Abstract
OBJECTIVE
Human herpesvirus 6 (HHV-6) is associated with a variety of complications in immunocompromised patients, but no studies have systematically and comprehensively assessed the impact of HHV-6 reactivation, and its interaction with cytomegalovirus (CMV), in intensive care unit (ICU) patients.
DESIGN
We prospectively assessed HHV-6 and CMV viremia by twice-weekly plasma PCR in a longitudinal cohort study of 115 adult, immunocompetent ICU patients. The association of HHV-6 and CMV reactivation with death or continued hospitalization by day 30 (primary endpoint) was assessed by multivariable logistic regression analyses.
SETTING
This study was performed in trauma, medical, surgical, and cardiac ICU’s at two separate hospitals of a large tertiary care academic medical center.
PATIENTS
A total of 115 CMV seropositive, immunocompetent adults with critical illness were enrolled in this study.
INTERVENTIONS
None.
MEASUREMENTS AND MAIN RESULTS
HHV-6 viremia occurred in 23% of patients at a median of 10 days. HHV-6B was the species detected in eight samples available for testing. Most patients with HHV-6 reactivation also reactivated CMV (70%). Severity of illness was not associated with viral reactivation. Mechanical ventilation, burn ICU, major infection, HHV-6 reactivation, and CMV reactivation were associated with the primary endpoint in unadjusted analyses. In a multivariable model adjusting for mechanical ventilation and ICU type, only co-reactivation of HHV-6 and CMV was significantly associated with the primary endpoint (adjusted odds ratio, 7.5; 95% CI, 1.9-29.9; p=0.005) compared to patients with only HHV-6, only CMV, or no viral reactivation.
CONCLUSIONS
Co-reactivation of both HHV-6 and CMV in ICU patients is associated with worse outcome than reactivation of either virus alone. Future studies should define the underlying mechanism(s) and determine whether prevention or treatment of viral reactivation improves clinical outcome.
Keywords: Herpes, cytomegalovirus, HHV-6, CMV, ICU, immunocompetent
INTRODUCTION
Human herpesvirus 6 (HHV-6) is a ubiquitous β-herpesvirus that infects the majority of the population and establishes life-long latency in a wide variety of host cells (1). Reactivation from latency rarely occurs in immunocompetent hosts but is common in immunocompromised patients, where it is associated with a variety of adverse events including fever, rash, central nervous system disease, graft rejection, pneumonitis, increased risk for infection, and increased all-cause mortality (2–5). Patients with critical illness can develop immunologic impairment that facilitates reactivation of latent herpes viruses (6). Cytomegalovirus (CMV), a closely related β-herpesvirus, has been shown to frequently reactivate in intensive care unit (ICU) patients and is associated with adverse outcomes (7–18). Multiple studies in transplant patients have demonstrated co-reactivation of HHV-6 and CMV (19, 20). However, no prospective studies have comprehensively evaluated the clinical impact of HHV-6, and its interaction with CMV, in critically ill ICU patients.
Two cross-sectional studies reported frequent HHV-6 reactivation in ICU patients (53%-54%) but did not find an independent association with adverse outcomes (21, 22). A recent prospective study assessing reactivation of multiple viruses in ICU patients also found frequent HHV-6 reactivation that was associated with longer ICU length of stay but not mortality (23). These three studies are the only reported investigations of HHV-6 in ICU patients to our knowledge and have important limitations that include study design, restrictive patient populations, qualitative or less sensitive PCR assays, use of cellular blood samples that may contain latent virus in lymphocytes (1), possible inclusion of patients with chromosomally integrated HHV-6, and lack of comprehensive multivariable statistical analyses to control for potential confounders. Only one study performed HHV-6 species typing and reported all reactivation events to be due to species A (21). Given that HHV-6A and B were recently classified as distinct species with unique biologic and epidemiologic characteristics (24), species classification is important for the study of these viruses.
To more accurately define the incidence, risk factors, characteristics, and significance of HHV-6 reactivation in a diverse population of critically ill adults, as well as the association of HHV-6 and CMV co-reactivation with clinically important outcomes, we tested for HHV-6 viremia in a previously characterized prospective cohort of CMV seropositive, immunocompetent adult ICU patients.
MATERIALS AND METHODS
Study design
We assessed HHV-6 viremia in twice-weekly collected plasma samples obtained from a previously characterized prospective cohort of 120 immunocompetent adults admitted to six ICUs (Burn [BICU], Cardiac Care [CICU], Medical [MICU], and Trauma [TICU]) at two hospitals of a large US tertiary care academic medical center between 2004 and 2006 (12). In the original cohort, twice weekly quantitative plasma PCR testing for CMV and prospective assessment of clinical endpoints were performed from time of admission to ICU until hospital discharge. Plasma samples were stored at −70°C for subsequent testing. Samples were available for HHV-6 testing in 115 patients for this secondary analysis.
