Abstract
Background
Whether biomechanical force on the heart can induce exosome secretion to modulate cardiovascular function is not known. We investigated the secretion and activity of exosomes containing a key receptor in cardiovascular function, the Angiotensin II Type I Receptor (AT1R).
Methods and Results
Exosomes containing AT1Rs were isolated from the media overlying AT1R-overexpressing cells exposed to osmotic stretch and from sera of mice undergoing cardiac pressure overload. The presence of AT1Rs in exosomes was confirmed by both electron microscopy and radioligand receptor binding assays, and shown to require β-arrestin2, a multifunctional adaptor protein essential for receptor trafficking. We show that functional AT1Rs are transferred via exosomes in an in vitro model of cellular stretch. Using mice with global and cardiomyocyte conditional deletion of β-arrestin2, we show that under conditions of in vivo pressure overload the cellular source for the exocytosis of exosomes containing AT1R is the cardiomyocyte. Exogenous administered AT1R-enriched exosomes target cardiomyocytes, skeletal myocytes and mesenteric resistance vessels, and is sufficient to confer blood pressure responsiveness to angiotensin II infusion in AT1R knockout mice.
Conclusions
This work reveals that AT1R-enriched exosomes are released from the heart under conditions of in vivo cellular stress to likely modulate vascular responses to neurohormonal stimulation. In the context of the whole organism, the concept of G protein-coupled receptor trafficking should consider circulating exosomes as part of the reservoir of functional AT1Rs.
Keywords: Exosomes, Angiotensin II Type I Receptor, Signaling, Osmotic Stretch, Pressure overload, Hypertension, β-arrestin
Introduction
Exosomes are extracellular nanovesicles of 30–100 nm in size that are released into the extracellular space by reverse budding of multivesicular bodies containing intraluminal vesicles.1 Exosomes were initially described as cell-secreted vesicles that eliminate obsolete molecules such as the transferrin receptors in reticulocytes,2 but are now known to be released by many different cell types and can be found in most bodily fluids.1, 3 The biogenesis of exosomes begins when early endosomes are processed by endosomal sorting mechanisms to form intraluminal vesicles within larger multivesicular bodies.1, 4 These multivesicular bodies containing intraluminal vesicles then traffic to, and fuse with, the plasma membrane to release their intraluminal vesicles (now termed extracellular vesicles or exosomes) into the extracellular microenvironment.1, 4, 5
Exosomes are structurally distinct from other shed particles, such as microparticles and ectosomes and vary in their abundance, size and composition. Exosomes are enriched in molecules derived from the parent cells including, but not limited to, adhesion and membrane trafficking molecules, signal transduction proteins, chemokines, mRNAs, non-coding RNAs and microRNAs, heat shock proteins, growth factors, and G protein-coupled receptors (GPCRs) such as the somatostatin receptor 2.1, 3, 5, 6 Intriguingly, exosome transfer can confer new functions on target cells and represent an important mechanism for intercellular communication and signaling.3, 7 This has particular relevance in cardiovascular physiology given the central role of neurohormonal signaling that occurs through the Angiotensin II Type 1 receptor (AT1R), a GPCR known to be critically involved in the maintenance of blood pressure and heart function. However, it remains unknown whether exosomes contain functional AT1Rs.
It is now appreciated that AT1Rs can be activated either by the endogenous agonist, angiotensin II (AngII), or by biomechanical stress.8–10 Interestingly, biomechanical stress induces AT1Rs to recruit the adaptor protein β-arrestin, internalize, and activate intracellular signaling in the absence of ligand.10, 11 Since it has been shown that stress conditions can lead to the cellular release of exosomes,12, 13 a potentially important functional outcome of stretch-activated AT1R trafficking could be the induction and release of exosomes containing AT1R into the microenvironment and systemic circulation.
In this study we tested the hypothesis that biomechanical stress induced in vitro by osmotic stretch, and in vivo by cardiac pressure overload, can induce secretion of AT1R enriched exosomes. Using radioligand binding, nanoparticle tracking of purified exosomes and mice with conditional deletion of β-arrestin2, we quantify the number of AT1Rs contained within exosomes, demonstrate the cellular source and mechanism for their release, and show functionality by testing their capacity to restore AT1R signaling in vitro and in vivo in AT1R KO mice.
Materials and Methods
Detailed material and methods are described in Supplemental Material.
Exosomes isolation
Prior to stimulation, cells were washed with PBS and then placed in serum free media for 30 minutes. Exosomes were isolated from conditioned media overlying ~4 ×108 cells after 30 minutes of either hypotonic conditions (143 mOsm/kg, Osmotic Stretch), Angiotensin II 10 µM (AngII), or no stimulation. Exosomes were purified using previously described methods.14
Nanoparticle Tracking Analysis (NTA)
Exosome preparations were diluted in PBS to obtain a particle concentration between 2–20 × 108/ml and examined with constant flow injection as described.15 (Figure S1, Figure S2).
AT1R radioligand binding assay
Modification of the radioligand binding assay was used to quantify AT1R density in exosomes.16
Experimental animals
Eight- to 12-week old mice of either sex of the following genotypes were used: C57/B6 wild type (WT), AT1R-KO,17 global β-arrestin1 KO,18, 19 global β-arrestin2 KO20 and β-arrestin2flox/flox.21 β-arrestin2flox/flox mice were generated by flanking exon 2 of the mouse β-arrestin2 gene (Arrb2) with LoxP sites21 and subsequent backcrossing into a C57/B6 genetic background for 7 generations. Conditional cardiomyocyte deletion of β-arrestin2 was generated by crossing β-arrestin2flox/flox mice with transgenic mice expressing a tamoxifen-inducible Mer-Cre-Mer recombinase under the control of the α-myosin heavy chain promoter,22 (Jackson lab, stock #005650) to generate β-arrestin2flox/+/αMHCMerCreMer mice and then crossed with β-arrestin2flox/flox to yield the genotypes used in the study (Figure S3).
At 12 weeks of age, β-arrestin2flox/flox/αMHCMerCreMer and β-arrestin2flox/flox mice of either sex were placed on a tamoxifen chow diet (400 mg/kg) for 7 days, followed by regular chow for an additional 21 days.
Statistical analysis
Data are expressed as median with 1st and 3rd quartile. Statistical significance was determined by Kruskal-Wallis. Correction for multiple comparisons was made using a Dunn’s correction. Analysis of blood pressure (Supplemental Material) comparing Basal vs Ang II was performed using Wilcoxon signed rank test for repeated measurements within each group, while comparison of % changes of hemodynamic parameters between 3 or more independent groups was assessed by Kruskal Wallis. QT-PCR data were analyzed by Mann-Whitney t test (Supplemental Material). A threshold value of p<0.05 was considered statistically significant. All analyses were performed with GraphPad Prism version 6.01.
