic events contribute to build the mature final nerve architecture that consists of myelinated fibers and Remak bundles properly surrounded by extracellular matrix and blood vessels.
Communication between immature SCs and other cellular components in nerves
During early development, SCPs and axons are the only cellular constituents of future peripheral nerves. When immature SCs are generated, they signal to surrounding mesenchymal cells to promote their differentiation into arterial and perineurial cells, and their organization into arteries and the perineurial/epineurial sheaths that surround mature nerves, respectively (Mukouyama et al. 2005; Parmantier et al. 1999). To promote arterial differentiation, SCs secrete VEGF, which interacts with neuropilin 1 on endothelial cells (Mukouyama et al. 2005), while to promote perineurial differentiation, SCs secrete Desert hedgehog (Dhh) that signals via patched receptors on mesenchymal cells (Parmantier et al. 1999). DHH mutations in mice and humans cause a peripheral neuropathy (OMIM #607080) with aberrant perineurial formation and increased nerve permeability, microfasciculation, and eventually axonal degeneration (Sharghi-Namini et al. 2006; Umehara et al. 2000). Immature SCs also likely communicate with endoneurial fibroblasts, another neural crest derivative that might also originate from SCPs (Joseph et al. 2004). In vitro, fibroblasts promote the deposition of basal lamina (Obremski et al. 1993), and after injury, fibroblasts and SC communicate via ephrin-B/EphB2 signaling (Parrinello et al. 2010), suggesting that similar communication may occur during development.
Radial sorting and the transition to promyelinating SC
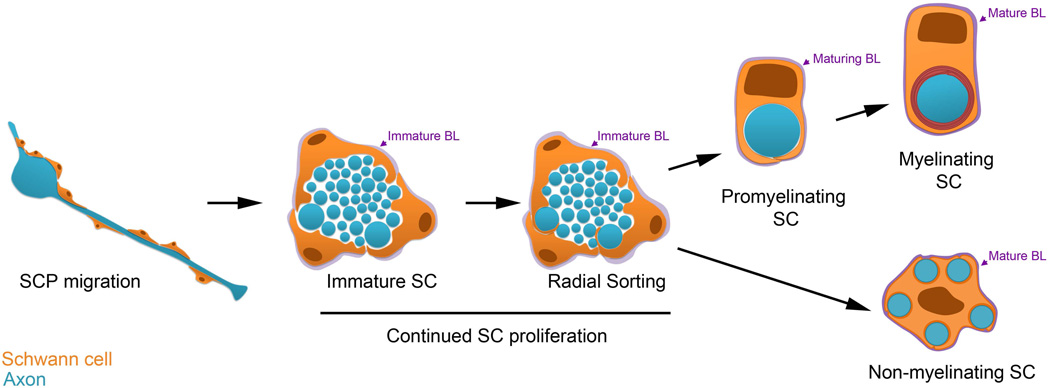
Radial sorting of axons by SCs starts perinatally and continues until about post-natal day 10 in rodents; this event serves to separate large caliber axons destined to be myelinated away from small caliber axons, which will ultimately reside in Remak bundles. SCs can myelinate only one axon segment, and only after reaching a 1:1 relationship with the axon (now termed a promyelinating SC) (Figure 1). In contrast, non–myelin forming SCs termed Remak SCs ensheathe several small axons without making myelin. Radial sorting enables immature SCs to achieve the proper relationship with axons, which is required for further differentiation. Our understanding of the events leading to radial sorting has been greatly informed by electron microscopy reconstructions of serial sections by Henry deF Webster (Webster et al. 1973), which showed that the process begins with immature SCs that infiltrate within the nerve, and subdivide axons into bundles surrounded by a “family” of 3–8 SCs that deposit a common basal lamina.
Figure 1. Development of Myelinating and Non-myelinating Schwann Cells.
Cartoon depicting Schwann cell (SC) development. SCs are orange and axons are blue. From left, SC precursors (SCPs), depicted longitudinally, are proliferative and migrate with growing axons. The remaining stages are depicted in cross-section. SCPs become immature SCs, which are associated with many axons. Immature SCs have ceased migration, remain proliferative, and form an immature basal lamina (BL, purple). The BL persists and matures through subsequent stages of SC development. During radial sorting, immature SCs interdigitate their cytoplasmic processes into the axon bundle, and promyelinating SCs are associated with a single axonal segment. Myelinating SCs spiral their membrane many times around the axonal segment to form the myelin sheath. Immature SCs can also develop into Non-myelinating SCs. A Remak SC, entheathing multiple small caliber axons is depicted.
Next, immature SCs send lamellipodia-like processes that interact with large caliber axons and segregate them to the periphery of the bundle (Figure 1). At this stage, it is thought that an immature SC divides, still within a common basal lamina, and a daughter SC remains associated via a long axial process to one axon and then separates it from the bundle (defasiculation), reconstituting its own basal lamina (Webster et al. 1973). Only at this point can the promyelinating SC, surrounded by its own basal lamina, begin to myelinate. When all of the large caliber axons have been separated, immature SC that embrace the remaining small caliber axons differentiate into Remak SC. This occurs and the end of radial sorting, starting around P15 in rodents.
Molecular control of radial sorting
Several steps must occur for proper radial sorting to be completed. Immature SCs must deposit ECM components and organize them into a basal lamina. Next, they must establish polarity between the basal lamina and axons, extend cytoplasmic processes to contact and recognize axons, proliferate, defasciculate axons, and re-establish correct polarization and its own basal lamina. Spontaneous and targeted mutants have revealed that many molecules that operate in these fundamental cellular processes in other cells, when mutated impair or arrest radial sorting in SCs. Hence, ECM components and components of signaling pathways that govern polarity, the formation of polarized cellular protrusions, cytoskeletal dynamics, and proliferation are all required in SCs for radial sorting of axons.
Deletion or inactivation of the ECM components Laminin 211, Laminin 411, or Collagen XV or of their receptors Integrins α6β1, α7β1, and Dystroglycan, or of the Dystroglycan glycosylation enzyme Fukutin all impair radial sorting (Berti et al. 2011; Feltri et al. 2002; Occhi et al. 2005; Patton et al. 2008; Pellegatta et al. 2013; Rasi et al. 2010; Saito et al. 2007; Saito et al. 2003; Wallquist et al. 2005; Yang et al. 2005), probably due to defects in basal lamina signaling to control SC proliferation, polarization, and formation of cellular protrusion. In keeping with this idea, deletion of signaling molecules downstream of laminin receptors causes similar defects, such as those seen in mutants with deletion of ILK, FAK, the Rho GTPases Rac1 and Cdc42, Profilin, Merlin/Nf2, and N-WASp (Benninger et al. 2007; Grove et al. 2007; Guo et al. 2012; Guo et al. 2013b; Jin et al. 2011; Montani et al. 2014; Nodari et al. 2007; Novak et al. 2011; Pereira et al. 2009). These molecules likely are required for SCs to regulate the actin cytoskeleton in order to generate cytoplasmic protrusions and to interact with axons. Indeed, SC processes that ensheathe and subdivide axons are often absent in many of these mutants (Benninger et al. 2007; Guo et al. 2012; Guo et al. 2013b; Jin et al. 2011; Montani et al. 2014; Pereira et al. 2009). Cdc42 and other Rho GTPases are also required for cell polarization, and although it has not been directly proven, it is likely that laminin and axonal signaling together initiate SC polarization during axonal sorting. In agreement with this idea, SC lineage deletion of Liver kinase B1 (Lkb1), which regulates SC metabolism and polarity, causes a transient delay in radial sorting (Beirowski et al. 2014; Shen et al. 2014), and it is likely that members of Par polarity complexes will also be involved (Chan et al. 2006; Lewallen et al. 2011). In contrast to SC-derived signals, only a few axonal molecules that control radial sorting have been identified. These include several Wnt and Respondins that are expressed on axons and may signal to Schwann cells in a paracrine manner via β-catenin/Respondin, Lgr receptors, and Lrp/Frizzled complexes on Schwann cells (Grigoryan et al. 2013; Lewallen et al. 2011).
The appropriate balance between proliferation and survival to control SC numbers is also crucial to coordinate the segregation of axons with the generation of enough daughter SCs that can ensheathe them. This must be followed by timely withdraw of SCs from the cell cycle to promote the formation of a promyelinating SC and subsequent differentiation. Proliferation and survival of SCs at the time of radial sorting is reduced in a number of radial sorting mutants, including those due to the deletion of Fak and Cdc42 in SCs, confirming the importance of generating enough SCs to engage in the proper relationship with axons. It should be noted, however, that Schwann cell deletion of Notch results in significant reduction in proliferation without modifying the rate by which axons are sorted (Woodhoo et al. 2009). Moreover, FAK and Cdc42 mutant SCs have reduced contact with axons, and thus reduced access to juxtacrine mitogenic and survival signals. Together, these observations suggest that the reduced number of SCs in these mutants might be the consequence, rather than the cause, of the radial sorting arrest.
On the other side of the coin, SCs must exit the cell cycle to complete radial sorting and differentiate (reviewed in (Stevens and Fields 2002)), and inhibitors of proliferation are equally important in the direct control of radial sorting. For example, SC deletion of nuclear Jun activation domain–binding protein 1 (Jab1) impairs radial sorting by regulating the levels of p27Kip1 (Porrello et al. 2014), which in turn promotes SC exit from the cell cycle and differentiation (Li et al. 2011). This transition is also controlled by levels of cAMP in SCs (Morgan et al. 1991), and recent studies have revealed that deletion of the regulatory subunit of PKA, which causes excessive PKA activation, arrests radial sorting, (Guo et al. 2013a). Similarly, GPR126 (ADGRG6), an adhesion class G protein-coupled receptor (GPCR) that binds collagen IV (Paavola et al. 2014) and Laminin 211 (Petersen et al. 2015) is required for radial sorting (Mogha et al. 2013; Monk et al. 2011; Petersen et al. 2015). Interestingly, this function appears to be at least in part distinct from a separate key role for GPR126 in controlling SC cAMP levels (discussed below). Jab1 integrates signals from laminin and ErbB2 in cancer cells (discussed in (Porrello et al. 2014)), suggesting that Laminin 211/α6β1+α7β1 integrins and the GPR126/cAMP/PKA axis might converge with signals from Neuregulins on Jab1/p27Kip1 to coordinate exit from the cell cycle during radial sorting.
