Abstract
Forces arising from contractile actomyosin filaments help shape tissue form during morphogenesis. Developmental events that result from actomyosin contractility include tissue elongation, bending, budding, and collective migration. Here, we highlight recent insights into these morphogenetic processes from the perspective of actomyosin contractility as a key regulator. Emphasis is placed on a range of results obtained through live imaging, culture, and computational methods. Combining these approaches in the future has the potential to generate a robust, quantitative understanding of tissue morphodynamics.
Keywords: mechanical force, actin, stress
Introduction
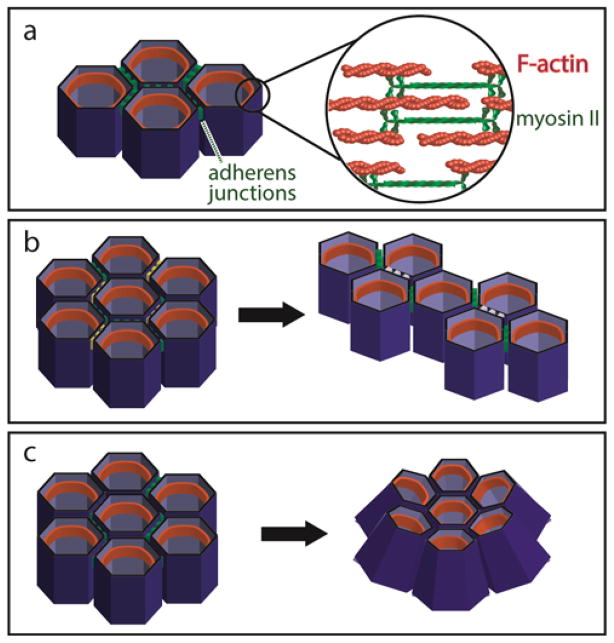
The generation of tissue form during morphogenesis is coordinated across multiple length scales, from molecular interactions to cell-level deformations to tissue-level changes in shape. These length scales are often bridged by the transmission of cytoskeletal tension, which arises from the movement of myosin motors along actin filaments (Figure 1a). At the molecular scale, this actomyosin contractility is regulated by several signaling pathways, including those downstream of Rho kinase (ROCK) and myosin light chain kinase (MLCK). When forces produced by locally-activated actomyosin are transmitted along greater length scales via junctional domains, a cell can do work on or move within its surrounding tissue. For example, planar polarized contractility can instruct convergent extension (Figure 1b), whereas actomyosin contractility localized to apical cellular surfaces drives apical constriction (Figure 1c). Proteins that are sensitive to local stresses and strains (e.g. stretch-activated ion channels and other mechanosensing proteins) can also convert mechanical information back into molecular instructions (reviewed in [1]). In this way, actomyosin contractility regulates and can be regulated by tissue morphogenesis.
Figure 1. Contraction of actomyosin filaments generates tissue-scale changes in shape.
(a) Filamentous actin, crosslinking proteins (not shown), and non-muscle myosin II form contractile actomyosin filaments, shown in the zonula adherens belt (orange structures) within an epithelium. (b) Local increases in planar polarized contractility (see yellow cell borders) result in preferential remodeling of cell junctions (to white cell borders) during convergent extension. (c) Apically-localized actomyosin contractility decreases the area of the apical membrane to drive budding and epithelial sheet bending.
Given the architectural complexity of native epithelial tissues, protein signaling, and cellular biophysics, quantitative information about morphogenesis has been gleaned from a combination of in vivo imaging, experiments in culture, and computational modeling. Here, we review recent work that has used these techniques to elucidate the mechanics and dynamics of tissue morphogenesis, from the perspective of actomyosin contractility as a key regulator of these processes. The interested reader is directed to more comprehensive reviews describing the forces in morphogenetic patterning [2], contractility-induced changes in tissue shape [3,4], and epithelial junctional dynamics [5]. Combining computational, culture, and in vivo methods in the future will be important for generating a more robust, quantitative understanding of tissue morphodynamics.