Results
Membrane stretch and cardiac pressure overload increase exosome secretion
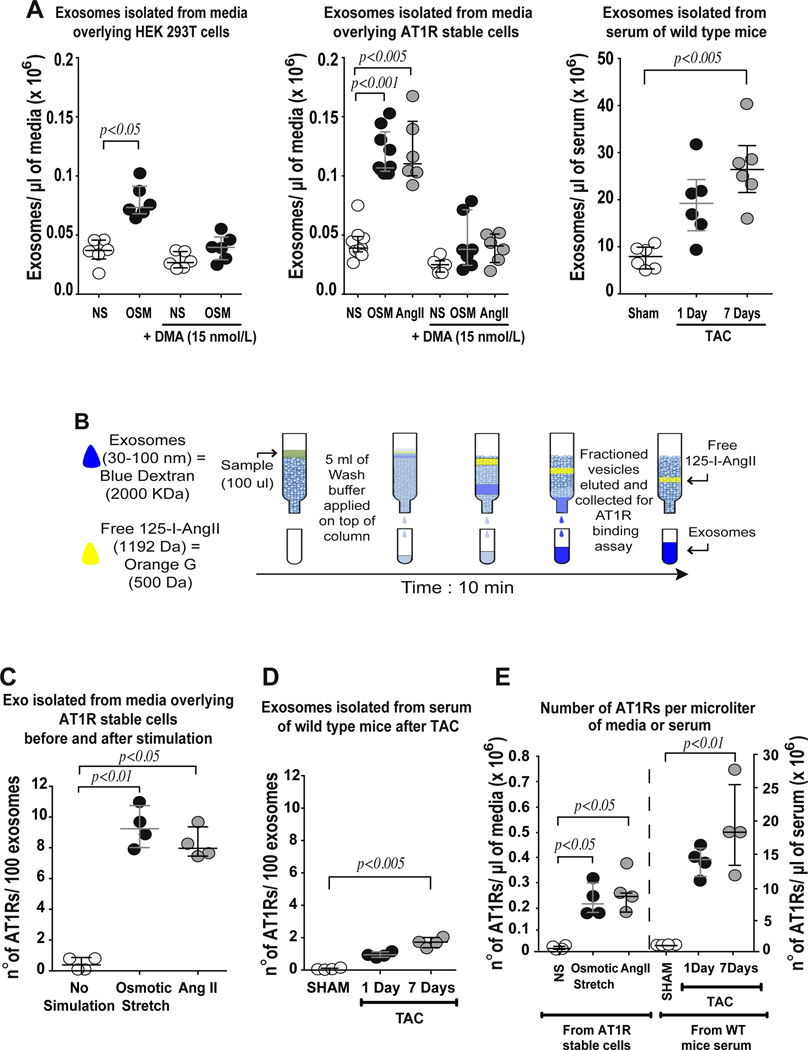
We used nanoparticle tracking analysis15 to determine whether exosomes are released into the media in vitro, and into the circulation in vivo, under conditions of mechanical overload. We found ~50,000 exosomes/µl of media overlying wild type HEK293T cells and cells stably overexpressing AT1Rs (receptor density 2.0 ± 0.22 pmol/mg protein) (Figure 1A). When stimulated by hypotonicity (OSM, osmotic stretch) or by AngII, there was approximately a two fold increase in the number of exosomes released from AT1R-expressing cells into the overlaying media (Figure 1A). This was abrogated with the addition of 15 nM dimethyl amiloride (DMA), an inhibitor of exosome release (Figure 1A). We next tested whether exosome release occurs in vivo under conditions of pressure overload in the intact animal. Wild-type mice were subjected to either transverse aortic constriction (TAC) or sham surgery and serum was harvested after 1 or 7 days. We found that under basal conditions, 1 µl of serum contains ~9 million exosomes, which increased 3 fold to ~28 million exosomes/µl of serum after 7 days of pressure overload (Figure 1A).
Figure 1.
Osmotic stretch significantly augments secretion of AT1R-enriched exosomes. A) Nanotracking particle analysis (NTA) was performed to determine concentration of exosomes isolated from conditioned media overlying HEK 293T cells or sera from mice. Left panel: Hypotonic conditioned media (osmotic stretch, OSM) significantly increased exosome concentration compared to isotonic media (NS) of HEK 293T cells; the addition of dimethyl amiloride (DMA, an inhibitor of exosome release) prevented the shedding of particles. Middle panel: Conditioned media from AT1R stable cells after stimulation with osmotic stretch or Angiotensin II (AngII) showed significantly increased concentration of exosomes that was also blocked by DMA treatment. Right panel: Exosomes isolated from sera of mice increased after pressure overload (transverse aortic constriction, TAC) compared to SHAM operated mice. B) Diagram of the size exclusion radioligand binding technique used to measure AT1R density in exosomes. C) Radioligand binding was used to measure AT1R density in isolated exosomes. AT1R density in exosomes isolated from overlying media after osmotic stretch and AngII stimulation were significantly increased compared to the basal no stimulation condition D) AT1R density of exosomes derived from sera of WT mice after TAC was significantly greater than sham serum. E) The number of AT1Rs calculated from receptor binding data was normalized to the starting volume of overlying media of AT1R stable cells stimulated with either Osmotic stretch (open bar) or AngII (filled bar); the same normalization was performed considering the starting volume mice serum after 1 day (open bar) or 7 days TAC (filled bar). A to E) Statistical significance was determined by Kruskal-Wallis with Dunn’s test comparing each group versus control non-stimulated (NS) or SHAM group. Data are represented as median with 1st and 3rd quartile.
We characterized exosomes isolated from HEK293T cells by transmission electron microscopy and immunoblotting (Figure S1A, B, C) and show the presence of common exosome markers such as CD9, CD63 and Alix.1, 3 Calnexin was not detected in the exosome fraction, demonstrating the lack of contaminating endoplasmic reticulum proteins. In addition, using immuno-gold labeling we demonstrate the presence of CD9 and tagged AT1R (AT1R-HA) on exosomes from media overlying AT1R-HA stably expressing cells (Figure S1D). We analyzed the size distribution in exosomes isolated from cell media after osmotic stress and from sera of mice after 7 days of TAC. Exosomes ranged in size from 30 nm to 100 nm with a mean size ~60–65 nm (Figure S1E, Figure S2), confirming the efficacy of our exosome isolation procedure with little contamination from larger microvesicles.