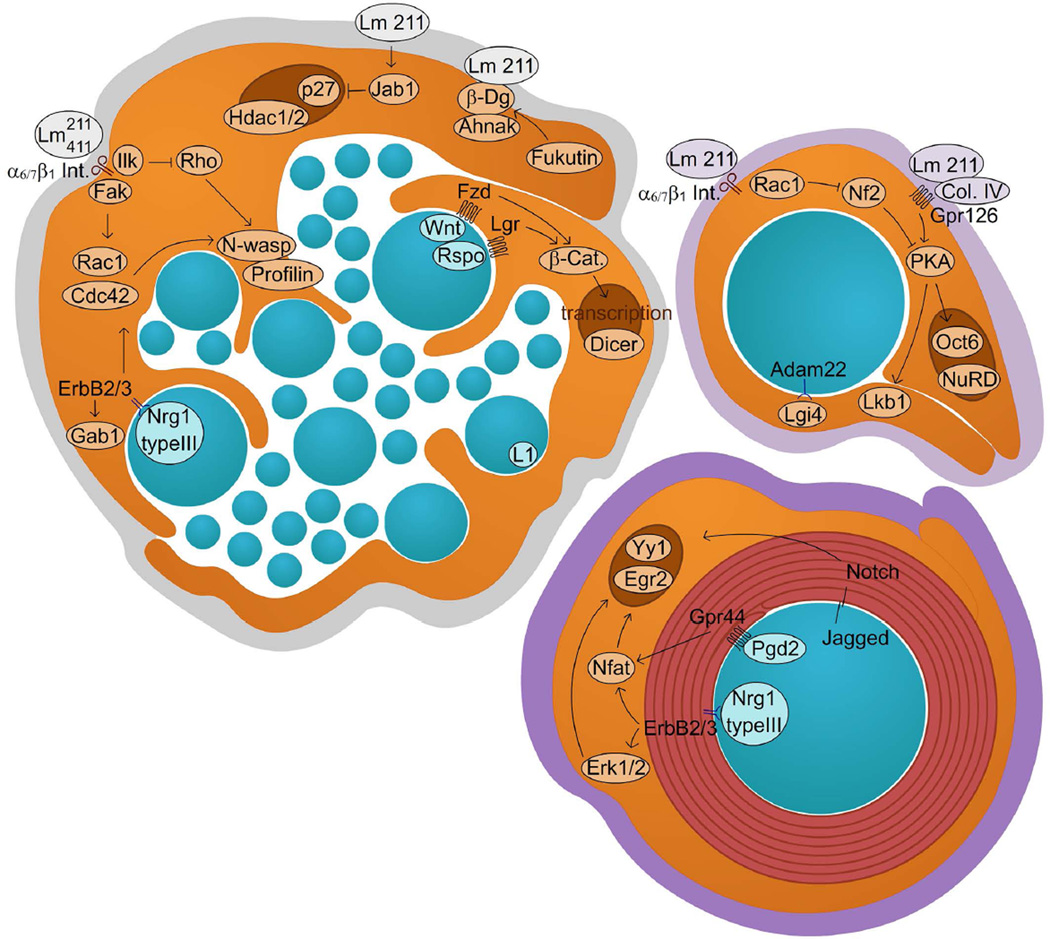
Nuclear, transcriptional, and epigenetic mechanisms also certainly operate during radial sorting, because deletion of HDAC1 and HDAC2 in SC delays radial sorting (Chen et al. 2011; Jacob et al. 2011). Furthermore, the signalosome component Jab1 controls radial sorting by entering the nucleus, where it binds p27Kip1 and favors its degradation (Porrello et al. 2014; Tomoda et al. 1999). However, apart from these two examples of nuclear control, little is known to date regarding transcription factors that control the gene expression program required during radial sorting. Interestingly, deletion of Dicer in Schwann cells delays radial sorting and arrests myelination, but the specific microRNAs involved in these processes have not yet been identified (Pereira et al. 2010; Yun et al. 2010). Some of the molecules discussed here and implicated in radial sorting are depicted in Figure 2.
Figure 2. Axo-glial interactions during radial sorting and myelination: novel concepts.
The image depicts molecules that were discovered recently and mediate signaling between Schwann cells and axons, or Schwann cells and the ECM, during radial sorting and myelination.
Pathologies associated with impaired radial sorting
If SCs do not acquire the proper relationship with axons, they cannot differentiate into either myelinating or non-myelinating SCs. Thus, radial sorting is a prerequisite for myelination and for differentiation of Remak bundles, and when arrested in humans, can result in dysmyelinating neuropathies. An example are the peripheral neuropathies due mutations in the LAMA2 gene, which encodes the α2 chain of Laminin 211. Loss-of-function mutations in LAMA2 cause Merosin-deficient Congenital Muscular Dystrophy (MDC1A, OMIM #607855) and a dysmyelinating neuropathy in 80% of MDC1A patients (Mercuri et al. 1996; Quijano-Roy et al. 2004; Shorer et al. 1995). Similar defects are seen in spontaneous mutations of Lama2 gene in other species, such as the dystrophic mouse mutants Dy/Dy and Dy2J/2J (Biscoe et al. 1974; Bradley and Jenkison 1973). The neuropathy of dystrophic mice was the first in which a radial sorting defect was described, and it began to focus attention on this morphogenetic process. In the case of Laminin 211 deficiency, radial sorting defects are characterized by the presence of groups of naked, unsorted axons, more severe in the spinal roots and the proximal part of the peripheral nerves, (Stirling 1975). These axons are myelinated mainly by boundary cap cell-derived SCs, which may have properties that make them more vulnerable to Laminin 211 deficiency (Maro et al. 2004).
In pathological situations that lead to defects in radials sorting, such as the Lama mutations, SC can begin to differentiate and even myelinate before reaching the promyelinating stage, giving rise to “polyaxonal myelination”. This is a pathological abnormality in which smaller bundles of multiple axons are wrapped by one SC that makes compact myelin around them. This suggests that there are inhibitory factors that actively prevent premature myelination. Certainly, we know that several signaling pathways in Schwann cells negatively regulate radial sorting, such as excessive PKA (Guo et al. 2013a) or GSK3-beta (da Silva et al. 2014) and ILK-RhoA-ROCK activation (Pereira et al. 2009). It is likely that these and/or other signals modulate the threshold of the response of the SC to NRG1 signaling, and this net outcome determines the final ensheathment fate of axons. For a complete review on the molecular control of radial sorting and associated pathologies, we refer the reader to: (Feltri et al. 2015).
The Transition from Promyelinating Schwann Cell to Myelinating Schwann Cell – Signals that Regulate Schwann Cell Myelination
Signaling Between Axons And Schwann Cells
Neuregulin 1 and secretases
Although it was well established that only axons above a certain diameter are myelinated (Friede 1972), the identity of the molecule(s) regulating such events and whether it was the axon dictating myelination or the SC, remained elusive for decades.
In the mid 1970s, elegant cross-anastomosis studies demonstrated that neurons control myelination. When myelinated axons were cross anastomosed into a non-myelinating environment, such as that of cervical ganglia, the axons became myelinated, indicating that axonal molecule(s) were responsible for the acquisition of the myelinating phenotype by the SC (Aguayo et al. 1976; Weinberg and Spencer 1975). Conditional ablation of ErbB2 in myelinating SCs showed that the NRG1/ErbB signaling pathway is crucial for PNS myelination (Garratt, et al., 2000). Among all the members of the NRG1 family of proteins (Mei and Xiong 2008), axonal NRG1 type III is the molecule determining the myelinating phenotype, although other molecules, like GPR126, Krox20 and Sox10, are also essential for PNS myelination, as we will describe later. When overexpressed in vivo in transgenic animals, NRG1 type III significantly increases the amount of myelin wrapping the axons, without altering its periodicity (Michailov et al. 2004). In addition to controlling the amount of myelin generated by a SC, NRG1 type III also determines the fate of SC differentiation. When ectopically expressed in non-myelinating superior cervical ganglia neurons in vitro, NRG1 type III changes their phenotype from non-myelinating and barely ensheathed (Obremski and Bunge 1995) to myelinated (Taveggia et al. 2005). These studies showed that NRG1 type III is the main growth factor involved in SC myelination. In addition to its role in promoting myelin formation, NRG1 type III is also required for the formation of Remak bundles (Fricker et al. 2009; Taveggia et al. 2005), indicating that NRG1 signaling is required for the specification of both myelinating and non-myelinating SCs.
Interestingly, although peripheral neurons express other NRG1 isoforms, they cannot substitute for NRG1 type III in PNS myelination (Taveggia et al. 2005). In mutants lacking NRG1 type III, SCs fail to migrate along neurites (Perlin et al. 2011; Wolpowitz et al. 2000), similar to ErbB2/ErbB3 mutants (Lyons et al. 2005; Riethmacher et al. 1997; Woldeyesus et al. 1999). Interestingly, in many of these models, the lack of SCs ultimately leads to cell death only at selected locations, for example in cervical and lumbar neurons (Woldeyesus et al. 1999), further underling the strict interconnection between these two cell types. The fact that NRG1 signaling is conserved in zebrafish (Lyons et al. 2005; Perlin et al. 2011) underscores the central role of this growth factor.
Although not much is known on the transcriptional regulation of NRG1 isoforms and in particular of NRG1 type III, the activities of NRG1 protein are controlled by post-translational modifications. Most notably, the extracellular domains of NRG1 proteins are processed by a complex network of secretases. NRG1 type III is activated by specific secretases that mediate extracellular cleavage, and this cleavage allows for subsequent classically regulated intra-membrane proteolysis. Several studies have identified the β secretase BACE-1 as a positive activator of NRG1 activity in both the PNS and CNS (Hu et al. 2006; Willem et al. 2006). BACE-1 modulation of myelination occurs via activation of the PI-3 kinase pathway, a downstream effector of NRG1 type III signaling (Taveggia et al. 2005). In addition, null BACE-1 transgenic mice do not fully remyelinate after an injury (Hu et al. 2008; Hu et al. 2015). This is in line with recent reports showing that NRG1 type I and type III is also crucial in early phases of remyelination (Fricker et al. 2013; Stassart et al. 2013), although NRG1 is rate limiting but not essential for PNS remyelination (Fricker et al. 2013). In addition to BACE-1, proteins belonging to the α-secretase family can cleave the extracellular region of NRG1 type III (Freese et al. 2009; La Marca et al. 2011; Luo et al. 2011; Yokozeki et al. 2007). While BACE-1 cleavage of NRG1 promotes myelination, α-secretase protein processing of NRG1 can have different outcomes. ADAM 17 cleavage of NRG1 inhibits myelination, suggesting that BACE-1 and ADAM 17 might compete for NRG1 cleavage and regulate the timing of myelination (La Marca et al. 2011). It has also been proposed that dual cleavage of NRG1 by BACE-1 and ADAM 17 releases a domain that activates ErbB receptors in vitro and which can suppress mutant bace-1 phenotypes in zebrafish (Fleck et al. 2013), suggesting that juxtacrine continuous communication between SCs and axons might not be necessary for myelination. Whether and how BACE-1 and ADAM 17 control NRG1 type III processing is still an open question. It is indeed not clear if they are differentially expressed in development, with ADAM 17 cleavage of NRG1 occurring in the embryo and BACE-1 acting later in development (Willem et al. 2006), or if the cleavages occur in different cellular organelles.