Actomyosin contractility and tissue elongation
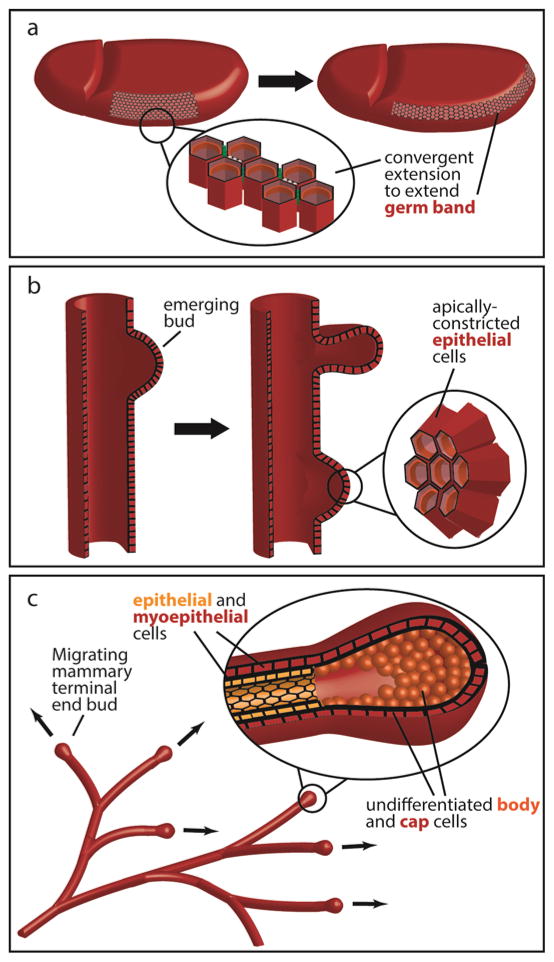
Cell intercalation (reviewed in [6]) is a morphogenetic process in which cells remodel their intercellular contacts to significantly rearrange their relative positions within an array of neighboring cells. One of the most well-studied types of intercalation in the context of tissue morphogenesis, convergent extension, occurs when the tissue lengthens along one axis while simultaneously narrowing along the perpendicular axis (Figure 1b), a process requiring asymmetric distributions of force along cell boundaries. During Drosophila germband extension (Figure 2a), for example, planar polarized actomyosin contractility shortens dorsoventral junctions to drive intercalation in the anteroposterior direction [7]. Planar cell polarity (PCP) proteins within Xenopus Laevis mesoderm also direct convert extension by compartmentalizing cortical contractility along mediolaterally-aligned junctional domains [8] and so it is likely that similar mechanisms occur in vertebrates.
Figure 2. Actomyosin contractility regulates many developmental processes.
(a) Convergent extension elongates the germband during Drosophila gastrulation. (b) Apical constriction initiates budding during airway branching morphogenesis in the embryonic chicken lung. (c) Contractility-dependent collective migration sculpts the 3D mammary ductal architecture during mammary branching morphogenesis.