Exosomes contain Angiotensin II Type 1 Receptors
We next used saturation radioligand binding to confirm the presence of AT1R in exosomes released by cells after stimulation. Using G50 size exclusion columns to isolate the exosomes containing AT1R bound to a saturating concentration of [125I]-SAR1-ILE8-AngII (Figure 1B, see Methods for details), we determined that exosomes isolated from AT1R stable cells stimulated with either osmotic stretch or AngII stimulation expressed ~8–9 receptors/100 exosomes (Figure 1C). In separate in vivo experiments, we found that the density of AT1Rs per exosome released into the serum of mice under basal conditions was <1 AT1R/100 exosomes and ~2 AT1Rs/100 exosomes after a 7-day induction of pressure overload (Figure 1D). Based on these data, we calculated the AT1R density under basal conditions in vitro to be ~6 × 103 receptors per µl of cell media and ~3 × 104 receptors per µl of serum in WT mice. After imposing a stress condition such as osmotic stretch in vitro or TAC in vivo, the AT1R density increased to ~0.2 × 106 per µl of media and ~20 × 106 per µl of serum, more than 10 and 100 fold respectively, compared to basal conditions (Figure 1E).
AT1Rs transferred by exosomes are biochemically functional
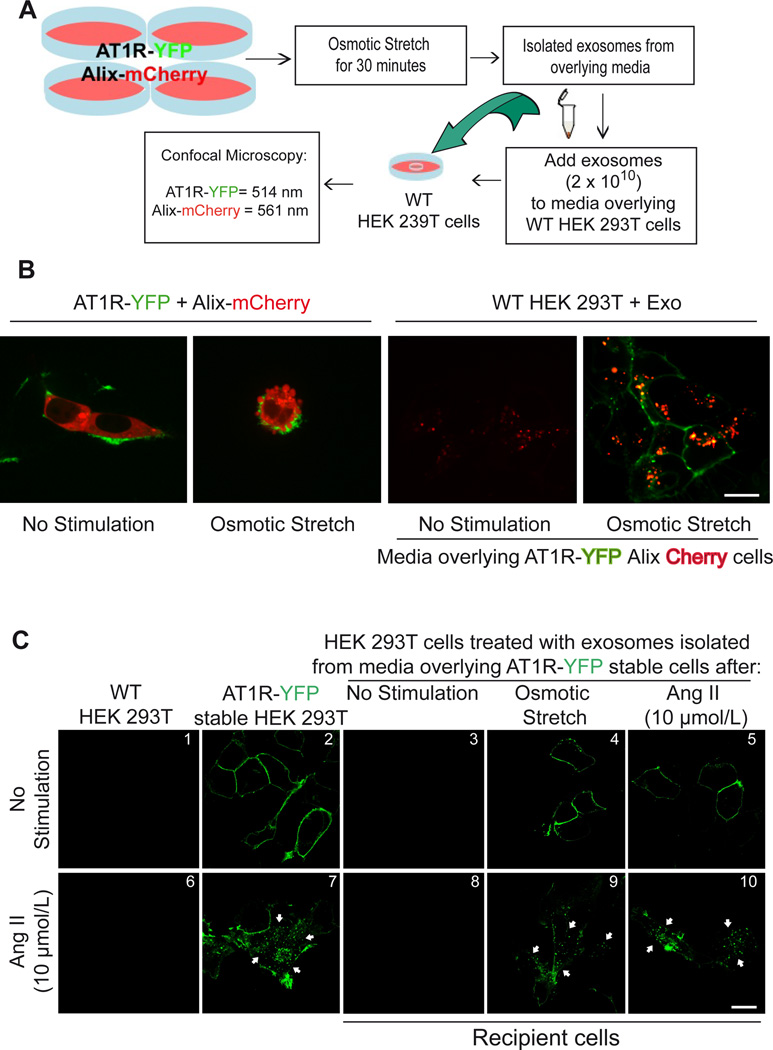
We next used confocal microscopy of YFP-tagged AT1Rs to test whether exosomes facilitate cell-to-cell communication by transferring functional AT1Rs (Figure 2A). In order to monitor for the presence of exosomes, we used donor cells overexpressing mCherry-tagged Alix, a commonly used exosome marker protein.1 AT1R-YFP cells transfected with Alix-mCherry were stimulated by osmotic stretch and ~40% of cells under these hypotonic conditions showed cell membrane surface budding (Figure 2B, middle panel), a process previously shown to involve both microvesicle23 and exosomal secretion.24 Confocal microscopy of wild type HEK 293T cells 12 hours after being exposed to exosomes derived from hypotonic-stimulated conditioned media overlying these AT1R-YFP and Alix-mCherry expressing cells showed transfer of Alix-mCherry to the inside of cells and AT1R-YFP to the plasma membrane (Figure 2B, right panel). To determine whether the AT1Rs transferred through exosomes were functional, we tested whether transferred AT1Rs would undergo internalization following AngII stimulation. Co-culturing wild type HEK 293T cells with exosomes isolated from osmotic stretch or AngII conditioned media of AT1R-YFP cells resulted in the transfer of AT1Rs to recipient cells that were localized to the plasma membrane at basal conditions (Figure 2C, panels 4 and 5). Exposure to 15 mins of AngII resulted in internalization of AT1Rs thus demonstrating that the exosome-mediated transfer of receptors retained their ability to traffic inside the cell after agonist stimulation (Figure 2C, panels 9 and 10, white arrows).
Figure 2.
HEK 293T cells respond to osmotic stretch and Angiotensin II by secreting AT1R enriched vesicles. A) Diagram of method used to show GPCR transfer by exosomes. B) confocal microscopy analysis of HEK 293T cells expressing AT1R-YFP and Alix-mCherry under basal conditions (panel I). 30 minutes of osmotic stress cells there is budding on the surface of cells and Alix (mCherry) and AT1R (YFP) enriched vesicles appear on the surface (panel II). Wild type HEK 293T cells 12 hours after being exposed to exosomes derived from not stimulated media with low transfer of Alix-Cherry protein (panel III) while hypotonic-stimulated conditioned media (panel IV) shows transfer of Alix-mCherry to the inside of cells and AT1R-YFP to the plasma membrane. C) Exosomes collected from overlying media of cells expressing AT1R-YFP were transferred to recipient cells and tested for their ability to internalize after agonist stimulation. Confocal microscopy analysis show AT1R-YFP localized to the plasma membrane in cells stably expressing AT1R-YFP (panel 2), and in recipient HEK293 cells after incubation with exosomes derived from osmotic stretch and AngII stimulation (panels 4 and 5). No GFP fluorescence was observed in recipient cells when incubated with exosomes isolated from unstimulated AT1R-GFP stable cells (panel 3). Stimulation of the transferred receptors with 10 µmol/L AngII for 15 min induced robust AT1R internalization (panels 9 and 10, white arrows). B,C (Scale bar 15 µm), N=4 independent experiments, representative images shown. The cell confluence (~70%) for all confocal experiments was checked in bright field using lower magnification microscope.