Only patients who were newly admitted to the ICU from home or baseline residential setting were included. Patients underwent clinical assessments using standardized data collection forms. Clinical information was collected prospectively by study personnel who were blinded to HHV-6 and CMV results. Clinical teams caring for patients did not know HHV-6 or CMV PCR results; testing was performed after finalization of all clinical endpoints by the blinded data extractors. Patients were followed until death or hospital discharge. Deaths occurring within 90 days after discharge from the hospital were assessed using state and national death registry data. No CMV-specific therapy was given to study patients. The study was approved by the human subjects division at the University of Washington and written informed consent was obtained from study participants or their legally-authorized representatives.
Inclusion and exclusion criteria
See Supplementary Table 1 (12).
Definitions
See Supplementary Table 2 (12).
HHV-6 and CMV assays
DNA was extracted from plasma samples utilizing the QIAamp 96 DNA Blood Kit (Qiagen, Inc., Santa Clarita, CA). Detection of HHV-6 DNA was performed using a real-time quantitative fluorescent probe polymerase chain reaction (PCR) assay as previously described (25). Detection of 1 copy of HHV-6 DNA/reaction (25 copies/mL of plasma) was the lower limit of detection of our assay and considered a positive test. A highly conserved region of the U94 gene in HHV-6A and HHV-6B was amplified and used to distinguish species. Testing for chromosomally integrated HHV-6 was considered for patients with suggestive test results (viral load >3.5 log10 copies/mL or persistent levels >100 copies/mL in >80% of plasma samples) (26). CMV PCR test results from our original publication were used for this study, and testing was performed as previously described (27).
Statistical Analysis
Descriptive statistics for patient HHV-6 and CMV PCR results are presented using percentages or median and range values. Cumulative incidence estimates for reactivation of HHV-6 considered death or discharge from the hospital as competing risk events. First, we performed logistic regression analyses of risk factors for HHV-6 reactivation by considering baseline variables at time of ICU admission including age, race, unit, sex, Acute Physiology and Chronic Health Evaluation (APACHE) II score, blood transfusion, and ventilator use. A similar analysis was previously reported for CMV in the original cohort (12).
Next, we performed a univariate analysis of risk factors associated with a composite endpoint of continued hospitalization or death by day 30 (primary endpoint) using baseline and hospital stay variables that occurred prior to the endpoint. Our primary goal was to determine the association between viral reactivation and length of stay as an indirect metric for a clinically significant impact. Mortality was included in the endpoint to reduce the potential effect of early deaths on assessment of the relationship between viral reactivation and duration of hospitalization. Hospital variables consisted of HHV-6 or CMV DNA detection, occurrence of major infection, and number of hospital days during which blood transfusions or mechanical ventilation were administered. Proportion of days transfused or ventilated were calculated by dividing the sum of days a patient was transfused or ventilated by the total number of days of follow-up, up to a maximum of 30 days. We did not evaluate the quantitative association between viral load and the endpoint given that only six patients had HHV-6 viral load >1,000 copies/ml.
Multivariable models were built to assess the association of HHV-6 and CMV viremia with the primary endpoint, adjusting for baseline covariates (present at time of ICU admission). HHV-6 and CMV reactivation were modelled as dichotomous variables and coded as positive with any level of viral detection before the primary endpoint. Initial models were built examining the independent association of any HHV-6 or any CMV reactivation with the primary endpoint (HHV-6 and CMV covariates were not included in the same model in this analysis). Due to the high level of co-reactivation of these viruses (most patients with HHV-6 reactivation also had CMV reactivation), we fit a model with a combined covariate to assess the joint and separate effects of HHV-6 and CMV. This variable for HHV-6 and CMV reactivation had four levels: (1) patients with only HHV-6 and no CMV reactivation; (2) patients with only CMV and no HHV-6 reactivation; (3) patients with reactivation of both viruses; and (4) patients without reactivation of either virus. Since some of these categories had low numbers, we created an additional model that considered a variable for HHV-6 and CMV reactivation with only two levels: (1) patients with co-reactivation of both viruses and (2) patients with reactivation of only HHV-6, only CMV, or neither virus. Potential interaction was examined between HHV-6 and CMV.