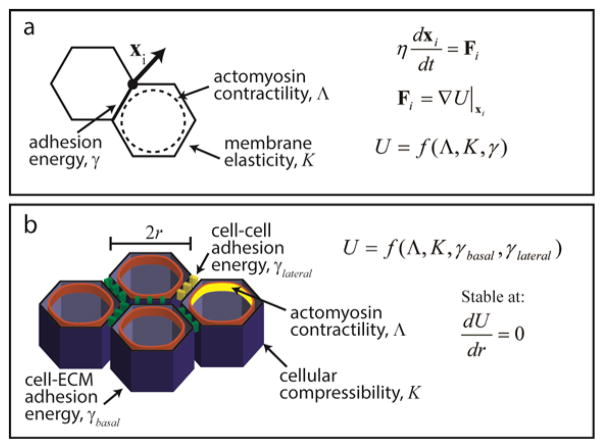
Obtaining a comprehensive, quantitative understanding of the dynamics of convergent extension remains an active area of research that has benefitted from computational modeling. Vertex models (reviewed in [9]) consider epithelial cells as polygons with the locations of the vertices specifying the state of the epithelial sheet (Figure 3a). Here, the equations of motion govern the movement of the vertices, and the forces associated with cellular contractility can be either specified explicitly or follow from the minimization of an energy function. The latter approach has been used to reveal that during germband extension, convergent extension results from the tissue minimizing its potential energy when driven by planar polarized contractility [10]. These cellular rearrangements occur with a characteristic, limiting velocity of ~2 μm/min, as calculated from a linear dependence of tissue cohesion, a function of actomyosin contractility and intercellular adhesion, on the resistance of the tissue to flow [11]. It will be interesting to determine how incorporating additional complexities, such as feedback between contractility and adhesion, competition between contractility and other cytoskeletal filaments [12], or mechanical communication with nearby morphogenetic events [13], into computational models quantitatively affects the final tissue form. In addition to elucidating rate-limiting steps in morphogenetic processes, such insight might clarify the degree to which certain mechanisms are context-dependent.
Figure 3. Computational modeling can quantify the underlying physical forces acting on cells during morphogenesis.
(a) In vertex models, cells are often modeled as 2D polygons representing a slice through the cell at the adherens belt. The movement of each vertex is related to the force acting on that vertex, which is a function of actomyosin contractility, adhesion to neighboring cells, and the elasticity of the membrane. See [9] for more information. (b) A recent model of epithelial morphology expands the force balance into 3D to represent changes in tissue shape as stable points in the underlying mechanical equations.
Axis elongation in Drosophila highlights the importance of controlled, spatiotemporal regulation of actomyosin contractility and the associated generation of force during tissue extension. Myosin II must be spatially and temporally regulated to achieve an efficient change in tissue shape [14], as increasing or decreasing its expression results in less efficient intercalation. This is consistent with studies in vitro in which it is seen that motor activity must be regulated to coordinate global contractions [15]. Otherwise, motor activity causes crosslinks to unbind, thereby driving an initially well-connected network to a critical, ruptured state. Thus, actomyosin contractility may be thought of as one regulator of a tissue-level potential energy function that, when properly maintained, drives cellular rearrangement and tissue extension.
Actomyosin contractility and tissue budding and bending
In addition to changes in tissue shape mediated by junctional remodeling, morphogenetic events also proceed when changes in cellular shape propagate into changes in tissue form. For example, apical constriction (reviewed in [16]) within adjacent cells produces a bend or bud within an epithelial sheet (Figure 1c). This change in cell shape, mediated by apically localized actomyosin contractility, is critical for many processes including initiation of monopodial branches in embryonic chicken lungs (Figure 2b) [17]. A similar role for actomyosin contractility has subsequently been suggested for domain branching during murine airway branching morphogenesis [18]. In this developing organ, the degree of budding scales with the degree of contractile activity [19] and bifurcation of lung buds is also driven by MLCK-regulated actomyosin contractility [20].
Live imaging has revealed many dynamic features of apical constriction, especially during Drosophila gastrulation. During formation of the ventral furrow, apical domain polarization localizes actomyosin activity in prospective mesodermal cells to drive apical constriction in a ratchet-like mechanism [21,22]. In this process, pulses of myosin II activation are associated with pulses of ROCK without a detectable lag time [23]. Forces generated by apical constriction in ventral furrow formation can then produce a tissue-scale hydrodynamic flow of cytoplasm to mediate tissue elongation and propagate forces deeper into the tissue [24].
Despite this tremendous insight, much less is known quantitatively about how force generation regulates apical constriction. Mechanical models have demonstrated that apical constriction is sufficient to give rise to budding [17] and folding of an epithelium [25], yet much remains to be learned about apically-localized contractility and how it varies in different contexts. It will be interesting to investigate force generation and transmission across multiple length scales during the ratchet-like mechanism of apical constriction with vertex or vertex-like models. Emerging techniques using fluorescence resonance energy transfer (FRET)-based force sensors [26,27] will also likely help provide quantitative data against which to test these models.