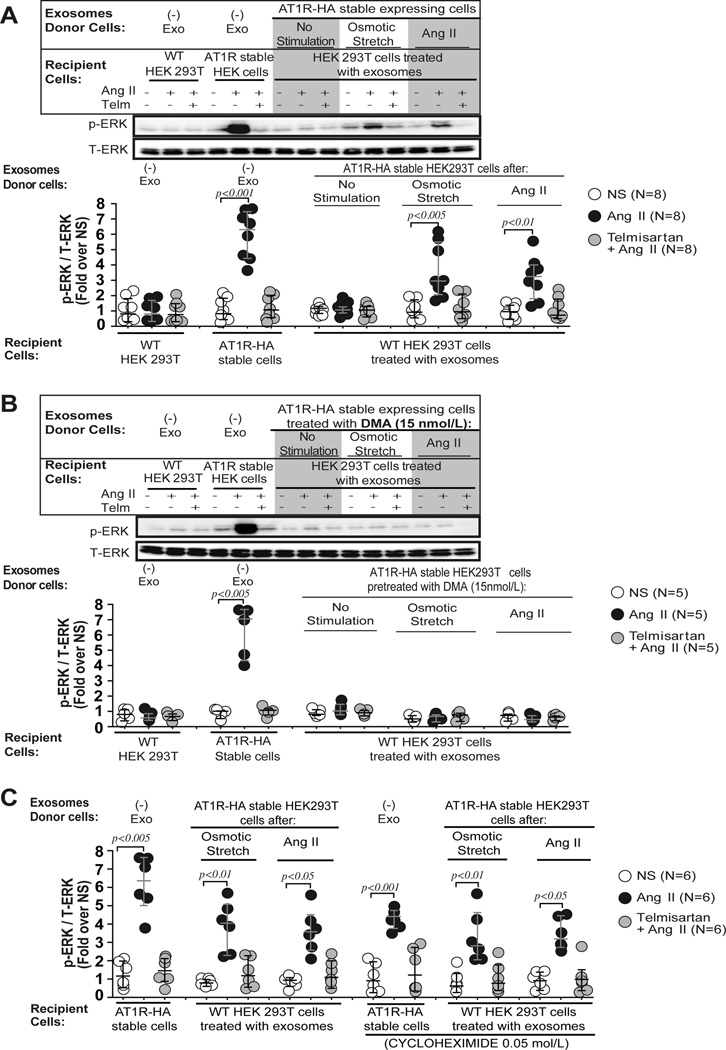
In order to test if exosome-transferred AT1Rs can activate agonist dependent signaling, we measured ERK1/2 phosphorylation in wild-type HEK 293T cells twelve hours after the addition of AT1R-HA containing exosomes (Figure 3A). AngII stimulation of exosome treated wild type 293T cells resulted in a 2–3 fold increase in ERK phosphorylation relative to T-ERK, that was completely blocked by pretreatment with the AT1R receptor blocker Telmisartan (Figure 3A). To test whether the transfer of functionally competent AT1Rs occurred via an exosome-dependent mechanism, we pretreated donor cells with the exosome release blocker, dimethyl amiloride to block secretion of exosomes. Recipient cells co-cultured with overlying media from dimethyl amiloride treated cells failed to respond to AngII stimulation (Figure 3B) indicating that in overlying media of stimulated cells the mechanism for AT1R responsiveness is most likely due to the transfer of AT1R contained in exosomes.
Figure 3.
Exosome derived AT1Rs are able to signal via classical GPCR pathways. A) HEK 293T cells preincubated with exosomes derived from conditioned media of AT1R-HA stable cells after osmotic stretch or AngII stimulation show a significant increase of p-ERK levels after AngII stimulation but not when cells were pre-incubated with exosomes derived from non-stimulated AT1R-HA stable cells. The AngII induced p-ERK response was blocked by the AT1R receptor blocker, Telmisartan (Telm). B) In a separate experiment AT1R-HA stable cells were pretreated with the inhibitor of exosome release, dimethyl amiloride (DMA,15 nmol/L), and exosomes were isolated from conditioned media of cells after stimulation with osmotic stretch or AngII. HEK 293T cells harvested with exosomes derived from AT1R-HA stable cells treated with DMA, failed to respond to AngII stimulation. A–B) Statistical significance was determined by 5 independent Kruskal-Wallis tests with post-hoc Dunn’s test comparing the p-ERK/T-ERK levels of AngII and Telm + AngII to the p-ERK/T-ERK levels of control non-stimulated (NS) in each group of recipient cells. C) HEK 293T cells preincubated with cycloheximide (0.05 mol/L) and exosomes derived from conditioned media of AT1R stable cells after osmotic stretch or AngII stimulation show a significant increase of p-ERK levels after AngII stimulation. Inhibiting protein synthesis with cycloheximide did not result in a reduction in AngII-induced p-ERK levels in recipient cells treated with exosomes. Statistical significance was determined by 6 independent Kruskal-Wallis tests with post-hoc Dunn’s test comparing the p-ERK/T-ERK levels of AngII and Telm + AngII to the p-ERK/T-ERK levels of control non-stimulated (NS) in each group of recipient cells. Additional comparison of the p-ERK/T-ERK levels of preselected pairs of Ang II columns with or without cycloheximide was performed in each 3 type of recipient cells (AT1R-HA stable cells no exo; HEK 293T cells + exo OSM; HEK 293T cells + exo AngII). Data are represented as median with 1st and 3rd quartile.
To exclude the possibility that transfer of AT1Rs resulted from new synthesis of transferred mRNAs, we incubated recipient cells with cycloheximide (0.05 mol/L) for 12 hours prior to AngII stimulation. While the level of ERK phosphorylation was slightly lower in the cells stably expressing AT1Rs treated with cycloheximide, no significant effect on ERK phosphorylation was observed in recipient cells (Figure 3C), suggesting that exosomes deliver functional AT1R protein and not mRNA.