Other members of the ADAM secretase family also process NRG1 proteins. In particular, ADAM 19 cleaves NRG1 type I (Yokozeki et al. 2007), ADAM 19 is upregulated upon axonal injury, and accordingly, ADAM 19 null mice have delayed remyelination (Wakatsuki et al. 2009). This is especially important in light of recent studies showing that SC-derived NRG1 type I promotes remyelination after injury (Stassart et al. 2013). In the future, it will be interesting to determine whether ADAM 19 specifically cleaves NRG1 type I in SCs. Finally, ADAM 10 also processes NRG1 type III, although the functional significance of this activity in peripheral nerve is not completely understood. Luo and colleagues implicated ADAM 10 in modulating NRG1 activity in vitro (Luo et al. 2011); however, transgenic mice overexpressing ADAM 10 or a dominant negative form of ADAM 10 myelinate normally in development (Freese et al. 2009).
Following extracellular cleavage, NRG1 type III undergoes an intra-membrane proteolysis event regulated by the γ-secretase complex. In the CNS, this cleavage promotes neuronal survival (Bao et al. 2003). In the PNS, it induces the expression of the prostaglandin D2 synthase (L-PGDS) in neurons, an enzyme that catalyzes the production of the prostaglandin PGD2. PGD2 then activates the GPCR GPR44, expressed on SCs, to promote myelination (discussed in more detail below). Thus, this newly discovered pathway reinforces NRG1 forward signaling during development and possibly also participates in myelin maintenance in adulthood (Trimarco et al. 2014).
ADAM 22 and Lgi4
The characterization of the genetic defect present in claw paw spontaneous mouse mutant led to the identification of essential molecules involved in SC-axon interactions and myelination. The claw paw phenotype is due to a mutation in the Lgi4 gene that prevents secretion of the protein and causes a severe PNS phenotype characterized by impaired axonal sorting and hypomyelination (Bermingham et al. 2006). In normal conditions, SCs secrete Lgi4, which binds to neuronal ADAM 22 (Nishino et al. 2010; Ozkaynak et al. 2010). The importance of this signaling system is further underscored by the fact that transgenic mice lacking ADAM 22 are hypomyelinated (Ozkaynak et al. 2010; Sagane et al. 2005). Although SCs express another ligand for Lgi4, ADAM 23, it has been recently shown that Lgi4 does not require ADAM 23 to drive PNS myelination (Kegel et al. 2014).
Notch-1
In PNS myelination, Notch-1 is another critical component of the SC-axon interaction network. While Notch-1 promotes SC maturation in development (discussed above), at the onset of myelination, Notch-1 expression is downregulated in an RBP-J independent manner by the transcription factor Krox-20, promoting myelination (Woodhoo et al. 2009). Interestingly, enforced NICD (Notch intracellular domain) expression in nerves promotes demyelination, suggesting that control of Notch-1 signaling is required to maintain myelin integrity (Woodhoo et al. 2009).
Neurotrophins and their receptors
Neurotrophins and their cognate receptors have been extensively investigated as potential source of signaling molecules in SC-axon interactions (Rosenberg et al. 2006). These molecules can signal in both directions: from axons to SCs and vice versa. Accordingly, two members of this family, brain derived neurotrophic factor (BDNF) and Neurotrophin 3 (NT-3), can mediate trophic support between SCs and axons (Rosenberg et al. 2006). In vitro studies have shown that axonal BDNF binds to SC p75 neurotrophin receptor to promote early stages of myelination. At later stages, however, BDNF inhibits myelination by binding to truncated tropomyosin receptor kinase (Trk) B on SCs (Cosgaya et al. 2002). Whether the lack of expression of p75 NTR by myelinating SCs is implicated in this switch is still unknown. In vitro, the related neurotrophin NT-3 promotes SC migration but inhibits myelination (Yamauchi et al. 2003). In vivo, axons from NT-3 null mice are hypomyelinated, (Woolley et al. 2008). Whether the hypomyelinating phenotype is due to a lack of SC recruitment, SC apoptosis, or the effects of other cross-signaling mechanisms that modulate NT-3 activity in vivo is unknown. In addition, SCs can release nerve growth factor (NGF), which promotes myelination in vitro (Chan et al. 2004). Neurotrophins also modulate myelination of nociceptive neurons. In particular, the glial-cell derived neurotrophic factor (GDNF) stimulates myelination, probably by binding to Ret receptors (Hoke et al. 2003).
Nectin-like proteins
Other molecules implicated in SC-axon interactions are members of the Nectin-like (Necl) family of proteins, also known as cell adhesion molecules (CADM). Indeed, SC-derived Necl-1/Cadm 3 and axonal Necl-4/Cadm 4 regulate PNS myelination in vitro (Maurel et al. 2007; Spiegel et al. 2007). Whether Necl proteins interact with the Par polarity complex it is still debated. Transgenic mice lacking Necl-1/Cadm3, Necl-2/Cadm1, or Necl-3/Cadm2 show no developmental defects in the PNS, while Necl-4/Cadm4 mutants develop focal hypermyelination (Golan et al. 2013). Interestingly, loss of Necl-1/Cadm3, Necl-2/Cadm1, or Necl-3/Cadm2 does not enhance Necl-4/Cadm4 mutant phenotypes, but whether other Necl proteins or unrelated adhesion molecules can compensate for the lack of Necls-1-3 is not known (Golan et al. 2013; Park et al. 2008; Pellissier et al. 2007).
G protein-coupled receptors
GPCRs are a family of cell surface receptors that integrate signaling from diverse stimuli including photons, ions, organic molecules, and proteins. GPCRs are characterized by a conserved seven transmembrane (7TM) domain and the ability to transduce signaling via heterotrimeric G proteins (Paavola and Hall 2012).
In the PNS, a small number of GPCRs has been thus far described to play an essential role in SC development and myelination. Studies in zebrafish and mouse showed that the adhesion GPCR GPR126 is required for PNS myelination in a SC autonomous way (Mogha et al. 2013; Monk et al. 2009). Adhesion GPCRs possess a long N-terminal extracellular fragment (NTF) in addition to the canonical 7TM. Although most adhesion GPCRs are orphan receptors, those with known ligands generally bind ECM proteins, and a given adhesion GPCR can have multiple endogenous binding partners (Langenhan et al. 2013; Liebscher et al. 2013). GPR126 acts through the Gαs subunit to increase cAMP levels, activate PKA, and initiate myelination (Glenn and Talbot 2013a; Mogha et al. 2013). We will discuss the role of cAMP elevation in myelination below in a dedicated section. Very recently, it has been shown that GPR126 contains an intramolecular agonist domain, the Stachel sequence, which stimulates substantial Gαs signaling and cAMP elevation in vitro and which is required for SC myelination in zebrafish in vivo (Liebscher et al. 2014) (Figure 3). As noted previously, loss of Gpr126 leads to radial sorting defects in addition to amyelination (Mogha et al. 2013; Monk et al. 2011; Petersen et al. 2015), and interestingly, it was recently shown that the function of GPR126 in radial sorting is mediated by the extracellular NTF domain, and not the 7TM signaling domain (Petersen et al. 2015). Recent studies have identified collagen IV as an activating ligand for GPR126 in vitro (Paavola et al. 2014); as a SC-secreted ECM protein, collagen IV likely functions in vivo to modulate GPR126 signaling and myelination. Indeed, the importance of ECM proteins in activating this adhesion GPCR has been confirmed in a separate study showing that Laminin 211 is an endogenous binding partner for GPR126 that can modulate the availability of the Stachel sequence (Petersen et al. 2015). In the future, it will be interesting to determine how GPR126 integrates signals from collagen IV, Laminin 211, and potentially other ECM molecules and their receptors, to control distinct stages of SC development and myelination.
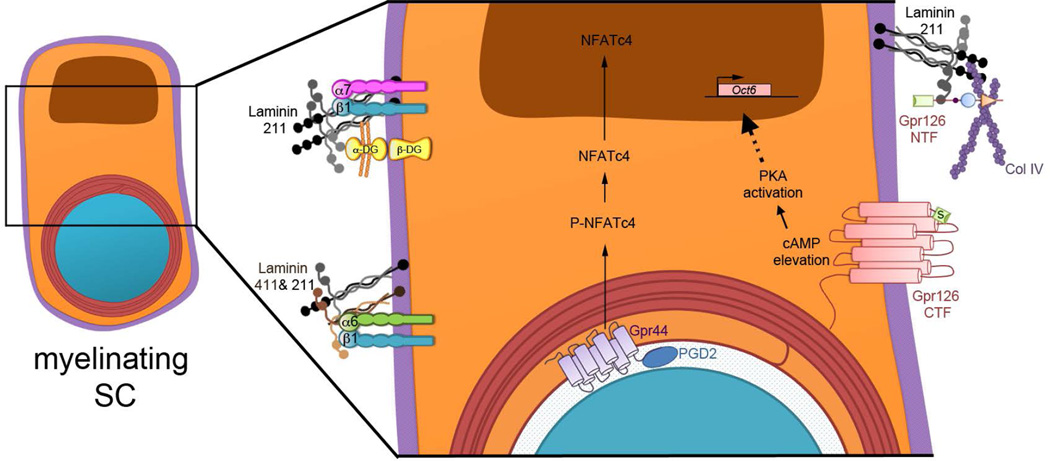
Figure 3. ECM Molecules and GPCRs in SC Myelination – Recent Advances.
From left, clockwise. In the SC basal lamina, Laminin 211 binds traditional (Integrins αββ1 and α7β1, dystroglycan) and unconventional (GPR126) receptors. Conversely, multiple ECM constituents bind the same receptor: integrin α6β1 can bind Laminin 211 and 411; The N-terminal fragment (NTF) of GPR126 can bind Laminin 211 and Col IV. The NTF must be modulated, perhaps by physical removal, in order to expose the Stachel tethered agonist (S) to activate the C-terminal fragment (CTF) of GPR126. CTF activation promotes cAMP accumulation, which activates PKA. This likely activates the cAMP-response element binding protein (CREB) pathway and phosphorylates NFkB, and a major downstream effector of cAMP in SCs is Oct-6. NRG1 type III intramembrane cleavage induces the expression of L-PGDS, which is released to produce prostaglandin D2 (PGD2). PGD2 then binds to GPR44 expressed on SCs membrane. Activation of GPR44 induces NFATc4 dephosphorylation and nuclear translocation to promote myelination.
As noted above, another GPCR implicated in PNS myelination is GPR44. This GPCR is expressed by SCs and is a receptor for the axonal prostaglandin PGD2. Unlike GPR126 which elevates cAMP and activates PKA (Glenn and Talbot 2013a; Mogha et al. 2013), GPR44 promotes PNS myelination in vitro and in vivo most likely by activating the transcription factor Nfatc4 downstream of NRG1 signaling (Trimarco et al. 2014) (Figure 3).