Actomyosin contractility is also an important regulator of epithelial bending, which can convert a two-dimensional (2D) sheet into a three-dimensional (3D) structure. Recent developmental studies of the chick heart tube [28], Drosophila wing disk [29] and eggshell [30] have implicated regulatory roles for cellular tension in producing bent, 3D tissue structures. It will be interesting to characterize the dynamics of such changes in shape in these and future cases with regard to recent models of epithelial morphogenesis (Figure 3b) [31]. Here, in vivo morphologies are understood as stable points of mechanical equations, with epithelial sheet curvature and bending resulting from cell adhesion and contractility. In the future, quantitative measurements of tension should be incorporated into such models. For example, pN-scale forces have been measured across focal adhesions [26] and adherens junctions [27]. Other emerging quantification tools include laser nanosurgery [32], laser ablation [33], and deformable microdroplets of fluorescent oil [34]. The latter method has been used to measure anisotropic stresses on the order of nN μm−2 in 3D aggregates.
Similar to tissue elongation, apical constriction requires precise regulation of the actomyosin force-generating machinery. During Drosophila dorsal closure, amnioserosa cells propel epidermal cell migration by rapidly fluctuating their apical membrane area. The cycle lengths of these fluctuations shorten with the onset of net tissue contraction, followed by a damping of fluctuation amplitude until the amnioserosa cells contract rapidly [35]. Here, a low level of myosin activity is required to generate efficient contraction, as increased myosin phosphorylation results in an apparently stiffer amnioserosa [36]. It will be interesting to study quantitatively how force generation, cytoskeletal architecture, and adhesion complex formation are optimized for this and similar morphogenetic events.
Actomyosin contractility and collective migration
As discussed above, a tissue can change shape through junctional rearrangements and cell deformations. Changes in tissue form also occur during collective cellular migration (reviewed in [37]). For example, collective epithelial cell movement through the mammary stroma during branching morphogenesis (Figure 2c) produces the characteristic mammary ductal architecture [38]. Here, the cells within the migrating cohort maintain junctional attachments to each other as the population remodels the surrounding tissue and extracellular matrix.
3D culture models have provided quantitative insight into how collective migration is regulated by actomyosin contractility. In a 3D model of mammary branching morphogenesis, tissue regions under higher mechanical stress (~0.1 kPa) show changes in gene expression [39] and molecular signaling [40], resulting in multicellular invasion into the surrounding matrix [41]. Additionally, contractility within the tissue generates surrounding mechanical heterogeneities [42] and locally aligns matrix fibrils to instruct ductal elongation [43]. These findings are congruent with recent observations of branching morphogenesis of the murine salivary gland, where actomyosin contractility regulates basement membrane remodeling [44]. Actomyosin contractility is thus central to branching morphogenesis, and so it will be exciting to uncover more quantitative details about how intercellular forces temporally vary as the branch extends.
A full understanding of the mechanics of collective migration will require quantitative observation into how the material properties and behavior of individual cells within a migrating cohort influence migration and morphogenesis. Indeed, as cell density increases in a migrating epithelial sheet, cell movements transition from ballistic (that is, the cellular mean square displacement is proportional to the square of the observation time, <r2> ~ t2) to sub-diffusive (<r2> ~ tα where α < 1) as the cells become trapped in cages formed by their neighbors [45]. This increases the stiffness of the epithelium, possibly due to increased transmission of stress between cells and a concomitant strengthening of the cytoskeleton. The formation of adherens junctions also coincides with an increase in the apparent stiffness of epithelial monolayers, reflecting the generation of tissue-level tension [46]. These observations provide further mechanical means by which actomyosin contractility is involved in regulating morphogenesis.