Exosomes containing AT1R can modulate blood pressure responses in vivo
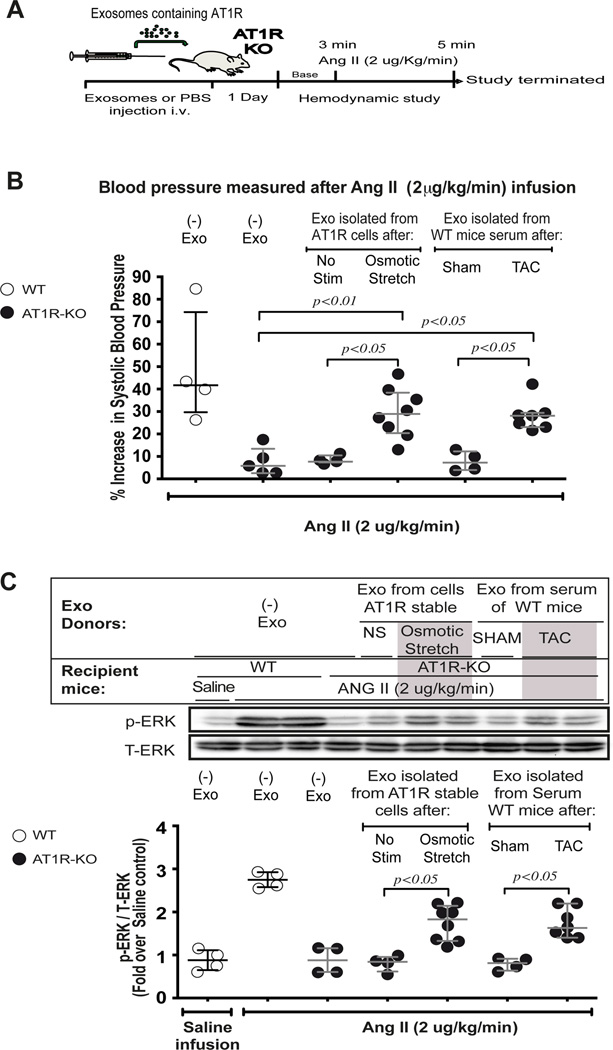
Previous studies have shown that exosomes contain functional molecules such as microRNAs, mRNAs and proteins, and when injected into animal models can lead to important physiologic effects on atherosclerosis formation13 or angiogenesis during peripartum cardiomyopathy.25 To determine whether AT1Rs transferred by exosomes could modulate a physiological response in vivo, we injected AT1R-enriched exosomes into the tail vein of AT1R-KO mice and 24 hours later measured the blood pressure response to AngII infusion (2 µg/Kg/min) (Figure 4A). AT1R-KO mice injected with PBS or exosomes isolated from media overlying non-stimulated cells, showed a minimal increase in blood pressure likely due to the infusion of volume (Figure 4 B, Table S1). However, when AT1R-KO mice received exosomes isolated from overlying media of hypotonic stimulated cells, or from serum of wild type mice 7 days after TAC, systolic blood pressure increased by ~30% (Fig. 4B, Table S1). We also found that heart lysates from AT1R-KO that received exosomes containing AT1R showed elevated levels of phosphorylated ERK when compared to either non-exosome treated or AT1R deficient exosome control hearts (Figure 4C). Using radioligand binding, we quantified AT1R expression in the heart, skeletal muscle, lung, and kidney tissue of WT and AT1R KO mice that received exogenous exosomes. We detected significant AT1R expression in both heart and skeletal muscle of exosome injected AT1R KO mice (~9 fmol/mg), which was approximately half the receptor density (15–19 fmol/mg protein) found in the heart and skeletal muscle of wild type mice (Table S2). Interestingly, AT1Rs were not detected in the kidney or lung of AT1R KO mice after intravenous exosome injection.
Figure 4.
Exosomes can serve as a means of receptor transfer in vivo: A) Diagram of in vivo study performed by injecting exosomes in tail vein of AT1R KO mice. The day following injection of exosomes into AT1R KO mice, responsiveness to AngII stimulation was assessed by invasive hemodynamic monitoring of blood pressure. B) In response to AngII infusion, systolic blood pressure in WT mice increased while no increase was detected in AT1R KO mice when injected with an exosomes free saline solution. Administration of exosomes derived from osmotic stretch treated AT1R stable cells or from serum of WT mice subjected to TAC restored the AngII-dependent increase in systolic blood pressure. C) After AngII infusion, hearts were removed to assess for AT1R mediated signaling. AngII-induced p-ERK signaling in the heart was restored in mice injected with AT1R enriched exosomes. B,C) Data are represented as median with 1st and 3rd quartile. Statistical significance was determined by Kruskal-Wallis with Dunn’s test for multi comparison.
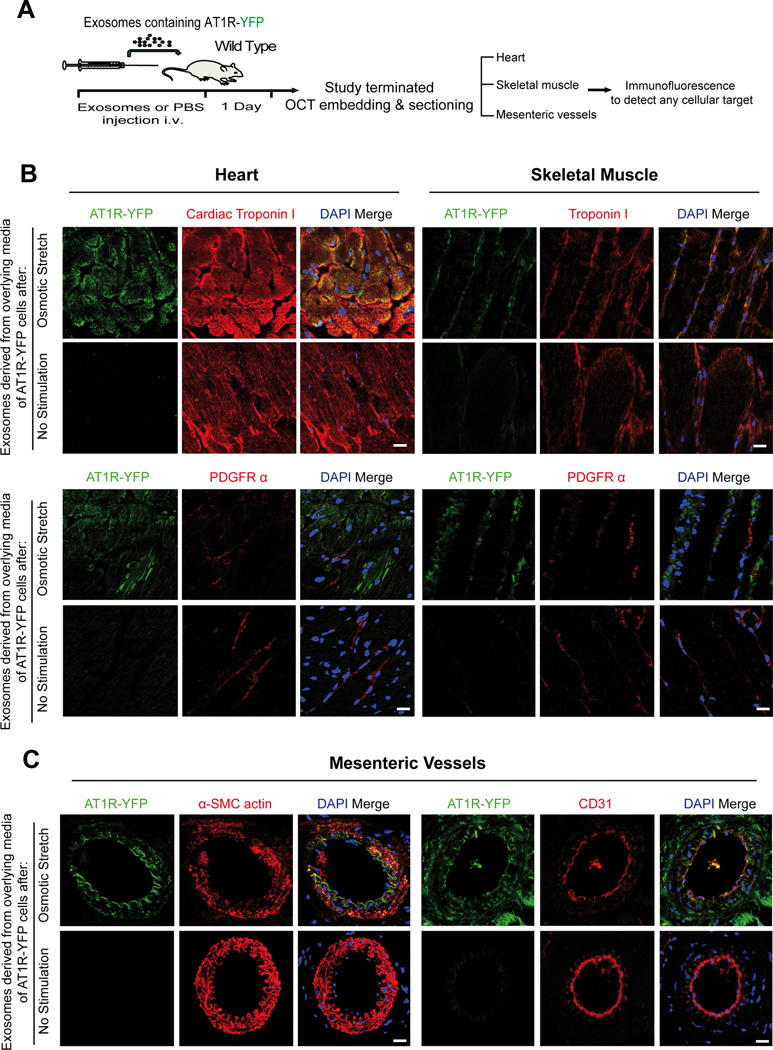
AT1Rs within circulating exosomes traffic to cardiac and skeletal myocytes, and resistance vessels
To investigate which cells in the heart and skeletal muscle uptake AT1Rs from circulating exosomes, we injected wild type mice with exosomes derived from media overlying AT1R-YFP cells with or without osmotic stretch (Figure 5A). Confocal microscopy of heart cryosections show the presence of fluorescently labeled AT1Rs only within troponin positive cardiomyocytes and not within PDGFR positive fibroblasts (Figure 5B left panel). A similar pattern was observed in skeletal muscle (Figure 5B right panel). These findings are supported by separate experiments of freshly isolated cardiomyocytes and fibroblasts from wild type mouse heart 24 hours after the intravenous injection of AT1R-YFP containing exosomes. Immunostaining for YFP, cardiac troponin I and PDGFR-α show incorporation of AT1R-YFP only in cardiomyocytes and not in fibroblasts (Figure S4).