In addition, the LPA1 receptor, a class A GPCR, signals through Gαi and Rac1 to promote SC migration and axonal sorting (Anliker et al. 2013). Although to date, only a small handful of GPCRs has been demonstrated to play roles in SC development and myelination, it is interesting to note that on average, a cell expresses ~20 GPCRs (Atwood et al. 2011). Therefore, future work will likely define other GPCRs with essential functions in SCs. Moreover, GPCR effectors can modulate signals from other key pathways. For example, low levels of cAMP favor NRG1-induced SC proliferation while high levels of cAMP promote NRG-1 induced SC differentiation in vitro (Arthur-Farraj et al. 2011). Interestingly, a recent model has emerged whereby Laminin 211/GPR126 interactions suppress cAMP accumulation during radial sorting stages and promote cAMP elevation at later stages (Petersen et al. 2015). Thus, it will also be important to understand how GPCR effectors and signaling pathways from many sources, discussed in more detail below, converge to control proper SC development.
Signaling pathways activated in Schwann cell myelination
The best studied signaling pathways involved in SC myelination are those activated downstream of NRG1 type III. Nonetheless, several of these pathways are also commonly activated by many of the molecules described above, suggesting that their activities could converge to promote myelination. What follows is a brief description of the role of these pathways in myelin formation, as comprehensive descriptions have been already reported in previous reviews (Glenn and Talbot 2013b; Pereira et al. 2012; Taveggia et al. 2010).
PI-3 kinase/AKT/mTOR pathway
Induction of the PI3-kinase pathway drives PDK1 phosphorylation of PtdIns (4,5) to PtdIns (3,4,5) and subsequent phosphorylation of AKT-1. The opposite reaction is favored by myotubularin phosphatases MTMR2 and MTMR13, which are required to limit myelination and are mutated in demyelinating Charcot-Marie-Tooth disease (reviewed in (Hnia et al. 2012; Previtali et al. 2007)). It has recently been proposed that the PI3-kinase pathway activates two different responses in SCs. The first depends upon NRG1 type III activation and induces myelination, whereas the second is controlled by laminin and may negatively influence myelination (Heller et al. 2014). Activation of PDK1 is counteracted by the action of PTEN. In agreement, conditional ablation of PTEN in SCs increases AKT-1 phosphorylation and causes hypermyelination of small fibers and aberrant myelination of large axons (Goebbels et al. 2010). Notably, these defects are ameliorated by rapamycin treatment, an inhibitor of mTORC1, indicating that mTORC1 acts downstream of PtdIns(3,4,5) (Goebbels et al. 2012). In agreement, mice lacking mTORC1 in SCs are hypomyelinated and SC longitudinal growth is delayed (Sherman et al. 2012). Very recently, these results were confirmed by a study demonstrating that only mTORC1, and not mTORC2, controls PNS myelination. Indeed, SC conditional ablation of Raptor, a specific mTORC1 adaptor, but not of the mTORC2 adapter Rictor, impairs PNS myelination and causes major sorting defects and abnormal Remak bundle formation. It has also been proposed that Raptor acts by controlling RXR-γ mediated transcription of SREPB1c and therefore affects lipid metabolism independent from ErbB receptor signaling (Norrmen et al. 2014).
MAPK pathway
While previous studies have implicated ERK signaling upon axonal injury to initiate the SC injury response (Harrisingh et al. 2004; Napoli et al. 2012; Yang et al. 2012), it is becoming increasingly evident that this pathway also actively participates in developmental PNS myelination downstream of NRG1. Sustained in vivo activation of ERK in myelinating SCs leads to hypermyelination (Ishii et al. 2013). Compellingly, SC-specific ablation of ERK proteins prevents PNS myelination (Newbern et al. 2011). Further, MEK dependent phosphorylation of the transcription factor YY1 is necessary for Krox-20 expression and myelination (He et al. 2010). In addition, conditional ablation of the tyrosine phosphatase Shp2 in SCs leads to hypomyelination and is associated with reduced Erk1/2 phosphorylation (Grossmann et al. 2009). Finally, recent studies suggest that termination of myelin growth can be overcome by sustained activation of the ERK signaling pathway, and that constitutively active ERK can replace NRG1/ErbB signaling in myelination (Sheean et al. 2014). This is in contrast to previous reports (see (Goebbels et al. 2010)), perhaps reflecting the importance of levels and extent of activation of the PI-3 kinase and MAPK pathways in myelination. How these differences are achieved is currently not known.
cAMP signaling
An essential role for cAMP in PNS myelination was first hypothesized when it was shown that increased levels of cAMP in SCs induces the expression of myelin transcription factors and lipid constituents and substitutes for axonal requirements (Lemke and Chao 1988; Mokuno et al. 1988; Monje et al. 2009; Monuki et al. 1989; Parkinson et al. 2003; Scherer et al. 1994; Sobue and Pleasure 1984). Notably, cAMP elevation also represses the expression of proteins involved in maintaining SCs in an immature state (Monje et al. 2009; Morgan et al. 1991). As discussed above, GPR126 is currently considered to be the main receptor that activates adenylate cyclase to generate cAMP in SCs. cAMP then phosphorylates PKA, which in turn likely activates the cAMP-response element binding protein (CREB) pathway and phosphorylates NFkB (Meijer 2009). Ultimately, major downstream effectors of cAMP in SCs are Oct-6 (Pou3f1) and Krox-20, key transcription factors for PNS myelination (Bermingham et al. 1996; Jaegle et al. 1996; Monuki et al. 1989; Scherer et al. 1994; Topilko et al. 1994).
Calcineurin/NFAT
Another signaling pathway elicited by NRG1 and implicated in PNS myelination is coupled to Ca2+/calcineurin activation. Addition of NRG1 to SCs in vitro triggers Ca2+ increase and is followed by NFATs dephosphorylation and nuclear translocation. Once in the nucleus, NFATs form a complex with Sox-10 to activate Krox-20 and P0 expression (Kao et al. 2009). Notably, this pathway depends upon NRG1 engagement of glial ErbB2/ErbB3 but is independent of PI-3 kinase or MAPK pathways (Kao et al. 2009). Interestingly, addition of PGD2 to SCs also promotes NFATs nuclear translocation, again independently of PI3-kinase and MAPK pathway activation (Trimarco et al. 2014). It has also been reported that cAMP synergizes with NFATs to upregulate Krox-20 in SCs in vitro (Kipanyula et al. 2013). Whether this is also required in vivo is not known.
Transcription Factors Important For Schwann Cell Myelination
Activation of the above-described pathways ultimately leads to transcriptional changes required to produce the significant amount of protein and lipid constituents of myelin. Several positive and negative transcriptional regulators have been characterized to date. The role exerted by these molecules in PNS myelination has been extensively reviewed elsewhere (Pereira et al. 2012; Svaren and Meijer 2008; Wegner and Stolt 2005). Here, we will briefly review the main roles of some key factors.
SRY-related HMG box (Sox) family of transcription factors
This class of genes contains both positive and negative regulators of myelination. Sox-10 is a main transcription factor responsible for SCP differentiation and SC maturation (see above) in addition to its more recently described function in myelin maintenance (Bremer et al. 2011). Importantly, it was also recently shown that dimeric Sox-10 synergizes with Oct-6 to induce Krox-20 expression (Jagalur et al. 2011).
While Sox-10 promotes SC development and myelination, Sox-2, which is expressed in immature SCs, inhibits myelin formation. In vitro experiments showed that Sox-2 expression is downregulated upon forced Krox-20 expression (Parkinson et al. 2008). Conversely, forced expression of Sox-2 blocks Krox-20 upregulation and myelination (Le et al. 2005a). Surprisingly, recent studies suggest that Sox-10 and Sox-2 cooperate to promote RNA polymerase transcriptional elongation, recruiting the transcription elongation factor P-TEFb to initiate myelin gene transcription in SCs (Arter and Wegner 2014).
POU domain transcription factors
Oct-6 is a POU domain transcription factor that controls Krox-20 expression (Blanchard et al. 1996; Ghislain et al. 2002). During mammalian development, Oct-6 is transiently expressed in promyelinating SCs, with highest expression during the transition to myelinating SCs. As soon as myelination begins, Oct-6 expression is downregulated (Arroyo et al. 1998; Scherer et al. 1994). Oct-6 expression in vivo is controlled by axonal signals, including NRG1 type III and cAMP elevation (Leimeroth et al. 2002; Monuki et al. 1989; Scherer et al. 1994). Although Oct-6 was originally described as an inhibitor of myelin protein gene expression in vitro (Monuki et al. 1989), in transgenic mice lacking Oct-6, myelin formation is transiently blocked and the expression of myelin genes repressed (Ghazvini et al. 2002; Jaegle et al. 1996).
A close relative of Oct-6, Brn-2 (Pou3f2), is expressed with similar kinetics in SCs. Single Brn-2 null mice do not show any impairment in SC development and PNS myelination (Jaegle et al. 2003); however, double Oct-6/Brn-2 knockout mice present a much more severe phenotype than single Oct-6 mutants. In these mice, SC development is stalled and myelination is further delayed, although not completely blocked. Notably, axons in these transgenic mice are hypomyelinated throughout life (Jaegle et al. 2003). Interestingly, it has also been shown that Brn-1 (Pou3f3), which is not normally expressed in SCs, can functionally substitute for Oct-6 in vivo (Friedrich et al. 2005).
Krox-20/Egr2
Krox-20 (Egr2) belongs to the family of early growth response genes and plays a crucial role in PNS myelination (Nagarajan et al. 2001; Topilko et al. 1994). Indeed, it is considered to be the master transcriptional regulator of SC myelination as ectopic expression in fibroblasts induces the expression of P0 in vitro (Parkinson et al. 2004). Axons in transgenic mice lacking Krox-20 are not myelinated and SCs in these mice are arrested at the pro-myelinating stage (Le et al. 2005a; Topilko et al. 1994). Interestingly, Krox-20 synergizes with Sox-10 to promote myelination (Svaren and Meijer 2008), and Krox-20 activity is controlled by a series of co-activators and co-repressors: the NAB proteins (Desmazieres et al. 2008; Le et al. 2005b; Nagarajan et al. 2001). Further, Krox-20, in conjunction with NAB proteins, directly represses negative regulators of PNS myelination, in particular the basic HLH transcription factor Id 2 (Stewart et al. 1997), to activate P0 protein expression in SCs (Mager et al. 2008). Importantly, mutations in KROX-20 are associated with Charcot Marie Tooth hereditary neuropathies in humans (Scherer and Wrabetz 2008).