Outlook and concluding remarks
Actomyosin contractility both regulates and is regulated by tissue morphogenesis, as is briefly described here for 2D tissue elongation, 3D budding and bending, and collective migration. A more complete understanding of the regulation of tissue morphodynamics will require better linking of molecular signaling to current mechanical models that describe changes in tissue shape based on localized contractility. A notable recent example combines kinetic and mechanics approaches to demonstrate oscillations of myosin contractile activity in the observed spatiotemporal pattern in the elongating Drosophila egg chamber [47]. Potential topics for mechanochemical modeling include a wingless-int chemical gradient specifying the precise domains of localized nonmuscle myosin II activity during chick feather morphogenesis [48] and feedback between ROCK and Shroom signaling to amplify planar polarized actomyosin contractility during Drosophila germband extension [49]. It will also be interesting to incorporate more complex feedback between intercellular forces and cellular biochemistry. For example, myosin-dependent contractility can decrease the mobility of cadherin molecules, thus concentrating them at adherens junctions in culture [50]. Finally, calcium channels also regulate force [51], indicating a further unexplored coupling between cellular bioelectrochemistry and tissue mechanics.
Recent advances have expanded our quantitative understanding of how contractility influences the dynamics of morphogenetic processes. Overcoming current challenges will require quantifying the forces generated by actomyosin filaments in tissues undergoing morphogenesis and incorporating these into more advanced models describing changes in tissue shape. Where this fails, better culture models must be developed to physiologically recapitulate in vivo development. Quantitative insight gained from these processes can then be used to test computational models that describe 3D tissue morphogenesis as a function of subcellular actomyosin regulation. Conversely, newly developed models should be able to aid in experimental design. By incorporating all three approaches, we will be able to use culture and modeling results to verify mechanisms postulated from in vivo data, while using in vivo observations to identify the physiological relevance of culture and modeling data. In vivo imaging, culture models, and computational approaches are thus well poised to generate a robust, quantitative understanding of tissue morphodynamics.
Acknowledgments
Work from the authors’ group was supported in part by grants from the NIH (GM083997, HL110335, HL118532, HL120142), the NSF (CMMI-1435853), the David & Lucile Packard Foundation, the Alfred P. Sloan Foundation, and the Camille & Henry Dreyfus Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. M.J.S. was supported in part by the NSF Graduate Research Fellowship Program.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- FRET
fluorescence resonance energy transfer
- MLCK
myosin light chain kinase
- PCP
planar cell polarity
- ROCK
Rho kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mammoto T, Mammoto A, Ingber DE. Mechanobiology and Developmental Control. Annu Rev Cell Dev Biol. 2013;29:27–61. doi: 10.1146/annurev-cellbio-101512-122340. [DOI] [PubMed] [Google Scholar]
- 2.Heisenberg CP, Bellaiche Y. Forces in Tissue Morphogenesis and Patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Munjal A, Lecuit T. Actomyosin networks and tissue morphogenesis. Development. 2014;141:1789–1793. doi: 10.1242/dev.091645. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecuit T, Guillot Cn. Mechanics of Epithelial Tissue Homeostasis and Morphogenesis. Science. 2013;340:1185–1189. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 6.Walck-Shannon E, Hardin J. Cell intercalation from top to bottom. Nat Rev Mol Cell Biol. 2014;15:34–48. doi: 10.1038/nrm3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 8.Shindo A, Wallingford J. PCP and Septins Compartmentalize Cortical Actomyosin to Direct Collective Cell Movement. Science. 2014;343:649–652. doi: 10.1126/science.1243126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. Vertex Models of Epithelial Morphogenesis. Biophys J. 2014;106:2291–2304. doi: 10.1016/j.bpj.2013.11.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda H, Nagai T, Tanemura M. Two different mechanisms of planar cell intercalation leading to tissue elongation. Dev Dyn. 2008;237:1826–1836. doi: 10.1002/dvdy.21609. [DOI] [PubMed] [Google Scholar]