Figure 5.
Circulating AT1Rs are taken up by cardiac and skeletal muscle myocytes and mesenteric vessels. A) Schematic of in vivo study performed by injecting in the tail vein of wild type mice exosomes derived from overlying media of cells stably overexpressing AT1R-YFP. The day following injection of exosomes heart, skeletal muscle and mesenteric vessels were embedded in OCT for sectioning. B) Tissue cryosections were immunostained for different cellular markers as indicated. AT1R green fluorescence was detected using a GFP antibody recognizing YFP. Cardiac and skeletal myocytes were detected by staining for cardiac troponin I or troponin I (top). Fibroblasts were detected by staining for PDGFRα (bottom). Green fluorescence was observed in cardiac and skeletal myocytes and not in fibroblasts. (Scale bar 15 µm), N=4 independent experiments, representative images shown. C) Mesenteric resistance vessels were immunostained for AT1R-YFP. Smooth muscle cells were detected with α-SMC actin (left panel) and endothelial cells with CD31 (right panel). (Scale bar 15 µm), N=4.
Since injection of exosomes containing AT1R resulted in the reconstitution of an AngII stimulated blood pressure response in AT1R KO mice (Figure 4A), we investigated whether the uptake of AT1Rs also occurred within cells of mesenteric resistance vessels. Consistent with their role in regulating blood pressure, we found the presence of AT1R-YFP within endothelial and smooth muscle cells of mesenteric vessels (Figure 5C).
β-arrestin is necessary for the packaging of AT1R cargo into exosomes
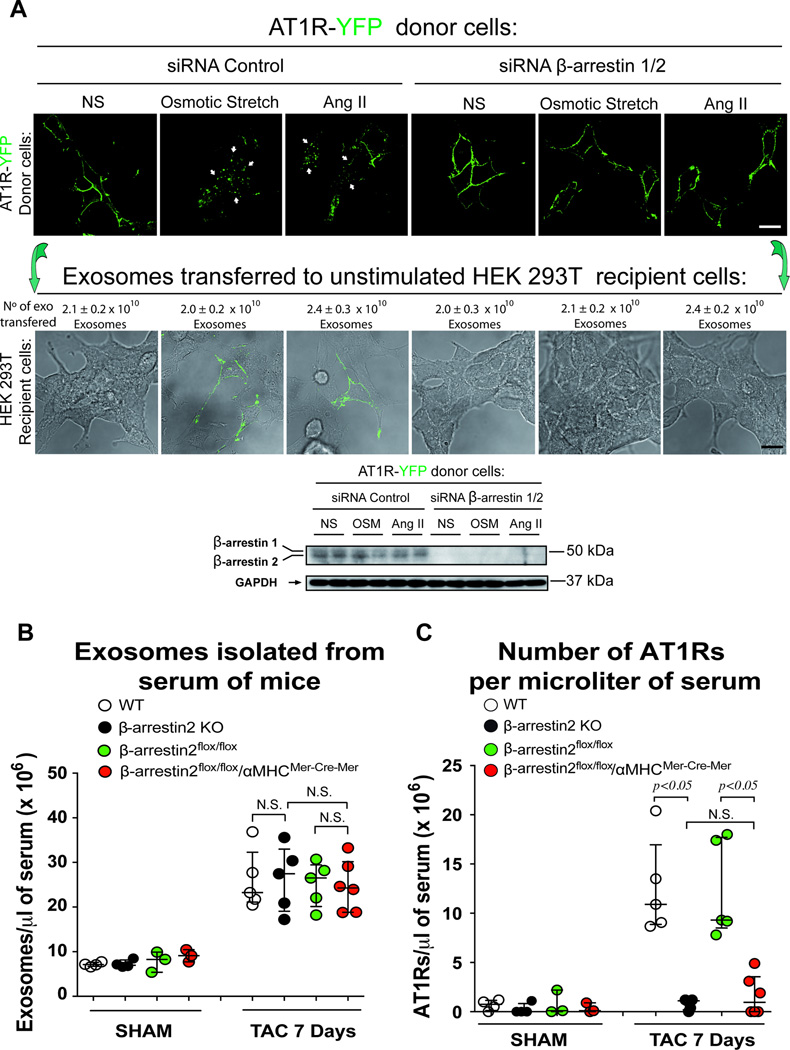
Since GPCR internalization requires the multifunctional adapter protein β-arrestin for efficient trafficking,26 we tested whether β-arrestin is necessary for the packaging of AT1R cargo into exosomes. Consistent with previous data for internalization and signaling,10, 27 silencing β-arrestin1 and 2 in AT1R-YFP stable expressing donor cells impaired both osmotic stretch and AngII induced AT1R internalization (Figure 6A). Importantly, when exosomes were isolated from media overlying AT1R-YFP overexpressing cells, silencing of β-arrestin1/2 blocked the exosomal transfer of AT1R-YFP to wild type HEK 293 recipient cells (Figure 6A). β-arrestin1/2 knock down did not affect the exosome concentration released by either osmotic or AngII (Figure 6A).
Figure 6.
Exosomes containing AT1Rs require β-arrestin for packaging and in vivo are secreted into the circulation by cardiomyocytes. A) siRNA targeting β-arrestin1/2 prevent AT1R internalization after osmotic stretch or AngII stimulation in AT1R-YFP donor cells (top panel). While siRNA targeting β-arrestin1/2 has no effect on the number of exosomes secreted, it prevented the enrichment of AT1Rs within exosomes leading to the inability to transfer ATR1R-YFP to recipient wild type HEK 293T cells (bottom panel). Representative Western blot showing silencing of β-arrestin1/2. B) Pressure overload induced a similar ~3 fold increase in circulating exosomes as measured by NTA in wild type, β-arrestin2 KO, β-arrestin2flox/flox, and β-arrestin2flox/flox/αMHCMerCreMer. C) Marked reduction in the number of AT1Rs in exosomes from sera of mice in β-arrestin2 KO and β-arrestin2flox/flox/αMHCMerCreMer mice compared to wild type and β-arrestin2flox/flox mice suggesting that the principal cellular source for AT1R-enriched exosome in the serum after TAC is the cardiomyocyte. Data are represented as median with 1st and 3rd quartile. Statistical significance was determined by Kruskal-Wallis with Dunn’s post-hoc test comparing different genotypes at 7 day TAC. p=NS β-arrestin2flox/flox/αMHCMerCreMer vs. β-arrestin 2 KO. Both the β-arrestin2flox/flox and β-arrestin2flox/flox/αMHCMerCreMer mice were treated with tamoxifen for 1 week followed by a 3 weeks wash out period.