NFkB
NRG1 activates the transcription factor NFkB (Limpert and Carter 2010), which further stimulates Oct-6 and, as a consequence, Krox-20. NFkB is required for SC myelination in vitro (Nickols et al. 2003), while its role in vivo has been recently questioned (Morton et al. 2013). It has been suggested that NFkB activation is mediated by chromatin remodeling complexes (Chen et al. 2011; Limpert et al. 2013).
Chromatin modifying complexes
Several studies have described the role of chromatin modifications in SC development and myelination. In particular, the histone deacetylase complexes HDAC1 and HDAC2 promote the activation of Sox-10 in SCs and subsequent myelination (Chen et al. 2011), in addition to their previously described role in SCP development. In agreement, it has been reported that Sox-10 recruits these complexes at Sox-10 and Krox-20 binding sites; however, only HDAC2 seems to synergize with Sox-10 to activate the transcriptional myelin program (Jacob et al. 2011).
Recently, it has also been shown that conditional inactivation of the NuRD (Nucleosome remodeling and deacetylase complex) complex in SCs impairs radial sorting and myelination and causes aberrant SC proliferation, suggesting that this chromatin remodeling complex is required for SC differentiation and myelination (Hung et al. 2012).
A final chromatin remodeling complex lately implicated in SC myelination downstream of NRG1 type III and NFkB is Brg1 (Limpert et al. 2013). Brg1 is the helicase of the mammalian SWI/SNF related complex, which is important for PNS myelination (Marathe et al. 2013) and is required for SC differentiation and myelination (Weider et al. 2012). Using loss and gain of function experiments, Limpert and colleagues elegantly showed that Brg1 recruits Sox-10 to the regulatory regions of Oct-6 and Krox-20 genes (Limpert et al. 2013).
Myelin Proteins and Lipids
The coordinated symphonies of signal transduction cascades, chromatin remodeling, and transcription factor expression converge to control the production of myelin proteins and lipids and iterative membrane wrapping. Myelin transcription factors induce the expression of genes encoding myelin-specific protein in SCs. These include structural and signaling proteins in compact (P0, Myelin Basic Protein, Peripheral Myelin Protein 22) and non-compact (Myelin Associated Glycoprotein, Connexin-32) myelin. Myelin proteins are crucial for terminal differentiation of myelin-forming SCs, as highlighted by their frequent involvement in Charcot-Marie-Tooth neuropathies. We direct the reader to extensive reviews on this important topic (e.g., (Rossor et al. 2013; Scherer and Wrabetz 2008)). Additionally, unlike most biological membranes, myelin has a higher ratio of lipids to proteins, and although there are no truly “myelin-specific” lipids, many (including cholesterol, cerebroside, and sphingomyelin) are enriched (Morrell and Quarles 1999). Interestingly, during development, the transcription of myelin genes and the synthesis of the enzymes required for lipid synthesis are synchronized, further underlying their strict interconnection in SC maturation and in diseases (Chrast et al. 2011; Schmitt et al. 2014). Moreover, Peripheral Myelin Protein 2 might also be involved in lipid transport (Zenker et al. 2014). The direct involvement of lipid biosynthesis in PNS myelination is further underscored by studies in rodents on transcription factors (SCA-SREBP cleavage-activating enzyme (Verheijen et al. 2009)) and enzymes involved in lipid synthesis (Lpin1 (Nadra et al. 2008) and cholesterol (Saher et al. 2009)). Finally, the spiral wrapping of SCs proceeds from the cytoplasmic “inner tongue” of membrane in contact with the axon (Bunge et al. 1989), though the biophysical mechanisms that govern myelination are largely unknown.
Non-Myelinating Schwann Cells
In the PNS, not all fibers are myelinated. As mentioned above, a fate determining signal controlling whether SCs will myelinate or not is axonal levels of NRG1 type III. There are several classes of non-myelinated fibers in the PNS, including C fiber nociceptors, post-ganglionic sympathetic and parasympathetic fibers, and motor nerve terminals at the neuromuscular synapses. All of these fibers are associated with non-myelinating SCs (NMSCs). This class of SCs comprises those associated with small caliber axons, known as Remak SCs, terminal/perisynaptic SCs at the neuromuscular junctions (NMJs), as well as NMSCs associated with Pacini and Meissner corpuscles (Griffin and Thompson 2008).
Morphological studies have shown that Remak SCs are located along the axons at a closer distance to one another than myelinating SCs (Aguayo et al. 1973), and that they usually ensheathe more than one axon. These axons are frequently contained in a single bundle surrounded by SC processes. Notably, the number of axons present in these bundles differs along the nerve and in different nerves (Murinson and Griffin 2004). Single Remak SCs can contain axons that are differentially responsive to different growth factors. For example, Remak fibers can express the neurotrophin receptor TrkA or the Ret receptor, thus responding to p75 neurotrophin or GDNF and artemin, respectively. Exciting recent data implicates Remak SCs in particular as playing vital roles in the trophic support of axons (Beirowski et al. 2014; Viader et al. 2011; Viader et al. 2013), further highlighting an emerging theme in axon-glial interactions (Funfschilling et al. 2012; Lee et al. 2012).
When Remak fibers enter the epidermis, they separate to reach a 1:1 association, similar to promyelinating SCs. Interestingly, fibers at this level lose contact with NMSC, so that only the axon passes through the dermal-epidermal junction (Hsieh et al. 1994). Unlike myelinated axons, these fibers are characterized by continuous axonal sprouting and growth, which is under the control of NGF released by NMSCs (Diamond et al. 1992). It has been hypothesized that this “plasticity” is required to respond to the continuous changes occurring in the epidermis. Given that immature SCs are essential for the proper epidermal-subepidermal positioning of sensory nerves in developing zebrafish (Raphael et al. 2010), it would be interesting to determine if NMSCs play a similar role in mammalian axon translocation to the epidermis.
Another class of NMSCs are those terminally associated with axons at NMJs. These NMSCs, called terminal or perisynaptic SCs, cover the entire synapse (Young et al. 2005; Zuo et al. 2004). Unlike other NMSCs, perisynaptic SCs extend process towards the axon but do not enwrap them. From a molecular point of view, perisynaptic SCs express neurotransmitter receptors, in particular muscarinic and purinergic receptors. Upon activation, these receptors induce the development of a Ca2+ wave that is modulated by BDNF present at the synapse (Todd et al. 2007). Whether perisynaptic SCs provide metabolic support to the NMJ axons similar to Remak SCs is not known. It has been proposed that perisynaptic SCs are important for developmental synapse rearrangement, especially for eliminating the extra branches formed at the NMJ during development (Smith et al. 2013; Turney and Lichtman 2012; Walsh and Lichtman 2003). Importantly, perisynaptic SCs are essential to guide axons remodeling after injury at the site of re-innervation (Kang et al. 2014). In addition to the aforementioned functions, recent work has opened the possibility that NMSCs may also play a role in the bone marrow hematopoietic stem cell niche (Yamazaki et al. 2011). Future work will continue to define new functions for NMSCs and to further elucidate their remarkable plasticity.
Conclusions
Recent advances in the study of SCs have highlighted previously unknown factors, SC signaling events, and ECM signals that regulate development and myelination. These advances open new questions that remain to be answered including: How is the binding of Laminin 211 on multiple receptors (integrins, dystroglycan and GPR126) coordinated? How are signals from axonal and basal lamina molecules integrated? Given that SCs must be polarized on multiple axes, how is polarity established during myelination? In addition to NRG1 type III and ADAM 22, which other axonal signals govern myelination? Are there negative signals on unmyelinated fibers that prevent myelination? What is the effect of other cell types, such as endothelial cells and endoneurial fibroblasts on SC development? Future work will surely continue to shed light on the genetic and molecular mechanisms that govern each developmental progression and ultimate myelination.
Main Points.
Schwann cells generate myelin in the peripheral nervous system and are essential for proper neuronal function.
Cell-cell and cell-matrix events are required for Schwann cell development and myelination.
Acknowledgements
The work in our laboratories was supported by National Institutes of Health Grants NS045630 and HD075363 (M.L.F.) and NS079445 and NS083052 (K.R.M.). Support was also provided by the European Community FP7-EU (M.L.F), the Muscular Dystrophy Association (K.R.M.), the Italian Minister of Health (C.T), and Telethon Italy (C.T. M.L.F). We are indebted to Sarah Ackerman and Yannick Poitelon for creating the figures.