Logistic regression models with odds ratios (OR) and corresponding 95% confidence intervals (CI) were used for these analyses. Risk factors that were significant at P < 0.1 in univariate analysis were entered into multivariable models that were limited to three or four clinically relevant factors due to the number of events or subjects. Statistical significance was defined as P <0.05. SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Study population
A total of 115 CMV seropositive adult ICU patients were included in the final analysis; five patients from the original cohort (12) did not have samples available for testing and were excluded. Characteristics of the study population are summarized in Table 1. The primary endpoint of continued hospitalization or death by 30 days occurred in 44 (38%) patients.
Table 1. Characteristics of the Study Population.
| Characteristic | All n = 115 | BICU n = 19 | CICU n = 19 | MICU n = 37 | TICU n = 40 |
|---|---|---|---|---|---|
| Age in years, median (range) | 52 (18-90) | 45 (19-80) | 59 (42-90) | 55 (19-80) | 42 (18-87) |
| Male Sex, n (%) | 71 (62) | 14 (74) | 12 (63) | 22 (60) | 23 (58) |
| Caucasian Race, n (%) | 92 (80) | 18 (95) | 14 (74) | 27 (73) | 33 (83) |
| APACHE II score, median (range) | 21 (7-36) | 20 (11-33) | 16 (7-34) | 28 (10-36) | 20 (11-30) |
| Transfusion within 24hr of admission, n (%) | 5 (4) | 0 (0) | 0 (0) | 2 (5) | 3 (8) |
| Ventilator use at admission, n (%) | 88 (77) | 16 (84) | 8 (42) | 26 (70) | 38 (95) |
| Major Infection, n (%) | 41 (36) | 15 (79) | 1 (5) | 11 (30) | 14 (35) |
| Hospital length of stay in days, median (range) |
17 (2-181) | 58 (8-181) | 6 (2-41) | 13 (4-94) | 18 (6-86) |
| ICU length of stay in days, median (range) | 11 (1-126) | 43 (8-126) | 5 (1-18) | 10 (3-55) | 10 (3-56) |
| Deceased by day 30 post-enrollment, n (%) | 10 (9) | 2 (11) | 5 (26) | 2 (5) | 1 (3) |
| Hospitalized at day 30 post-enrollment, n (%) | 34 (30) | 16 (84) | 1 (5) | 6 (16) | 11 (28) |
| In ICU at day 30 post-enrollment, n (%) | 20 (17) | 13 (68) | 0 (0) | 2 (5) | 5 (13) |
Characteristics of HHV-6 and CMV reactivation
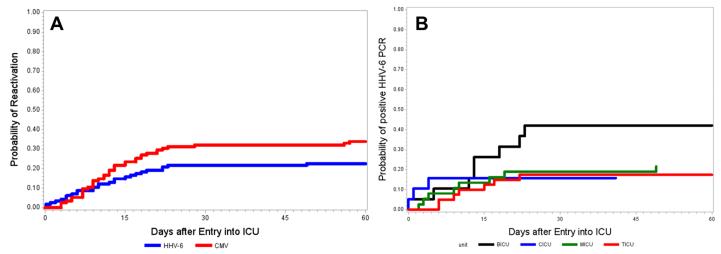
The characteristics of HHV-6 and CMV reactivation are displayed in Table 2. The cumulative incidence estimate of HHV-6 viremia at any level was 23% (27/115; 95% CI, 16-32) and occurred at a median of 10 days (range, 0-75 days). Although duration of consecutive positive tests for HHV-6 from the time of first detection was short (median, 1 day; range, 1-4) compared to CMV, 22% of these patients had subsequent detection of HHV-6 during the study period. When stratified by ICU, the cumulative incidence estimates of HHV-6 viremia at any level were 42% (95% CI, 20-67), 16% (95% CI, 3-40), 22% (95% CI, 10-38), and 20% (95% CI, 9-36) in the BICU, CICU, MICU, and TICU, respectively (Figure 1). Most patients with HHV-6 reactivation also reactivated CMV (70%), and 49% with CMV reactivation also reactivated HHV-6 (Figure 2). Time to first detection of HHV-6 and CMV was similar (Table 2). Only 5% of patients had HHV-6 viral load >1,000 copies/ml. HHV-6 species typing was performed in eight patients and all had species B. No patients had virologic findings suggestive of chromosomally integrated HHV-6 based on previously published criteria (26).
Table 2. Incidence and Quantitation of HHV-6 and CMV Viremia.
| All ICUs n = 115 | ||
|---|---|---|
|
| ||
| Variable | HHV-6 | CMV |
| Viremia at any level, n (%) | 27 (23) | 39 (34) |
| Viremia >1000 copies/ml, n (%) | 6 (5) | 24 (21) |
| Maximum viral load (log10 PCR copies), median (range) | 2.4 (1.4-4.1) | 3.3 (1.8-5.5) |
| Days to first detectable viremia, median (range) | 10 (0-75)a | 12 (3-57) |
| Duration of viremia in days, median (range)b | 1 (1-4) | 14 (1-38) |
| Reactivation by ICU, n (%)c | ||
| BICU | 8 (40) | 11 (55) |
| CICU | 3 (15) | 3 (15) |
| MICU | 8 (20) | 10 (25) |
| TICU | 8 (20) | 15 (38) |
Two patients had HHV-6 detected on the day of admission to the hospital.