Cardiomyocytes are the principal source of AT1R-enriched exosomes secreted during pressure overload
Based on our observation that packaging of AT1Rs into exosomes requires β-arrestin, we next tested whether this is also true for exosomes released in vivo into the circulation. In experiments using β-arrestin1 and 2 global knock out mice, we found that after pressure overload the total number of exosomes released into the circulation was the same as wild type for both β-arrestin1 and β-arrestin2 KO mice (Figure 6B and S5A). However, only the β-arrestin2 KO mice showed a markedly reduced number of AT1Rs in the serum after TAC (Figure 6C and S5 B,C,D) indicating that β-arrestin2 is needed for the in vivo packaging of AT1Rs into exosomes and is also consistent with the known role of β-arrestin2, and not β-arrestin1, in mechanical stretch induced-AT1R signaling.10
We next sought to identify the cellular source of AT1R-enriched exosomes after cardiac pressure overload. We crossed β-arrestin2flox/flox mice with αMHCMerCreMer transgenic driver mice and after tamoxifen treatment achieved approximately an 80% reduction in β-arrestin2 mRNA in cardiomyocytes (Figure S3). After 1 week of pressure overload, we observed a marked reduction in circulating AT1Rs in the serum of β-arrestin2flox/flox/αMHCMerCreMer compared to tamoxifen treated β-arrestin2flox/flox lacking the αMHCMerCreMer transgene (Figure 6C,S5D). These data suggest that the cellular source of AT1Rs released into the circulation under conditions of pressure overload is principally from cardiomyocytes.
Discussion
In this study we show that mechanical stress induces the release of exosomes containing AT1R in vitro under hypotonic conditions and in vivo with pressure overload. Using confocal microscopy and radioligand binding we show that exocytosis of exosomes by AT1R expressing cells after osmotic stretch or AngII contain functional AT1Rs. We demonstrate that the in vivo injection of AT1R-containing exosomes reconstitutes AngII-induced blood pressure response and cardiac ERK signaling in AT1R knock out animals, and that cardiac and skeletal myocytes, and mesenteric resistance vessels are the principal targets for exosomal delivery of AT1Rs. Most strikingly, we show that the trafficking and sorting of AT1Rs into exosomes requires the multifunctional adaptor protein, β-arrestin, and that the cellular source for AT1R-enriched exosomes released into the circulation after pressure overload is predominantly the cardiomyocyte. Our study supports the concept that exosomes contain functional AT1Rs and provide a means of intercellular communication at both the local tissue level, and at a distance, to maintain cardiovascular homeostasis.
It is now appreciated that cells continuously release exosomes containing proteins and RNAs as an important mechanism for cell-to-cell communication in multi-cellular organisms.1, 28, 29 A wide variety of cell types have been reported to release exosomes under normal or pathological conditions including astrocytes,30 microglial cells,31 tumor cells,32 endothelial cells13, 25 and cardiomyocytes.33 In this study we show that cardiomyocytes are the principal cellular source of AT1R-containing exosomes released into the circulation following TAC, since cardiomyocyte-specific deletion of β-arrestin2 in mice did not affect the total number of exosomes released (Figure 6B), but markedly impaired the packaging of AT1Rs into secreted exosomes (Figure 6C). Interestingly, the total number of exosomes released into the circulation was not reduced (Figure 6B) suggesting that β-arrestin is not required for intracellular maturation and exocytosis of exosomes, but is required for the sorting and packaging of GPCRs into the exosome. These data suggest a novel role for the multifunctional adaptor protein β-arrestin2, which not only regulates the intracellular trafficking and signaling of an agonist-stimulated GPCR,34 but also is an important mechanism regulating the intracellular pathways that lead to packaging of a GPCR into exosomes in response to a biomechanical stress.
While virtually all cells release microvesicles and nanovesicles (exosomes), the main distinction is particle size, with nanovesicles ranging between 30–100 nm and microvesicles ranging between 100 and 1000 nm in diameter.3 Therefore, distinguishing exosomes from the other types of microparticles secreted by cells can be technically challenging if rigorous methods are not employed.3 In this study we used a number of methods to purify and quantify high quality exosomes such as nanoparticle tracking, electron microscopy, differential centrifugation, size exclusion chromatography and ultracentrifugation. Together with our modification of the radioligand binding assay, we show that while only a few receptors can be detected per 100 exosomes, the number of exosomes released into the circulation increases 3 fold with hemodynamic overload, leading to the striking assessment that a microliter of serum contains ~10–20 million receptors. Furthermore, the number of circulating exosomes in our mouse studies (~2 × 109 per mL) is consistent with data in humans showing approximately 1010 exosomes per ml of plasma.35
Previous studies have shown that the release of exosomes containing growth factor receptors into the tumor microenvironment may be a mechanism to promote metastatic spread of tumor cells.1, 36 A proteomic study of plasma from healthy donors showed microvesicles contain proteins mostly associated with the complement and coagulation signal-transduction cascade and the cytoskeletal and integrin complex.37 Indeed, exosomes secreted from platelets confer pro-adhesive properties to endothelial cells38 or tumor cells39 by the transfer of adhesion molecules such as CD41. While it has been shown that AT1R agonist stimulation can increase secretion of functionally active microparticles to promote ROS production and proinflammatory response in endothelial cells in an EGFR dependent manner,40 it was not investigated whether AngII stimulation increased secretion of exosomes enriched with AT1Rs. Here we show that cardiomyocytes release AT1Rs containing exosomes in response to pressure overload and likely play an important role in cardiovascular homeostasis.
Our findings extend the physiological relevance of exosomal transfer of proteins to include the cardiovascular system. Previous in vitro studies have shown that adenosine 2A receptors and dopamine 2 receptors are released within microvesicles and can be transferred to acceptor cells retaining their ability to respond to agonist stimulation.41 Since hypotonic osmotic stretch in vitro is not comparable to the complex physiology of in vivo pressure overload, we tested whether exosomes secreted in vivo contain functional G protein-coupled receptors and could facilitate intercellular signaling. We speculate that under pathological conditions of increased circulating AngII levels or elevated cardiac filling pressures due to cardiac dysfunction or hypertension, enhanced vesicle secretion from cardiomyocytes and transfer of AT1Rs to distant cellular sites may represent a mechanism to offset the physiologic downregulation after agonist stimulation. Importantly, this may aggravate the cardiac function during blood pressure overload promoting ROS production or proinflammatory response in AT1R-dependent manner and lead to aggravation of cardiac remodeling during pressure overload.