- 11.David R, Luu O, Damm EW, Wen JWH, Nagel M, Winklbauer R. Tissue cohesion and the mechanics of cell rearrangement. Development. 2014;141:3672–3682. doi: 10.1242/dev.104315. [DOI] [PubMed] [Google Scholar]
- 12.Dush MK, Nascone-Yoder NM. Jun N-terminal kinase maintains tissue integrity during cell rearrangement in the gut. Development. 2013;140:1457–1466. doi: 10.1242/dev.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller E, Kumar KV, Grill S, Fuchs E. Forces Generated by Cell Intercalation Tow Epidermal Sheets in Mammalian Tissue Morphogenesis. Dev Cell. 2014;28:617–632. doi: 10.1016/j.devcel.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasza KE, Farrell DL, Zallen JA. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc Natl Acad Sci U S A. 2014;111:11732–11737. doi: 10.1073/pnas.1400520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarado J, Sheinman M, Sharma A, MacKintosh FC, Koenderink GH. Molecular motors robustly drive active gels to a critically connected state. Nat Phys. 2013;9:591–597. [Google Scholar]
- 16.Martin AC, Goldstein B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 2014;141:1987–1998. doi: 10.1242/dev.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ““17.Kim HY, Varner VD, Nelson CM. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development. 2013;140:3146–3155. doi: 10.1242/dev.093682. In a study of airway branching morphogenesis within the embryonic chicken, the authors provide the first demonstration that apical constriction of the epithelium induces branching. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM, Morrisey EE. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci U S A. 2014;111:12444–12449. doi: 10.1073/pnas.1406639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 20.Schnatwinkel C, Niswander L. Multiparametric image analysis of lung-branching morphogenesis. Dev Dyn. 2013;242:622–637. doi: 10.1002/dvdy.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “21.Mason FM, Tworoger M, Martin AC. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat Cell Biol. 2013;15:926–U358. doi: 10.1038/ncb2796. The authors show that radial-polarized, medioapical ROCK recruits myosin II to drive ratchet-like apical constriction of mesoderm cells during Drosophila gastrulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasquez CG, Tworoger M, Martin AC. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J Cell Biol. 2014;206:435–450. doi: 10.1083/jcb.201402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ““24.He B, Doubrovinski K, Polyakov O, Wieschaus E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature. 2014;508:392–396. doi: 10.1038/nature13070. Interpreting live imaging results in terms of low Reynolds number fluid behavior, the authors show that stresses generated at the tissue surface can be transmitted deeper in the tissue through the hydrodynamic properties of the cytoplasm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones G, Chapman SJ. Modelling apical constriction in epithelia using elastic shell theory. Biomech Model Mechanobiol. 2010;9:247–261. doi: 10.1007/s10237-009-0174-1. [DOI] [PubMed] [Google Scholar]
- 26.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell–cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varner VD, Taber LA. Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development. 2012;139:1680–1690. doi: 10.1242/dev.073486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldaz S, Escudero LM, Freeman M. Dual role of myosin II during Drosophila imaginal disc metamorphosis. Nat Commun. 2013;4:1761. doi: 10.1038/ncomms2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “30.Osterfield M, Du X, Schüpbach T, Wieschaus E, Shvartsman Stanislav Y. Three-Dimensional Epithelial Morphogenesis in the Developing Drosophila Egg. Dev Cell. 2013;24:400–410. doi: 10.1016/j.devcel.2013.01.017. The authors combine computational modeling and live imaging results to quantitatively describe the epithelial bending and intercalations involved in converting a 2D sheet into the 3D respiratory appendages of the developing Drosophila egg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ““31.Hannezo E, Prost J, Joanny J-F. Theory of epithelial sheet morphology in three dimensions. Proc Natl Acad Sci U S A. 2014;111:27–32. doi: 10.1073/pnas.1312076111. The authors describe 3D epithelial sheet morphology in terms of mechanical equilibrium within the tissue. This single framework can describe many developmental processes, including epithelial bending, tubulogenesis, and buckling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombelli J, Solon J. Force communication in multicellular tissues addressed by laser nanosurgery. Cell Tissue Res. 2013;352:133–147. doi: 10.1007/s00441-012-1445-1. [DOI] [PubMed] [Google Scholar]