22% of patients had subsequent detection of HHV-6 after the initial period of detection
Number of reactivation events per ICU
Figure 1.
Cumulative incidence of (A) any HHV-6 or CMV reactivation in the entire cohort, and (B) HHV-6 reactivation stratified by ICU. Abbreviations: BICU, burn intensive care unit; CICU, cardiac intensive care unit; MICU, medical intensive care unit; TICU, thoracic intensive care unit.
Figure 2.
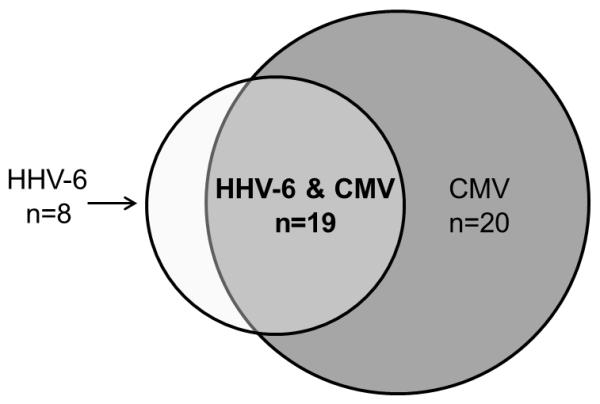
Comparison of isolated HHV-6 reactivation, isolated CMV reactivation, and co-reactivation of both HHV-6 and CMV.
The primary endpoint occurred in 50% of patients with isolated HHV-6, 45% of patients with isolated CMV, and 76% of patients with HHV-6 and CMV.
Risk factors for HHV-6 reactivation
Univariate logistic regression analysis of baseline characteristics identified only male sex as a significant risk factor for HHV-6 reactivation (OR, 3.5; 95% CI, 1.2-10.1; p=0.02; data not shown), as was previously shown for CMV reactivation. APACHE II score at admission was not associated with either HHV-6 or CMV reactivation. No other variables met criteria for inclusion into a multivariable model.
Risk factors for death or continued hospitalization
Descriptively, the primary composite endpoint of death or continued hospitalization by 30 days occurred in 50% of patients who reactivated only HHV-6, 45% of patients who reactivated only CMV, 76% of patients who reactivated both HHV-6 and CMV, and 41% of those who never reactivated either virus. Only HHV-6 and/or CMV reactivation prior to the endpoint was considered in these analyses. A univariate analysis examining associations of baseline and hospital stay variables with the primary endpoint showed significantly elevated risks for subjects with BICU admission (OR, 48.4; 95% CI, 6.2-381.1; p<0.001), mechanical ventilation at baseline (OR, 4.8; 95% CI, 1.5-15; p=0.007), development of a major infection (OR, 2.4; 95% CI, 1.0-5.4; p=0.04), HHV-6 viremia at any level (OR, 5.0; 95% CI, 1.9-12.9; p=0.001), and CMV viremia at any level (OR, 3.7; 95% CI, 1.6-8.5; p=0.002) (Table 3).
Table 3. Risk Factors for Continued Hospitalization or Death at 30 Days After Admission to ICU. Odds Ratio (OR) and 95% Confidence Intervals (CI) Estimated by Logistic Regression Models.