The identification of cardiomyocytes as the principal cellular source that exocytose AT1R-enriched exosomes into the circulation with pressure overload, with subsequent uptake by skeletal muscle and resistance vessels, suggests that the current concept of GPCR trafficking may need to be expanded when considering the whole organism. The accepted model for GPCR trafficking is a multistep process that begins with the processing and folding of newly synthesized receptors within the endoplasmic reticulum, followed by transit through the Golgi into vesicles that become targeted to the plasma membrane.42 Once on the cell surface, ligand exposure promotes the endocytosis of receptors and involves a number of dynamic protein-protein interactions that is initiated by the recruitment of β-arrestin. β-arrestin facilitates the formation of clathrin-coated vesicles and subsequent interaction with multiple trafficking proteins leads to the formation of intracellular endosomes containing receptor.26, 42 Internalized GPCRs within endosomes may experience several fates: 1) they are recycled back to the plasma membrane to once again be available for stimulation by ligand; 2) they activate signaling pathways as signalosomes; or 3) they are targeted for lysosomal degradation.42 The data from our study suggest an additional fate should be considered, particularly under conditions of biomechanical stress: the trafficking of GPCRs into exosomes for release into the circulation. Thus, we propose the concept that the trafficking of AT1Rs should not be limited to the cell, but expanded to include the whole organism where the total reservoir of functional AT1Rs include both those in the cell and those circulating as exosomes. For the AT1R, this includes the β-arrestin2-dependent extrusion of functional receptors into exosomes from cardiomyocytes, which then circulate and are incorporated into endothelial and smooth muscle cells of resistance vessels and skeletal myocytes, and available to respond to neurohormonal stimulation.
The precise mechanism of how exosomes in the circulation cross the capillary endothelium to release their cargo into underlying cells is not clearly defined. Studies using fluorescently labeled ovalbumin within vesicles have suggested that an equilibrium between endocytosis and subsequent exocytosis occurs in the capillary endothelium. This process has been termed transcytosis whereby endothelial cells can endocytose proteins across the endothelium.43 Additionally, other mechanisms of intercellular communication have been described such as the formation of short membranous nanotubes that allow for the transfer of B cell antigen receptor to bystander B cells thereby increasing the pool of antigen presenting cells.44 Nonetheless, our data demonstrate the remote transfer of AT1Rs by means of exosomes from cardiomyocytes to endothelial and smooth muscle cells of mesenteric vessels. Although we cannot be certain of whether or not exosomes are really the mediator of AT1R density changes in cardiomyocytes of mice subjected to TAC in vivo since the limitation of our experiments.
Supplementary Material
Acknowledgments
We sincerely thank Dr. Thomas M. Coffman for kindly providing AT1R-KO mice and Dr. Sudha K. Shenoy for her gift of the YFP-tagged AT1R stable cells. We thank Dr. Julia Walker and Barbara Theriot for maintaining, backcrossing and providing the β-arrestin2Flox/Flox mice. We thank Dr. Robert J. Lefkowitz for his considerable support in the development of the β-arrestin2Flox/Flox mice. Requests for these mice should be addressed to R.J.L. or W.C.
Funding Sources: This work was supported by National Institutes of Health grants HL56687 and HL75443 to H.A.R.; the institutional T32 HL007101 to L.J.W.; Clinical Oncology Research Center Development Grant 5K12-CA100639-08 to M.C.; R01-CA172570 to W.C.
Footnotes
Disclosures: H.A.R. is a scientific cofounder for Trevena Inc., a company that is developing G protein-coupled receptor targeted drugs.
References
- 1.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res. 2014;114:325–332. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 4.Waldenstrom A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114:315–324. doi: 10.1161/CIRCRESAHA.114.300584. [DOI] [PubMed] [Google Scholar]
- 5.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Estelles A, Sperinde J, Roulon T, Aguilar B, Bonner C, LePecq JB, Delcayre A. Exosome nanovesicles displaying G protein-coupled receptors for drug discovery. Int J Nanomedicine. 2007;2:751–760. [PMC free article] [PubMed] [Google Scholar]
- 7.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mederos y, Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 10.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric modulation of beta-arrestin-biased angiotensin II type 1 receptor signaling by membrane stretch. J Biol Chem. 2014;289:28271–28283. doi: 10.1074/jbc.M114.585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 14.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 15.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strachan RT, Sun JP, Rominger DH, Violin JD, Ahn S, Rojas Bie Thomsen A, Zhu X, Kleist A, Costa T, Lefkowitz RJ. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR) J Biol Chem. 2014;289:14211–14224. doi: 10.1074/jbc.M114.548131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 19.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Hegde A, Choi YH, Theriot BS, Premont RT, Chen W, Walker JK. Genetic Deletion of beta-arrestin-2 Mitigates Established Airway Hyperresponsiveness in a Murine Asthma Model. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 23.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123:2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci U S A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: Regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. Epub 2014 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- 32.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. Epub 2014 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Momen-Heravi F, Balaj L, Alian S, Tigges J, Toxavidis V, Ericsson M, Distel RJ, Ivanov AR, Skog J, Kuo WP. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3:354. doi: 10.3389/fphys.2012.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 37.Little KM, Smalley DM, Harthun NL, Ley K. The plasma microparticle proteome. Semin Thromb Hemost. 2010;36:845–856. doi: 10.1055/s-0030-1267038. [DOI] [PubMed] [Google Scholar]
- 38.Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 40.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–1907. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 41.Guescini M, Leo G, Genedani S, Carone C, Pederzoli F, Ciruela F, Guidolin D, Stocchi V, Mantuano M, Borroto-Escuela DO, Fuxe K, Agnati LF. Microvesicle and tunneling nanotube mediated intercellular transfer of g-protein coupled receptors in cell cultures. Exp Cell Res. 2012;318:603–613. doi: 10.1016/j.yexcr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 43.Williams SK, Greener DA, Solenski NJ. Endocytosis and exocytosis of protein in capillary endothelium. J Cell Physiol. 1984;120:157–162. doi: 10.1002/jcp.1041200208. [DOI] [PubMed] [Google Scholar]
- 44.Quah BJ, Barlow VP, McPhun V, Matthaei KI, Hulett MD, Parish CR. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc Natl Acad Sci U S A. 2008;105:4259–4264. doi: 10.1073/pnas.0800259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.