- 33.Angelo J, Tremblay K. Laser-mediated cell ablation during post-implantation mouse development. Dev Dyn. 2013;242:1202–1209. doi: 10.1002/dvdy.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campas O, Mammoto T, Hasso S, Sperling RA, O’Connell D, Bischof AG, Maas R, Weitz DA, Mahadevan L, Ingber DE. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat Met. 2014;11:183–189. doi: 10.1038/nmeth.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanchard GB, Murugesu S, Adams RJ, Martinez-Arias A, Gorfinkiel N. Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development. 2010;137:2743–2752. doi: 10.1242/dev.045872. [DOI] [PubMed] [Google Scholar]
- 36.Fischer SC, Blanchard GB, Duque J, Adams RJ, Arias AM, Guest SD, Gorfinkiel N. Contractile and Mechanical Properties of Epithelia with Perturbed Actomyosin Dynamics. PLoS One. 2014:9. doi: 10.1371/journal.pone.0095695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rorth P. Fellow travellers: emergent properties of collective cell migration. EMBO Rep. 2012;13:984–991. doi: 10.1038/embor.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nat Rev Mol Cell Biol. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Gjorevski N, Boghaert E, Radisky DC, Nelson CM. Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. EMBO J. 2011;30:2662–2674. doi: 10.1038/emboj.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Nelson CM. PI3K regulates branch initiation and extension of cultured mammary epithelia via Akt and Rac1 respectively. Dev Biol. 2013;379:235–245. doi: 10.1016/j.ydbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol. 2010;2:424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gjorevski N, Nelson CM. Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues. Biophys J. 2012;103:152–162. doi: 10.1016/j.bpj.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes C, Speroni L, Quinn K, Montevil M, Saetzler K, Bode Animashaun G, McKerr G, Georgakoudi I, Downes CS, Sonnenschein C, et al. From Single Cells to Tissues: Interactions between the Matrix and Human Breast Cells in Real Time. PLoS One. 2014;9:e93325. doi: 10.1371/journal.pone.0093325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev Biol. 2014;394:197–205. doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nnetu KD, Knorr M, Pawlizak S, Fuhs T, Kas JA. Slow and anomalous dynamics of an MCF-10A epithelial cell monolayer. Soft Matter. 2013;9:9335–9341. [Google Scholar]
- 46.Harris AR, Daeden A, Charras GT. Formation of adherens junctions leads to the emergence of a tissue-level tension in epithelial monolayers. J Cell Sci. 2014;127:2507–2517. doi: 10.1242/jcs.142349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koride S, He L, Xiong L-P, Lan G, Montell DJ, Sun SX. Mechanochemical regulation of oscillatory follicle cell dynamics in the developing Drosophila egg chamber. Mol Biol Cell. 2014;25:3709–3716. doi: 10.1091/mbc.E14-04-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li A, Chen M, Jiang TX, Wu P, Nie Q, Widelitz R, Chuong CM. Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation. Proc Natl Acad Sci U S A. 2013;110:E1452–E1461. doi: 10.1073/pnas.1219813110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simoes SD, Mainieri A, Zallen JA. Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J Cell Biol. 2014;204:575–589. doi: 10.1083/jcb.201307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat Cell Biol. 2014;16:587–594. doi: 10.1038/ncb2973. [DOI] [PubMed] [Google Scholar]
- 51.Hunter G, Crawford J, Genkins J, Kiehart D. Ion channels contribute to the regulation of cell sheet forces during Drosophila dorsal closure. Development. 2014;141:325–334. doi: 10.1242/dev.097097. [DOI] [PMC free article] [PubMed] [Google Scholar]