| Comparison | Univariate OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|---|
| Baseline Variables | |||||
| Age | 10-year increments | 1.1 (0.9-1.3) | 0.52 | -- | -- |
| Unit | Other | 1.0 (ref) | 1.0 | ||
| BICU | 48.4 (6.2-381.1) | <0.001 | 66.0 (7.1-613.9)a | <0.001 | |
| Race | Caucasian | 1.0 | |||
| Other | .4 (0.1-1.1) | 0.08 | -- | -- | |
| Sex | Female | 1.0 | |||
| Male | 1.0 (0.5-2.2) | 0.95 | -- | -- | |
| APACHE II score quartile | <16 | 1.0 | |||
| ≥16, <21 | .9 (0.3-3.5) | 0.94 | -- | -- | |
| ≥21, <27.5 | 1.0 (0.3-3.8) | 0.99 | -- | -- | |
| ≥ 27.5 | 1.5 (0.4-5.4) | 0.54 | -- | -- | |
| Transfusion | No | 1.0 | |||
| Yes | 2.5 (0.4-15.7) | 0.32 | -- | -- | |
| Mechanical Ventilation | No | 1.0 | 1.0 | ||
| Yes | 4.8 (1.5-15.0) | 0.007 | 7.5 (1.5-36.2)b | 0.01 | |
| Hospital Stay Variables | |||||
| Major infection | No | 1.0 | 1.0 | ||
| Yes | 2.4 (1.0-5.4) | 0.04 | 2.1 (0.8-5.6)c | 0.14 | |
| Proportion of transfusion days | 10% increments | 1.2 (0.8-1.7) | 0.43 | -- | -- |
| Proportion of ventilator days | 10% increments | 1.3 (1.0-1.7) | 0.02 | 1.6 (1.1-2.2) c | 0.01 |
| HHV-6 viremia at any level | No | 1.0 | 1.0 | ||
| Yes | 5.0 (1.9-12.9) | 0.001 | 3.5 (1.2-10.3) c | 0.03 | |
| CMV viremia at any level | No | 1.0 | 1.0 | ||
| Yes | 3.7 (1.6-8.5) | 0.002 | 3.4 (1.3-8.9) c | 0.01 | |
Adjusted for mechanical ventilation at baseline
Adjusted for ICU type
Adjusted for baseline variables of mechanical ventilation and ICU type
In a multivariable logistic regression model adjusting for ICU type and mechanical ventilation at baseline, HHV-6 reactivation at any level, without adjustment for CMV, was associated with the primary endpoint (adjusted [a]OR, 3.5; 95% CI, 1.2-10.3; p=0.03; Table 3). Similarly, CMV reactivation at any level, without adjustment for HHV-6, was associated with the primary endpoint as we previously demonstrated (aOR, 3.4; 95% CI, 1.3-8.9; p=0.01).
However, after adjusting for HHV-6 reactivation, any CMV reactivation was no longer significantly associated with the endpoint (data not shown). Interaction between CMV reactivation and HHV-6 reactivation was not significant (p=0.30). Since a large proportion of subjects reactivated both HHV-6 and CMV (Figure 2), we used two approaches to better understand the associations of each virus vs. combined viruses with the primary endpoint. First, we used a model incorporating a viral reactivation variable stratified into four levels, including: (1) only HHV-6 reactivation (n=8); (2) only CMV reactivation (n=20); (3) co-reactivation of both viruses (n=19); (4) or reactivation of neither virus (n=68). In this model, only co-reactivation of both viruses was significantly associated with the primary endpoint (aOR, 7.5; 95% CI, 1.9-29.9; p=0.005; Table 4). Second, since there were relatively small numbers in some levels of this model, we also created a model using a viral reactivation variable with only two levels: (1) patients with co-reactivation of HHV-6 and CMV and (2) a combined category that included patients with reactivation of only HHV-6, only CMV, or neither virus. This model also showed a significant association of co-reactivation of HHV-6 and CMV with the primary endpoint (aOR, 6.5; 95% CI, 1.7-24.7; p=0.006; Table 4) compared to patients with only HHV-6, only CMV, or no viral reactivation.
Table 4.
Results of Two Multivariable Logistic Regression Models Evaluating HHV-6 and CMV Reactivation as Risk Factors for Continued Hospitalization or Death at 30 Days After Admission to ICU.
| Variable | Adjusted OR (95% CI) | P value |
|---|---|---|
| Model 1a | ||
| Neither virus | 1.0 (ref) | |
| HHV-6 only | 1.1 (0.2-7.4) | 0.9 |
| CMV only | 1.8 (0.5-6.4) | 0.3 |
| HHV-6 and CMV | 7.5 (1.9-29.9) | 0.005 |
| Model 2a | ||
| HHV-6 only or CMV only or neither virus |
1.0 (ref) | |
| HHV-6 and CMV | 6.5 (1.7-24.7) | 0.006 |
Adjusted for baseline variables of mechanical ventilation and ICU type, which retained significance in both models.
DISCUSSION
In this prospective study of a diverse cohort of immunocompetent ICU patients, we demonstrate frequent HHV-6 reactivation and the novel finding that patients with co-reactivation of both HHV-6 and CMV have the greatest risk for death or continued hospitalization by day 30. Eight tested samples were HHV-6B, which is the species identified in the majority of reactivation events in immunocompromised patients (1, 3). Co-reactivation of CMV occurred in the majority of patients with HHV-6 reactivation. This updated analysis of our initial study, which showed an association of CMV reactivation with prolonged hospitalization or death (12), advances understanding of particularly high-risk patient groups by considering HHV-6 in the models. Increased rates of adverse events in patients with co-reactivation of HHV-6 and CMV have been reported in other settings and are speculated to be due to mechanistic interactions between the two viruses (28, 29).