References
- Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976;104:1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- Aguayo AJ, Peyronnard JM, Bray GM. A quantitative ultrastructural study of regeneration from isolated proximal stumps of transected unmyelinated nerves. J Neuropath Experim Neurol. 1973;32:256–270. doi: 10.1097/00005072-197304000-00006. [DOI] [PubMed] [Google Scholar]
- Anliker B, Choi JW, Lin ME, Gardell SE, Rivera RR, Kennedy G, Chun J. Lysophosphatidic acid (LPA) and its receptor, LPA1, influence embryonic schwann cell migration, myelination, and cell-to-axon segregation. Glia. 2013;61:2009–2022. doi: 10.1002/glia.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo EJ, Bermingham JR, Jr, Rosenfeld MG, Scherer SS. Promyelinating Schwann cells express Tst-1/SCIP/Oct-6. J Neurosci. 1998;18:7891–7902. doi: 10.1523/JNEUROSCI.18-19-07891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arter J, Wegner M. Transcription factors Sox10 and Sox2 functionally interact with positive transcription elongation factor b in Schwann cells. J Neurochem. 2015;13:384–393. doi: 10.1111/jnc.13013. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj P, Wanek K, Hantke J, Davis CM, Jayakar A, Parkinson DB, Mirsky R, Jessen KR. Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–733. doi: 10.1002/glia.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC genomics. 2011;12:14. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini A, Varum S, Mateos JM, Bettosini D, John N, Bonalli M, Ziegler U, Dimou L, Clevers H, Furrer R, Sommer L. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell. 2015;16:314–322. doi: 10.1016/j.stem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Golden JP, Chen YJ, Yang K, Gross RW, Patti GJ, Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat Neurosc. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Revlas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Scherer SS, O'Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Shearin H, Pennington J, O'Moore J, Jaegle M, Driegen S, van Zon A, Darbas A, Ozkaynak E, Ryu EJ, Milbrandt J, Meijer D. The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nat Neurosci. 2006;9:76–84. doi: 10.1038/nn1598. [DOI] [PubMed] [Google Scholar]
- Berti C, Bartesaghi L, Ghidinelli M, Zambroni D, Figlia G, Chen ZL, Quattrini A, Wrabetz L, Feltri ML. Non-redundant function of dystroglycan and beta1 integrins in radial sorting of axons. Development. 2011;138:4025–4037. doi: 10.1242/dev.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Caddy KW, Pallot DJ, Pehrson UM, Stirling CA. The neurological lesion in the dystrophic mouse. Brain Res. 1974;76:534–536. doi: 10.1016/0006-8993(74)90830-0. [DOI] [PubMed] [Google Scholar]
- Blanchard AD, Sinanan A, Parmantier E, Zwart R, Broos L, Meijer D, Meier C, Jessen KR, Mirsky R. Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20, and Pax-3. J Neurosci Res. 1996;46:630–640. doi: 10.1002/(SICI)1097-4547(19961201)46:5<630::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bradley WG, Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973;18:227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Bremer M, Frob F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022–1032. doi: 10.1002/glia.21173. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, Bates M. Movements of the Schwann cell nucleus implicate progression of the inner (axon-related) Schwann cell process during myelination. J Cell Biol. 1989;109:273–284. doi: 10.1083/jcb.109.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang H, Yoon SO, Xu X, Hottiger MO, Svaren J, Nave KA, Kim HA, Olson EN, Lu QR. HDAC-mediated deacetylation of NF-kappaB is critical for Schwann cell myelination. Nat Neurosci. 2011;14:437–441. doi: 10.1038/nn.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrast R, Saher G, Nave KA, Verheijen MH. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lip Res. 2011;52:419–434. doi: 10.1194/jlr.R009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- Coulpier F, Le Crom S, Maro GS, Manent J, Giovannini M, Maciorowski Z, Fischer A, Gessler M, Charnay P, Topilko P. Novel features of boundary cap cells revealed by the analysis of newly identified molecular markers. Glia. 2009;57:1450–1457. doi: 10.1002/glia.20862. [DOI] [PubMed] [Google Scholar]
- da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, Avila RL, Wanders RJ, Just WW, Kirschner DA, Sousa MM, Brites P. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J Clin Inv. 2014;124:2560–2570. doi: 10.1172/JCI72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmazieres A, Decker L, Vallat JM, Charnay P, Gilardi-Hebenstreit P. Disruption of Krox20-Nab interaction in the mouse leads to peripheral neuropathy with biphasic evolution. J Neurosci. 2008;28:5891–5900. doi: 10.1523/JNEUROSCI.5187-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddrell RD, Dun XP, Moate RM, Jessen KR, Mirsky R, Parkinson DB. Regulation of Schwann cell differentiation and proliferation by the Pax-3 transcription factor. Glia. 2012;60:1269–1278. doi: 10.1002/glia.22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Dyachuk V, Furlan A, Shahidi MK, Giovenco M, Kaukua N, Konstantinidou C, Pachnis V, Memic F, Marklund U, Muller T, Birchmeier C, Freid K, Ernfors P, Adameyko I. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science. 2014;345:82–87. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I, Outin E, Picard CA, Chettouh Z, Dymecki S, Consalez GG, Coppola E, Brunet JF. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science. 2014;345:87–90. doi: 10.1126/science.1253286. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, Poitelon Y, Previtali SC. How Schwann Cells Sort Axons: New Concepts. Neuroscientist. 2015 doi: 10.1177/1073858415572361. pii: 1073858415572361 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, Kremmer E, Tahirovic S, Edbauer D, Lichtenthaler SF, Schmid B, Willem M, Haass C. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Sanes JR. Lineage of neurons and glia in chick dorsal root ganglia: analysis in vivo with a recombinant retrovirus. Development. 1991;111:895–908. doi: 10.1242/dev.111.4.895. [DOI] [PubMed] [Google Scholar]
- Freese C, Garratt AN, Fahrenholz F, Endres K. The effects of alpha-secretase ADAM10 on the proteolysis of neuregulin-1. Febs J. 2009;276:1568–1580. doi: 10.1111/j.1742-4658.2009.06889.x. [DOI] [PubMed] [Google Scholar]
- Fricker FR, Antunes-Martins A, Galino J, Paramsothy R, La Russa F, Perkins J, Goldberg R, Brelstaff J, Zhu N, McMahon SB, Orengo C, Garratt AN, Birchmeier C, Bennett DL. Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain. 2013;136:2279–2297. doi: 10.1093/brain/awt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker FR, Zhu N, Tsantoulas C, Abrahamsen B, Nassar MA, Thakur M, Garratt AN, Birchmeier C, McMahon SB, Wood JN, Bennett DL. Sensory axon-derived neuregulin-1 is required for axoglial signaling and normal sensory function but not for long-term axon maintenance. J Neurosci. 2009;29:7667–7678. doi: 10.1523/JNEUROSCI.6053-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL. Control of myelin formation by axon caliber (with a model of the control mechanism) J Comp Neurol. 1972;144:233–252. doi: 10.1002/cne.901440207. [DOI] [PubMed] [Google Scholar]
- Friedrich RP, Schlierf B, Tamm ER, Bosl MR, Wegner M. The class III POU domain protein Brn-1 can fully replace the related Oct-6 during schwann cell development and myelination. Mol Cell Biol. 2005;25:1821–1829. doi: 10.1128/MCB.25.5.1821-1829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini M, Mandemakers W, Jaegle M, Piirsoo M, Driegen S, Koutsourakis M, Smit X, Grosveld F, Meijer D. A cell type-specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. EMBO J. 2002;21:4612–4620. doi: 10.1093/emboj/cdf475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M. Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development. 2002;129:155–166. doi: 10.1242/dev.129.1.155. [DOI] [PubMed] [Google Scholar]
- Glenn TD, Talbot WS. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development. 2013a;140:3167–3175. doi: 10.1242/dev.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TD, Talbot WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol. 2013b;23:1041–1048. doi: 10.1016/j.conb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Wolfer S, Wieser GL, Nientiedt T, Pieper A, Ruhwedel T, Groszer M, Sereda MW, Nave KA. Genetic disruption of Pten in a novel mouse model of tomaculous neuropathy. EMBO Mol Med. 2012;4:486–499. doi: 10.1002/emmm.201200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan N, Kartvelishvily E, Spiegel I, Salomon D, Sabanay H, Rechav K, Vainshtein A, Frechter S, Maik-Rachline G, Eshed-Eisenbach Y, Momoi T, Peles E. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J Neurosci. 2013;33:10950–10961. doi: 10.1523/JNEUROSCI.0571-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56:1518–1531. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Stein S, Qi J, Wende H, Garratt AN, Nave KA, Birchmeier C, Birchmeier W. Wnt/Rspondin/beta-catenin signals control axonal sorting and lineage progression in Schwann cell development. Proc Natl Acad Sci USA. 2013;110:18174–18179. doi: 10.1073/pnas.1310490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Peles E, Selbach M, Birchmeier W, Birchmeier C. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci USA. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Lee AA, Rizvi TA, Ratner N, Kirschner LS. The protein kinase A regulatory subunit R1A (Prkar1a) plays critical roles in peripheral nerve development. J Neurosci. 2013a;33:17967–17975. doi: 10.1523/JNEUROSCI.0766-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. J Neurosci. 2012;32:17251–17261. doi: 10.1523/JNEUROSCI.2461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moon C, Zheng Y, Ratner N. Cdc42 regulates Schwann cell radial sorting and myelin sheath folding through NF2/merlin-dependent and independent signaling. Glia. 2013b;61:1906–1921. doi: 10.1002/glia.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evolution & development. 2000;2:3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, Svaren J, Casaccia P. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci. 2010;13:1472–1480. doi: 10.1038/nn.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller BA, Ghidinelli M, Voelkl J, Einheber S, Smith R, Grund E, Morahan G, Chandler D, Kalaydjieva L, Giancotti F, King RH, Fejes-Toth AN, Fejes-Toth G, Feltri ML, Lang F, Salzer JL. Functionally distinct PI 3-kinase pathways regulate myelination in the peripheral nervous system. J Cell Biol. 2014;204:1219–1236. doi: 10.1083/jcb.201307057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnia K, Vaccari I, Bolino A, Laporte J. Myotubularin phosphoinositide phosphatases: cellular functions and disease pathophysiology. Trends Mol Med. 2012;18:317–327. doi: 10.1016/j.molmed.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh ST, Kidd GJ, Crawford TO, Xu Z, Lin WM, Trapp BD, Cleveland DW, Griffin JW. Regional modulation of neurofilament organization by myelination in normal axons. J Neurosci. 1994;14:6392–6401. doi: 10.1523/JNEUROSCI.14-11-06392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Hu X, Hu J, Dai L, Trapp B, Yan R. Axonal and Schwann cell Bace1 is equally required for remyelination of peripheral nerves. J Neurosci. 2015;35:3806–3814. doi: 10.1523/JNEUROSCI.5207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung H, Kohnken R, Svaren J. The nucleosome remodeling and deacetylase chromatin remodeling (NuRD) complex is required for peripheral nerve myelination. J Neurosci. 2012;32:1517–1527. doi: 10.1523/JNEUROSCI.2895-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, Lotscher P, Ozcelik M, Tricaud N, Meijer D, Yamaguchi T, Matthias P, Suter U. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nature neuroscience. 2011;14:429–436. doi: 10.1038/nn.2762. [DOI] [PubMed] [Google Scholar]