There are several biologically plausible mechanisms to explain frequent co-reactivation of HHV-6 and CMV and a possible causal association with prolonged hospitalization or death. One of the original descriptions of concurrent detection of multiple herpesviruses was reported in 1979 for CMV and EBV in healthy patients with mononucleosis syndromes (30). The authors postulated that depressed cellular immunity due to infection with one virus, as well as non-specificity of viral DNA polymerase activity, might facilitate reactivation of the other. Similarly, HHV-6 has been associated with an increased risk for CMV reactivation (3) and clinically manifest CMV disease (31). HHV-6 and CMV are closely related β-herpesviruses that can cause disease of a variety of organ systems in immunocompetent and immunocompromised patients. Direct virally-mediated tissue injury (e.g. pneumonia) (32) and immunomodulatory effects leading to an increased risk for secondary infections (33, 34) are some mechanisms through which CMV could mediate worse clinical outcomes in ICU patients. Similar mechanisms are possible for HHV-6, which has also been associated with lung injury (4, 5) and immune dysfunction (33, 34) in immunocompromised patients. HHV-6 might also contribute to ICU delirium, which has been associated with prolonged ICU length of stay and overall hospitalization, as well as long-term cognitive impairment and increased mortality (35, 36). This potential mechanistic link is supported by results from a prospective trial in hematopoietic cell transplantation (HCT) recipients showing an independent association between HHV-6 reactivation and delirium (37). The cognitive deficits and pathologic changes seen in ICU patients with delirium are similar to the manifestations of HHV-6-associated central nervous system disease in HCT recipients (1, 38). Since HHV-6 and CMV each have similar pathogenic effects, it follows that co-reactivation of both viruses might be associated with worse outcomes than reactivation of either virus alone, as demonstrated in this study. Testing for viral detection in other compartments (e.g. bronchoalveolar lavage fluid, cerebrospinal fluid) may be important in future studies.
This study has several strengths including the prospective design, inclusion of diverse ICU populations, use of a sensitive and quantitative PCR method, use of plasma to avoid detection of latent virus, species typing, exclusion of chromosomally integrated HHV-6, blinded clinical endpoints to minimize bias, and comprehensive statistical analyses with an adequate number of clinically relevant endpoints. The primary endpoint considered events occurring up to 30 days after ICU admission because all patients had equal follow-up assessments for viral reactivation during this period, and it took into consideration a biologically-relevant time-lag for HHV-6 or CMV effects.Furthermore, establishing a discrete endpoint of 30 days minimized a potential spurious association between viral reactivation and increased length of stay as a consequence of increased opportunity for detection in people with longer hospitalizations. The study also has potential limitations. Although >98% of people are seropositive for HHV-6 (1), only CMV-seropositive patients were included; however, this still captured 65% of all screened patients (data not shown). Future studies could focus on CMV seronegative patients to better understand the impact of HHV-6 in the absence of CMV. Given the lack of an international standard for HHV-6 viral DNA detection, extrapolation of viral load data remains a limitation in all studies of HHV-6. It is possible that some patients had late undetected HHV-6 reactivation since we did not monitor patients after hospital discharge. Although this would not affect our results, it would mitigate our ability to ascribe a biologically significant impact of HHV-6 viremia in this study. However, we think this is unlikely, as HHV-6 viremia is uncommon outside the setting of critical illness or immunosuppression (21, 23, 39), and the vast majority of first reactivation events occurred before day 30. Lastly, we recognize the heterogeneity in the ICU patients included in this study, and particularly the BICU patients, who have longer length of stays and may represent a unique population. However, we were unable to evaluate the BICU patients separately due to sample size.
Our data should be interpreted in the context of the study design and are hypothesis generating. We are careful to emphasize that it is not possible to infer a causal link between HHV-6 and CMV co-reactivation and the composite endpoint of this study. Although reactivation of herpesviruses in ICU patients has been hypothesized to represent a marker of illness severity, we did not find an association between at least one widely used marker for illness severity (Apache II score) and reactivation of either HHV-6 or CMV, suggesting that factors beyond illness severity might be important. Given that additional viruses may reactivate in critically ill patients (23) and the association of viral co-reactivation with adverse clinical outcomes reported here, future studies in larger CMV seropositive and seronegative ICU cohorts should be performed to define the mechanism(s) underlying this relationship and to determine if prevention or treatment of reactivating viruses leads to improved outcomes.