- Jacob C, Lotscher P, Engler S, Baggiolini A, Varum Tavares S, Brugger V, John N, Buchmann-Moller S, Snider PL, Conway SJ, Yamaguchi T, Matthias P, Sommer L, Mantei N, Suter U. HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia. J Neurosci. 2014;34:6112–6122. doi: 10.1523/JNEUROSCI.5212-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, Levavasseur F, Raghoenath S, Grosveld F, Meijer D. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17:1380–1391. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, Grosveld F, Meijer D. The POU factor Oct-6 and Schwann cell differentiation. Science. 1996;273:507–510. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jagalur NB, Ghazvini M, Mandemakers W, Driegen S, Maas A, Jones EA, Jaegle M, Grosveld F, Svaren J, Meijer D. Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. J Neurosci. 2011;31:8585–8594. doi: 10.1523/JNEUROSCI.0659-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jin F, Dong B, Georgiou J, Jiang Q, Zhang J, Bharioke A, Qiu F, Lommel S, Feltri ML, Wrabetz L, Roder JC, Eyer J, Chen X, Peterson AC, Siminovitch KA. N-WASp is required for Schwann cell cytoskeletal dynamics, normal myelin gene expression and peripheral nerve myelination. Development. 2011;138:1329–1337. doi: 10.1242/dev.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Tian L, Mikesh M, Lichtman JW, Thompson WJ. Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury. J Neurosci. 2014;34:6323–6333. doi: 10.1523/JNEUROSCI.4673-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel L, Jaegle M, Driegen S, Aunin E, Leslie K, Fukata Y, Watanabe M, Fukata M, Meijer D. Functional phylogenetic analysis of LGI proteins identifies an interaction motif crucial for myelination. Development. 2014;141:1749–1756. doi: 10.1242/dev.107995. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Eisen JS. The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives. Development. 2000;127:515–525. doi: 10.1242/dev.127.3.515. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Gross MK, Gruss P. Pax3: a paired domain gene as a regulator in PNS myelination. Neuron. 1995;15:553–562. doi: 10.1016/0896-6273(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Kipanyula MJ, Woodhoo A, Rahman M, Payne D, Jessen KR, Mirsky R. Calcineurin-nuclear factor of activated T cells regulation of Krox-20 expression in Schwann cells requires elevation of intracellular cyclic AMP. J Neurosci Res. 2013;91:105–115. doi: 10.1002/jnr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca R, Cerri F, Horiuchi K, Bachi A, Feltri ML, Wrabetz L, Blobel CP, Quattrini A, Salzer JL, Taveggia C. TACE (ADAM17) inhibits Schwann cell myelination. Nat Neurosci. 2011;14:857–865. doi: 10.1038/nn.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T, Aust G, Hamann J. Sticky signaling--adhesion class G protein-coupled receptors take the stage. Science signaling. 2013;6:re3. doi: 10.1126/scisignal.2003825. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The ontogeny of the neural crest in avian embryo chimaeras. Nature. 1980;286:663–669. doi: 10.1038/286663a0. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005a;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J. Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci. 2005b;8:932–940. doi: 10.1038/nn1490. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Dev Biol. 2002;246:245–258. doi: 10.1006/dbio.2002.0670. [DOI] [PubMed] [Google Scholar]
- Lemke G, Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988;102:499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Lewallen KA, Shen YA, De la Torre AR, Ng BK, Meijer D, Chan JR. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31:3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yang H, Liu Y, Huan W, Zhang S, Wu G, Lu Q, Wang Q, Wang Y. The cyclin-dependent kinase inhibitor p27(Kip1) is a positive regulator of Schwann cell differentiation in vitro. J Mol Neurosci. 2011;45:277–283. doi: 10.1007/s12031-011-9518-2. [DOI] [PubMed] [Google Scholar]
- Liebscher I, Schon J, Petersen SC, Fischer L, Auerbach N, Demberg LM, Mogha A, Coster M, Simon KU, Rothemund S, Monk KR, Schoneberg T. A Tethered Agonist within the Ectodomain Activates the Adhesion G Protein-Coupled Receptors GPR126 and GPR133. Cell Rep. 2014;9:2018–2026. doi: 10.1016/j.celrep.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher I, Schoneberg T, Promel S. Progress in demystification of adhesion G protein-coupled receptors. Biol Chem. 2013;394:937–950. doi: 10.1515/hsz-2013-0109. [DOI] [PubMed] [Google Scholar]
- Limpert AS, Bai S, Narayan M, Wu J, Yoon SO, Carter BD, Lu QR. NF-kappaB forms a complex with the chromatin remodeler BRG1 to regulate Schwann cell differentiation. J Neurosci. 2013;33:2388–2397. doi: 10.1523/JNEUROSCI.3223-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpert AS, Carter BD. Axonal neuregulin 1 type III activates NF-kappaB in Schwann cells during myelin formation. J Biol Chem. 2010;285:16614–16622. doi: 10.1074/jbc.M109.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Prior M, He W, Hu X, Tang X, Shen W, Yadav S, Kiryu-Seo S, Miller R, Trapp BD, Yan R. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem. 2011;286:23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–524. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Mager GM, Ward RM, Srinivasan R, Jang SW, Wrabetz L, Svaren J. Active gene repression by the Egr2.NAB complex during peripheral nerve myelination. J Biol Chem. 2008;283:18187–18197. doi: 10.1074/jbc.M803330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe HG, Mehta G, Zhang X, Datar I, Mehrotra A, Yeung KC, de la Serna IL. SWI/SNF enzymes promote SOX10- mediated activation of myelin gene expression. PLoS One. 2013;8:e69037. doi: 10.1371/journal.pone.0069037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–874. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D. Neuroscience. Went fishing, caught a snake. Science. 2009;325:1353–1354. doi: 10.1126/science.1180103. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Pennock J, Goodwin F, Sewry C, Cowan F, Dubowitz L, Dubowitz V, Muntoni F. Sequential study of central and peripheral nervous system involvement in an infant with merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 1996;6:425–429. doi: 10.1016/s0960-8966(96)00383-5. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33:17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuno K, Sobue G, Reddy UR, Wurzer J, Kreider B, Hotta H, Baron P, Ross AH, Pleasure D. Regulation of Schwann cell nerve growth factor receptor by cyclic adenosine 3',5'-monophosphate. J Neurosci Res. 1988;21:465–472. doi: 10.1002/jnr.490210237. [DOI] [PubMed] [Google Scholar]
- Monje PV, Rendon S, Athauda G, Bates M, Wood PM, Bunge MB. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia. 2009;57:947–961. doi: 10.1002/glia.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani L, Buerki-Thurnherr T, de Faria JP, Pereira JA, Dias NG, Fernandes R, Goncalves AF, Braun A, Benninger Y, Bottcher RT, Costell M, Nave KA, Franklin RJ, Meijer D, Suter U, Revlas JB. Profilin 1 is required for peripheral nervous system myelination. Development. 2014;141:1553–1561. doi: 10.1242/dev.101840. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Weinmaster G, Kuhn R, Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989;3:783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell P, Quarles RH. Myelin Formation, Structure, and Biochemistry. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry. 6th edition. Chapter 4. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- Morton PD, Dellarole A, Theus MH, Walters WM, Berge SS, Bethea JR. Activation of NF-kappaB in Schwann cells is dispensable for myelination in vivo. J Neurosci. 2013;33:9932–9936. doi: 10.1523/JNEUROSCI.2483-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Murinson BB, Griffin JW. C-fiber structure varies with location in peripheral nerve. J Neuropath Exper Neurol. 2004;63:246–254. doi: 10.1093/jnen/63.3.246. [DOI] [PubMed] [Google Scholar]
- Nadra K, de Preux Charles AS, Medard JJ, Hendriks WT, Han GS, Gres S, Carman GM, Saulnier-Blache JS, Verheijen MH, Chrast R. Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 2008;22:1647–1661. doi: 10.1101/gad.1638008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30:355–368. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Seminars in cell & Dev Biol. 2010;21:922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM. Molecular control of the neural crest and peripheral nervous system development. Curr Top Dev Biol. 2015;111:201–231. doi: 10.1016/bs.ctdb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, Landreth GE, Snider WD. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–167. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Nishino J, Saunders TL, Sagane K, Morrison SJ. Lgi4 promotes the proliferation and differentiation of glial lineage cells throughout the developing peripheral nervous system. J Neurosci. 2010;30:15228–15240. doi: 10.1523/JNEUROSCI.2286-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Figlia G, Lebrun-Julien F, Pereira JA, Trotzmuller M, Kofeler HC, Rantanen V, Wessig C, van Deijk AL, Smit AB, Verheijen MH, Ruegg MA, Hall MN, Suter U. mTORC1 Controls PNS Myelination along the mTORC1-RXRgamma-SREBP-Lipid Biosynthesis Axis in Schwann Cells. Cell Rep. 2014;9:646–660. doi: 10.1016/j.celrep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Novak N, Bar V, Sabanay H, Frechter S, Jaegle M, Snapper SB, Meijer D, Peles E. N-WASP is required for membrane wrapping and myelination by Schwann cells. J Cell Biol. 2011;192:243–250. doi: 10.1083/jcb.201010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obremski VJ, Bunge MB. Addition of purified basal lamina molecules enables Schwann cell ensheathment of sympathetic neurites in culture. Dev Biol. 1995;168:124–137. doi: 10.1006/dbio.1995.1066. [DOI] [PubMed] [Google Scholar]
- Obremski VJ, Wood PM, Bunge MB. Fibroblasts promote Schwann cell basal lamina deposition and elongation in the absence of neurons in culture. Dev Biol. 1993;160:119–134. doi: 10.1006/dbio.1993.1291. [DOI] [PubMed] [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, Di Muzio A, Uncini A, Wrabetz L, Feltri ML. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J Neurosci. 2005;25:9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Abello G, Jaegle M, van Berge L, Hamer D, Kegel L, Driegen S, Sagane K, Bermingham JR, Jr, Meijer D. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. J Neurosci. 2010;30:3857–3864. doi: 10.1523/JNEUROSCI.6287-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Hall RA. Adhesion G protein-coupled receptors: signaling, pharmacology, and mechanisms of activation. Mol Pharm. 2012;82:777–783. doi: 10.1124/mol.112.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Science Sig. 2014;7:ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Liu B, Chen T, Li H, Hu X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J, Peng X, Qiu M. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–12819. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Dickinson S, Bhaskaran A, Kinsella MT, Brophy PJ, Sherman DL, Sharghi-Namini S, Duran Alonso MB, Mirsky R, Jessen KR. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol Cell Neurosci. 2003;23:13–27. doi: 10.1016/s1044-7431(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron. 1999;23:713–724. doi: 10.1016/s0896-6273(01)80030-1. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Wang B, Tarumi YS, Seburn KL, Burgess RW. A single point mutation in the LN domain of LAMA2 causes muscular dystrophy and peripheral amyelination. J Cell Sci. 2008;121:1593–1604. doi: 10.1242/jcs.015354. [DOI] [PubMed] [Google Scholar]
- Pellegatta M, De Arcangelis A, D'Urso A, Nodari A, Zambroni D, Ghidinelli M, Matafora V, Williamson C, Georges-Labouesse E, Kreidberg J, Mayer U, McKee KK, Yurchenco PD, Quattrini A, Wrabetz L, Feltri ML. alpha6beta1 and alpha7beta1 integrins are required in Schwann cells to sort axons. J Neurosci. 2013;33:17995–18007. doi: 10.1523/JNEUROSCI.3179-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier F, Gerber A, Bauer C, Ballivet M, Ossipow V. The adhesion molecule Necl-3/SynCAM-2 localizes to myelinated axons, binds to oligodendrocytes and promotes cell adhesion. BMC Neurosci. 2007;8:90. doi: 10.1186/1471-2202-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Baumann R, Norrmen C, Somandin C, Miehe M, Jacob C, Luhmann T, Hall-Bozic H, Mantei N, Meijer D, Suter U. Dicer in Schwann cells is required for myelination and axonal integrity. J Neurosci. 2010;30:6763–6775. doi: 10.1523/JNEUROSCI.0801-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Goncalves AF, Ozcelik M, Thurnherr T, Tricaud N, Meijer D, Fassler R, Suter U, Revlas JB. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol. 2009;185:147–161. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35:123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Perlin JR, Lush ME, Stephens WZ, Piotrowski T, Talbot WS. Neuronal Neuregulin 1 type III directs Schwann cell migration. Development. 2011;138:4639–4648. doi: 10.1242/dev.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schoneberg T, Piao X, Monk KR. The Adhesion GPCR GPR126 Has Distinct, Domain-Dependent Functions in Schwann Cell Development Mediated by Interaction with Laminin-211. Neuron. 2015;85:755–769. doi: 10.1016/j.neuron.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello E, Rivellini C, Dina G, Triolo D, Del Carro U, Ungaro D, Panattoni M, Feltri ML, Wrabetz L, Pardi R, Quattrini A, Previtali SC. Jab1 regulates Schwann cell proliferation and axonal sorting through p27. J Exp Med. 2014;211:29–43. doi: 10.1084/jem.20130720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast A, Raible DW. Neural Crest Cells: Evolution, Development, and Disease. Oxford, UK: Elsevier Press; 2014. Neural Crest Cells and Peripheral Nervous System Development; pp. 255–285. [Google Scholar]
- Previtali SC, Quattrini A, Bolino A. Charcot-Marie-Tooth type 4B demyelinating neuropathy: deciphering the role of MTMR phosphatases. Expert Rev Mol Med. 2007;9:1–16. doi: 10.1017/S1462399407000439. [DOI] [PubMed] [Google Scholar]
- Quijano-Roy S, Renault F, Romero N, Guicheney P, Fardeau M, Estournet B. EMG and nerve conduction studies in children with congenital muscular dystrophy. Muscle & nerve. 2004;29:292–299. doi: 10.1002/mus.10544. [DOI] [PubMed] [Google Scholar]
- Raphael AR, Perlin JR, Talbot WS. Schwann cells reposition a peripheral nerve to isolate it from postembryonic remodeling of its targets. Development. 2010;137:3643–3649. doi: 10.1242/dev.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael AR, Talbot WS. New insights into signaling during myelination in zebrafish. Curr Top Dev Biol. 2011;97:1–19. doi: 10.1016/B978-0-12-385975-4.00007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi K, Hurskainen M, Kallio M, Staven S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T. Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. J Neurosci. 2010;30:14490–14501. doi: 10.1523/JNEUROSCI.2644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol. 2013;203:835–847. doi: 10.1083/jcb.201305050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Ng BK, Chan JR. The quest for remyelination: a new role for neurotrophins and their receptors. Brain Path. 2006;16:288–294. doi: 10.1111/j.1750-3639.2006.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor AM, Polke JM, Houlden H, Reilly MM. Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat Rev Neurol. 2013;9:562–571. doi: 10.1038/nrneurol.2013.179. [DOI] [PubMed] [Google Scholar]
- Sagane K, Hayakawa K, Kai J, Hirohashi T, Takahashi E, Miyamoto N, Ino M, Oki T, Yamazaki K, Nagasu T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Quintes S, Mobius W, Wehr MC, Kramer-Albers EM, Brugger B, Nave KA. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci. 2009;29:6094–6104. doi: 10.1523/JNEUROSCI.0686-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Masaki T, Saito Y, Nakamura A, Takeda S, Shimizu T, Toda T, Matsumura K. Defective peripheral nerve myelination and neuromuscular junction formation in fukutin-deficient chimeric mice. J Neurochem. 2007;101:1712–1722. doi: 10.1111/j.1471-4159.2007.04462.x. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Wang DY, Kuhn R, Lemke G, Wrabetz L, Kamholz J. Axons regulate Schwann cell expression of the POU transcription factor SCIP. J Neurosci. 1994;14:1930–1942. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Wrabetz L. Molecular mechanisms of inherited demyelinating neuropathies. Glia. 2008;56:1578–1589. doi: 10.1002/glia.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Cantuti Castelvetri L, Simons M. Metabolism and functions of lipids in myelin. Biochimica et biophysica acta. 2014:pii: S1388–pii: S1981. doi: 10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Sharghi-Namini S, Turmaine M, Meier C, Sahni V, Umehara F, Jessen KR, Mirsky R. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J Neurosci. 2006;26:6364–6376. doi: 10.1523/JNEUROSCI.0157-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheean ME, McShane E, Cheret C, Walcher J, Muller T, Wulf-Goldenberg A, Hoelper S, Garratt AN, Kruger M, Rajewsky K, Meijer D, Birchmeier W, Lewin GR, Selbach M, Birchmeier C. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28:290–303. doi: 10.1101/gad.230045.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YA, Chen Y, Dao DQ, Mayoral SR, Wu L, Meijer D, Ullian EM, Chan JR, Lu QR. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nat Comm. 2014;5:4991. doi: 10.1038/ncomms5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Krols M, Wu LM, Grove M, Nave KA, Gangloff YG, Brophy PJ. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J Neurosci. 2012;32:1817–1825. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorer Z, Philpot J, Muntoni F, Sewry C, Dubowitz V. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. J Child Neurol. 1995;10:472–475. doi: 10.1177/088307389501000610. [DOI] [PubMed] [Google Scholar]
- Smith IW, Mikesh M, Lee Y, Thompson WJ. Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination. J Neurosci. 2013;33:17724–17736. doi: 10.1523/JNEUROSCI.3339-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Hall BK. A developmental model for the evolution of vertebrate exoskeleton and teeth. In: Hecht MK, MacIntyre RJ, Clegg MT, editors. Evolutionary Biology. New York: Springer US; 1993. pp. 387–448. [Google Scholar]
- Sobue G, Pleasure D. Schwann cell galactocerebroside induced by derivatives of adenosine 3',5'-monophosphate. Science. 1984;224:72–74. doi: 10.1126/science.6322307. [DOI] [PubMed] [Google Scholar]
- Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN, Peles E. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci. 2007;10:861–869. doi: 10.1038/nn1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Regulation of the cell cycle in normal and pathological glia. Neuroscientist. 2002;8:93–97. doi: 10.1177/107385840200800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart HJ, Zoidl G, Rossner M, Brennan A, Zoidl C, Nave KA, Mirsky R, Jessen KR. Helix-loop-helix proteins in Schwann cells: a study of regulation and subcellular localization of Ids, REB, and E12/47 during embryonic and postnatal development. J Neurosci Res. 1997;50:684–701. doi: 10.1002/(SICI)1097-4547(19971201)50:5<684::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Stirling CA. Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. J Anat. 1975;119:169–180. [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, Garcia-Castro MI. Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366:34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Auld DS, Robitaille R. Neurotrophins modulate neuron-glia interactions at a vertebrate synapse. Eur J Neurosci. 2007;25:1287–1296. doi: 10.1111/j.1460-9568.2007.05385.x. [DOI] [PubMed] [Google Scholar]
- Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–1605. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, Dina G, Pieragostino D, Sacchetta P, Urade Y, Boizet-Bonhoure B, Martinello Boneschi F, Quattrini A, Taveggia C. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat Neurosci. 2014;17:1682–1692. doi: 10.1038/nn.3857. [DOI] [PubMed] [Google Scholar]
- Turney SG, Lichtman JW. Reversing the outcome of synapse elimination at developing neuromuscular junctions in vivo: evidence for synaptic competition and its mechanism. PLoS Biol. 2012;10:e1001352. doi: 10.1371/journal.pbio.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Gen. 2000;67:1302–1305. doi: 10.1016/s0002-9297(07)62958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen MH, Camargo N, Verdier V, Nadra K, de Preux Charles AS, Medard JJ, Luoma A, Crowther M, Inouye H, Shimano H, Chen S, Brouwers JF, Helms JB, Feltri ML, Wrabetz L, Kirchner D, Chrast R, Smit AB. SCAP is required for timely and proper myelin membrane synthesis. Proc Natl Acad Sci USA. 2009;106:21383–21388. doi: 10.1073/pnas.0905633106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, Golden JP, Baloh RH, Schmidt RE, Hunter DA, Milbrandt J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J Neurosci. 2011;31:10128–10140. doi: 10.1523/JNEUROSCI.0884-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K, Gross RW, Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886–898. doi: 10.1016/j.neuron.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki S, Yumoto N, Komatsu K, Araki T, Sehara-Fujisawa A. Roles of meltrin-beta/ADAM19 in progression of Schwann cell differentiation and myelination during sciatic nerve regeneration. J Biol Chem. 2009;284:2957–2966. doi: 10.1074/jbc.M803191200. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lannergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, Tryggvason K, Culheim S. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J Neurosci. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Webster HD, Martin R, O'Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973;32:401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Weider M, Kuspert M, Bischof M, Vogl MR, Hornig J, Loy K, Kosian T, Muller J, Hillgartner S, Tamm ER, Metzger D, Wegner M. Chromatin-remodeling factor Brg1 is required for Schwann cell differentiation and myelination. Dev Cell. 2012;23:193–201. doi: 10.1016/j.devcel.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Weinberg HJ, Spencer PS. Studies on the control of myelinogenesis. I. Myelination of regenerating axons after entry into a foreign unmyelinated nerve. J Neurocyt. 1975;4:395–418. doi: 10.1007/BF01261372. [DOI] [PubMed] [Google Scholar]
- Weston JA. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev Biol. 1963;6:279–310. doi: 10.1016/0012-1606(63)90016-2. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D'Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- Woolley AG, Tait KJ, Hurren BJ, Fisher L, Sheard PW, Duxson MJ. Developmental loss of NT-3 in vivo results in reduced levels of myelin-specific proteins, a reduced extent of myelination and increased apoptosis of Schwann cells. Glia. 2008;56:306–317. doi: 10.1002/glia.20614. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci USA. 2003;100:14421–14426. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol. 2005 doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DP, Kim J, Syed N, Tung YJ, Bhaskaran A, Mindos T, Mirsky R, Jessen KR, Maurel P, Parkinson DB, Kim HA. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 2012;32:7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozeki T, Wakatsuki S, Hatsuzawa K, Black RA, Wada I, Sehara-Fujisawa A. Meltrin beta (ADAM19) mediates ectodomain shedding of Neuregulin beta1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells. 2007;12:329–343. doi: 10.1111/j.1365-2443.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- Young P, Nie J, Wang X, McGlade CJ, Rich MM, Feng G. LNX1 is a perisynaptic Schwann cell specific E3 ubiquitin ligase that interacts with ErbB2. Mol Cell Neurosci. 2005;30:238–248. doi: 10.1016/j.mcn.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Yun B, Anderegg A, Menichella D, Wrabetz L, Feltri ML, Awatramani R. MicroRNA-deficient Schwann cells display congenital hypomyelination. J Neurosci. 2010;30:7722–7728. doi: 10.1523/JNEUROSCI.0876-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, Stettner M, Ruskamo S, Domenech-Estevez E, Baloui H, Medard JJ, Verheijen MH, Brouwers JF, Kursula P, Kieseier BC, Chrast R. A role of peripheral myelin protein 2 in lipid homeostasis of myelinating Schwann cells. Glia. 2014;62:1502–1512. doi: 10.1002/glia.22696. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci. 2004;24:10999–11009. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]