CONCLUSION
In summary, HHV-6 reactivation occurs frequently in immunocompetent ICU patients, often concurrently with CMV, and co-reactivation of both viruses is independently associated with worse outcomes compared to no viral reactivation or reactivation of either virus alone. HHV-6B appeared to be the predominant species in our cohort. Although there are biologically plausible mechanisms by which viral reactivation could contribute to morbidity and/or mortality, randomized controlled treatment or prevention trials using agents active against both HHV-6 and CMV are needed to explore a causal link between viral reactivation and adverse outcomes in ICU patients.
Supplementary Material
Key Points.
This prospective study demonstrates that HHV-6 reactivation occurs frequently in immunocompetent intensive care unit patients and primarily among those with CMV reactivation. Co-reactivation of both viruses has greater association with worse clinical outcome than reactivation of either virus alone.
Acknowledgements
We gratefully acknowledge the expert research coordination of Lucretia Granger, Carol Eubanks, and Sarah W. Johnson.
Financial Support: This work was supported by the National Institutes of Health [grant numbers 1U01HL102547-01 (to MB, APL), K24 HL093294 (to MB)], and an unrestricted investigator-initiated study grant from Roche (APL).
Copyright form disclosures:
Dr. Hill received grant support from the National Institutes of Health (NIH) K23. His institution received grant support from Roche (Unrestricted investigator-initiated grant). Dr. Leisenring received support for article research from the NIH. Her institution received grant support from Roche (Unrestricted investigator-initiated grant), the NIH, and Merck. Dr. Jerome lectured for Roche (Lecture Honorarium). His institution received grant support from Roche (Unrestricted investigator-initiated grant). Dr. Boeckh consulted for Roche/Genentech, Chimerix Inc, Clinigen, Merck, and Astellas. His institution received grant support from Roche/Genentech (Unrestricted investigator-initiated grant). Dr. Limaye received support for article research from the NIH. His institution received grant support from Roche (Unrestricted investigator-initiated grant). Dr. Roa, Kirby , Huang, and Santo’s institutions received grant support from Roche (Unrestricted investigator-initiated grant).
Footnotes
Author Contributions: AL had full access to the data. The study was designed jointly by AL and MB. AL was responsible for patient recruitment and data collection. MH, TS, and KJ performed the HHV-6 and CMV PCR assays. KK, WL, JHill, PR, AL, and MB analyzed the data. The paper was drafted by PR and JH with input from all other authors. All authors reviewed and approved the final version.
Conflicts of interest: P. L. R. has a Río Hortega contract (CM11/00142) from the Instituto de Salud Carlos III-FIS. M. B. has served as a consultant and has received research support from Chimerix Inc., Astellas, Genentech/Roche, Merck and Gilead in addition to consulting for Clinigen and receiving research support from Viropharma. A.P. L. has served as a consultant and has received Research support from Viropharma, Genentech, and Astellas. W.ML has received research support from Merck. All other authors report no potential conflicts.
Presented in abstract form at IDWeek 2014, Philadelphia, PA. 2014: Abstract 45349.
References
- 1.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217–45. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill JA, Zerr DM. Roseoloviruses in transplant recipients: clinical consequences and prospects for treatment and prevention trials. Curr Opin Virol. 2014 doi: 10.1016/j.coviro.2014.09.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerr DM, Boeckh M, Delaney C, et al. HHV-6 Reactivation and Associated Sequelae after Hematopoietic Cell Transplant. Biol Blood Marrow Transplant. 2012;18:1700–1708. doi: 10.1016/j.bbmt.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchbinder S, Elmaagacli AH, Schaefer UW, et al. Human herpesvirus 6 is an important pathogen in infectious lung disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;26:639–44. doi: 10.1038/sj.bmt.1702569. [DOI] [PubMed] [Google Scholar]
- 5.Carrigan DR, Drobyski WR, Russler SK, et al. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991;338:147–9. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- 6.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heininger A, Jahn G, Engel C, et al. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29:541–7. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Heininger A, Haeberle H, Fischer I, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care. 2011;15:R77. doi: 10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook CH, Martin LC, Yenchar JK, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003;31:1923–9. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- 10.Von Müller L, Klemm A, Weiss M, et al. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006;12:1517–22. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaber S, Chanques G, Borry J, et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127:233–41. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 12.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–22. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemann M, Sedemund-Adib B, Reiland P, et al. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. 2008;36:3145–50. doi: 10.1097/CCM.0b013e31818f3fc4. [DOI] [PubMed] [Google Scholar]
- 14.Chiche L, Forel J-M, Roch A, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37:1850–7. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 15.Chilet M, Aguilar G, Benet I, et al. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. J Med Virol. 2010;82:1384–91. doi: 10.1002/jmv.21825. [DOI] [PubMed] [Google Scholar]
- 16.De Vlieger G, Meersseman W, Lagrou K, et al. Cytomegalovirus serostatus and outcome in nonimmunocompromised critically ill patients. Crit Care Med. 2012;40:36–42. doi: 10.1097/CCM.0b013e31822b50ae. [DOI] [PubMed] [Google Scholar]
- 17.Osawa R, Singh N. Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care. 2009;13:R68. doi: 10.1186/cc7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009;37:2350–8. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- 19.DesJardin J a, Gibbons L, Cho E, et al. Human herpesvirus 6 reactivation is associated with cytomegalovirus infection and syndromes in kidney transplant recipients at risk for primary cytomegalovirus infection. J Infect Dis. 1998;178:1783–6. doi: 10.1086/314510. [DOI] [PubMed] [Google Scholar]
- 20.Van Leer-Buter CC, Sanders JSF, Vroom HEJ, et al. Human herpesvirus-6 DNAemia is a sign of impending primary CMV infection in CMV sero-discordant renal transplantations. J Clin Virol. 2013 doi: 10.1016/j.jcv.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Razonable RR, Fanning C, Brown RA, et al. Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts. J Infect Dis. 2002;185:110–3. doi: 10.1086/324772. [DOI] [PubMed] [Google Scholar]
- 22.Desachy a, Ranger-Rogez S, François B, et al. Reactivation of human herpesvirus type 6 in multiple organ failure syndrome. Clin Infect Dis. 2001;32:197, 203. doi: 10.1086/318474. [DOI] [PubMed] [Google Scholar]
- 23.Walton AH, Muenzer JT, Rasche D, et al. Reactivation of Multiple Viruses in Patients with Sepsis. PLoS One. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159:863–70. doi: 10.1007/s00705-013-1902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zerr DM, Gupta D, Huang M-L, et al. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:309–17. doi: 10.1086/338044. [DOI] [PubMed] [Google Scholar]
- 26.Potenza L, Barozzi P, Masetti M, et al. Prevalence of human herpesvirus-6 chromosomal integration (CIHHV-6) in Italian solid organ and allogeneic stem cell transplant patients. Am J Transplant. 2009;9:1690–7. doi: 10.1111/j.1600-6143.2009.02685.x. [DOI] [PubMed] [Google Scholar]
- 27.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–8. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratnamohan VM, Chapman J, Howse H, et al. Cytomegalovirus and human herpesvirus 6 both cause viral disease after renal transplantation. Transplantation. 1998;66:877–82. doi: 10.1097/00007890-199810150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Loutfy SA, Fawzy M, El-Wakil M, et al. Presence of human herpes virus 6 (HHV6) in pediatric lymphomas: impact on clinical course and association with cytomegalovirus infection. Virol J. 2010;7:287. doi: 10.1186/1743-422X-7-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemon SM, Hutt LM, Huang YT, et al. Simultaneous infection with multiple herpesviruses. Am J Med. 1979;66:270–6. doi: 10.1016/0002-9343(79)90544-8. [DOI] [PubMed] [Google Scholar]
- 31.Humar A, Malkan G, Moussa G, et al. Human herpesvirus-6 is associated with cytomegalovirus reactivation in liver transplant recipients. J Infect Dis. 2000;181:1450–3. doi: 10.1086/315391. [DOI] [PubMed] [Google Scholar]
- 32.Travi G, Pergam SA. Cytomegalovirus Pneumonia in Hematopoietic Stem Cell Recipients. J Intensive Care Med. 2013;29:200–212. doi: 10.1177/0885066613476454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeckh M, Nichols WG. Immunosuppressive effects of beta-herpesviruses. Herpes. 2003;10:12–6. [PubMed] [Google Scholar]
- 34.Humar A, Kumar D, Caliendo AM, et al. Clinical impact of human herpesvirus 6 infection after liver transplantation. Transplantation. 2002;73:599–604. doi: 10.1097/00007890-200202270-00021. [DOI] [PubMed] [Google Scholar]
- 35.Pandharipande PP, Girard TD, Jackson JC, et al. Long-Term Cognitive Impairment after Critical Illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomason JWW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–81. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zerr DM, Fann JR, Breiger D, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117:5243–9. doi: 10.1182/blood-2010-10-316083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol. 2006;37(Suppl 1):S52–6. doi: 10.1016/S1386-6532(06)70012-9. [DOI] [PubMed] [Google Scholar]
- 39.Huang LM, Kuo PF, Lee CY, et al. Detection of human herpesvirus-6 DNA by polymerase chain reaction in serum or plasma. J Med Virol. 1992;38:7–10. doi: 10.1002/jmv.1890380103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.